Importance of Heparan Sulfate Proteoglycans in Pancreatic Islets and β-Cells
Abstract
1. Introduction
2. Glycosaminoglycans with Heparin/Heparan Sulfate in Pancreatic Islets and β-Cells
3. HSPG Core Proteins in Pancreatic Islets and β-Cells
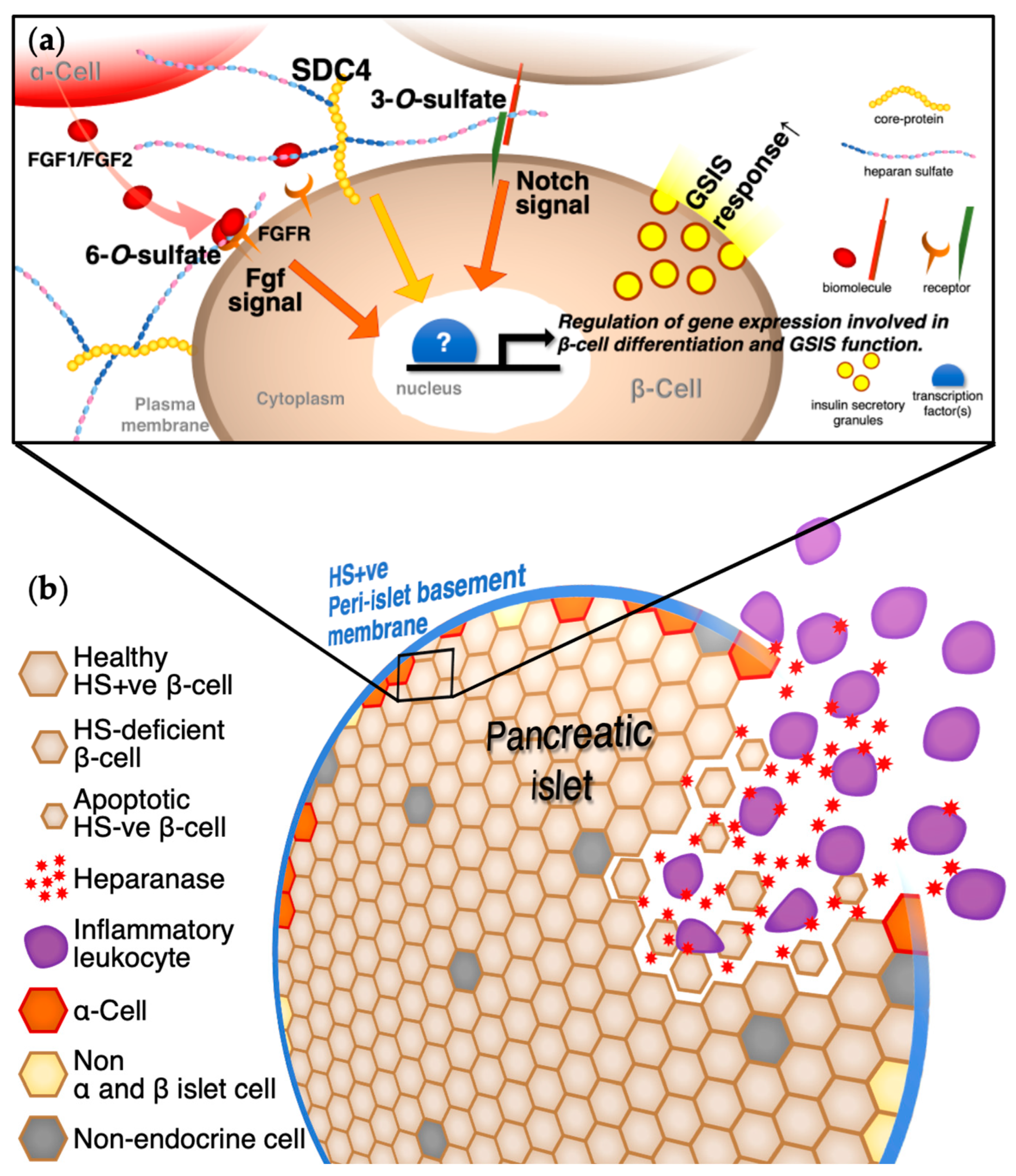
4. Sulfotransferases, Heparanases, Sulfatases, and Signaling Pathways in Pancreatic Islets and β-Cells
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kadowaki, T.; Miyake, Y.; Hagura, R.; Akanuma, Y.; Kajinuma, H.; Kuzuya, N.; Takaku, F.; Kosaka, K. Risk factors for worsening to diabetes in subjects with impaired glucose tolerance. Diabetologia 1984, 26, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.I.; Accili, D.; Imai, Y. Insulin resistance or insulin deficiency. Which is the primary cause of NIDDM? Diabetes 1994, 43, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Kadowaki, T. Insights into insulin resistance and type 2 diabetes from knockout mouse models. J. Clin. Investig. 2000, 106, 459–465. [Google Scholar] [CrossRef][Green Version]
- Franks, P.W.; Pearson, E.; Florez, J.C. Gene-environment and gene-treatment interactions in type 2 diabetes: Progress, pitfalls, and prospects. Diabetes Care. 2013, 36, 1413–1421. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, J.; Hamet, P. Environmental and genetic contributions to diabetes. Metabolism 2019, 100S, 153952. [Google Scholar] [CrossRef]
- Komatsu, M.; Takei, M.; Ishii, H.; Sato, Y. Glucose-stimulated insulin secretion: A newer perspective. J. Diabetes Investig. 2013, 27, 511–516. [Google Scholar] [CrossRef]
- Takahashi, I.; Noguchi, N.; Nata, K.; Yamada, S.; Kaneiwa, T.; Mizumoto, S.; Ikeda, T.; Sugihara, K.; Asano, M.; Yoshikawa, T.; et al. Important role of heparan sulfate in postnatal islet growth and insulin secretion. Biochem. Biophys. Res. Commun. 2009, 383, 113–118. [Google Scholar] [CrossRef]
- Takahashi, I. Important Role of Heparan Sulfate in the Morphogenesis, Β-Cell Proliferation, and Insulin Secretion of Mouse Pancreatic Islet. Ph.D. Thesis, Tohoku University Graduate School of Medicine, Sendai, Japan, 2009. [Google Scholar]
- Takahashi, I.; Ohashi, K.; Nata, K. Involvement of heparan sulfate 3-O-sulfotransferase isoform-1 in the insulin secretion pathway. J. Diabetes Investig. 2012, 3, 362–370. [Google Scholar] [CrossRef]
- Takahashi, I.; Yamada, S.; Nata, K. Effects of heparan sulfate proteoglycan syndecan-4 on the insulin secretory response in a mouse pancreatic β-cell line, MIN6. Mol. Cell. Endocrinol. 2018, 470, 142–150. [Google Scholar] [CrossRef]
- Matsuzawa, T.; Yoshikawa, T.; Iida, T.; Kárpáti, A.; Kitano, H.; Harada, R.; Nakamura, T.; Sugawara, A.; Yamaguchi, Y.; Yanai, K. Heparan sulfate in pancreatic β-cells contributes to normal glucose homeostasis by regulating insulin secretion. Biochem. Biophys. Res. Commun. 2018, 499, 688–695. [Google Scholar] [CrossRef]
- Ziolkowski, A.F.; Popp, S.K.; Freeman, C.; Parish, C.R.; Simeonovic, C.J. Heparan sulfate and heparanase play key roles in mouse β cell survival and autoimmune diabetes. J. Clin. Investig. 2012, 122, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Bernelot Moens, S.J.; Mooij, H.L.; Hassing, H.C.; Kruit, J.K.; Witjes, J.J.; van de Sande, M.A.; Nederveen, A.J.; Xu, D.; Dallinga-Thie, G.M.; Esko, J.D.; et al. Carriers of loss-of-function mutations in EXT display impaired pancreatic beta-cell reserve due to smaller pancreas volume. PLoS ONE 2014, 9, e115662. [Google Scholar] [CrossRef] [PubMed]
- Theodoraki, A.; Hu, Y.; Poopalasundaram, S.; Oosterhof, A.; Guimond, S.E.; Disterer, P.; Khoo, B.; Hauge-Evans, A.C.; Jones, P.M.; Turnbull, J.E.; et al. Distinct patterns of heparan sulphate in pancreatic islets suggest novel roles in paracrine islet regulation. Mol. Cell. Endocrinol. 2015, 339, 296–310. [Google Scholar] [CrossRef] [PubMed]
- Simeonovic, C.J.; Popp, S.K.; Starrs, L.M.; Brown, D.J.; Ziolkowski, A.F.; Ludwig, B.; Bornstein, S.R.; Wilson, J.D.; Pugliese, A.; Kay, T.W.H.; et al. Loss of intra-islet heparan sulfate is a highly sensitive marker of type 1 diabetes progression in humans. PLoS ONE 2018, 13, e0191360. [Google Scholar] [CrossRef] [PubMed]
- Dhounchak, S.; Popp, S.K.; Brown, D.J.; Laybutt, D.R.; Biden, T.J.; Bornstein, S.R.; Parish, C.R.; Simeonovic, C.J. Heparan sulfate proteoglycans in beta cells provide a critical link between endoplasmic reticulum stress, oxidative stress and type 2 diabetes. PLoS ONE 2021, 16, e0252607. [Google Scholar] [CrossRef] [PubMed]
- Song, W.Y.; Jiang, X.H.; Ding, Y.; Wang, Y.; Zhou, M.X.; Xia, Y.; Zhang, C.Y.; Yin, C.C.; Qiu, C.; Li, K.; et al. Inhibition of heparanase protects against pancreatic beta cell death in streptozotocin-induced diabetic mice via reducing intra-islet inflammatory cell infiltration. Br. J. Pharmacol. 2020, 177, 4433–4447. [Google Scholar] [CrossRef] [PubMed]
- Coulson-Thomas, V.J. The role of heparan sulphate in development: The ectodermal story. Int. J. Exp. Pathol. 2016, 97, 213–229. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.P.; Stephens, R.S. Mechanism of C. trachomatis attachment to eukaryotic host cells. Cell 1992, 69, 861–869. [Google Scholar] [CrossRef]
- Wuppermann, F.N.; Hegemann, J.H.; Jantos, C.A. Heparan sulfate-like glycosaminoglycan is a cellular receptor for Chlamydia pneumoniae. J. Infect. Dis. 2001, 184, 181–187. [Google Scholar] [CrossRef]
