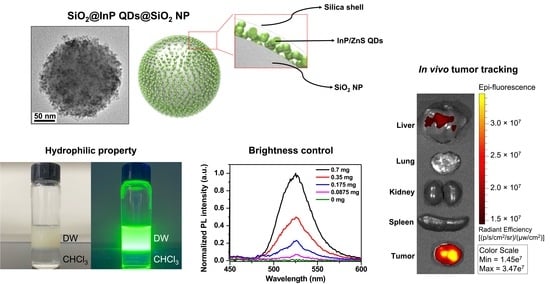
Highly Bright Silica-Coated InP/ZnS Quantum Dot-Embedded Silica Nanoparticles as Biocompatible Nanoprobes
Abstract
:1. Introduction
2. Results and Discussion
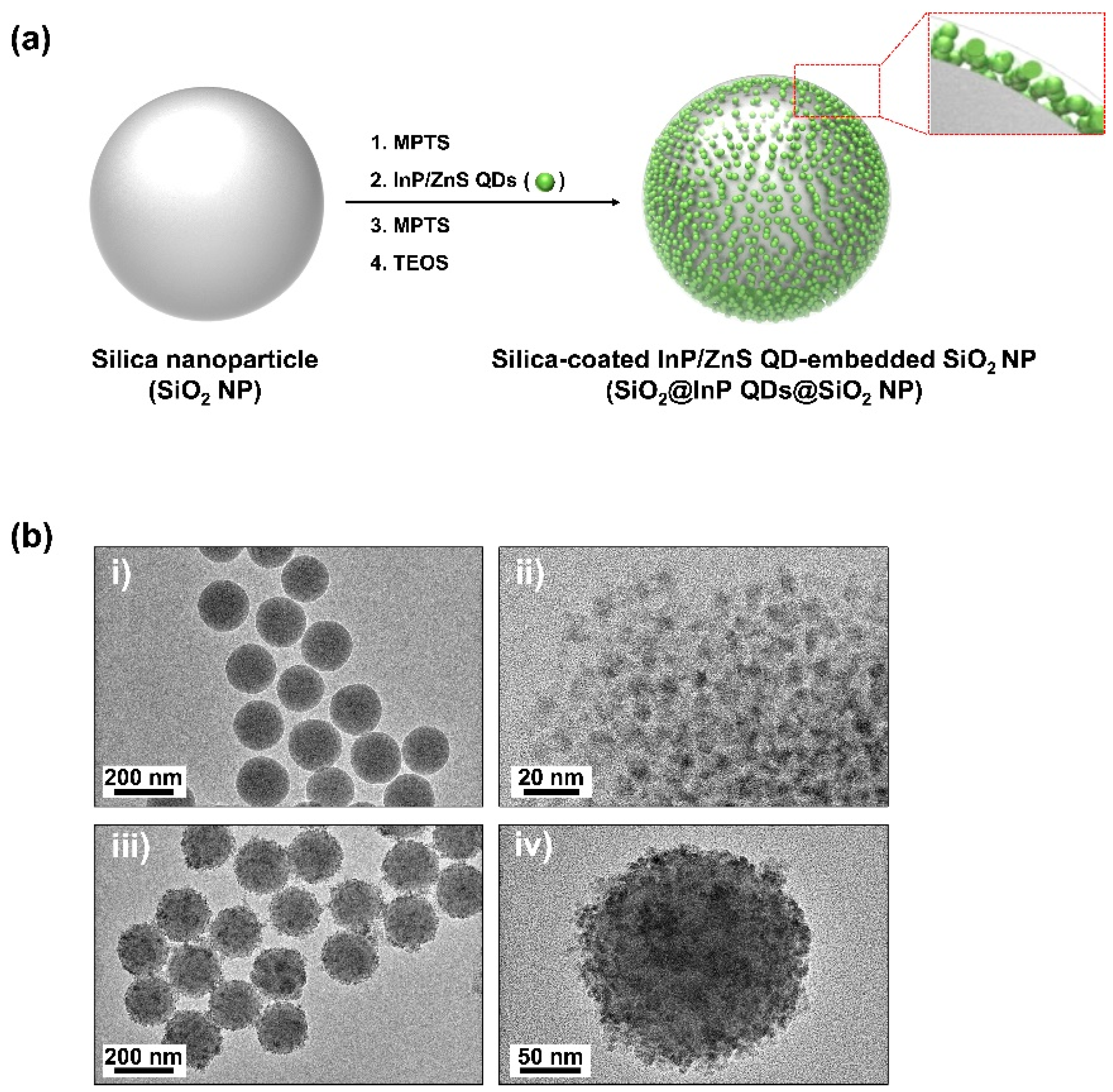
2.1. Fabrication of SiO2@InP QDs@SiO2 NPs
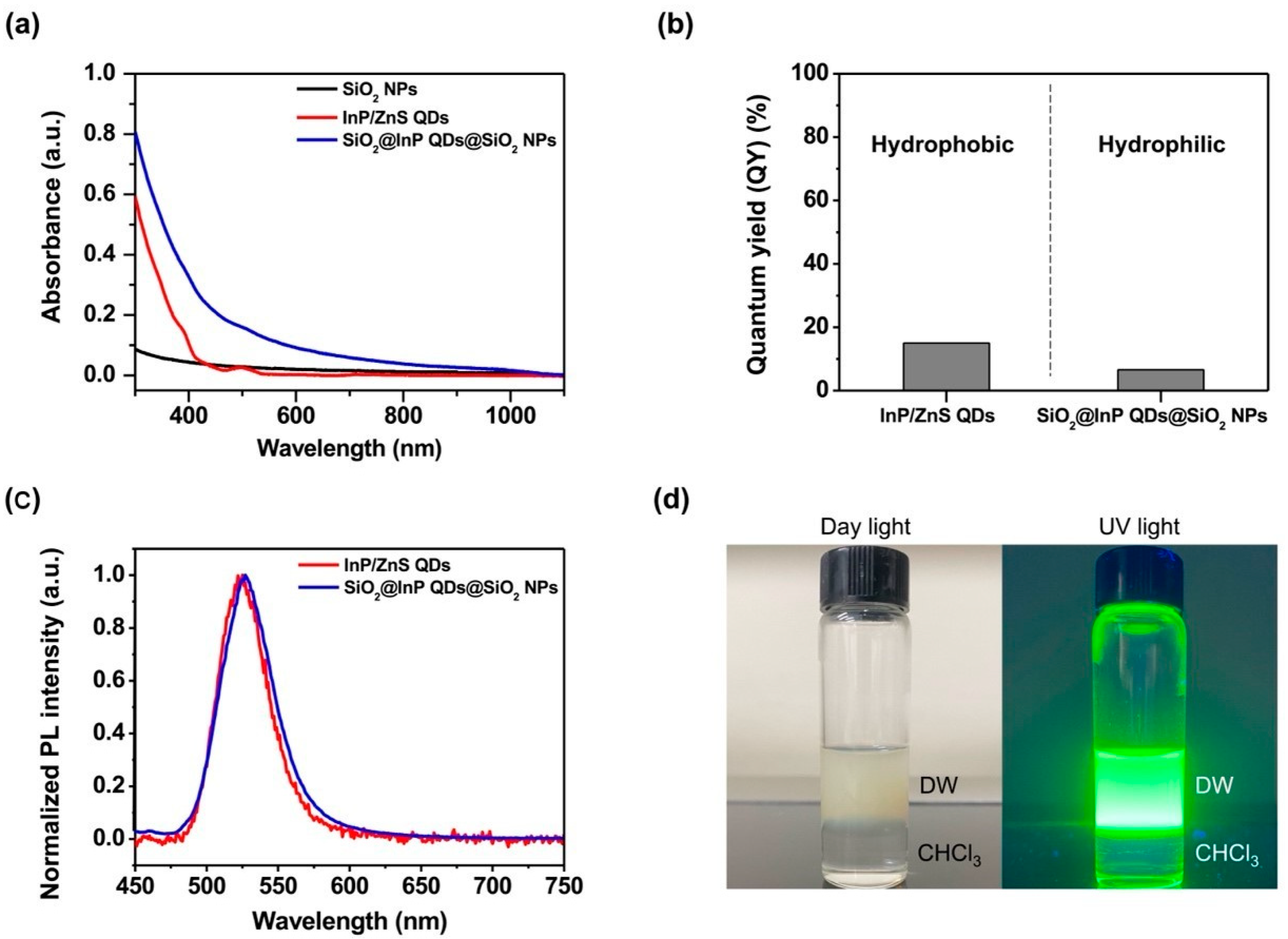
2.2. Characterization of SiO2@InP QDs@SiO2 NPs
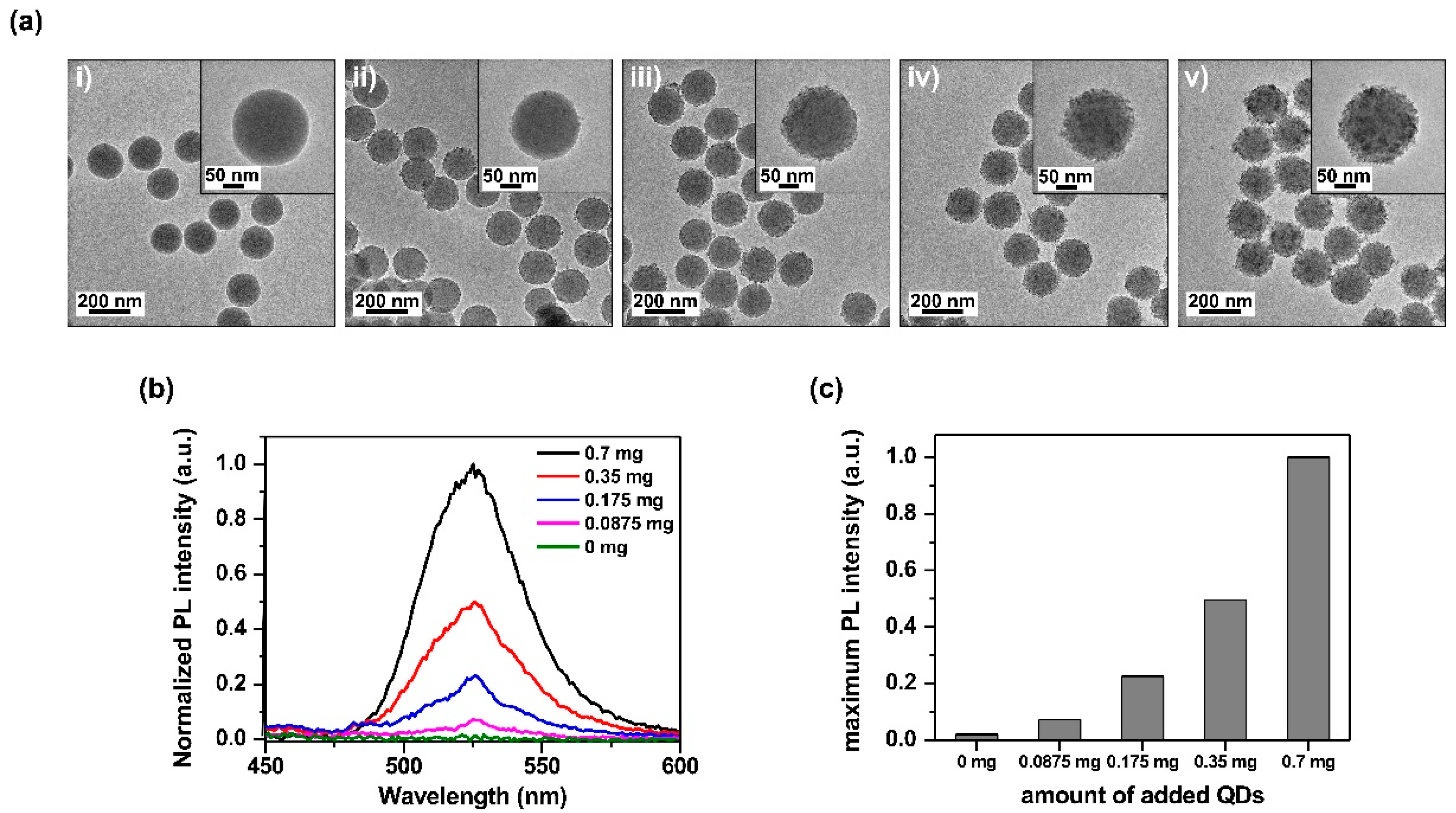
2.3. Brightness Control of SiO2@InP QDs@SiO2 NPs
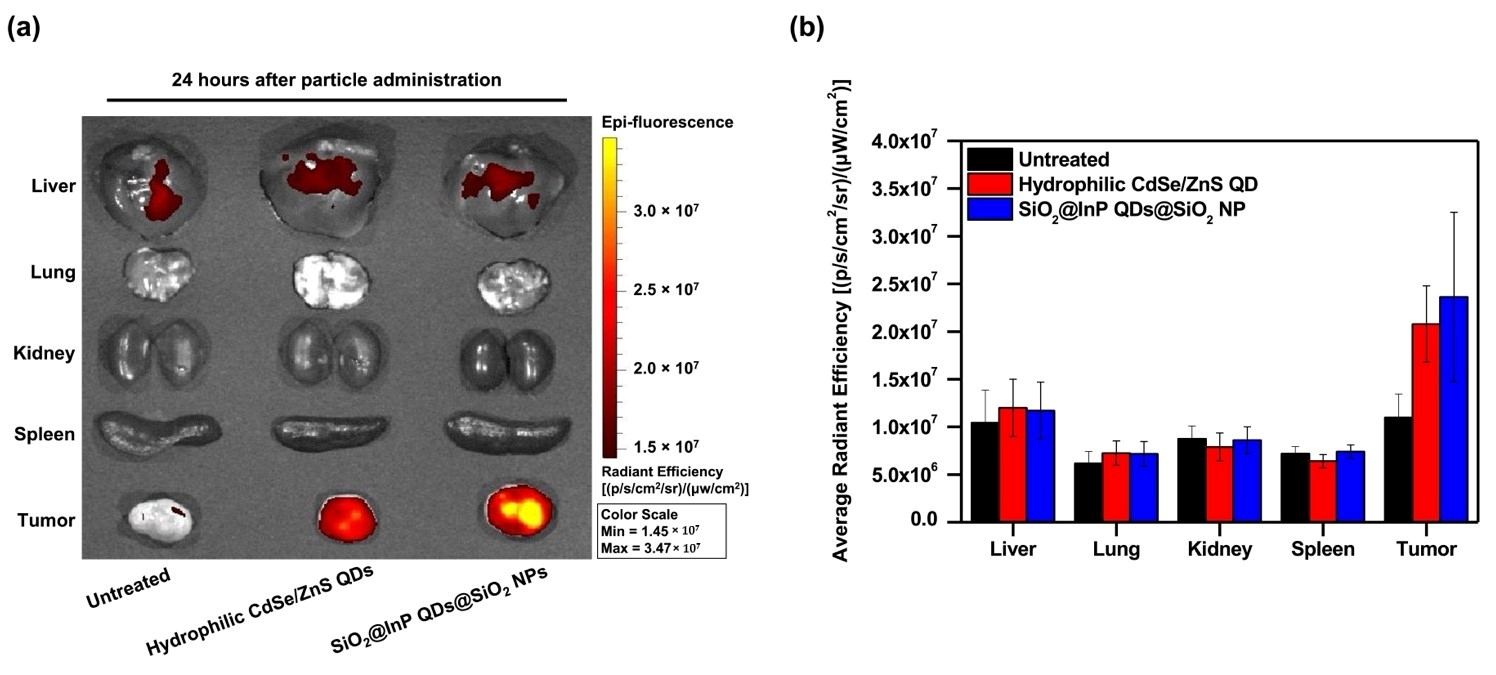
2.4. Cytotoxicity Investigation and In Vivo Biodistribution of SiO2@InP QDs@SiO2 NPs
3. Materials and Methods
3.1. Materials
3.2. Preparation of Thiol-Modified Silica Nanoparticles
3.3. Fabrication of Silica-Coated InP/ZnS QD-Embedded SiO2 NPs (SiO2@InP QDs@ SiO2 NPs)
3.4. Surface Modification of CdSe/ZnS QDs
3.5. Fabrication of SiO2@InP QDs@ SiO2 NPs with Different Number of Embedded QDs
3.6. Characterization of SiO2@InP QDs@ SiO2 NPs
3.7. Cytotoxicity Investigation of SiO2@InP QDs@ SiO2 NPs
3.8. In Vivo Biodistribution of SiO2@InP QDs@ SiO2 NPs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bruchez, M., Jr.; Moronne, M.; Gin, P.; Weiss, S.; Alivisatos, A.P. Semiconductor nanocrystals as fluorescent biological labels. Science 1998, 281, 2013–2016. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.C.; Nie, S. Quantum dot bioconjugates for ultrasensitive nonisotopic detection. Science 1998, 281, 2016–2018. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.C.; Maxwell, D.J.; Gao, X.; Bailey, R.E.; Han, M.; Nie, S. Luminescent quantum dots for multiplexed biological detection and imaging. Curr. Opin. Biotechnol. 2002, 13, 40–46. [Google Scholar] [CrossRef]
