Severe Natural Outbreak of Cryptocaryon irritans in Gilthead Seabream Produces Leukocyte Mobilization and Innate Immunity at the Gill Tissue
Abstract
:1. Introduction
2. Results
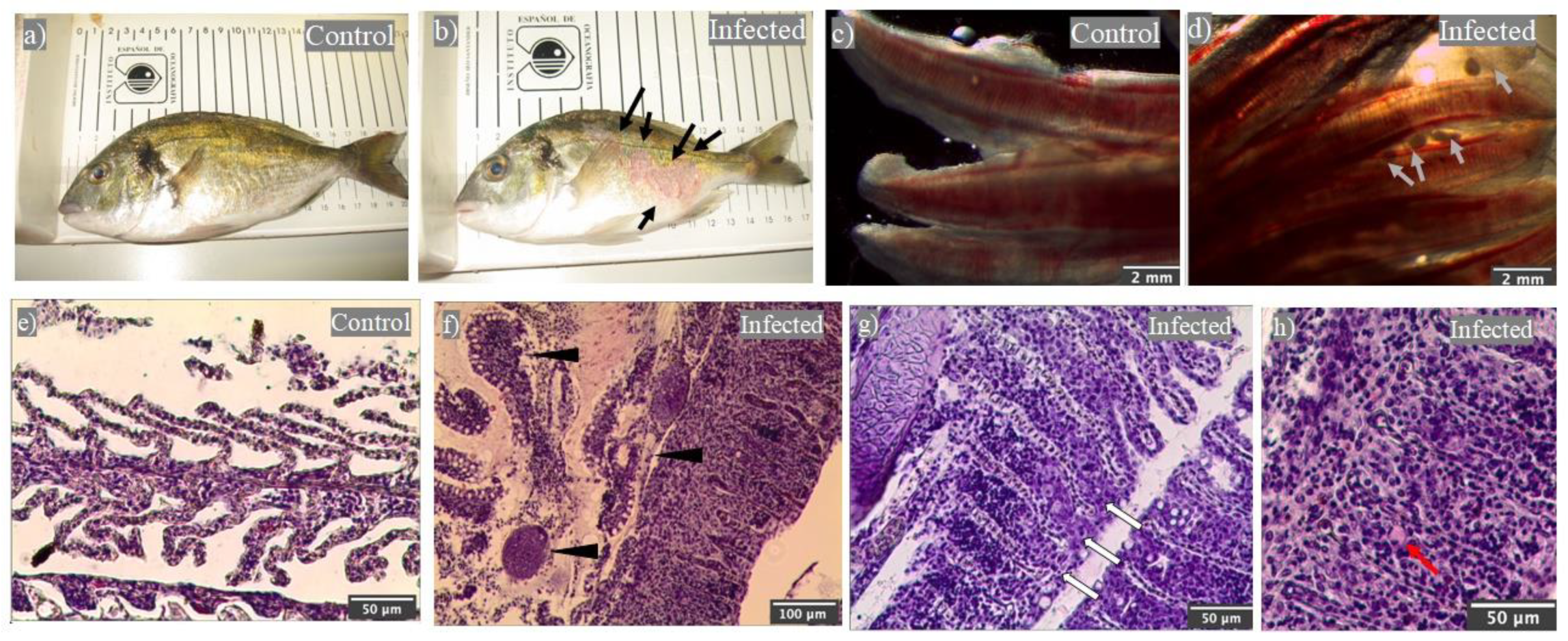
2.1. Natural Outbreak of Cryptocaryon irritans Produced Gilthead Seabream Mortality
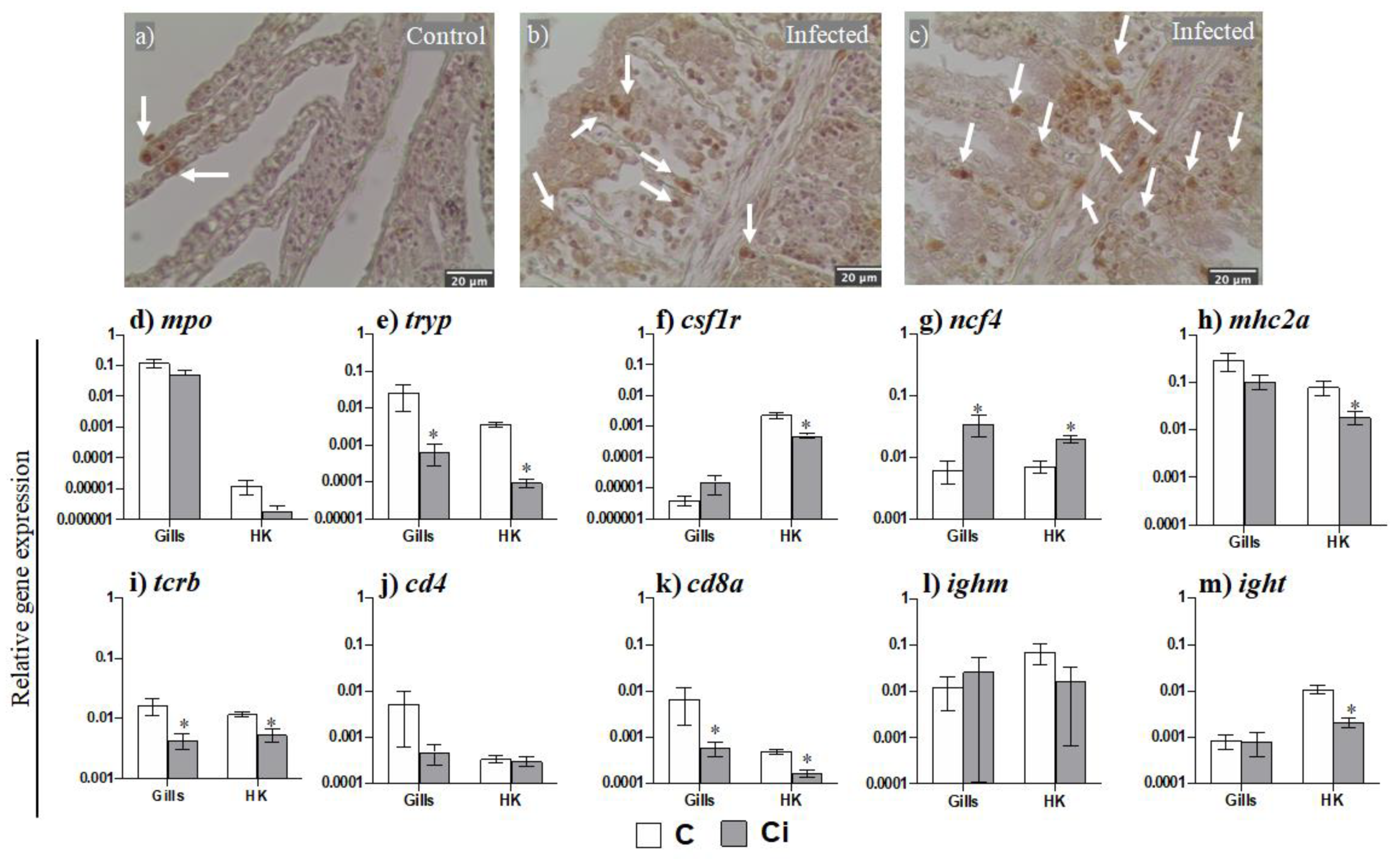
2.2. Leukocytes, Namely Acidophilic Granulocytes, Are Mobilized upon Infection
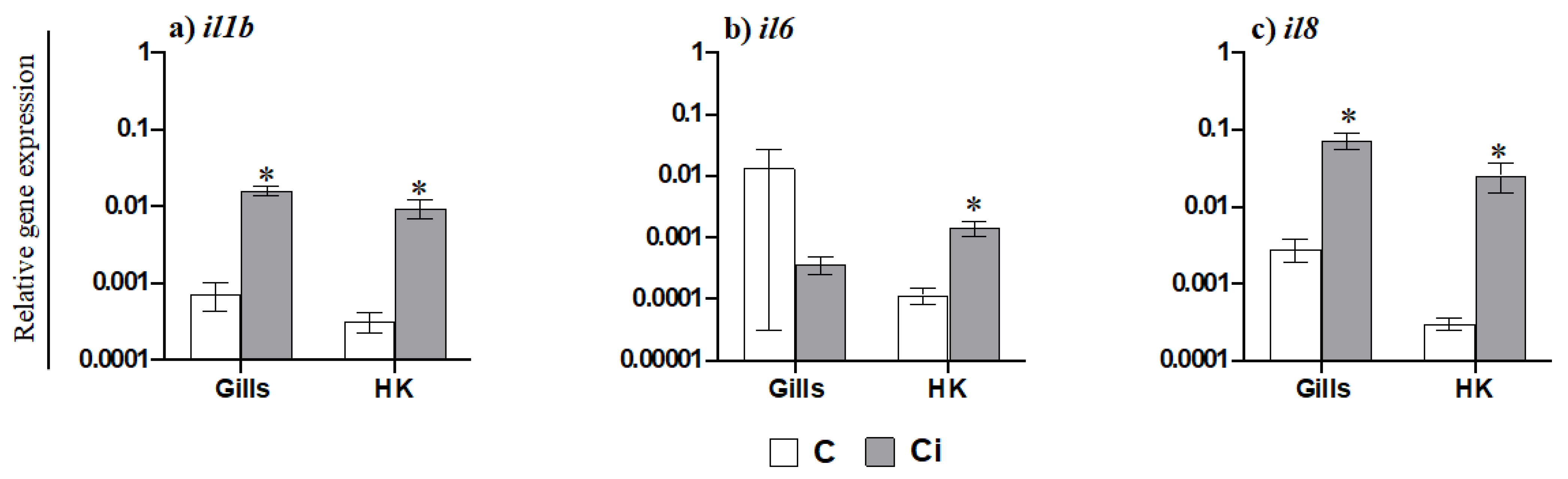
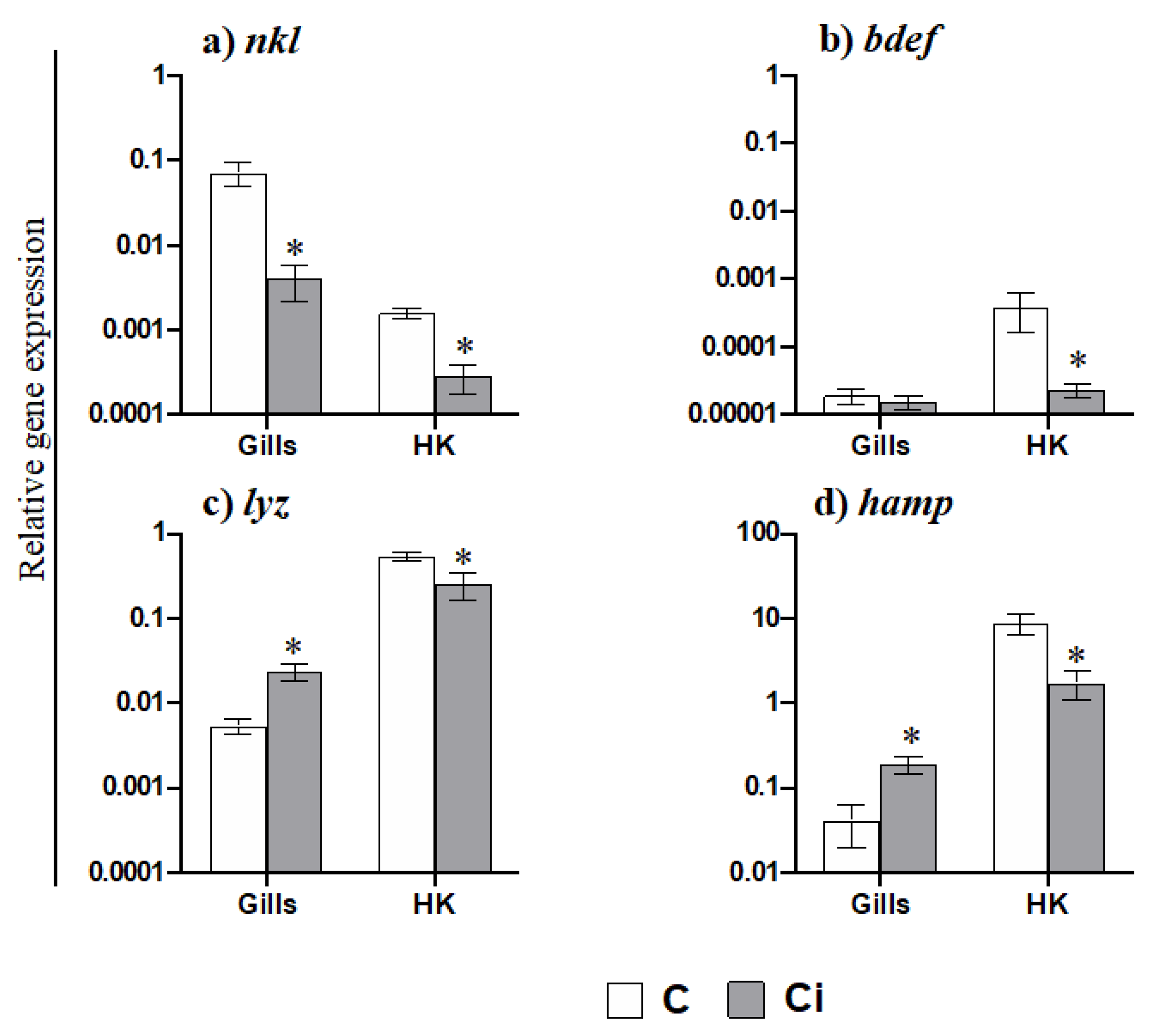
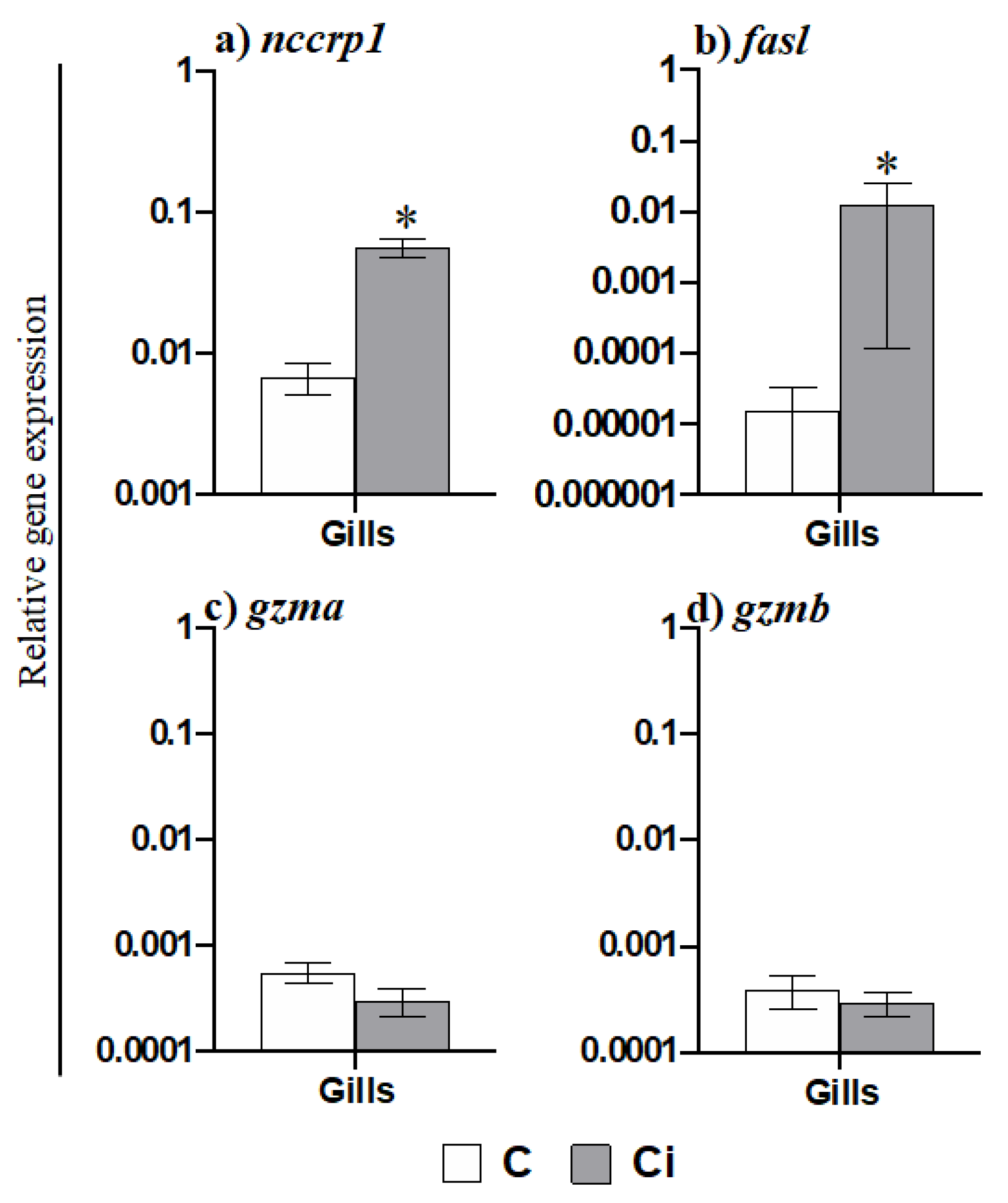
2.3. C. irritans Infection Produces Inflammation and Activation of the AMP and CMC Responses in the Gills
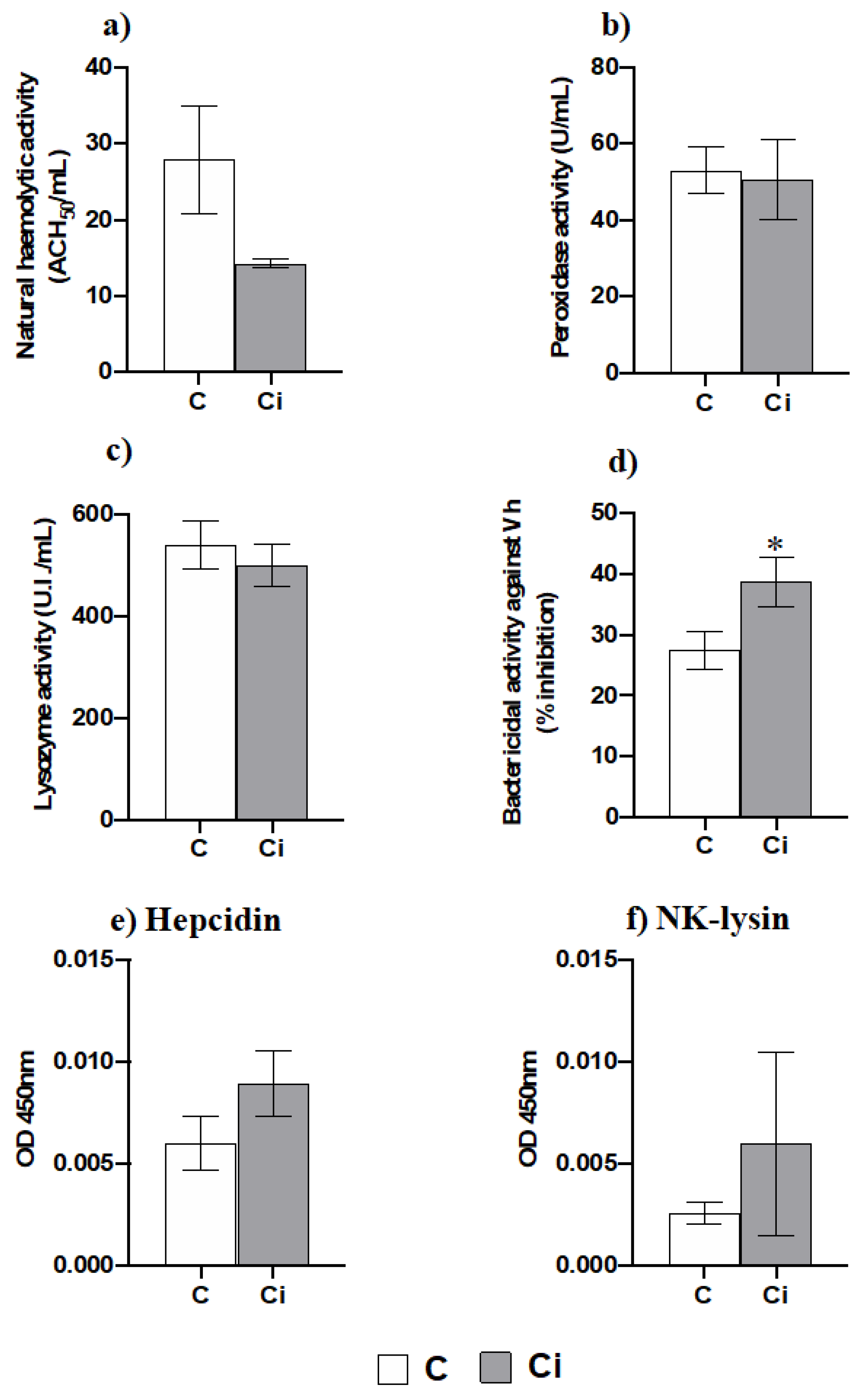
2.4. Infection with C. irritans Produces Little Changes in the Serum Innate Immunity but Increases the Bactericidal Activity
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Natural Outbreak and Sampling
4.3. Light Microscopy and Immunohistochemistry (IHC)
4.4. Hepcidin and NK-Lysin Quantification by ELISA
4.5. Analysis of Gene Expression by Real-Time PCR
4.6. Antimicrobial Activities in Serum
4.6.1. Natural Haemolytic Complement Activity
4.6.2. Lysozyme Activity
4.6.3. Bactericidal Activity
4.6.4. Peroxidase Activity
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture 2020; Food and Agriculture Organization of the United Nations: Rome, Italy, 2020; ISBN 9789251326923. [Google Scholar]
- APROMAR. Aquaculture in Spain 2020; Apromar Asociación Empresarial de Acuicultura de España: Cádiz, Spain, 2020; ISBN 9788578110796. [Google Scholar]
- Colorni, A. Aspects of the biology of Cryptocaryon irritans and hyposalinity as a control measure in cultured gilthead seabream Sparus aurata. Dis. Aquat. Organ. 1985, 1, 19–22. [Google Scholar] [CrossRef]
- Wright, A.-D.; Colorni, A. Taxonomic re-assignment of Cryptocaryon irritans, a marine fish parasite. Eur. J. Protistol. 2002, 37, 375–378. [Google Scholar] [CrossRef]
- Diggles, B.K.; Lester, R.J.G. Influence of temperature and host species on the development of Cryptocaryon irritans. J. Parasitol. 1996, 82, 45. [Google Scholar] [CrossRef] [PubMed]
