Gemcitabine–Paclitaxel Chemotherapy for Patients with Advanced Urothelial Cancer Refractory to Cisplatin-Based Chemotherapy: Predictive Role of PGK1 for Treatment Response to Cytotoxic Chemotherapy
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics
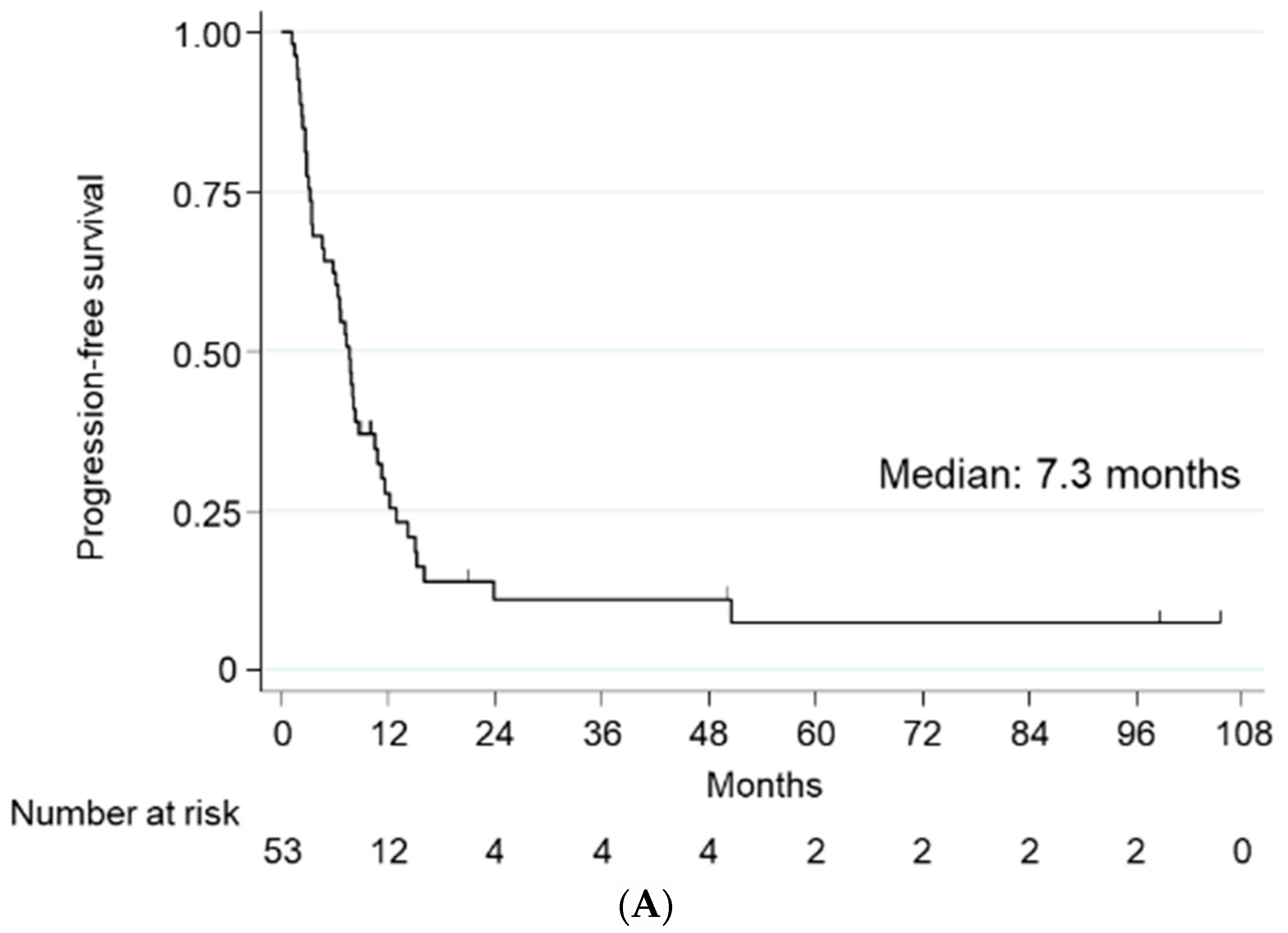
2.2. Treatment Efficacy
2.3. Adverse Events
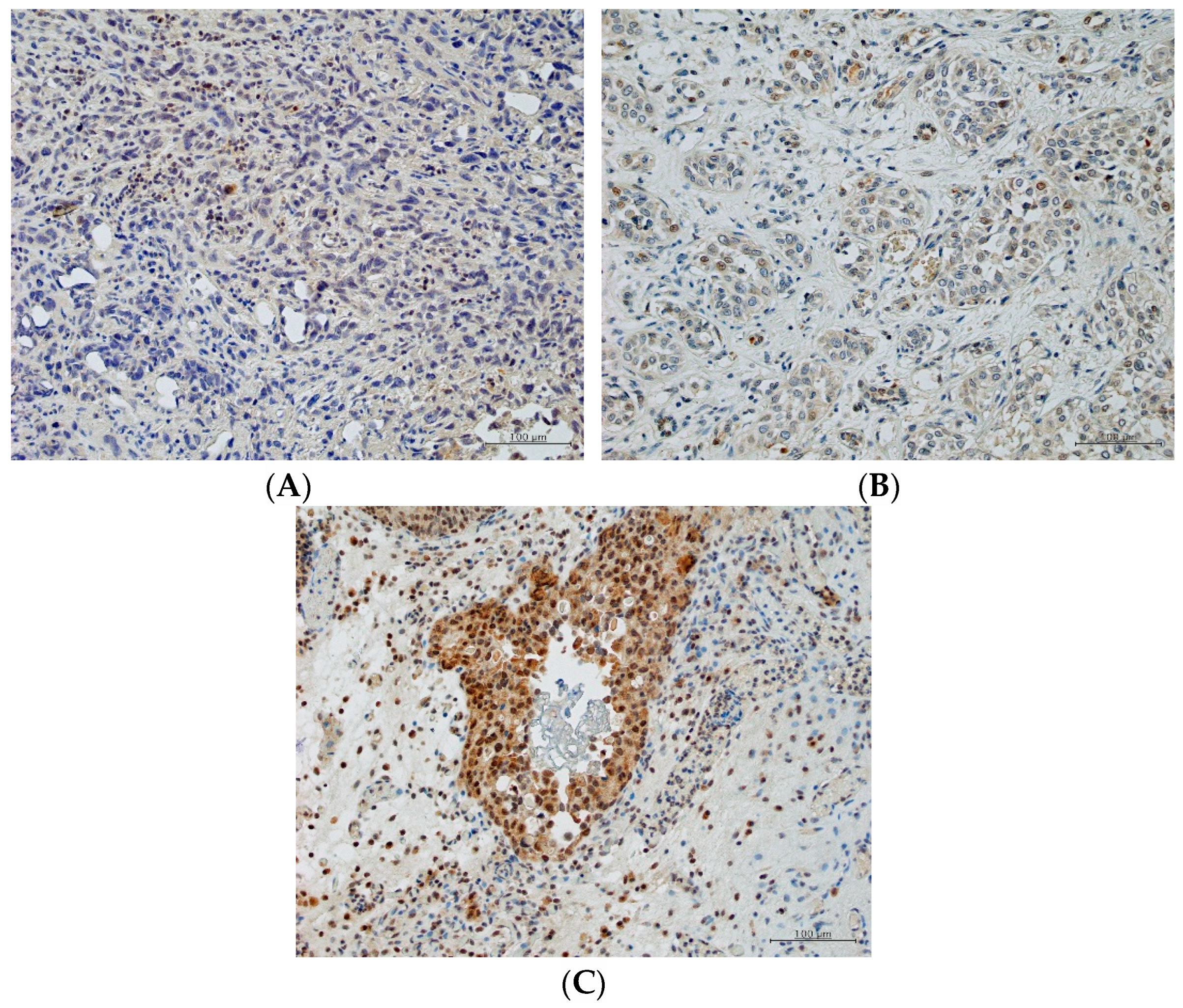
2.4. Predictive Role of PGK1
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Chemotherapy Regimen
4.3. Treatment Evaluation
4.4. Immunohistochemistry and Scoring of PGK1
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dougherty, D.W.; Gonsorcik, V.K.; Harpster, L.E.; Trussell, J.C.; Drabick, J.J. Superficial bladder cancer metastatic to the lungs: Two case reports and review of the literature. Urology 2009, 73, 210.E3–210.E5. [Google Scholar] [CrossRef] [PubMed]
- Margulis, V.; Shariat, S.F.; Matin, S.F.; Kamat, A.M.; Zigeuner, R.; Kikuchi, E.; Lotan, Y.; Weizer, A.; Raman, J.D.; Wood, C.G. Outcomes of radical nephroureterectomy: A series from the Upper Tract Urothelial Carcinoma Collaboration. Cancer 2009, 115, 1224. [Google Scholar] [CrossRef] [PubMed]
- Svatek, R.S.; Siefker-Radtke, A.; Dinney, C.P. Management of metastatic urothelial cancer: The role of surgery as an adjunct to chemotherapy. Can. Urol. Assoc. J. 2009, 3, S228–S231. [Google Scholar] [CrossRef] [PubMed]
- von der Maase, H.; Hansen, S.W.; Roberts, J.T.; Dogliotti, L.; Oliver, T.; Moore, M.J.; Bodrogi, I.; Albers, P.; Knuth, A.; Lippert, C.M.; et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin and cisplatin in advanced or metastatic bladder cancer: Results of a large, randomized, multinational, multicenter, phase III study. J. Clin. Oncol. 2000, 18, 3068–3077. [Google Scholar] [CrossRef]
- von der Maase, H.; Hansen, S.W.; Roberts, J.T.; Dogliotti, L.; Oliver, T.; Moore, M.J.; Bodrogi, I.; Albers, P.; Knuth, A.; Lippert, C.M.; et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N. Engl. J. Med. 2017, 376, 1015–1026. [Google Scholar]
- Jiang, Y.; Zhan, H. Communication between EMT and PD-L1 signaling: New insights into tumor immune evasion. Cancer Lett. 2019, 468, 72–81. [Google Scholar] [CrossRef]
