Fullerenol C60(OH)36 Protects the Antioxidant Enzymes in Human Erythrocytes against Oxidative Damage Induced by High-Energy Electrons
Abstract
1. Introduction
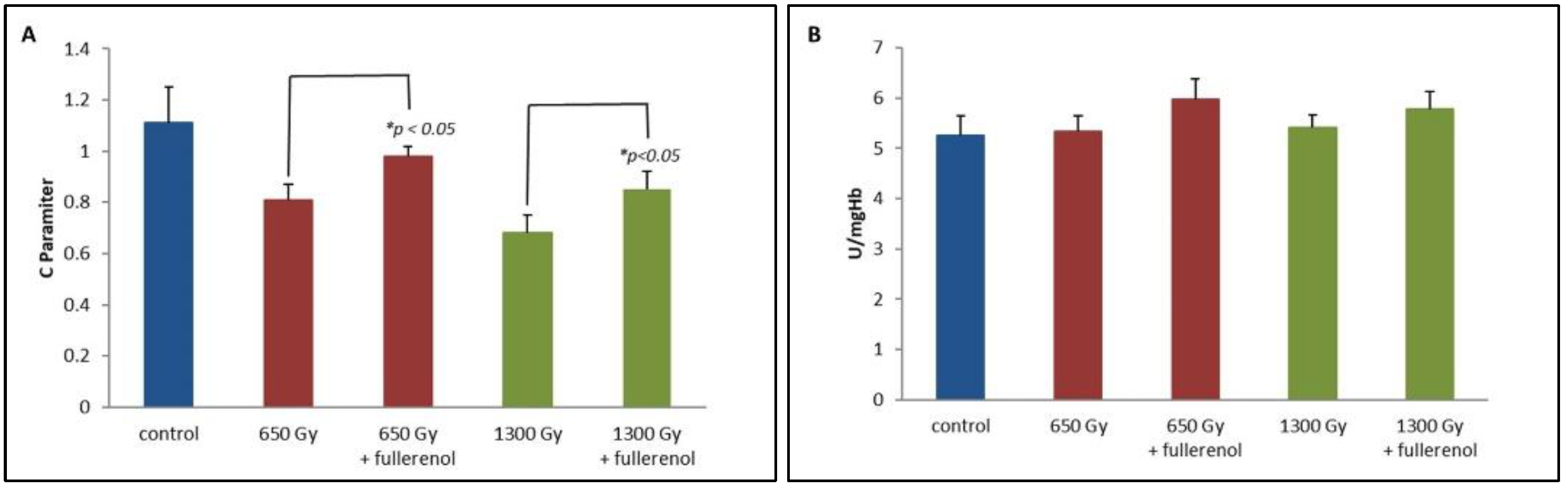
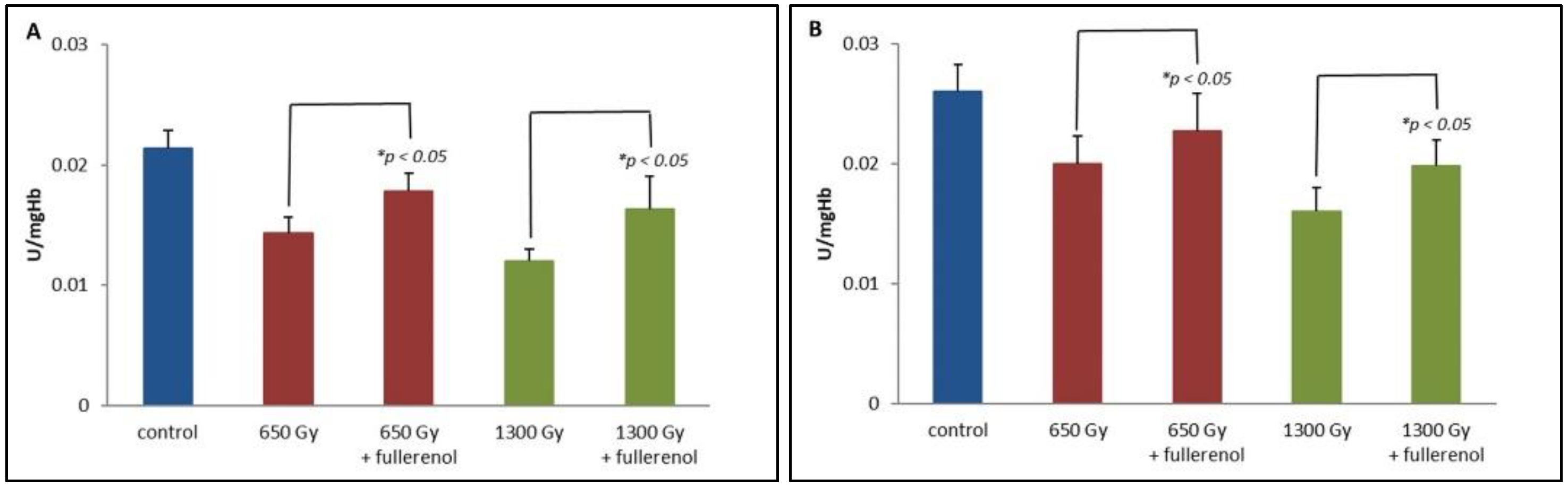
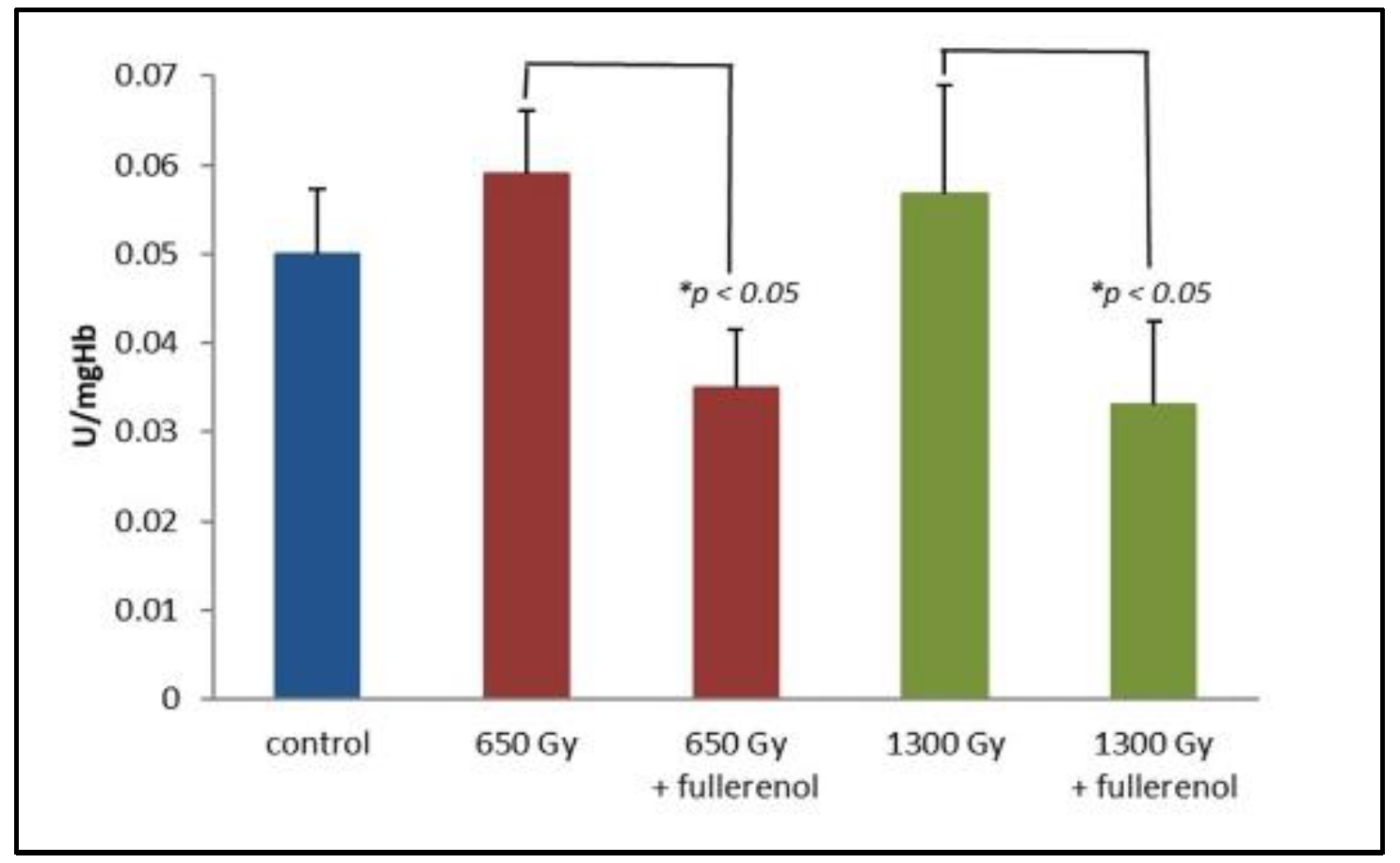
2. Results
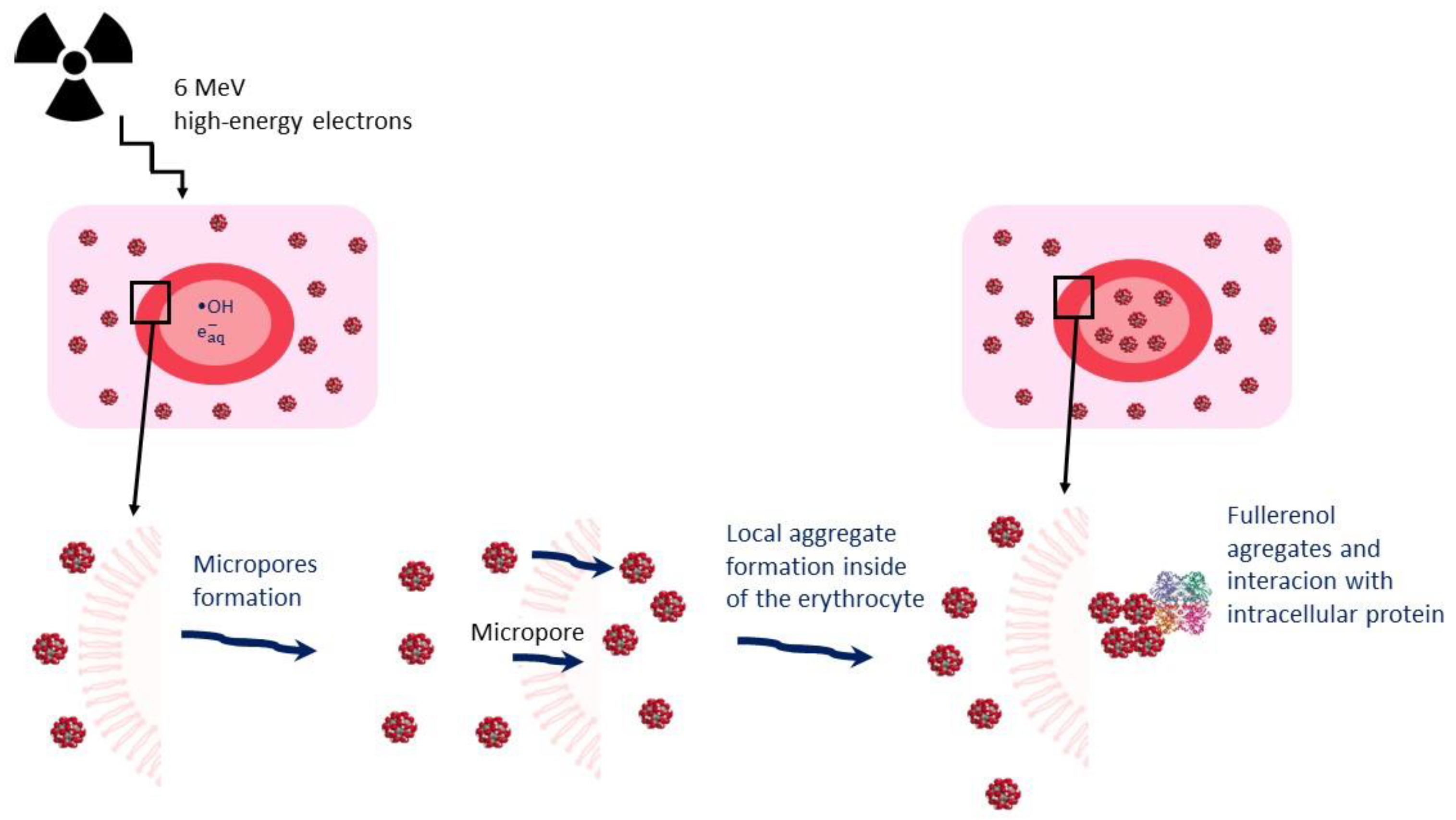
3. Discussion
4. Materials and Methods
4.1. Chemicals
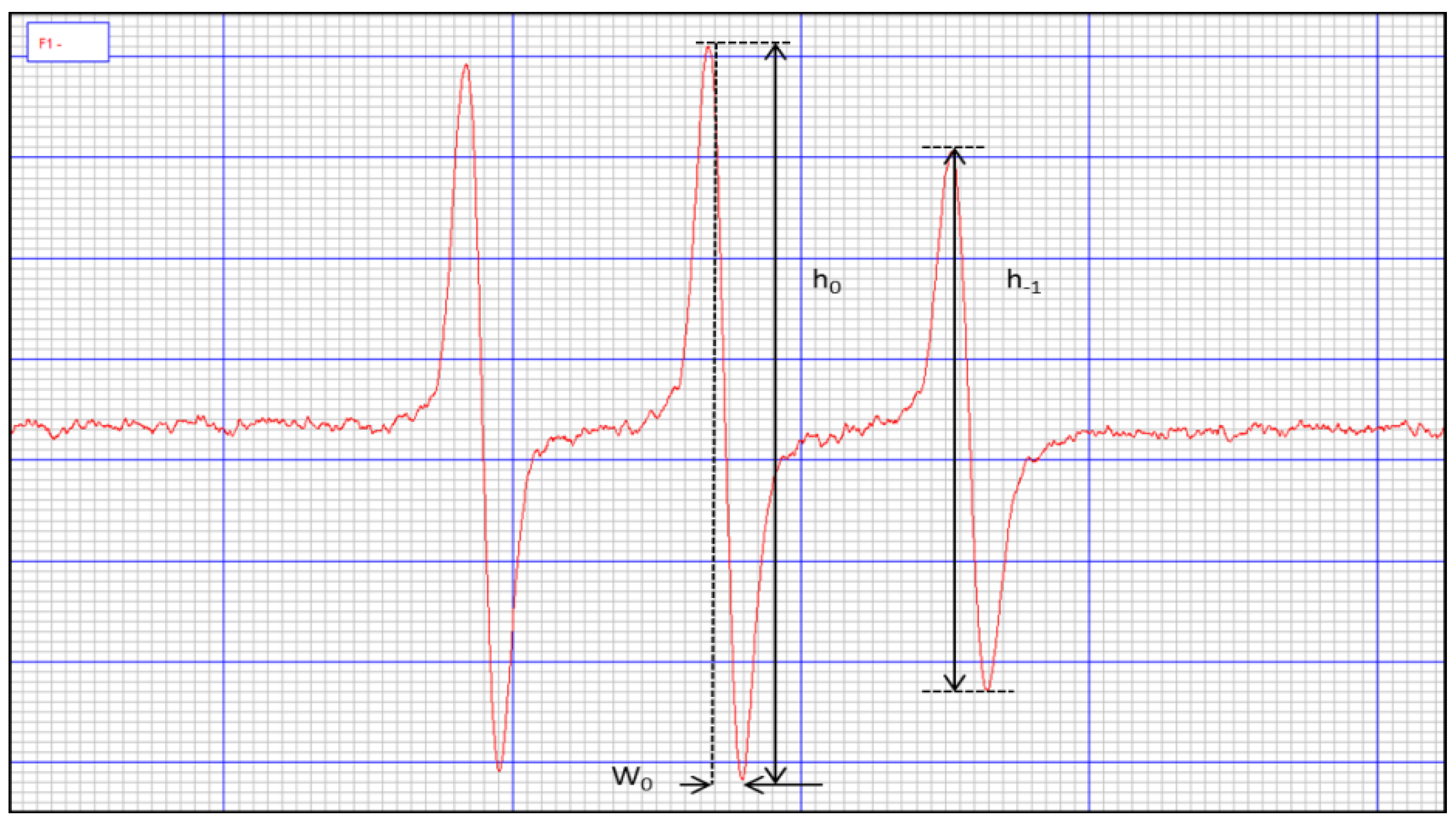
4.2. Irradiation Conditions
4.3. Sample Preparation
4.4. CAT Activity Determination
4.5. Glutathione Peroxidase Activity Determination
4.6. Determination of Glutathione Transferase Activity
4.7. Determination of Glutathione Reductase Activity
4.8. Erythrocyte Microviscosity Identification
4.9. Total Concentration of -SH Groups in Erythrocytes
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Donya, M.; Radford, M.; ElGuindy, A.; Firmin, D.; Yacoub, M.H. Radiation in medicine: Origins, risks and aspirations. Glob. Cardiol. Sci. Pract. 2014, 2014, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Buonanno, M.; de Toledo, S.M.; Pain, D.; Azzam, E.I. Long-term consequences of radiation-induced bystander effects depend on radiation quality and dose and correlate with oxidative stress. Radiat. Res. 2011, 175, 405–415. [Google Scholar] [CrossRef]
- Nuszkiewicz, J.; Woźniak, A.; Szewczyk-Golec, K. Ionizing Radiation as a Source of Oxidative Stress—The Protective Role of Melatonin and Vitamin D. Int. J. Mol. Sci. 2020, 21, 5804. [Google Scholar] [CrossRef] [PubMed]
- Santacruz-Gomez, K.; Sarabia-Sainz, A.; Acosta-Elias, M.; Sarabia-Sainz, M.; Janetanakit, W.; Khosla, N.; Melendrez, R.; Montero, M.P.; Lal, R. Antioxidant activity of hydrated carboxylated nanodiamonds and its influence on water γ-radiolysis. Nanotechnology 2018, 29, 125707. [Google Scholar] [CrossRef] [PubMed]
