Anti-Inflammatory Effects of Rhamnetin on Bradykinin-Induced Matrix Metalloproteinase-9 Expression and Cell Migration in Rat Brain Astrocytes
Abstract
:1. Introduction
2. Results
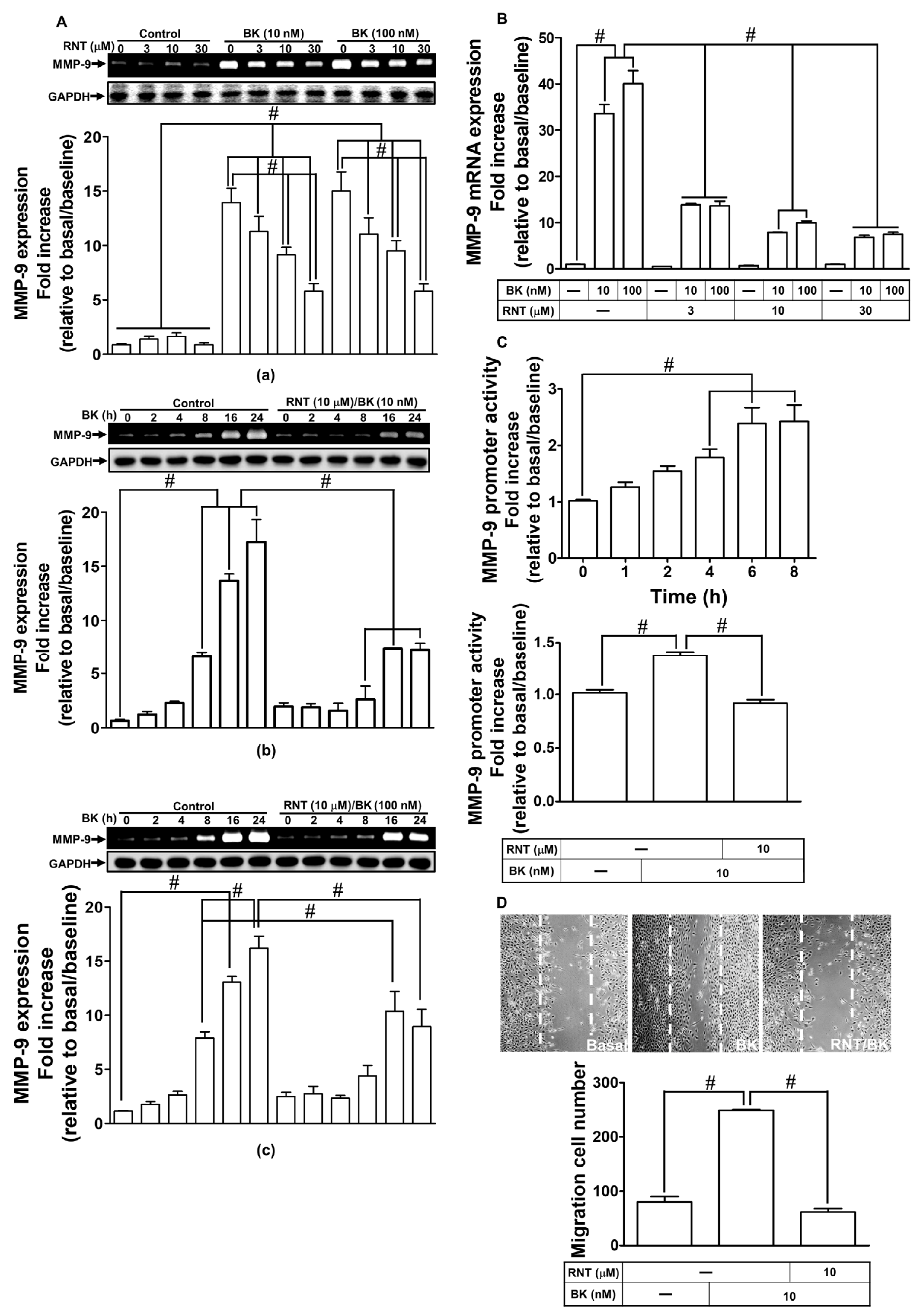
2.1. RNT Inhibited BK-Induced MMP-9 Expression and Cell Migration in RBA-1 Cells
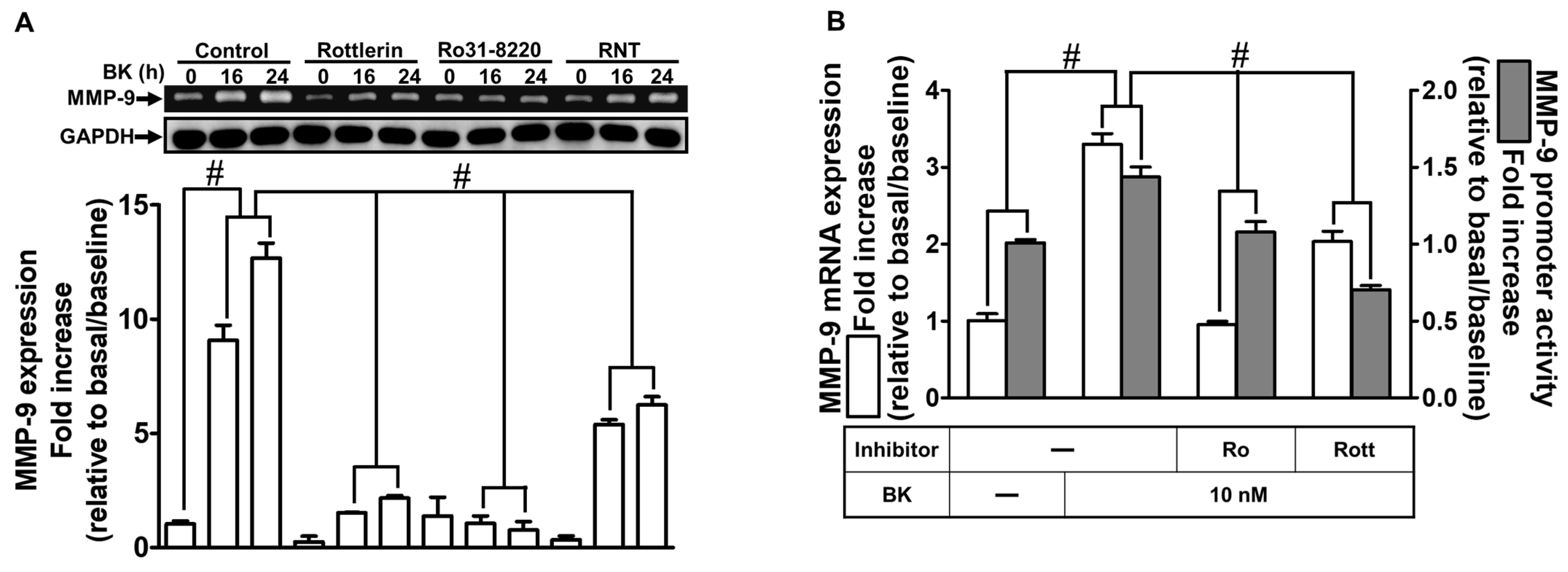
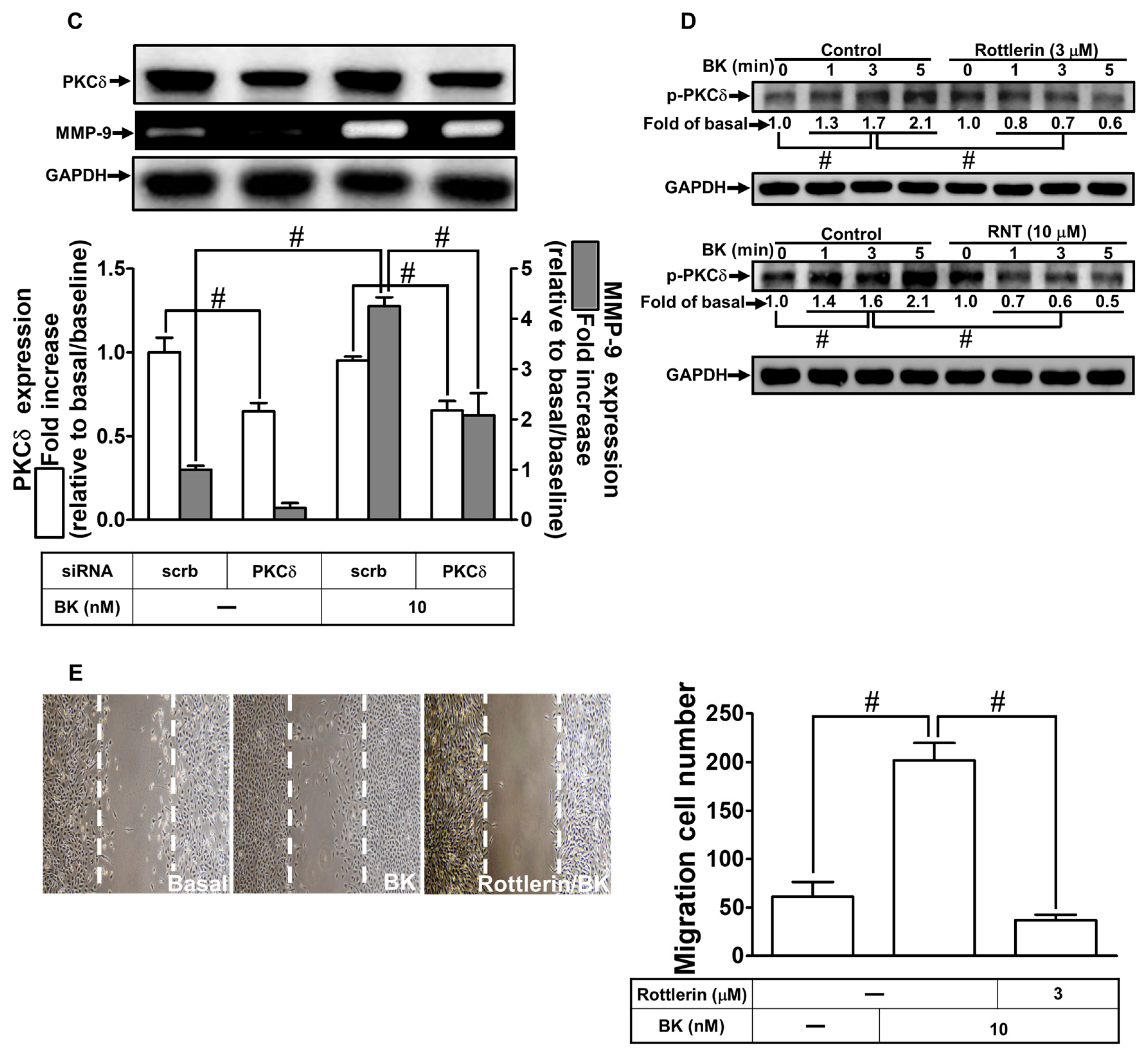
2.2. The Inhibitory Effects of RNT on BK-Induced MMP-9 Expression via Suppressing PKCδ
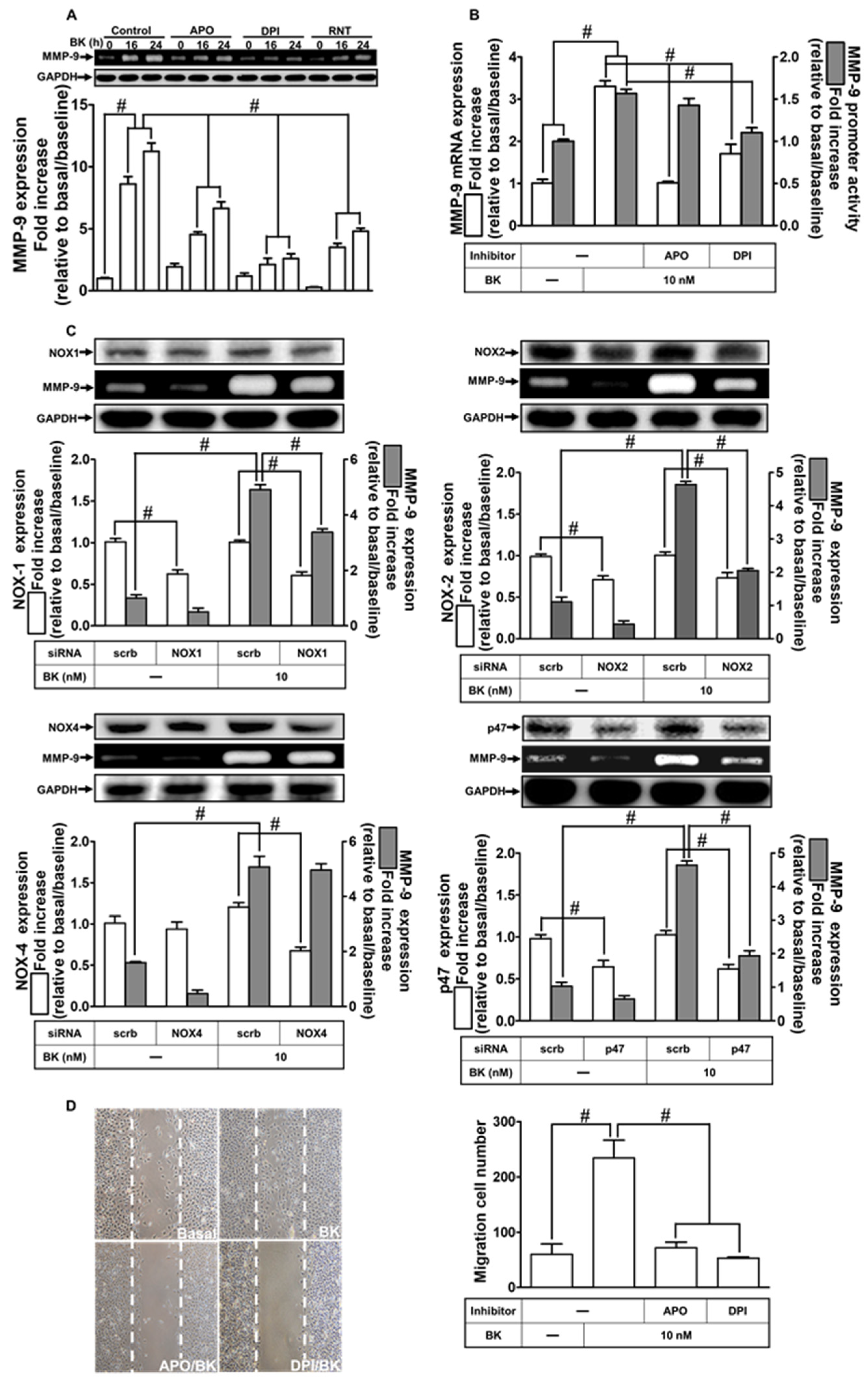
2.3. NOXs Participate in BK-Induced MMP-9 Expression and Cell Migration
2.4. RNT Attenuates BK-Induced MMP-9 Expression via Reducing ROS Generation
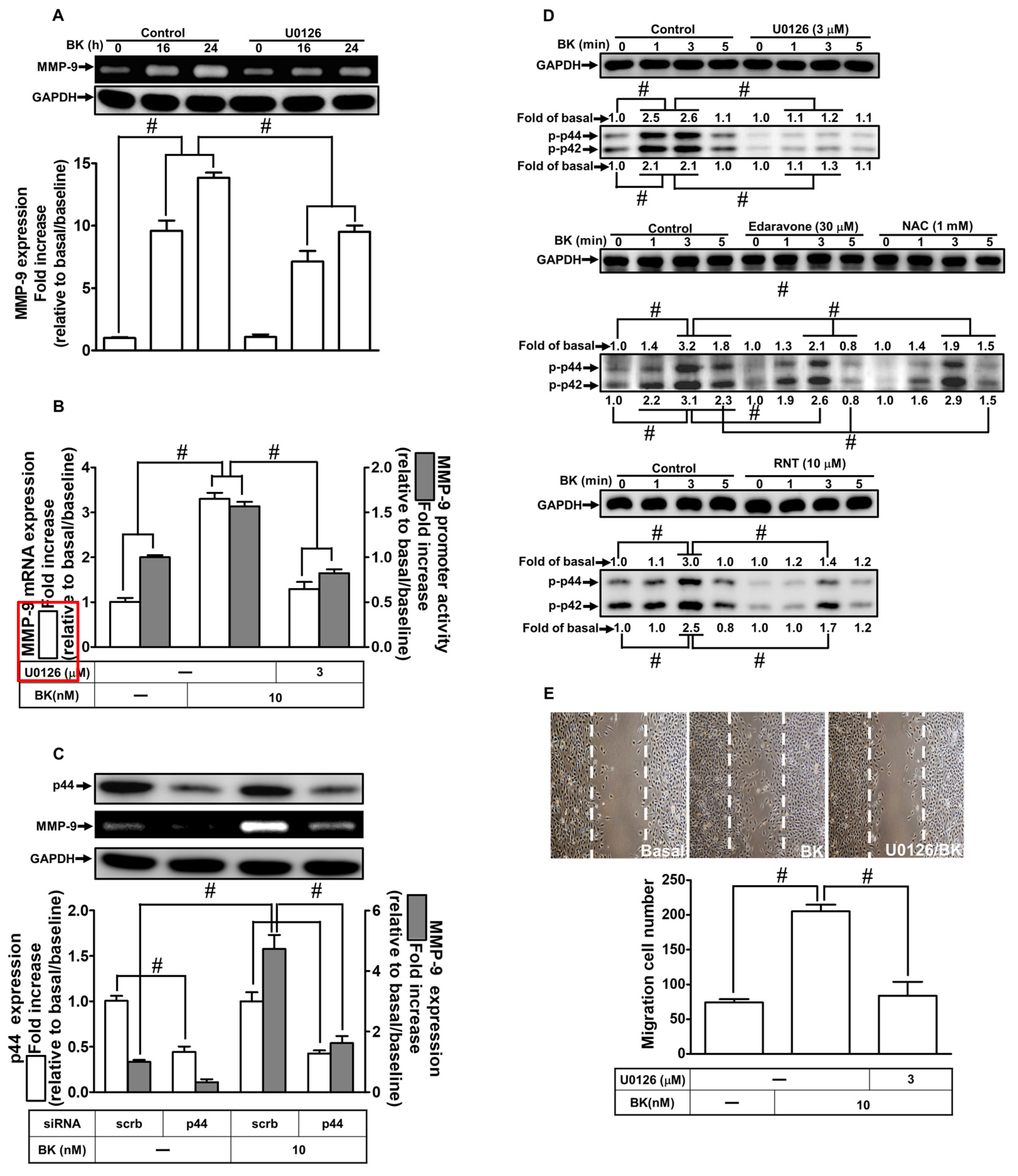
2.5. RNT Attenuates BK-Induced MMP-9 Expression Mediated via Suppressing ERK1/2
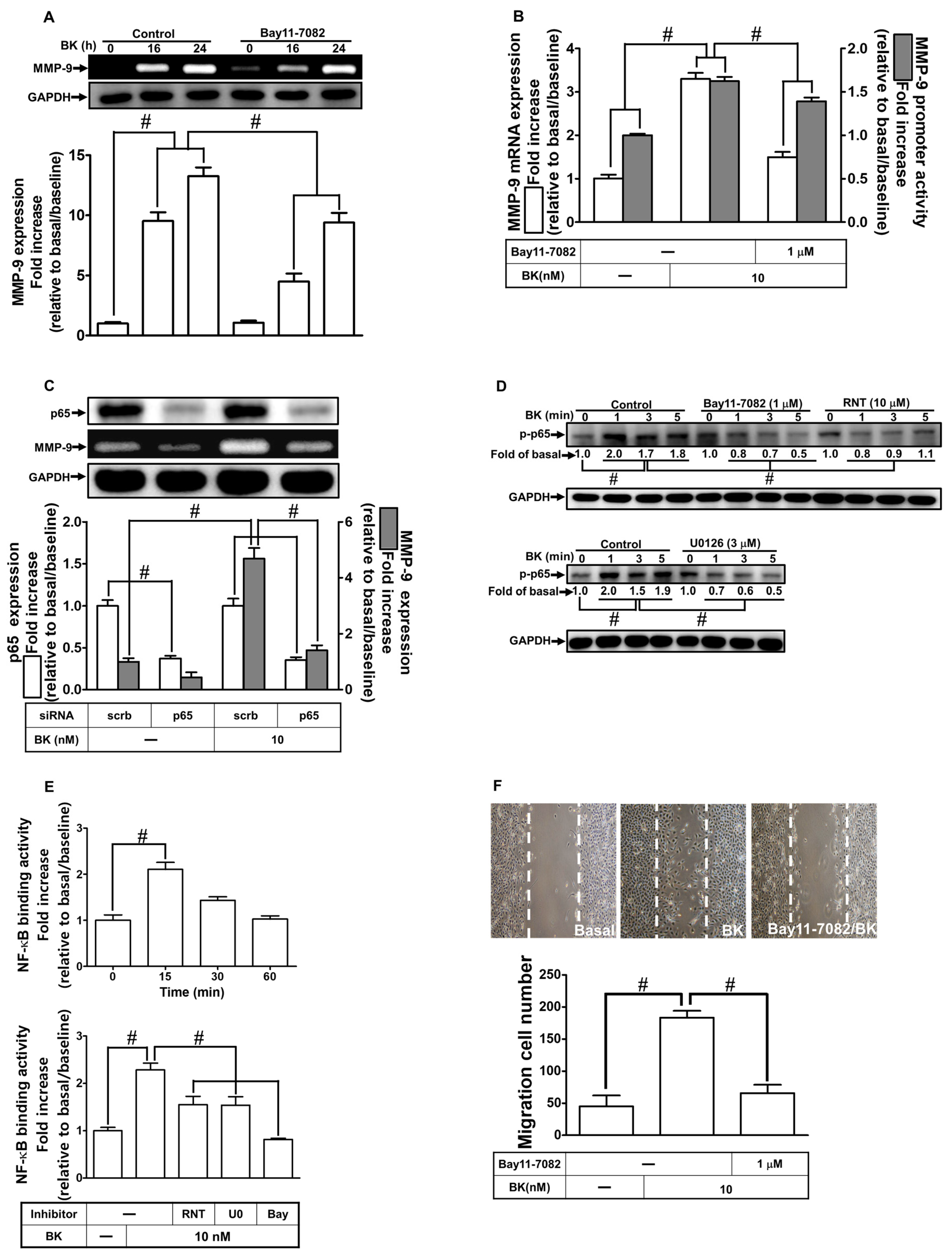
2.6. RNT Inhibits BK-Induced MMP-9 Expression via Attenuating NF-κB Activation
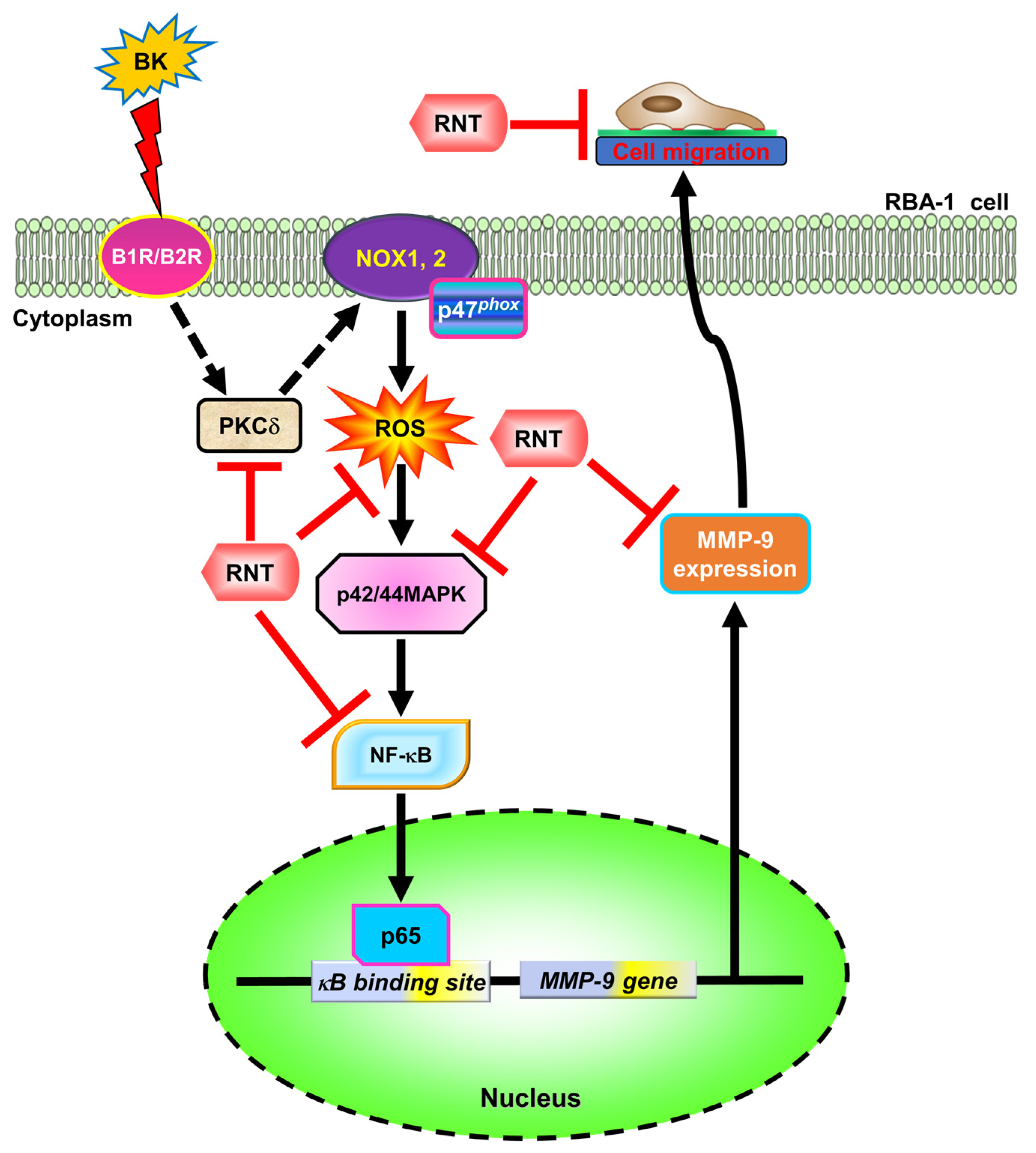
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture and Treatment
4.3. Protein Preparation and Western Blotting
4.4. MMP Gelatin Zymography
4.5. Total RNA Extraction, and Real-Time PCR Analysis
4.6. Transient siRNA Transfection
4.7. Rat MMP-9 Promoter Reporter Gene Assay
4.8. Chromatin Immunoprecipitation (ChIP) Assay
4.9. Intracellular ROS Measurement
4.10. Cell Migration Assay
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Homkajorn, B.; Sims, N.R.; Muyderman, H. Connexin 43 regulates astrocytic migration and proliferation in response to injury. Neurosci. Lett. 2010, 486, 197–201. [Google Scholar] [CrossRef]
- Lagos-Cabré, R.; Burgos-Bravo, F.; Avalos, A.M.; Leyton, L. Connexins in astrocyte migration. Front. Pharmacol. 2019, 10, 1546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kunz, M.; Nussberger, J.; Holtmannspötter, M.; Bitterling, H.; Plesnila, N.; Zausinger, S. Bradykinin in blood and cerebrospinal fluid after acute cerebral lesions: Correlations with cerebral edema and intracranial pressure. J. Neurotrauma 2013, 30, 1638–1644. [Google Scholar] [CrossRef]
- Kamiya, T.; Katayama, Y.; Kashiwagi, F.; Terashi, A. The role of bradykinin in mediating ischemic brain edema in rats. Stroke 1993, 24, 571–575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dobrivojević, M.; Špiranec, K.; Sinđić, A. Involvement of bradykinin in brain edema development after ischemic stroke. Pflügers Arch.-Eur. J. Physiol. 2015, 467, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Chao, H.; Liu, Y.; Lin, C.; Xu, X.; Li, Z.; Bao, Z.; Fan, L.; Tao, C.; Zhao, L.; Liu, Y.; et al. Activation of bradykinin B2 receptor induced the inflammatory responses of cytosolic phospholipase A2 after the early traumatic brain injury. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2018, 1864, 2957–2971. [Google Scholar] [CrossRef]