- Takahashi, I. Role of Heparan Sulfate Proteoglycans in Insulin-producing Pancreatic β-cell Function. Trends Glycosci. Glycotechnol. 2021, 195, E109–E114. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, F.; Linhardt, R.J. Glycosaminoglycans. In The Role of Glycosylation in Health and Disease; Akmačić, I.T., Lauc, G., Eds.; Springer: Berlin/Heidelberg, Germany, 2021; Volume 1325, pp. 103–116. [Google Scholar] [CrossRef]
- Lodish, H.F.; Berk, A.; Kaiser, C.A.; Krieger, M.; Bretscher, A.; Ploegh, H.L.; Martin, K.C.; Yaffe, M.B.; Amon, A. Molecular Cell Biology, 9th ed.; Macmillan Learning: New York, NY, USA, 2020; pp. 932–942. [Google Scholar]
- Fraser, J.R.; Laurent, T.C.; Laurent, U.B. Hyaluronan: Its nature, distribution, functions and turnover. J. Intern. Med. 1997, 242, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Sasarman, F.; Maftei, C.; Campeau, P.M.; Brunel-Guitton, C.; Mitchell, G.A.; Allard, P. Biosynthesis of glycosaminoglycans: Associated disorders and biochemical tests. J. Inherit. Metab. Dis. 2016, 39, 173–188. [Google Scholar] [CrossRef] [PubMed]
- Sarrazin, S.; Lamanna, W.C.; Esko, J.D. Heparan sulfate proteoglycans. Cold Spring Harb. Perspect. Biol. 2011, 3, a004952. [Google Scholar] [CrossRef] [PubMed]
- Lindahl, U. What else can ‘Heparin’ do? Haemostasis 1999, 29, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Templin, A.T.; Mellati, M.; Soininen, R.; Hogan, M.F.; Esser, N.; Castillo, J.J.; Zraika, S.; Kahn, S.E.; Hull, R.L. Loss of perlecan heparan sulfate glycosaminoglycans lowers body weight and decreases islet amyloid deposition in human islet amyloid polypeptide transgenic mice. Protein Eng. Des. Sel. 2019, 32, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Choong, F.J.; Freeman, C.; Parish, C.R.; Simeonovic, C.J. Islet heparan sulfate but not heparan sulfate proteoglycan core protein is lost during islet isolation and undergoes recovery post-islet transplantation. Am. J. Transplant. 2015, 15, 2851–2864. [Google Scholar] [CrossRef]
- Cheng, J.Y.; Whitelock, J.; Poole-Warren, L. Syndecan-4 is associated with beta-cells in the pancreas and the MIN6 beta-cell line. Histochem. Cell Biol. 2012, 138, 933–944. [Google Scholar] [CrossRef]
- Ping Lu, Y.; Ishiwata, T.; Asano, G. Lumican expression in alpha cells of islets in pancreas and pancreatic cancer cells. J. Pathol. 2002, 196, 324–330. [Google Scholar] [CrossRef]
- Ishiwata, T.; Cho, K.; Kawahara, K.; Yamamoto, T.; Fujiwara, Y.; Uchida, E.; Tajiri, T.; Naito, Z. Role of lumican in cancer cells and adjacent stromal tissues in human pancreatic cancer. Oncol. Rep. 2007, 18, 537–543. [Google Scholar] [CrossRef]
- Yamamoto, T.; Matsuda, Y.; Kawahara, K.; Ishiwata, T.; Naito, Z. Effects Secreted 70 kDa lumican stimulates growth and inhibits invasion of human pancreatic cancer. Cancer Lett. 2012, 320, 31–39. [Google Scholar] [CrossRef]
- Yang, Z.X.; Lu, C.Y.; Yang, Y.L.; Dou, K.F.; Tao, K.S. Lumican expression in pancreatic ductal adenocarcinoma. Hepatogastroenterology 2013, 60, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Truty, M.A.; Kang, Y.; Chopin-Laly, X.; Zhang, R.; Roife, D.; Chatterjee, D.; Lin, E.; Thomas, R.M.; Wang, H.; et al. Extracellular lumican inhibits pancreatic cancer cell growth and is associated with prolonged survival after surgery. Clin. Cancer Res. 2014, 20, 6529–6540. [Google Scholar] [CrossRef] [PubMed]
- Hayes, A.J.; Melrose, J. Keratan Sulphate in the Tumour Environment. Adv. Exp. Med. Biol. 2020, 1245, 39–66. [Google Scholar] [CrossRef]
- Bollyky, P.L.; Bogdani, M.; Bollyky, J.B.; Hull, R.L.; Wight, T.N. The role of hyaluronan and the extracellular matrix in islet inflammation and immune regulation. Curr. Diab. Rep. 2012, 12, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Hull, R.L.; Bogdani, M.; Nagy, N.; Johnson, P.Y.; Wight, T.N. Hyaluronan: A Mediator of Islet Dysfunction and Destruction in Diabetes? J. Histochem. Cytochem. 2015, 63, 592–603. [Google Scholar] [CrossRef] [PubMed]
- Nagy, N.; Kaber, G.; Johnson, P.Y.; Gebe, J.A.; Preisinger, A.; Falk, B.A.; Sunkari, V.G.; Gooden, M.D.; Vernon, R.B.; Bogdani, M.; et al. Inhibition of hyaluronan synthesis restores immune tolerance during autoimmune insulitis. J. Clin. Investig. 2015, 125, 3928–3940. [Google Scholar] [CrossRef]
- Bogdani, M. Thinking Outside the Cell: A Key Role for Hyaluronan in the Pathogenesis of Human Type 1 Diabetes. Diabetes 2016, 65, 2105–2114. [Google Scholar] [CrossRef]
- Kojima, N. Production of type 1 diabetes model islets by a design method for multicellular spheroid. Manuf. Technol. 2020, 72, 13–18. [Google Scholar]
- Gomes, A.M.; Kozlowski, E.O.; Pomin, V.H.; de Barros, C.M.; Zaganeli, J.L.; Pavão, M.S. Unique extracellular matrix heparan sulfate from the bivalve Nodipecten nodosus (Linnaeus, 1758) safely inhibits arterial thrombosis after photochemically induced endothelial lesion. J. Biol. Chem. 2010, 285, 7312–7323. [Google Scholar] [CrossRef]
- Vieira, R.P.; Mulloy, B.; Mourão, P.A. Structure of a fucose-branched chondroitin sulfate from sea cucumber. Evidence for the presence of 3-O-sulfo-beta-D-glucuronosyl residues. J. Biol. Chem. 1991, 266, 13530–13536. [Google Scholar] [CrossRef]
- Sugahara, K.; Tanaka, Y.; Yamada, S.; Seno, N.; Kitagawa, H.; Haslam, S.M.; Morris, H.R.; Dell, A. Novel sulfated oligosaccharides containing 3-O-sulfated glucuronic acid from king crab cartilage chondroitin sulfate K. Unexpected degradation by chondroitinase ABC. J. Biol. Chem. 1996, 271, 26745–26754. [Google Scholar] [CrossRef] [PubMed]