- Harikrishnan, R.; Balasundaram, C.; Heo, M.S. Molecular studies, disease status and prophylactic measures in grouper aquaculture: Economic importance, diseases and immunology. Aquaculture 2010, 309, 1–14. [Google Scholar] [CrossRef]
- Li, Y.W.; Dan, X.M.; Zhang, T.W.; Luo, X.C.; Li, A.X. Immune-related genes expression profile in orange-spotted grouper during exposure to Cryptocaryon irritans. Parasite Immunol. 2011, 33, 679–987. [Google Scholar] [CrossRef] [PubMed]
- Maha, I.F.; Xie, X.; Zhou, S.; Yu, Y.; Liu, X.; Zahid, A.; Lei, Y.; Ma, R.; Yin, F.; Qian, D. Skin metabolome reveals immune responses in yellow drum Nibea albiflora to Cryptocaryon irritans infection. Fish Shellfish Immunol. 2019, 94, 661–674. [Google Scholar] [CrossRef]
- Picón-Camacho, S.M.; Ruiz de Ybáñez, M.R.; Holzer, A.S.; Arizcun Arizcun, M.; Muñoz, P. In vitro treatments for the theront stage of the ciliate protozoan Cryptocaryon irritans. Dis. Aquat. Organ. 2011, 94, 167–172. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Jiang, B.; Mo, Z.; Li, A.; Dan, X. Cryptocaryon irritans (Brown, 1951) is a serious threat to aquaculture of marine fish. Rev. Aquac. 2021, 14, 218–236. [Google Scholar] [CrossRef]
- Colorni, A.; Burgess, P. Cryptocaryon irritans Brown 1951, the cause of ‘white spot disease’ in marine fish: An update. Aquar. Sci. Conserv. 1997, 1, 217–238. [Google Scholar] [CrossRef]
- Colorni, A.; Diamant, A. Ultrastructural features of Cryptocaryon irritans, a ciliate parasite of marine fish. Eur. J. Protistol. 1993, 29, 425–434. [Google Scholar] [CrossRef]
- Álvarez-Pellitero, P. Fish immunity and parasite infections: From innate immunity to immunoprophylactic prospects. Vet. Immunol. Immunopathol. 2008, 126, 171–198. [Google Scholar] [CrossRef]
- de Melo Souza, D.C.; dos Santos, M.C.; Chagas, E.C. Immune response of teleost fish to helminth parasite infection. Rev. Bras. Parasitol. Vet. 2019, 28, 533–547. [Google Scholar] [CrossRef]
- Sitja-Bobadilla, A. Living off a fish: A trade-off between parasites and the immune system. Fish Shellfish Immunol. 2008, 25, 358–372. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Luo, X.; Dan, X.; Huang, X.; Qiao, W. Orange-spotted grouper (Epinephelus coioides) TLR2, MyD88 and IL-1b involved in anti-Cryptocaryon irritans response. Fish Shellfish Immunol. 2011, 30, 1230–1240. [Google Scholar] [CrossRef]