- Hsieh, H.-L.; Wu, C.-Y.; Yang, C.-M. Bradykinin induces matrix metalloproteinase-9 expression and cell migration through a PKC-δ-dependent ERK/Elk-1 pathway in astrocytes. Glia 2008, 56, 619–632. [Google Scholar] [CrossRef]
- Hsieh, H.-L.; Yen, M.-H.; Jou, M.-J.; Yang, C.-M. Intracellular signalings underlying bradykinin-induced matrix metalloproteinase-9 expression in rat brain astrocyte-1. Cell. Signal. 2004, 16, 1163–1176. [Google Scholar] [CrossRef]
- Lin, C.-C.; Hsieh, H.-L.; Shih, R.-H.; Chi, P.-L.; Cheng, S.-E.; Chen, J.-C.; Yang, C.-M. NADPH oxidase 2-derived reactive oxygen species signal contributes to bradykinin-induced matrix metalloproteinase-9 expression and cell migration in brain astrocytes. Cell Commun. Signal. 2012, 10, 35. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.-M.; Hsieh, H.-L.; Lin, C.-C.; Shih, R.-H.; Chi, P.-L.; Cheng, S.-E.; Hsiao, L.-D. Multiple factors from bradykinin-challenged astrocytes contribute to the neuronal apoptosis: Involvement of astroglial ROS, MMP-9, and HO-1/CO system. Mol. Neurobiol. 2013, 47, 1020–1033. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, H.-L.; Wang, H.-H.; Wu, C.-Y.; Yang, C.-M. Reactive oxygen species-dependent c-Fos/activator protein 1 induction upregulates heme oxygenase-1 expression by bradykinin in brain astrocytes. Antioxid. Redox Signal. 2010, 13, 1829–1844. [Google Scholar] [CrossRef]
- Ramos-Fernandez, M.; Bellolio, M.F.; Stead, L.G. Matrix metalloproteinase-9 as a marker for acute ischemic stroke: A systematic review. J. Stroke. Cereb. Dis. 2011, 20, 47–54. [Google Scholar] [CrossRef]
- Lorenzl, S.; Albers, D.S.; Relkin, N.; Ngyuen, T.; Hilgenberg, S.L.; Chirichigno, J.; Cudkowicz, M.E.; Beal, M.F. Increased plasma levels of matrix metalloproteinase-9 in patients with Alzheimer’s disease. Neurochem. Int. 2003, 43, 191–196. [Google Scholar] [CrossRef]
- Huang, H. Matrix metalloproteinase-9 (MMP-9) as a cancer biomarker and MMP-9 biosensors: Recent advances. Sensors 2018, 18, 3249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ende, C.; Gebhardt, R. Inhibition of matrix metalloproteinase-2 and -9 activities by selected flavonoids. Planta Med. 2004, 70, 1006–1008. [Google Scholar] [CrossRef] [PubMed]