- Volkova, P.Y.; Geras’kin, S.A.; Kazakova, E.A. Radiation exposure in the remote period after the Chernobyl accident caused oxidative stress and genetic effects in Scots pine populations. Sci. Rep. 2017, 7, 43009. [Google Scholar] [CrossRef]
- Kelley, M.; Paulines, M.J.; Yoshida, G.; Myers, R.; Jora, M.; Levoy, J.P.; Addepalli, B.; Benoit, J.B.; Limbach, P.A. Ionizing radiation and chemical oxidant exposure impacts on Cryptococcus neoformans transfer RNAs. PLoS ONE 2022, 17, e0266239. [Google Scholar] [CrossRef]
- Cai, X.; Hao, J.; Zhang, X.; Yu, B.; Ren, J.; Luo, C.; Li, Q.; Huang, Q.; Shi, X.; Li, W.; et al. The polyhydroxylated fullerene derivative C60(OH)24 protects mice from ionizing-radiation-induced immune and mitochondrial dysfunction. Toxicol. Appl. Pharmacol. 2010, 243, 27–34. [Google Scholar] [CrossRef]
- Remigante, A.; Morabito, R.; Marino, A. Band 3 protein function and oxidative stress in erythrocytes. J. Cell. Physiol. 2021, 236, 6225–6234. [Google Scholar] [CrossRef]
- Grebowski, J.; Kazmierska-Grebowska, P.; Cichon, N.; Piotrowski, P.; Litwinienko, G. The Effect of Fullerenol C60(OH)36 on the Antioxidant Defense System in Erythrocytes. Int. J. Mol. Sci. 2021, 23, 119. [Google Scholar] [CrossRef]
- Grebowski, J.; Kazmierska, P.; Krokosz, A. Fullerenols as a new therapeutic approach in nanomedicine. Biomed. Res. Int. 2013, 2013, 751913. [Google Scholar] [CrossRef]
- Grebowski, J.; Kazmierska, P.; Litwinienko, G.; Lankoff, A.; Wolszczak, M.; Krokosz, A. Fullerenol C60(OH)36 protects human erythrocyte membrane against high-energy electrons. Biochim. Biophys. Acta Biomembr. 2018, 1860, 1528–1536. [Google Scholar] [CrossRef] [PubMed]
- Grebowski, J.; Litwinienko, G. Metallofullerenols in biomedical applications. Eur. J. Med. Chem. 2022, 238, 114481. [Google Scholar] [CrossRef] [PubMed]
