Impact of Environmental Risk Factors on Mitochondrial Dysfunction, Neuroinflammation, Protein Misfolding, and Oxidative Stress in the Etiopathogenesis of Parkinson’s Disease
Abstract
1. Introduction
2. MPTP
3. Rotenone
4. Paraquat
5. DDT
6. Dieldrin
7. Manganese
8. Vanadium
9. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sveinbjornsdottir, S. The clinical symptoms of Parkinson’s disease. J. Neurochem. 2016, 139 (Suppl. S1), 318–324. [Google Scholar] [CrossRef] [PubMed]
- Schapira, A.H.V.; Chaudhuri, K.R.; Jenner, P. Non-Motor features of Parkinson disease. Nat. Rev. Neurosci. 2017, 18, 435–450. [Google Scholar] [CrossRef] [PubMed]
- Marras, C.; Beck, J.C.; Bower, J.H.; Roberts, E.; Ritz, B.; Ross, G.W.; Abbott, R.D.; Savica, R.; Van Den Eeden, S.K.; Willis, A.W.; et al. Prevalence of Parkinson’s disease across North America. NPJ Parkinson’s Dis. 2018, 4, 21. [Google Scholar] [CrossRef] [PubMed]
- Blauwendraat, C.; Nalls, M.A.; Singleton, A.B. The genetic architecture of Parkinson’s disease. Lancet Neurol. 2020, 19, 170–178. [Google Scholar] [CrossRef]
- Lunati, A.; Lesage, S.; Brice, A. The genetic landscape of Parkinson’s disease. Rev. Neurol. 2018, 174, 628–643. [Google Scholar] [CrossRef]
- Bellou, V.; Belbasis, L.; Tzoulaki, I.; Evangelou, E.; Ioannidis, J.P. Environmental risk factors and Parkinson’s disease: An umbrella review of meta-analyses. Parkinsonism Relat Disord 2016, 23, 1–9. [Google Scholar] [CrossRef]
- Dick, F.D.; De Palma, G.; Ahmadi, A.; Scott, N.W.; Prescott, G.J.; Bennett, J.; Semple, S.; Dick, S.; Counsell, C.; Mozzoni, P.; et al. Environmental risk factors for Parkinson’s disease and parkinsonism: The Geoparkinson study. Occup. Environ. Med. 2007, 64, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Pezzoli, G.; Cereda, E. Exposure to pesticides or solvents and risk of Parkinson disease. Neurology 2013, 80, 2035–2041. [Google Scholar] [CrossRef]
- Priyadarshi, A.; Khuder, S.A.; Schaub, E.A.; Priyadarshi, S.S. Environmental risk factors and Parkinson’s disease: A metaanalysis. Environ. Res. 2001, 86, 122–127. [Google Scholar] [CrossRef]
- Breckenridge, C.B.; Berry, C.; Chang, E.T.; Sielken, R.L., Jr.; Mandel, J.S. Association between Parkinson’s Disease and Cigarette Smoking, Rural Living, Well-Water Consumption, Farming and Pesticide Use: Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0151841. [Google Scholar] [CrossRef]
- Silver, M.R.; Racette, B.A.; Dube, U.; Faust, I.M.; Nielsen, S.S. Well Water and Parkinson’s Disease in Medicare Beneficiaries: A Nationwide Case-Control Study. J. Park. Dis. 2020, 10, 693–705. [Google Scholar] [CrossRef] [PubMed]
- Dorsey, E.R.; Elbaz, A.; Nichols, E.; Abbasi, N.; Abd-Allah, F.; Abdelalim, A.; Adsuar, J.C.; Ansha, M.G.; Brayne, C.; Choi, J.Y.; et al. Global, regional, and national burden of Parkinson’s disease, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018, 17, 939–953. [Google Scholar] [CrossRef]
- Langston, J.W. The MPTP Story. J. Parkinson’s Dis. 2017, 7, S11–S19. [Google Scholar] [CrossRef] [PubMed]
- Tanner, C.M.; Kamel, F.; Ross, G.W.; Hoppin, J.A.; Goldman, S.M.; Korell, M.; Marras, C.; Bhudhikanok, G.S.; Kasten, M.; Chade, A.R.; et al. Rotenone, paraquat, and Parkinson’s disease. Environ. Health Perspect. 2011, 119, 866–872. [Google Scholar] [CrossRef] [PubMed]
- Giguère, N.; Burke Nanni, S.; Trudeau, L.E. On Cell Loss and Selective Vulnerability of Neuronal Populations in Parkinson’s Disease. Front. Neurol. 2018, 9, 455. [Google Scholar] [CrossRef] [PubMed]
- Minakaki, G.; Krainc, D.; Burbulla, L.F. The Convergence of Alpha-Synuclein, Mitochondrial, and Lysosomal Pathways in Vulnerability of Midbrain Dopaminergic Neurons in Parkinson’s Disease. Front. Cell Dev. Biol. 2020, 8, 580634. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Scarselli, M.; Fasciani, I.; Maggio, R.; Giorgi, F. Dichlorodiphenyltrichloroethane (DDT) induced extracellular vesicle formation: A potential role in organochlorine increased risk of Parkinson’s disease. Acta Neurobiol. Exp. 2017, 77, 113–117. [Google Scholar] [CrossRef]
- Hatcher, J.M.; Delea, K.C.; Richardson, J.R.; Pennell, K.D.; Miller, G.W. Disruption of dopamine transport by DDT and its metabolites. NeuroToxicology 2008, 29, 682–690. [Google Scholar] [CrossRef]
- Song, C.; Kanthasamy, A.; Anantharam, V.; Sun, F.; Kanthasamy, A.G. Environmental Neurotoxic Pesticide Increases Histone Acetylation to Promote Apoptosis in Dopaminergic Neuronal Cells: Relevance to Epigenetic Mechanisms of Neurodegeneration. Mol. Pharmacol. 2010, 77, 621–632. [Google Scholar] [CrossRef]
- Saminathan, H.; Asaithambi, A.; Anantharam, V.; Kanthasamy, A.G.; Kanthasamy, A. Environmental neurotoxic pesticide dieldrin activates a non receptor tyrosine kinase to promote pkcδ-mediated dopaminergic apoptosis in a dopaminergic neuronal cell model. NeuroToxicology 2011, 32, 567–577. [Google Scholar] [CrossRef]
- Yan, D.; Zhang, Y.; Liu, L.; Shi, N.; Yan, H. Pesticide exposure and risk of Parkinson’s disease: Dose-response meta-analysis of observational studies. Regul. Toxicol. Pharmacol. 2018, 96, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, T.-H.; Chen, J.-J.J.; Chen, L.-H.; Chiang, P.-T.; Lee, H.-Y. Time-Course gait analysis of hemiparkinsonian rats following 6-hydroxydopamine lesion. Behav. Brain Res. 2011, 222, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Rokad, D.; Malovic, E.; Luo, J.; Harischandra, D.S.; Jin, H.; Anantharam, V.; Huang, X.; Lewis, M.; Kanthasamy, A.; et al. Manganese activates NLRP3 inflammasome signaling and propagates exosomal release of ASC in microglial cells. Sci. Signal. 2019, 12, 563. [Google Scholar] [CrossRef] [PubMed]
