Peroxisome Proliferator-Activated Receptor-α (PPARα) Expression in a Clinical Population of Pakistani Patients with Type 2 Diabetes and Dyslipidemia
Abstract
1. Introduction
2. Results
2.1. Participant Characteristics
2.1.1. Physical Parameters
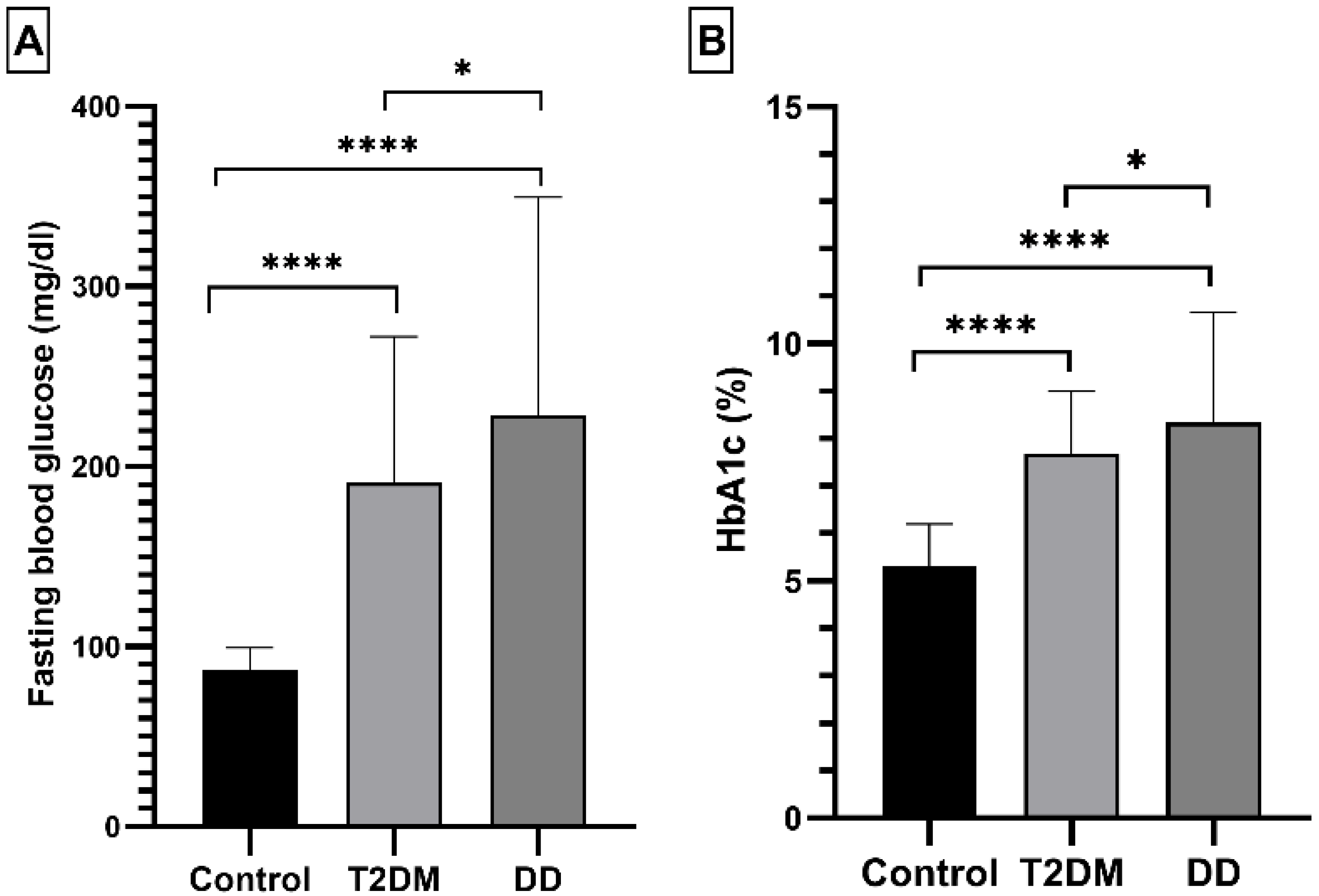
2.1.2. Fasting Blood Glucose and HbA1c Levels
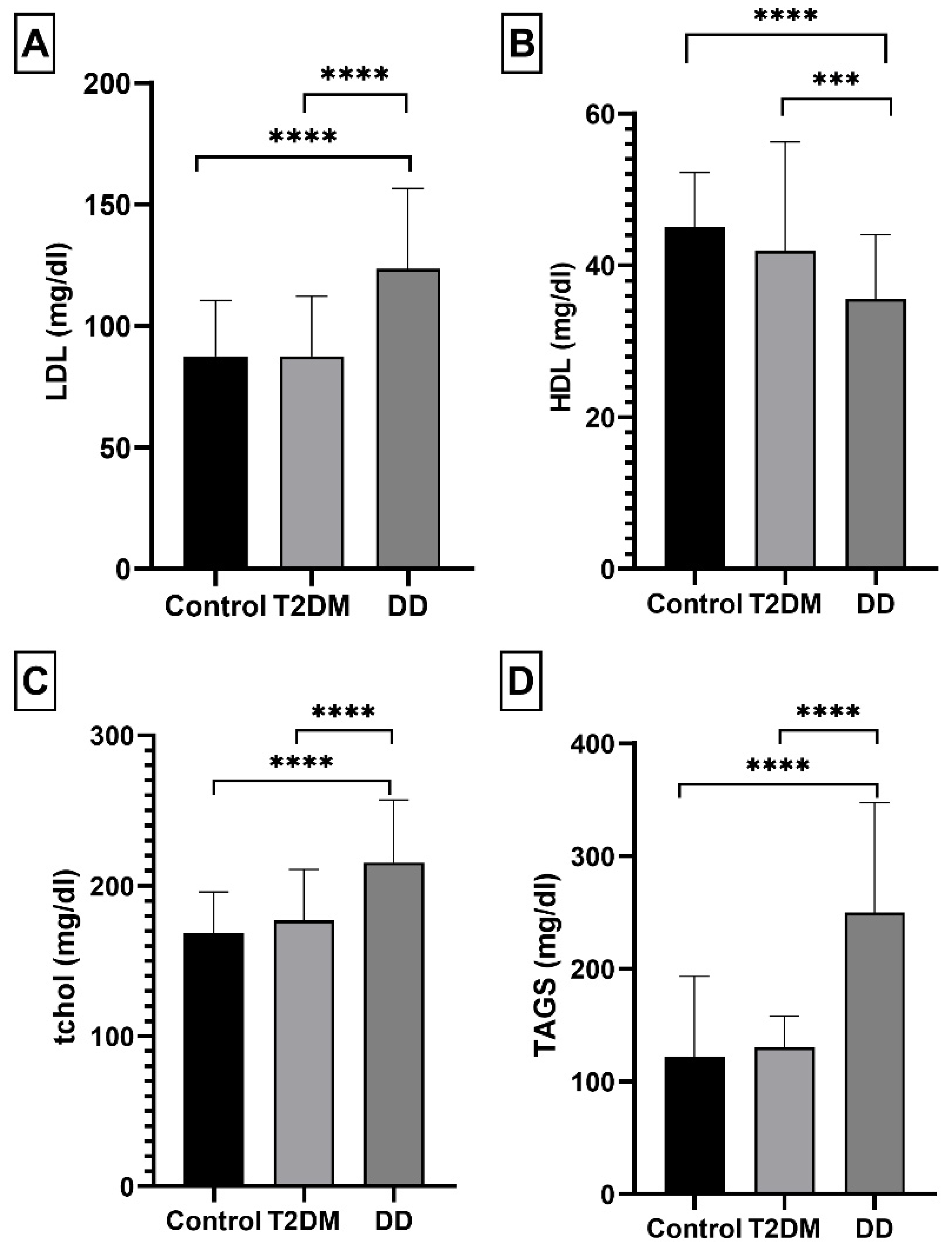
2.1.3. Lipid Panel Levels
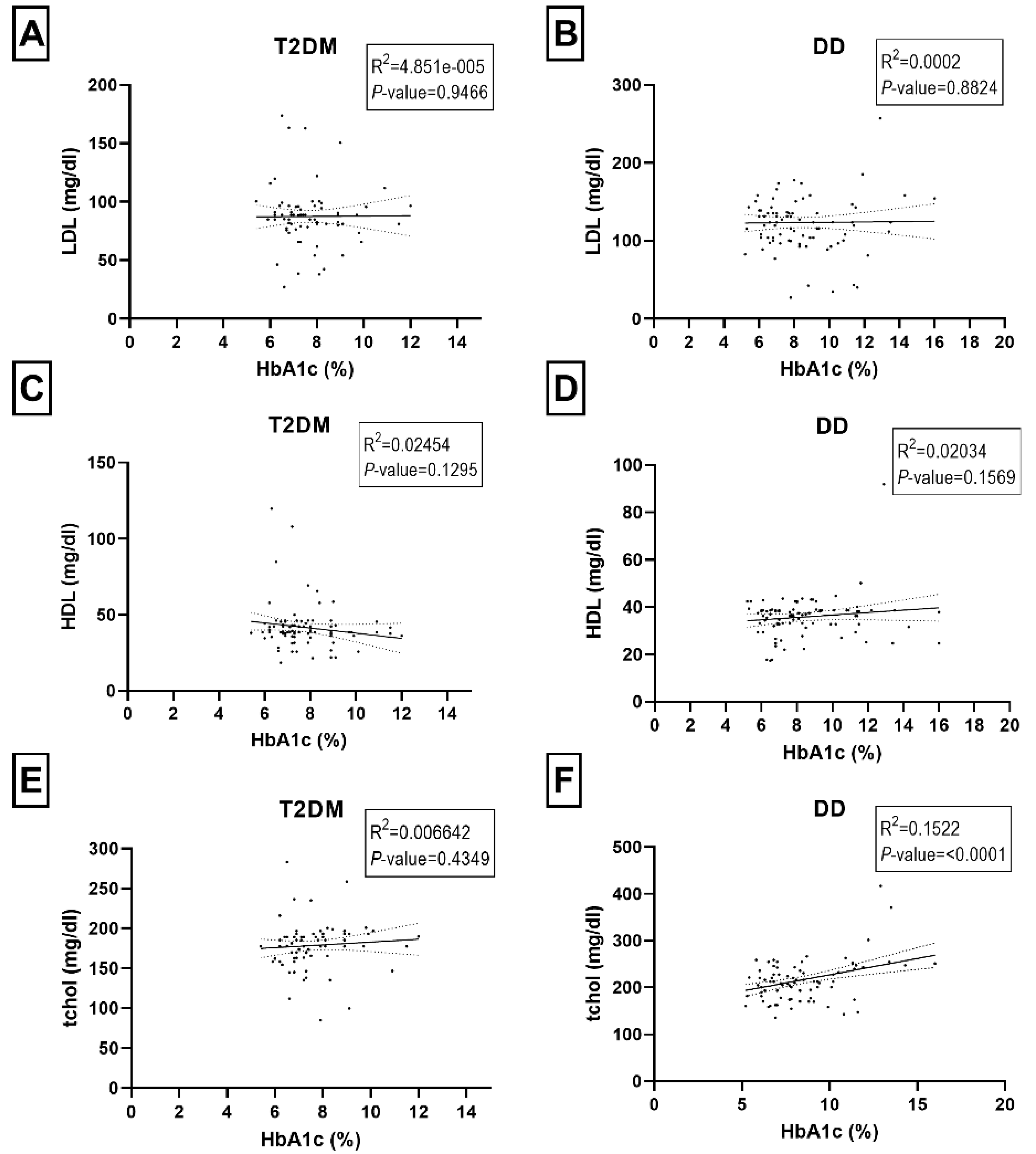
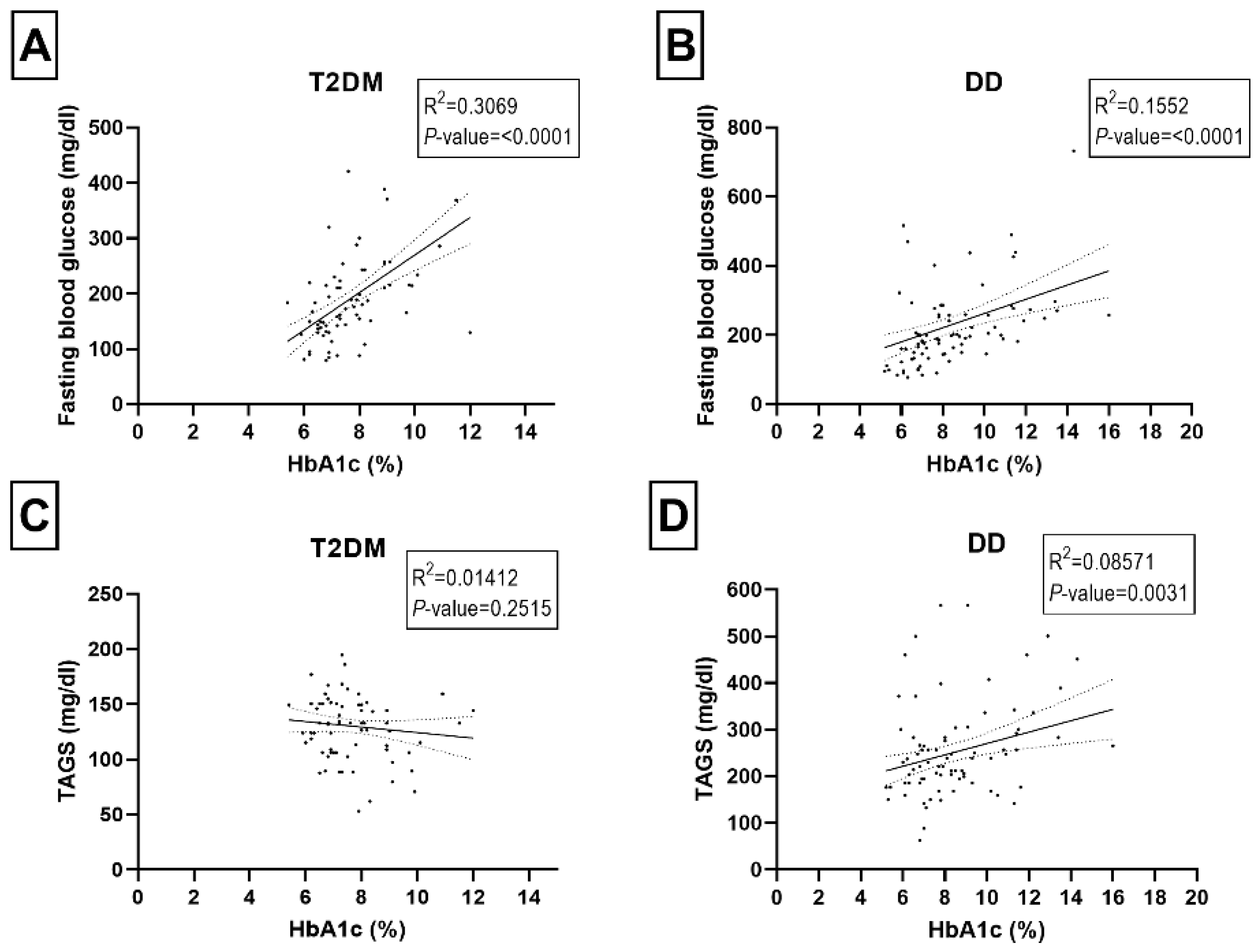
2.2. Correlations among HbAc1, Fasting Blood Glucose, and Blood Lipids Panel
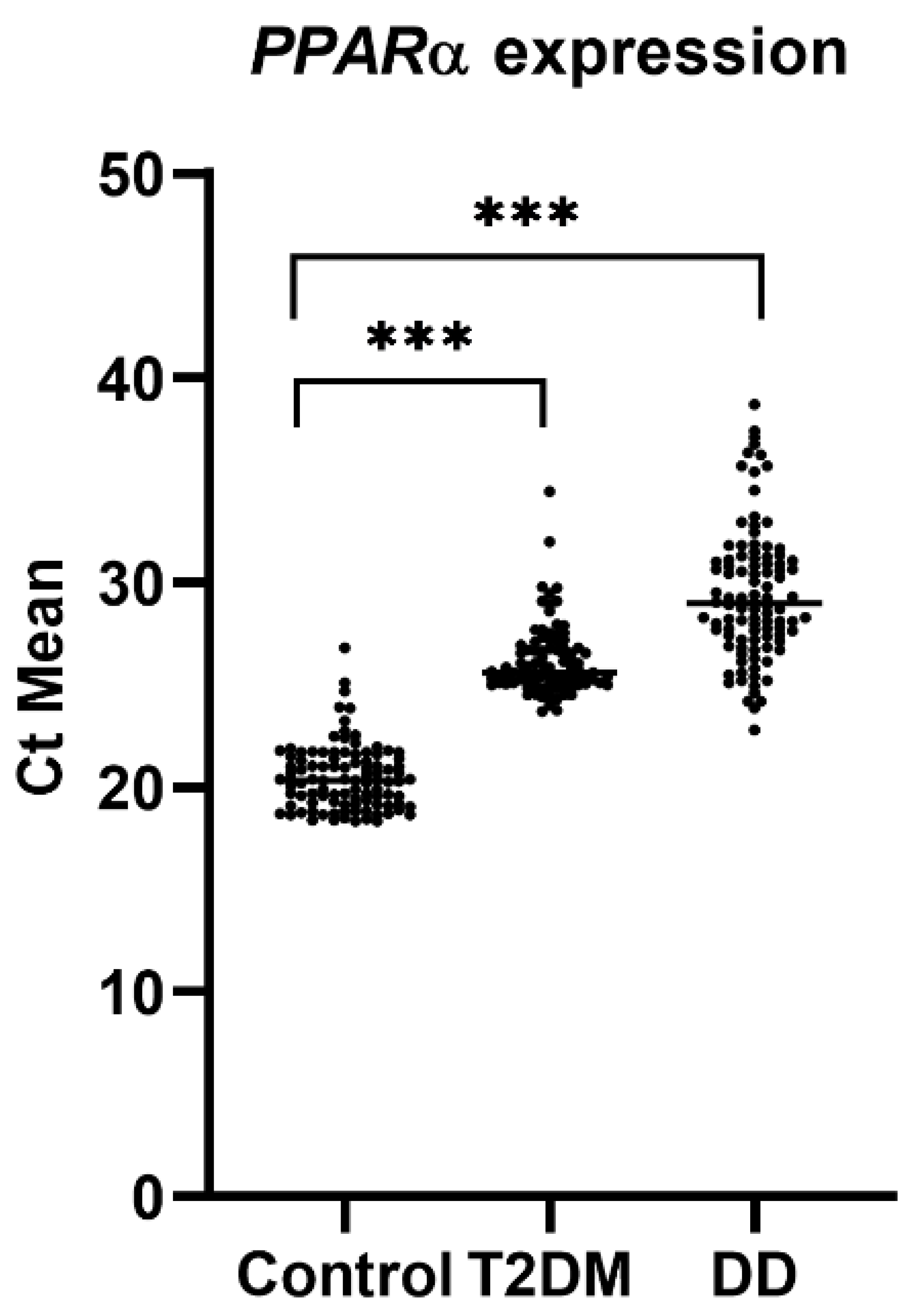
2.3. PPARα Gene Expression
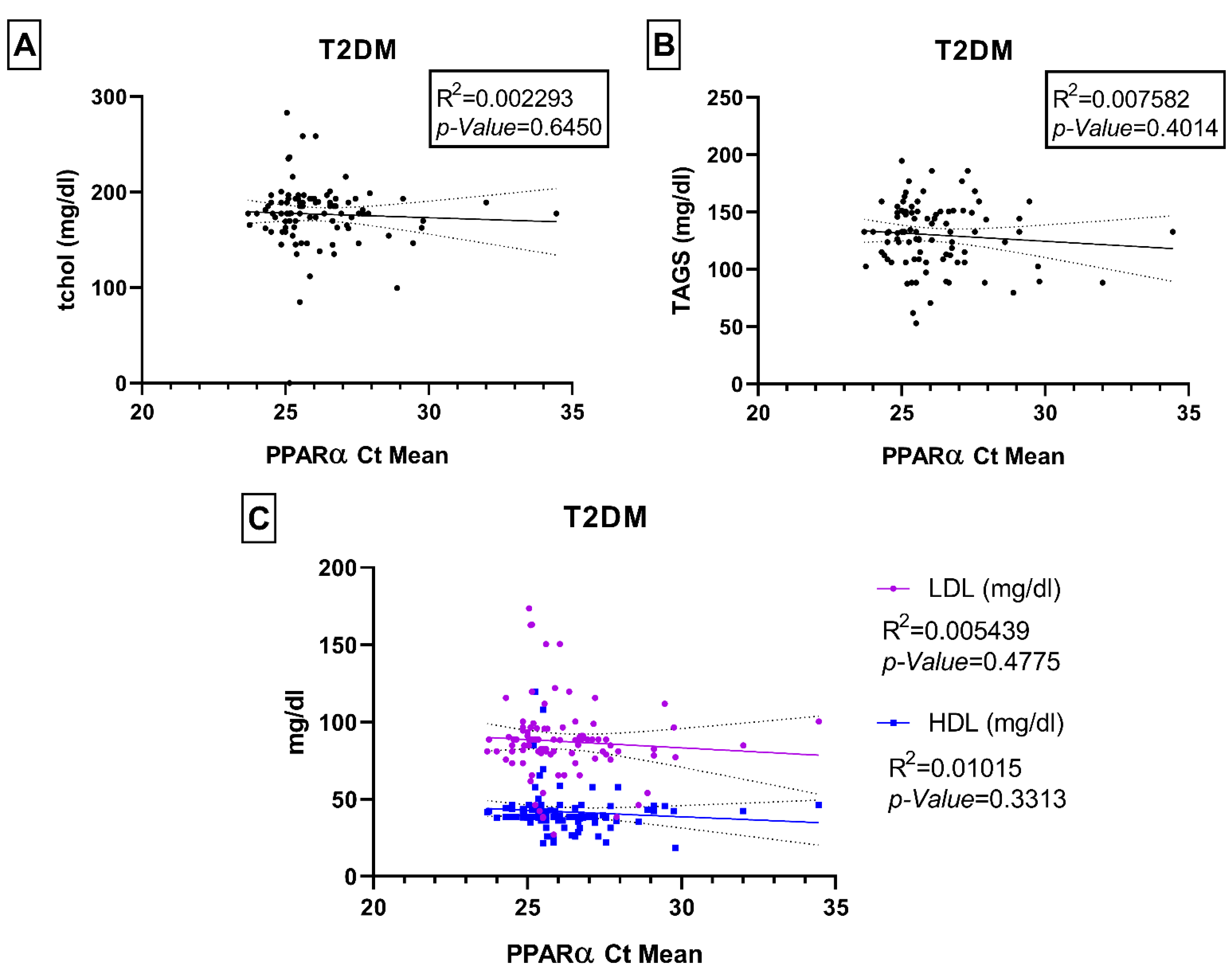
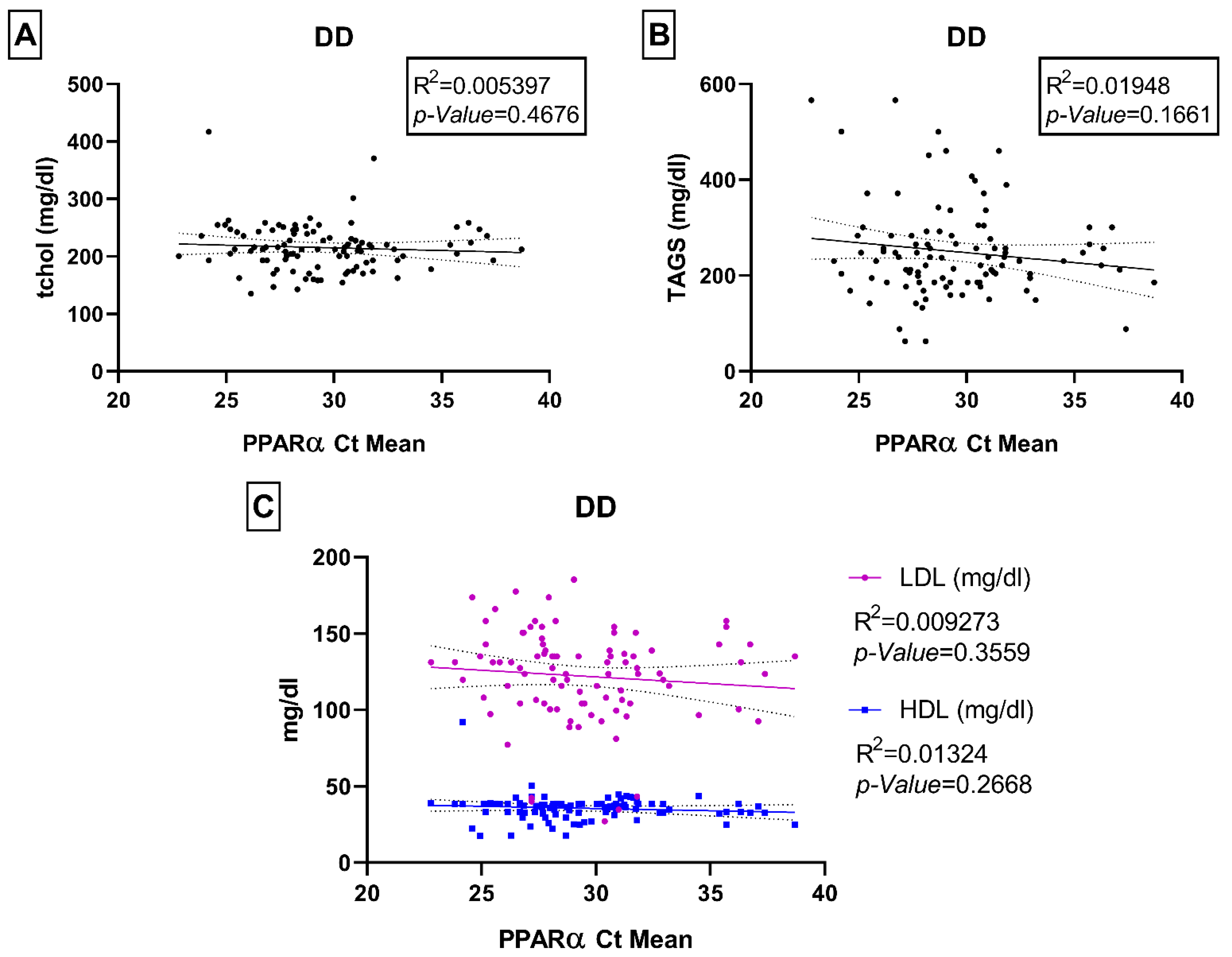
Associations of PPARα Gene Expression with Age, HbA1c, Fasting Blood Glucose, and Lipid Panel
3. Discussion
4. Materials and Methods
4.1. Study Ethics and Approval
4.2. Study Participants
4.3. Sample Collection and Biochemical Levels
4.4. RNA Isolation, cDNA Synthesis, and Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
4.5. Statistical Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kamalova, F.R.; Yarieva, O.O.; Akhmedov, I.I. Risk factors for oral diseases in children with diabetes mellitus. J. Nat. Remedies 2021, 22, 57–63. [Google Scholar]
