Developmental Formation of the GABAergic and Glycinergic Networks in the Mouse Spinal Cord
Abstract
:1. Introduction
2. GABAergic and Glycinergic Network in the Mature Spinal Cord
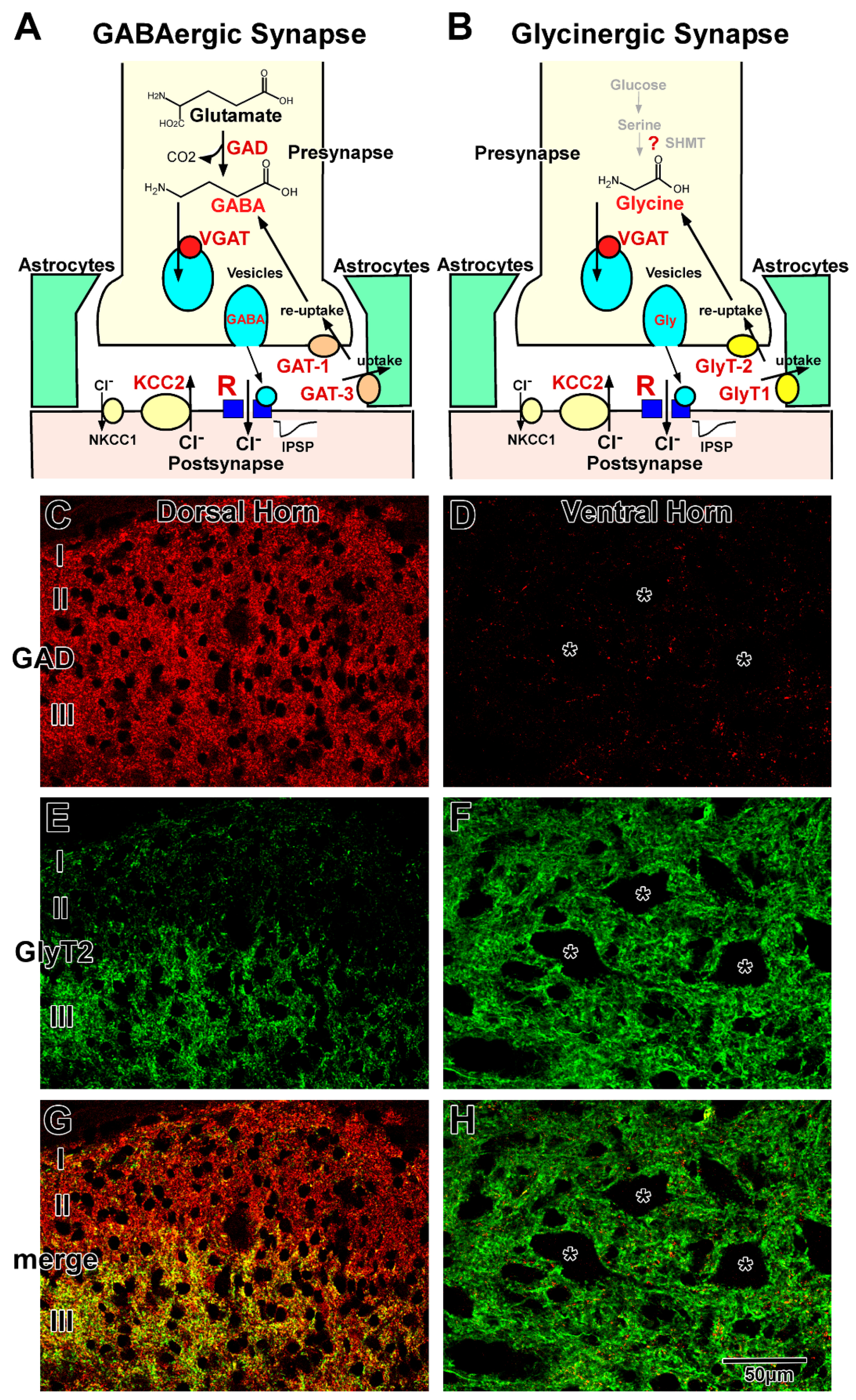
2.1. GABAergic Transmission
2.2. Glycinergic Transmission
2.3. GABAergic and Glycinergic Transmission in the Mature Spinal Cord
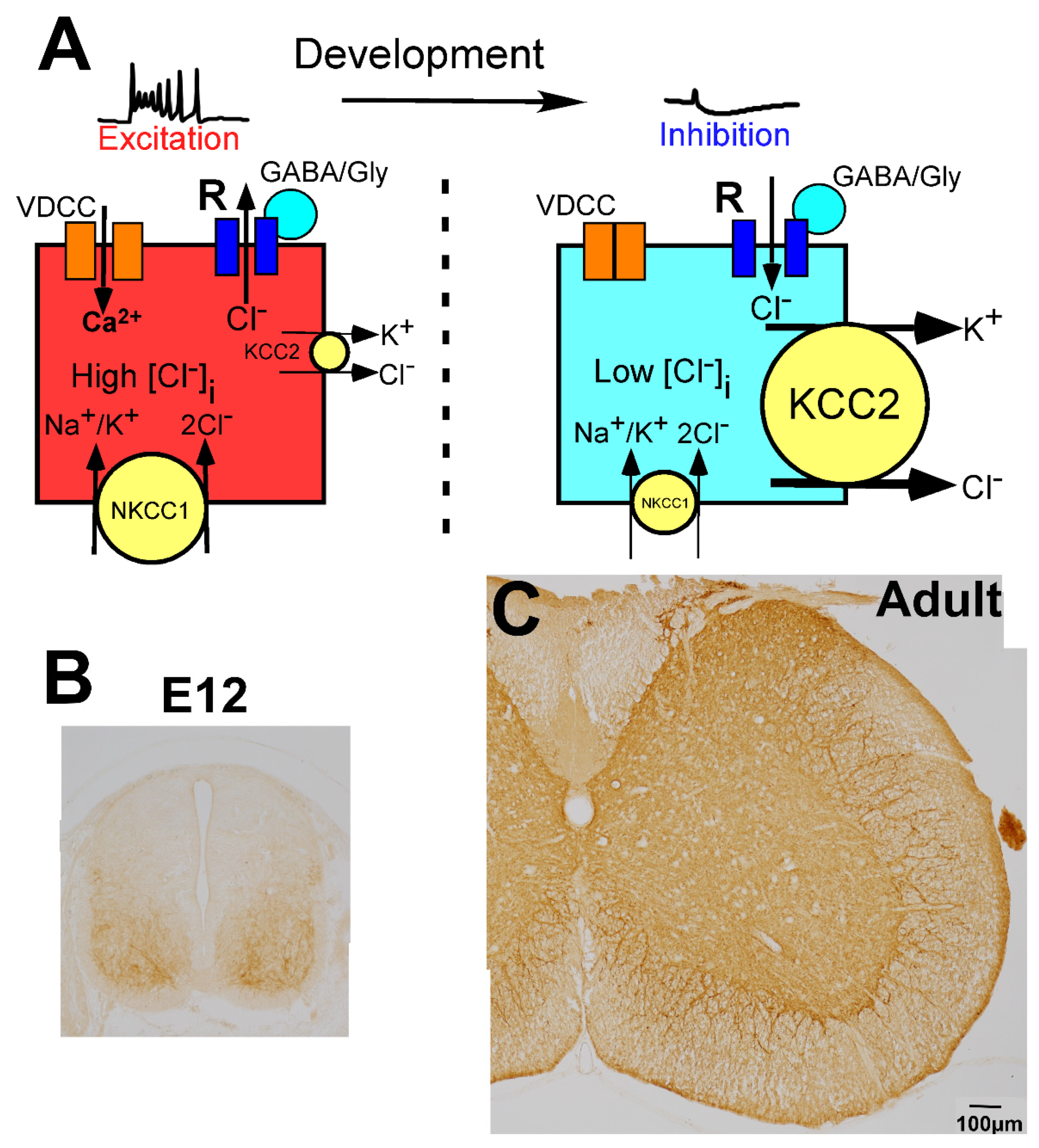
2.4. Regulation of GABAergic and Glycinergic Action by Chloride Transporters
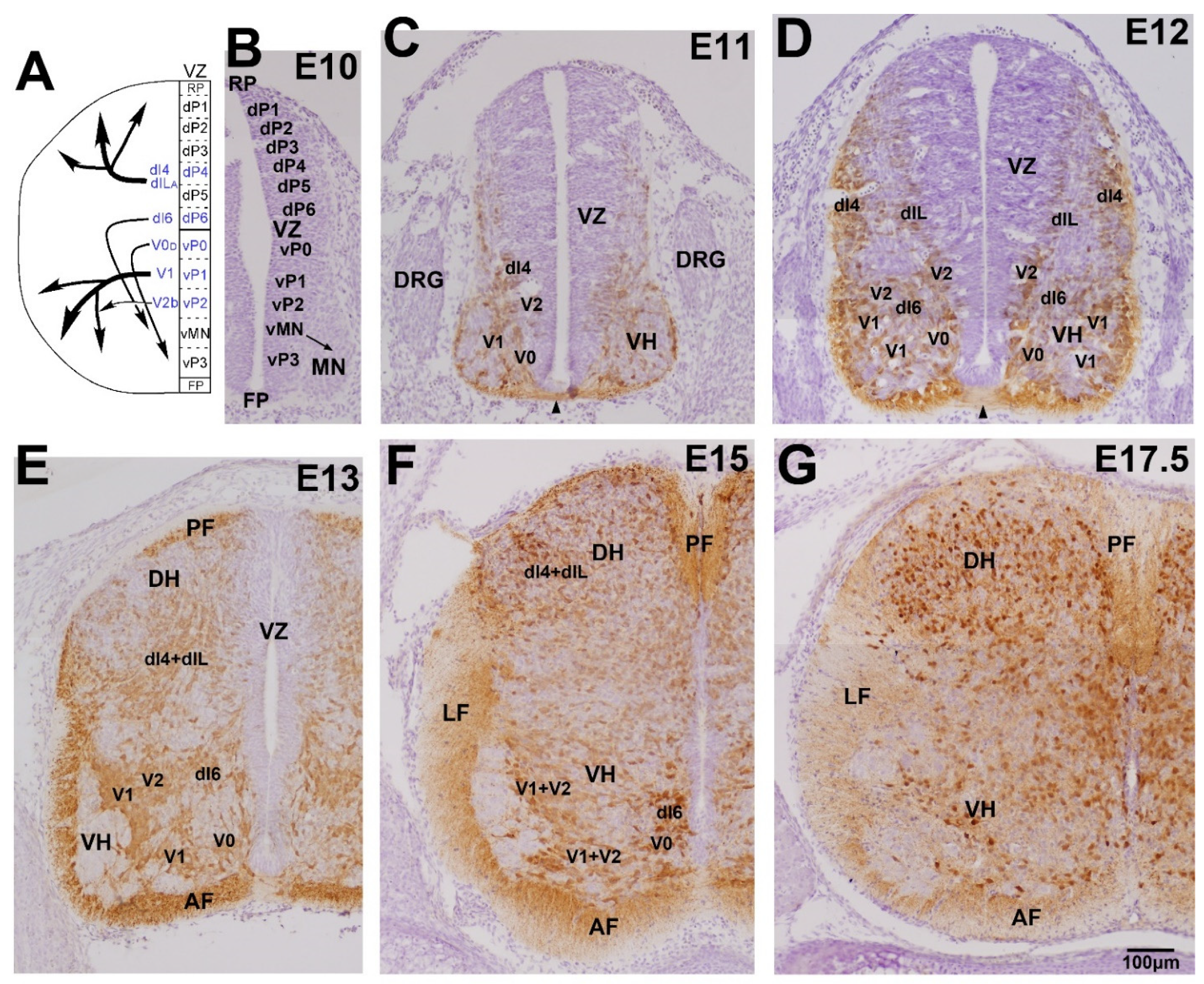
3. Development of GABAergic and Glycinergic Neurons and Their Axon Terminals
3.1. Early Development of the GABAergic and Glycinergic Neurons
3.2. Development in the Ventral Horn
3.3. Development in the Dorsal Horn
3.4. Developmental Formation of Total Inhibitory Terminals
4. Developmental Changes in Ionotropic GABA and Glycine Receptors
4.1. GABAA Receptor
4.2. GABAB Receptor
4.3. GABAC Receptor
4.4. Glycine Receptors
4.5. Developmental Formation of GABA and Glycine Removal System
4.6. Uptake into the Presynaptic Terminals
4.7. Reuptake into the Astrocytes
5. Developmental Changes in GABAergic and Glycinergic Action
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AF | anterior funiculus |
| CNS | central nervous system |
| [Cl−]i | intracellular chloride ion concentration |
| CNS | central nervous system |
| DH | dorsal horn |
| DRG | dorsal root ganglion |
| dI1–6, dIL, dILA, dILB, MN, V0–3, V0D, V0V, V2a, and V2b | neuronal groups, classes, derived from their distinct domains of spinal cord ventricular zone |
| dP1–dP6, vMN, and vP0–vP3 | domains of the ventricular zone in embryonic spinal cord |
| E | embryonic day |
| FP | floor plate |
| GABA | γ-aminobutyric acid |
| GAD | glutamic acid decarboxylase |
| GAT-1, GAT-2, and GAT-3 | GABA transporter 1, 2, and 3 |
| Gly | glycine |
| GlyT1 and GlyT2 | glycine transporter 1 and 2 |
| GFP | green fluorescence protein |
| IPSP | inhibitory postsynaptic potential |
| KCC2 | potassium (K+), chloride (Cl−) cotransporter 2 |
| LF | lateral funiculus |
| NKCC1 | sodium (Na+)-K+-2Cl− cotransporter 1 |
| P | postnatal day |
| PF | posterior funiculus |
| R | GABAA and glycine receptor |
| RP | roof plate |
| SHMT | serine hydroxy-methyltransferase |
| VDCC | voltage-dependent calcium channel |
| VIAAT | vesicular inhibitory amino acid transporter |
| VGAT | vesicular GABA transporter |
| VH | ventral horn |
| VZ | ventricular zone |
| I-IX | laminar number of the gray matter |
| 1 | GAT-1 |
| 3 | GAT-3 |
References
- Macdonald, R.L.; Olsen, R.W. GABAA receptor channels. Annu. Rev. Neurosci. 1994, 17, 569–602. [Google Scholar] [CrossRef]
- Olsen, R.W.; Tobin, A.J. Molecular biology of GABAA receptors. FASEB J. 1990, 4, 1469–1480. [Google Scholar] [CrossRef]
- Kirsch, J. Glycinergic transmission. Cell Tissue Res. 2006, 326, 535–540. [Google Scholar] [CrossRef]
- Legendre, P. The glycinergic inhibitory synapse. Cell. Mol. Life Sci. 2001, 58, 760–793. [Google Scholar] [CrossRef]
- Tritsch, N.X.; Granger, A.J.; Sabatini, B.L. Mechanisms and functions of GABA co-release. Nat. Rev. Neurosci. 2016, 17, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Vaaga, C.E.; Borisovska, M.; Westbrook, G.L. Dual-transmitter neurons: Functional implications of co-release and co-transmission. Curr. Opin. Neurobiol. 2014, 29, 25–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jonas, P.; Bischofberger, J.; Sandkuhler, J. Corelease of two fast neurotransmitters at a central synapse. Science 1998, 281, 419–424. [Google Scholar] [CrossRef] [Green Version]
- Hossaini, M.; French, P.J.; Holstege, J.C. Distribution of glycinergic neuronal somata in the rat spinal cord. Brain Res. 2007, 1142, 61–69. [Google Scholar] [CrossRef]
- Todd, A.J.; Sullivan, A.C. Light microscope study of the coexistence of GABA-like and glycine-like immunoreactivities in the spinal cord of the rat. J. Comp. Neurol. 1990, 296, 496–505. [Google Scholar] [CrossRef]
- Zeilhofer, H.U.; Studler, B.; Arabadzisz, D.; Schweizer, C.; Ahmadi, S.; Layh, B.; Bosl, M.R.; Fritschy, J.M. Glycinergic neurons expressing enhanced green fluorescent protein in bacterial artificial chromosome transgenic mice. J. Comp. Neurol. 2005, 482, 123–141. [Google Scholar] [CrossRef] [PubMed]
- Barker, J.L.; Behar, T.; Li, Y.X.; Liu, Q.Y.; Ma, W.; Maric, D.; Maric, I.; Schaffner, A.E.; Serafini, R.; Smith, S.V.; et al. GABAergic cells and signals in CNS development. Perspect. Dev. Neurobiol. 1998, 5, 305–322. [Google Scholar] [PubMed]
- Zafra, F.; Aragon, C.; Gimenez, C. Molecular biology of glycinergic neurotransmission. Mol. Neurobiol. 1997, 14, 117–142. [Google Scholar] [CrossRef]
- Martin, D.L.; Rimvall, K. Regulation of gamma-aminobutyric acid synthesis in the brain. J. Neurochem. 1993, 60, 395–407. [Google Scholar] [CrossRef]
- Varju, P.; Katarova, Z.; Madarasz, E.; Szabo, G. GABA signalling during development: New data and old questions. Cell Tissue Res. 2001, 305, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Bak, L.K.; Schousboe, A.; Waagepetersen, H.S. The glutamate/GABA-glutamine cycle: Aspects of transport, neurotransmitter homeostasis and ammonia transfer. J. Neurochem. 2006, 98, 641–653. [Google Scholar] [CrossRef] [PubMed]
- Bedet, C.; Isambert, M.F.; Henry, J.P.; Gasnier, B. Constitutive phosphorylation of the vesicular inhibitory amino acid transporter in rat central nervous system. J. Neurochem. 2000, 75, 1654–1663. [Google Scholar] [CrossRef] [Green Version]