- Krokosz, A.; Grebowski, J.; Rodacka, A.; Pasternak, B.; Puchala, M. The effect of fullerenol C60(OH)~30 on the alcohol dehydrogenase activity irradiated with X-rays. Radiat. Phys. Chem. 2014, 97, 102–106. [Google Scholar] [CrossRef]
- Trajković, S.; Dobrić, S.; Jaćević, V.; Dragojević-Simić, V.; Milovanović, Z.; Dordević, A. Tissue-protective effects of fullerenol C60(OH)24 and amifostine in irradiated rats. Colloids Surf. B Biointerfaces 2007, 58, 39–43. [Google Scholar] [CrossRef]
- Zhao, Q.; Li, Y.; Xu, J.; Liu, R.; Li, W. Radioprotection by fullerenols of Stylonychia mytilus exposed to gamma-rays. Int. J. Radiat. Biol. 2005, 81, 169–175. [Google Scholar] [CrossRef]
- Grebowski, J.; Krokosz, A.; Konarska, A.; Wolszczak, M.; Puchala, M. Rate constants of highly hydroxylated fullerene C60 interacting with hydroxyl radicals and hydrated electrons. Pulse radiolysis study. Radiat. Phys. Chem. 2014, 103, 146–152. [Google Scholar] [CrossRef]
- Grębowski, J.; Kaźmierska, P.; Krokosz, A. Fullerenol—Properties and applications in biomedical sciences. Postepy Hig. Med. Dosw. 2013, 67, 859–872. [Google Scholar] [CrossRef]
- Sushko, E.S.; Vnukova, N.G.; Churilov, G.N.; Kudryasheva, N.S. Endohedral Gd-Containing Fullerenol: Toxicity, Antioxidant Activity, and Regulation of Reactive Oxygen Species in Cellular and Enzymatic Systems. Int. J. Mol. Sci. 2022, 23, 5152. [Google Scholar] [CrossRef]
- Proskurnina, E.V.; Mikheev, I.V.; Savinova, E.A.; Ershova, E.S.; Veiko, N.N.; Kameneva, L.V.; Dolgikh, O.A.; Rodionov, I.V.; Proskurnin, M.A.; Kostyuk, S.V. Effects of Aqueous Dispersions of C60, C70 and Gd@C82 Fullerenes on Genes Involved in Oxidative Stress and Anti-Inflammatory Pathways. Int. J. Mol. Sci. 2021, 22, 6130. [Google Scholar] [CrossRef]
- Mikheev, I.V.; Sozarukova, M.M.; Proskurnina, E.V.; Kareev, I.E.; Proskurnin, M.A. Non-Functionalized Fullerenes and Endofullerenes in Aqueous Dispersions as Superoxide Scavengers. Molecules 2020, 25, 2506. [Google Scholar] [CrossRef]