- Khoo, C.K.; Abdul-Murad, A.M.; Kua, B.C.; Mohd-Adnan, A. Cryptocaryon irritans infection induces the acute phase response in Lates calcarifer: A transcriptomic perspective. Fish Shellfish Immunol. 2012, 33, 788–794. [Google Scholar] [CrossRef]
- Mohd-Shaharuddin, N.; Mohd-Adnan, A.; Kua, B.C.; Nathan, S. Expression profile of immune-related genes in Lates calcarifer infected by Cryptocaryon irritans. Fish Shellfish Immunol. 2013, 34, 762–769. [Google Scholar] [CrossRef]
- Xie, J.; Obiefuna, V.; Hodgkinson, J.W.; McAllister, M.; Belosevic, M. Teleost antimicrobial peptide hepcidin contributes to host defense of goldfish (Carassius auratus L.) against Trypanosoma carassii. Dev. Comp. Immunol. 2019, 94, 11–15. [Google Scholar] [CrossRef]
- Zheng, L.B.; Mao, Y.; Wang, J.; Chen, R.N.; Su, Y.Q.; Hong, Y.Q.; Hong, Y.J.; Hong, Y.C. Excavating differentially expressed antimicrobial peptides from transcriptome of Larimichthys crocea liver in response to Cryptocaryon irritans. Fish Shellfish Immunol. 2018, 75, 109–114. [Google Scholar] [CrossRef]
- Zhou, Q.J.; Wang, J.; Liu, M.; Qiao, Y.; Hong, W.S.; Su, Y.Q.; Han, K.H.; Ke, Q.Z.; Zheng, W.Q. Identification, expression and antibacterial activities of an antimicrobial peptide NK-lysin from a marine fish Larimichthys crocea. Fish Shellfish Immunol. 2016, 55, 195–202. [Google Scholar] [CrossRef]
- Lama, R.; Pereiro, P.; Costa, M.M.; Encinar, J.A.; Medina-Gali, R.M.; Pérez, L.; Lamas, J.; Leiro, J.; Figueras, A.; Novoa, B. Turbot (Scophthalmus maximus) NK-lysin induces protection against the pathogenic parasite Philasterides dicentrarchi via membrane disruption. Fish Shellfish Immunol. 2018, 82, 190–199. [Google Scholar] [CrossRef] [Green Version]
- Cuesta, A.; Salinas, I.; Rodríguez, A.; Muñoz, P.; Sitjà-Bobadilla, A.; Álvarez-Pellitero, P.; Meseguer, J.; Esteban, M.Á.; Cabanes, R. De Cell-mediated cytotoxicity is the main innate immune mechanism involved in the cellular defence of gilthead seabream (Teleostei: Sparidae) against Enteromyxum leei (Myxozoa). Parasite Immunol. 