- Bennet, W.; Groth, C.G.; Larsson, R.; Nilsson, B.; Korsgren, O. Isolated human islets trigger an instant blood mediated inflammatory reaction: Implications for intraportal islet transplantation as a treatment for patients with type 1 diabetes. Ups. J. Med. Sci. 2000, 105, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Bennet, W.; Sundberg, B.; Groth, C.G.; Brendel, M.D.; Brandhorst, D.; Brandhorst, H.; Bretzel, R.G.; Elgue, G.; Larsson, R.; Nilsson, B.; et al. Incompatibility between human blood and isolated islets of Langerhans: A finding with implications for clinical intraportal islet transplantation? Diabetes 1999, 48, 1907–1914. [Google Scholar] [CrossRef]
- Cabric, S.; Sanchez, J.; Lundgren, T.; Foss, A.; Felldin, M.; Källen, R.; Salmela, K.; Tibell, A.; Tufveson, G.; Larsson, R.; et al. Islet surface heparinization prevents the instant blood-mediated inflammatory reaction in islet transplantation. Diabetes 2007, 56, 2008–2015. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N.; Gerber, H.P.; LeCouter, J. The biology of VEGF and its receptors. Nat. Med. 2003, 9, 669–676. [Google Scholar] [CrossRef]
- Schlessinger, J.; Plotnikov, A.N.; Ibrahimi, O.A.; Eliseenkova, A.V.; Yeh, B.K.; Yayon, A.; Linhardt, R.J.; Mohammadi, M. Crystal structure of a ternary FGF-FGFR-heparin complex reveals a dual role for heparin in FGFR binding and dimerization. Mol. Cell. 2000, 6, 743–750. [Google Scholar] [CrossRef]
- McGadey, J. A staining sequence for the differentiation of A-, B-, and D-cells of the islets of guinea-pig pancreas. Acta Diabetol. Lat. 1979, 16, 243–246. [Google Scholar] [CrossRef]
- Joyce, J.A.; Freeman, C.; Meyer-Morse, N.; Parish, C.R.; Hanahan, D. A functional heparan sulfate mimetic implicates both heparanase and heparan sulfate in tumor angiogenesis and invasion in a mouse model of multistage cancer. Oncogene 2005, 24, 4037–4051. [Google Scholar] [CrossRef]
- Irving-Rodgers, H.F.; Ziolkowski, A.F.; Parish, C.R.; Sado, Y.; Ninomiya, Y.; Simeonovic, C.J.; Rodgers, R.J. Molecular composition of the peri-islet basement membrane in NOD mice: A barrier against destructive insulitis. Diabetologia 2008, 51, 1680–1688. [Google Scholar] [CrossRef][Green Version]
- Sladek, R.; Rocheleau, G.; Rung, J.; Dina, C.; Shen, L.; Serre, D.; Boutin, P.; Vincent, D.; Belisle, A.; Hadjadj, S.; et al. A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature 2007, 445, 881–885. [Google Scholar] [CrossRef]
- Lind, T.; Tufaro, F.; McCormick, C.; Lindahl, U.; Lidholt, K. The putative tumor suppressors EXT1 and EXT2 are glycosyltransferases required for the biosynthesis of heparan sulfate. J. Biol. Chem. 1998, 273, 26265–26268. [Google Scholar] [CrossRef] [PubMed]
- Wuyts, W.; Van Hul, W. Molecular basis of multiple exostoses: Mutations in the EXT1 and EXT2 genes. Hum. Mutat. 2000, 15, 220–227. [Google Scholar] [CrossRef]
- David, G.; Bai, X.M.; Van der Schueren, B.; Cassiman, J.J.; Van den Berghe, H. Developmental changes in heparan sulfate expression: In situ detection with mAbs. J. Cell. Biol. 1992, 119, 961–975. [Google Scholar] [CrossRef] [PubMed]
- Bernfield, M.; Götte, M.; Park, P.W.; Reizes, O.; Fitzgerald, M.L.; Lincecum, J.; Zako, M. Functions of Cell Surface Heparan Sulfate Proteoglycans. Annu. Rev. Biochem. 1999, 68, 729–777. [Google Scholar] [CrossRef]
- Kramer, K.L.; Yost, H.J. Heparan sulfate core proteins in cell-cell signaling. Annu. Rev. Genet. 2003, 37, 461–484. [Google Scholar] [CrossRef]
- Dreyfuss, J.L.; Regatieri, C.V.; Jarrouge, T.R.; Cavalheiro, R.P.; Sampaio, L.O.; Nader, H.B. Heparan sulfate proteoglycans: Structure, protein interactions and cell signaling. An. Acad. Bras. Cienc. 2009, 81, 409–429. [Google Scholar] [CrossRef]
- Irving-Rodgers, H.F.; Choong, F.J.; Hummitzsch, K.; Parish, C.R.; Rodgers, R.J.; Simeonovic, C.J. Pancreatic islet basement membrane loss and remodeling after mouse islet isolation and transplantation: Impact for allograft rejection. Cell Transplant. 2014, 23, 59–72. [Google Scholar] [CrossRef]
- Nikpour, M.; Nilsson, J.; Persson, A.; Noborn, F.; Vorontsov, E.; Larson, G. Proteoglycan profiling of human, rat and mouse insulin-secreting cells. Glycobiology 2021, 31, 916–930. [Google Scholar] [CrossRef]
- Miyazaki, J.; Araki, K.; Yamato, E.; Ikegami, H.; Asano, T.; Shibasaki, Y.; Oka, Y.; Yamamura, K. Establishment of a pancreatic beta cell line that retains glucose-inducible insulin secretion: Special reference to expression of glucose transporter isoforms. Endocrinology 1990, 127, 126–132. [Google Scholar] [CrossRef]
- Ishihara, H.; Asano, T.; Tsukuda, K.; Katagiri, H.; Inukai, K.; Anai, M.; Kikuchi, M.; Yazaki, Y.; Miyazaki, J.I.; Oka, Y. Pancreatic beta cell line MIN6 exhibits characteristics of glucose metabolism and glucose-stimulated insulin secretion similar to those of normal islets. Diabetologia 1993, 36, 1139–1145. [Google Scholar] [CrossRef]
- Minami, K.; Yano, H.; Miki, T.; Nagashima, K.; Wang, C.Z.; Tanaka, H.; Miyazaki, J.I.; Seino, S. Insulin secretion and differential gene expression in glucose-responsive and -unresponsive MIN6 sublines. Am. J. Physiol. Endocrinol. Metab. 2000, 279, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Lilla, V.; Webb, G.; Rickenbach, K.; Maturana, A.; Steiner, D.F.; Halban, P.A.; Irminger, J.C. Differential gene expression in well-regulated and dysregulated pancreatic beta-cell (MIN6) sublines. Endocrinology 2013, 144, 1368–1379. [Google Scholar] [CrossRef] [PubMed]