- Ngwa, H.A.; Ay, M.; Jin, H.; Anantharam, V.; Kanthasamy, A.; Kanthasamy, A.G. Neurotoxicity of Vanadium. Adv. Neurobiol. 2017, 18, 287–301. [Google Scholar] [CrossRef] [PubMed]
- Packer, M.; Miesel, R.; Murphy, M. Exposure to the parkinsonian neurotoxin 1-methyl-4-phenylpyridinium (MPP+) and nitric oxide simultaneously causes cyclosporin A-sensitive mitochondrial calcium efflux and depolarisation. Biochem. Pharmacol. 1996, 51, 267–273. [Google Scholar] [CrossRef]
- Sun, Y.; Sukumaran, P.; Selvaraj, S.; Cilz, N.I.; Schaar, A.; Lei, S.; Singh, B.B. TRPM2 Promotes Neurotoxin MPP+/MPTP-Induced Cell Death. Mol. Neurobiol. 2016, 55, 409–420. [Google Scholar] [CrossRef]
- Zaidi, A.; Fernandes, D.; Bean, J.L.; Michaelis, M.L. Effects of paraquat-induced oxidative stress on the neuronal plasma membrane Ca2+-ATPase. Free Radic. Biol. Med. 2009, 47, 1507–1514. [Google Scholar] [CrossRef]
- Singh, Y.; El-Hadidi, M.; Admard, J.; Wassouf, Z.; Schulze-Hentrich, J.; Kohlhofer, U.; Quintanilla-Martinez, L.; Huson, D.; Riess, O.; Casadei, N. Enriched Environmental Conditions Modify the Gut Microbiome Composition and Fecal Markers of Inflammation in Parkinson’s Disease. Front. Neurosci. 2019, 13, 1032. [Google Scholar] [CrossRef]
- Ghaisas, S.; Maher, J.; Kanthasamy, A. Gut microbiome in health and disease: Linking the microbiome–gut–brain axis and environmental factors in the pathogenesis of systemic and neurodegenerative diseases. Pharmacol. Ther. 2015, 158, 52–62. [Google Scholar] [CrossRef]
- Delic, V.; Beck, K.D.; Pang, K.C.H.; Citron, B.A. Biological links between traumatic brain injury and Parkinson’s disease. Acta Neuropathol. Commun. 2020, 8, 45. [Google Scholar] [CrossRef]
- Sarkar, S.; Malovic, E.; Harishchandra, D.S.; Ghaisas, S.; Panicker, N.; Charli, A.; Palanisamy, B.N.; Rokad, D.; Jin, H.; Anantharam, V.; et al. Mitochondrial impairment in microglia amplifies NLRP3 inflammasome proinflammatory signaling in cell culture and animal models of Parkinson’s disease. Npj Park. Dis. 2017, 3, 30. [Google Scholar] [CrossRef] [PubMed]
- Gordon, R.; Neal, M.L.; Luo, J.; Langley, M.; Harischandra, D.S.; Panicker, N.; Charli, A.; Jin, H.; Anantharam, V.; Woodruff, T.M.; et al. Prokineticin-2 upregulation during neuronal injury mediates a compensatory protective response against dopaminergic neuronal degeneration. Nat. Commun. 2016, 7, 12932. [Google Scholar] [CrossRef] [PubMed]
- Harischandra, D.S.; Rokad, D.; Neal, M.L.; Ghaisas, S.; Manne, S.; Sarkar, S.; Panicker, N.; Zenitsky, G.; Jin, H.; Lewis, M.; et al. Manganese promotes the aggregation and prion-like cell-to-cell exosomal transmission of α-synuclein. Sci. Signal. 2019, 12, 572. [Google Scholar] [CrossRef] [PubMed]
- Langley, M.; Ghaisas, S.; Ay, M.; Luo, J.; Palanisamy, B.N.; Jin, H.; Anantharam, V.; Kanthasamy, A.; Kanthasamy, A.G. Manganese exposure exacerbates progressive motor deficits and neurodegeneration in the MitoPark mouse model of Parkinson’s disease: Relevance to gene and environment interactions in metal neurotoxicity. NeuroToxicology 2017, 64, 240–255. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Ghebremedhin, E.; Rüb, U.; Bratzke, H.; Del Tredici, K. Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res. 2004, 318, 121–134. [Google Scholar] [CrossRef]
- Schildknecht, S.; Di Monte, D.A.; Pape, R.; Tieu, K.; Leist, M. Tipping Points and Endogenous Determinants of Nigrostriatal Degeneration by MPTP. Trends Pharmacol. Sci. 2017, 38, 541–555. [Google Scholar] [CrossRef]
- Kotake, Y.; Ohta, S. MPP+ analogs acting on mitochondria and inducing neuro-degeneration. Curr. Med. Chem. 2003, 10, 2507–2516. [Google Scholar] [CrossRef]
- Schober, A. Classic toxin-induced animal models of Parkinson’s disease: 6-OHDA and MPTP. Cell Tissue Res. 2004, 318, 215–224. [Google Scholar] [CrossRef]
- Przedborski, S.; Jackson-Lewis, V.; Naini, A.B.; Jakowec, M.; Petzinger, G.; Miller, R.; Akram, M. The parkinsonian toxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP): A technical review of its utility and safety. J. Neurochem. 2001, 76, 1265–1274. [Google Scholar] [CrossRef]
- McGeer, P.L.; McGeer, E.G. Glial reactions in Parkinson’s disease. Mov. Disord. 2008, 23, 474–483. [Google Scholar] [CrossRef]
- Han, N.-R.; Kim, Y.-K.; Ahn, S.; Hwang, T.-Y.; Lee, H.; Park, H.-J. A Comprehensive Phenotype of Non-motor Impairments and Distribution of Alpha-Synuclein Deposition in Parkinsonism-Induced Mice by a Combination Injection of MPTP and Probenecid. Front. Aging Neurosci. 2021, 12, 599045. [Google Scholar] [CrossRef] [PubMed]
- Carbone, D.L.; Popichak, K.A.; Moreno, J.A.; Safe, S.; Tjalkens, R.B. Suppression of 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine-Induced Nitric-Oxide Synthase 2 Expression in Astrocytes by a Novel Diindolylmethane Analog Protects Striatal Neurons against Apoptosis. Mol. Pharmacol. 2008, 75, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Saporito, M.; Brown, E.M.; Miller, M.S.; Carswell, S. CEP-1347/KT-7515, an inhibitor of c-jun N-terminal kinase activation, attenuates the 1-methyl-4-phenyl tetrahydropyridine-mediated loss of nigrostriatal dopaminergic neurons In vivo. J. Pharmacol. Exp. Ther. 1999, 288, 421–427. [Google Scholar] [PubMed]
- Vila, M.; Jackson-Lewis, V.; Vukosavic, S.; Djaldetti, R.; Liberatore, G.; Offen, D.; Korsmeyer, S.J.; Przedborski, S. Bax ablation prevents dopaminergic neurodegeneration in the 1-methyl- 4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson’s disease. Proc. Natl. Acad. Sci. USA 2001, 98, 2837–2842. [Google Scholar] [CrossRef]