- Bae, M.J.; Karadeniz, F.; Oh, J.H.; Yu, G.H.; Jang, M.-S.; Nam, K.-H.; Seo, Y.; Kong, C.-S. MMP-inhibitory effects of flavonoid glycosides from edible medicinal halophyte limonium tetragonum. Evid.-Based Complement. Altern. Med. 2017, 2017, 6750274. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.-C.; Tsai, P.-H.; Lin, C.-Y.; Cheng, C.-H.; Lin, T.-H.; Lee, K.P.H.; Huang, K.-Y.; Chen, S.-H.; Hwang, J.-J.; Kandaswami, C.C.; et al. Impact of flavonoids on matrix metalloproteinase secretion and invadopodia formation in highly invasive A431-III cancer cells. PLoS ONE 2013, 8, e71903. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-W.; Hou, W.-C.; Shen, S.-C.; Juan, S.-H.; Ko, C.-H.; Wang, L.-M.; Chen, Y.-C. Quercetin inhibition of tumor invasion via suppressing PKCδ/ERK/AP-1-dependent matrix metalloproteinase-9 activation in breast carcinoma cells. Carcinogenesis 2008, 29, 1807–1815. [Google Scholar] [CrossRef] [PubMed]
- Majtan, J.; Bohova, J.; Garcia-Villalba, R.; Tomas-Barberan, F.A.; Madakova, Z.; Majtan, T.; Majtan, V.; Klaudiny, J. Fir honeydew honey flavonoids inhibit TNF-α-induced MMP-9 expression in human keratinocytes: A new action of honey in wound healing. Arch. Dermatol. Res. 2013, 305, 619–627. [Google Scholar] [CrossRef]
- Lan, L.; Wang, Y.; Pan, Z.; Wang, B.; Yue, Z.; Jiang, Z.; Li, L.; Wang, C.; Tang, H. Rhamnetin induces apoptosis in human breast cancer cells via the miR-34a/Notch-1 signaling pathway. Oncol. Lett. 2019, 17, 676–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, E.-S.; Kang, J.C.; Jang, Y.C.; Park, J.S.; Jang, S.Y.; Kim, D.-E.; Kim, B.; Shin, H.-S. Cardioprotective effects of rhamnetin in H9c2 cardiomyoblast cells under H2O2-induced apoptosis. J. Ethnopharmacol. 2014, 153, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, B.; Guo, Y.; Bai, Y.; Wang, T.; Fu, K.; Sun, G. Rhamnetin attenuates cognitive deficit and inhibits hippocampal inflammatory response and oxidative stress in rats with traumatic brain injury. Cent. Eur. J. Immunol. 2015, 40, 35–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, W.; Liu, Q.; Jiang, N.; Li, M.; Shi, L. Isorhamnetin inhibited migration and invasion via suppression of Akt/ERK-mediated epithelial-to-mesenchymal transition (EMT) in A549 human non-small-cell lung cancer cells. Biosci. Rep. 2019, 39, BSR20190159. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.H.; Shin, B.Y.; Han, J.Y.; Kim, M.G.; Wi, J.E.; Kim, Y.W.; Cho, I.J.; Kim, S.C.; Shin, S.M.; Ki, S.H.J.T.; et al. Isorhamnetin protects against oxidative stress by activating Nrf2 and inducing the expression of its target genes. Toxicol. Appl. Pharmacol. 2014, 274, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.P.; Kim, J.-E.; Park, W.-H. Cytoprotective effect of rhamnetin on miconazole-induced H9c2 cell damage. Nutr. Res. Pract. 2015, 9, 586–591. [Google Scholar] [CrossRef] [Green Version]
- Pandey, A.K.; Bhattacharya, P.; Paul, S.; Patnaik, R. Rhamnetin attenuates oxidative stress and matrix metalloproteinase in animal model of ischemia/reperfusion: A possible antioxidant therapy in stroke. Am. J. Neuroprotection Neuroregeneration 2013, 5, 49–55. [Google Scholar] [CrossRef]