- Dabbousi, B.O.; Rodriguez-Viejo, J.; Mikulec, F.V.; Heine, J.R.; Mattoussi, H.; Ober, R.; Jensen, K.F.; Bawendi, M.G. (CdSe) ZnS core–shell quantum dots: Synthesis and characterization of a size series of highly luminescent nanocrystallites. J. Phys. Chem. B 1997, 101, 9463–9475. [Google Scholar] [CrossRef]
- Lim, Y.T.; Kim, S.; Nakayama, A.; Stott, N.E.; Bawendi, M.G.; Frangioni, J.V. Selection of quantum dot wavelengths for biomedical assays and imaging. Mol. Imaging 2003, 2, 15353500200302163. [Google Scholar] [CrossRef]
- Park, S.-M.; Aalipour, A.; Vermesh, O.; Yu, J.H.; Gambhir, S.S. Towards clinically translatable in vivo nanodiagnostics. Nat. Rev. Mater. 2017, 2, 17014. [Google Scholar] [CrossRef]
- Tarrahi, R.; Movafeghi, A.; Khataee, A.; Rezanejad, F.; Gohari, G. Evaluating the toxic impacts of cadmium selenide nanoparticles on the aquatic plant Lemna minor. Molecules 2019, 24, 410. [Google Scholar] [CrossRef]
- Soenen, S.J.; Rivera-Gil, P.; Montenegro, J.-M.; Parak, W.J.; De Smedt, S.C.; Braeckmans, K. Cellular toxicity of inorganic nanoparticles: Common aspects and guidelines for improved nanotoxicity evaluation. Nano Today 2011, 6, 446–465. [Google Scholar] [CrossRef]
- Tsoi, K.M.; Dai, Q.; Alman, B.A.; Chan, W.C. Are quantum dots toxic? Exploring the discrepancy between cell culture and animal studies. Acc. Chem. Res. 2013, 46, 662–671. [Google Scholar] [CrossRef]
- Brunetti, V.; Chibli, H.; Fiammengo, R.; Galeone, A.; Malvindi, M.A.; Vecchio, G.; Cingolani, R.; Nadeau, J.L.; Pompa, P.P. InP/ZnS as a safer alternative to CdSe/ZnS core/shell quantum dots: In vitro and in vivo toxicity assessment. Nanoscale 2013, 5, 307–317. [Google Scholar] [CrossRef]
- Wu, T.; Tang, M. Toxicity of quantum dots on respiratory system. Inhal. Toxicol. 2014, 26, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Grim, J.Q.; Manna, L.; Moreels, I. A sustainable future for photonic colloidal nanocrystals. Chem. Soc. Rev. 2015, 44, 5897–5914. [Google Scholar] [CrossRef] [PubMed]