- Viswanath, V.; Wu, Y.; Boonplueang, R.; Chen, S.; Stevenson, F.F.; Yantiri, F.; Yang, L.; Beal, M.F.; Andersen, J.K. Caspase-9 Activation Results in Downstream Caspase-8 Activation and Bid Cleavage in 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine-Induced Parkinson’s Disease. J. Neurosci. 2001, 21, 9519–9528. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.S.; Heng, Y.; Mou, Z.; Huang, J.Y.; Yuan, Y.H.; Chen, N.H. Reassessment of subacute MPTP-treated mice as animal model of Parkinson’s disease. Acta Pharmacol. Sin. 2017, 38, 1317–1328. [Google Scholar] [CrossRef] [PubMed]
- Chuang, J.-I.; Pan, I.-L.; Hsieh, C.-Y.; Huang, C.-Y.; Chen, P.-C.; Shin, J.W. Melatonin prevents the dynamin-related protein 1-dependent mitochondrial fission and oxidative insult in the cortical neurons after 1-methyl-4-phenylpyridinium treatment. J. Pineal Res. 2016, 61, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Su, B.; Liu, W.; He, X.; Gao, Y.; Castellani, R.J.; Perry, G.; Smith, M.A.; Zhu, X. DLP1-dependent mitochondrial fragmentation mediates 1-methyl-4-phenylpyridinium toxicity in neurons: Implications for Parkinson’s disease. Aging Cell 2011, 10, 807–823. [Google Scholar] [CrossRef]
- Rao, S.P.; Sharma, N.; Kalivendi, S.V. Embelin averts MPTP-induced dysfunction in mitochondrial bioenergetics and biogenesis via activation of SIRT1. Biochim. Biophys. Acta 2020, 1861, 148157. [Google Scholar] [CrossRef]
- Palmer, G.; Horgan, D.J.; Tisdale, H.O.; Singer, T.P.; Beinert, H. Studies on the respiratory chain-linked reduced nicotinamide adenine dinucleotide dehydrogenase. XIV. Location of the sites of inhibition of rotenone, barbiturates, and piericidin by means of electron paramagnetic resonance spectroscopy. J. Biol. Chem. 1968, 243, 844–847. [Google Scholar] [CrossRef]
- Li, N.; Ragheb, K.; Lawler, G.; Sturgis, J.; Rajwa, B.; Melendez, J.A.; Robinson, J.P. Mitochondrial complex I inhibitor rotenone induces apoptosis through enhancing mitochondrial reactive oxygen species production. J. Biol. Chem. 2003, 278, 8516–8525. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-W.; Lin, K.-M.; Hung, T.-Y.; Chuang, Y.-C.; Wu, S.-N. Multiple Actions of Rotenone, an Inhibitor of Mitochondrial Respiratory Chain, on Ionic Currents and Miniature End-Plate Potential in Mouse Hippocampal (mHippoE-14) Neurons. Cell. Physiol. Biochem. 2018, 47, 330–343. [Google Scholar] [CrossRef] [PubMed]
- Passmore, J.B.; Pinho, S.; Gomez-Lazaro, M.; Schrader, M. The respiratory chain inhibitor rotenone affects peroxisomal dynamics via its microtubule-destabilising activity. Histochem. Cell Biol. 2017, 148, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.E.; Bobrovskaya, L. An update on the rotenone models of Parkinson’s disease: Their ability to reproduce the features of clinical disease and model gene–environment interactions. NeuroToxicology 2015, 46, 101–116. [Google Scholar] [CrossRef] [PubMed]
- Betarbet, R.; Sherer, T.B.; MacKenzie, G.; Garcia-Osuna, M.; Panov, A.V.; Greenamyre, J.T. Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat. Neurosci. 2000, 3, 1301–1306. [Google Scholar] [CrossRef] [PubMed]
- Wrangel, C.V.; Schwabe, K.; John, N.; Krauss, J.K.; Alam, M. The rotenone-induced rat model of Parkinson’s disease: Behavioral and electrophysiological findings. Behav. Brain Res. 2015, 279, 52–61. [Google Scholar] [CrossRef]
- Zhang, Z.N.; Zhang, J.S.; Xiang, J.; Yu, Z.H.; Zhang, W.; Cai, M.; Li, X.T.; Wu, T.; Li, W.W.; Cai, D.F. Subcutaneous rotenone rat model of Parkinson’s disease: Dose exploration study. Brain Res. 2017, 1655, 104–113. [Google Scholar] [CrossRef]
- Cannon, J.R.; Tapias, V.; Na, H.M.; Honick, A.S.; Drolet, R.E.; Greenamyre, J.T. A highly reproducible rotenone model of Parkinson’s disease. Neurobiol. Dis. 2009, 34, 279–290. [Google Scholar] [CrossRef]
- Carriere, C.H.; Kang, N.H.; Niles, L.P. Chronic low-dose melatonin treatment maintains nigrostriatal integrity in an intrastriatal rotenone model of Parkinson’s disease. Brain Res. 2016, 1633, 115–125. [Google Scholar] [CrossRef]
- Chu, C.T.; Ji, J.; Dagda, R.K.; Jiang, J.F.; Tyurina, Y.Y.; Kapralov, A.A.; Tyurin, V.A.; Yanamala, N.; Shrivastava, I.H.; Mohammadyani, D.; et al. Cardiolipin externalization to the outer mitochondrial membrane acts as an elimination signal for mitophagy in neuronal cells. Nat. Cell Biol. 2013, 15, 1197–1205. [Google Scholar] [CrossRef]
- Chu, C.T.; Bayır, H.; Kagan, V.E. LC3 binds externalized cardiolipin on injured mitochondria to signal mitophagy in neurons: Implications for Parkinson disease. Autophagy 2014, 10, 376–378. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Liang, Y.; Jing, X.; Lin, D.; Chen, Y.; Zhou, T.; Peng, S.; Zheng, D.; Zeng, Z.; Lei, M.; et al. Rifampicin Prevents SH-SY5Y Cells from Rotenone-Induced Apoptosis via the PI3K/Akt/GSK-3β/CREB Signaling Pathway. Neurochem. Res. 2018, 43, 86–893. [Google Scholar] [CrossRef] [PubMed]
- Rokad, D.; Ghaisas, S.; Harischandra, D.; Jin, H.; Anantharam, V.; Kanthasamy, A.; Kanthasamy, A.G. Role of neurotoxicants and traumatic brain injury in α-synuclein protein misfolding and aggregation. Brain Res. Bull. 2016, 133, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, M.; Huh, Y.-J.; Lee, Y.-I. The Impairments of α-Synuclein and Mechanistic Target of Rapamycin in Rotenone-Induced SH-SY5Y Cells and Mice Model of Parkinson’s Disease. Front. Neurosci. 2019, 13, 1028. [Google Scholar] [CrossRef]
