Intrinsic Expression of Coagulation Factors and Protease Activated Receptor 1 (PAR1) in Photoreceptors and Inner Retinal Layers
Abstract
1. Introduction
2. Results
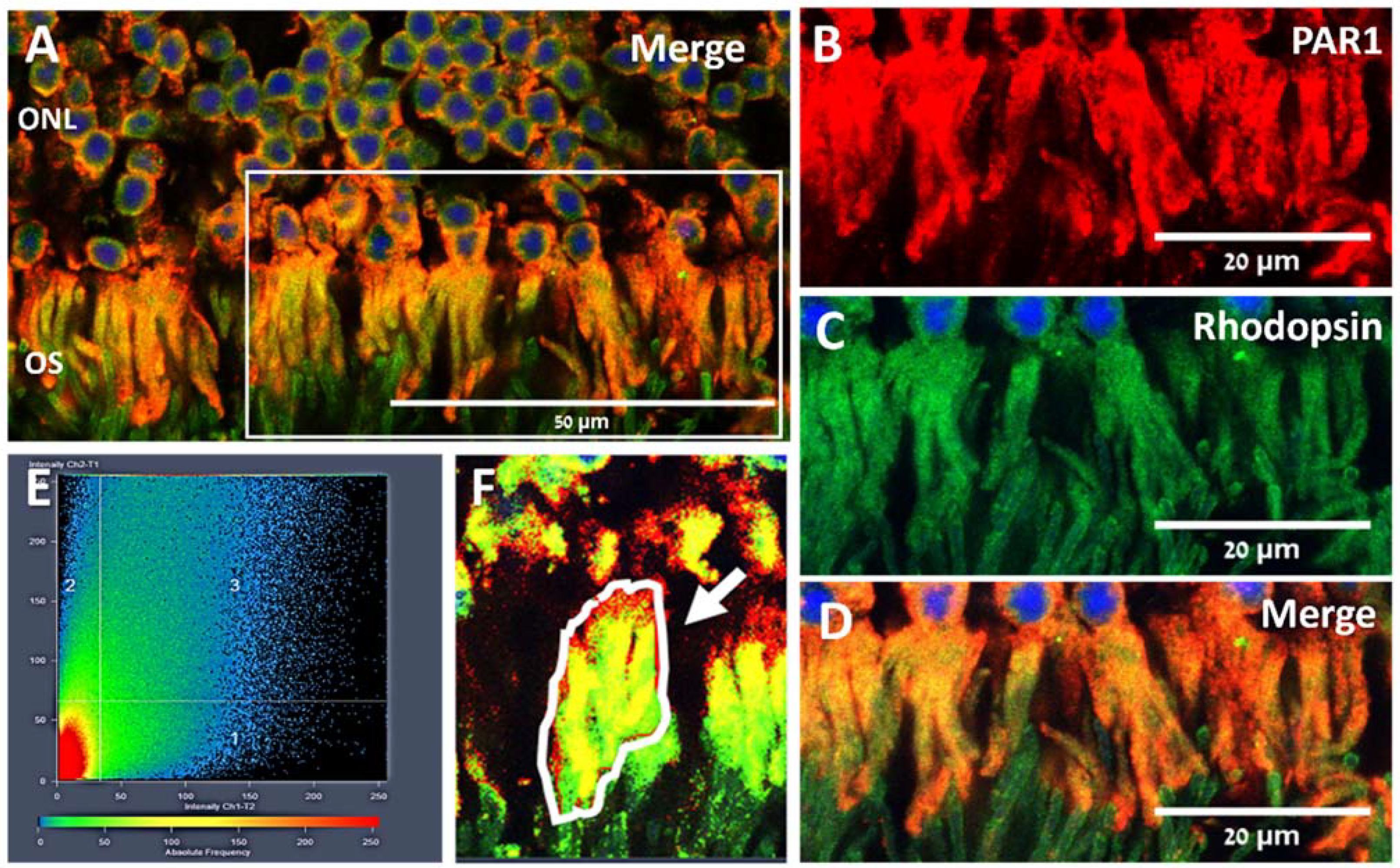
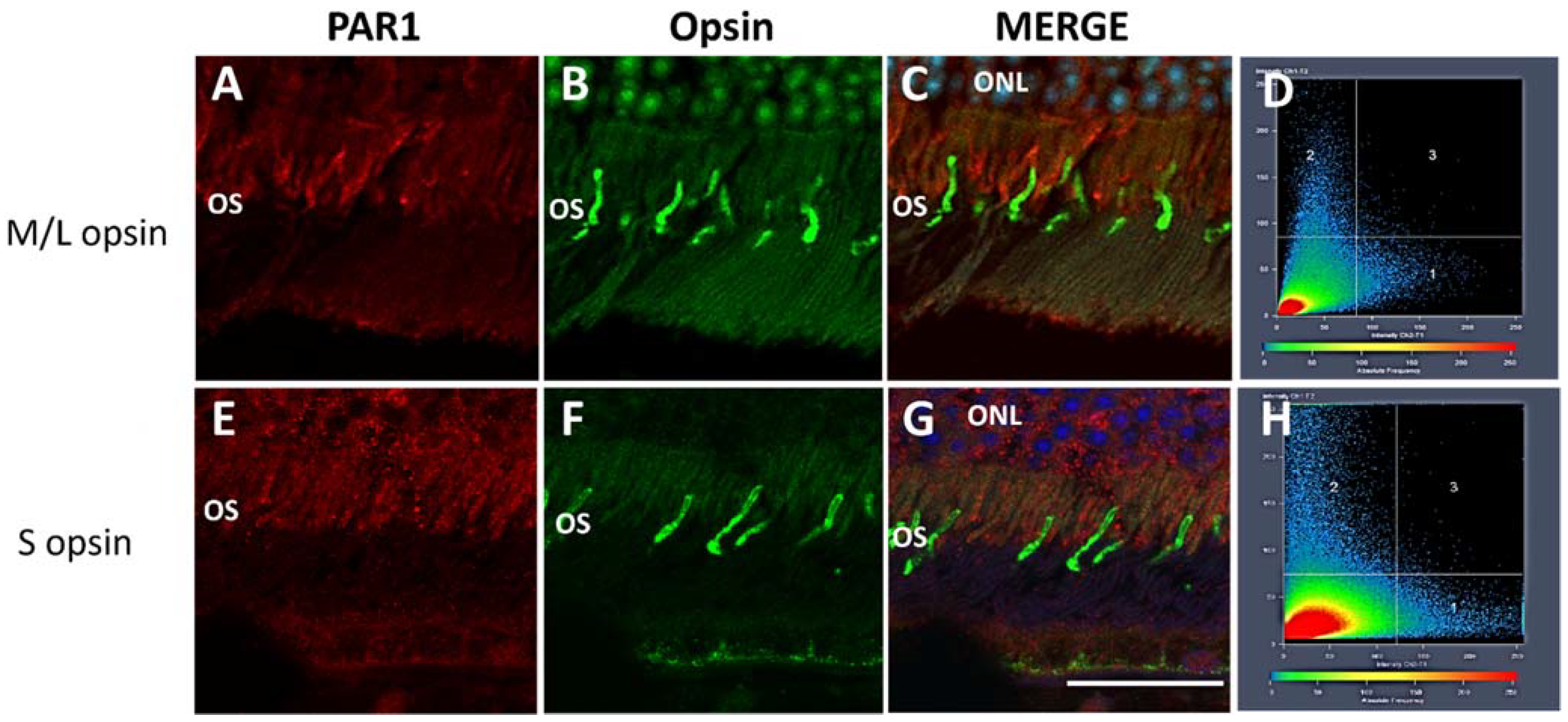
2.1. PAR1 Expression Pattern in Mouse Neuroretina by Immunofluorescence
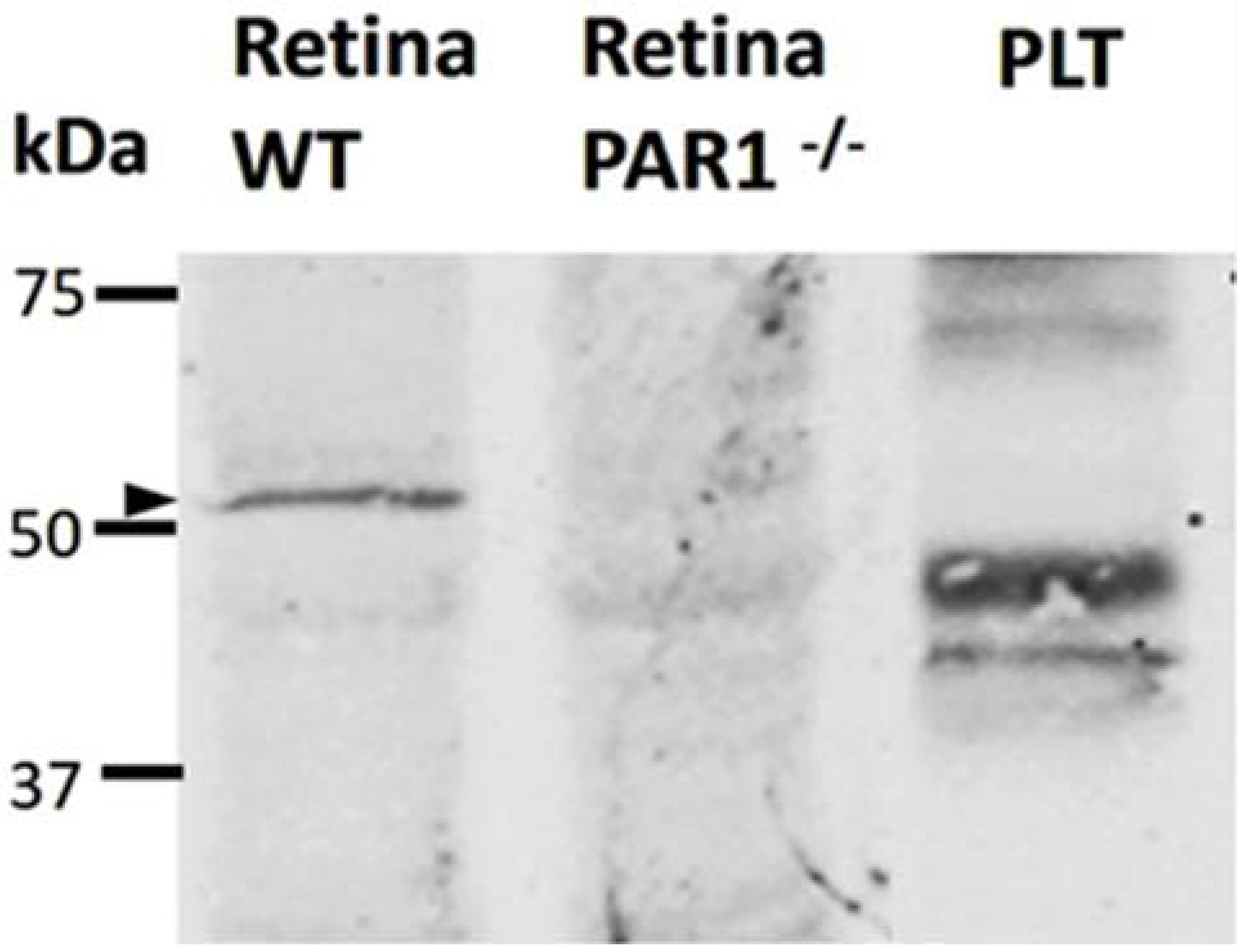
2.2. PAR1 Expression in the Neuroretina by Western Blot Analysis
2.3. Expression of PAR1, Coagulation Factor X, and Prothrombin mRNA in Mouse Neuroretina
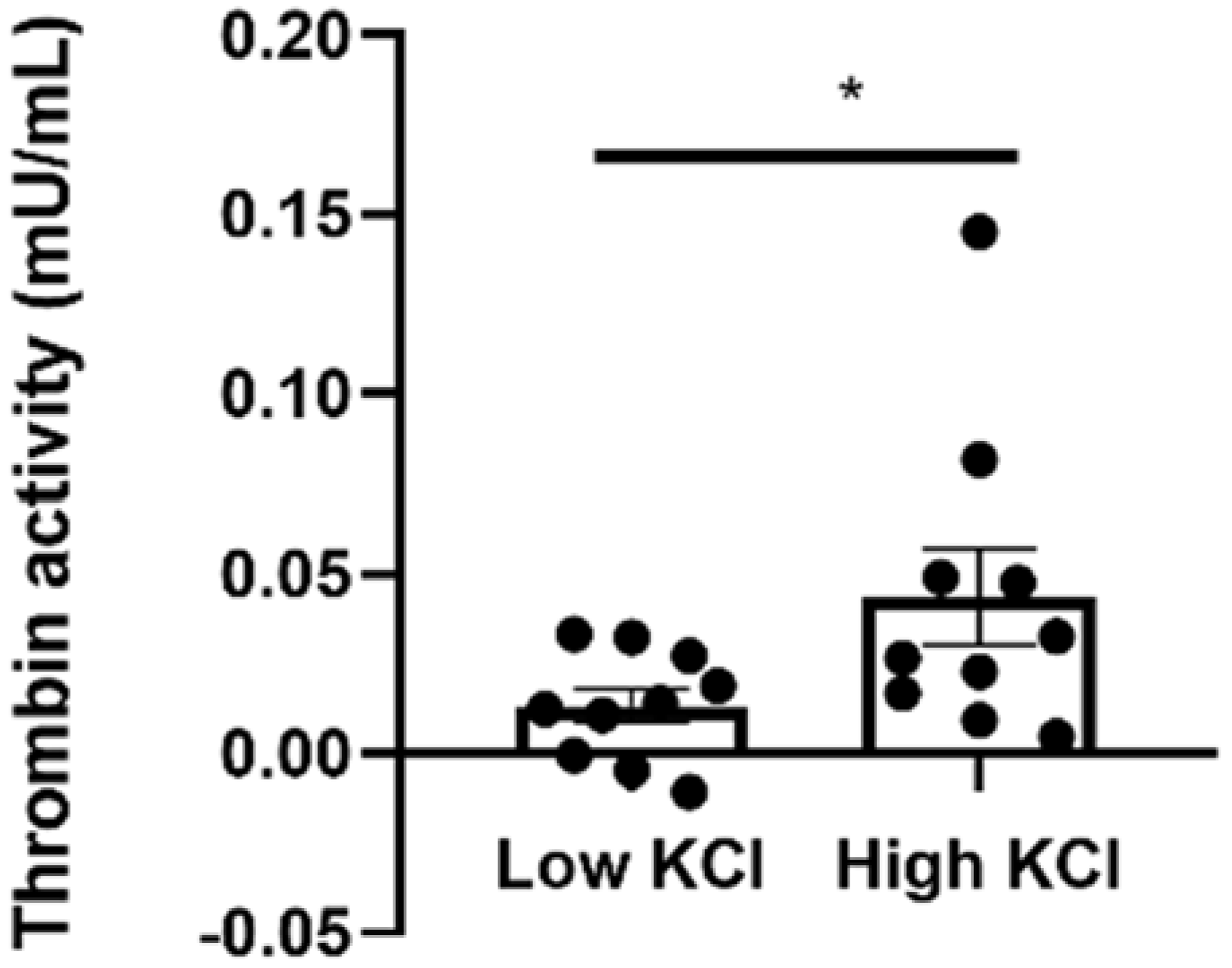
2.4. Thrombin Activity Is Elevated in Isolated Neuroretinas Ex Vivo following KCl Depolarization
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Animals
5.2. Immunofluorescence Staining
5.3. Co-Localization Analysis
5.4. Isolation of Mouse Neuroretinas
5.5. Protein Extraction from Mouse Neuroretinas and Platelets
5.6. Western Blot Analysis
5.7. Quantitative Reverse Transcription Real-Time PCR (qRT-PCR)
5.8. Thrombin Activity Assay
5.9. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rohatgi, T.; Sedehizade, F.; Sabel, B.A.; Reiser, G. Protease-activated receptor subtype expression in developing eye and adult retina of the rat after optic nerve crush. J. Neurosci. Res. 2003, 73, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Arora, P.; Ricks, T.K.; Trejo, J. Protease-activated receptor signalling, endocytic sorting and dysregulation in cancer. J. Cell Sci. 2007, 120, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.-J.; Woo, M.-S.; Moon, P.-G.; Baek, M.-C.; Choi, I.-Y.; Kim, W.-K.; Junn, E.; Kim, H.-S. Alpha-synuclein activates microglia by inducing the expressions of matrix metalloproteinases and the subsequent activation of protease-activated receptor-1. J. Immunol. 2010, 185, 615–623. [Google Scholar] [CrossRef]