- Naeem, N.; Butt, A.; Zafar, A.B.; Fawwad, A.; Tahir, B.; Basit, A. Dyslipidemia Pattern Among Newly Diagnosed and Known Type 2 Diabetics: A Comparative Analysis from a Tertiary Care Hospital of Karachi, Pakistan. Pak. J. Med. Res. 2020, 59, 45–50. [Google Scholar]
- Hussain, A.; Ghafar, A.; Raza, M.A. The association of plasma glucose levels with lipid profile in type 2 diabetes mellitus. Indo Am. J. Pharm. Sci. 2021, 8, 767–771. [Google Scholar]
- Suruga, K.; Miyoshi, T.; Kotani, K.; Ichikawa, K.; Miki, T.; Osawa, K.; Ejiri, K.; Toda, H.; Nakamura, K.; Morita, H.; et al. Higher oxidized high-density lipoprotein to apolipoprotein A-I ratio is associated with high-risk coronary plaque characteristics determined by CT angiography. Int. J. Cardiol. 2021, 324, 193–198. [Google Scholar] [CrossRef]
- Sharif, S.; Raza, M.T.; Mushtaq, S.; Afreen, B.; Azam, B.; Hashmi, A.; Ali, M.H. Frequency of Depression in Patients with Type 2 Diabetes Mellitus and its Relationship with Glycemic Control and Diabetic Microvascular Complications. Cureus 2019, 11, e5145. [Google Scholar] [CrossRef]
- Poorani, K.; Khokhar, N.A.; Kumar, P.; Goswami, P. Diabetic dyslipidemia and association of glycemic status with lipid profile, body mass index and abdominal obesity. RMJ 2020, 45, 265–268. [Google Scholar]