- Yoffe, A.D. Semiconductor quantum dots and related systems: Electronic, optical, luminescence and related properties of low dimensional systems. Adv. Phys. 2001, 50, 1–208. [Google Scholar] [CrossRef]
- Chen, B.; Li, D.; Wang, F. InP quantum dots: Synthesis and lighting applications. Small 2020, 16, 2002454. [Google Scholar] [CrossRef]
- Adam, S.; Talapin, D.; Borchert, H.; Lobo, A.; McGinley, C.; De Castro, A.; Haase, M.; Weller, H.; Möller, T. The effect of nanocrystal surface structure on the luminescence properties: Photoemission study of HF-etched InP nanocrystals. J. Chem. Phys. 2005, 123, 084706. [Google Scholar] [CrossRef] [PubMed]
- Reiss, P.; Carriere, M.; Lincheneau, C.; Vaure, L.; Tamang, S. Synthesis of semiconductor nanocrystals, focusing on nontoxic and earth-abundant materials. Chem. Rev. 2016, 116, 10731–10819. [Google Scholar] [CrossRef]
- Haubold, S.; Haase, M.; Kornowski, A.; Weller, H. Strongly luminescent InP/ZnS core–shell nanoparticles. ChemPhysChem 2001, 2, 331–334. [Google Scholar] [CrossRef]
- Xie, R.; Battaglia, D.; Peng, X. Colloidal InP nanocrystals as efficient emitters covering blue to near-infrared. J. Am. Chem. Soc. 2007, 129, 15432–15433. [Google Scholar] [CrossRef]
- Xu, S.; Ziegler, J.; Nann, T. Rapid synthesis of highly luminescent InP and InP/ZnS nanocrystals. J. Mater. Chem. 2008, 18, 2653–2656. [Google Scholar] [CrossRef]
- Fan, G.; Wang, C.; Fang, J. Solution-based synthesis of III–V quantum dots and their applications in gas sensing and bio-imaging. Nano Today 2014, 9, 69–84. [Google Scholar] [CrossRef]
