Biomarkers Related to Synaptic Dysfunction to Discriminate Alzheimer’s Disease from Other Neurological Disorders
Abstract
1. Introduction
2. Results
2.1. Participants’ Features
2.2. Differences in CSF Ng and α-Syn Levels between AD and n-ND Patients
2.3. Biomarkers Related to Synaptic Dysfunctions and AD-Core Biomarkers to Discriminate AD from n-AD
2.4. Biomarkers Related to Synaptic Dysfunctions and AD-Core Biomarkers in AD Subgroups
2.5. Effect of ApoE4 Genotype on CSF Ng and α-Syn Concentrations in AD Group
2.6. Correlations of CSF Levels of Synaptic-Related Biomarkers with Clinical Features in AD Group
2.7. Relationship of Synaptic-Related Biomarkers with Clinical Features and AD-Core Biomarkers
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Neuropsychological Assessment
4.3. FDG-PET
4.4. CSF Sampling, Processing and Analyses
4.5. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tzioras, M.; Davies, C.; Newman, A.; Jackson, R.; Spires-Jones, T. Invited Review: APOE at the interface of inflammation, neurodegeneration and pathological protein spread in Alzheimer’s disease. Neuropathol. Appl. Neurobiol. 2019, 45, 327–346. [Google Scholar] [CrossRef] [PubMed]
- Deture, M.A.; Dickson, D.W. The neuropathological diagnosis of Alzheimer’s disease. Mol. Neurodegener. 2019, 14, 32. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.R.; Knopman, D.S.; Jagust, W.J.; Shaw, L.M.; Aisen, P.S.; Weiner, M.W.; Petersen, R.C.; Trojanowski, J.Q. Hypothetical Pathological Cascade in Alheimer’s Disease. Lancet Neurol. 2010, 9, 1–20. [Google Scholar] [CrossRef]
- Agnello, L.; Piccoli, T.; Vidali, M.; Cuffaro, L.; Lo Sasso, B.; Iacolino, G.; Giglio, V.R.; Lupo, F.; Alongi, P.; Bivona, G.; et al. Diagnostic accuracy of cerebrospinal fluid biomarkers measured by chemiluminescent enzyme immunoassay for Alzheimer disease diagnosis. Scand. J. Clin. Lab. Investig. 2020, 80, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Virgilio, E.; De Marchi, F.; Contaldi, E.; Dianzani, U.; Cantello, R.; Mazzini, L.; Comi, C. The Role of Tau beyond Alzheimer’s Disease: A Narrative Review. Biomedicines 2022, 10, 760. [Google Scholar] [CrossRef]
- Jack, C.R., Jr.; Knopman, D.S.; Jagust, W.J.; Shaw, L.M.; Aisen, P.S.; Weiner, M.W.; Petersen, R.C.; Trojanowski, J.Q. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010, 9, 119–128. [Google Scholar] [CrossRef]
- Perani, D.; Cerami, C.; Caminiti, S.P.; Santangelo, R.; Coppi, E.; Ferrari, L.; Pinto, P.; Passerini, G.; Falini, A.; Iannaccone, S.; et al. Cross-validation of biomarkers for the early differential diagnosis and prognosis of dementia in a clinical setting. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 499–508. [Google Scholar] [CrossRef]
- Villemagne, V.L.; Chételat, G. Neuroimaging biomarkers in Alzheimer’s disease and other dementias. Ageing Res. Rev. 2016. Published online. [Google Scholar] [CrossRef]
- Iaccarino, L.; Chiotis, K.; Alongi, P.; Almkvist, O.; Wall, A.; Cerami, C.; Bettinardi, V.; Gianolli, L.; Nordberg, A.; Perani, D. A Cross-Validation of FDG-and Amyloid-PET Biomarkers in Mild Cognitive Impairment for the Risk Prediction to Dementia due to Alzheimer’s Disease in a Clinical Setting. J. Alzheimer’s Dis. 2017, 59, 603–614. [Google Scholar] [CrossRef]