- Heeren, J.; Scheja, L. Metabolic-associated fatty liver disease and lipoprotein metabolism. Mol. Metab. 2021, 50, 101238. [Google Scholar] [CrossRef]
- Montaigne, D.; Butruille, L.; Staels, B. PPAR control of metabolism and cardiovascular functions. Nat. Rev. Cardiol. 2021, 18, 809–823. [Google Scholar] [CrossRef] [PubMed]
- Karimi, N.; Karami Tehrani, F.S. Expression of SR-B1 receptor in breast cancer cell lines, MDAMB-468 and MCF-7: Effect on cell proliferation and apoptosis. Iran. J. Basic Med. Sci. 2021, 24, 1069–1077. [Google Scholar]
- Thomas, D.T.; Del Cimmuto, N.R.; Flack, K.D.; Stec, D.E.; Hinds, T.D., Jr. Reactive Oxygen Species (ROS) and Antioxidants as Immunomodulators in Exercise: Implications for Heme Oxygenase and Bilirubin. Antioxidants 2022, 11, 179. [Google Scholar] [CrossRef]
- Arif, M.; Siddique, K.; Majeed, A. Comparison of expression of PPARα and SCARB1 gene in type 2 diabetic and type 2 diabetic dyslipidemia patients. Medicina 2022. submitted. [Google Scholar]
- Zhang, H.; Kwapong, W.R.; Shao, M.M.; Yan, J.Y.; Lin, X.D.; Chen, B.B.; Chen, K.Y. Predictors of the Prevalence of Dyslipidemia and Influencing Factors for Young Health Examination Cohort: A Cross-Sectional Survey. Front. Public Health 2020, 8, 400. [Google Scholar] [CrossRef]