- Yamato, E.; Tashiro, F.; Miyazaki, J. Microarray analysis of novel candidate genes responsible for glucose-stimulated insulin secretion in mouse pancreatic β cell line MIN6. PLoS ONE 2013, 8, e61211. [Google Scholar] [CrossRef] [PubMed]
- Echtermeyer, F.; Streit, M.; Wilcox-Adelman, S.; Saoncella, S.; Denhez, F.; Detmar, M.; Goetinck, P. Delayed wound repair and impaired angiogenesis in mice lacking syndecan-4. J. Clin. Investig. 2001, 107, R9–R14. [Google Scholar] [CrossRef]
- Ishiguro, K.; Kadomatsu, K.; Kojima, T.; Muramatsu, H.; Nakamura, E.; Ito, M.; Nagasaka, T.; Kobayashi, H.; Kusugami, K.; Saito, H.; et al. Syndecan-4 deficiency impairs the fetal vessels in the placental labyrinth. Dev. Dyn. 2000, 219, 539–544. [Google Scholar] [CrossRef]
- Ishiguro, K.; Kadomatsu, K.; Kojima, T.; Muramatsu, H.; Matsuo, S.; Kusugami, K.; Saito, H.; Muramatsu, T. Syndecan-4 deficiency increases susceptibility to kappa-carrageenan-induced renal damage. Lab. Investig. 2001, 81, 509–516. [Google Scholar] [CrossRef][Green Version]
- Takahashi, I.; Yamada, S.; Nata, K. Deletion of heparan sulfate proteoglycan syndecan-4 impairs pancreatic β-cell function in mice. In Proceedings of the 11th International Conference on Proteoglycans, Kanazawa, Japan, 28 September–3 October 2009. [Google Scholar]
- Ito, M.; Kondo, Y.; Nakatani, A.; Hayashi, K.; Naruse, A. Characterization of low dose streptozotocin-induced progressive diabetes in mice. Environ. Toxicol. Pharmacol. 2001, 9, 71–78. [Google Scholar] [CrossRef]
- Yang, C.; Chang, T.J.; Chang, J.C.; Liu, M.W.; Tai, T.Y.; Hsu, W.H.; Chuang, L.M. Rosiglitazone (BRL 49653) enhances insulin secretory response via phosphatidylinositol 3-kinase pathway. Diabetes 2001, 50, 2598–2602. [Google Scholar] [CrossRef][Green Version]
- Santini, E.; Fallahi, P.; Ferrari, S.M.; Masoni, A.; Antonelli, A.; Ferrannini, E. Effect of PPAR-gamma activation and inhibition on glucose-stimulated insulin release in INS-1e cells. Diabetes 2004, 53 (Suppl. 3), S79–S83. [Google Scholar] [CrossRef]
- Kim, H.S.; Noh, J.H.; Hong, S.H.; Hwang, Y.C.; Yang, T.Y.; Lee, M.S.; Kim, K.W.; Lee, M.K. Rosiglitazone stimulates the release and synthesis of insulin by enhancing GLUT-2, glucokinase and BETA2/NeuroD expression. Biochem. Biophys. Res. Commun. 2008, 367, 623–629. [Google Scholar] [CrossRef]
- Kim, H.S.; Hwang, Y.C.; Koo, S.H.; Park, K.S.; Lee, M.S.; Kim, K.W.; Lee, M.K. PPAR-γ activation increases insulin secretion through the up-regulation of the free fatty acid receptor GPR40 in pancreatic β-cells. PLoS ONE 2013, 8, e50128. [Google Scholar] [CrossRef]
- Nakamichi, Y.; Kikuta, T.; Ito, E.; Ohara-Imaizumi, M.; Nishiwaki, C.; Ishida, H.; Nagamatsu, S. PPAR-gamma overexpression suppresses glucose-induced proinsulin biosynthesis and insulin release synergistically with pioglitazone in MIN6 cells. Biochem. Biophys. Res. Commun. 2008, 306, 832–836. [Google Scholar] [CrossRef]
- Bollheimer, L.C.; Troll, S.; Landauer, H.; Wrede, C.E.; Schölmerich, J.; Buettner, R. Insulin-sparing effects of troglitazone in rat pancreatic islets. J. Mol. Endocrinol. 2003, 31, 61–69. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ito, E.; Ozawa, S.; Takahashi, K.; Tanaka, T.; Katsuta, H.; Yamaguchi, S.; Maruyama, M.; Takizawa, M.; Katahira, H.; Yoshimoto, K.; et al. PPAR-gamma overexpression selectively suppresses insulin secretory capacity in isolated pancreatic islets through induction of UCP-2 protein. Biochem. Biophys. Res. Commun. 2004, 324, 810–814. [Google Scholar] [CrossRef] [PubMed]
- Ravnskjaer, K.; Boergesen, M.; Rubi, B.; Larsen, J.K.; Nielsen, T.; Fridriksson, J.; Maechler, P.; Mandrup, S. Peroxisome proliferator-activated receptor alpha (PPARalpha) potentiates, whereas PPARgamma attenuates, glucose-stimulated insulin secretion in pancreatic beta-cells. Endocrinology 2005, 146, 3266–3276. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, L.; Shao, L.; Qian, L.; Fu, X.; Li, G.; Luo, T.; Gu, Y.; Li, F.; Li, J.; et al. Troglitazone acutely activates AMP-activated protein kinase and inhibits insulin secretion from beta cells. Life Sci. 2007, 81, 160–165. [Google Scholar] [CrossRef]
- Habuchi, O. Diversity and functions of glycosaminoglycan sulfotransferases. Biochim. Biophys. Acta 2000, 1474, 115–127. [Google Scholar] [CrossRef]
- Esko, J.D.; Lindahl, U. Molecular diversity of heparan sulfate. J. Clin. Investig. 2001, 108, 169–173. [Google Scholar] [CrossRef]
- Lin, X. Functions of heparan sulfate proteoglycans in cell signaling during development. Development 2004, 131, 6009–6021. [Google Scholar] [CrossRef]
- Fux, L.; Ilan, N.; Sanderson, R.D.; Vlodavsky, I. Heparanase: Busy at the cell surface. Trends Biochem. Sci. 2009, 34, 511–519. [Google Scholar] [CrossRef]
- Ai, X.; Kitazawa, T.; Do, A.T.; Kusche-Gullberg, M.; Labosky, P.A.; Emerson, C.P., Jr. SULF1 and SULF2 regulate heparan sulfate-mediated GDNF signaling for esophageal innervation. Development 2007, 134, 3327–3338. [Google Scholar] [CrossRef] [PubMed]
- Habuchi, H.; Habuchi, O.; Kimata, K. Sulfation pattern in glycosaminoglycan: Does it have a code? Glycoconj. J. 2004, 21, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Kreuger, J.; Spillmann, D.; Li, J.P.; Lindahl, U. Interactions between heparan sulfate and proteins: The concept of specificity. J. Cell Biol. 2006, 174, 323–327. [Google Scholar] [CrossRef]