2006, 28, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Piazzon, M.C.; Estensoro, I.; Calduch-Giner, J.A.; Pozo, R.; Picard-Sánchez, A.; Pérez-Sánchez, J.; Sitja-Bobadilla, A. Hints on T cell responses in a fish-parasite model: Enteromyxum leei induces differential expression of T cell signature molecules depending on the organ and the infection status. Parasites Vectors 2018, 11, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaso-Friedmann, L.; Leary, J.H.; Evans, D.L. The non-specific cytotoxic cell receptor (NCCRP-1): Molecular organization and signaling properties. Dev. Comp. Immunol. 2001, 25, 701–711. [Google Scholar] [CrossRef]
- Nakanishi, T.; Shibasaki, Y.; Matsuura, Y. T Cells in Fish. Biology 2015, 4, 640–663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuesta, A.; Muñoz, P.; Rodríguez, A.; Salinas, I.; Sitjà-Bobadilla, A.; Álvarez-Pellitero, P.; Esteban, M.A.; Meseguer, J. Gilthead seabream (Sparus aurata L.) innate defence against the parasite Enteromyxum leei (Myxozoa). Parasitology 2006, 132, 95–104. [Google Scholar] [CrossRef]
- Yambot, A.V.; Song, Y.L. Immunization of grouper, Epinephelus coioides, confers protection against a protozoan parasite, Cryptocaryon irritans. Aquaculture 2006, 260, 1–9. [Google Scholar] [CrossRef]
- Yu, Y.Y.; Kong, W.; Yin, Y.X.; Dong, F.; Huang, Z.Y.; Yin, G.M.; Dong, S.; Salinas, I.; Zhang, Y.A.; Xu, Z. Mucosal immunoglobulins protect the olfactory organ of teleost fish against parasitic infection. PLoS Pathog. 2018, 14, e1007251. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Yu, Y.; Zhang, X.; Xu, Z. Immune responses of fish to Ichthyophthirius multifiliis (Ich): A model for understanding immunity against protozoan parasites. Dev. Comp. Immunol. 2019, 93, 93–102. [Google Scholar] [CrossRef]
- Zhang, Y.-A.; Salinas, I.; Li, J.; Parra, D.; Bjork, S.; Xu, Z.; LaPatra, S.E.; Bartholomew, J.; Sunyer, J.O. IgT, a primitive immunoglobulin class specialized in mucosal immunity. Nat. Immunol. 2010, 11, 827–835. [Google Scholar] [CrossRef]
- Parra, D.; Korytár, T.; Takizawa, F.; Sunyer, J.O. B cells and their role in the teleost gut. Dev. Comp. Immunol. 2016, 64, 150–166. [Google Scholar] [CrossRef] [Green Version]