- Mahesar, A.H.; Soomro, M.H.; Magsi, M.; Baloch, A.A.; Soomro, M.A. Dyslipidaemia in ischaemic stroke patients: Results from a tertiary care teaching hospital of Pakistan. J. Coll. Community Phys. Sri Lanka 2021, 27, 435–441. [Google Scholar] [CrossRef]
- Zaid, M.; Hasnain, S. Plasma lipid abnormalities in Pakistani population: Trends, associated factors, and clinical implications. Braz J. Med. Biol. Res. 2018, 51, e7239. [Google Scholar] [CrossRef] [PubMed]
- Zahid, N.; Claussen, B.; Hussain, A. High prevalence of obesity, dyslipidemia and metabolic syndrome in a rural area in Pakistan. Diabetes Metab. Syndr. Clin. Res. Rev. 2008, 2, 13–19. [Google Scholar] [CrossRef]
- Sarfraz, M.; Sajid, S.; Ashraf, M.A. Prevalence and pattern of dyslipidemia in hyperglycemic patients and its associated factors among Pakistani population. Saudi J. Biol. Sci. 2016, 23, 761–766. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.; Majeed, A.; Rashid, A. The Risk Factors Associated with Diabetic Dyslipidemia and Anthropometric Parameters Linked with and without Dyslipidemia in Type 2 Diabetes Mellitus. PAFMJ 2020, 70, 628–633. Available online: https://www.pafmj.org/index.php/PAFMJ/article/view/4245 (accessed on 11 May 2022).