- Lee, J.S.; Chien, C.B. When sugars guide axons: Insights from heparan sulphate proteoglycan mutants. Nat. Rev. Genet. 2004, 5, 923–935. [Google Scholar] [CrossRef] [PubMed]
- Bishop, J.R.; Schuksz, M.; Esko, J.D. Heparan sulphate proteoglycans fine-tune mammalian physiology. Nature 2007, 446, 1030–1037. [Google Scholar] [CrossRef]
- Lamanna, W.C.; Kalus, I.; Padva, M.; Baldwin, R.J.; Merry, C.L.; Dierks, T. The heparanome—The enigma of encoding and decoding heparan sulfate sulfation. J. Biotechnol. 2007, 129, 290–307. [Google Scholar] [CrossRef] [PubMed]
- Afratis, N.; Gialeli, C.; Nikitovic, D.; Tsegenidis, T.; Karousou, E.; Theocharis, A.D.; Pavão, M.S.; Tzanakakis, G.N.; Karamanos, N.K. Glycosaminoglycans: Key players in cancer cell biology and treatment. FEBS J. 2012, 279, 1177–1197. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Li, J.P. Heparan sulfate proteoglycan—A common receptor for diverse cytokines. Cell. Signal. 2019, 54, 115–121. [Google Scholar] [CrossRef]
- Hart, A.W.; Baeza, N.; Apelqvist, A.; Edlund, H. Attenuation of FGF signalling in mouse beta-cells leads to diabetes. Nature 2000, 408, 864–868. [Google Scholar] [CrossRef]
- Smart, N.G.; Apelqvist, A.A.; Gu, X.; Harmon, E.B.; Topper, J.N.; MacDonald, R.J.; Kim, S.K. Conditional expression of Smad7 in pancreatic beta cells disrupts TGF-beta signaling and induces reversible diabetes mellitus. PLoS Biol. 2006, 4, e39. [Google Scholar] [CrossRef]
- Fujino, T.; Asaba, H.; Kang, M.J.; Ikeda, Y.; Sone, H.; Takada, S.; Kim, D.H.; Ioka, R.X.; Ono, M.; Tomoyori, H.; et al. Low-density lipoprotein receptor-related protein 5 (LRP5) is essential for normal cholesterol metabolism and glucose-induced insulin secretion. Proc. Natl. Acad. Sci. USA 2003, 100, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Luo, S.; Jia, H.; Feng, L.; Lu, X.; Zhou, L.; Cai, J. Wnt/β-catenin signaling may be involved with the maturation, but not the differentiation, of insulin-producing cells. Biomed. Pharmacother. 2013, 67, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Billiard, F.; Karaliota, S.; Wang, B.; Stellas, D.; Serafimidis, I.; Manousopoulou, A.; Koutmani, Y.; Ninou, E.; Golubov, J.; DaNave, A.; et al. Delta-like Ligand-4-Notch Signaling Inhibition Regulates Pancreatic Islet Function and Insulin Secretion. Cell Rep. 2018, 22, 895–904. [Google Scholar] [CrossRef]
- Rubey, M.; Chhabra, N.F.; Gradinger, D.; Sanz-Moreno, A.; Lickert, H.; Przemeck, G.K.H.; de Angelis, M.H. DLL1- and DLL4-Mediated Notch Signaling Is Essential for Adult Pancreatic Islet Homeostasis. Diabetes 2020, 69, 915–926. [Google Scholar] [CrossRef] [PubMed]
- Humphries, D.E.; Silbert, J.E. Chlorate: A reversible inhibitor of proteoglycan sulfation. Biochem. Biophys. Res. Commun. 1988, 154, 365–371. [Google Scholar] [CrossRef]
- Zertal-Zidani, S.; Bounacer, A.; Scharfmann, R. Regulation of pancreatic endocrine cell differentiation by sulphated proteoglycans. Diabetologia 2007, 50, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Lindahl, U.; Bäckström, G.; Thunberg, L.; Leder, I.G. Evidence for a 3-O-sulfated D-glucosamine residue in the antithrombin-binding sequence of heparin. Proc. Natl. Acad. Sci. USA 1980, 77, 6551–6555. [Google Scholar] [CrossRef]
- Petitou, M.; Duchaussoy, P.; Lederman, I.; Choay, J.; Sinaÿ, P. Binding of heparin to antithrombin III: A chemical proof of the critical role played by a 3-sulfated 2-amino-2-deoxy-D-glucose residue. Carbohydr Res. 1988, 179, 163–172. [Google Scholar] [CrossRef]
- Petitou, M.; Casu, B.; Lindahl, U. 1976–1983, a critical period in the history of heparin: The discovery of the antithrombin binding site. Biochimie 2003, 85, 83–89. [Google Scholar] [CrossRef]
- Borjigin, J.; Deng, J.; Sun, X.; De Jesus, M.; Liu, T.; Wang, M.M. Diurnal pineal 3-O-sulphotransferase 2 expression controlled by beta-adrenergic repression. J. Biol. Chem. 2003, 278, 16315–16319. [Google Scholar] [CrossRef]
- Denys, A.; Allain, F. The Emerging Roles of Heparan Sulfate 3-O-Sulfotransferases in Cancer. Front Oncol. 2019, 9, 507–16319. [Google Scholar] [CrossRef] [PubMed]
- Tecle, E.; Diaz-Balzac, C.A.; Bülow, H.E. Distinct 3-O-sulfated heparan sulfate modification patterns are required for kal-1-dependent neurite branching in a context-dependent manner in Caenorhabditis elegans. G3: Genes Genomes Genet. 2013, 3, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.N.; Lombaert, I.M.; Cowherd, S.N.; Shworak, N.W.; Xu, Y.; Liu, J.; Hoffman, M.P. Hs3st3-modified heparan sulfate controls KIT+ progenitor expansion by regulating 3-O-sulfotransferases. Dev. Cell 2014, 29, 662–673. [Google Scholar] [CrossRef] [PubMed]
- Kamimura, K.; Rhodes, J.M.; Ueda, R.; McNeely, M.; Shukla, D.; Kimata, K.; Spear, P.G.; Shworak, N.W.; Nakato, H. Regulation of Notch signaling by Drosophila heparan sulfate 3-O sulfotransferase. J. Cell Biol. 2004, 166, 1069–1079. [Google Scholar] [CrossRef]
- Guo, Y.; Feng, Y.; Li, Z.; Lin, X. Drosophila heparan sulfate 3-O sulfotransferase B null mutant is viable and exhibits no defects in Notch signaling. J. Genet. Genom. 2014, 41, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Thacker, B.E.; Xu, D.; Lawrence, R.; Esko, J.D. Heparan sulfate 3-O-sulfation: A rare modification in search of a function. Matrix Biol. 2014, 35, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Girardin, E.P.; Hajmohammadi, S.; Birmele, B.; Helisch, A.; Shworak, N.W.; de Agostini, A.I. Synthesis of anticoagulantly active heparan sulfate proteoglycans by glomerular epithelial cells involves multiple 3-O-sulfotransferase isoforms and a limiting precursor pool. J. Biol. Chem. 2005, 280, 38059–38070. [Google Scholar] [CrossRef]