- Shavit-Stein, E.; Sheinberg, E.; Golderman, V.; Sharabi, S.; Wohl, A.; Gofrit, S.G.; Zivli, Z.; Shelestovich, N.; Last, D.; Guez, D.; et al. A novel compound targeting protease receptor 1 activators for the treatment of glioblastoma. Front. Neurol. 2018, 9, 1087. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Garton, H.J.L.; Hua, Y.; Keep, R.F.; Xi, G. The role of thrombin in brain injury after hemorrhagic and ischemic stroke. Transl. Stroke Res. 2020, 12, 496–511. [Google Scholar] [CrossRef]
- Shavit-Stein, E.; Abu Rahal, I.; Bushi, D.; Gera, O.; Sharon, R.; Gofrit, S.G.; Pollak, L.; Mindel, K.; Maggio, N.; Kloog, Y.; et al. Brain protease activated receptor 1 pathway: A therapeutic target in the superoxide dismutase 1 (SOD1) mouse model of amyotrophic lateral sclerosis. Int. J. Mol. Sci. 2020, 21, 3419. [Google Scholar] [CrossRef]
- Gera, O.; Shavit-Stein, E.; Bushi, D.; Harnof, S.; Shimon, M.B.; Weiss, R.; Golderman, V.; Dori, A.; Maggio, N.; Finegold, K.; et al. Thrombin and protein C pathway in peripheral nerve Schwann cells. Neuroscience 2016, 339, 587–598. [Google Scholar] [CrossRef] [PubMed]
- Shavit, E.; Beilin, O.; Korczyn, A.D.; Sylantiev, C.; Aronovich, R.; Drory, V.E.; Gurwitz, D.; Horresh, I.; Bar-Shavit, R.; Peles, E.; et al. Thrombin receptor PAR-1 on myelin at the node of Ranvier: A new anatomy and physiology of conduction block. Brain 2008, 131, 1113–1122. [Google Scholar] [CrossRef] [PubMed]