- Sagne, C.; El Mestikawy, S.; Isambert, M.F.; Hamon, M.; Henry, J.P.; Giros, B.; Gasnier, B. Cloning of a functional vesicular GABA and glycine transporter by screening of genome databases. FEBS Lett. 1997, 417, 177–183. [Google Scholar] [CrossRef] [Green Version]
- Mehta, A.K.; Ticku, M.K. An update on GABAA receptors. Brain Res. Brain Res. Rev. 1999, 29, 196–217. [Google Scholar] [CrossRef]
- Sieghart, W. Structure and pharmacology of gamma-aminobutyric acidA receptor subtypes. Pharmacol. Rev. 1995, 47, 181–234. [Google Scholar]
- Laurie, D.J.; Seeburg, P.H.; Wisden, W. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. II. Olfactory bulb and cerebellum. J. Neurosci. 1992, 12, 1063–1076. [Google Scholar] [CrossRef]
- Wisden, W.; Gundlach, A.L.; Barnard, E.A.; Seeburg, P.H.; Hunt, S.P. Distribution of GABAA receptor subunit mRNAs in rat lumbar spinal cord. Brain Res. Mol. Brain Res. 1991, 10, 179–183. [Google Scholar] [CrossRef]
- Wisden, W.; Laurie, D.J.; Monyer, H.; Seeburg, P.H. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. I. Telencephalon, diencephalon, mesencephalon. J. Neurosci. 1992, 12, 1040–1062. [Google Scholar] [CrossRef] [Green Version]
- Pritchett, D.B.; Sontheimer, H.; Shivers, B.D.; Ymer, S.; Kettenmann, H.; Schofield, P.R.; Seeburg, P.H. Importance of a novel GABAA receptor subunit for benzodiazepine pharmacology. Nature 1989, 338, 582–585. [Google Scholar] [CrossRef] [PubMed]
- Vicini, S. New perspectives in the functional role of GABA(A) channel heterogeneity. Mol. Neurobiol. 1999, 19, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Bormann, J.; Feigenspan, A. GABAC receptors. Trends Neurosci. 1995, 18, 515–519. [Google Scholar] [CrossRef]
- Bormann, J. The ‘ABC’ of GABA receptors. Trends Pharmacol. Sci. 2000, 21, 16–19. [Google Scholar] [CrossRef]
- Bormann, J. Electrophysiology of GABAA and GABAB receptor subtypes. Trends Neurosci. 1988, 11, 112–116. [Google Scholar] [CrossRef]
- Connors, B.W.; Malenka, R.C.; Silva, L.R. Two inhibitory postsynaptic potentials, and GABAA and GABAB receptor-mediated responses in neocortex of rat and cat. J. Physiol. 1988, 406, 443–468. [Google Scholar] [CrossRef]
- LeVine, H., 3rd. Structural features of heterotrimeric G-protein-coupled receptors and their modulatory proteins. Mol. Neurobiol. 1999, 19, 111–149. [Google Scholar] [CrossRef] [PubMed]
- Nicoll, R.A. The coupling of neurotransmitter receptors to ion channels in the brain. Science 1988, 241, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Jembrek, M.J.; Vlainic, J. GABA Receptors: Pharmacological Potential and Pitfalls. Curr. Pharm. Des. 2015, 21, 4943–4959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evenseth, L.S.M.; Gabrielsen, M.; Sylte, I. The GABAB Receptor-Structure, Ligand Binding and Drug Development. Molecules 2020, 25, 3093. [Google Scholar] [CrossRef]
- Cherubini, E.; Conti, F. Generating diversity at GABAergic synapses. Trends Neurosci. 2001, 24, 155–162. [Google Scholar] [CrossRef]
- Kanner, B.I. Sodium-coupled neurotransmitter transport: Structure, function and regulation. J. Exp. Biol. 1994, 196, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Gadea, A.; Lopez-Colome, A.M. Glial transporters for glutamate, glycine, and GABA: II. GABA transporters. J. Neurosci. Res. 2001, 63, 461–468. [Google Scholar] [CrossRef]
- Conti, F.; Zuccarello, L.V.; Barbaresi, P.; Minelli, A.; Brecha, N.C.; Melone, M. Neuronal, glial, and epithelial localization of gamma-aminobutyric acid transporter 2, a high-affinity gamma-aminobutyric acid plasma membrane transporter, in the cerebral cortex and neighboring structures. J. Comp. Neurol. 1999, 409, 482–494. [Google Scholar] [CrossRef]
- Minelli, A.; Brecha, N.C.; Karschin, C.; DeBiasi, S.; Conti, F. GAT-1, a high-affinity GABA plasma membrane transporter, is localized to neurons and astroglia in the cerebral cortex. J. Neurosci. 1995, 15, 7734–7746. [Google Scholar] [CrossRef]
- Minelli, A.; DeBiasi, S.; Brecha, N.C.; Zuccarello, L.V.; Conti, F. GAT-3, a high-affinity GABA plasma membrane transporter, is localized to astrocytic processes, and it is not confined to the vicinity of GABAergic synapses in the cerebral cortex. J. Neurosci. 1996, 16, 6255–6264. [Google Scholar] [CrossRef] [Green Version]
- Itouji, A.; Sakai, N.; Tanaka, C.; Saito, N. Neuronal and glial localization of two GABA transporters (GAT1 and GAT3) in the rat cerebellum. Brain Res. Mol. Brain Res. 1996, 37, 309–316. [Google Scholar] [CrossRef]
- Takayama, C.; Inoue, Y. Developmental expression of GABA transporter-1 and 3 during formation of the GABAergic synapses in the mouse cerebellar cortex. Brain Res. Dev. Brain Res. 2005, 158, 41–49. [Google Scholar] [CrossRef] [Green Version]
- Kolker, S. Metabolism of amino acid neurotransmitters: The synaptic disorder underlying inherited metabolic diseases. J. Inherit. Metab. Dis. 2018, 41, 1055–1063. [Google Scholar] [CrossRef] [PubMed]
- Verleysdonk, S.; Martin, H.; Willker, W.; Leibfritz, D.; Hamprecht, B. Rapid uptake and degradation of glycine by astroglial cells in culture: Synthesis and release of serine and lactate. Glia 1999, 27, 239–248. [Google Scholar] [CrossRef]