- Yong, K.-T.; Ding, H.; Roy, I.; Law, W.-C.; Bergey, E.J.; Maitra, A.; Prasad, P.N. Imaging pancreatic cancer using bioconjugated InP quantum dots. ACS Nano 2009, 3, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Hosokawa, C.; Suzuki, M.; Taguchi, T.; Murase, N. Preparation and biomedical applications of bright robust silica nanocapsules with multiple incorporated InP/ZnS quantum dots. New J. Chem. 2018, 42, 18951–18960. [Google Scholar] [CrossRef]
- Xu, Y.; Lv, Y.; Wu, R.; Li, J.; Shen, H.; Yang, H.; Zhang, H.; Li, L.S. Sensitive Immunoassay based on biocompatible and robust silica-coated Cd-free InP-based quantum dots. Inorg. Chem. 2021, 60, 6503–6513. [Google Scholar] [CrossRef]
- Watanabe, T.; Iso, Y.; Isobe, T.; Sasaki, H. Photoluminescence color stability of green-emitting InP/ZnS core/shell quantum dots embedded in silica prepared via hydrophobic routes. RSC Adv. 2018, 8, 25526–25533. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yang, X.-Q.; An, J.; Zhao, S.-D.; Zhao, T.-Y.; Tan, F.; Cao, Y.-C.; Zhao, Y.-D. In vivo tumor active cancer targeting and CT-fluorescence dual-modal imaging with nanoprobe based on gold nanorods and InP/ZnS quantum dots. J. Mater. Chem. B 2018, 6, 2574–2583. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Brown, S.; Walter, G.; Santra, S.; Moudgil, B. Nanoparticles for bioimaging. Adv. Colloid Interface Sci. 2006, 123, 471–485. [Google Scholar] [CrossRef]
- Brown, R.P.; Gallagher, M.J.; Fairbrother, D.H.; Rosenzweig, Z. Synthesis and degradation of cadmium-free InP and InPZn/ZnS quantum dots in solution. Langmuir 2018, 34, 13924–13934. [Google Scholar] [CrossRef]
- Kim, J.; Hwang, D.W.; Jung, H.S.; Kim, K.W.; Pham, X.-H.; Lee, S.-H.; Byun, J.W.; Kim, W.; Kim, H.-M.; Hahm, E. High-quantum yield alloy-typed core/shell CdSeZnS/ZnS quantum dots for bio-applications. J. Nanobiotechnol. 2022, 20, 22. [Google Scholar] [CrossRef]
- Pham, X.-H.; Park, S.-M.; Ham, K.-M.; Kyeong, S.; Son, B.S.; Kim, J.; Hahm, E.; Kim, Y.-H.; Bock, S.; Kim, W. Synthesis and application of silica-coated quantum dots in biomedicine. Int. J. Mol. Sci. 2021, 22, 10116. [Google Scholar] [CrossRef]