- Hussain, A.; Ali, I.; Ijaz, M.; Rahi, A. Correlation between hemoglobin A1c and serum lipid profile in Afghani patients with type 2 diabetes: Hemoglobin A1c prognosticates dyslipidemia. Ther. Adv. Endocrinol. Metab. 2017, 8, 51–57. [Google Scholar] [CrossRef]
- Basit, A.; Sabir, S.; Riaz, M.; Fawwad, A.; NDSP members. NDSP 05: Prevalence and pattern of dyslipidemia in urban and rural areas of Pakistan; a sub analysis from second National Diabetes Survey of Pakistan (NDSP) 2016-2017. J. Diabetes Metab. Disord. 2020, 19, 1215–1225. [Google Scholar] [CrossRef]
- Saeed, A.; Virani, S.S.; Mulukutla, S.; Chow, C.K. Dyslipidemia and Cardiovascular Disease Prevention in South Asians: A Review and Discussion of Causes, Challenges and Management Strategies. Curr. Diabetes Rev. 2021, 17, e011221190238. [Google Scholar] [CrossRef]
- Cuzzocrea, S.; Mazzon, E.; Di Paola, R.; Peli, A.; Bonato, A.; Britti, D.; Genovese, T.; Muià, C.; Crisafulli, C.; Caputi, A.P. The role of the peroxisome proliferator-activated receptor-alpha (PPAR-alpha) in the regulation of acute inflammation. J. Leukoc. Biol. 2006, 79, 999–1010. [Google Scholar] [CrossRef] [PubMed]