- Caminiti, S.P.; Ballarini, T.; Sala, A.; Cerami, C.; Presotto, L.; Santangelo, R.; Fallanca, F.; Vanoli, E.G.; Gianolli, L.; Iannaccone, S.; et al. FDG-PET and CSF biomarker accuracy in prediction of conversion to different dementias in a large multicentre MCI cohort. NeuroImage Clin. 2018, 18, 167–177. [Google Scholar] [CrossRef]
- McKhann, G.M.; Knopman, D.S.; Chertkow, H.; Hyman, B.T.; Jack, C.R., Jr.; Kawas, C.H.; Klunk, W.E.; Koroshetz, W.J.; Manly, J.J.; Mayeux, R.; et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011, 7, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Albert, M.S.; DeKosky, S.T.; Dickson, D.; Dubois, B.; Feldman, H.H.; Fox, N.C.; Gamst, A.; Holtzman, D.M.; Jagust, W.J.; Petersen, R.C.; et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011, 7, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Chételat, G.; Arbizu, J.; Barthel, H.; Garibotto, V.; Law, I.; Morbelli, S.; van de Giessen, E.; Agosta, F.; Barkhof, F.; Brooks, D.J.; et al. Amyloid-PET and 18F-FDG-PET in the diagnostic investigation of Alzheimer’s disease and other dementias. Lancet Neurol. 2020, 19, 951–962. [Google Scholar] [CrossRef]
- Blennow, K.; Hampel, H.; Weiner, M.; Zetterberg, H. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nat. Rev. Neurol. 2010, 6, 131–144. [Google Scholar] [CrossRef]
- Oeckl, P.; Jardel, C.; Salachas, F.; Lamari, F.; Andersen, P.M.; Bowser, R.; de Carvalho, M.; Costa, J.; Van Damme, P.; Gray, E.; et al. Multicenter validation of CSF neurofilaments as diagnostic biomarkers for ALS. Amyotroph. Lateral Scler. Front. Degener. 2016, 17, 404–413. [Google Scholar] [CrossRef]
- Ashton, N.J.; Leuzy, A.; Karikari, T.K.; Mattsson-Carlgren, N.; Dodich, A.; Boccardi, M.; Corre, J.; Drzezga, A.; Nordberg, A.; Ossenkoppele, R.; et al. The validation status of blood biomarkers of amyloid and phospho-tau assessed with the 5-phase development framework for AD biomarkers. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2140–2156. [Google Scholar] [CrossRef]
- Ferreira, D.; Nordberg, A.; Westman, E. Biological subtypes of Alzheimer disease A systematic review and meta-analysis. Neurology 2020, 94, 436–448. [Google Scholar] [CrossRef]
- Duara, R.; Barker, W. Heterogeneity in Alzheimer’s Disease Diagnosis and Progression Rates: Implications for Therapeutic Trials. Neurotherapeutics 2022, 19, 8–25. [Google Scholar] [CrossRef]
- Boyle, P.A.; Yu, L.; Wilson, R.S.; Leurgans, S.E.; Schneider, J.A.; Bennett, D.A. Person-Specific Contribution of Neuropathologies to Cognitive Loss in Old Age. Ann. Neurol. 2018, 83, 74–83. [Google Scholar] [CrossRef]
- White, L.R.; Edland, S.D.; Hemmy, L.S.; Montine, K.S.; Zarow, C.; Sonnen, J.A.; Uyehara-Lock, J.H.; Gelber, R.P.; Ross, G.W.; Petrovitch, H.; et al. Neuropathologic comorbidity and cognitive impairment in the Nun and Honolulu-Asia Aging Studies. Neurology 2016, 86, 1000–1008. [Google Scholar] [CrossRef]
- Coulthard, E.J.; Love, S. A broader view of dementia: Multiple co-pathologies are the norm. Brain 2018, 141, 1894–1897. [Google Scholar] [CrossRef] [PubMed]