- Pompili, E.; De Franchis, V.; Giampietri, C.; Leone, S.; De Santis, E.; Fornai, F.; Fumagalli, L.; Fabrizi, C. Protease activated receptor 1 and its ligands as main regulators of the regeneration of peripheral nerves. Biomolecules 2021, 11, 1668. [Google Scholar] [CrossRef]
- Sakamoto, T.; Sakamoto, H.; Sheu, S.J.; Gabrielian, K.; Ryan, S.J.; Hinton, D.R. Intercellular gap formation induced by thrombin in confluent cultured bovine retinal pigment epithelial cells. Investig. Ophthalmol. Vis. Sci. 1994, 35, 720–729. [Google Scholar]
- Abu El-Asrar, A.M.; Alam, K.; Nawaz, M.I.; Mohammad, G.; Van den Eynde, K.; Siddiquei, M.M.; Mousa, A.; De Hertogh, G.; Opdenakker, G. Upregulation of Thrombin/Matrix Metalloproteinase-1/Protease-Activated Receptor-1 Chain in proliferative diabetic retinopathy. Curr. Eye Res. 2016, 41, 1590–1600. [Google Scholar] [CrossRef]
- Hollborn, M.; Petto, C.; Steffen, A.; Trettner, S.; Bendig, A.; Wiedemann, P.; Bringmann, A.; Kohen, L. Effects of thrombin on RPE cells are mediated by transactivation of growth factor receptors. Investig. Ophthalmol. Vis. Sci. 2009, 50, 4452–4459. [Google Scholar] [CrossRef][Green Version]
- Hammes, H.P.; Lin, J.; Bretzel, R.G.; Brownlee, M.; Breier, G. Upregulation of the vascular endothelial growth factor/vascular endothelial growth factor receptor system in experimental background diabetic retinopathy of the rat. Diabetes 1998, 47, 401–406. [Google Scholar] [CrossRef]
- Aguilar-Solis, E.D.; Lee-Rivera, I.; Álvarez-Arce, A.; López, E.; López-Colomé, A.M. FAK phosphorylation plays a central role in thrombin-induced RPE cell migration. Cell. Signal. 2017, 36, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Bastiaans, J.; van Meurs, J.C.; van Holten-Neelen, C.; Nijenhuis, M.S.