- Kasai, T.; Miyauchi, K.; Yokoyama, T.; Aihara, K.; Daida, H. Efficacy of peroxisome proliferative activated receptor (PPAR)-alpha ligands, fenofibrate, on intimal hyperplasia and constrictive remodeling after coronary angioplasty in porcine models. Atherosclerosis 2006, 188, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Gheorghe, G.; Toth, P.P.; Bungau, S.; Behl, T.; Ilie, M.; Pantea Stoian, A.; Bratu, O.G.; Bacalbasa, N.; Rus, M.; Diaconu, C.C. Cardiovascular Risk and Statin Therapy Considerations in Women. Diagnostics 2020, 10, 483. [Google Scholar] [CrossRef] [PubMed]
- Arruda, A.C.; Perilhão, M.S.; Santos, W.A.; Gregnani, M.F.; Budu, A.; Neto, J.; Estrela, G.R.; Araujo, R.C. PPARα-Dependent Modulation by Metformin of the Expression of OCT-2 and MATE-1 in the Kidney of Mice. Molecules 2020, 25, 392. [Google Scholar] [CrossRef] [PubMed]
- Sunil, B.; Ashraf, A.P. Dyslipidemia in Pediatric Type 2 Diabetes Mellitus. Curr. Diabetes Rep. 2020, 20, 53. [Google Scholar] [CrossRef]
- Vesa, C.M.; Behl, T.; Nemeth, S.; Bratu, O.G.; Diaconu, C.C.; Moleriu, R.D.; Negrut, N.; Zaha, D.C.; Bustea, C.; Radu, F.I.; et al. Prediction of NAFLD occurrence in prediabetes patients. Exp. Ther. Med. 2020, 20, 190. [Google Scholar] [CrossRef]
- Gu, S.J.; Guo, Z.R.; Zhou, Z.Y.; Hu, X.S.; Wu, M. PPAR α and PPAR γ polymorphisms as risk factors for dyslipidemia in a Chinese Han population. Lipids Health Dis. 2014, 13, 23. [Google Scholar] [CrossRef] [PubMed]