- Kamimura, K.; Koyama, T.; Habuchi, H.; Ueda, R.; Masu, M.; Kimata, K.; Nakato, H. Specific and flexible roles of heparan sulfate modifications in Drosophila FGF signaling. J. Biol. Chem. 2006, 174, 773–778. [Google Scholar] [CrossRef]
- Gorsi, B.; Stringer, S.E. Tinkering with heparan sulfate sulfation to steer development. Trends Cell Biol. 2007, 17, 173–177. [Google Scholar] [CrossRef]
- Takahashi, I.; Ohashi, K.; Shervani, N.J.; Nata, K. Involvement of heparan sulphate 3-O-sulfotransferase isoform-1 for insulin secretion pathway. Diabetologia 2011, 54, S116. [Google Scholar]
- Dam, G.B.T.; Kurup, S.; van de Westerlo, E.M.; Versteeg, E.M.; Lindahl, U.; Spillmann, D.; van Kuppevelt, T.H. 3-O-sulfated oligosaccharide structures are recognized by anti-heparan sulfate antibody HS4C3. J. Biol. Chem. 2006, 281, 4654–4662. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, H.; Futatsumori, H.; Suzuki, E.; Kimata, K. A quantitative method to detect non-antithrombin-binding 3-O-sulfated units in heparan sulfate. J. Biol. Chem. 2021, 296, 100115. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Pedersen, L.C. Emerging chemical and biochemical tools for studying 3-O-sulfated heparan sulfate. Am. J. Physiol. Cell Physiol. 2022, 322, C1166–C1175. [Google Scholar] [CrossRef] [PubMed]
- Vlodavsky, I.; Friedmann, Y.; Elkin, M.; Aingorn, H.; Atzmon, R.; Ishai-Michaeli, R.; Bitan, M.; Pappo, O.; Peretz, T.; Michal, I.; et al. Mammalian heparanase: Gene cloning, expression and function in tumor progression and metastasis. Nat. Med. 1999, 5, 793–802. [Google Scholar] [CrossRef]
- Hulett, M.D.; Freeman, C.; Hamdorf, B.J.; Baker, R.T.; Harris, M.J.; Parish, C.R. Cloning of mammalian heparanase, an important enzyme in tumor invasion and metastasis. Nat. Med. 1999, 5, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Toyoshima, M.; Nakajima, M. Human heparanase. Purification, characterization, cloning, and expression. J. Biol. Chem. 1999, 274, 24153–24160. [Google Scholar] [CrossRef]
- Kussie, P.H.; Hulmes, J.D.; Ludwig, D.L.; Patel, S.; Navarro, E.C.; Seddon, A.P.; Giorgio, N.A.; Bohlen, P. Cloning and functional expression of a human heparanase gene. Biochem. Biophys. Res. Commun. 1999, 261, 183–187. [Google Scholar] [CrossRef]
- Fairbanks, M.B.; Mildner, A.M.; Leone, J.W.; Cavey, G.S.; Mathews, W.R.; Drong, R.F.; Slightom, J.L.; Bienkowski, M.J.; Smith, C.W.; Bannow, C.A.; et al. Processing of the human heparanase precursor and evidence that the active enzyme is a heterodimer. J. Biol. Chem. 1999, 274, 29587–29590. [Google Scholar] [CrossRef]
- Höök, M.; Wasteson, A.; Oldberg, A. A heparan sulfate-degrading endoglycosidase from rat liver tissue. Biochem. Biophys. Res. Commun. 1975, 67, 1422–1428. [Google Scholar] [CrossRef]
- Nakajima, M.; Irimura, T.; Nicolson, G.L. Heparanases and tumor metastasis. J. Biol. Chem. 1988, 36, 157–167. [Google Scholar] [CrossRef]
- Goldshmidt, O.; Nadav, L.; Aingorn, H.; Irit, C.; Feinstein, N.; Ilan, N.; Zamir, E.; Geiger, B.; Vlodavsky, I.; Katz, B.Z. Human heparanase is localized within lysosomes in a stable form. Exp. Cell Res. 2002, 281, 50–62. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nadav, L.; Eldor, A.; Yacoby-Zeevi, O.; Zamir, E.; Pecker, I.; Ilan, N.; Geiger, B.; Vlodavsky, I.; Katz, B.Z. Activation, processing and trafficking of extracellular heparanase by primary human fibroblasts. J. Cell Sci. 2002, 115, 2179–2187. [Google Scholar] [CrossRef] [PubMed]
- Gingis-Velitski, S.; Zetser, A.; Kaplan, V.; Ben-Zaken, O.; Cohen, E.; Levy-Adam, F.; Bashenko, Y.; Flugelman, M.Y.; Vlodavsky, I.; Ilan, N. Heparanase uptake is mediated by cell membrane heparan sulfate proteoglycans. J. Biol. Chem. 2004, 279, 44084–44092. [Google Scholar] [CrossRef] [PubMed]
- Zetser, A.; Levy-Adam, F.; Kaplan, V.; Gingis-Velitski, S.; Bashenko, Y.; Schubert, S.; Flugelman, M.Y.; Vlodavsky, I.; Ilan, N. Processing and activation of latent heparanase occurs in lysosomes. J. Cell Sci. 2004, 117, 2249–2258. [Google Scholar] [CrossRef] [PubMed]
- Abboud-Jarrous, G.; Atzmon, R.; Peretz, T.; Palermo, C.; Gadea, B.B.; Joyce, J.A.; Vlodavsky, I. Cathepsin L is responsible for processing and activation of proheparanase through multiple cleavages of a linker segment. J. Biol. Chem. 2008, 283, 18167–18176. [Google Scholar] [CrossRef]
- Parish, C.R. The role of heparan sulphate in inflammation. Nat. Rev. Immunol. 2006, 6, 633–643. [Google Scholar] [CrossRef]
- Ilan, N.; Elkin, M.; Vlodavsky, I. Regulation, function and clinical significance of heparanase in cancer metastasis and angiogenesis. Int. J. Biochem. Cell Biol. 2006, 38, 2018–2039. [Google Scholar] [CrossRef]
- Mita, T.; Watada, H.; Uchino, H.; Shimizu, T.; Hirose, T.; Tanaka, Y.; Kawamori, R. Association of C-reactive protein with early-stage carotid atherosclerosis in Japanese patients with early-state type 2 diabetes mellitus. Endocr. J. 2006, 53, 693–698. [Google Scholar] [CrossRef]
- Calle, M.C.; Fernandez, M.L. Inflammation and type 2 diabetes. Diabetes Metab. 2012, 38, 183–191. [Google Scholar] [CrossRef]