- Huang, L.; Liao, T.; Wang, J.; Ao, L.; Su, W.; Hu, J. Brilliant pitaya-type silica colloids with central–radial and high-density quantum dots incorporation for ultrasensitive fluorescence immunoassays. Adv. Funct. Mater. 2018, 28, 1705380. [Google Scholar] [CrossRef]
- Li, D.-Y.; He, X.-W.; Chen, Y.; Li, W.-Y.; Zhang, Y.-K. Novel hybrid structure silica/CdTe/molecularly imprinted polymer: Synthesis, specific recognition, and quantitative fluorescence detection of bovine hemoglobin. ACS Appl. Mater. Interfaces 2013, 5, 12609–12616. [Google Scholar] [CrossRef] [PubMed]
- Ha, Y.; Jung, H.S.; Jeong, S.; Kim, H.M.; Kim, T.H.; Cha, M.G.; Kang, E.J.; Pham, X.H.; Jeong, D.H.; Jun, B.H. Fabrication of Remarkably Bright QD Densely-Embedded Silica Nanoparticle. Bull. Korean Chem. Soc. 2019, 40, 9–13. [Google Scholar] [CrossRef]
- Jun, B.H.; Hwang, D.W.; Jung, H.S.; Jang, J.; Kim, H.; Kang, H.; Kang, T.; Kyeong, S.; Lee, H.; Jeong, D.H. Ultrasensitive, Biocompatible, Quantum-Dot-Embedded Silica Nanoparticles for Bioimaging. Adv. Funct. Mater. 2012, 22, 1843–1849. [Google Scholar] [CrossRef]
- Kim, H.-M.; Oh, C.; An, J.; Baek, S.; Bock, S.; Kim, J.; Jung, H.-S.; Song, H.; Kim, J.-W.; Jo, A. Multi-quantum dots-embedded silica-encapsulated nanoparticle-based lateral flow assay for highly sensitive exosome detection. Nanomaterials 2021, 11, 768. [Google Scholar] [CrossRef] [PubMed]
- Bock, S.; Kim, H.-M.; Kim, J.; An, J.; Choi, Y.-S.; Pham, X.-H.; Jo, A.; Ham, K.-M.; Song, H.; Kim, J.-W. Lateral Flow Immunoassay with Quantum-Dot-Embedded Silica Nanoparticles for Prostate-Specific Antigen Detection. Nanomaterials 2021, 12, 33. [Google Scholar] [CrossRef]