-T.; Kolijn-Couwenberg, M.J.; van Hagen, P.M.; Kuijpers, R.W.A.M.; Hooijkaas, H.; Dik, W.A. Factor Xa and thrombin stimulate proinflammatory and profibrotic mediator production by retinal pigment epithelial cells: A role in vitreoretinal disorders? Graefes Arch. Clin. Exp. Ophthalmol. 2013, 251, 1723–1733. [Google Scholar] [CrossRef]
- Kowluru, R.A.; Odenbach, S. Role of interleukin-1beta in the pathogenesis of diabetic retinopathy. Br. J. Ophthalmol. 2004, 88, 1343–1347. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Wang, Y.; Reiser, G. Two types of protease-activated receptors (PAR-1 and PAR-2) mediate calcium signaling in rat retinal ganglion cells RGC-5. Brain Res. 2005, 1047, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Lee-Rivera, I.; López, E.; Carranza-Pérez, M.G.; López-Colomé, A.M. PKC-ζ regulates thrombin-induced proliferation of human Müller glial cells. Investig. Ophthalmol. Vis. Sci. 2016, 57, 3769–3779. [Google Scholar] [CrossRef] [PubMed]
- Arachiche, A.; de la Fuente, M.; Nieman, M.T. Platelet specific promoters are insufficient to express protease activated receptor 1 (PAR1) transgene in mouse platelets. PLoS ONE 2014, 9, e97724. [Google Scholar] [CrossRef]
- Massey, S.C.; Neal, M.J. Release of [3H]-acetylcholine from the isolated retina of the rat by potassium depolarization: Dependence on high affinity choline uptake. Br. J. Pharmacol. 1979, 65, 271–276. [Google Scholar] [CrossRef]
- Ackerman, J.M.; Gnegy, M.E. Desensitization of the dopaminergic system in bovine retina following incubation with high potassium. Brain Res. 1990, 521, 296–302. [Google Scholar] [CrossRef]
