OCTN1: A Widely Studied but Still Enigmatic Organic Cation Transporter Linked to Human Pathology and Drug Interactions
Abstract
:1. Introduction
2. Tissue Expression/Localization of OCTN1
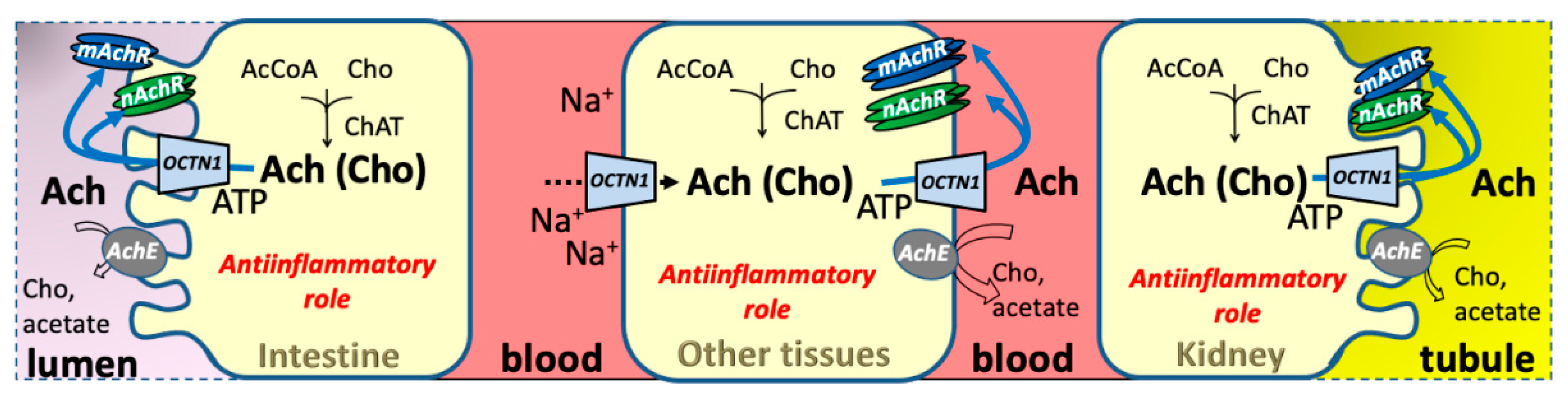
2.1. OCTN1 Expression in Relationships with Physiological Roles
3. Functional and Structural Aspects of OCTN1
3.1. OCTN1 Substrate Specificity and Relationships with Human Physiology
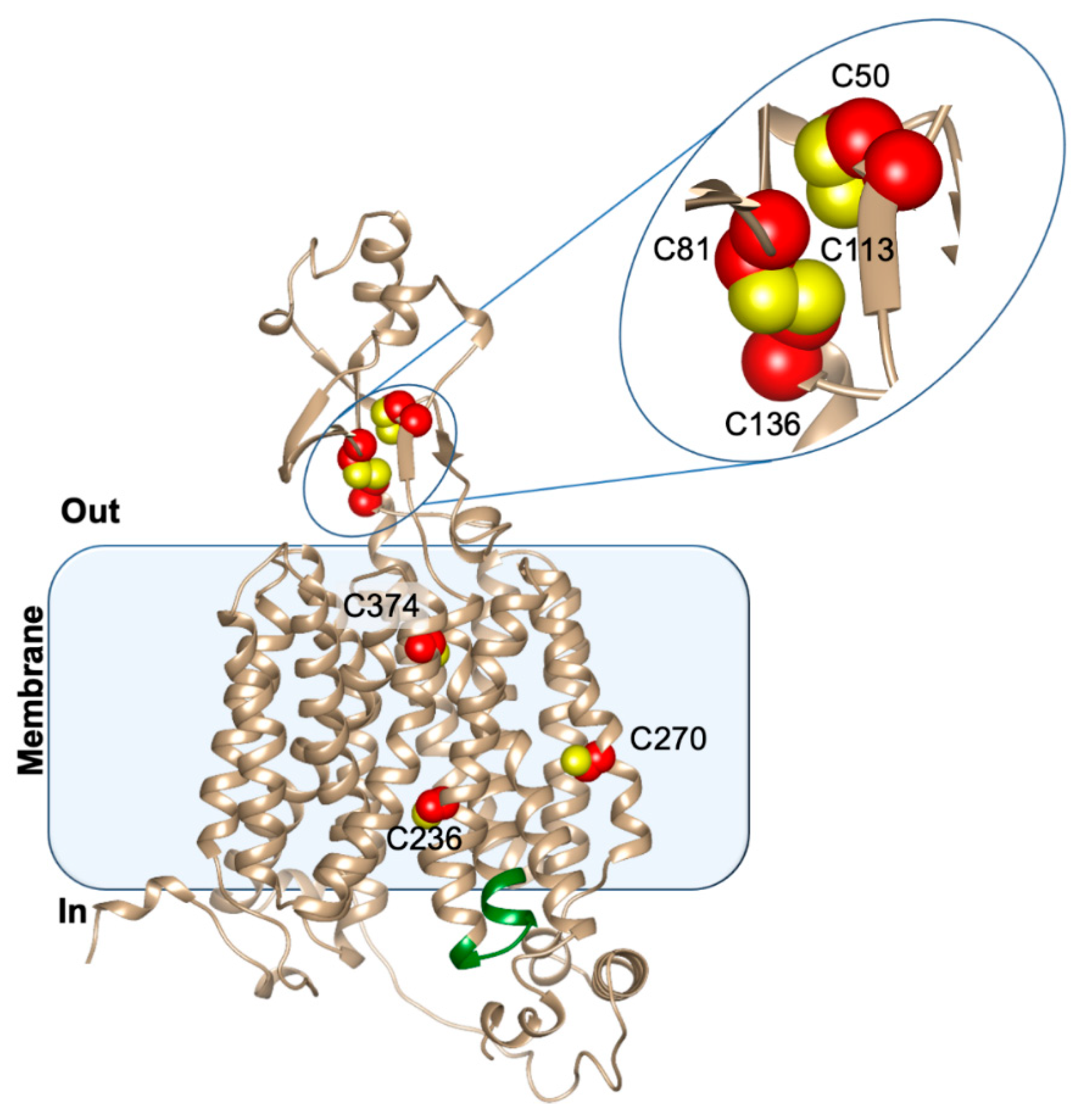
3.2. Structure/Function Relationship
4. Interaction with Endogenous/Exogenous Substrates
4.1. OCTN1 Substrate Specificity
4.1.1. Carnitine
4.1.2. Ergothioneine
4.1.3. Acetylcholine
4.2. Knocking Out OCTN1 to Understand Substrate Selectivity
5. Interaction with Drugs
5.1. Controversial Data on Antineoplastic Drug Transport
5.2. Specificity of OCTN1–Drug Interactions
5.3. Toxicokinetics and Drug–Drug Interactions (DDI)
5.4. OCTN1 Polymorphisms and Drug Transport
5.5. Influence of Xenobiotics and Natural Compounds on OCTN1 Expression
6. Relevance to Human Pathology
6.1. Pathologies Modulating OCTN1 Expression
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Eraly, S.A.; Monte, J.C.; Nigam, S.K. Novel slc22 transporter homologs in fly, worm, and human clarify the phylogeny of organic anion and cation transporters. Physiol. Genom. 2004, 18, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, C.; Bach, M.; Bauer, T.; da Ponte, J.C.; Schömig, E.; Gründemann, D. Knockout of the ergothioneine transporter ETT in zebrafish results in increased 8-oxoguanine levels. Free. Radic. Biol. Med. 2015, 83, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Kitsanayanyong, L.; Pahila, J.; Ishikawa, Y.; Koyama, T.; Kiron, V.; Ohshima, T. Functional identification of ergothioneine transporter in rainbow trout (Oncorhynchus mykiss). Comp. Biochem. Physiol. Part B: Biochem. Mol. Biol. 2021, 256, 110631. [Google Scholar] [CrossRef]
- Gai, Y.; Liu, Z.; Cervantes-Sandoval, I.; Davis, R.L. Drosophila SLC22A Transporter Is a Memory Suppressor Gene that Influences Cholinergic Neurotransmission to the Mushroom Bodies. Neuron 2016, 90, 581–595. [Google Scholar] [CrossRef]
- Liu, D.; Zeng, X.; Li, L.; Ou, Z.-L. Carnitine promotes recovery from oxidative stress and extends lifespan in C. elegans. Aging 2020, 13, 813–830. [Google Scholar] [CrossRef] [PubMed]
- Pochini, L.; Galluccio, M.; Scalise, M.; Console, L.; Indiveri, C. OCTN: A Small Transporter Subfamily with Great Relevance to Human Pathophysiology, Drug Discovery, and Diagnostics. SLAS Discov. Adv. Sci. Drug Discov. 2018, 24, 89–110. [Google Scholar] [CrossRef]
- Koepsell, H. Organic Cation Transporters in Health and Disease. Pharmacol. Rev. 2019, 72, 253–319. [Google Scholar] [CrossRef]
- Karahoda, R.; Ceckova, M.; Staud, F. The inhibitory effect of antiretroviral drugs on the L-carnitine uptake in human placenta. Toxicol. Appl. Pharmacol. 2019, 368, 18–25. [Google Scholar] [CrossRef]
- Pochini, L.; Scalise, M.; Galluccio, M.; Indiveri, C. OCTN Cation Transporters in Health and Disease: Role as Drug Targets and Assay Development. SLAS Discov. Adv. Sci. Drug Discov. 2013, 18, 851–867. [Google Scholar] [CrossRef]
- Gründemann, D.; Harlfinger, S.; Golz, S.; Geerts, A.; Lazar, A.; Berkels, R.; Jung, N.; Rubbert, A.; Schömig, E. Discovery of the ergothioneine transporter. Proc. Natl. Acad. Sci. USA 2005, 102, 5256–5261. [Google Scholar] [CrossRef]
- Pochini, L.; Scalise, M.; Galluccio, M.; Pani, G.; Siminovitch, K.A.; Indiveri, C. The human OCTN1 (SLC22A4) reconstituted in liposomes catalyzes acetylcholine transport which is defective in the mutant L503F associated to the Crohn's disease. Biochim. et Biophys. Acta (BBA)-Biomembr. 2012, 1818, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Pochini, L.; Scalise, M.; Indiveri, C. Immuno-detection of OCTN1 (SLC22A4) in HeLa cells and characterization of transport function. Int. Immunopharmacol. 2015, 29, 21–26. [Google Scholar] [CrossRef]
- Pochini, L.; Scalise, M.; Di Silvestre, S.; Belviso, S.; Pandolfi, A.; Arduini, A.; Bonomini, M.; Indiveri, C. Acetylcholine and acetylcarnitine transport in peritoneum: Role of the SLC22A4 (OCTN1) transporter. Biochim. et Biophys. Acta (BBA)-Biomembr. 2016, 1858, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Masuo, Y.; Ohba, Y.; Yamada, K.; Al-Shammari, A.H.; Seba, N.; Nakamichi, N.; Ogihara, T.; Kunishima, M.; Kato, Y. Combination Metabolomics Approach for Identifying Endogenous Substrates of Carnitine/Organic Cation Transporter OCTN1. Pharm. Res. 2018, 35, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Yabuuchi, H.; Tamai, I.; Nezu, J.; Sakamoto, K.; Oku, A.; Shimane, M.; Sai, Y.; Tsuji, A. Novel membrane transporter OCTN1 mediates multispecific, bidirectional, and pH-dependent transport of organic cations. Experiment 1999, 289, 768–773. [Google Scholar]