- Beyoglu, D.; Idle, J.R. The glycine deportation system and its pharmacological consequences. Pharmacol. Ther. 2012, 135, 151–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeilhofer, H.U.; Wildner, H.; Yevenes, G.E. Fast synaptic inhibition in spinal sensory processing and pain control. Physiol. Rev. 2012, 92, 193–235. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.L.; Gong, N. Glycine and glycine receptor signaling in hippocampal neurons: Diversity, function and regulation. Prog. Neurobiol. 2010, 91, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Gomeza, J.; Ohno, K.; Hulsmann, S.; Armsen, W.; Eulenburg, V.; Richter, D.W.; Laube, B.; Betz, H. Deletion of the mouse glycine transporter 2 results in a hyperekplexia phenotype and postnatal lethality. Neuron 2003, 40, 797–806. [Google Scholar] [CrossRef] [Green Version]
- Latal, A.T.; Kremer, T.; Gomeza, J.; Eulenburg, V.; Hulsmann, S. Development of synaptic inhibition in glycine transporter 2 deficient mice. Mol. Cell. Neurosci. 2010, 44, 342–352. [Google Scholar] [CrossRef]
- Chalphin, A.V.; Saha, M.S. The specification of glycinergic neurons and the role of glycinergic transmission in development. Front. Mol. Neurosci. 2010, 3, 11. [Google Scholar] [CrossRef] [Green Version]
- Laube, B.; Maksay, G.; Schemm, R.; Betz, H. Modulation of glycine receptor function: A novel approach for therapeutic intervention at inhibitory synapses? Trends Pharmacol. Sci. 2002, 23, 519–527. [Google Scholar] [CrossRef] [Green Version]
- Lynch, J.W. Molecular structure and function of the glycine receptor chloride channel. Physiol. Rev. 2004, 84, 1051–1095. [Google Scholar] [CrossRef]
- Lynch, J.W. Native glycine receptor subtypes and their physiological roles. Neuropharmacology 2009, 56, 303–309. [Google Scholar] [CrossRef] [Green Version]
- Eulenburg, V.; Armsen, W.; Betz, H.; Gomeza, J. Glycine transporters: Essential regulators of neurotransmission. Trends Biochem. Sci. 2005, 30, 325–333. [Google Scholar] [CrossRef]
- Sunagawa, M.; Shimizu-Okabe, C.; Kim, J.; Kobayashi, S.; Kosaka, Y.; Yanagawa, Y.; Matsushita, M.; Okabe, A.; Takayama, C. Distinct development of the glycinergic terminals in the ventral and dorsal horns of the mouse cervical spinal cord. Neuroscience 2017, 343, 459–471. [Google Scholar] [CrossRef] [Green Version]
- Zafra, F.; Aragon, C.; Olivares, L.; Danbolt, N.C.; Gimenez, C.; Storm-Mathisen, J. Glycine transporters are differentially expressed among CNS cells. J. Neurosci. 1995, 15, 3952–3969. [Google Scholar] [CrossRef]
- Sato, K.; Yoshida, S.; Fujiwara, K.; Tada, K.; Tohyama, M. Glycine cleavage system in astrocytes. Brain Res. 1991, 567, 64–70. [Google Scholar] [CrossRef]
- Kikuchi, G.; Motokawa, Y.; Yoshida, T.; Hiraga, K. Glycine cleavage system: Reaction mechanism, physiological significance, and hyperglycinemia. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2008, 84, 246–263. [Google Scholar] [CrossRef] [Green Version]
- Kosaka, Y.; Kin, H.; Tatetsu, M.; Uema, I.; Takayama, C. Distinct development of GABA system in the ventral and dorsal horns in the embryonic mouse spinal cord. Brain Res. 2012, 1486, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Todd, A.J.; Maxwell, D.J. GABA in the mammalian spinal cord. In GABA in the Nervous System; Martin, D.L., Olsen, R.W., Eds.; Lippincotto Willams & Wilkins: Philadelphia, PA, USA, 2000; pp. 439–457. [Google Scholar]
- Ottersen, O.P.; Storm-Mathisen, J. Glutamate- and GABA-containing neurons in the mouse and rat brain, as demonstrated with a new immunocytochemical technique. J. Comp. Neurol. 1984, 229, 374–392. [Google Scholar] [CrossRef]
- Tamamaki, N.; Yanagawa, Y.; Tomioka, R.; Miyazaki, J.; Obata, K.; Kaneko, T. Green fluorescent protein expression and colocalization with calretinin, parvalbumin, and somatostatin in the GAD67-GFP knock-in mouse. J. Comp. Neurol. 2003, 467, 60–79. [Google Scholar] [CrossRef] [PubMed]
- Campistron, G.; Buijs, R.M.; Geffard, M. Glycine neurons in the brain and spinal cord. Antibody production and immunocytochemical localization. Brain Res. 1986, 376, 400–405. [Google Scholar] [CrossRef] [Green Version]
- Allain, A.E.; Bairi, A.; Meyrand, P.; Branchereau, P. Expression of the glycinergic system during the course of embryonic development in the mouse spinal cord and its co-localization with GABA immunoreactivity. J. Comp. Neurol. 2006, 496, 832–846. [Google Scholar] [CrossRef] [PubMed]
- Wojcik, S.M.; Katsurabayashi, S.; Guillemin, I.; Friauf, E.; Rosenmund, C.; Brose, N.; Rhee, J.S. A shared vesicular carrier allows synaptic corelease of GABA and glycine. Neuron 2006, 50, 575–587. [Google Scholar] [CrossRef] [Green Version]
- Ishibashi, H.; Yamaguchi, J.; Nakahata, Y.; Nabekura, J. Dynamic regulation of glycine-GABA co-transmission at spinal inhibitory synapses by neuronal glutamate transporter. J. Physiol. 2013, 591, 3821–3832. [Google Scholar] [CrossRef] [PubMed]
- Ornung, G.; Shupliakov, O.; Linda, H.; Ottersen, O.P.; Storm-Mathisen, J.; Ulfhake, B.; Cullheim, S. Qualitative and quantitative analysis of glycine- and GABA-immunoreactive nerve terminals on motoneuron cell bodies in the cat spinal cord: A postembedding electron microscopic study. J. Comp. Neurol. 1996, 365, 413–426. [Google Scholar] [CrossRef]
- Dougherty, K.J.; Sawchuk, M.A.; Hochman, S. Phenotypic diversity and expression of GABAergic inhibitory interneurons during postnatal development in lumbar spinal cord of glutamic acid decarboxylase 67-green fluorescent protein mice. Neuroscience 2009, 163, 909–919. [Google Scholar] [CrossRef] [Green Version]