- Bogdanović, V.; Stankov, K.; Icević, I.; Zikic, D.; Nikolić, A.; Solajić, S.; Djordjević, A.; Bogdanović, G. Fullerenol C60(OH)24 effects on antioxidative enzymes activity in irradiated human erythroleukemia cell line. J. Radiat. Res. 2008, 49, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Wang, C.; Xie, J.; Ji, C.; Gu, Z. Eco-Friendly and Scalable Synthesis of Fullerenols with High Free Radical Scavenging Ability for Skin Radioprotection. Small 2021, 17, e2102035. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhao, M.; Xie, J.; Ji, C.; Leng, Z.; Gu, Z. Fullerenol@nano-montmorillonite nanocomposite as an efficient radioprotective agent for ameliorating radioactive duodenal injury. Chem. Eng. J. 2022, 427, 131725. [Google Scholar] [CrossRef]
- Hamblin, M.R. Fullerenes as photosensitizers in photodynamic therapy: Pros and cons. Photochem. Photobiol. Sci. 2018, 17, 1515–1533. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Yoshimura, T.; Miwa, N. Radiosensitization by Liposome-Encapsulated Fullerenes to Mitochondria/DNA-Damages on Human Melanoma Cells. J. Nanosci. Nanotechnol. 2018, 18, 3775–3786. [Google Scholar] [CrossRef]
- Kamat, J.P.; Devasagayam, T.P.; Priyadarsini, K.I.; Mohan, H. Reactive oxygen species mediated membrane damage induced by fullerene derivatives and its possible biological implications. Toxicology 2000, 155, 55–61. [Google Scholar] [CrossRef]
- Morse II, P.D. Use of the spin label tempamine for measuring the internal viscosity of red blood cells. Biochem. Biophys. Res. Commun. 1977, 77, 1486–1491. [Google Scholar] [CrossRef]
- Bartosz, G.; Gaczyńska, M.; Grzelińska, E.; Judkiewicz, L. A spin-label study of membrane proteins and internal microviscosity of erythrocytes in hereditary spherocytosis. Life Sci. 1987, 41, 2285–2288. [Google Scholar] [CrossRef]