- Pochini, L.; Scalise, M.; Galluccio, M.; Amelio, L.; Indiveri, C. Reconstitution in liposomes of the functionally active human OCTN1 (SLC22A4) transporter overexpressed in Escherichia coli. Biochem. J. 2011, 439, 227–233. [Google Scholar] [CrossRef]
- Drenberg, C.D.; Gibson, A.A.; Pounds, S.B.; Shi, L.; Rhinehart, D.P.; Li, L.; Hu, S.; Du, G.; Nies, A.T.; Schwab, M.; et al. OCTN1 Is a High-Affinity Carrier of Nucleoside Analogues. Cancer Res 2017, 77, 2102–2111. [Google Scholar] [CrossRef]
- Nishiyama, M.; Nakamichi, N.; Yoshimura, T.; Masuo, Y.; Komori, T.; Ishimoto, T.; Matsuo, J.I.; Kato, Y. Homostachydrine is a Xenobiotic Substrate of OCTN1/SLC22A4 and Potentially Sensitizes Pentylenetetrazole-Induced Seizures in Mice. Neurochem. Res. 2020, 45, 2664–2678. [Google Scholar] [CrossRef]
- Engelhart, D.C.; Granados, J.C.; Shi, D.; Saier Jr, M.H., Jr.; Baker, M.E.; Abagyan, R.; Nigam, S.K. Systems Biology Analysis Reveals Eight SLC22 Transporter Subgroups, Including OATs, OCTs, and OCTNs. Int. J. Mol. Sci. 2020, 21, 1791. [Google Scholar] [CrossRef]
- Rosenthal, S.B.; Bush, K.T.; Nigam, S.K. A Network of SLC and ABC Transporter and DME Genes Involved in Remote Sensing and Signaling in the Gut-Liver-Kidney Axis. Sci. Rep. 2019, 9, 1–19. [Google Scholar] [CrossRef]
- Koepsell, H. Update on drug-drug interaction at organic cation transporters: Mechanisms, clinical impact, and proposal for advanced in vitro testing. Expert Opin. Drug Metab. Toxicol. 2021, 17, 635–653. [Google Scholar] [CrossRef] [PubMed]
- Veiga-Matos, J.; Remiaão, F.; Motales, A. Pharmacokinetics and Toxicokinetics Roles of Membrane Transporters at Kidney Level. J. Pharm. Pharm. Sci. 2020, 23, 333–356. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos Pereira, J.N.; Tadjerpisheh, S.; Abu Abed, M.; Saadatmand, A.R.; Weksler, B.; Romero, I.; Couraud, P.-O.; Brockmöller, J.; Tzvetkov, M.V. The Poorly Membrane Permeable Antipsychotic Drugs Amisulpride and Sulpiride Are Substrates of the Organic Cation Transporters from the SLC22 Family. AAPS J. 2014, 16, 1247–1258. [Google Scholar] [CrossRef] [PubMed]
- Futatsugi, A.; Masuo, Y.; Kawabata, S.; Nakamichi, N.; Kato, Y. L503F variant of carnitine/organic cation transporter 1 efficiently transports metformin and other biguanides. J. Pharm. Pharmacol. 2016, 68, 1160–1169. [Google Scholar] [CrossRef]
- Zeng, Q.; Bai, M.; Li, C.; Lu, S.; Ma, Z.; Zhao, Y.; Zhou, H.; Jiang, H.; Sun, D.; Zheng, C. Multiple Drug Transporters Contribute to the Placental Transfer of Emtricitabine. Antimicrob. Agents Chemother. 2019, 63, e00199-19. [Google Scholar] [CrossRef]
- Yang, X.; Ma, Z.; Zhou, S.; Weng, Y.; Lei, H.; Zeng, S.; Li, L.; Jiang, H. Multiple Drug Transporters Are Involved in Renal Secretion of Entecavir. Antimicrob. Agents Chemother. 2016, 60, 6260–6270. [Google Scholar] [CrossRef]
- Parvez, M.M.; Kaisar, N.; Shin, H.J.; Lee, Y.J.; Shin, J.G. Comprehensive Substrate Characterization of 22 Antituberculosis Drugs for Multiple Solute Carrier (SLC) Uptake Transporters In Vitro. Antimicrob. Agents Chemother. 2018, 62, e00512-18. [Google Scholar] [CrossRef]
- Urban, T.; Brown, C.; Castro, R.; Shah, N.; Mercer, R.; Huang, Y.; Brett, C.; Burchard, E.; Giacomini, K. Effects of Genetic Variation in the Novel Organic Cation Transporter, OCTN1, on the Renal Clearance of Gabapentin. Clin. Pharmacol. Ther. 2007, 83, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Nakanishi, T.; Haruta, T.; Shirasaka, Y.; Keogh, J.P.; Tamai, I. Transport of Ipratropium, an Anti-Chronic Obstructive Pulmonary Disease Drug, Is Mediated by Organic Cation/Carnitine Transporters in Human Bronchial Epithelial Cells: Implications for Carrier-Mediated Pulmonary Absorption. Mol. Pharm. 2009, 7, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Nakamichi, N.; Shima, H.; Asano, S.; Ishimoto, T.; Sugiura, T.; Matsubara, K.; Kusuhara, H.; Sugiyama, Y.; Sai, Y.; Miyamoto, K.; et al. Involvement of carnitine/organic cation transporter OCTN1/SLC22A4 in gastrointestinal absorption of metformin. J. Pharm. Sci. 2013, 102, 3407–3417. [Google Scholar] [CrossRef] [PubMed]
- Jong, N.N.; Nakanishi, T.; Liu, J.J.; Tamai, I.; McKeage, M.J. Oxaliplatin Transport Mediated by Organic Cation/Carnitine Transporters OCTN1 and OCTN2 in Overexpressing Human Embryonic Kidney 293 Cells and Rat Dorsal Root Ganglion Neurons. Experiment 2011, 338, 537–547. [Google Scholar] [CrossRef]