- Yong, V.W.; Krekoski, C.A.; Forsyth, P.A.; Bell, R.; Edwards, D.R. Matrix metalloproteinases and diseases of the CNS. Trends Neurosci. 1998, 21, 75–80. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, G.-M.; Zhu, Y.; Peng, X.-Y.; Liu, L.-M.; Li, T. Bradykinin induces vascular contraction after hemorrhagic shock in rats. J. Surg. Res. 2015, 193, 334–343. [Google Scholar] [CrossRef]
- Sternlicht, M.D.; Werb, Z. How matrix metalloproteinases regulate cell behavior. Annu. Rev. Cell Dev. Biol. 2001, 17, 463–516. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.-M.; Hsieh, H.-L.; Yu, P.-H.; Lin, C.-C.; Liu, S.-W. IL-1β induces MMP-9-dependent brain astrocytic migration via transactivation of PDGF receptor/NADPH oxidase 2-derived reactive oxygen species signals. Mol. Neurobiol. 2015, 52, 303–317. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.H.; Moon, Y.J.; Seo, J.-E.; Lee, Y.; Kim, K.H.; Chung, J.H. Reactive oxygen species produced by NADPH oxidase, xanthine oxidase, and mitochondrial electron transport system mediate heat shock-induced MMP-1 and MMP-9 expression. Free. Radic. Biol. Med. 2008, 44, 635–645. [Google Scholar] [CrossRef]
- Yang, C.-C.; Lin, C.-C.; Hsiao, L.-D.; Yang, C.-M. Galangin inhibits thrombin-induced MMP-9 expression in SK-N-SH cells via protein kinase-dependent NF-κB phosphorylation. Int. J. Mol. Sci. 2018, 19, 4084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Courtois, G.; Gilmore, T.D. Mutations in the NF-κB signaling pathway: Implications for human disease. Oncogene 2006, 25, 6831–6843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vafadari, B.; Salamian, A.; Kaczmarek, L. MMP-9 in translation: From molecule to brain physiology, pathology, and therapy. J. Neurochem. 2016, 139 (Suppl. 2), 91–114. [Google Scholar] [CrossRef] [Green Version]
- Rempe, R.G.; Hartz, A.M.S.; Bauer, B. Matrix metalloproteinases in the brain and blood–brain barrier: Versatile breakers and makers. J. Cereb. Blood Flow Metab. 2016, 36, 1481–1507. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.-W.; Lin, C.-C.; Lin, W.-N.; Liang, K.-C.; Luo, S.-F.; Wu, C.-B.; Wang, S.-W.; Yang, C.-M. TNF-α induces MMP-9 expression via activation of Src/EGFR, PDGFR/PI3K/Akt cascade and promotion of NF-κB/p300 binding in human tracheal smooth muscle cells. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2007, 292, L799–L812. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.-Y.; Hsieh, H.-L.; Sun, C.-C.; Tseng, C.-P.; Yang, C.-M. IL-1β induces proMMP-9 expression via c-Src-dependent PDGFR/PI3K/Akt/p300 cascade in rat brain astrocytes. J. Neurochem. 2008, 105, 1499–1512. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-C.; Lee, I.-T.; Wu, W.-B.; Liu, C.-J.; Hsieh, H.-L.; Hsiao, L.-D.; Yang, C.-C.; Yang, C.-M. Thrombin mediates migration of rat brain astrocytes via PLC, Ca2+, CaMKII, PKCα, and AP-1-dependent matrix metalloproteinase-9 expression. Mol. Neurobiol. 2013, 48, 616–630. [Google Scholar] [CrossRef]