- Meluch, A.A.; Greco, F.A.; Burris, H.A.; O’Rourke, T.; Ortega, G.; Steis, R.G.; Morrissey, L.H.; Johnson, V.; Hainsworth, J.D. Paclitaxel and gemcitabine chemotherapy for advanced transitional-cell carcinoma of the urothelial tract: A phase II trial of the Minnie pearl cancer research network. J. Clin. Oncol. 2001, 15, 3018–3024. [Google Scholar] [CrossRef]
- Matsumoto, K.; Irie, A.; Satoh, T.; Okazaki, M.; Iwamura, M.; Baba, S. Gemcitabine and paclitaxel chemotherapy as a second-line treatment for advanced or metastatic urothelial carcinoma. Int. J. Urol. 2007, 14, 1000–1004. [Google Scholar] [CrossRef]
- Ikeda, M.; Matsumoto, K.; Tabata, K.; Minamida, S.; Fujita, T.; Satoh, T.; Iwamura, M.; Baba, S. Combination of gemcitabine and paclitaxel is a favorable option for patients with advanced or metastatic urothelial carcinoma previously treated with cisplatin-based chemotherapy. Jpn. J. Clin. Oncol. 2011, 41, 1214–1220. [Google Scholar] [CrossRef]
- Sternberg, C.N.; Calabro, F.; Pizzocaro, G.; Marini, L.; Schnetzer, S.; Sella, A. Chemotherapy with an every-2-week regimen of gemcitabine and paclitaxel in patients with transitional cell carcinoma who have received prior cisplatinbased therapy. Cancer 2001, 92, 2993–2998. [Google Scholar] [CrossRef]
- Suyama, T.; Ueda, T.; Fukasawa, S.; Imamura, Y.; Nakamura, K.; Miyasaka, K.; Sazuka, T.; Egoshi, K.; Nihei, N.; Hamano, M.; et al. Combination of gemcitabine and paclitaxel as second-line chemotherapy for advanced urothelial carcinoma. Jpn. J. Clin. Oncol. 2009, 39, 244–250. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kanai, K.; Kikuchi, E.; Ohigashi, T.; Miyajima, K.; Nakagawa, K.; Nakashima, J.; Oya, M. Gemcitabine and paclitaxel chemotherapy for advanced urothelial carcinoma in patients who have received prior cisplatin-based chemotherapy. Int. J. Clin. Oncol. 2008, 13, 510–514. [Google Scholar] [CrossRef] [PubMed]
- Harada, M.; Tomisaki, I.; Minato, A.; Onishi, R.; Terado, M.; Inatomi, H.; Fujimoto, N. Combination therapy with paclitaxel and gemcitabine after platinum-based chemotherapy in patients with advanced urothelial cancer. Int. J. Urol. 2021, 28, 970–974. [Google Scholar] [CrossRef] [PubMed]