- Harrach, S.; Edemir, B.; Schmidt-Lauber, C.; Pap, T.; Bertrand, J.; Ciarimboli, G. Importance of the novel organic cation transporter 1 for tyrosine kinase inhibition by saracatinib in rheumatoid arthritis synovial fibroblasts. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef]
- Basit, A.; Radi, Z.; Vaidya, V.S.; Karasu, M.; Prasad, B. Kidney Cortical Transporter Expression across Species Using Quantitative Proteomics. Drug Metab. Dispos. 2019, 47, 802–808. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.; Meier-Abt, F. Differential expression of hydroxyurea transporters in normal and polycythemia vera hematopoietic stem and progenitor cell subpopulations. Exp. Hematol. 2021, 97, 47–56.e45. [Google Scholar] [CrossRef] [PubMed]
- Samodelov, S.L.; Kullak-Ublick, G.A.; Gai, Z.; Visentin, M. Organic Cation Transporters in Human Physiology, Pharmacology, and Toxicology. Int. J. Mol. Sci. 2020, 21, 7890. [Google Scholar] [CrossRef] [PubMed]
- Borodina, I.; Kenny, L.C.; McCarthy, C.M.; Paramasivan, K.; Pretorius, E.; Roberts, T.; Van Der Hoek, S.A.; Kell, D.B. The biology of ergothioneine, an antioxidant nutraceutical. Nutr. Res. Rev. 2020, 33, 190–217. [Google Scholar] [CrossRef] [PubMed]
- Tamai, I. Pharmacological and pathophysiological roles of carnitine/organic cation transporters (OCTNs: SLC22A4, SLC22A5 and Slc22a21). Biopharm. Drug Dispos. 2012, 34, 29–44. [Google Scholar] [CrossRef]
- O’Hagan, S.; Wright Muelas, M.; Day, P.J.; Lundberg, E.; Kell, D.B. GeneGini: Assessment via the Gini Coefficient of Reference “Housekeeping” Genes and Diverse Human Transporter Expression Profiles. Cell Syst. 2018, 6, 230–244.e231. [Google Scholar] [CrossRef]
- Peltekova, V.D.; Wintle, R.; Rubin, L.A.; Amos, C.I.; Huang, Q.; Gu, X.; Newman, W.; Van Oene, M.; Cescon, D.; Greenberg, G.; et al. Functional variants of OCTN cation transporter genes are associated with Crohn disease. Nat. Genet. 2004, 36, 471–475. [Google Scholar] [CrossRef]
- Ahbara, A.M.; Rouatbi, M.; Gharbi, M.; Rekik, M.; Haile, A.; Rischkowsky, B.; Mwacharo, J.M. Genome-wide insights on gastrointestinal nematode resistance in autochthonous Tunisian sheep. Sci. Rep. 2021, 11, 9250. [Google Scholar] [CrossRef]
- Sugiura, T.; Kato, S.; Shimizu, T.; Wakayama, T.; Nakamichi, N.; Kubo, Y.; Iwata, D.; Suzuki, K.; Soga, T.; Asano, M.; et al. Functional expression of carnitine/organic cation transporter OCTN1/SLC22A4 in mouse small intestine and liver. Drug Metab. Dispos. 2010, 38, 1665–1672. [Google Scholar] [CrossRef] [PubMed]
- Shinozaki, Y.; Furuichi, K.; Toyama, T.; Kitajima, S.; Hara, A.; Iwata, Y.; Sakai, N.; Shimizu, M.; Kaneko, S.; Isozumi, N.; et al. Impairment of the carnitine/organic cation transporter 1-ergothioneine axis is mediated by intestinal transporter dysfunction in chronic kidney disease. Kidney Int. 2017, 92, 1356–1369. [Google Scholar] [CrossRef] [PubMed]
- Harwood, M.D.; Zhang, M.; Pathak, S.M.; Neuhoff, S. The Regional-Specific Relative and Absolute Expression of Gut Transporters in Adult Caucasians: A Meta-Analysis. Drug Metab. Dispos. 2019, 47, 854–864. [Google Scholar] [CrossRef] [PubMed]
- Nakamichi, N.; Kato, Y. Physiological Roles of Carnitine/Organic Cation Transporter OCTN1/SLC22A4 in Neural Cells. Biol. Pharm. Bull. 2017, 40, 1146–1152. [Google Scholar] [CrossRef]
- Billington, S.; Salphati, L.; Hop, C.E.C.A.; Chu, X.; Evers, R.; Burdette, D.; Rowbottom, C.; Lai, Y.; Xiao, G.; Humphreys, W.G.; et al. Interindividual and Regional Variability in Drug Transporter Abundance at the Human Blood–Brain Barrier Measured by Quantitative Targeted Proteomics. Clin. Pharmacol. Ther. 2019, 106, 228–237. [Google Scholar] [CrossRef]
- Li, Q.; Barres, B.A. Microglia and macrophages in brain homeostasis and disease. Nat. Rev. Immunol. 2017, 18, 225–242. [Google Scholar] [CrossRef]
- Anoshchenko, O.; Prasad, B.; Neradugomma, N.K.; Wang, J.; Mao, Q.; Unadkat, J.D. Gestational Age-Dependent Abundance of Human Placental Transporters as Determined by Quantitative Targeted Proteomics. Drug Metab. Dispos. 2020, 48, 735–741. [Google Scholar] [CrossRef]
- García-Lino, A.M.; Álvarez-Fernández, I.; Blanco-Paniagua, E.; Merino, G.; Álvarez, A.I. Transporters in the Mammary Gland—Contribution to Presence of Nutrients and Drugs into Milk. Nutrients 2019, 11, 2372. [Google Scholar] [CrossRef]
- Hau, R.K.; Miller, S.R.; Wright, S.H.; Cherrington, N.J. Generation of a hTERT-Immortalized Human Sertoli Cell Model to Study Transporter Dynamics at the Blood-Testis Barrier. Pharmaceutics 2020, 12, 5. [Google Scholar] [CrossRef]
- Meyer, M.J.; Tuerkova, A.; Römer, S.; Wenzel, C.; Seitz, T.; Gaedcke, J.; Oswald, S.; Brockmöller, J.; Zdrazil, B.; Tzvetkov, M.V. Differences in Metformin and Thiamine Uptake between Human and Mouse Organic Cation Transporter 1: Structural Determinants and Potential Consequences for Intrahepatic Concentrations. Drug Metab. Dispos. 2020, 48, 1380–1392. [Google Scholar] [CrossRef] [PubMed]