- Angeline, M.S.; Chaterjee, P.; Anand, K.; Ambasta, R.K.; Kumar, P. Rotenone-Induced parkinsonism elicits behavioral impairments and differential expression of parkin, heat shock proteins and caspases in the rat. Neuroscience 2012, 220, 291–301. [Google Scholar] [CrossRef]
- Gao, H.M.; Liu, B.; Hong, J.S. Critical role for microglial NADPH oxidase in rotenone-induced degeneration of dopaminergic neurons. J. Neurosci. Off. J. Soc. Neurosci. 2003, 23, 6181–6187. [Google Scholar] [CrossRef]
- Drechsel, D.A.; Patel, M. Role of reactive oxygen species in the neurotoxicity of environmental agents implicated in Parkinson’s disease. Free. Radic. Biol. Med. 2008, 44, 1873–1886. [Google Scholar] [CrossRef]
- Kanthasamy, A.; Jin, H.; Charli, A.; Vellareddy, A.; Kanthasamy, A. Environmental neurotoxicant-induced dopaminergic neurodegeneration: A potential link to impaired neuroinflammatory mechanisms. Pharmacol. Ther. 2019, 197, 61–82. [Google Scholar] [CrossRef]
- Lawana, V.; Singh, N.; Sarkar, S.; Charli, A.; Jin, H.; Anantharam, V.; Kanthasamy, A.G.; Kanthasamy, A. Involvement of c-Abl Kinase in Microglial Activation of NLRP3 Inflammasome and Impairment in Autolysosomal System. J. Neuroimmune Pharmacol. Off. J. Soc. NeuroImmune Pharmacol. 2017, 12, 624–660. [Google Scholar] [CrossRef]
- Liu, C.; Ye, Y.; Zhou, Q.; Zhang, R.; Zhang, H.; Liu, W.; Xu, C.; Liu, L.; Huang, S.; Chen, L. Crosstalk between Ca2+ signaling and mitochondrial H2O2 is required for rotenone inhibition of mTOR signaling pathway leading to neuronal apoptosis. Oncotarget 2016, 7, 7534–7549. [Google Scholar] [CrossRef]
- Peng, K.; Tao, Y.; Zhang, J.; Wang, J.; Ye, F.; Dan, G.; Zhao, Y.; Cai, Y.; Zhao, J.; Wu, Q.; et al. Resveratrol Regulates Mitochondrial Biogenesis and Fission/Fusion to Attenuate Rotenone-Induced Neurotoxicity. Oxidative Med. Cell. Longev. 2016, 2016, 6705621. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.; Yang, L.; Wang, J.; Ye, F.; Dan, G.; Zhao, Y.; Cai, Y.; Cui, Z.; Ao, L.; Liu, J.; et al. The Interaction of Mitochondrial Biogenesis and Fission/Fusion Mediated by PGC-1α Regulates Rotenone-Induced Dopaminergic Neurotoxicity. Mol. Neurobiol. 2016, 54, 3783–3797. [Google Scholar] [CrossRef] [PubMed]
- Castello, P.R.; Drechsel, D.A.; Patel, M. Mitochondria Are a Major Source of Paraquat-induced Reactive Oxygen Species Production in the Brain. J. Biol. Chem. 2007, 282, 14186–14193. [Google Scholar] [CrossRef] [PubMed]
- Rappold, P.M.; Cui, M.; Chesser, A.S.; Tibbett, J.; Grima, J.C.; Duan, L.; Sen, N.; Javitch, J.A.; Tieu, K. Paraquat neurotoxicity is mediated by the dopamine transporter and organic cation transporter-3. Proc. Natl. Acad. Sci. USA 2011, 108, 20766–20771. [Google Scholar] [CrossRef]
- McCarthy, S.; Somayajulu, M.; Sikorska, M.; Borowy-Borowski, H.; Pandey, S. Paraquat induces oxidative stress and neuronal cell death; neuroprotection by water-soluble Coenzyme Q10. Toxicol. Appl. Pharmacol. 2004, 201, 21–31. [Google Scholar] [CrossRef]
- Somayajulu-Niţu, M.; Sandhu, J.K.; Cohen, J.; Sikorska, M.; Sridhar, T.; Matei, A.; Borowy-Borowski, H.; Pandey, S. Paraquat induces oxidative stress, neuronal loss in substantia nigra region and Parkinsonism in adult rats: Neuroprotection and amelioration of symptoms by water-soluble formulation of Coenzyme Q10. BMC Neurosci. 2009, 10, 88. [Google Scholar] [CrossRef]
- Song, C.; Kanthasamy, A.; Jin, H.; Anantharam, V. Paraquat induces epigenetic changes by promoting histone acetylation in cell culture models of dopaminergic degeneration. NeuroToxicology 2011, 32, 586–595. [Google Scholar] [CrossRef]
- See, W.Z.C.; Naidu, R.; Tang, K.S. Cellular and Molecular Events Leading to Paraquat-Induced Apoptosis: Mechanistic Insights into Parkinson’s Disease Pathophysiology. Mol. Neurobiol. 2022, 59, 3353–3369. [Google Scholar] [CrossRef]
- Manning-Bog, A.B.; McCormack, A.L.; Li, J.; Uversky, V.N.; Fink, A.L.; Di Monte, D.A. The herbicide paraquat causes up-regulation and aggregation of alpha-synuclein in mice: Paraquat and alpha-synuclein. J. Biol. Chem. 2002, 277, 1641–1644. [Google Scholar] [CrossRef]
- Harada, T.; Takeda, M.; Kojima, S.; Tomiyama, N. Toxicity and Carcinogenicity of Dichlorodiphenyltrichloroethane (DDT). Toxicol. Res. 2016, 32, 21–33. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, C.; Zhang, C.; Wen, Y.; Liu, W. Enantioselective Cytotoxicity Profile of o,p’-DDT in PC 12 Cells. PLoS ONE 2012, 7, e43823. [Google Scholar] [CrossRef]
- Kajta, M.; Litwa, E.; Rzemieniec, J.; Wnuk, A.; Lason, W.; Zelek-Molik, A.; Nalepa, I.; Grzegorzewska-Hiczwa, M.; Tokarski, K.; Golas, A.; et al. Isomer-nonspecific action of dichlorodiphenyltrichloroethane on aryl hydrocarbon receptor and G-protein-coupled receptor 30 intracellular signaling in apoptotic neuronal cells. Mol. Cell. Endocrinol. 2014, 392, 90–105. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, F.M.; Madeira, V.M.; Moreno, A.J. Interactions of 2,2-bis(p-chlorophenyl)-1,1-dichloroethylene with Mitochondrial Oxidative Phosphorylation. Biochem. Pharmacol. 1997, 53, 299–308. [Google Scholar] [CrossRef]
- Moreno, A.J.; Madeira, V.M. Mitochondrial bioenergetics as affected by DDT. Biochim. Biophys. Acta 1991, 1060, 166–174. [Google Scholar] [CrossRef]
- Kanthasamy, A.G.; Kitazawa, M.; Kanthasamy, A.; Anantharam, V. Dieldrin-Induced Neurotoxicity: Relevance to Parkinson’s Disease Pathogenesis. NeuroToxicology 2005, 26, 701–719. [Google Scholar] [CrossRef] [PubMed]
- American Chemical Society Pesticide Exposure Could Increase Risk of Early Onset of Parkinson’s Disease. Available online: https://www.sciencedaily.com/releases/2006/09/060914194700.htm (accessed on 15 September 2006).