- Feidantsis, K.; Pörtner, H.O.; Vlachonikola, E.; Antonopoulou, E.; Michaelidis, B. Seasonal changes in metabolism and cellular stress phenomena in the gilthead sea bream (Sparus aurata). Physiol. Biochem. Zool. 2018, 91, 878–895. [Google Scholar] [CrossRef]
- Feidantsis, K.; Pörtner, H.O.; Lazou, A.; Kostoglou, B.; Michaelidis, B. Metabolic and molecular stress responses of the gilthead seabream Sparus aurata during long-term exposure to increasing temperatures. Mar. Biol. 2009, 156, 797–809. [Google Scholar] [CrossRef]
- Picard-Sánchez, A.; Estensoro, I.; del Pozo, R.; Piazzon, M.C.; Palenzuela, O.; Sitjà-Bobadilla, A. Acquired protective immune response in a fish-myxozoan model encompasses specific antibodies and inflammation resolution. Fish Shellfish Immunol. 2019, 90, 349–362. [Google Scholar] [CrossRef]
- Ma, R.; Yu, Y.; Liu, X.; Lei, Y.; Zhou, S.; Xie, X.; Jin, S.; Qian, D.; Yin, F. Transcriptomic analysis of Nibea albiflora skin in response to infection by Cryptocaryon irritans. Fish Shellfish Immunol. 2020, 98, 819–831. [Google Scholar] [CrossRef]
- Monteiro, S.M.; Oliveira, E.; Fontaínhas-Fernandes, A.; Sousa, M. Fine structure of the branchial epithelium in the teleost Oreochromis niloticus. J. Morphol. 2010, 271, 621–633. [Google Scholar] [CrossRef]
- Dezfuli, B.S.; Lui, A.; Boldrini, P.; Pironi, F.; Giari, L. The inflammatory response of fish to helminth parasites. Parasite 2008, 15, 426–433. [Google Scholar] [CrossRef] [Green Version]
- Dezfuli, B.S.; Lui, A.; Giari, L.; Pironi, F.; Manera, M.; Lorenzoni, M.; Noga, E.J. Piscidins in the intestine of European perch, Perca fluviatilis, naturally infected with an enteric worm. Fish Shellfish Immunol. 2013, 35, 1539–1546. [Google Scholar] [CrossRef] [PubMed]
- Dezfuli, B.S.; Pironi, F.; Giari, L.; Noga, E.J. Immunocytochemical localization of piscidin in mast cells of infected seabass gill. Fish Shellfish Immunol. 2010, 28, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Dezfuli, B.S.; Giari, L.; Lui, A.; Lorenzoni, M.; Noga, E.J. Mast cell responses to Ergasilus (Copepoda), a gill ectoparasite of sea bream. Fish Shellfish Immunol. 2011, 30, 1087–1094. [Google Scholar] [CrossRef] [PubMed]