- Scalise, M.; Console, L.; Galluccio, M.; Pochini, L.; Indiveri, C. Chemical Targeting of Membrane Transporters: Insights into Structure/Function Relationships. ACS Omega 2020, 5, 2069–2080. [Google Scholar] [CrossRef] [PubMed]
- Bacher, P.; Giersiefer, S.; Bach, M.; Fork, C.; Schömig, E.; Gründemann, D. Substrate discrimination by ergothioneine transporter SLC22A4 and carnitine transporter SLC22A5: Gain-of-function by interchange of selected amino acids. Biochim. et Biophys. Acta (BBA)-Biomembr. 2009, 1788, 2594–2602. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.T.; Hu, S.; Fu, Q.; Baker, S.D.; Sparreboom, A. Role of equilibrative nucleoside transporter 1 (ENT1) in the disposition of cytarabine in mice. Pharmacol. Res. Perspect. 2019, 7, e00534. [Google Scholar] [CrossRef]
- Tucker, R.A.; Cheah, I.K.; Halliwell, B. Specificity of the ergothioneine transporter natively expressed in HeLa cells. Biochem. Biophys. Res. Commun. 2019, 513, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Kummer, W.; Krasteva-Christ, G. Non-neuronal cholinergic airway epithelium biology. Curr. Opin. Pharmacol. 2014, 16, 43–49. [Google Scholar] [CrossRef]
- Koepsell, H. Multiple binding sites in organic cation transporters require sophisticated procedures to identify interactions of novel drugs. Biol. Chem. 2018, 400, 195–207. [Google Scholar] [CrossRef]
- Keller, T.; Gorboulev, V.; Mueller, T.D.; Dotsch, V.; Bernhard, F.; Koepsell, H. Rat Organic Cation Transporter 1 Contains Three Binding Sites for Substrate 1-Methyl-4-phenylpyridinium per Monomer. Mol. Pharmacol. 2018, 95, 169–182. [Google Scholar] [CrossRef]
- Zhang, L.; Gui, T.; Console, L.; Scalise, M.; Indiveri, C.; Hausler, S.; Kullak-Ublick, G.A.; Gai, Z.; Visentin, M. Cholesterol stimulates the cellular uptake of L-carnitine by the carnitine/organic cation transporter novel 2 (OCTN2). J. Biol. Chem. 2021, 296, 100204. [Google Scholar] [CrossRef]
- Pochini, L.; Pappacoda, G.; Galluccio, M.; Pastore, F.; Scalise, M.; Indiveri, C. Effect of Cholesterol on the Organic Cation Transporter OCTN1 (SLC22A4). Int. J. Mol. Sci. 2020, 21, 1091. [Google Scholar] [CrossRef]
- Brast, S.; Grabner, A.; Sucic, S.; Sitte, H.H.; Hermann, E.; Pavenstädt, H.; Schlatter, E.; Ciarimboli, G. The cysteines of the extracellular loop are crucial for trafficking of human organic cation transporter 2 to the plasma membrane and are involved in oligomerization. FASEB J. 2011, 26, 976–986. [Google Scholar] [CrossRef]
- Galluccio, M.; Pochini, L.; Peta, V.; Iannì, M.; Scalise, M.; Indiveri, C. Functional and Molecular Effects of Mercury Compounds on the Human OCTN1 Cation Transporter: C50 and C136 Are the Targets for Potent Inhibition. Toxicol. Sci. 2014, 144, 105–113. [Google Scholar] [CrossRef]
- Keller, T.; Egenberger, B.; Gorboulev, V.; Bernhard, F.; Uzelac, Z.; Gorbunov, D.; Wirth, C.; Koppatz, S.; Dötsch, V.; Hunte, C.; et al. The Large Extracellular Loop of Organic Cation Transporter 1 Influences Substrate Affinity and Is Pivotal for Oligomerization. J. Biol. Chem. 2011, 286, 37874–37886. [Google Scholar] [CrossRef]
- Cheah, I.K.; Halliwell, B. Ergothioneine, recent developments. Redox Biol. 2021, 42, 101868. [Google Scholar] [CrossRef]
- Halliwell, B.; Cheah, I.K.; Drum, C.L. Ergothioneine, an adaptive antioxidant for the protection of injured tissues? A hypothesis. Biochem. Biophys. Res. Commun. 2016, 470, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Gyawali, A.; Hyeon, S.; Ryu, H.; Kang, Y.-S. The Alteration of L-Carnitine Transport and Pretreatment Effect under Glutamate Cytotoxicity on Motor Neuron-Like NSC-34 Lines. Pharmaceutics 2021, 13, 551. [Google Scholar] [CrossRef] [PubMed]
- Smeets, N.J.L.; Litjens, C.H.C.; van den Heuvel, J.J.M.W.; Van Hove, H.; van den Broek, P.; Russel, F.G.M.; Koenderink, J.B.; De Wildt, S.N. Completing the Enalaprilat Excretion Pathway—Renal Handling by the Proximal Tubule. Pharmaceutics 2020, 12, 935. [Google Scholar] [CrossRef]
- Bakhtiarizadeh, M.R.; Alamouti, A.A. RNA-Seq based genetic variant discovery provides new insights into controlling fat deposition in the tail of sheep. Sci. Rep. 2020, 10, 13525. [Google Scholar] [CrossRef]
- Matsuda, Y.; Ozawa, N.; Shinozaki, T.; Wakabayashi, K.-I.; Suzuki, K.; Kawano, Y.; Ohtsu, I.; Tatebayashi, Y. Ergothioneine, a metabolite of the gut bacterium Lactobacillus reuteri, protects against stress-induced sleep disturbances. Transl. Psychiatry 2020, 10, 170. [Google Scholar] [CrossRef]