- Ghorbel, I.; Maktouf, S.; Kallel, C.; Ellouze Chaabouni, S.; Boudawara, T.; Zeghal, N. Disruption of erythrocyte antioxidant defense system, hematological parameters, induction of pro-inflammatory cytokines and DNA damage in liver of co-exposed rats to aluminium and acrylamide. Chem. Biol. Interact. 2015, 236, 31–40. [Google Scholar] [CrossRef]
- Bernabucci, U.; Ronchi, B.; Lacetera, N.; Nardone, A. Markers of oxidative status in plasma and erythrocytes of transition dairy cows during hot season. J. Dairy Sci. 2002, 85, 2173–2179. [Google Scholar] [CrossRef]
- Remigante, A.; Spinelli, S.; Basile, N.; Caruso, D.; Falliti, G.; Dossena, S.; Marino, A.; Morabito, R. Oxidation Stress as a Mechanism of Aging in Human Erythrocytes: Protective Effect of Quercetin. Int. J. Mol. Sci. 2022, 23, 7781. [Google Scholar] [CrossRef] [PubMed]
- Remigante, A.; Spinelli, S.; Straface, E.; Gambardella, L.; Caruso, D.; Falliti, G.; Dossena, S.; Marino, A.; Morabito, R. Açaì (Euterpe oleracea) Extract Protects Human Erythrocytes from Age-Related Oxidative Stress. Cells 2022, 11, 2391. [Google Scholar] [CrossRef] [PubMed]
- Nazıroğlu, M.; Akkuş, S.; Çelik, H. Levels of lipid peroxidation and antioxidant vitamins in plasma and erythrocytes of patients with ankylosing spondylitis. Clin. Biochem. 2011, 44, 1412–1415. [Google Scholar] [CrossRef] [PubMed]
- Baldacchino, G.; Brun, E.; Denden, I.; Bouhadoun, S.; Roux, R.; Khodja, H.; Sicard-Roselli, C. Importance of radiolytic reactions during high-LET irradiation modalities: LET effect, role of O2 and radiosensitization by nanoparticles. Cancer Nanotechnol. 2019, 10, 3. [Google Scholar] [CrossRef]
- Gebicki, J.M.; Nauser, T. Initiation and Prevention of Biological Damage by Radiation-Generated Protein Radicals. Int. J. Mol. Sci. 2021, 23, 396. [Google Scholar] [CrossRef]