- Cuesta, A.; Meseguer, J.; Esteban, M.Á. The antimicrobial peptide hepcidin exerts an important role in the innate immunity against bacteria in the bony fish gilthead seabream. Mol. Immunol. 2008, 45, 2333–2342. [Google Scholar] [CrossRef]
- Chaves-Pozo, E.; Muñoz, P.; López-Muñoz, A.; Pelegrín, P.; García Ayala, A.; Mulero, V.; Meseguer, J. Early innate immune response and redistribution of inflammatory cells in the bony fish gilthead seabream experimentally infected with Vibrio anguillarum. Cell Tissue Res. 2005, 320, 61–68. [Google Scholar] [CrossRef]
- Cabas, I.; Chaves-Pozo, E.; Alcázar, A.G.; Meseguer, J.; Mulero, V.; García-Ayala, A. Dietary intake of 17α-ethinylestradiol promotes leukocytes infiltration in the gonad of the hermaphrodite gilthead seabream. Mol. Immunol. 2011, 48, 2079–2086. [Google Scholar] [CrossRef]
- Jiang, B.; Li, Y.-W.; Hu, Y.-Z.; Luo, H.-L.; Li, A.-X. Characterization and expression analysis of six interleukin-17 receptor genes in grouper (Epinephelus coioides) after Cryptocaryon irritans infection. Fish Shellfish Immunol. 2017, 69, 46–51. [Google Scholar] [CrossRef]
- Mo, Z.-Q.; Chen, R.-A.; Li, Y.-W.; Huang, X.-Z.; Li, A.-X.; Luo, X.-C.; Dan, X.-M. Characterization and expression analysis of two novel CCR6 chemokine receptors and their three potential ligands CCL20Ls of grouper (Epinephelus coioides) post Cryptocaryon irritans infection. Fish Shellfish Immunol. 2015, 47, 280–288. [Google Scholar] [CrossRef]
- Zheng, L.; Qiu, J.; Chen, J.; Zheng, W.; Pan, Y. Histopathological changes and piscidin 5-like location in infected Larimichthys crocea with parasite Cryptocaryon irritans. Fish Shellfish Immunol. 2020, 99, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhou, Y.; Cao, Z.; Sun, Y.; Chen, Y.; Xiang, Y.; Wang, L.; Zhang, S.; Guo, W. Comparative analysis of the expression patterns of IL-1 β, IL-11, and IL-34 in golden pompano (Trachinotus ovatus) following different pathogens challenge. Fish Shellfish Immunol. 2019, 93, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Mo, Z.Q.; Yang, M.; Wang, H.Q.; Xu, Y.; Huang, M.Z.; Lao, G.F.; Li, Y.W.; Li, A.X.; Luo, X.C.; Dan, X.M. Grouper (Epinephelus coioides) BCR signaling pathway was involved in response against Cryptocaryon irritans infection. Fish Shellfish Immunol. 