- Kato, Y.; Kubo, Y.; Iwata, D.; Kato, S.; Sudo, T.; Sugiura, T.; Kagaya, T.; Wakayama, T.; Hirayama, A.; Sugimoto, M.; et al. Gene Knockout and Metabolome Analysis of Carnitine/Organic Cation Transporter OCTN1. Pharm. Res. 2010, 27, 832–840. [Google Scholar] [CrossRef] [PubMed]
- Makiishi, S.; Furuichi, K.; Yamamura, Y.; Sako, K.; Shinozaki, Y.; Toyama, T.; Kitajima, S.; Iwata, Y.; Sakai, N.; Shimizu, M.; et al. Carnitine/organic cation transporter 1 precipitates the progression of interstitial fibrosis through oxidative stress in diabetic nephropathy in mice. Sci. Rep. 2021, 11, 9093. [Google Scholar] [CrossRef] [PubMed]
- Nakamichi, N.; Masuo, Y.; Kato, Y. Possible Treatment of Neuropsychiatric Disorders by Promotion of Neuronal Differentiation through Organic Cation Transporters. Yakugaku Zasshi J. Pharm. Soc. Jpn. 2019, 139, 847–852. [Google Scholar] [CrossRef]
- Yee, S.W.; Buitrago, D.; Stecula, A.; Ngo, H.X.; Chien, H.; Zou, L.; Koleske, M.L.; Giacomini, K.M. Deorphaning a solute carrier 22 family member, SLC22A15, through functional genomic studies. FASEB J. 2020, 34, 15734–15752. [Google Scholar] [CrossRef]
- Grando, S.A.; Kawashima, K.; Wessler, I. A historic perspective on the current progress in elucidation of the biologic significance of non-neuronal acetylcholine. Int. Immunopharmacol. 2020, 81, 106289. [Google Scholar] [CrossRef]
- Angelini, S.; Soverini, S.; Ravegnini, G.; Barnett, M.; Turrini, E.; Thornquist, M.; Pane, F.; Hughes, T.; White, D.; Radich, J.; et al. Association between imatinib transporters and metabolizing enzymes genotype and response in newly diagnosed chronic myeloid leukemia patients receiving imatinib therapy. Haematologica 2012, 98, 193–200. [Google Scholar] [CrossRef]
- Harrach, S.; Barz, V.; Pap, T.; Pavenstädt, H.; Schlatter, E.; Edemir, B.; Distler, J.; Ciarimboli, G.; Bertrand, J. Notch Signaling Activity Determines Uptake and Biological Effect of Imatinib in Systemic Sclerosis Dermal Fibroblasts. J. Investig. Dermatol. 2018, 139, 439–447. [Google Scholar] [CrossRef]
- Hu, S.; Franke, R.M.; Filipski, K.K.; Hu, C.; Orwick, S.J.; de Bruijn, E.A.; Burger, H.; Baker, S.D.; Sparreboom, A. Interaction of Imatinib with Human Organic Ion Carriers. Clin. Cancer Res. 2008, 14, 3141–3148. [Google Scholar] [CrossRef]
- Tschirka, J.; Kreisor, M.; Betz, J.; Gründemann, D. Substrate Selectivity Check of the Ergothioneine Transporter. Drug Metab. Dispos. 2018, 46, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, H.; Kubo, H.; Washio, I.; Staub, A.Y.; Nedachi, S.; Ishiguro, N.; Nakanishi, T.; Tamai, I. Rat Kidney Slices for Evaluation of Apical Membrane Transporters in Proximal Tubular Cells. J. Pharm. Sci. 2019, 108, 2798–2804. [Google Scholar] [CrossRef]
- Buelow, D.R.; Anderson, J.T.; Pounds, S.B.; Shi, L.; Lamba, J.K.; Hu, S.; Gibson, A.A.; Goodwin, E.A.; Sparreboom, A.; Baker, S.D. DNA Methylation-Based Epigenetic Repression of SLC22A4 Promotes Resistance to Cytarabine in Acute Myeloid Leukemia. Clin. Transl. Sci. 2020, 14, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Bankar, A.; Siriwardena, T.P.; Rizoska, B.; Rydergård, C.; Kylefjord, H.; Rraklli, V.; Eneroth, A.; Pinho, P.; Norin, S.; Bylund, J.; et al. The novel L-nucleoside analogue, 5-Fluorotroxacitabine, displays potent efficacy against acute myeloid leukemia. Haematologica 2019, 106, 574–579. [Google Scholar] [CrossRef] [PubMed]
- Xin, Z.; Li-Fang, W.; Jia-Qi, C.; Bo-Lin, Z.; Xiu-Hong, C.; Jun, L.I.; Peng-Fei, T.U.; Jin-Ling, W. Absorption mechanism of dragon's blood phenolic extracts in Caco-2 cells. Zhongguo Zhong Yao Za Zhi 2020, 45, 4889–4895. [Google Scholar]
- Gyawali, A.; Kim, M.-H.; Kang, Y.-S. A novel organic cation transporter involved in paeonol transport across the inner blood-retinal barrier and changes in uptake in high glucose conditions. Exp. Eye Res. 2020, 202, 108387. [Google Scholar] [CrossRef]
- Krieter, P.; Chiang, C.N.; Gyaw, S.; Skolnick, P.; Snyder, R. Pharmacokinetic Interaction between Naloxone and Naltrexone Following Intranasal Administration to Healthy Subjects. Drug Metab. Dispos. 2019, 47, 690–698. [Google Scholar] [CrossRef] [PubMed]
- Sekhar, G.N.; Fleckney, A.L.; Boyanova, S.T.; Rupawala, H.; Lo, R.; Wang, H.; Farag, D.B.; Rahman, K.M.; Broadstock, M.; Reeves, S.; et al. Region-specific blood–brain barrier transporter changes leads to increased sensitivity to amisulpride in Alzheimer’s disease. Fluids Barriers CNS 2019, 16, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, C.; Yi, Y.; Du, W.; Jiang, H.; Zeng, S.; Zhou, H. Organic Cation Transporter 1 and 3 Contribute to the High Accumulation of Dehydrocorydaline in the Heart. Drug Metab. Dispos. 2020, 48, 1074–1083. [Google Scholar] [CrossRef] [PubMed]