- Bardoni, R.; Takazawa, T.; Tong, C.K.; Choudhury, P.; Scherrer, G.; Macdermott, A.B. Pre- and postsynaptic inhibitory control in the spinal cord dorsal horn. Ann. N. Y. Acad. Sci. 2013, 1279, 90–96. [Google Scholar] [CrossRef]
- Basbaum, A.I.; Bautista, D.M.; Scherrer, G.; Julius, D. Cellular and molecular mechanisms of pain. Cell 2009, 139, 267–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cioffi, C.L. Modulation of Glycine-Mediated Spinal Neurotransmission for the Treatment of Chronic Pain. J. Med. Chem. 2018, 61, 2652–2679. [Google Scholar] [CrossRef]
- Goulding, M. Circuits controlling vertebrate locomotion: Moving in a new direction. Nat. Rev. Neurosci. 2009, 10, 507–518. [Google Scholar] [CrossRef]
- Paul, J.; Zeilhofer, H.U.; Fritschy, J.M. Selective distribution of GABA(A) receptor subtypes in mouse spinal dorsal horn neurons and primary afferents. J. Comp. Neurol. 2012, 520, 3895–3911. [Google Scholar] [CrossRef]
- Malosio, M.L.; Marqueze-Pouey, B.; Kuhse, J.; Betz, H. Widespread expression of glycine receptor subunit mRNAs in the adult and developing rat brain. EMBO J. 1991, 10, 2401–2409. [Google Scholar] [CrossRef] [PubMed]
- Todd, A.J.; Watt, C.; Spike, R.C.; Sieghart, W. Colocalization of GABA, glycine, and their receptors at synapses in the rat spinal cord. J. Neurosci. 1996, 16, 974–982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dorfman, V.B.; Vega, M.C.; Coirini, H. Age-related changes of the GABA-B receptor in the lumbar spinal cord of male rats and penile erection. Life Sci. 2006, 78, 1529–1534. [Google Scholar] [CrossRef] [PubMed]
- Sands, S.A.; Purisai, M.G.; Chronwall, B.M.; Enna, S.J. Ontogeny of GABA(B) receptor subunit expression and function in the rat spinal cord. Brain Res. 2003, 972, 197–206. [Google Scholar] [CrossRef]
- Rozzo, A.; Armellin, M.; Franzot, J.; Chiaruttini, C.; Nistri, A.; Tongiorgi, E. Expression and dendritic mRNA localization of GABAC receptor rho1 and rho2 subunits in developing rat brain and spinal cord. Eur. J. Neurosci. 2002, 15, 1747–1758. [Google Scholar] [CrossRef]
- Kim, J.; Kosaka, Y.; Shimizu-Okabe, C.; Niizaki, A.; Takayama, C. Characteristic development of the GABA-removal system in the mouse spinal cord. Neuroscience 2014, 262, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Jursky, F.; Nelson, N. Localization of glycine neurotransmitter transporter (GLYT2) reveals correlation with the distribution of glycine receptor. J. Neurochem. 1995, 64, 1026–1033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Owens, D.F.; Kriegstein, A.R. Is there more to GABA than synaptic inhibition? Nat. Rev. Neurosci. 2002, 3, 715–727. [Google Scholar] [CrossRef]
- Ben-Ari, Y. Excitatory actions of gaba during development: The nature of the nurture. Nat. Rev. Neurosci. 2002, 3, 728–739. [Google Scholar] [CrossRef] [PubMed]
- Payne, J.A.; Rivera, C.; Voipio, J.; Kaila, K. Cation-chloride co-transporters in neuronal communication, development and trauma. Trends Neurosci. 2003, 26, 199–206. [Google Scholar] [CrossRef]
- Baccei, M.L.; Fitzgerald, M. Development of GABAergic and glycinergic transmission in the neonatal rat dorsal horn. J. Neurosci. 2004, 24, 4749–4757. [Google Scholar] [CrossRef]
- Sagner, A.; Briscoe, J. Establishing neuronal diversity in the spinal cord: A time and a place. Development 2019, 146, dev182154. [Google Scholar] [CrossRef] [Green Version]
- Hernandez-Miranda, L.R.; Muller, T.; Birchmeier, C. The dorsal spinal cord and hindbrain: From developmental mechanisms to functional circuits. Dev. Biol. 2017, 432, 34–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Briscoe, J.; Ericson, J. Specification of neuronal fates in the ventral neural tube. Curr. Opin. Neurobiol. 2001, 11, 43–49. [Google Scholar] [CrossRef]
- Jessell, T.M. Neuronal specification in the spinal cord: Inductive signals and transcriptional codes. Nat. Rev. Genet. 2000, 1, 20–29. [Google Scholar] [CrossRef]
- Lee, K.J.; Jessell, T.M. The specification of dorsal cell fates in the vertebrate central nervous system. Annu. Rev. Neurosci. 1999, 22, 261–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helms, A.W.; Johnson, J.E. Specification of dorsal spinal cord interneurons. Curr. Opin. Neurobiol. 2003, 13, 42–49. [Google Scholar] [CrossRef]
- Hori, K.; Hoshino, M. GABAergic neuron specification in the spinal cord, the cerebellum, and the cochlear nucleus. Neural Plast. 2012, 2012, 921732. [Google Scholar] [CrossRef] [Green Version]
- Goulding, M.; Lamar, E. Neuronal patterning: Making stripes in the spinal cord. Curr. Biol. 2000, 10, R565–R568. [Google Scholar] [CrossRef] [Green Version]
- Tran, T.S.; Alijani, A.; Phelps, P.E. Unique developmental patterns of GABAergic neurons in rat spinal cord. J. Comp. Neurol. 2003, 456, 112–126. [Google Scholar] [CrossRef]
- Allain, A.E.; Bairi, A.; Meyrand, P.; Branchereau, P. Ontogenic changes of the GABAergic system in the embryonic mouse spinal cord. Brain Res. 2004, 1000, 134–147. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Chen, J.; Wang, W.; Wei, Y.Y.; Cai, G.H.; Tamamaki, N.; Li, Y.Q.; Wu, S.X. Birthdate study of GABAergic neurons in the lumbar spinal cord of the glutamic acid decarboxylase 67-green fluorescent protein knock-in mouse. Front. Neuroanat. 2013, 7, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delile, J.; Rayon, T.; Melchionda, M.; Edwards, A.; Briscoe, J.; Sagner, A. Single cell transcriptomics reveals spatial and temporal dynamics of gene expression in the developing mouse spinal cord. Development 2019, 146, dev.173807. [Google Scholar] [CrossRef] [Green Version]