- Azzam, E.I.; Jay-Gerin, J.-P.; Pain, D. Ionizing radiation-induced metabolic oxidative stress and prolonged cell injury. Cancer Lett. 2012, 327, 48–60. [Google Scholar] [CrossRef]
- Krokosz, A.; Szweda-Lewandowska, Z. Prehemolytic changes in human erythrocyte membranes induced by gamma radiation under air and nitrous oxide. Curr. Top. Biophys. 1996, 20, 154–157. [Google Scholar]
- Gudkov, S.V.; Guryev, E.L.; Gapeyev, A.B.; Sharapov, M.G.; Bunkin, N.F.; Shkirin, A.V.; Zabelina, T.S.; Glinushkin, A.P.; Sevost’yanov, M.A.; Belosludtsev, K.N.; et al. Unmodified hydrated C60 fullerene molecules exhibit antioxidant properties, prevent damage to DNA and proteins induced by reactive oxygen species and protect mice against injuries caused by radiation-induced oxidative stress. Nanomedicine 2019, 15, 37–46. [Google Scholar] [CrossRef]
- Ðordević, A.; Bogdanović, G. Fullerenol—A new nanopharmaceutic? Arch. Oncol. 2008, 16, 42–45. [Google Scholar] [CrossRef]
- Wade, R.S.; Castro, C.E. Oxidation of heme proteins by alkyl halides. J. Am. Chem. Soc. 1973, 95, 231–234. [Google Scholar] [CrossRef]
- Poulos, T.L. Heme enzyme structure and function. Chem. Rev. 2014, 114, 3919–3962. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.D.; Bi, Y.H.; Zhang, W.D.; Luo, Q.M. The interface behavior of hemoglobin at carbon nanotube and the detection for H2O2. Talanta 2005, 65, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Reisz, J.A.; Bansal, N.; Qian, J.; Zhao, W.; Furdui, C.M. Effects of ionizing radiation on biological molecules—Mechanisms of damage and emerging methods of detection. Antioxid. Redox Signal. 2014, 21, 260–292. [Google Scholar] [CrossRef] [PubMed]
- López-Canizales, A.M.; Angulo-Molina, A.; Garibay-Escobar, A.; Silva-Campa, E.; Mendez-Rojas, M.A.; Santacruz-Gómez, K.; Acosta-Elías, M.; Castañeda-Medina, B.; Soto-Puebla, D.; Álvarez-Bajo, O.; et al. Nanoscale Changes on RBC Membrane Induced by Storage and Ionizing Radiation: A Mini-Review. Front. Physiol. 2021, 12, 802. [Google Scholar] [CrossRef]