- Lopez, O.L.; Maillard, P. Vascular disease and cerebral amyloid deposition. Neurology 2018, 90, 635–636. [Google Scholar] [CrossRef]
- Scott, G.; Ramlackhansingh, A.F.; Edison, P.; Hellyer, P.; Cole, J.; Veronese, M.; Leech, R.; Greenwood, R.J.; Turkheimer, F.E.; Gentleman, S.M.; et al. Amyloid pathology and axonal injury after brain trauma. Neurology 2016, 86, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Blennow, K.; Zetterberg, H. The Past and the Future of Alzheimer’s Disease Fluid Biomarkers. J. Alzheimer’s Dis. 2018, 62, 1125–1140. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Fu, A.K.Y.; Ip, N.Y. Synaptic dysfunction in Alzheimer’s disease: Mechanisms and therapeutic strategies. Pharmacol. Ther. 2019, 195, 186–198. [Google Scholar] [CrossRef] [PubMed]
- Scheff, S.W.; Price, D.A. Alzheimer’s disease-related alterations in synaptic density: Neocortex and hippocampus. J. Alzheimers Dis. 2006, 9 (Suppl. S3), 101–115. [Google Scholar] [CrossRef]
- Scheff, S.W.; Price, D.A.; Schmitt, F.A.; DeKosky, S.T.; Mufson, E.J. Synaptic alterations in CA1 in mild Alzheimer disease and mild cognitive impairment. Neurology 2007, 68, 1501–1508. [Google Scholar] [CrossRef]
- Spires-Jones, T.L.; Hyman, B.T. The Intersection of Amyloid Beta and Tau at Synapses in Alzheimer’s Disease. Neuron 2014, 82, 756–771. [Google Scholar] [CrossRef]
- Agnello, L.; Gambino, C.M.; Lo Sasso, B.; Bivona, G.; Milano, S.; Ciaccio, A.M.; Piccoli, T.; La Bella, V.; Ciaccio, M. Neurogranin as a Novel Biomarker in Alzheimer’s Disease. Lab. Med. 2020, 52, 188–196. [Google Scholar] [CrossRef]
- Agnello, L.; Lo Sasso, B.; Vidali, M.; Scazzone, C.; Piccoli, T.; Gambino, C.M.; Bivona, G.; Giglio, R.V.; Ciaccio, A.M.; La Bella, V.; et al. Neurogranin as a reliable biomarker for synaptic dysfunction in alzheimer’s disease. Diagnostics 2021, 11, 2339. [Google Scholar] [CrossRef]
- Xiang, Y.; Xin, J.; Le, W.; Yang, Y. Neurogranin: A Potential Biomarker of Neurological and Mental Diseases. Front. Aging Neurosci. 2020, 12, 584743. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yang, J.; Zhu, W.; Tang, Y.; Jia, J. The synaptic marker neurogranin as a disease state biomarker in Alzheimer’s disease: A systematic review and meta-analysis. Int. J. Neurosci. 2021, 0, 1–9. [Google Scholar] [CrossRef]
- Cousins, K.A.; Burke, S.E.; Ballinger, S.; Irwin, D.J.; Shaw, L.M.; Wolk, D.A.; Trojanowski, J.Q.; Lee, E.B.; Grossman, M. Cerebrospinal fluid neurogranin in non-amnestic and amnestic Alzheimer’s disease. Alzheimer’s Dement. 2021, 17, 53884. [Google Scholar] [CrossRef]
- Mavroudis, I.A.; Petridis, F.; Chatzikonstantinou, S.; Kazis, D. A meta-analysis on CSF neurogranin levels for the diagnosis of Alzheimer’s disease and mild cognitive impairment. Aging Clin. Exp. Res. 2020, 32, 1639–1646. [Google Scholar] [CrossRef]
- Sanfilippo, C.; Forlenza, O.; Zetterberg, H.; Blennow, K. Increased neurogranin concentrations in cerebrospinal fluid of Alzheimer’s disease and in mild cognitive impairment due to AD. J. Neural. Transm. 2016, 123, 1443–1447. [Google Scholar] [CrossRef]