- Borromeo, M.D.; Meredith, D.M.; Castro, D.S.; Chang, J.C.; Tung, K.C.; Guillemot, F.; Johnson, J.E. A transcription factor network specifying inhibitory versus excitatory neurons in the dorsal spinal cord. Development 2014, 141, 2803–2812. [Google Scholar] [CrossRef] [Green Version]
- Restrepo, C.E.; Lundfald, L.; Szabo, G.; Erdelyi, F.; Zeilhofer, H.U.; Glover, J.C.; Kiehn, O. Transmitter-phenotypes of commissural interneurons in the lumbar spinal cord of newborn mice. J. Comp. Neurol. 2009, 517, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.; Pfaff, S.L. Transcriptional networks regulating neuronal identity in the developing spinal cord. Nat. Neurosci. 2001, 4, 1183–1191. [Google Scholar] [CrossRef]
- Stachowski, N.J.; Dougherty, K.J. Spinal Inhibitory Interneurons: Gatekeepers of Sensorimotor Pathways. Int. J. Mol. Sci. 2021, 22, 2667. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.S.; Cohen-Cory, S.; Phelps, P.E. Embryonic GABAergic spinal commissural neurons project rostrally to mesencephalic targets. J. Comp. Neurol. 2004, 475, 327–339. [Google Scholar] [CrossRef]
- Moran-Rivard, L.; Kagawa, T.; Saueressig, H.; Gross, M.K.; Burrill, J.; Goulding, M. Evx1 is a postmitotic determinant of v0 interneuron identity in the spinal cord. Neuron 2001, 29, 385–399. [Google Scholar] [CrossRef] [Green Version]
- Joshi, K.; Lee, S.; Lee, B.; Lee, J.W.; Lee, S.K. LMO4 controls the balance between excitatory and inhibitory spinal V2 interneurons. Neuron 2009, 61, 839–851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lundfald, L.; Restrepo, C.E.; Butt, S.J.; Peng, C.Y.; Droho, S.; Endo, T.; Zeilhofer, H.U.; Sharma, K.; Kiehn, O. Phenotype of V2-derived interneurons and their relationship to the axon guidance molecule EphA4 in the developing mouse spinal cord. Eur. J. Neurosci. 2007, 26, 2989–3002. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, F.J.; Jonas, P.C.; Sapir, T.; Hartley, R.; Berrocal, M.C.; Geiman, E.J.; Todd, A.J.; Goulding, M. Postnatal phenotype and localization of spinal cord V1 derived interneurons. J. Comp. Neurol. 2005, 493, 177–192. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Lanuza, G.M.; Britz, O.; Wang, Z.; Siembab, V.C.; Zhang, Y.; Velasquez, T.; Alvarez, F.J.; Frank, E.; Goulding, M. V1 and v2b interneurons secure the alternating flexor-extensor motor activity mice require for limbed locomotion. Neuron 2014, 82, 138–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Condie, B.G.; Bain, G.; Gottlieb, D.I.; Capecchi, M.R. Cleft palate in mice with a targeted mutation in the gamma-aminobutyric acid-producing enzyme glutamic acid decarboxylase 67. Proc. Natl. Acad. Sci. USA 1997, 94, 11451–11455. [Google Scholar] [CrossRef] [Green Version]
- Ding, R.; Tsunekawa, N.; Obata, K. Cleft palate by picrotoxin or 3-MP and palatal shelf elevation in GABA-deficient mice. Neurotoxicol. Teratol. 2004, 26, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Ji, F.; Kanbara, N.; Obata, K. GABA and histogenesis in fetal and neonatal mouse brain lacking both the isoforms of glutamic acid decarboxylase. Neurosci. Res. 1999, 33, 187–194. [Google Scholar] [CrossRef]
- Gao, B.X.; Stricker, C.; Ziskind-Conhaim, L. Transition from GABAergic to glycinergic synaptic transmission in newly formed spinal networks. J. Neurophysiol. 2001, 86, 492–502. [Google Scholar] [CrossRef]
- Singer, J.H.; Berger, A.J. Development of inhibitory synaptic transmission to motoneurons. Brain Res. Bull. 2000, 53, 553–560. [Google Scholar] [CrossRef]
- Kotak, V.C.; Korada, S.; Schwartz, I.R.; Sanes, D.H. A developmental shift from GABAergic to glycinergic transmission in the central auditory system. J. Neurosci. 1998, 18, 4646–4655. [Google Scholar] [CrossRef] [Green Version]
- Nabekura, J.; Katsurabayashi, S.; Kakazu, Y.; Shibata, S.; Matsubara, A.; Jinno, S.; Mizoguchi, Y.; Sasaki, A.; Ishibashi, H. Developmental switch from GABA to glycine release in single central synaptic terminals. Nat. Neurosci. 2004, 7, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Nerlich, J.; Rubsamen, R.; Milenkovic, I. Developmental Shift of Inhibitory Transmitter Content at a Central Auditory Synapse. Front. Cell. Neurosci. 2017, 11, 211. [Google Scholar] [CrossRef]
- McMenamin, C.A.; Anselmi, L.; Travagli, R.A.; Browning, K.N. Developmental regulation of inhibitory synaptic currents in the dorsal motor nucleus of the vagus in the rat. J. Neurophysiol. 2016, 116, 1705–1714. [Google Scholar] [CrossRef] [Green Version]
- Ma, W.; Saunders, P.A.; Somogyi, R.; Poulter, M.O.; Barker, J.L. Ontogeny of GABAA receptor subunit mRNAs in rat spinal cord and dorsal root ganglia. J. Comp. Neurol. 1993, 338, 337–359. [Google Scholar] [CrossRef]
- Laurie, D.J.; Wisden, W.; Seeburg, P.H. The distribution of thirteen GABAA receptor subunit mRNAs in the rat brain. III. Embryonic and postnatal development. J. Neurosci. 1992, 12, 4151–4172. [Google Scholar] [CrossRef]