- Gong, F.; Peng, X.; Zeng, Z.; Yu, M.; Zhao, Y.; Tong, A. Proteomic analysis of cisplatin resistance in human ovarian cancer using 2-DE method. Mol. Cell Biochem. 2011, 348, 141–147. [Google Scholar] [CrossRef]
- Taoka, Y.; Matsumoto, K.; Ohashi, K.; Minamida, S.; Hagiwara, M.; Nagi, S.; Saito, T.; Kodera, Y.; Iwamura, M. Protein expression profile related to cisplatin resistance in bladder cancer cell lines detected by two-dimensional gel electrophoresis. Biomed. Res. 2015, 36, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.F.; Chuang, H.W.; Kuo, W.T.; Lin, B.S.; Chang, Y.C. Current development and application of anaerobic glycolytic enzymes in urothelial cancer. Int. J. Mol. Sci. 2021, 22, 10612. [Google Scholar] [CrossRef] [PubMed]
- Shao, F.; Yang, X.; Wang, W.; Wang, J.; Guo, W.; Feng, X.; Shi, S.; Xue, Q.; Gao, S.; Gao, Y.; et al. Associations of PGK1 promoter hypomethylation and PGK1-mediated PDHK1 phosphorylation with cancer stage and prognosis: A TCGA pan-cancer analysis. Cancer Commun. 2019, 39, 54. [Google Scholar] [CrossRef]
- Perez-Gracia, J.L.; Loriot, Y.; Rosenberg, J.E.; Powles, T.; Necchi, A.; Hussain, S.A.; Morales-Barrera, R.; Retz, M.M.; Niegisch, G.; Durán, I.; et al. Atezolizumab in platinum-treated locally advanced or metastatic urothelial carcinoma: Outcomes by prior number of regimens. Eur. Urol. 2018, 73, 462–468. [Google Scholar] [CrossRef]
- Bersanelli, M.; Buti, S.; Cortellini, A.; Bandini, M.; Banna, G.L.; Pederzoli, F.; Farè, E.; Raggi, D.; Giannatempo, P.; De Giorgi, U.; et al. Clinical outcomes of patients with metastatic urothelial carcinoma after progression to immune checkpoint inhibitors: A retrospective analysis by the Meet-Uro Group (Meet-URO 1 Study). Clin. Med. Insights Oncol. 2021, 8, 11795549211021667. [Google Scholar] [CrossRef]
- Curran, C.; Adib, E.; Kazakova, V.; Grivas, P.; Diamantopoulos, L.N.; Alva, A.S.; Su, C.; Jain, R.K.; Tandon, A.; Necchi, A.; et al. Outcomes of metastatic urothelial carcinoma following discontinuation of enfortumab vedotin. Clin. Genitourin. Cancer 2022, 20, 11–16. [Google Scholar] [CrossRef]