- Yang, C.-C.; Lin, C.-C.; Hsiao, L.-D.; Kuo, J.-M.; Tseng, H.-C.; Yang, C.-M. Lipopolysaccharide-induced matrix metalloproteinase-9 expression associated with cell migration in rat brain astrocytes. Int. J. Mol. Sci. 2020, 21, 259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lutz, J.A.; Carter, M.; Fields, L.; Barron, S.; Littleton, J.M. The dietary flavonoid rhamnetin inhibits both inflammation and excitotoxicity during ethanol withdrawal in rat organotypic hippocampal slice cultures. Alcohol. Clin. Exp. Res. 2015, 39, 2345–2353. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Zhang, Y.; Liu, S.; Liu, Y.; Yang, X.; Liu, G.; Shimizu, T.; Ikenaka, K.; Fan, K.; Ma, J. Cathepsin C promotes microglia M1 polarization and aggravates neuroinflammation via activation of Ca2+-dependent PKC/p38MAPK/NF-κB pathway. J. Neuroinflammation 2019, 16, 10. [Google Scholar] [CrossRef]
- Ye, Y.; He, X.; Lu, F.; Mao, H.; Zhu, Z.; Yao, L.; Luo, W.; Sun, X.; Wang, B.; Qian, C.; et al. A lincRNA-p21/miR-181 family feedback loop regulates microglial activation during systemic LPS- and MPTP- induced neuroinflammation. Cell Death Dis. 2018, 9, 803. [Google Scholar] [CrossRef] [Green Version]
- Machida, T.; Dohgu, S.; Takata, F.; Matsumoto, J.; Kimura, I.; Koga, M.; Nakamoto, K.; Yamauchi, A.; Kataoka, Y. Role of thrombin-PAR1-PKCθ/δ axis in brain pericytes in thrombin-induced MMP-9 production and blood–brain barrier dysfunction in vitro. Neuroscience 2017, 350, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Akter, H.; Park, M.; Kwon, O.-S.; Song, E.J.; Park, W.-S.; Kang, M.-J. Activation of matrix metalloproteinase-9 (MMP-9) by neurotensin promotes cell invasion and migration through ERK pathway in gastric cancer. Tumor Biol. 2015, 36, 6053–6062. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Han, J.; Lee, S.K.; Choi, M.-Y.; Kim, J.; Lee, J.; Jung, S.P.; Kim, J.S.; Kim, J.-H.; Choe, J.-H.; et al. Berberine suppresses the TPA-induced MMP-1 and MMP-9 expressions through the inhibition of PKC-α in breast cancer cells. J. Surg. Res. 2012, 176, e21–e29. [Google Scholar] [CrossRef] [PubMed]
- Tammela, P.; Ekokoski, E.; García-Horsman, J.A.; Talman, V.; Finel, M.; Tuominen, R.; Vuorela, P. Screening of natural compounds and their derivatives as potential protein kinase C inhibitors. Drug Dev. Res. 2004, 63, 76–87. [Google Scholar] [CrossRef]