- Bergen, W.G. The In Vitro Effect of Dieldrin on Respiration of Rat Liver Mitochondria. Exp. Biol. Med. 1971, 136, 732–735. [Google Scholar] [CrossRef]
- Hatcher, J.M.; Richardson, J.; Guillot, T.S.; McCormack, A.L.; Di Monte, D.; Jones, D.P.; Pennell, K.; Miller, G.W. Dieldrin exposure induces oxidative damage in the mouse nigrostriatal dopamine system. Exp. Neurol. 2007, 204, 619–630. [Google Scholar] [CrossRef]
- Kanthasamy, A.G.; Kitazawa, M.; Yang, Y.; Anantharam, V.; Kanthasamy, A. Environmental neurotoxin dieldrin induces apoptosis via caspase-3-dependent proteolytic activation of protein kinase C delta (PKCdelta): Implications for neurodegeneration in Parkinson’s disease. Mol. Brain 2008, 1, 12. [Google Scholar] [CrossRef]
- Sun, F.; Anantharam, V.; Latchoumycandane, C.; Kanthasamy, A.; Kanthasamy, A. Dieldrin Induces Ubiquitin-Proteasome Dysfunction in α-Synuclein Overexpressing Dopaminergic Neuronal Cells and Enhances Susceptibility to Apoptotic Cell Death. J. Pharmacol. Exp. Ther. 2005, 315, 69–79. [Google Scholar] [CrossRef]
- Harischandra, D.S.; Ghaisas, S.; Zenitsky, G.; Jin, H.; Kanthasamy, A.; Anantharam, V.; Kanthasamy, A.G. Manganese-Induced Neurotoxicity: New Insights Into the Triad of Protein Misfolding, Mitochondrial Impairment, and Neuroinflammation. Front. Neurosci. 2019, 13, 654. [Google Scholar] [CrossRef]
- Fitsanakis, V.A.; Au, C.; Erikson, K.M.; Aschner, M. The effects of manganese on glutamate, dopamine and gamma-aminobutyric acid regulation. Neurochem. Int. 2006, 48, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Kwakye, G.F.; Paoliello, M.M.; Mukhopadhyay, S.; Bowman, A.B.; Aschner, M. Manganese-Induced Parkinsonism and Parkinson’s Disease: Shared and Distinguishable Features. Int. J. Environ. Res. Public Health 2015, 12, 7519–7540. [Google Scholar] [CrossRef]
- Gonzalez-Cuyar, L.F.; Nelson, G.; Criswell, S.R.; Ho, P.; Lonzanida, J.A.; Checkoway, H.; Seixas, N.; Gelman, B.B.; Evanoff, B.A.; Murray, J.; et al. Quantitative neuropathology associated with chronic manganese exposure in South African mine workers. NeuroToxicology 2013, 45, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Antunes, M.B.; Bowler, R.; Doty, R.L. San Francisco/Oakland Bay Bridge Welder Study: Olfactory function. Neurology 2007, 69, 1278–1284. [Google Scholar] [CrossRef] [PubMed]
- Bowler, R.M.; Roels, H.A.; Nakagawa, S.; Drezgic, M.; Diamond, E.; Park, R.; Koller, W.; Mergler, D.; Bouchard, M.; Smith, D.; et al. Dose-effect relationships between manganese exposure and neurological, neuropsychological and pulmonary function in confined space bridge welders. Occup. Environ. Med. 2007, 64, 167–177. [Google Scholar] [CrossRef]
- Bowler, R.M.; Gocheva, V.; Harris, M.; Ngo, L.; Abdelouahab, N.; Wilkinson, J.; Doty, R.L.; Park, R.; Roels, H.A. Prospective study on neurotoxic effects in manganese-exposed bridge construction welders. NeuroToxicology 2011, 32, 596–605. [Google Scholar] [CrossRef]
- Stagg, C.J.; Bestmann, S.; Constantinescu, A.O.; Moreno, L.M.; Allman, C.; Mekle, R.; Woolrich, M.; Near, J.; Johansen-Berg, H.; Rothwell, J. Relationship between physiological measures of excitability and levels of glutamate and GABA in the human motor cortex. J. Physiol. 2011, 589, 5845–5855. [Google Scholar] [CrossRef]
- Guarneros, M.; Ortiz-Romo, N.; Alcaraz-Zubeldia, M.; Drucker-Colín, R.; Hudson, R. Nonoccupational Environmental Exposure to Manganese is Linked to Deficits in Peripheral and Central Olfactory Function. Chem. Senses 2013, 38, 783–791. [Google Scholar] [CrossRef]
- Lucchini, R.G.; Guazzetti, S.; Zoni, S.; Donna, F.; Peter, S.; Zacco, A.; Salmistraro, M.; Bontempi, E.; Zimmerman, N.J.; Smith, D.R. Tremor, olfactory and motor changes in Italian adolescents exposed to historical ferro-manganese emission. NeuroToxicology 2012, 33, 687–696. [Google Scholar] [CrossRef]
- Iannilli, E.; Gasparotti, R.; Hummel, T.; Zoni, S.; Benedetti, C.; Fedrighi, C.; Tang, C.Y.; Van Thriel, C.; Lucchini, R.G. Effects of Manganese Exposure on Olfactory Functions in Teenagers: A Pilot Study. PLoS ONE 2016, 11, e0144783. [Google Scholar] [CrossRef]
- Rolle-McFarland, D.; Liu, Y.; Mostafaei, F.; Zauber, S.E.; Zhou, Y.; Li, Y.; Fan, Q.; Zheng, W.; Nie, L.H.; Wells, E.M. The association of bone, fingernail and blood manganese with cognitive and olfactory function in Chinese workers. Sci. Total Environ. 2019, 666, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Aschner, M.; Erikson, K.M.; Herrero Hernández, E.; Hernández, E.H.; Tjalkens, R. Manganese and its role in Parkinson’s disease: From transport to neuropathology. Neuromolecular Med. 2009, 11, 252–266. [Google Scholar] [CrossRef] [PubMed]
- Ngwa, H.A.; Kanthasamy, A.; Gu, Y.; Fang, N.; Anantharam, V.; Kanthasamy, A.G. Manganese nanoparticle activates mitochondrial dependent apoptotic signaling and autophagy in dopaminergic neuronal cells. Toxicol. Appl. Pharmacol. 2011, 256, 227–240. [Google Scholar] [CrossRef]