- Wellington, H.; Paterson, R.W.; Suárez-González, A.; Poole, T.; Frost, C.; Sjöbom, U.; Slattery, C.F.; Magdalinou, N.K.; Lehmann, M.; Portelius, E.; et al. CSF neurogranin or tau distinguish typical and atypical Alzheimer disease. Ann. Clin. Transl. Neurol. 2018, 5, 162–171. [Google Scholar] [CrossRef]
- Tarawneh, R.; D’Angelo, G.; Crimmins, D.; Herries, E.; Griest, T.; Fagan, A.M.; Zipfel, G.J.; Ladenson, J.H.; Morris, J.C.; Holtzman, D.M. Diagnostic and Prognostic Utility of the Synaptic Marker Neurogranin in Alzheimer Disease. JAMA Neurol. 2016, 73, 561–571. [Google Scholar] [CrossRef]
- Fyfe, I. Neurogranin in CSF identifies Creutzfeldt–Jakob disease. Nat. Rev. Neurol. 2019, 15, 434–435. [Google Scholar] [CrossRef]
- Blennow, K.; Diaz-Lucena, D.; Zetterberg, H.; Villar-Pique, A.; Karch, A.; Vidal, E.; Hermann, P.; Schmitz, M.; Abizanda, I.F.; Zerr, I.; et al. CSF neurogranin as a neuronal damage marker in CJD: A comparative study with AD. J. Neurol. Neurosurg. Psychiatry 2019, 129, 846–853. [Google Scholar] [CrossRef]
- Bereczki, E.; Bogstedt, A.; Höglund, K.; Tsitsi, P.; Brodin, L.; Ballard, C.; Svenningsson, P.; Aarsland, D. Synaptic proteins in CSF relate to Parkinson’s disease stage markers. Npj Parkinson’s Dis. 2017, 3, 7. [Google Scholar] [CrossRef]
- Waxman, E.A.; Giasson, B.I. Molecular mechanisms of $α$-synuclein neurodegeneration. Biochim. Biophys. Acta-Mol. Basis Dis. 2009, 1792, 616–624. [Google Scholar] [CrossRef] [PubMed]
- Goedert, M. Alpha-synuclein and neurodegenerative diseases. Nat. Rev. Neurosci. 2001, 2, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, P.; Lashuel, H.A. Alpha-synuclein as a Potential Biomarker for Parkinson’s Disease and Other Synucleinopathies: Gaps, Challenges, and Opportunities. Available online: https://infoscience.epfl.ch/record/292668 (accessed on 13 September 2022).
- Twohig, D.; Nielsen, H.M. α-synuclein in the pathophysiology of Alzheimer’s disease. Mol. Neurodegener. 2019, 14, 23. [Google Scholar] [CrossRef] [PubMed]
- Twohig, D.; Rodriguez-Vieitez, E.; Sando, S.B.; Berge, G.; Lauridsen, C.; Møller, I.; Grøntvedt, G.R.; Bråthen, G.; Patra, K.; Bu, G.; et al. The relevance of cerebrospinal fluid α-synuclein levels to sporadic and familial Alzheimer’s disease. Acta Neuropathol. Commun. 2018, 6, 130. [Google Scholar] [CrossRef] [PubMed]
- Coskuner-Weber, O.; Uversky, V.N. Insights into the molecular mechanisms of alzheimer’s and parkinson’s diseases with molecular simulations: Understanding the roles of artificial and pathological missense mutations in intrinsically disordered proteins related to pathology. Int. J. Mol. Sci. 2018, 19, 336. [Google Scholar] [CrossRef] [PubMed]
- Clinton, L.K.; Blurton-Jones, M.; Myczek, K.; Trojanowski, J.Q.; LaFerla, F.M. Synergistic interactions between Aβ, tau, and α-synuclein: Acceleration of neuropathology and cognitive decline. J. Neurosci. 2010, 30, 7281–7289. [Google Scholar] [CrossRef]