2016, 57, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Olsen, M.M.; Kania, P.W.; Heinecke, R.D.; Skjoedt, K.; Rasmussen, K.J.; Buchmann, K. Cellular and humoral factors involved in the response of rainbow trout gills to Ichthyophthirius multifiliis infections: Molecular and immunohistochemical studies. Fish Shellfish Immunol. 2011, 30, 859–869. [Google Scholar] [CrossRef]
- Graves, S.; Evans, D.L.; Dawe, D.L. Antiprotozoan activity of nonspecific cytotoxic cells (NCC) from the channel catfish (Ictalurus punctatus). J. Immunol. 1985, 134, 78–85. [Google Scholar] [PubMed]
- Huang, X.Z.; Li, Y.W.; Mai, Y.Z.; Luo, X.C.; Dan, X.M.; Li, A.X. Molecular cloning of NCCRP-1 gene from orange-spotted grouper (Epinephelus coioides) and characterization of NCCRP-1+ cells post Cryptocaryon irritans infection. Dev. Comp. Immunol. 2014, 46, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Lima, T.S.; Lodoen, M.B. Mechanisms of human innate immune evasion by Toxoplasma gondii. Front. Cell. Infect. Microbiol. 2019, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Valle, A.; Leiro, J.M.; Pereiro, P.; Figueras, A.; Novoa, B.; Dirks, R.P.H.; Lamas, J. Interactions between the parasite Philasterides dicentrarchi and the immune system of the turbot Scophthalmus maximus. A transcriptomic analysis. Biology 2020, 9, 337. [Google Scholar] [CrossRef]
- Barrett, D.E.; Bartholomew, J.L. A tale of two fish: Comparative transcriptomics of resistant and susceptible steelhead following exposure to Ceratonova shasta highlights differences in parasite recognition. PLoS ONE 2021, 16, e0234837. [Google Scholar] [CrossRef]
- Zhang, M.; Li, M.F.; Sun, L. NKLP27: A teleost NK-lysin peptide that modulates immune response, induces degradation of bacterial DNA, and inhibits bacterial and viral infection. PLoS ONE 2014, 9, e106543. [Google Scholar] [CrossRef] [PubMed]
- Valero, Y.; Chaves-Pozo, E.; Cuesta, A. NK-lysin is highly conserved in European sea bass and gilthead seabream but differentially modulated during the immune response. Fish Shellfish Immunol. 2020, 99, 435–441. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, 2002–2007. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 254, 248–254. [Google Scholar] [CrossRef]