- Tarale, P.; Daiwile, A.P.; Sivanesan, S.; Stöger, R.; Bafana, A.; Naoghare, P.K.; Parmar, D.; Chakrabarti, T.; Krishnamurthi, K. Manganese exposure: Linking down-regulation of miRNA-7 and miRNA-433 with α-synuclein overexpression and risk of idiopathic Parkinson’s disease. Toxicol. In Vitro 2018, 46, 94–101. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, Z.; Wang, Q.; Zhang, J.; Wang, L.; Zhang, Q.; Li, H.; Wu, S. Manganese chloride induces histone acetylation changes in neuronal cells: Its role in manganese-induced damage. NeuroToxicology 2018, 65, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Tarale, P.; Stöger, R.; Bafana, A.; Parmar, D.; Chakrabarti, T.; Kannan, K.; Daiwile, A.P.; Naoghare, P.K.; Sivanesan, S. Global DNA methylation profiling of manganese-exposed human neuroblastoma SH-SY5Y cells reveals epigenetic alterations in Parkinson’s disease-associated genes. Arch. Toxicol. 2016, 91, 2629–2641. [Google Scholar] [CrossRef]
- Wang, L.; Shiraki, A.; Itahashi, M.; Akane, H.; Abe, H.; Mitsumori, K.; Shibutani, M. Aberration in Epigenetic Gene Regulation in Hippocampal Neurogenesis by Developmental Exposure to Manganese Chloride in Mice. Toxicol. Sci. 2013, 136, 154–165. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Zhang, X.; Tarantini, L.; Nordio, F.; Bonzini, M.; Angelici, L.; Marinelli, B.; Rizzo, G.; Cantone, L.; Apostoli, P.; et al. Ambient PM exposure and DNA methylation in tumor suppressor genes: A cross-sectional study. Part. Fibre Toxicol. 2011, 8, 25. [Google Scholar] [CrossRef]
- Lim, J.; Bang, Y.; Choi, H.J. Abnormal hippocampal neurogenesis in Parkinson’s disease: Relevance to a new therapeutic target for depression with Parkinson’s disease. Arch. Pharmacal Res. 2018, 41, 943–954. [Google Scholar] [CrossRef]
- Han, M.-H.; Lee, E.-H.; Koh, S.-H. Current Opinion on the Role of Neurogenesis in the Therapeutic Strategies for Alzheimer Disease, Parkinson Disease, and Ischemic Stroke; Considering Neuronal Voiding Function. Int. Neurourol. J. 2016, 20, 276–287. [Google Scholar] [CrossRef]
- Adamson, S.X.-F.; Shen, X.; Jiang, W.; Lai, V.; Wang, X.; Shannahan, J.H.; Cannon, J.R.; Chen, J.; Zheng, W. Subchronic Manganese Exposure Impairs Neurogenesis in the Adult Rat Hippocampus. Toxicol. Sci. 2018, 163, 592–608. [Google Scholar] [CrossRef] [PubMed]
- Ijomone, O.M.; Aluko, O.M.; Okoh, C.O.A.; Martins, A.C., Jr.; Aschner, M. Role for calcium signaling in manganese neurotoxicity. J. Trace Elem. Med. Biol. Organ Soc. Miner. Trace Elem. (GMS) 2019, 56, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Malovic, E.; Harischandra, D.S.; Ngwa, H.A.; Ghosh, A.; Hogan, C.; Rokad, D.; Zenitsky, G.; Jin, H.; Anantharam, V.; et al. Manganese exposure induces neuroinflammation by impairing mitochondrial dynamics in astrocytes. NeuroToxicology 2017, 64, 204–218. [Google Scholar] [CrossRef]
- Avila-Costa, M.R.; Flores, E.M.; Colin-Barenque, L.; Ordoñez, J.L.; Gutiérrez, A.L.; Niño-Cabrera, H.G.; Mussali-Galante, P.; Fortoul, T.I. Nigrostriatal Modifications After Vanadium Inhalation: An Immunocytochemical and Cytological Approach. Neurochem. Res. 2004, 29, 1365–1369. [Google Scholar] [CrossRef] [PubMed]
- Avila-Costa, M.R.; Fortoul, T.; Niño-Cabrera, G.; Colín-Barenque, L.; Bizarro-Nevares, P.; Gutiérrez-Valdez, A.L.; Ordóñez-Librado, J.L.; Rodríguez-Lara, V.; Mussali-Galante, P.; Díaz-Bech, P.; et al. Hippocampal cell alterations induced by the inhalation of vanadium pentoxide (V2O5) promote memory deterioration. NeuroToxicology 2006, 27, 1007–1012. [Google Scholar] [CrossRef]
- Avila-Costa, M.R.; Colín-Barenque, L.; Zepeda-Rodríguez, A.; Antuna, S.B.; Saldivar, O.L.; Espejel-Maya, G.; Mussali-Galante, P.; del Carmen Avila-Casado, M.; Reyes-Olivera, A.; Anaya-Martinez, V.; et al. Ependymal epithelium disruption after vanadium pentoxide inhalation. A mice experimental model. Neurosci. Lett. 2005, 381, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Ngwa, H.A.; Kanthasamy, A.; Jin, H.; Anantharam, V.; Kanthasamy, A.G. Vanadium exposure induces olfactory dysfunction in an animal model of metal neurotoxicity. NeuroToxicology 2013, 43, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Folarin, O.R.; Snyder, A.M.; Peters, D.G.; Olopade, F.; Connor, J.R.; Olopade, J.O. Brain Metal Distribution and Neuro-Inflammatory Profiles after Chronic Vanadium Administration and Withdrawal in Mice. Front. Neuroanat. 2017, 11, 58. [Google Scholar] [CrossRef]
- Cuesta, S.; Francés, D.; García, G.B. ROS formation and antioxidant status in brain areas of rats exposed to sodium metavanadate. Neurotoxicology Teratol. 2011, 33, 297–302. [Google Scholar] [CrossRef]