- Cong, J.; Ruan, Y.; Lyu, Q.; Qin, X.; Qi, X.; Liu, W.; Kang, L.; Zhang, J.; Wu, C. A proton-coupled organic cation antiporter is involved in the blood-brain barrier transport of Aconitum alkaloids. J. Ethnopharmacol. 2020, 252, 112581. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Mizutani, A.; Okamoto, T.; Muranaka, Y.; Nishi, K.; Nishii, R.; Shikano, N.; Nakanishi, T.; Tamai, I.; Kleinerman, E.S.; et al. Assessment of drug transporters involved in the urinary secretion of [99mTc]dimercaptosuccinic acid. Nucl. Med. Biol. 2021, 94, 92–97. [Google Scholar] [CrossRef]
- Hasegawa, N.; Furugen, A.; Ono, K.; Koishikawa, M.; Miyazawa, Y.; Nishimura, A.; Umazume, T.; Narumi, K.; Kobayashi, M.; Iseki, K. Cellular uptake properties of lamotrigine in human placental cell lines: Investigation of involvement of organic cation transporters (SLC22A1–5). Drug Metab. Pharmacokinet. 2020, 35, 266–273. [Google Scholar] [CrossRef]
- Fujita, S.; Hirota, T.; Sakiyama, R.; Baba, M.; Ieiri, I. Identification of drug transporters contributing to oxaliplatin-induced peripheral neuropathy. J. Neurochem. 2018, 148, 373–385. [Google Scholar] [CrossRef]
- Yi, Y.; Li, L.; Song, F.; Li, P.; Chen, M.; Ni, S.; Zhang, H.; Zhou, H.; Zeng, S.; Jiang, H. L-tetrahydropalmatine reduces oxaliplatin accumulation in the dorsal root ganglion and mitochondria through selectively inhibiting the transporter-mediated uptake thereby attenuates peripheral neurotoxicity. Toxicology 2021, 459, 152853. [Google Scholar] [CrossRef]
- Costa, A.C.C.; Yamamotos, P.A.; Lauretti, G.R.; Benzi, J.R.; Zanelli, C.F.; Barz, V.; Ciarimboli, G.; De Moraes, N.V. Cetirizine Reduces Gabapentin Plasma Concentrations and Effect: Role of Renal Drug Transporters for Organic Cations. J. Clin. Pharmacol. 2020, 60, 1076–1086. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.C.C.; de Lima Benzi, J.R.; Yamamoto, P.A.; de Freitas, M.C.F.; de Paula, F.J.A.; Zanelli, C.F.; Lauretti, G.R.; de Moraes, N.V. Population pharmacokinetics of gabapentin in patients with neuropathic pain: Lack of effect of diabetes or glycaemic control. Br. J. Clin. Pharmacol. 2020, 87, 1981–1989. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, P.A.; Benzi, J.; Azeredo, F.J.; Dach, F.; Júnior, E.I.; Zanelli, C.; De Moraes, N.V. Pharmacogenetics-based population pharmacokinetic analysis of gabapentin in patients with chronic pain: Effect of OCT 2 and OCTN 1 gene polymorphisms. Basic Clin. Pharmacol. Toxicol. 2018, 124, 266–272. [Google Scholar] [CrossRef]
- Yoon, H.; Cho, H.-Y.; Yoo, H.-D.; Kim, S.-M.; Lee, Y.-B. Influences of Organic Cation Transporter Polymorphisms on the Population Pharmacokinetics of Metformin in Healthy Subjects. AAPS J. 2013, 15, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Jiang, Z.-P.; Zeng, J.; Zhu, Y.; Cai, H.-L.; Xiang, D.-X.; He, Q.; Shi, X.-L.; Zhong, A.-N.; Zhao, X.-L.; et al. Effects of Trough Concentration and Solute Carrier Polymorphisms on Imatinib Efficacy in Chinese Patients with Chronic Myeloid Leukemia. J. Pharm. Pharm. Sci. 2020, 23, 1–9. [Google Scholar] [CrossRef]
- Stanke-Labesque, F.; Gautier-Veyret, E.; Chhun, S.; Guilhaumou, R.; French Society of, P.; Therapeutics. Inflammation is a major regulator of drug metabolizing enzymes and transporters: Consequences for the personalization of drug treatment. Pharmacol. Ther. 2020, 215, 107627. [Google Scholar] [CrossRef]
- Jinno, N.; Furugen, A.; Kurosawa, Y.; Kanno, Y.; Narumi, K.; Kobayashi, M.; Iseki, K. Effects of single and repetitive valproic acid administration on the gene expression of placental transporters in pregnant rats: An analysis by gestational period. Reprod. Toxicol. 2020, 96, 47–56. [Google Scholar] [CrossRef]
- Qian, X.; Wang, X.; Luo, J.; Liu, Y.; Pang, J.; Zhang, H.; Xu, Z.; Xie, J.; Jiang, X.; Ling, W. Hypouricemic and nephroprotective roles of anthocyanins in hyperuricemic mice. Food Funct. 2019, 10, 867–878. [Google Scholar] [CrossRef]
- Vagnerová, K.; Ergang, P.; Soták, M.; Balounová, K.; Kvapilová, P.; Vodička, M.; Pácha, J. Diurnal expression of ABC and SLC transporters in jejunum is modulated by adrenalectomy. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2019, 226, 108607. [Google Scholar] [CrossRef]
- Oppedisano, F.; Muscoli, C.; Musolino, V.; Carresi, C.; Macrì, R.; Giancotta, C.; Bosco, F.; Maiuolo, J.; Scarano, F.; Paone, S.; et al. The Protective Effect of Cynara Cardunculus Extract in Diet-Induced NAFLD: Involvement of OCTN1 and OCTN2 Transporter Subfamily. Nutrients 2020, 12, 1435. [Google Scholar] [CrossRef]