- Muramaki, M.; So, A.; Hayashi, N.; Sowery, R.; Miyake, H.; Fujisawa, M.; Gleave, M.E. Chemosensitization of gemcitabine-resistant human bladder cancer cell line both in vitro and in vivo using antisense oligonucleotide targeting the anti-apoptotic gene, clusterin. BJU Int. 2009, 103, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Rosenberg, J.E.; Sonpavde, G.P.; Loriot, Y.; Durán, I.; Lee, J.L.; Matsubara, N.; Vulsteke, C.; Castellano, D.; Wu, C.; et al. Enfortumab Vedotin in previously treated advanced urothelial carcinoma. N. Engl. J. Med. 2021, 25, 1125–1135. [Google Scholar] [CrossRef]
- Zuazo, M.; Arasanz, H.; Fernández-Hinojal, G.; García-Granda, M.J.; Gato, M.; Bocanegra, A.; Martínez, M.; Hernández, B.; Teijeira, L.; Morilla, I.; et al. Functional systemic CD4 immunity is required for clinical responses to PD-L1/PD-1 blockade therapy. EMBO Mol. Med. 2019, 11, e10293. [Google Scholar] [CrossRef] [PubMed]
- De Palma, M.; Jain, R.K. CD4+ T Cell activation and vascular normalization: Two sides of the same coin? Immunity 2017, 46, 773–775. [Google Scholar] [CrossRef] [PubMed]
- Lepleux, C.; Abeilard-Lemoisson, E.; Duval, M.; Icard, P.; Lincet, H. siPGK1 sensitizes chemoresistant human ovarian cancer cell lines to cisplatin. Anticancer Res. 2012, 32, 4277–4286. [Google Scholar] [PubMed]
- Gou, R.; Hu, Y.; Liu, O.; Dong, H.; Gao, L.; Wang, S.; Zheng, M.; Li, X.; Lin, B. PGK1 is a key target for anti-glycolytic therapy of ovarian cancer: Based on the comprehensive analysis of glycolysis-related genes. Front Oncol. 2021, 11, 682461. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Qian, X.; Jiang, H.; Xia, Y.; Zheng, Y.; Li, J.; Huang, B.J.; Fang, J.; Qian, C.N.; Jiang, T.; et al. Nuclear PGK1 alleviates ADP-dependent inhibition of CDC7 to promote DNA replication. Mol. Cell 2018, 72, 650–660. [Google Scholar] [CrossRef]