- Bassil, F.; Brown, H.J.; Pattabhiraman, S.; Iwasyk, J.E.; Maghames, C.M.; Meymand, E.S.; Cox, T.O.; Riddle, D.M.; Zhang, B.; Trojanowski, J.Q.; et al. Amyloid-Beta (Aβ) Plaques Promote Seeding and Spreading of Alpha-Synuclein and Tau in a Mouse Model of Lewy Body Disorders with Aβ Pathology. Neuron 2020, 105, 260–275. [Google Scholar] [CrossRef]
- Wang, H.; Stewart, T.; Toledo, J.B.; Ginghina, C.; Tang, L.; Atik, A.; Aro, P.; Shaw, L.M.; Trojanowski, J.Q.; Galasko, D.R.; et al. A longitudinal study of total and phosphorylated α-synuclein with other biomarkers in cerebrospinal fluid of Alzheimer’s disease and mild cognitive impairment. J. Alzheimer’s Dis. 2018, 61, 1541–1553. [Google Scholar] [CrossRef]
- Liu, W.; Lin, H.; He, X.; Chen, L.; Dai, Y.; Jia, W.; Xue, X.; Tao, J.; Chen, L. Neurogranin as a cognitive biomarker in cerebrospinal fluid and blood exosomes for Alzheimer’s disease and mild cognitive impairment. Transl. Psychiatry 2020, 10, 125. [Google Scholar] [CrossRef]
- Corder, E.H.; Saunders, A.M.; Strittmatter, W.J.; Schmechel, D.E.; Gaskell, P.C.; Small, G.; Roses, A.D.; Haines, J.L.; Pericak-Vance, M.A. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 1993, 261, 921–923. [Google Scholar] [CrossRef]
- Qian, J.; Betensky, R.A.; Hyman, B.T.; Serrano-Pozo, A. Association of APOE Genotype With Heterogeneity of Cognitive Decline Rate in Alzheimer Disease. Neurology 2021, 96, e2414–e2428. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.A.; Inman, C.E.; Wargel, Z.M.; Dube, U.; Freeberg, B.M.; Galluppi, A.; Haines, J.N.; Dhavale, D.D.; Miller, R.; Choudhury, F.A.; et al. APOE Genotype Regulates Pathology and Disease Progression in Synucleinopathy. Sci. Transl. Med. 2020, 12, eaay3069. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Gao, Y.; Therriault, J.; Luo, J.; Ba, M.; Zhang, H. The Effects of CSF Neurogranin and APOE ε4 on Cognition and Neuropathology in Mild Cognitive Impairment and Alzheimer’s Disease. Front. Aging Neurosci 2021, 13, 667899. [Google Scholar] [CrossRef]
- Mata, I.F.; Leverenz, J.B.; Weintraub, D.; Trojanowski, J.Q.; Hurtig, H.I.; Van Deerlin, V.M.; Ritz, B.; Rausch, R.; Rhodes, S.L.; Factor, S.A.; et al. APOE, MAPT, and SNCA genes and cognitive performance in Parkinson disease. JAMA Neurol. 2014, 71, 1405–1412. [Google Scholar] [CrossRef]
- Janelidze, S.; Hertze, J.; Zetterberg, H.; Landqvist Waldö, M.; Santillo, A.; Blennow, K.; Hansson, O. Cerebrospinal fluid neurogranin and YKL-40 as biomarkers of Alzheimer’s disease. Ann. Clin. Transl. Neurol. 2016, 3, 12–20. [Google Scholar] [CrossRef]
- Mucke, L.; Selkoe, D.J. Synaptic and Network Dysfunction. Cold Spring Harb. Perspect. Med. 2012, 2, a006338. [Google Scholar] [CrossRef] [PubMed]
- Sancesario, G.M.; Di Lazzaro, G.; Alwardat, M.; Biticchi, B.; Basile, V.; Salimei, C.; Colona, V.L.; Sinibaldi Salimei, P.; Bernardini, S.; Mercuri, N.B.; et al. Amyloid-β42/Neurogranin Ratio as a Potential Index for Cognitive Impairment in Parkinson’s Disease. J. Alzheimer’s Dis. 2020, 76, 1171–1178. [Google Scholar] [CrossRef]
- Perneczky, R.; Wagenpfeil, S.; Komossa, K.; Grimmer, T.; Diehl, J.; Kurz, A. Mapping scores onto stages: Mini-mental state examination and clinical dementia rating. Am. J. Geriatr. Psychiatry 2006, 14, 139–144. [Google Scholar] [CrossRef]