- Frazier-Wood, A.C.; Ordovas, J.M.; Straka, R.J.; Hixson, J.E.; Borecki, I.B.; Tiwari, H.K. Arnett DK. The PPAR alpha gene is associated with triglyceride, low-density cholesterol and inflammation marker response to fenofibrate intervention: The GOLDN study. Pharm. J. 2013, 13, 312–317. [Google Scholar]
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2021. Diabetes Care 2021, 44 (Suppl. 1), S15–S33. [Google Scholar] [CrossRef]
- Summary of the second report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel II). JAMA 1993, 269, 3015–3023. [CrossRef]
| Characteristics of the Patients | Control (n = 100) | T2DM (n = 95) | p-Value of T2DM Compared to Control | DD (n = 100) | p-Value of DD Compared to Control | p-Value T2DM vs. DD |
|---|---|---|---|---|---|---|
| Age (Y) | 43.26 ± 15.34 | 52.38 ± 14.96 | <0.0001 | 51.25 ± 10.27 | <0.0001 | 0.53 |
| Gender | M-53; F-47 | M-27; F-68 | 0.0005 | M-31; F-69 | 0.0016 | 0.69 |
| Weight (kg) | 67.81 ± 9.54 | 71.02 ± 10.00 | 0.0233 | 70.67 ± 9.34 | 0.0334 | 0.80 |
| BMI (kg/m2) | 23.83 ± 4.04 | 26.81 ± 4.42 | <0.0001 | 25.89 ± 3.16 | <0.0001 | 0.53 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arif, M.; Mondal, T.; Majeed, A.; Loffredo, C.A.; Korba, B.E.; Ghosh, S. Peroxisome