- Chang, Y.C.; Chan, M.H.; Li, C.H.; Yang, C.J.; Tseng, Y.W.; Tsai, H.F.; Chiou, J.; Hsiao, M. Metabolic protein phosphoglycerate kinase 1 confers lung cancer migration by directly binding HIV Tat specific factor 1. Cell Death Discov. 2021, 5, 135. [Google Scholar] [CrossRef]
- Fekete, J.T.; Ősz, Á.; Pete, I.; Nagy, G.R.; Vereczkey, I.; Győrffy, B. Predictive biomarkers of platinum and taxane resistance using the transcriptomic data of 1816 ovarian cancer patients. Gynecol. Oncol. 2020, 156, 654–661. [Google Scholar] [CrossRef]
- Duan, Z.; Lamendola, D.E.; Yusuf, R.Z.; Penson, R.T.; Prefer, F.I.; Seiden, M.V. Overexpression of human phosphoglycerate kinase 1 (PGK1) induces a multidrug resistance phenotype. Anticancer Res. 2002, 22, 1933–1941. [Google Scholar]
- Mazzoni, C.; Torella, M.; Petrera, A.; Palermo, V.; Falcone, C. PGK1, the gene encoding the glycolytic enzyme phosphoglycerate kinase, acts as a multicopy suppressor of apoptotic phenotypes in S. cerevisiae. Yeast 2009, 26, 31–37. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | Value |
|---|---|
| Age (years) | |
| Median | 68 |
| IQR | 64–74 |
| Sex (N [%]) | |
| Male | 42 (79.1) |
| Female | 11 (20.8) |
| Performance status (N [%]) | |
| 0 | 20 (37.3) |
| 1 | 26 (49.1) |
| 2 | 27 (13.2) |
| Primary organ (N [%]) | |
| Bladder | 32 (60.4) |
| Upper urinary tract | 21 (39.6) |
| Surgical management (N [%]) | |
| Radical cystectomy | 7 (13.2) |
| Partial cystectomy | 2 (3.8) |
| Radical nephroureterectomy | 14 (26.4) |
| Transurethral resection of bladder cancer | 18 (33.9) |
| Lymph node dissection | 2 (3.8) |
| Inoperable | 10 (18.9) |
| 1st-line chemotherapy regimen (N [%]) | |
| MVAC | 45 (84.9) |
| GC | 8 (15.1) |
| Metastatic sites (N [%]) | |
| Lymph node | 24 (42.1) |
| Lung | 20 (35.1) |
| Bone | 6 (10.5) |
| Liver | 4 (7.0) |
| Peritoneum | 2 (3.5) |
| Adrenal | 1 (1.8) |
| Response Type | Patients (N [%]) | Responded to Chemotherapy Used in 1st Line (N [%]) | |||||
|---|---|---|---|---|---|---|---|
| Yes | No | ||||||
| MVAC | GC | p Value | MVAC | GC | p Value | ||
| Complete response (CR) | 4 (7.5) | 14 (53.9) | 0 (0) | 0.045 | 4 (21.1) | 1 (25.0) | 0.86 |
| Partial response (PR) | 15 (28.3) | ||||||