- Konings, S.C.; Torres-Garcia, L.; Martinsson, I.; Gouras, G.K. Astrocytic and Neuronal Apolipoprotein E Isoforms Differentially Affect Neuronal Excitability. Front. Neurosci. 2021, 15, 1190. [Google Scholar] [CrossRef]
- Kwon, E.H.; Tennagels, S.; Gold, R.; Gerwert, K.; Beyer, L.; Tönges, L. Update on CSF Biomarkers in Parkinson’s Disease. Biomolecules 2022, 12, 329. [Google Scholar] [CrossRef]
- Pa, J.; Aslanyan, V.; Casaletto, K.B.; Renter, M.A. Effects of Sex APOE4, and Lifestyle Activities on Cognitive Reserve in Older Adults. Neurology 2022, 99, e789–e798. [Google Scholar] [CrossRef] [PubMed]
- Pohlan, J.; Leidel, B.A.; Lindner, T. Neurogranin. In Biomarkers for Traumatic Brain Injury; Alan, H.B., Wu, W., Frank Peacock, W., Eds.; Academic Press: Cambridge, MA, USA, 2020; Chapter 15; pp. 211–219. ISBN 978-0-12-816346-7. [Google Scholar] [CrossRef]
- Lista, S.; Toschi, N.; Baldacci, F.; Zetterberg, H.; Blennow, K.; Kilimann, I.; Teipel, S.J.; Cavedo, E.; Dos Santos, A.M.; Epelbaum, S.; et al. Cerebrospinal fluid neurogranin as a biomarker of neurodegenerative diseases: A cross-sectional study. J. Alzheimer’s Dis. 2017, 59, 1327–1334. [Google Scholar] [CrossRef] [PubMed]
- Dulewicz, M.; Kulczynska-Przybik, A.; Klimkowicz-Mrowiec, A.; Pera, J.; Borawska, R.; Slowik, A.; Mroczko, B. Neurogranin: A novel biomarker of Alzheimer’s disease. Alzheimer’s Dement. 2021, 17, e051279. [Google Scholar] [CrossRef]
- Kester, M.I.; Teunissen, C.E.; Crimmins, D.L.; Herries, E.M.; Ladenson, J.H.; Scheltens, P.; Van Der Flier, W.M.; Morris, J.C.; Holtzman, D.M.; Fagan, A.M. Neurogranin as a cerebrospinal fluid biomarker for synaptic loss in symptomatic Alzheimer disease. JAMA Neurol. 2015, 72, 1275–1280. [Google Scholar] [CrossRef] [PubMed]
- Casaletto, K.B.; Elahi, F.M.; Bettcher, B.M.; Neuhaus, J.; Bendlin, B.B.; Asthana, S.; Johnson, S.C.; Yaffe, K.; Carlsson, C.; Blennow, K.; et al. Neurogranin, a synaptic protein, is associated with memory independent of Alzheimer biomarkers. Neurology 2017, 89, 1782–1788. [Google Scholar] [CrossRef]
- Simonsen, A.H.; Kuiperij, B.; El-Agnaf, O.M.A.; Engelborghs, S.; Herukka, S.K.; Parnetti, L.; Rektorova, I.; Vanmechelen, E.; Kapaki, E.; Verbeek, M.; et al. The utility of α-synuclein as biofluid marker in neurodegenerative diseases: A systematic review of the literature. Biomark Med. 2016, 10, 19–34. [Google Scholar] [CrossRef]
- Berg, D.; Postuma, R.B.; Bloem, B.; Chan, P.; Dubois, B.; Gasser, T.; Goetz, C.G.; Halliday, G.M.; Hardy, J.; Lang, A.E.; et al. Time to redefine PD? Introductory statement of the MDS Task Force on the definition of Parkinson’s disease. Mov. Disord. 2014, 29, 454–462. [Google Scholar] [CrossRef]
- Magni, E.; Binetti, G.; Bianchetti, A.; Rozzini, R.; Trabucchi, M. Mini-Mental State Examination: A normative study in Italian elderly population. Eur. J. Neurol. 1996, 3, 198–202. [Google Scholar] [CrossRef]
- Nobili, F.; Arbizu, J.; Bouwman, F.; Drzezga, A.; Agosta, F.; Nestor, P.; Walker, Z.; Boccardi, M. European Association of Nuclear Medicine and European Academy of Neurology recommendations for the use of brain 18 F-fluorodeoxyglucose positron emission tomography in neurodegenerative cognitive impairment and dementia: Delphi consensus. Eur. J. Neurol. 2018, 25, 1201–1217. [Google Scholar] [CrossRef]