- LoTurco, J.J.; Owens, D.F.; Heath, M.J.; Davis, M.B.; Kriegstein, A.R. GABA and glutamate depolarize cortical progenitor cells and inhibit DNA synthesis. Neuron 1995, 15, 1287–1298. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.O.; Li, S.; Park, M.S.; Hornung, J.P. Early fetal expression of GABA(B1) and GABA(B2) receptor mRNAs on the development of the rat central nervous system. Brain Res. Dev. Brain Res. 2003, 143, 47–55. [Google Scholar] [CrossRef]
- Watanabe, E.; Akagi, H. Distribution patterns of mRNAs encoding glycine receptor channels in the developing rat spinal cord. Neurosci. Res. 1995, 23, 377–382. [Google Scholar] [CrossRef]
- Takahashi, T.; Momiyama, A.; Hirai, K.; Hishinuma, F.; Akagi, H. Functional correlation of fetal and adult forms of glycine receptors with developmental changes in inhibitory synaptic receptor channels. Neuron 1992, 9, 1155–1161. [Google Scholar] [CrossRef]
- Withers, M.D.; St John, P.A. Embryonic rat spinal cord neurons change expression of glycine receptor subtypes during development in vitro. J. Neurobiol. 1997, 32, 579–592. [Google Scholar] [CrossRef]
- Aguayo, L.G.; van Zundert, B.; Tapia, J.C.; Carrasco, M.A.; Alvarez, F.J. Changes on the properties of glycine receptors during neuronal development. Brain Res. Brain Res. Rev. 2004, 47, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, S.; Morud, J.; Pickering, C.; Adermark, L.; Ericson, M.; Soderpalm, B. Changes in glycine receptor subunit expression in forebrain regions of the Wistar rat over development. Brain Res. 2012, 1446, 12–21. [Google Scholar] [CrossRef]
- Aroeira, R.I.; Ribeiro, J.A.; Sebastiao, A.M.; Valente, C.A. Age-related changes of glycine receptor at the rat hippocampus: From the embryo to the adult. J. Neurochem. 2011, 118, 339–353. [Google Scholar] [CrossRef]
- Borden, L.A. GABA transporter heterogeneity: Pharmacology and cellular localization. Neurochem. Int. 1996, 29, 335–356. [Google Scholar] [CrossRef]
- Jursky, F.; Nelson, N. Developmental expression of GABA transporters GAT1 and GAT4 suggests involvement in brain maturation. J. Neurochem. 1996, 67, 857–867. [Google Scholar] [CrossRef]
- Takayama, C.; Inoue, Y. GABAergic signaling in the developing cerebellum. Anat. Sci. Int. 2004, 79, 124–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben-Ari, Y. The GABA excitatory/inhibitory developmental sequence: A personal journey. Neuroscience 2014, 279, 187–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hubner, C.A.; Stein, V.; Hermans-Borgmeyer, I.; Meyer, T.; Ballanyi, K.; Jentsch, T.J. Disruption of KCC2 reveals an essential role of K-Cl cotransport already in early synaptic inhibition. Neuron 2001, 30, 515–524. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Chen, C.X.; Liu, Y.J.; Aizenman, E.; Kandler, K. KCC2 expression in immature rat cortical neurons is sufficient to switch the polarity of GABA responses. Eur. J. Neurosci. 2005, 21, 2593–2599. [Google Scholar] [CrossRef] [Green Version]
- Rivera, C.; Voipio, J.; Kaila, K. Two developmental switches in GABAergic signalling: The K+-Cl− cotransporter KCC2 and carbonic anhydrase CAVII. J. Physiol. 2005, 562, 27–36. [Google Scholar] [CrossRef]
- Mahadevan, V.; Woodin, M.A. Regulation of neuronal chloride homeostasis by neuromodulators. J. Physiol. 2016, 594, 2593–2605. [Google Scholar] [CrossRef] [PubMed]
- Delpy, A.; Allain, A.E.; Meyrand, P.; Branchereau, P. NKCC1 cotransporter inactivation underlies embryonic development of chloride-mediated inhibition in mouse spinal motoneuron. J. Physiol. 2008, 586, 1059–1075. [Google Scholar] [CrossRef]
- Tatetsu, M.; Kim, J.; Kina, S.; Sunakawa, H.; Takayama, C. GABA/glycine signaling during degeneration and regeneration of mouse hypoglossal nerves. Brain Res. 2012, 1446, 22–33. [Google Scholar] [CrossRef]
- Kim, J.; Kobayashi, S.; Shimizu-Okabe, C.; Okabe, A.; Moon, C.; Shin, T.; Takayama, C. Changes in the expression and localization of signaling molecules in mouse facial motor neurons during regeneration of facial nerves. J. Chem. Neuroanat. 2018, 88, 13–21. [Google Scholar] [CrossRef]
- Nabekura, J.; Ueno, T.; Okabe, A.; Furuta, A.; Iwaki, T.; Shimizu-Okabe, C.; Fukuda, A.; Akaike, N. Reduction of KCC2 expression and GABAA receptor-mediated excitation after in vivo axonal injury. J. Neurosci. 2002, 22, 4412–4417. [Google Scholar] [CrossRef] [Green Version]
- Toyoda, H.; Ohno, K.; Yamada, J.; Ikeda, M.; Okabe, A.; Sato, K.; Hashimoto, K.; Fukuda, A. Induction of NMDA and GABAA receptor-mediated Ca2+ oscillations with KCC2 mRNA downregulation in injured facial motoneurons. J. Neurophysiol. 2003, 89, 1353–1362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stein, V.; Hermans-Borgmeyer, I.; Jentsch, T.J.; Hubner, C.A. Expression of the KCl cotransporter KCC2 parallels neuronal maturation and the emergence of low intracellular chloride. J. Comp. Neurol. 2004, 468, 57–64. [Google Scholar] [CrossRef]
- Stil, A.; Liabeuf, S.; Jean-Xavier, C.; Brocard, C.; Viemari, J.C.; Vinay, L. Developmental up-regulation of the potassium-chloride cotransporter type 2 in the rat lumbar spinal cord. Neuroscience 2009, 164, 809–821. [Google Scholar] [CrossRef] [PubMed]
- Vinay, L.; Jean-Xavier, C. Plasticity of spinal cord locomotor networks and contribution of cation-chloride cotransporters. Brain Res. Rev. 2008, 57, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Nishimaru, H.; Kakizaki, M. The role of inhibitory neurotransmission in locomotor circuits of the developing mammalian spinal cord. Acta Physiol. 2009, 197, 83–97. [Google Scholar] [CrossRef] [PubMed]