- Ortuño, J.; Esteban, M.A.; Meseguer, J. Effects of four anaesthetics on the innate immune response of gilthead seabream (Sparus aurata L.). Fish Shellfish Immunol. 2002, 12, 49–59. [Google Scholar] [CrossRef]
- Parry, R.M.; Chandan, R.C.; Shahani, K.M. A rapid and sensitive assay of muramidase. Proc. Soc. Exp. Biol. Med. 1965, 119, 384–386. [Google Scholar] [CrossRef]
- Sunyer, J.O.; Tort, L. Natural hemolytic and bactericidal activities of sea bream Sparus aurata serum are effected by the alternative complement pathway. Vet. Immunol. Immunopathol. 1995, 45, 333–345. [Google Scholar] [CrossRef]
- Quade, M.J.; Roth, J.A. A rapid, direct assay to measure degranulation of bovine neutrophil primary granules. Vet. Immunol. Immunopathol. 1997, 58, 239–248. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cervera, L.; González-Fernández, C.; Arizcun, M.; Cuesta, A.; Chaves-Pozo, E. Severe Natural Outbreak of Cryptocaryon irritans in Gilthead Seabream Produces Leukocyte Mobilization and Innate Immunity at the Gill Tissue. Int. J. Mol. Sci. 2022, 23, 937. https://doi.org/10.3390/ijms23020937
Cervera L, González-Fernández C, Arizcun M, Cuesta A, Chaves-Pozo E. Severe Natural Outbreak of Cryptocaryon irritans in Gilthead Seabream Produces Leukocyte Mobilization and Innate Immunity at the Gill Tissue. International Journal of Molecular Sciences. 2022; 23(2):937. https://doi.org/10.3390/ijms23020937
Chicago/Turabian StyleCervera, Laura, Carmen González-Fernández, Marta Arizcun, Alberto Cuesta, and Elena Chaves-Pozo. 2022. "Severe Natural Outbreak of Cryptocaryon irritans in Gilthead Seabream Produces Leukocyte Mobilization and Innate Immunity at the Gill Tissue" International Journal of Molecular Sciences 23, no. 2: 937. https://doi.org/10.3390/ijms23020937
APA StyleCervera, L., González-Fernández, C., Arizcun, M., Cuesta, A., & Chaves-Pozo, E. (2022). Severe Natural Outbreak of Cryptocaryon irritans in Gilthead Seabream Produces Leukocyte Mobilization and Innate Immunity at the Gill Tissue. International Journal of Molecular Sciences, 23(2), 937. https://doi.org/10.3390/ijms23020937