- Del Campo, M.; Mollenhauer, B.; Bertolotto, A.; Engelborghs, S.; Hampel, H.; Simonsen, A.H.; Kapaki, E.; Kruse, N.; Le Bastard, N.; Lehmann, S.; et al. Recommendations to standardize preanalytical confounding factors in Alzheimers and Parkinsons disease cerebrospinal fluid biomarkers: An update. Biomark Med. 2012, 6, 419–430. [Google Scholar] [CrossRef]
- Robin, X.; Turck, N.; Hainard, A.; Tiberti, N.; Lisacek, F.; Sanchez, J.C.; Müller, M. pROC: An open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinform. 2011, 12, 77. [Google Scholar] [CrossRef] [PubMed]
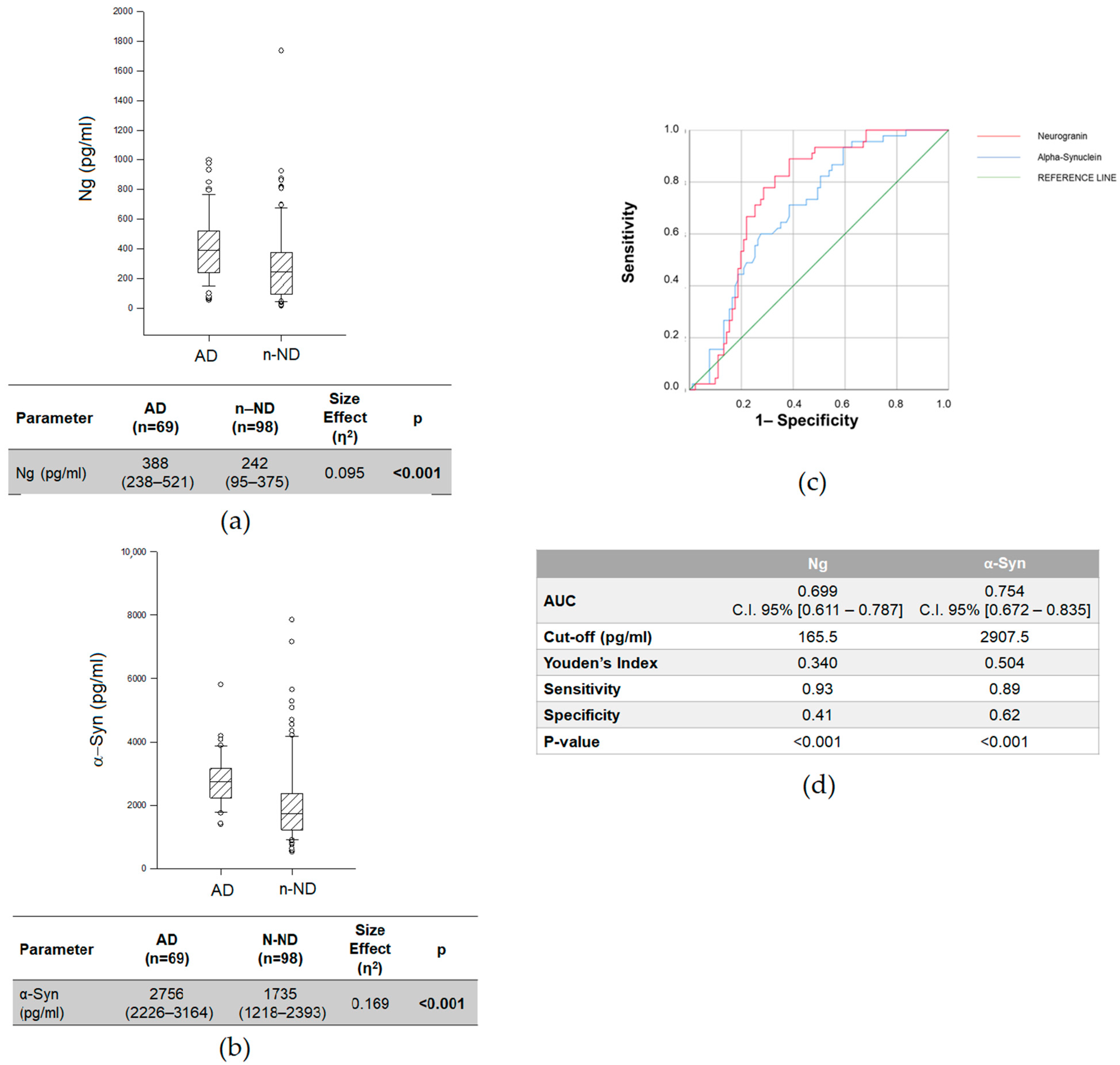
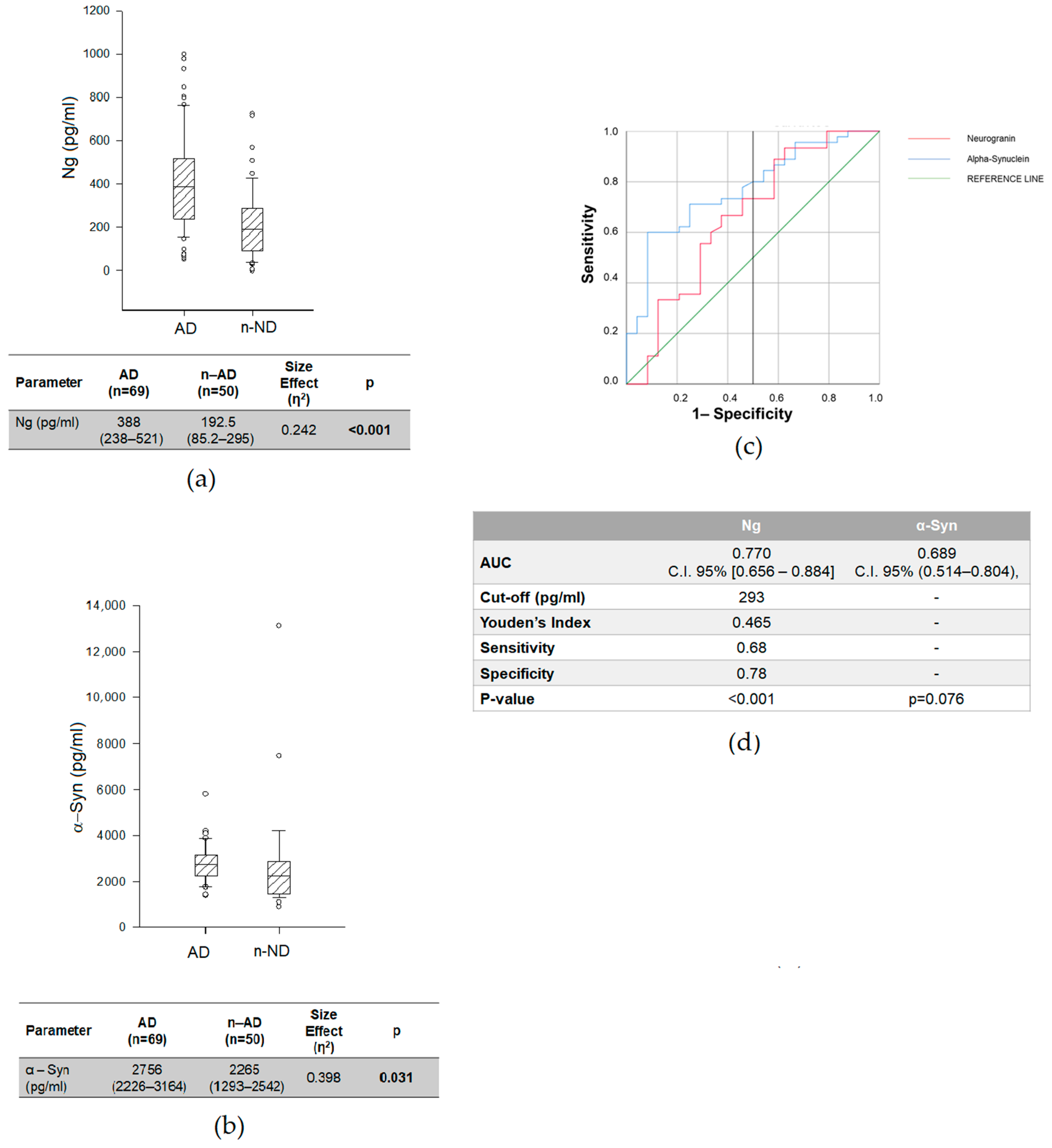
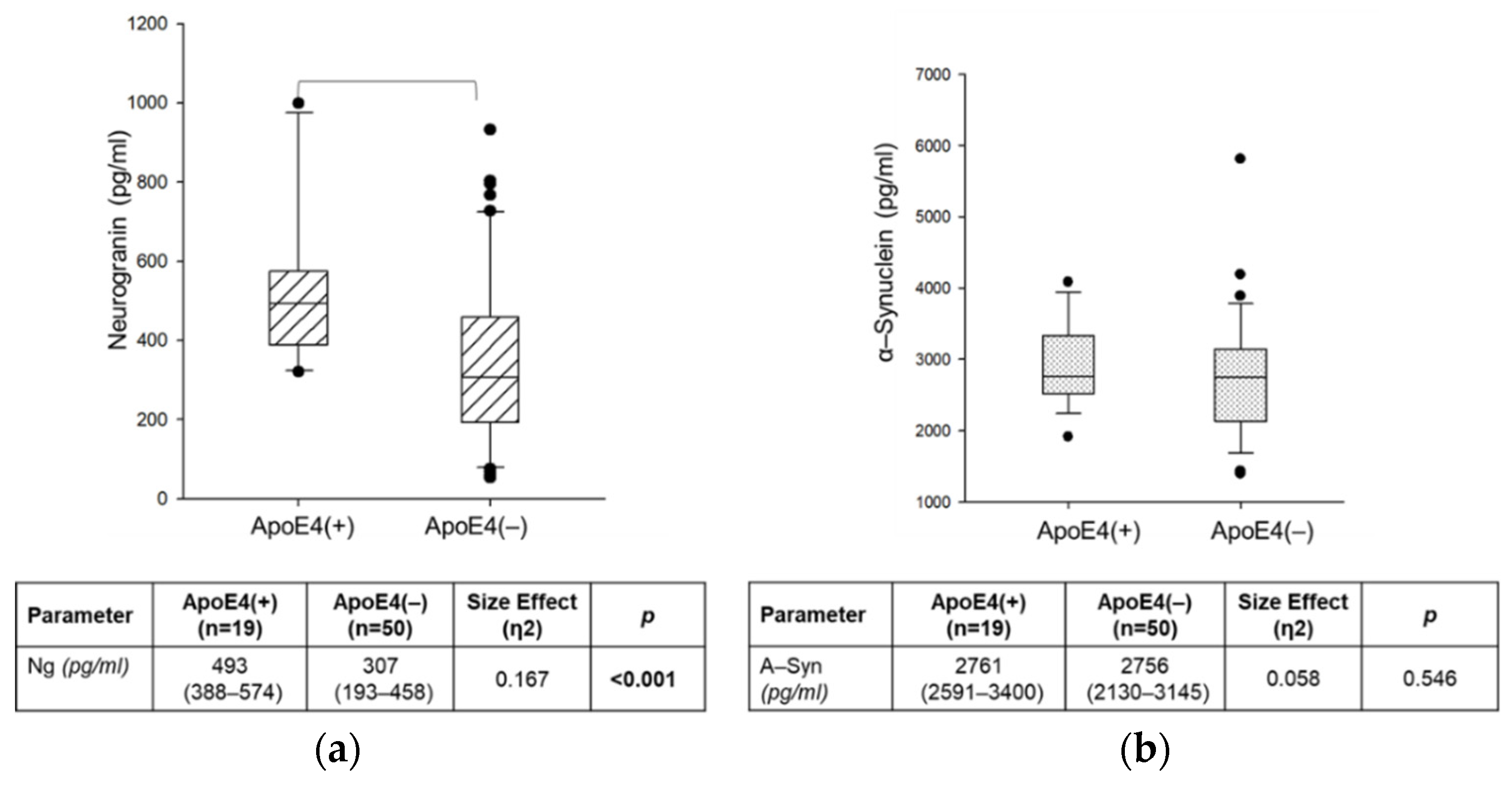
| Variables | AD (n = 69) | n-AD (n = 50) | n-ND (n = 98) | p |
|---|---|---|---|---|
| Demographic and clinical features | ||||
| Age at LP £ (years) | 72 (67.5–76) | 69 (59.5–73) | 63 (47–71) | <0.001A,**,*** |
| Age at onset (years) | 67 (62–71.5) | 63 (55–71) | 59 (44–68) | <0.001A,** |
| Gender (M/F) | 1.26 | 1.42 | 1.67 | 0.727 B |
| Education (years) | 8 (5–13) | 8 (5–8) | 8 (5–13) | 0.156 A |
| Diagnostic delay (years) | 4 (3–6) | 3 (2–5.5) | 2 (1–4) | <0.001 A,**,*** |
| MMSE scores (raw) | 23 (15–26) | 21 (17–28) | 29 (28–30) | 0.012 A,**, *** |
| CSF biochemical features | ||||
| Protein (mg/dL) | 39 (31.8–48.8) | 42.7 (31.1–56.4) | 45 (31–57) | 0.337 A |
| Glucose (mg/dL) | 59.3 (56–64) | 60 (53–67) | 62 (55–71) | 0.346 A |
| Cells (n/mmc) | 0.8 (0.6–2) | 0.8 (0.6–2) | 1.2 (0.8–2.4) | 0.058 A |
| AD (n = 69) | Non-AD (n = 50) | Size Effect (η2) | p | |
|---|---|---|---|---|
| Aβ 42/40 Ratio | 0.051 (0.040–0.079) | 0.098 (0.072–0.102) | 0.233 | <0.001 |
| Aβ 42/Neurogranin | 1.443 (0.822–2.861) | 4.170 (2.222–5.613) | 0.280 | <0.001 |
| Aβ 42/α-Synuclein | 0.231 (0.149–0.383) | 0.425 (0.298–0.480) | 0.131 | 0.001 |
| Variables | AUC | C.I. 95% | p | AUC Difference Versus Aβ42/Aβ40 (p-Value) |
|---|---|---|---|---|
| Aβ 42/40 Ratio | 0.802 | 0.672–0.933 | 0.002 | |
| Neurogranin | 0.768 | 0.682–0.853 | 0.004 | |
| A-Synuclein | 0.689 | 0.514–0.804 | 0.076 | |
| Aβ 42/Neurogranin | 0.814 | 0.684–0.944 | 0.001 | 0.005 (0.943) |
| Aβ 42/α-Synuclein | 0.710 | 0.572–0.848 | 0.004 | −0.066 (0.420) |
| Variables | MCI (n = 28) | Mild AD (n = 14) | Moderate AD (n = 27) | Size Effect (η2) | p |
|---|---|---|---|---|---|
| Aβ 42 (pg/mL) | 677 (495–971) | 599 (421–725) | 521 (413–674) | 0.041 | 0.093 |
| Aβ 42/40 Ratio | 0.060 (0.043–0.102) | 0.044 (0.036–0.059) | 0.046 (0.038–0.052) | 0.100 | 0.014 * |
| pTau (pg/mL) | 65.0 (32.4–88.0) | 79.2 (44.8–121.7) | 95.8 (65.1–154.6) | 0.080 | 0.026 * |
| tTau (pg/mL) | 550 (284–645) | 541 (412–810) | 678 (495–896) | 0.053 | 0.065 |
| Neurogranin (pg/mL) | 338 (235–492) | 417 (217–526) | 388 (260–526) | 0.000 | 0.852 |
| α-Synuclein (pg/mL) | 2754 (2135–3143) | 3082 (2266–3883) | 2556 (2377–3124) | 0.000 | 0.434 |