- Vinay, L.; Brocard, F.; Pflieger, J.F.; Simeoni-Alias, J.; Clarac, F. Perinatal development of lumbar motoneurons and their inputs in the rat. Brain Res. Bull. 2000, 53, 635–647. [Google Scholar] [CrossRef]
- Kahle, K.T.; Deeb, T.Z.; Puskarjov, M.; Silayeva, L.; Liang, B.; Kaila, K.; Moss, S.J. Modulation of neuronal activity by phosphorylation of the K-Cl cotransporter KCC2. Trends Neurosci. 2013, 36, 726–737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kahle, K.T.; Delpire, E. Kinase-KCC2 coupling: Cl− rheostasis, disease susceptibility, therapeutic target. J. Neurophysiol. 2016, 115, 8–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukuda, A.; Watanabe, M. Pathogenic potential of human SLC12A5 variants causing KCC2 dysfunction. Brain Res. 2019, 1710, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Come, E.; Heubl, M.; Schwartz, E.J.; Poncer, J.C.; Levi, S. Reciprocal Regulation of KCC2 Trafficking and Synaptic Activity. Front. Cell. Neurosci. 2019, 13, 48. [Google Scholar] [CrossRef]
- Tang, B.L. K(+)-Cl(−) co-transporter 2 (KCC2)—A membrane trafficking perspective. Mol. Membr. Biol. 2016, 33, 100–110. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.H.; Jurd, R.; Moss, S.J. Tyrosine phosphorylation regulates the membrane trafficking of the potassium chloride co-transporter KCC2. Mol. Cell. Neurosci. 2010, 45, 173–179. [Google Scholar] [CrossRef] [Green Version]
- Friedel, P.; Kahle, K.T.; Zhang, J.; Hertz, N.; Pisella, L.I.; Buhler, E.; Schaller, F.; Duan, J.; Khanna, A.R.; Bishop, P.N.; et al. WNK1-regulated inhibitory phosphorylation of the KCC2 cotransporter maintains the depolarizing action of GABA in immature neurons. Sci. Signal. 2015, 8, ra65. [Google Scholar] [CrossRef]
- Watanabe, M.; Zhang, J.; Mansuri, M.S.; Duan, J.; Karimy, J.K.; Delpire, E.; Alper, S.L.; Lifton, R.P.; Fukuda, A.; Kahle, K.T. Developmentally regulated KCC2 phosphorylation is essential for dynamic GABA-mediated inhibition and survival. Sci. Signal. 2019, 12, eaaw9315. [Google Scholar] [CrossRef]
- Connor, J.A.; Tseng, H.Y.; Hockberger, P.E. Depolarization- and transmitter-induced changes in intracellular Ca2+ of rat cerebellar granule cells in explant cultures. J. Neurosci. 1987, 7, 1384–1400. [Google Scholar] [CrossRef] [Green Version]
- Yuste, R.; Katz, L.C. Control of postsynaptic Ca2+ influx in developing neocortex by excitatory and inhibitory neurotransmitters. Neuron 1991, 6, 333–344. [Google Scholar] [CrossRef]
- Reichling, D.B.; Kyrozis, A.; Wang, J.; MacDermott, A.B. Mechanisms of GABA and glycine depolarization-induced calcium transients in rat dorsal horn neurons. J. Physiol. 1994, 476, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Leinekugel, X.; Tseeb, V.; Ben-Ari, Y.; Bregestovski, P. Synaptic GABAA activation induces Ca2+ rise in pyramidal cells and interneurons from rat neonatal hippocampal slices. J. Physiol. 1995, 487 Pt 2, 319–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obrietan, K.; van den Pol, A.N. Growth cone calcium elevation by GABA. J. Comp. Neurol. 1996, 372, 167–175. [Google Scholar] [CrossRef]
- Ben-Ari, Y.; Khazipov, R.; Leinekugel, X.; Caillard, O.; Gaiarsa, J.L. GABAA, NMDA and AMPA receptors: A developmentally regulated ‘menage a trois’. Trends Neurosci. 1997, 20, 523–529. [Google Scholar] [CrossRef]
- Serafini, R.; Ma, W.; Maric, D.; Maric, I.; Lahjouji, F.; Sieghart, W.; Barker, J.L. Initially expressed early rat embryonic GABA(A) receptor Cl- ion channels exhibit heterogeneous channel properties. Eur. J. Neurosci. 1998, 10, 1771–1783. [Google Scholar] [CrossRef] [PubMed]
- Eilers, J.; Plant, T.D.; Marandi, N.; Konnerth, A. GABA-mediated Ca2+ signalling in developing rat cerebellar Purkinje neurones. J. Physiol. 2001, 536, 429–437. [Google Scholar] [CrossRef]
- McCarthy, M.M.; Auger, A.P.; Perrot-Sinal, T.S. Getting excited about GABA and sex differences in the brain. Trends Neurosci. 2002, 25, 307–312. [Google Scholar] [CrossRef]
- Belhage, B.; Hansen, G.H.; Elster, L.; Schousboe, A. Effects of gamma-aminobutyric acid (GABA) on synaptogenesis and synaptic function. Perspect. Dev. Neurobiol. 1998, 5, 235–246. [Google Scholar]
- Kardos, J. Recent advances in GABA research. Neurochem. Int. 1999, 34, 353–358. [Google Scholar] [CrossRef]
- Haydar, T.F.; Wang, F.; Schwartz, M.L.; Rakic, P. Differential modulation of proliferation in the neocortical ventricular and subventricular zones. J. Neurosci. 2000, 20, 5764–5774. [Google Scholar] [CrossRef] [Green Version]
- Behar, T.N.; Schaffner, A.E.; Colton, C.A.; Somogyi, R.; Olah, Z.; Lehel, C.; Barker, J.L. GABA-induced chemokinesis and NGF-induced chemotaxis of embryonic spinal cord neurons. J. Neurosci. 1994, 14, 29–38. [Google Scholar] [CrossRef]
- Behar, T.N.; Li, Y.X.; Tran, H.T.; Ma, W.; Dunlap, V.; Scott, C.; Barker, J.L. GABA stimulates chemotaxis and chemokinesis of embryonic cortical neurons via calcium-dependent mechanisms. J. Neurosci. 1996, 16, 1808–1818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behar, T.N.; Schaffner, A.E.; Tran, H.T.; Barker, J.L. GABA-induced motility of spinal neuroblasts develops along a ventrodorsal gradient and can be mimicked by agonists of GABAA and GABAB receptors. J. Neurosci. Res. 1995, 42, 97–108. [Google Scholar] [CrossRef]