- Afeseh Ngwa, H.; Kanthasamy, A.; Anantharam, V.; Song, C.; Witte, T.; Houk, R.; Kanthasamy, A.G. Vanadium induces dopaminergic neurotoxicity via protein kinase Cdelta dependent oxidative signaling mechanisms: Relevance to etiopathogenesis of Parkinson’s disease. Toxicol. Appl. Pharmacol. 2009, 240, 273–285. [Google Scholar] [CrossRef]
- Zhao, Y.; Ye, L.; Liu, H.; Xia, Q.; Zhang, Y.; Yang, X.; Wang, K. Vanadium compounds induced mitochondria permeability transition pore (PTP) opening related to oxidative stress. J. Inorg. Biochem. 2010, 104, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, M.-J.; Shaki, F.; Ghazi-Khansari, M.; Pourahmad, J. Toxicity of vanadium on isolated rat liver mitochondria: A new mechanistic approach. Metallomics 2013, 5, 152–166. [Google Scholar] [CrossRef] [PubMed]
- Tysnes, O.B.; Storstein, A. Epidemiology of Parkinson’s disease. J. Neural Transm. 2017, 124, 901–905. [Google Scholar] [CrossRef] [PubMed]
- Langston, J.W.; Ballard, P.; Tetrud, J.W.; Irwin, I. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science 1983, 219, 979–980. [Google Scholar] [CrossRef] [PubMed]
- Dauer, W.; Przedborski, S. Parkinson’s disease: Mechanisms and models. Neuron 2003, 39, 889–909. [Google Scholar] [CrossRef]
- Sugumar, M.; Sevanan, M.; Sekar, S. Neuroprotective effect of naringenin against MPTP-induced oxidative stress. Int. J. Neurosci. 2018, 129, 534–539. [Google Scholar] [CrossRef]
- Del Zompo, M.; Piccardi, M.; Ruiu, S.; Corsini, G.; Vaccari, A. High-affinity binding of [3H]1-methyl-4-phenyl-2,3-dihydropyridinium ion to mouse striatal membranes: Putative vesicular location. Eur. J. Pharmacol. 1991, 202, 293–294. [Google Scholar] [CrossRef]
- Del Zompo, M.; Piccardi, M.; Ruiu, S.; Corsini, G.; Vaccari, A. Characterization of a putatively vesicular binding site for [3H]MPP+ in mouse striatal membranes. Brain Res. 1992, 571, 354–357. [Google Scholar] [CrossRef]
- Peter, D.; Jimenez, J.; Liu, Y.; Kim, J.; Edwards, R.H. The chromaffin granule and synaptic vesicle amine transporters differ in substrate recognition and sensitivity to inhibitors. J. Biol. Chem. 1994, 269, 7231–7237. [Google Scholar] [CrossRef]
- Lotharius, J.; Brundin, P. Pathogenesis of Parkinson’s disease: Dopamine, vesicles and alpha-synuclein. Nat. Rev. Neurosci. 2002, 3, 932–942. [Google Scholar] [CrossRef]
- Munoz-Manchado, A.B.; Villadiego, J.; Romo-Madero, S.; Suarez-Luna, N.; Bermejo-Navas, A.; Rodriguez-Gomez, J.A.; Garrido-Gil, P.; Labandeira-Garcia, J.L.; Echevarria, M.; Lopez-Barneo, J.; et al. Chronic and progressive Parkinson’s disease MPTP model in adult and aged mice. J. Neurochem. 2016, 136, 373–387. [Google Scholar] [CrossRef] [PubMed]
- Masilamoni, G.J.; Smith, Y. Chronic MPTP administration regimen in monkeys: A model of dopaminergic and non-dopaminergic cell loss in Parkinson’s disease. J. Neural Transm. 2017, 125, 337–363. [Google Scholar] [CrossRef] [PubMed]
- Rotenone as an Insecticide. Nature 1933, 132, 167. [CrossRef]
- Isman, M. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu. Rev. Entomol. 2006, 51, 45–66. [Google Scholar] [CrossRef] [PubMed]
- Soloway, S.B. Naturally occurring insecticides. Environ. Health Perspect. 1976, 14, 109–117. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. International Programme on Chemical Safety, Rotenone: Health and Safety Guide; World Health Organization: Geneva, Switerland, 1992.
- Nandipati, S.; Litvan, I. Environmental Exposures and Parkinson’s Disease. Int. J. Environ. Res. Public Health 2016, 13, 881. [Google Scholar] [CrossRef]
- US Environmental Protection Agency. Reregistration Eligibility Decision for Rotenone; US Environmental Protection Agency: Dallas, TX, USA, 2007.
- Newsome, W.H.; Shields, J.B. Residues of rotenone and rotenolone on lettuce and tomato fruit after treatment in the field with rotenone formulations. J. Agric. Food Chem. 1980, 28, 722–724. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, N.; Wang, K.; Li, W.; Li, H.; Zhang, Z. Dissipation and Residue of Rotenone in Cabbage and Soil Under Field Conditions. Bull. Environ. Contam. Toxicol. 2013, 91, 251–255. [Google Scholar] [CrossRef]
- Cabras, P.; Caboni, P.; Cabras, M.; Angioni, A.; Russo, M. Rotenone residues on olives and in olive oil. J. Agric. Food Chem. 2002, 50, 2576–2580. [Google Scholar] [CrossRef]
- Cavoski, I.; Caboni, P.; Sarais, G.; Miano, T. Degradation and persistence of rotenone in soils and influence of temperature variations. J. Agric. Food Chem. 2008, 56, 8066–8073. [Google Scholar] [CrossRef]
- US Environmental Protection Agency. Product Cancellation Order for Certain Pesticide Registrations. Fed. Regist. 2011, 76, 16415–16417. [Google Scholar]
- US Environmental Protection Agency. Tolerance Actions. Fed. Regist. 2012, 77, 59120. [Google Scholar]
- Baker, B. Rotenone Use in Organic Farming|Hygeia Analytics. Available online: https://hygeia-analytics.com/2017/01/04/rotenone-use-in-organic-farming/ (accessed on 4 January 2017).