Proliferator-Activated Receptor-α (PPARα) Expression in a Clinical Population of Pakistani Patients with Type 2 Diabetes and Dyslipidemia. Int. J. Mol. Sci. 2022, 23, 10847. https://doi.org/10.3390/ijms231810847
Arif M, Mondal T, Majeed A, Loffredo CA, Korba BE, Ghosh S. Peroxisome Proliferator-Activated Receptor-α (PPARα) Expression in a Clinical Population of Pakistani Patients with Type 2 Diabetes and Dyslipidemia. International Journal of Molecular Sciences. 2022; 23(18):10847. https://doi.org/10.3390/ijms231810847
Chicago/Turabian StyleArif, Maria, Tanmoy Mondal, Asifa Majeed, Christopher A. Loffredo, Brent E. Korba, and Somiranjan Ghosh. 2022. "Peroxisome Proliferator-Activated Receptor-α (PPARα) Expression in a Clinical Population of Pakistani Patients with Type 2 Diabetes and Dyslipidemia" International Journal of Molecular Sciences 23, no. 18: 10847. https://doi.org/10.3390/ijms231810847
APA StyleArif, M., Mondal, T., Majeed, A., Loffredo, C. A., Korba, B. E., & Ghosh, S. (2022). Peroxisome Proliferator-Activated Receptor-α (PPARα) Expression in a Clinical Population of Pakistani Patients with Type 2 Diabetes and Dyslipidemia. International Journal of Molecular Sciences, 23(18), 10847. https://doi.org/10.3390/ijms231810847