- Jo, A.; Kim, T.H.; Kim, D.-M.; Kim, H.-M.; Seong, B.; Kim, J.; Pham, X.-H.; Jung, H.S.; Lee, S.H.; Jeong, D.H. Sensitive detection of virus with broad dynamic range based on highly bright quantum dot-embedded nanoprobe and magnetic beads. J. Ind. Eng. Chem. 2020, 90, 319–326. [Google Scholar] [CrossRef]
- Chen, L.; Chen, C.; Li, R.; Li, Y.; Liu, S. CdTe quantum dot functionalized silica nanosphere labels for ultrasensitive detection of biomarker. Chem. Commun. 2009, 19, 2670–2672. [Google Scholar] [CrossRef]
- Huang, L.; Wu, Q.; Wang, J.; Foda, M.; Liu, J.; Cai, K.; Han, H. A brilliant sandwich type fluorescent nanostructure incorporating a compact quantum dot layer and versatile silica substrates. Chem. Commun. 2014, 50, 2896–2899. [Google Scholar] [CrossRef]
- Li, J.; Lv, Y.; Li, N.; Wu, R.; Xing, M.; Shen, H.; Li, L.S.; Chen, X. Robust synthesis of bright multiple quantum dot-embedded nanobeads and its application to quantitative immunoassay. Chem. Eng. J. 2019, 361, 499–507. [Google Scholar] [CrossRef]
- Hu, Y.; Fu, A.; Miao, Z.; Zhang, X.; Wang, T.; Kang, A.; Shan, J.; Zhu, D.; Li, W. Fluorescent ligand fishing combination with in-situ imaging and characterizing to screen Hsp 90 inhibitors from Curcuma longa L. based on InP/ZnS quantum dots embedded mesoporous nanoparticles. Talanta 2018, 178, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Iso, Y.; Isobe, T. Critical Review—Photostable Fluorescent Cd-Free Quantum Dots Transparently Embedded in Monolithic Silica. ECS J. Solid State Sci. Technol. 2019, 9, 016005. [Google Scholar] [CrossRef]
- Miao, Z.; Hu, Y.; Zhang, X.; Yang, X.; Tang, Y.; Kang, A.; Zhu, D. Screening and identification of ligand-protein interactions using functionalized heat shock protein 90-fluorescent mesoporous silica-indium phosphide/zinc sulfide quantum dot nanocomposites. J. Chromatogr. A 2018, 