- Jung, E.S.; Park, H.J.; Kong, K.A.; Choi, J.H.; Cheon, J.H. Association study between OCTN1 functional haplotypes and Crohn's disease in a Korean population. Korean J. Physiol. Pharmacol. 2017, 21, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Martini, M.; Ferrara, A.M.; Giachelia, M.; Panieri, E.; Siminovitch, K.; Galeotti, T.; Larocca, L.M.; Pani, G. Association of the OCTN1/1672T variant with increased risk for colorectal cancer in young individuals and ulcerative colitis patients. Inflamm. Bowel Dis. 2012, 18, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Petito, V.; Fidaleo, M.; Pani, G.; Putignani, L.; Gasbarrini, A.; Scaldaferri, F. Tumor necrosis factor-α and solute carrier family 22 member 4 gene polymorphisms as potential determinants of intestinal dysbiosis. Dig. Liver Dis. 2020, 52, 691–693. [Google Scholar] [CrossRef] [PubMed]
- Console, L.; Scalise, M.; Mazza, T.; Pochini, L.; Galluccio, M.; Giangregorio, N.; Tonazzi, A.; Indiveri, C. Carnitine Traffic in Cells. Link With Cancer. Front. Cell Dev. Biol. 2020, 8. [Google Scholar] [CrossRef]
- Friedman, J.R.; Richbart, S.D.; Merritt, J.C.; Brown, K.C.; Nolan, N.A.; Akers, A.T.; Lau, J.K.; Robateau, Z.R.; Miles, S.L.; Dasgupta, P. Acetylcholine signaling system in progression of lung cancers. Pharmacol. Ther. 2018, 194, 222–254. [Google Scholar] [CrossRef]
- Lamhonwah, A.M.; Tein, I. Novel localization of OCTN1, an organic cation/carnitine transporter, to mammalian mitochondria. Biochem. Biophys. Res. Commun. 2006, 345, 1315–1325. [Google Scholar] [CrossRef]
- Balderas, P.M.D.O. Mitochondria–plasma membrane interactions and communication. J. Biol. Chem. 2021, 297. [Google Scholar] [CrossRef]
- Li, D.; Qi, C.; Zhou, J.; Wen, Z.; Zhu, X.; Xia, H.; Song, J. LPS-induced inflammation delays the transportation of ASP(+) due to down-regulation of OCTN1/2 in alveolar epithelial cells. J. Drug Target. 2020, 28, 437–447. [Google Scholar] [CrossRef]
- Li, X.; Bian, L.; Xu, F.; Qu, Z.; Yang, F.; Jiang, Z. Analysis of tumor heterogeneity at the molecular level: A case report of efficacy separation in metastatic breast cancer. Transl. Breast Cancer Res. 2020, 1, 1–8. [Google Scholar] [CrossRef]
- Meisel, P.; Pagels, S.; Grube, M.; Jedlitschky, G.; Volzke, H.; Kocher, T. Correction to: Tooth loss and adiposity: Possible role of carnitine transporter (OCTN1/2) polymorphisms in women but not in men. Clin. Oral Investig. 2021, 25, 5119. [Google Scholar] [CrossRef]
- Souissi, A.; Ben Said, M.; Ben Ayed, I.; Elloumi, I.; Bouzid, A.; Mosrati, M.A.; Hasnaoui, M.; Belcadhi, M.; Idriss, N.; Kamoun, H.; et al. Novel pathogenic mutations and further evidence for clinical relevance of genes and variants causing hearing impairment in Tunisian population. J. Adv. Res. 2021, 31, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Chiereghin, C.; Robusto, M.; Mauri, L.; Primignani, P.; Castorina, P.; Ambrosetti, U.; Duga, S.; Asselta, R.; Solda, G. SLC22A4 Gene in Hereditary Non-syndromic Hearing Loss: Recurrence and Incomplete Penetrance of the p.C113Y Mutation in Northwest Africa. Front. Genet. 2021, 12, 606630. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.; Zhou, J.; Wang, Z.; Fang, X.; Li, D.; Jin, Y.; Song, J. Cigarette smoke extract combined with lipopolysaccharide reduces OCTN1/2 expression in human alveolar epithelial cells in vitro and rat lung in vivo under inflammatory conditions. Int. Immunopharmacol. 2020, 87, 106812. [Google Scholar] [CrossRef] [PubMed]
- Ostheim, P.; Majewski, M.; Gluzman-Poltorak, Z.; Vainstein, V.; A Basile, L.; Lamkowski, A.; Schüle, S.; Kaatsch, H.L.; Haimerl, M.; Stroszczynski, C.; et al. Predicting the Radiation Sensitivity of Male and Female Rhesus Macaques Using Gene Expression. Radiat. Res. 2020, 195, 25–37. [Google Scholar] [CrossRef]
- Lamhonwah, A.-M.; Tein, I. Expression of the organic cation/carnitine transporter family (Octn1,-2 and-3) in mdx muscle and heart: Implications for early carnitine therapy in Duchenne muscular dystrophy to improve cellular carnitine homeostasis. Clin. Chim. Acta 2020, 505, 92–97. [Google Scholar] [CrossRef]
- Maeda, T.; Hirayama, M.; Kobayashi, D.; Miyazawa, K.; Tamai, I. Mechanism of the Regulation of Organic Cation/Carnitine Transporter 1 (SLC22A4) by Rheumatoid Arthritis-Associated Transcriptional Factor RUNX1 and Inflammatory Cytokines. Drug Metab. Dispos. 2006, 35, 394–401. [Google Scholar] [CrossRef]
- Ishimoto, T.; Nakamichi, N.; Nishijima, H.; Masuo, Y.; Kato, Y. Carnitine/Organic Cation Transporter OCTN1 Negatively Regulates Activation in Murine Cultured Microglial Cells. Neurochem. Res. 2018, 43, 116–128. [Google Scholar] [CrossRef]