- Gwoździński, K. Ionizing radiation-induced structural modification of human red blood cells. Radiat. Environ. Biophys. 1991, 30, 45–52. [Google Scholar] [CrossRef]
- Raza, H. Dual localization of glutathione S-transferase in the cytosol and mitochondria: Implications in oxidative stress, toxicity and disease. FEBS J. 2011, 278, 4243–4251. [Google Scholar] [CrossRef]
- Doukali, H.; Ben Salah, G.; Hamdaoui, L.; Hajjaji, M.; Tabebi, M.; Ammar-Keskes, L.; Masmoudi, M.E.; Kamoun, H. Oxidative stress and glutathione S-transferase genetic polymorphisms in medical staff professionally exposed to ionizing radiation. Int. J. Radiat. Biol. 2017, 93, 697–704. [Google Scholar] [CrossRef]
- Cholon, A.; Giaccia, A.J.; Lewis, A.D.; Hickson, I.; Brown, J.M. What role do glutathione S-transferases play in the cellular response to ionizing radiation? Int. J. Radiat. Oncol. Biol. Phys. 1992, 22, 759–763. [Google Scholar] [CrossRef]
- Bocedi, A.; Noce, A.; Marrone, G.; Noce, G.; Cattani, G.; Gambardella, G.; Di Lauro, M.; Di Daniele, N.; Ricci, G. Glutathione Transferase P1-1 an Enzyme Useful in Biomedicine and as Biomarker in Clinical Practice and in Environmental Pollution. Nutrients 2019, 11, 1741. [Google Scholar] [CrossRef]
- Marchewka, Z.; Piwowar, A.; Ruzik, S.; Długosz, A. Glutathione S—Transferases class Pi and Mi and their significance in oncology. Postepy Hig. Med. Dosw. 2017, 71, 541–550. [Google Scholar] [CrossRef]
- Grebowski, J.; Konopko, A.; Krokosz, A.; DiLabio, G.A.; Litwinienko, G. Antioxidant activity of highly hydroxylated fullerene C60 and its interactions with the analogue of α-tocopherol. Free. Radic. Biol. Med. 2020, 160, 734–744. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Tian, Z.; Dong, Y.; Wang, X.; Zhan, L. Current advances in nanomaterials affecting morphology, structure, and function of erythrocytes. RSC Adv. 2021, 11, 6958–6971. [Google Scholar] [CrossRef] [PubMed]