- Cabrera, S.M.; Henschel, A.M.; Hessner, M.J. Innate inflammation in type 1 diabetes. Transl. Res. 2016, 167, 214–227. [Google Scholar] [CrossRef]
- Tsalamandris, S.; Antonopoulos, A.S.; Oikonomou, E.; Papamikroulis, G.A.; Vogiatzi, G.; Papaioannou, S.; Deftereos, S.; Tousoulis, D. The Role of Inflammation in Diabetes: Current Concepts and Future Perspectives. Eur. Cardiol. 2019, 14, 50–59. [Google Scholar] [CrossRef]
- Simeonovic, C.J.; Ziolkowski, A.F.; Wu, Z.; Choong, F.J.; Freeman, C.; Parish, C.R. Heparanase and autoimmune diabetes. Front. Immunol. 2013, 4, 471. [Google Scholar] [CrossRef] [PubMed]
- Simeonovic, C.J.; Popp, S.K.; Brown, D.J.; Li, F.J.; Lafferty, A.R.A.; Freeman, C.; Parish, C.R. Heparanase and Type 1 Diabetes. Adv. Exp. Med. Biol. 2020, 1221, 607–630. [Google Scholar] [CrossRef] [PubMed]
- Dhoot, G.K.; Gustafsson, M.K.; Ai, X.; Sun, W.; Standiford, D.M.; Emerson, C.P., Jr. Regulation of Wnt signaling and embryo patterning by an extracellular sulfatase. Science 2001, 293, 1663–1666. [Google Scholar] [CrossRef] [PubMed]
- Ohto, T.; Uchida, H.; Yamazaki, H.; Keino-Masu, K.; Matsui, A.; Masu, M. Identification of a novel nonlysosomal sulphatase expressed in the floor plate, choroid plexus and cartilage. Genes Cells 2002, 7, 173–185. [Google Scholar] [CrossRef]
- Morimoto-Tomita, M.; Uchimura, K.; Werb, Z.; Hemmerich, S.; Rosen, S.D. Cloning and characterization of two extracellular heparin-degrading endosulfatases in mice and humans. J. Biol. Chem. 2002, 227, 49175–49185. [Google Scholar] [CrossRef]
- Saad, O.M.; Ebel, H.; Uchimura, K.; Rosen, S.D.; Bertozzi, C.R.; Leary, J.A. Compositional profiling of heparin/heparan sulfate using mass spectrometry: Assay for specificity of a novel extracellular human endosulfatase. Glycobiology 2005, 15, 818–826. [Google Scholar] [CrossRef]
- Ai, X.; Do, A.T.; Lozynska, O.; Kusche-Gullberg, M.; Lindahl, U.; Emerson, C.P., Jr. QSulf1 remodels the 6-O sulfation states of cell surface heparan sulfate proteoglycans to promote Wnt signaling. J. Biol. Chem. 2003, 162, 341–351. [Google Scholar] [CrossRef]
- Viviano, B.L.; Paine-Saunders, S.; Gasiunas, N.; Gallagher, J.; Saunders, S. Domain-specific modification of heparan sulfate by Qsulf1 modulates the binding of the bone morphogenetic protein antagonist Noggin. J. Biol. Chem. 2004, 279, 5604–5611. [Google Scholar] [CrossRef]
- Tran, T.H.; Shi, X.; Zaia, J.; Ai, X. Heparan sulfate 6-O-endosulfatases (Sulfs) coordinate the Wnt signaling pathways to regulate myoblast fusion during skeletal muscle regeneration. J. Biol. Chem. 2012, 287, 32651–32664. [Google Scholar] [CrossRef]
- Higginson, J.R.; Thompson, S.M.; Santos-Silva, A.; Guimond, S.E.; Turnbull, J.E.; Barnett, S.C. Differential sulfation remodelling of heparan sulfate by extracellular 6-O-sulfatases regulates fibroblast growth factor-induced boundary formation by glial cells: Implications for glial cell transplantation. J. Neurosci. 2012, 32, 15902–15912. [Google Scholar] [CrossRef]
- Dhanasekaran, R.; Nakamura, I.; Hu, C.; Chen, G.; Oseini, A.M.; Seven, E.S.; Miamen, A.G.; Moser, C.D.; Zhou, W.; van Kuppevelt, T.H.; et al. Activation of the transforming growth factor-β/SMAD transcriptional pathway underlies a novel tumor-promoting role of sulfatase 1 in hepatocellular carcinoma. Hepatology 2015, 61, 1269–1283. [Google Scholar] [CrossRef]
- Nakamura, I.; Asumda, F.Z.; Moser, C.D.; Kang, Y.N.N.; Lai, J.P.; Roberts, L.R. Sulfatase-2 Regulates Liver Fibrosis through the TGF-β Signaling Pathway. Cancers 2021, 3, 5279. [Google Scholar] [CrossRef]
- Xian, X.; Gopal, S.; Couchman, J.R. Syndecans as receptors and organizers of the extracellular matrix. Cell Tissue Res. 2010, 339, 31–46. [Google Scholar] [CrossRef]
- Elfenbein, A.; Simons, M. Syndecan-4 signaling at a glance. J. Cell Sci. 2013, 26, 3799–3804. [Google Scholar] [CrossRef]
- Cheng, B.; Montmasson, M.; Terradot, L.; Rousselle, P. Syndecans as Cell Surface Receptors in Cancer Biology. A Focus on their Interaction with PDZ Domain Proteins. Front. Pharmacol. 2016, 7, 10. [Google Scholar] [CrossRef]
- Gondelaud, F.; Ricard-Blum, S. Structures and interactions of syndecans. FEBS J. 2019, 286, 2994–3007. [Google Scholar] [CrossRef]
- Parish, C.R.; Freeman, C.; Brown, K.J.; Francis, D.J.; Cowden, W.B. Identification of sulfated oligosaccharide-based inhibitors of tumor growth and metastasis using novel in vitro assays for angiogenesis and heparanase activity. Cancer Res. 1999, 59, 3433–3441. [Google Scholar]
- Ferro, V.; Dredge, K.; Liu, L.; Hammond, E.; Bytheway, I.; Li, C.; Johnstone, K.; Karoli, T.; Davis, K.; Copeman, E.; et al. PI-88 and novel heparan sulfate mimetics inhibit angiogenesis. Semin. Thromb. Hemost. 2007, 33, 557–568. [Google Scholar] [CrossRef]
- Courtney, S.M.; Hay, P.A.; Buck, R.T.; Colville, C.S.; Phillips, D.J.; Scopes, D.I.; Pollard, F.C.; Page, M.J.; Bennett, J.M.; Hircock, M.L.; et al. Furanyl-1,3-thiazol-2-yl and benzoxazol-5-yl acetic acid derivatives: Novel classes of heparanase inhibitor. Bioorg. Med. Chem. Lett. 2005, 19, 2295–2299. [Google Scholar] [CrossRef]
- Ishihara, M.; Ono, K. Structure and Function of Heparin and Heparan Sulfate; Heparinoid Library and Modification of FGF-Activities. Trends Glycosci. Glycotechnol. 1998, 10, 223–233. [Google Scholar] [CrossRef]