- Perhal, A.; Wolf, S.; Jamous, Y.F.; Langer, A.; Alla, J.A.; Quitterer, U. Increased reactive oxygen species generation contributes to the atherogenic activity of the B2 bradykinin receptor. Front. Med. 2019, 6, 32. [Google Scholar] [CrossRef]
- Zekry, D.; Epperson, T.K.; Krause, K.-H. A role for NOX NADPH oxidases in Alzheimer’s disease and other types of dementia? IUBMB Life 2003, 55, 307–313. [Google Scholar] [CrossRef]
- Velarde, V.; De La Cerda, P.M.; Duarte, C.; Arancibia, F.; Abbott, E.; González, A.; Moreno, F.; Jaffa, A.A. Role of reactive oxygen species in bradykinin-induced proliferation of vascular smooth muscle cells. Biol. Res. 2004, 37, 419–430. [Google Scholar] [CrossRef] [Green Version]
- Vacek, T.P.; Gillespie, W.; Tyagi, N.; Vacek, J.C.; Tyagi, S.C. Hydrogen sulfide protects against vascular remodeling from endothelial damage. Amino Acids 2010, 39, 1161–1169. [Google Scholar] [CrossRef]
- Diebold, I.; Petry, A.; Burger, M.; Hess, J.; Görlach, A. NOX4 mediates activation of FoxO3a and matrix metalloproteinase-2 expression by urotensin-II. Mol. Biol. Cell 2011, 22, 4424–4434. [Google Scholar] [CrossRef]
- Liu, F.; Gomez Garcia, A.M.; Meyskens, F.L., Jr. NADPH oxidase 1 overexpression enhances invasion via matrix metalloproteinase-2 and epithelial–mesenchymal transition in melanoma cells. J. Investig. Dermatol. 2012, 132, 2033–2041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velimirović, M.; Dožudić, G.J.; Selaković, V.; Stojković, T.; Puškaš, N.; Zaletel, I.; Živković, M.; Dragutinović, V.; Nikolić, T.; Jelenković, A.; et al. Effects of vitamin D3 on the NADPH oxidase and matrix metalloproteinase 9 in an animal model of global cerebral ischemia. Oxidative Med. Cell. Longev. 2018, 2018, 3273654. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, R.; Lopez-Sepulveda, R.; Romero, M.; Toral, M.; Cogolludo, A.; Perez-Vizcaino, F.; Duarte, J. Quercetin and its metabolites inhibit the membrane NADPH oxidase activity in vascular smooth muscle cells from normotensive and spontaneously hypertensive rats. Food Funct. 2015, 6, 409–414. [Google Scholar] [CrossRef]
- Walker, F.; Kato, A.; Gonez, L.J.; Hibbs, M.L.; Pouliot, N.; Levitzki, A.; Burgess, A.W. Activation of the Ras/mitogen-activated protein kinase pathway by kinase-defective epidermal growth factor receptors results in cell survival but not proliferation. Mol. Cell. Biol. 1998, 18, 7192–7204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhan, Y.; Kim, S.; Izumi, Y.; Izumiya, Y.; Nakao, T.; Miyazaki, H.; Iwao, H. Role of JNK, p38, and ERK in platelet-derived growth factor–induced vascular proliferation, migration, and gene expression. Arter. Thromb. Vasc. Biol. 2003, 23, 795–801. [Google Scholar] [CrossRef] [Green Version]