| η | SD | |
|---|---|---|
| [Pa·s] | ||
| 2 h after the irradiation | ||
| Control | 4.72 | ±0.22 |
| 0.65 kGy | 4.31 | ±0.29 |
| 0.65 kGy + C60(OH)36 | 4.62 | ±0.18 |
| 1.3 kGy | 3.91 * | ±0.22 |
| 1.3 kGy + C60(OH)36 | 4.42 | ±0.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grebowski, J.; Kazmierska-Grebowska, P.; Cichon, N.; Konarska, A.; Wolszczak, M.; Litwinienko, G. Fullerenol C60(OH)36 Protects the Antioxidant Enzymes in Human Erythrocytes against Oxidative Damage Induced by High-Energy Electrons. Int. J. Mol. Sci. 2022, 23, 10939. https://doi.org/10.3390/ijms231810939
Grebowski J, Kazmierska-Grebowska P, Cichon N, Konarska A, Wolszczak M, Litwinienko G. Fullerenol C60(OH)36 Protects the Antioxidant Enzymes in Human Erythrocytes against Oxidative Damage Induced by High-Energy Electrons. International Journal of Molecular Sciences. 2022; 23(18):10939. https://doi.org/10.3390/ijms231810939
Chicago/Turabian StyleGrebowski, Jacek, Paulina Kazmierska-Grebowska, Natalia Cichon, Anna Konarska, Marian Wolszczak, and Grzegorz Litwinienko. 2022. "Fullerenol C60(OH)36 Protects the Antioxidant Enzymes in Human Erythrocytes against Oxidative Damage Induced by High-Energy Electrons" International Journal of Molecular Sciences 23, no. 18: 10939. https://doi.org/10.3390/ijms231810939
APA StyleGrebowski, J., Kazmierska-Grebowska, P., Cichon, N., Konarska, A., Wolszczak, M., & Litwinienko, G. (2022). Fullerenol C60(OH)36 Protects the Antioxidant Enzymes in Human Erythrocytes against Oxidative Damage Induced by High-Energy Electrons. International Journal of Molecular Sciences, 23(18), 10939. https://doi.org/10.3390/ijms231810939