| Aβ 42/Neurogranin | 2.055 (1.015–3.639) | 1.221 (0.838–2.522) | 0.865 (0.400–1.578) | 0.126 | 0.006 * |
| Aβ 42/α-Synuclein | 0.313 (0.187–0.423) | 0.162 (0.144–0.280) | 0.202 (0.141–0.249) | 0.051 | 0.068 |
| Variables | Neurogranin | α-Synuclein | Aβ-42/Neurogranin | Aβ-42/α-Synuclein |
|---|---|---|---|---|
| Age at LP | 0.250; p = 0.002 | 0.280; p < 0.001 | −0.107; p = 0.393 | 0.397; p < 0.001 |
| Age at Onset | 0.187; p = 0.008 | 0.236; p = 0.003 | 0.063; p = 0.594 | 0.321; p < 0.001 |
| Education | −0.157; p = 0.157 | −0.113; p = 0.360 | 0.044; p = 0.723 | −0.043; p = 0.731 |
| MMSE | −0.215; p = 0.037 | −0.174; p = 0.170 | 0.427; p < 0.001 | 0.368; p = 0.003 |
| Model | Formula | AIC | BIC | R2 | Adjusted R2 | ΔR2 |
|---|---|---|---|---|---|---|
| Ng | ||||||
| Base | ~Age at LP + Gender + Education + MMSE | 263.919 | 270.209 | 0.511 | 0.417 | 0.004 |
| 1 | ~Base + Aβ42/40 | 259.499 | 267.048 | 0.618 | 0.522 | 0.028 |
| α-Syn | ||||||
| Base | ~Age at LP + Gender + Education + MMSE | 275.412 | 280.635 | 0.290 | 0.113 | 0.290 |
| 1 | ~Base + Aβ42/40 | 272.220 | 278.487 | 0.446 | 0.261 | 0.156 |
| Aβ 42/Ng | ||||||
| Base | ~Age at LP + Gender + Education + MMSE + AD-PET + ApoE4 | 17.856 | 26.663 | 0.620 | 0.500 | 0.620 |
| 1 | ~Base + Aβ42/40 | 4.280 | 14.344 | 0.791 | 0.710 | 0.171 |
| 2 | ~Base + Aβ42/40 + pTau | 2.128 | 13.451 | 0.822 | 0.738 | 0.031 |
| Aβ 42/α-Syn | ||||||
| Base | ~Age at LP + Gender + Education + MMSE + AD-PET | −209.986 | −204.011 | 0.494 | 0.313 | 0.494 |
| 1 | ~Base + Aβ42/40 | −219.376 | −212.406 | 0.714 | 0.582 | 0.220 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piccoli, T.; Blandino, V.; Maniscalco, L.; Matranga, D.; Graziano, F.; Guajana, F.; Agnello, L.; Lo Sasso, B.; Gambino, C.M.; Giglio, R.V.; et al. Biomarkers Related to Synaptic Dysfunction to Discriminate Alzheimer’s Disease from Other Neurological Disorders. Int. J. Mol. Sci. 2022, 23, 10831. https://doi.org/10.3390/ijms231810831
Piccoli T, Blandino V, Maniscalco L, Matranga D, Graziano F, Guajana F, Agnello L, Lo Sasso B, Gambino CM, Giglio RV, et al. Biomarkers Related to Synaptic Dysfunction to Discriminate Alzheimer’s Disease from Other Neurological Disorders. International Journal of Molecular Sciences. 2022; 23(18):10831. https://doi.org/10.3390/ijms231810831
Chicago/Turabian StylePiccoli, Tommaso, Valeria Blandino, Laura Maniscalco, Domenica Matranga, Fabiola Graziano, Fabrizio Guajana, Luisa Agnello, Bruna Lo Sasso, Caterina Maria Gambino, Rosaria Vincenza Giglio, and et al. 2022. "Biomarkers Related to Synaptic Dysfunction to Discriminate Alzheimer’s Disease from Other Neurological Disorders" International Journal of Molecular Sciences 23, no. 18: 10831. https://doi.org/10.3390/ijms231810831
APA StylePiccoli, T., Blandino, V., Maniscalco, L., Matranga, D., Graziano, F., Guajana, F., Agnello, L., Lo Sasso, B., Gambino, C. M., Giglio, R. V., La Bella, V., Ciaccio, M., & Colletti, T. (2022). Biomarkers Related to Synaptic Dysfunction to Discriminate Alzheimer’s Disease from Other Neurological Disorders. International Journal of Molecular Sciences, 23(18), 10831. https://doi.org/10.3390/ijms231810831