- Yamada, S.; Sugahara, K.; Ozbek, S. Evolution of glycosaminoglycans: Comparative biochemical study. Commun. Integr. Biol. 2011, 4, 150–158. [Google Scholar] [CrossRef]
- Potter, K.J.; Werner, I.; Denroche, H.C.; Montane, J.; Plesner, A.; Chen, Y.; Lei, D.; Soukhatcheva, G.; Warnock, G.L.; Oberholzer, J.; et al. Amyloid Formation in Human Islets Is Enhanced by Heparin and Inhibited by Heparinase. Am. J. Transplant. 2015, 15, 1519–1530. [Google Scholar] [CrossRef]
- Oskarsson, M.E.; Singh, K.; Wang, J.; Vlodavsky, I.; Li, J.P.; Westermark, G.T. Heparan Sulfate Proteoglycans Are Important for Islet Amyloid Formation and Islet Amyloid Polypeptide-induced Apoptosis. J. Biol. Chem. 2015, 290, 15121–15132. [Google Scholar] [CrossRef]
- Takahashi, I.; Yamada, S.; Nata, K. Analysis of Syndecan-4 gene expression control mechanism in MIN6 cells. J. Diabetes Investig. 2017, 8, S44. [Google Scholar]
- Yoneda, A.; Asada, M.; Oda, Y.; Suzuki, M.; Imamura, T. Engineering of an FGF-proteoglycan fusion protein with heparin-independent, mitogenic activity. Nat. Biotechnol. 2000, 18, 641–644. [Google Scholar] [CrossRef]
- Imamura, T.; Friedman, S.A.; Gamble, S.; Tokita, Y.; Opalenik, S.R.; Thompson, J.A.; Maciag, T. Identification of the domain within fibroblast growth factor-1 responsible for heparin-dependence. Biochim. Biophys. Acta 1995, 1266, 124–130. [Google Scholar] [CrossRef]
- Asada, M.; Yoneda, A.; Imamura, T. Engineering of a Heparin-Binding Growth Factor with Heparan Sulfate Sugar Chains. Trends Glycosci. Glycotechnol. 2001, 13, 385–394. [Google Scholar] [CrossRef]
- Nakayama, F.; Hagiwara, A.; Umeda, S.; Asada, M.; Goto, M.; Oki, J.; Suzuki, M.; Imamura, T.; Akashi, M. Post treatment with an FGF chimeric growth factor enhances epithelial cell proliferation to improve recovery from radiation-induced intestinal damage. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 860–867. [Google Scholar] [CrossRef]
- Maeda, T.; Yamamoto, T.; Imamura, T.; Tsuboi, R. Impaired wound healing in bleomycin-induced murine scleroderma: A new model of wound retardation. Arch. Dermatol. Res. 2016, 308, 78–94. [Google Scholar] [CrossRef]
| Disaccharide Structures | Link between GAGs and Pancreatic Islets and β-Cells |
|---|---|
Heparin/Heparan sulfate (HS)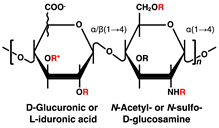 | Impaired postnatal islet growth, β-cell differentiation, and insulin secretion in HS biosynthesis enzyme knock out mice [7,8,9,10,11]. Islet-protective effects of heparanase inhibitors or HS in diabetic mouse models (avoidance of β-cell death and HS loss through the inhibition of heparanase activity) [12,17]. Contribution to β-cell mass and insulin secretion capacity in hereditary multiple exostosis subjects [13]. Different sulfate modification pattern features HS in α-cells and HS in β-cells of rat and human pancreatic islets [14]. Loss of HS and the heparanase expression through islet-infiltrating leukocytes in human type 1 diabetes mellitus (T1DM) islets [15]. Reduction of islet amyloid deposition by perlecan depletion in human islet amyloid polypeptide transgenic mice [28]. Contribution of HS to antioxidant activity and viability increased in transplanted islets [29]. |
Chondroitin/dermatan sulfate (CS/DS) | CS/DS is below the detection limit by high-performance liquid chromatography and immunostaining methods in mouse and rat islets [8,14,21,30]. |
Keratan sulfate (KS) | No reports of detection of KS chains in pancreatic islets to date. Lumican, a KS core-protein, is expressed in human pancreaticα- and human pancreatic ductal adenocarcinoma cells [31,32,33,34,35,36]. |
Hyaluronan (HA)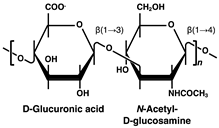 | HA accumulation in pancreatic islets with T1DM progressing and the following inflammation and β-cell destruction [37,38,39,40]. HA directly impaired in the insulin secretory function of β-cells in vitro [41]. |
| Antibody | Binding Sites of Antibodies in Pancreatic Islets | HS Modifications Required for Antibody Binding |
|---|---|---|
| 10E4 | Mouse intra-islet-β-cells [12], human intra-β-cells [14,15], and rat islets basement membranes [14] | N-acetylation/sulfation |
| RB4EA12 | Human and rat intra-β-cells [14] | N-sulfation, 2-O-, 6-O-, C5-epimerization |
| AO4B08 | Human and rat intra-β-cells [14] | N-acetylation/sulfation, 6-O-sulfation |
| HepSS-1 | Mouse islet-β-cell surface [12] | N-sulfation |
| EV3C3 * | Human and rat α-cells [14] | N-sulfation, 2-O-sulfation, C5-epimerization |
| HS4E4 ** | Human and rat α-cells [14] | N-acetylation/sulfation, C5-epimerization |
| HS4C3 | Nuclei of the cells [14] | N-sulfation, 2-O-, 6-O-, 3-O-sulfation |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takahashi, I. Importance of Heparan Sulfate Proteoglycans in Pancreatic Islets and β-Cells. Int. J. Mol. Sci. 2022, 23, 12082. https://doi.org/10.3390/ijms232012082
Takahashi I. Importance of Heparan Sulfate Proteoglycans in Pancreatic Islets and β-Cells. International Journal of Molecular Sciences. 2022; 23(20):12082. https://doi.org/10.3390/ijms232012082
Chicago/Turabian StyleTakahashi, Iwao. 2022. "Importance of Heparan Sulfate Proteoglycans in Pancreatic Islets and β-Cells" International Journal of Molecular Sciences 23, no. 20: 12082. https://doi.org/10.3390/ijms232012082
APA StyleTakahashi, I. (2022). Importance of Heparan Sulfate Proteoglycans in Pancreatic Islets and β-Cells. International Journal of Molecular Sciences, 23(20), 12082. https://doi.org/10.3390/ijms232012082