1562, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Perton, F.; Harlepp, S.; Follain, G.; Parkhomenko, K.; Goetz, J.G.; Bégin-Colin, S.; Mertz, D. Wrapped stellate silica nanocomposites as biocompatible luminescent nanoplatforms assessed in vivo. J. Colloid Interface Sci. 2019, 542, 469–482. [Google Scholar] [CrossRef]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Kim, S.; Bawendi, M.G. Oligomeric ligands for luminescent and stable nanocrystal quantum dots. J. Am. Chem. Soc. 2003, 125, 14652–14653. [Google Scholar] [CrossRef]
- Rogach, A.L.; Nagesha, D.; Ostrander, J.W.; Giersig, M.; Kotov, N.A. “Raisin bun”-type composite spheres of silica and semiconductor nanocrystals. Chem. Mater. 2000, 12, 2676–2685. [Google Scholar] [CrossRef]
- Shibu, E.S.; Hamada, M.; Nakanishi, S.; Wakida, S.-I.; Biju, V. Photoluminescence of CdSe and CdSe/ZnS quantum dots: Modifications for making the invisible visible at ensemble and single-molecule levels. Coord. Chem. Rev. 2014, 263, 2–12. [Google Scholar] [CrossRef]
- Green, M. Semiconductor quantum dots as biological imaging agents. Angew. Chem. Int. Ed. 2004, 43, 4129–4131. [Google Scholar] [CrossRef]
- Wagner, A.M.; Knipe, J.M.; Orive, G.; Peppas, N.A. Quantum dots in biomedical applications. Acta Biomater. 2019, 94, 44–63. [Google Scholar] [CrossRef]
- Pong, B.-K.; Trout, B.L.; Lee, J.-Y. Modified ligand-exchange for efficient solubilization of CdSe/ZnS quantum dots in water: A procedure guided by computational studies. Langmuir 2008, 24, 5270–5276. [Google Scholar] [CrossRef]
- Kalyane, D.; Raval, N.; Maheshwari, R.; Tambe, V.; Kalia, K.; Tekade, R.K. Employment of enhanced permeability and retention effect (EPR): Nanoparticle-based precision tools for targeting of therapeutic and diagnostic agent in cancer. Mater. Sci. Eng. C 2019, 98, 1252–1276. [Google Scholar] [CrossRef] [PubMed]