- Calderón, F.; Rodríguez, G.; López, E.; López-Colomé, A.M. Calcium-independent release of [3H]spermine from chick retina. Brain Res. 2000, 854, 1–5. [Google Scholar] [CrossRef]
- Smith, L.F.; Pycock, C.J. Potassium-stimulated release of radiolabelled taurine and glycine from the isolated rat retina. J. Neurochem. 1982, 39, 653–658. [Google Scholar] [CrossRef]
- Haider, S.Z.; Sadanandan, N.P.; Joshi, P.G.; Mehta, B. Early diabetes induces changes in mitochondrial physiology of inner retinal neurons. Neuroscience 2019, 406, 140–149. [Google Scholar] [CrossRef]
- Blackshaw, S.; Harpavat, S.; Trimarchi, J.; Cai, L.; Huang, H.; Kuo, W.P.; Weber, G.; Lee, K.; Fraioli, R.E.; Cho, S.-H.; et al. Genomic analysis of mouse retinal development. PLoS Biol. 2004, 2, e247. [Google Scholar] [CrossRef] [PubMed]
- Carter-Dawson, L.D.; LaVail, M.M. Rods and cones in the mouse retina. I. Structural analysis using light and electron microscopy. J. Comp. Neurol. 1979, 188, 245–262. [Google Scholar] [CrossRef]
- Ingram, N.T.; Sampath, A.P.; Fain, G.L. Why are rods more sensitive than cones? J. Physiol. 2016, 594, 5415–5426. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, Y.; Morigiwa, K.; Ueda, M.; Sterling, P. Microcircuits for night vision in mouse retina. J. Neurosci. 2001, 21, 8616–8623. [Google Scholar] [CrossRef] [PubMed]
- Puro, D.G.; Stuenkel, E.L. Thrombin-induced inhibition of potassium currents in human retinal glial (Müller) cells. J. Physiol. 1995, 485 Pt 2, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Rauen, T.; Taylor, W.R.; Kuhlbrodt, K.; Wiessner, M. High-affinity glutamate transporters in the rat retina: A major role of the glial glutamate transporter GLAST-1 in transmitter clearance. Cell Tissue Res. 1998, 291, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Barnett, N.L.; Pow, D.V. Antisense Knockdown of GLAST, a Glial Glutamate Transporter, Compromises Retinal Function | IOVS | ARVO Journals. Investig. Ophthalmol. Vis. Sci. 2000, 41, 585–591. [Google Scholar]
- Brew, H.; Attwell, D. Electrogenic glutamate uptake is a major current carrier in the membrane of axolotl retinal glial cells. Nature 1987, 327, 707–709. [Google Scholar] [CrossRef] [PubMed]
- Barbour, B.; Brew, H.; Attwell, D. Electrogenic glutamate uptake in glial cells is activated by intracellular potassium. Nature 1988, 335, 433–435. [Google Scholar] [CrossRef] [PubMed]
- Sarantis, M.; Attwell, D. Glutamate uptake in mammalian retinal glia is voltage- and potassium-dependent. Brain Res. 1990, 516, 322–325. [Google Scholar] [CrossRef]
- Ulbricht, E.; Pannicke, T.; Hollborn, M.; Raap, M.; Goczalik, I.; Iandiev, I.; Härtig, W.; Uhlmann, S.; Wiedemann, P.; Reichenbach, A.; et al. Proliferative gliosis causes mislocation and inactivation of inwardly rectifying K(+) (Kir) channels in rabbit retinal glial cells. Exp. Eye Res. 2008, 86, 305–313. [Google Scholar] [CrossRef]
- Francke, M.; Pannicke, T.; Biedermann, B.; Faude, F.; Wiedemann, P.; Reichenbach, A.; Reichelt, W. Loss of inwardly rectifying potassium currents by human retinal glial cells in diseases of the eye. Glia 1997, 20, 210–218. [Google Scholar] [CrossRef]
- Maggio, N.; Itsekson, Z.; Dominissini, D.; Blatt, I.; Amariglio, N.; Rechavi, G.; Tanne, D.; Chapman, J. Thrombin regulation of synaptic plasticity: Implications for physiology and pathology. Exp. Neurol. 2013, 247, 595–604. [Google Scholar] [CrossRef]