| Stable disease (SD) | 18 (34.0) | 12 (46.1) | 4 (100) | 15 (78.9) | 3 (75.0) | ||
| Progressive disease (PD) | 16 (30.2) | ||||||
| Overall response rate (CR+PR) | 19 (35.8) | ||||||
| Disease control (CR+PR+SD) | 37 (69.8) | ||||||
| Variable | Comparator | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | p Value | HR | 95% CI | p Value | ||
| Age | ≥70 | 0.99 | 0.56 to 1.75 | 0.43 | 0.92 | 0.50 to 1.69 | 0.78 |
| ≤69 | 1.0 | 1.0 | |||||
| Performance status | 2 | 6.16 | 2.55 to 12.83 | <0.001 | 6.73 | 2.64 to 17.2 | <0.001 |
| ≤1 | 1.0 | 1.0 | |||||
| Visceral metastasis | Present | 0.92 | 0.53 to 1.58 | 0.88 | 1.01 | 0.54 to 1.86 | 0.99 |
| Absent | 1.0 | 1.0 | |||||
| Responded to 1st-line chemotherapy | No | 2.07 | 1.17 to 3.68 | 0.013 | 1.92 | 1.02 to 3.63 | 0.044 |
| Yes | 1.0 | 1.0 | |||||
| Responded to GP | No | 3.10 | 1.61 to 5.97 | 0.006 | 2.85 | 1.37 to 5.94 | 0.005 |
| Yes | 1.0 | 1.0 | |||||
| Variable | Comparator | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | p Value | HR | 95% CI | p Value | ||
| Age | ≥70 | 0.94 | 0.49 to 1.82 | 0.86 | 0.51 | 0.25 to 1.04 | 0.07 |
| ≤69 | 1.0 | 1.0 | |||||
| Performance status | 2 | 4.08 | 1.82 to 9.16 | 0.004 | 2.84 | 1.19 to 6.81 | 0.019 |
| ≤1 | 1.0 | 1.0 | |||||
| Visceral metastasis | Present | 0.91 | 0.48 to 1.7 | 0.92 | 0.80 | 0.39 to 1.64 | 0.54 |
| Absent | 1.0 | 1.0 | |||||
| Response to 1st-line chemotherapy | No | 1.40 | 0.71 to 2.81 | 0.33 | 2.55 | 1.12 to 5.77 | 0.025 |
| Yes | 1.0 | 1.0 | |||||
| Response to GP | No | 2.59 | 1.19 to 5.65 | 0.017 | 1.57 | 0.70 to 3.56 | 0.28 |
| Yes | 1.0 | 1.0 | |||||
| Adverse Event | Grade (N [%]) | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | All | |
| Neutropenia | 4 | 7 | 28 | 8 | 47 (88.7) |
| Anemia | 17 | 16 | 11 | 3 | 47 (88.7) |
| Thrombocytopenia | 14 | 7 | 9 | — | 30 (56.6) |
| Febrile neutropenia | — | — | 5 | — | 5 (9.4) |
| Neuropathy | 11 | 10 | — | — | 21 (39.6) |
| Skin rash | 1 | 3 | — | — | 4 (7.5) |
| Nausea/vomiting | 7 | 1 | — | — | 8 (15.1) |
| Alopecia | 21 | 1 | — | — | 22 (41.5) |
| Liver dysfunction | 9 | 1 | — | — | 10 (18.9) |
| Characteristics (N [%]) | LE (n = 12) | HE (n = 16) | p Value |