- Abraham, J.H.; Schousboe, A. Effects of taurine on cell morphology and expression of low-affinity GABA receptors in cultured cerebellar granule cells. Neurochem. Res. 1989, 14, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Elster, L.; Hansen, G.H.; Belhage, B.; Fritschy, J.M.; Mohler, H.; Schousboe, A. Differential distribution of GABAA receptor subunits in soma and processes of cerebellar granule cells: Effects of maturation and a GABA agonist. Int. J. Dev. Neurosci. 1995, 13, 417–428. [Google Scholar] [CrossRef]
- Gao, X.B.; van den Pol, A.N. GABA release from mouse axonal growth cones. J. Physiol. 2000, 523 Pt 3, 629–637. [Google Scholar] [CrossRef]
- Carlson, B.X.; Belhage, B.; Hansen, G.H.; Elster, L.; Olsen, R.W.; Schousboe, A. Expression of the GABA(A) receptor alpha6 subunit in cultured cerebellar granule cells is developmentally regulated by activation of GABA(A) receptors. J. Neurosci. Res. 1997, 50, 1053–1062. [Google Scholar] [CrossRef]
- Carlson, B.X.; Elster, L.; Schousboe, A. Pharmacological and functional implications of developmentally-regulated changes in GABA(A) receptor subunit expression in the cerebellum. Eur. J. Pharmacol. 1998, 352, 1–14. [Google Scholar] [CrossRef]
- Mellor, J.R.; Merlo, D.; Jones, A.; Wisden, W.; Randall, A.D. Mouse cerebellar granule cell differentiation: Electrical activity regulates the GABAA receptor alpha 6 subunit gene. J. Neurosci. 1998, 18, 2822–2833. [Google Scholar] [CrossRef] [Green Version]
- Moss, S.J.; Smart, T.G. Constructing inhibitory synapses. Nat. Rev. Neurosci. 2001, 2, 240–250. [Google Scholar] [CrossRef]
- Meier, E.; Jorgensen, O.S. Gamma-aminobutyric acid affects the developmental expression of neuron-associated proteins in cerebellar granule cell cultures. J. Neurochem. 1986, 46, 1256–1262. [Google Scholar] [CrossRef] [PubMed]
- Meier, E.; Jorgensen, O.S.; Schousboe, A. Effect of repeated treatment with a gamma-aminobutyric acid receptor agonist on postnatal neural development in rats. J. Neurochem. 1987, 49, 1462–1470. [Google Scholar] [CrossRef]
- Wolff, J.R.; Joo, F.; Dames, W. Plasticity in dendrites shown by continuous GABA administration in superior cervical ganglion of adult rat. Nature 1978, 274, 72–74. [Google Scholar] [CrossRef]
- Spoerri, P.E. Neurotrophic effects of GABA in cultures of embryonic chick brain and retina. Synapse 1988, 2, 11–22. [Google Scholar] [CrossRef]
- Barbin, G.; Pollard, H.; Gaiarsa, J.L.; Ben-Ari, Y. Involvement of GABAA receptors in the outgrowth of cultured hippocampal neurons. Neurosci. Lett. 1993, 152, 150–154. [Google Scholar] [CrossRef]
- Mitchell, C.K.; Redburn, D.A. GABA and GABA-A receptors are maximally expressed in association with cone synaptogenesis in neonatal rabbit retina. Brain Res. Dev. Brain Res. 1996, 95, 63–71. [Google Scholar] [CrossRef]
- Kim, H.Y.; Sapp, D.W.; Olsen, R.W.; Tobin, A.J. GABA alters GABAA receptor mRNAs and increases ligand binding. J. Neurochem. 1993, 61, 2334–2337. [Google Scholar] [CrossRef]
- Saito, K.; Kakizaki, T.; Hayashi, R.; Nishimaru, H.; Furukawa, T.; Nakazato, Y.; Takamori, S.; Ebihara, S.; Uematsu, M.; Mishina, M.; et al. The physiological roles of vesicular GABA transporter during embryonic development: A study using knockout mice. Mol. Brain 2010, 3, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujii, M.; Arata, A.; Kanbara-Kume, N.; Saito, K.; Yanagawa, Y.; Obata, K. Respiratory activity in brainstem of fetal mice lacking glutamate decarboxylase 65/67 and vesicular GABA transporter. Neuroscience 2007, 146, 1044–1052. [Google Scholar] [CrossRef]
- Yamada, M.H.; Nishikawa, K.; Kubo, K.; Yanagawa, Y.; Saito, S. Impaired glycinergic synaptic transmission and enhanced inflammatory pain in mice with reduced expression of vesicular GABA transporter (VGAT). Mol. Pharmacol. 2012, 81, 610–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kakizaki, T.; Oriuchi, N.; Yanagawa, Y. GAD65/GAD67 double knockout mice exhibit intermediate severity in both cleft palate and omphalocele compared with GAD67 knockout and VGAT knockout mice. Neuroscience 2015, 288, 86–93. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shimizu-Okabe, C.; Kobayashi, S.; Kim, J.; Kosaka, Y.; Sunagawa, M.; Okabe, A.; Takayama, C. Developmental Formation of the GABAergic and Glycinergic Networks in the Mouse Spinal Cord. Int. J. Mol. Sci. 2022, 23, 834. https://doi.org/10.3390/ijms23020834
Shimizu-Okabe C, Kobayashi S, Kim J, Kosaka Y, Sunagawa M, Okabe A, Takayama C. Developmental Formation of the GABAergic and Glycinergic Networks in the Mouse Spinal Cord. International Journal of Molecular Sciences. 2022; 23(2):834. https://doi.org/10.3390/ijms23020834
Chicago/Turabian StyleShimizu-Okabe, Chigusa, Shiori Kobayashi, Jeongtae Kim, Yoshinori Kosaka, Masanobu Sunagawa, Akihito Okabe, and Chitoshi Takayama. 2022. "Developmental Formation of the GABAergic and Glycinergic Networks in the Mouse Spinal Cord" International Journal of Molecular Sciences 23, no. 2: 834. https://doi.org/10.3390/ijms23020834
APA StyleShimizu-Okabe, C., Kobayashi, S., Kim, J., Kosaka, Y., Sunagawa, M., Okabe, A., & Takayama, C. (2022). Developmental Formation of the GABAergic and Glycinergic Networks in the Mouse Spinal Cord. International Journal of Molecular Sciences, 23(2), 834. https://doi.org/10.3390/ijms23020834