- Cellier, E.; Mage, M.; Duchêne, J.; Pécher, C.; Couture, R.; Bascands, J.-L.; Girolami, J.-P. Bradykinin reduces growth factor-induced glomerular ERK1/2 phosphorylation. Am. J. Physiol.-Ren. Physiol. 2003, 284, F282–F292. [Google Scholar] [CrossRef] [Green Version]
- Molina, L.; Matus, C.E.; Astroza, A.; Pavicic, F.; Tapia, E.; Toledo, C.; Perez, J.A.; Nualart, F.; Gonzalez, C.B.; Burgos, R.A.; et al. Stimulation of the bradykinin B 1 receptor induces the proliferation of estrogen-sensitive breast cancer cells and activates the ERK1/2 signaling pathway. Breast Cancer Res. Treat. 2009, 118, 499–510. [Google Scholar] [CrossRef]
- Sun, D.-P.; Lee, Y.-W.; Chen, J.-T.; Lin, Y.-W.; Chen, R.-M. The bradykinin-BDKRB1 axis regulates aquaporin 4 gene expression and consequential migration and invasion of malignant glioblastoma cells via a Ca2+-MEK1-ERK1/2-NF-κB mechanism. Cancers 2020, 12, 667. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.-C.; Lin, C.-C.; Jou, M.-J.; Hsiao, L.-D.; Yang, C.-M. RTA 408 inhibits interleukin-1β-induced MMP-9 expression via suppressing protein kinase-dependent NF-κB and AP-1 activation in rat brain astrocytes. Int. J. Mol. Sci. 2019, 20, 2826. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Yang, D.; Zhao, Y.; Qiu, Y.; Cao, X.; Yu, Y.; Guo, H.; Gu, X.; Yin, X. Inhibitory effects of isorhamnetin on the invasion of human breast carcinoma cells by downregulating the expression and activity of matrix metalloproteinase-2/9. Nutr. Cancer 2015, 67, 1191–1200. [Google Scholar] [CrossRef]
- Muscella, A.; Cossa, L.G.; Vetrugno, C.; Marsigliante, S. Bradykinin stimulates prostaglandin E2 release in human skeletal muscular fibroblasts. Mol. Cell. Endocrinol. 2020, 507, 110771. [Google Scholar] [CrossRef] [PubMed]
- Guarneri, C.; Bevelacqua, V.; Polesel, J.; Falzone, L.; Cannavò, P.S.; Spandidos, D.A.; Malaponte, G.; Libra, M. NF-κB inhibition is associated with OPN/MMP-9 downregulation in cutaneous melanoma. Oncol. Rep. 2017, 37, 737–746. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Jin, X.; Liao, Y.; Sun, Q.; Luo, C.; Wang, G.; Zhao, F.; Jin, Y. Association of NF-κB and AP-1 with MMP-9 overexpression in 2-chloroethanol exposed rat astrocytes. Cells 2018, 7, 96. [Google Scholar] [CrossRef] [Green Version]
- Shih, R.-H.; Wang, C.-Y.; Yang, C.-M. NF-κB signaling pathways in neurological inflammation: A mini review. Front. Mol. Neurosci. 2015, 8, 77. [Google Scholar] [CrossRef] [Green Version]