|---|---|---|---|
| Primary organ | |||
| Bladder | 7 (58.3) | 9 (56.3) | 0.91 |
| Ureter | 5 (41.7) | 7 (43.7) | |
| 1st-line chemotherapy | |||
| MVAC | 10 (83.3) | 13 (76.9) | 0.89 |
| GC | 2 (16.7) | 3 (23.1) | |
| Non response | |||
| 1st-line chemotherapy | 2 (16.7) | 11 (69.7) | 0.006 |
| 2nd-line chemotherapy | 7 (58.3) | 11 (69.7) | 0.57 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koguchi, D.; Matsumoto, K.; Ikeda, M.; Shimizu, Y.; Nakamura, M.; Shiono, Y.; Katsumata, H.; Sato, Y.; Iwamura, M. Gemcitabine–Paclitaxel Chemotherapy for Patients with Advanced Urothelial Cancer Refractory to Cisplatin-Based Chemotherapy: Predictive Role of PGK1 for Treatment Response to Cytotoxic Chemotherapy. Int. J. Mol. Sci. 2022, 23, 12119. https://doi.org/10.3390/ijms232012119
Koguchi D, Matsumoto K, Ikeda M, Shimizu Y, Nakamura M, Shiono Y, Katsumata H, Sato Y, Iwamura M. Gemcitabine–Paclitaxel Chemotherapy for Patients with Advanced Urothelial Cancer Refractory to Cisplatin-Based Chemotherapy: Predictive Role of PGK1 for Treatment Response to Cytotoxic Chemotherapy. International Journal of Molecular Sciences. 2022; 23(20):12119. https://doi.org/10.3390/ijms232012119
Chicago/Turabian StyleKoguchi, Dai, Kazumasa Matsumoto, Masaomi Ikeda, Yuriko Shimizu, Marie Nakamura, Yutaka Shiono, Hiroki Katsumata, Yuichi Sato, and Masatsugu Iwamura. 2022. "Gemcitabine–Paclitaxel Chemotherapy for Patients with Advanced Urothelial Cancer Refractory to Cisplatin-Based Chemotherapy: Predictive Role of PGK1 for Treatment Response to Cytotoxic Chemotherapy" International Journal of Molecular Sciences 23, no. 20: 12119. https://doi.org/10.3390/ijms232012119
APA StyleKoguchi, D., Matsumoto, K., Ikeda, M., Shimizu, Y., Nakamura, M., Shiono, Y., Katsumata, H., Sato, Y., & Iwamura, M. (2022). Gemcitabine–Paclitaxel Chemotherapy for Patients with Advanced Urothelial Cancer Refractory to Cisplatin-Based Chemotherapy: Predictive Role of PGK1 for Treatment Response to Cytotoxic Chemotherapy. International Journal of Molecular Sciences, 23(20), 12119. https://doi.org/10.3390/ijms232012119