- Stylianopoulos, T. EPR-effect: Utilizing size-dependent nanoparticle delivery to solid tumors. Ther. Deliv. 2013, 4, 421–423. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Rho, S.; Stiles, W.R.; Hu, S.; Baek, Y.; Hwang, D.W.; Kashiwagi, S.; Kim, M.S.; Choi, H.S. Size-dependent EPR effect of polymeric nanoparticles on tumor targeting. Adv. Healthc. Mater. 2020, 9, 1901223. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ham, K.-M.; Kim, M.; Bock, S.; Kim, J.; Kim, W.; Jung, H.S.; An, J.; Song, H.; Kim, J.-W.; Kim, H.-M.; et al. Highly Bright Silica-Coated InP/ZnS Quantum Dot-Embedded Silica Nanoparticles as Biocompatible Nanoprobes. Int. J. Mol. Sci. 2022, 23, 10977. https://doi.org/10.3390/ijms231810977
Ham K-M, Kim M, Bock S, Kim J, Kim W, Jung HS, An J, Song H, Kim J-W, Kim H-M, et al. Highly Bright Silica-Coated InP/ZnS Quantum Dot-Embedded Silica Nanoparticles as Biocompatible Nanoprobes. International Journal of Molecular Sciences. 2022; 23(18):10977. https://doi.org/10.3390/ijms231810977
Chicago/Turabian StyleHam, Kyeong-Min, Minhee Kim, Sungje Bock, Jaehi Kim, Wooyeon Kim, Heung Su Jung, Jaehyun An, Hobeom Song, Jung-Won Kim, Hyung-Mo Kim, and et al. 2022. "Highly Bright Silica-Coated InP/ZnS Quantum Dot-Embedded Silica Nanoparticles as Biocompatible Nanoprobes" International Journal of Molecular Sciences 23, no. 18: 10977. https://doi.org/10.3390/ijms231810977
APA StyleHam, K.-M., Kim, M., Bock, S., Kim, J., Kim, W., Jung, H. S., An, J., Song, H., Kim, J.-W., Kim, H.-M., Rho, W.-Y., Lee, S. H., Park, S.-m., Kim, D.-E., & Jun, B.-H. (2022). Highly Bright Silica-Coated InP/ZnS Quantum Dot-Embedded Silica Nanoparticles as Biocompatible Nanoprobes. International Journal of Molecular Sciences, 23(18), 10977. https://doi.org/10.3390/ijms231810977