- Hämäläinen, M.; Nieminen, R.; Vuorela, P.; Heinonen, M.; Moilanen, E. Anti-inflammatory effects of flavonoids: Genistein, kaempferol, quercetin, and daidzein inhibit STAT-1 and NF-κB activations, whereas flavone, isorhamnetin, naringenin, and pelargonidin inhibit only NF-κB activation along with their inhibitory effect on iNOS expression and NO production in activated macrophages. Mediat. Inflamm. 2007, 2007, 45673. [Google Scholar] [CrossRef] [Green Version]
- Lee, T.-H.; Chen, J.-L.; Liu, P.-S.; Tsai, M.-M.; Wang, S.-J.; Hsieh, H.-L. Rottlerin, a natural polyphenol compound, inhibits upregulation of matrix metalloproteinase-9 and brain astrocytic migration by reducing PKC-δ-dependent ROS signal. J. Neuroinflammation 2020, 17, 177. [Google Scholar] [CrossRef]
- Jou, T.C.; Jou, M.J.; Chen, J.Y.; Lee, S.Y. Properties of rat brain astrocytes in long-term culture. Taiwan Yi Xue Hui Za Zhi. J. Formos. Med. Assoc. 1985, 84, 865–881. [Google Scholar]
- Yang, C.-C.; Hsiao, L.-D.; Tseng, H.-C.; Kuo, C.-M.; Yang, C.-M. Pristimerin inhibits MMP-9 expression and cell migration through attenuating NOX/ROS-dependent NF-κB activation in rat brain astrocytes challenged with LPS. J. Inflamm. Res. 2020, 13, 325–341. [Google Scholar] [CrossRef]
- Eberhardt, W.; Akool, E.-S.; Rebhan, J.; Frank, S.; Beck, K.-F.; Franzen, R.; Hamada, F.M.A.; Pfeilschifter, J. Inhibition of cytokine-induced matrix metalloproteinase 9 expression by peroxisome proliferator-activated receptor α agonists is indirect and due to a NO-mediated reduction of mRNA stability. J. Biol. Chem. 2002, 277, 33518–33528. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.-C.; Hsiao, L.-D.; Shih, Y.-F.; Yu, Z.-Y.; Yang, C.-M. Anti-Inflammatory Effects of Rhamnetin on Bradykinin-Induced Matrix Metalloproteinase-9 Expression and Cell Migration in Rat Brain Astrocytes. Int. J. Mol. Sci. 2022, 23, 609. https://doi.org/10.3390/ijms23020609
Yang C-C, Hsiao L-D, Shih Y-F, Yu Z-Y, Yang C-M. Anti-Inflammatory Effects of Rhamnetin on Bradykinin-Induced Matrix Metalloproteinase-9 Expression and Cell Migration in Rat Brain Astrocytes. International Journal of Molecular Sciences. 2022; 23(2):609. https://doi.org/10.3390/ijms23020609
Chicago/Turabian StyleYang, Chien-Chung, Li-Der Hsiao, Ya-Fang Shih, Zih-Yao Yu, and Chuen-Mao Yang. 2022. "Anti-Inflammatory Effects of Rhamnetin on Bradykinin-Induced Matrix Metalloproteinase-9 Expression and Cell Migration in Rat Brain Astrocytes" International Journal of Molecular Sciences 23, no. 2: 609. https://doi.org/10.3390/ijms23020609
APA StyleYang, C.-C., Hsiao, L.-D., Shih, Y.-F., Yu, Z.-Y., & Yang, C.-M. (2022). Anti-Inflammatory Effects of Rhamnetin on Bradykinin-Induced Matrix Metalloproteinase-9 Expression and Cell Migration in Rat Brain Astrocytes. International Journal of Molecular Sciences, 23(2), 609. https://doi.org/10.3390/ijms23020609