- Chavkin, N.W.; Walsh, K.; Hirschi, K.K. Isolation of highly purified and viable retinal endothelial cells. J. Vasc. Res. 2021, 58, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Edelshtain, V.; Peled, A.; Tzameret, A.; Pri Chen, S.; Ziv, H.; Derazne, E.; Harats, D.; Greenberg, R.; Harari, A.; Shaish, A.; et al. Long-term treatment with 9-cis-β-carotene rich alga Dunaliella bardawil ameliorates photoreceptor degeneration in a mouse model of retinoid cycle defect. Algal Res. 2019, 43, 101607. [Google Scholar] [CrossRef]
- Tzameret, A.; Sher, I.; Belkin, M.; Treves, A.J.; Meir, A.; Nagler, A.; Levkovitch-Verbin, H.; Barshack, I.; Rosner, M.; Rotenstreich, Y. Transplantation of human bone marrow mesenchymal stem cells as a thin subretinal layer ameliorates retinal degeneration in a rat model of retinal dystrophy. Exp. Eye Res. 2014, 118, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Bushi, D.; Gera, O.; Kostenich, G.; Shavit-Stein, E.; Weiss, R.; Chapman, J.; Tanne, D. A novel histochemical method for the visualization of thrombin activity in the nervous system. Neuroscience 2016, 320, 93–104. [Google Scholar] [CrossRef]
- Bushi, D.; Chapman, J.; Katzav, A.; Shavit-Stein, E.; Molshatzki, N.; Maggio, N.; Tanne, D. Quantitative detection of thrombin activity in an ischemic stroke model. J. Mol. Neurosci. 2013, 51, 844–850. [Google Scholar] [CrossRef]
- Itsekson-Hayosh, Z.; Shavit-Stein, E.; Last, D.; Goez, D.; Daniels, D.; Bushi, D.; Gera, O.; Zibly, Z.; Mardor, Y.; Chapman, J.; et al. Thrombin activity and thrombin receptor in rat glioblastoma model: Possible markers and targets for intervention? J. Mol. Neurosci. 2015, 56, 644–651. [Google Scholar] [CrossRef] [PubMed]