- Pour, N.K.; McColl, E.R.; Piquette-Miller, M. Impact of Viral Inflammation on the Expression of Renal Drug Transporters in Pregnant Rats. Pharmaceutics 2019, 11, 624. [Google Scholar] [CrossRef]
- Pour, N.K.; Piquette-Miller, M. Dysregulation of renal transporters in a rodent model of viral Infection. Int. Immunopharmacol. 2020, 80, 106135. [Google Scholar] [CrossRef]
- Nwafor, J.G.; Nowik, M.; Anzai, N.; Endou, H.; Wagner, C.A. Metabolic Acidosis Alters Expression of Slc22 Transporters in Mouse Kidney. Kidney Blood Press. Res. 2020, 45, 263–274. [Google Scholar] [CrossRef]
- Ling, B.; Alcorn, J. LPS-induced inflammation downregulates mammary gland glucose, fatty acid, and l-carnitine transporter expression at different lactation stages. Res. Veter- Sci. 2010, 89, 200–202. [Google Scholar] [CrossRef] [PubMed]
- Ciarimboli, G. Physiology, Biochemistry, and Pharmacology of Transporters for Organic Cations. Int. J. Mol. Sci. 2021, 22, 732. [Google Scholar] [CrossRef] [PubMed]
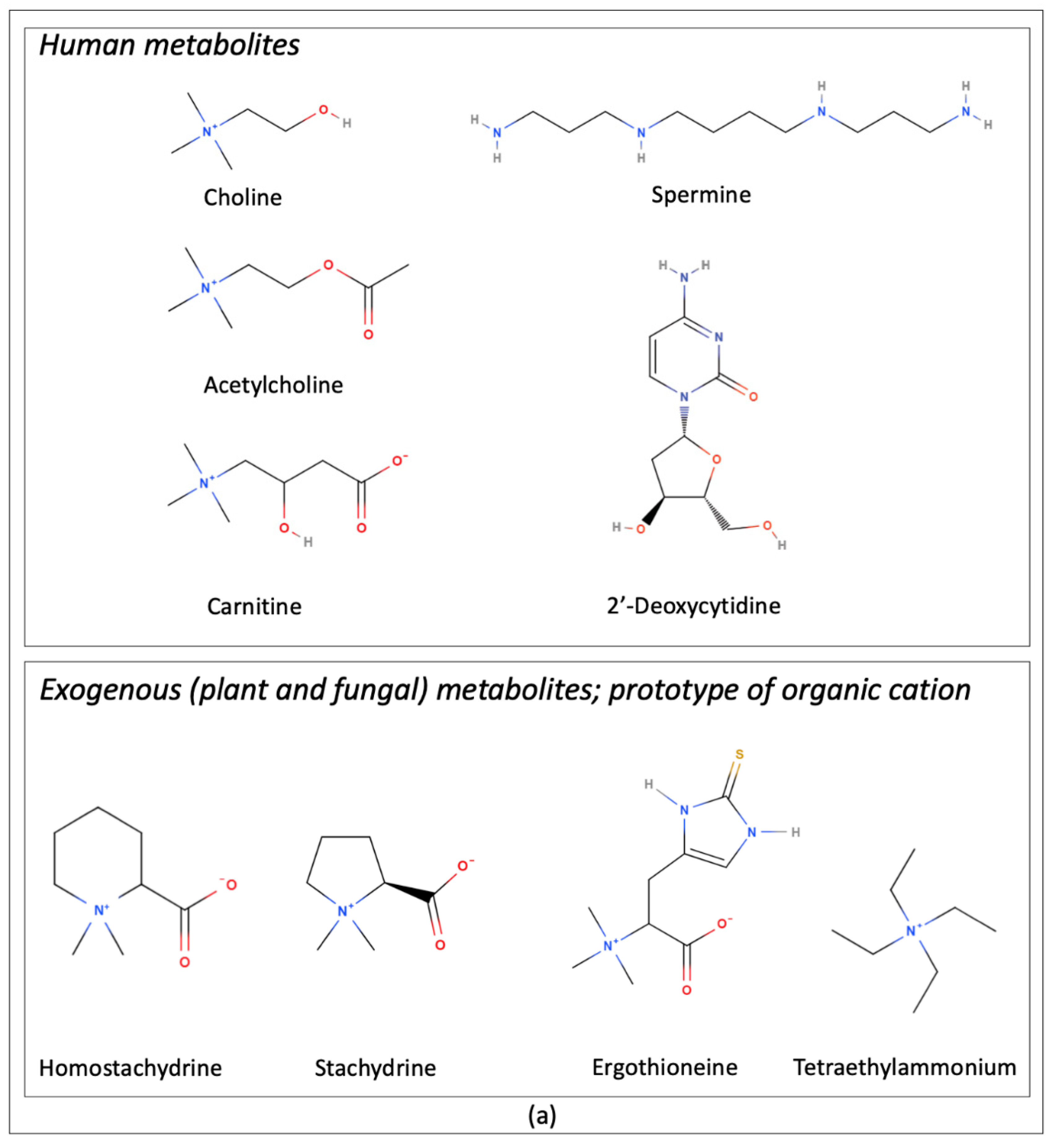
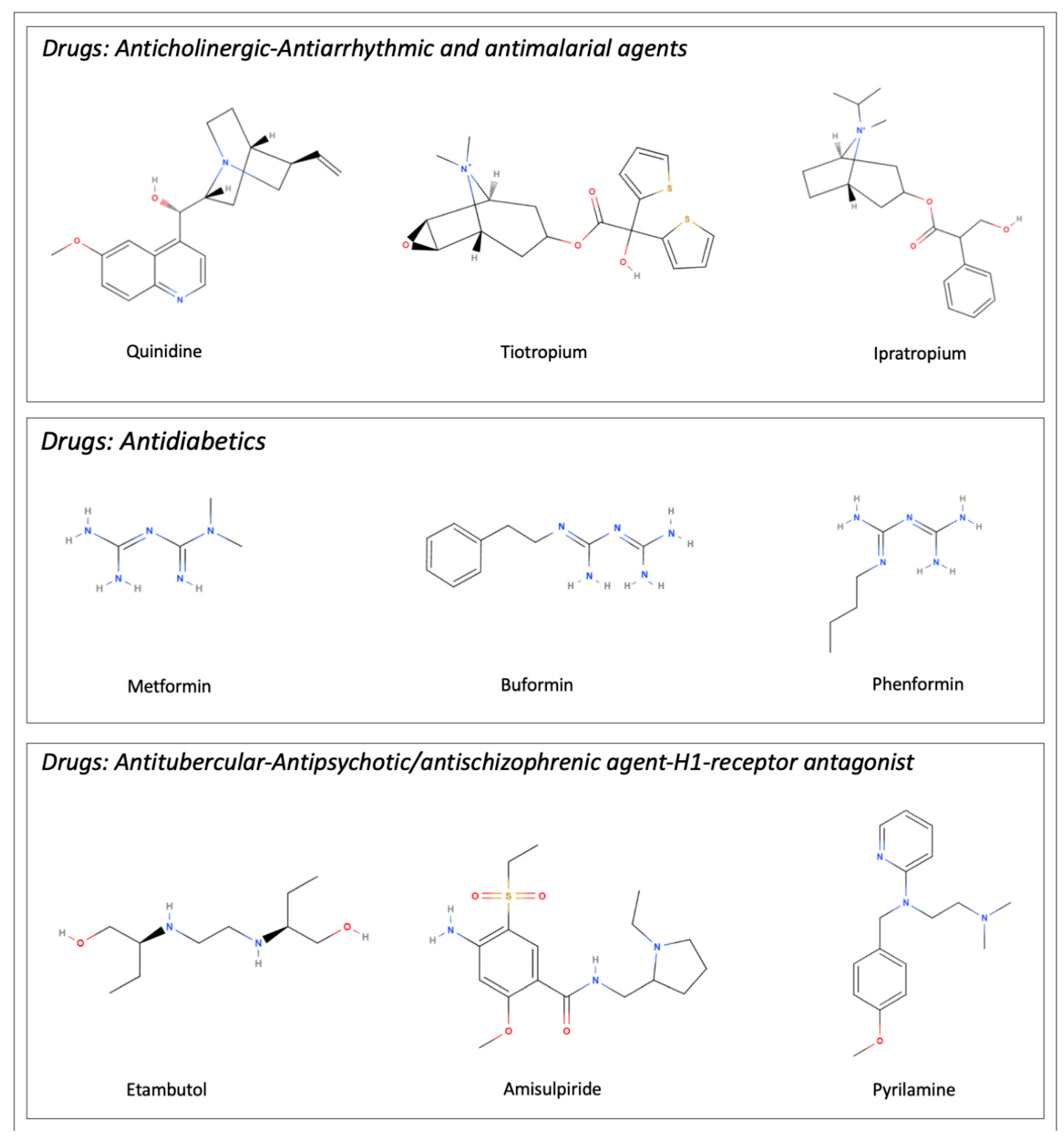
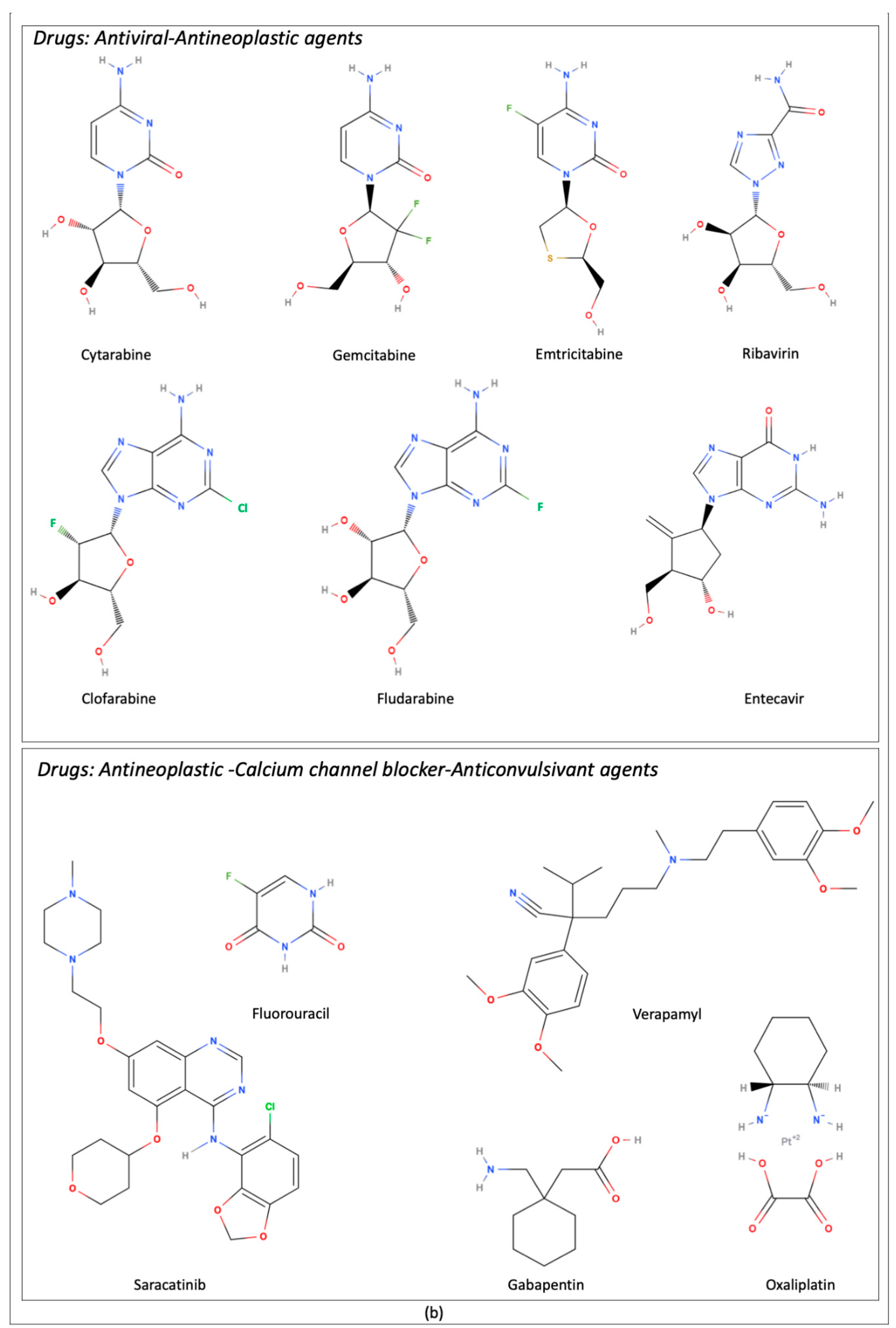

| Transported Endogenous/Natural Substrates | Description/Role From Pubmed Compound Database | References |
|---|---|---|
| Acetylcholine | Acetate ester and an acylcholine. Human metabolite | [11] |
| Carnitine | Amino-acid betaine. Human metabolite | [15,16] |
| Choline | Parent compound of the cholines class. Human metabolite | [12] |
| 2′-Deoxycytidine | Cytidine analogue. Human metabolite | [17] |
| Ergothioneine | L-histidine derivative. Fungal metabolite | [10] |
| Homostachydrine | Ammonium betaine. Plant metabolite | [18] |
| Spermine | Polyazaalkane. Antioxidant, immunosuppressive agent and human metabolite | [14] |
| Stachydrine | L-proline betaine. Plant metabolite | [10] |
| Transported Drugs | Description/Role From Pubmed Compound Database | References |
|---|---|---|
| Amisulpiride | Member of pyrrolidines. Antipsychotic/antischizophrenic agent | [23] |
| Buformin | Class of biguanides. Antidiabetic drug | [24] |
| Clofarabine | Adenosine analogue. Antineoplastic drug | [17] |
| Cytarabine | Cytidine analogue. Antiviral and antineoplastic drug | [17] |
| Emtricitabine | Nucleoside analogue. Antiviral drug | [25] |
| Entecavir | Nucleoside analogue. Antiviral drug | [26] |
| Ethambutol | Ethylenediamine derivative. Antitubercular drug | [27] |
| Fludarabine | Adenosine analogue. Antineoplastic drug | [17] |
| 5-Fluorouracil | Pyrimidine analogue. Antineoplastic activity | [17] |
| Gabapentin | γ-amino acid. Anticonvulsivant, treatment of neuropathic pain | [24,28] |
| Gemcitabine | Cytidine analogue. Antineoplastic drug | [17] |
| Ipratropium | Quaternary ammonium ion. Anticholinergic drug | [29] |
| Metformin | Class of guanidines. Hypoglycemic drug | [24,30] |
| Oxaliplatin | Organoplatinum complex. Antineoplastic drug | [31] |
| Phenformin | Class of biguanides. Antidiabetic drug | [24] |
| Pyrilamine | Ethylenediamine derivative. H1-receptor antagonist | [15] |
| Quinidine | Cinchona alkaloid. Antiarrhythmic and antimalarial effects | [15] |
| Ribavirin | Guanosine analogue. Antiviral drug | [17] |
| Saracatinib | Class of quinazolines. Antitumor activity | [32] |
| Tea | Quaternary ammonium ion. Experimental drug | [15,16] |
| Tiotropium | Quaternary ammonium ion. Muscarinic antagonist and bronchodilator drug | [29] |
| Verapamil | Tertiary amino compound. Calcium channel blocker | [15] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pochini, L.; Galluccio, M.; Scalise, M.; Console, L.; Pappacoda, G.; Indiveri, C. OCTN1: A Widely Studied but Still Enigmatic Organic Cation Transporter Linked to Human Pathology and Drug Interactions. Int. J. Mol. Sci. 2022, 23, 914. https://doi.org/10.3390/ijms23020914
Pochini L, Galluccio M, Scalise M, Console L, Pappacoda G, Indiveri C. OCTN1: A Widely Studied but Still Enigmatic Organic Cation Transporter Linked to Human Pathology and Drug Interactions. International Journal of Molecular Sciences. 2022; 23(2):914. https://doi.org/10.3390/ijms23020914
Chicago/Turabian StylePochini, Lorena, Michele Galluccio, Mariafrancesca Scalise, Lara Console, Gilda Pappacoda, and Cesare Indiveri. 2022. "OCTN1: A Widely Studied but Still Enigmatic Organic Cation Transporter Linked to Human Pathology and Drug Interactions" International Journal of Molecular Sciences 23, no. 2: 914. https://doi.org/10.3390/ijms23020914
APA StylePochini, L., Galluccio, M., Scalise, M., Console, L., Pappacoda, G., & Indiveri, C. (2022). OCTN1: A Widely Studied but Still Enigmatic Organic Cation Transporter Linked to Human Pathology and Drug Interactions. International Journal of Molecular Sciences, 23(2), 914. https://doi.org/10.3390/ijms23020914