- Tzameret, A.; Ketter-Katz, H.; Edelshtain, V.; Sher, I.; Corem-Salkmon, E.; Levy, I.; Last, D.; Guez, D.; Mardor, Y.; Margel, S.; et al. In vivo MRI assessment of bioactive magnetic iron oxide/human serum albumin nanoparticle delivery into the posterior segment of the eye in a rat model of retinal degeneration. J. Nanobiotechnol. 2019, 17, 3. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goldberg, Z.; Sher, I.; Qassim, L.; Chapman, J.; Rotenstreich, Y.; Shavit-Stein, E. Intrinsic Expression of Coagulation Factors and Protease Activated Receptor 1 (PAR1) in Photoreceptors and Inner Retinal Layers. Int. J. Mol. Sci. 2022, 23, 984. https://doi.org/10.3390/ijms23020984
Goldberg Z, Sher I, Qassim L, Chapman J, Rotenstreich Y, Shavit-Stein E. Intrinsic Expression of Coagulation Factors and Protease Activated Receptor 1 (PAR1) in Photoreceptors and Inner Retinal Layers. International Journal of Molecular Sciences. 2022; 23(2):984. https://doi.org/10.3390/ijms23020984
Chicago/Turabian StyleGoldberg, Zehavit, Ifat Sher, Lamis Qassim, Joab Chapman, Ygal Rotenstreich, and Efrat Shavit-Stein. 2022. "Intrinsic Expression of Coagulation Factors and Protease Activated Receptor 1 (PAR1) in Photoreceptors and Inner Retinal Layers" International Journal of Molecular Sciences 23, no. 2: 984. https://doi.org/10.3390/ijms23020984
APA StyleGoldberg, Z., Sher, I., Qassim, L., Chapman, J., Rotenstreich, Y., & Shavit-Stein, E. (2022). Intrinsic Expression of Coagulation Factors and Protease Activated Receptor 1 (PAR1) in Photoreceptors and Inner Retinal Layers. International Journal of Molecular Sciences, 23(2), 984. https://doi.org/10.3390/ijms23020984