- Imamura, K.; Takeshima, T.; Kashiwaya, Y.; Nakaso, K.; Nakashima, K. D-beta-hydroxybutyrate protects dopaminergic SH-SY5Y cells in a rotenone model of Parkinson’s disease. J. Neurosci. Res. 2006, 84, 1376–1384. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, P.; Panda, D. Rotenone inhibits mammalian cell proliferation by inhibiting microtubule assembly through tubulin binding. FEBS J. 2007, 274, 4788–4801. [Google Scholar] [CrossRef] [PubMed]
- Schapira, A.H. Mitochondria in the aetiology and pathogenesis of Parkinson’s disease. Lancet Neurol. 2008, 7, 97–109. [Google Scholar] [CrossRef]
- Dhillon, A.S.; Tarbutton, G.L.; Levin, J.L.; Plotkin, G.M.; Lowry, L.K.; Nalbone, J.T.; Shepherd, S. Pesticide/Environmental Exposures and Parkinson’s Disease in East Texas. J. Agromed. 2008, 13, 37–48. [Google Scholar] [CrossRef]
- Spivey, A. Rotenone and Paraquat Linked to Parkinson’s Disease: Human Exposure Study Supports Years of Animal Studies. Environ. Health Perspect. 2011, 119, A259. [Google Scholar] [CrossRef]
- Furlong, M.; Tanner, C.M.; Goldman, S.M.; Bhudhikanok, G.S.; Blair, A.; Chade, A.; Comyns, K.; Hoppin, J.A.; Kasten, M.; Korell, M.; et al. Protective glove use and hygiene habits modify the associations of specific pesticides with Parkinson’s disease. Environ. Int. 2015, 75, 144–150. [Google Scholar] [CrossRef]
- Pouchieu, C.; Piel, C.; Carles, C.; Gruber, A.; Helmer, C.; Tual, S.; Marcotullio, E.; Lebailly, P.; Baldi, I. Pesticide use in agriculture and Parkinson’s disease in the AGRICAN cohort study. Int. J. Epidemiol. 2018, 47, 299–310. [Google Scholar] [CrossRef]
- Zeng, X.-S.; Geng, W.-S.; Jia, J.-J. Neurotoxin-Induced Animal Models of Parkinson Disease: Pathogenic Mechanism and Assessment. ASN Neuro 2018, 10, 1759091418777438. [Google Scholar] [CrossRef]
- Inden, M.; Kitamura, Y.; Abe, M.; Tamaki, A.; Takata, K.; Taniguchi, T. Parkinsonian rotenone mouse model: Reevaluation of long-term administration of rotenone in C57BL/6 mice. Biol. Pharm. Bull. 2011, 34, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Liu, W.; Jiang, H.; Jiang, Q.; Feng, J. Selective vulnerability of dopaminergic neurons to microtubule depolymerization. J. Biol. Chem. 2005, 280, 34105–34112. [Google Scholar] [CrossRef] [PubMed]
- Xiong, N.; Xiong, J.; Jia, M.; Liu, L.; Zhang, X.; Chen, Z.; Huang, J.; Zhang, Z.; Hou, L.; Luo, Z.; et al. The role of autophagy in Parkinson’s disease: Rotenone-based modeling. Behav. Brain Funct. BBF 2013, 9, 13. [Google Scholar] [CrossRef] [PubMed]
- Tabata, Y.; Imaizumi, Y.; Sugawara, M.; Andoh-Noda, T.; Banno, S.; Chai, M.; Sone, T.; Yamazaki, K.; Ito, M.; Tsukahara, K.; et al. T-type Calcium Channels Determine the Vulnerability of Dopaminergic Neurons to Mitochondrial Stress in Familial Parkinson Disease. Stem Cell Rep. 2018, 11, 1171–1184. [Google Scholar] [CrossRef]
- Yuan, Y.H.; Yan, W.F.; Sun, J.D.; Huang, J.Y.; Mu, Z.; Chen, N.H. The molecular mechanism of rotenone-induced α-synuclein aggregation: Emphasizing the role of the calcium/GSK3β pathway. Toxicol. Lett. 2015, 233, 163–171. [Google Scholar] [CrossRef]
- Silva, B.A.; Einarsdóttir, O.; Fink, A.L.; Uversky, V.N. Biophysical Characterization of α-Synuclein and Rotenone Interaction. Biomolecules 2013, 3, 703–732. [Google Scholar] [CrossRef]
- Arnold, B.; Cassady, S.; Van Laar, V.S.; Berman, S.B. Integrating multiple aspects of mitochondrial dynamics in neurons: Age-related differences and dynamic changes in a chronic rotenone model. Neurobiol. Dis. 2011, 41, 189–200. [Google Scholar] [CrossRef]
- Zhang, X.; Du, L.; Zhang, W.; Yang, Y.; Zhou, Q.; Du, G. Therapeutic effects of baicalein on rotenone-induced Parkinson’s disease through protecting mitochondrial function and biogenesis. Sci. Rep. 2017, 7, 9968. [Google Scholar] [CrossRef]
- Barsoum, M.J.; Yuan, H.; Gerencser, A.A.; Liot, G.; Kushnareva, Y.; Gräber, S.; Kovacs, I.; Lee, W.D.; Waggoner, J.; Cui, J.; et al. Nitric oxide-induced mitochondrial fission is regulated by dynamin-related GTPases in neurons. EMBO J. 2006, 25, 3900–3911. [Google Scholar] [CrossRef]
- Staiff, D.C.; Comer, S.W.; Armstrong, J.F.; Wolfe, H.R. Exposure to the herbicide, paraquat. Bull Environ. Contam. Toxicol. 1975, 14, 334–340. [Google Scholar] [CrossRef]
- Smith, J. Paraquat Poisoning by Skin Absorption: A Review. Hum. Toxicol. 1988, 7, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Baharudin, M.R.; Sahid, I.B.; Noor, M.A.B.M.; Sulaiman, N.; Othman, F. Pesticide risk assessment: A study on inhalation and dermal exposure to 2,4-D and paraquat among Malaysian paddy farmers. J. Environ. Sci. Health Part B 2011, 46, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Dinis-Oliveira, R.J.; Remião, F.; Carmo, H.; Duarte, J.A.; Navarro, A.S.; Bastos, M.L.; Carvalho, F. Paraquat exposure as an etiological factor of Parkinson’s disease. Neurotoxicology 2006, 27, 1110–1122. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H.; Souders, C.L.; Zhao, Y.H.; Martyniuk, C.J. Paraquat affects mitochondrial bioenergetics, dopamine system expression, and locomotor activity in zebrafish (Danio rerio). Chemosphere 2018, 191, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Li, Y.; Wu, K.; Yan, W.; Tian, T.; Wang, Y.; Yang, H. Paraquat modulates microglia M1/M2 polarization via activation of TLR4-mediated NF-κB signaling pathway. Chem. Biol. Interact. 2019, 310, 108743. [Google Scholar] [CrossRef] [PubMed]
- McCormack, A.; Atienza, J.; Langston, J.; Di Monte, D. Decreased susceptibility to oxidative stress underlies the resistance of specific dopaminergic cell populations to paraquat-induced degeneration. Neuroscience 2006, 141, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Konthonbut, P.; Kongtip, P.; Nankongnab, N.; Tipayamongkholgul, M.; Yoosook, W.; Woskie, S. Paraquat Exposure of Pregnant Women and Neonates in Agricultural Areas in Thailand. Int. J. Environ. Res. Public Health 2018, 15, 1163. [Google Scholar] [CrossRef]
- Goldman, S.M.; Kamel, F.; Ross, G.W.; Bhudhikanok, G.S.; Hoppin, J.A.; Korell, M.; Marras, C.; Meng, C.; Umbach, D.M.; Kasten, M.; et al. Genetic modification of the association of paraquat and Parkinson’s disease. Mov. Disord. 2012, 27, 1652–1658. [Google Scholar] [CrossRef]
- Thiruchelvam, M.; McCormack, A.; Richfield, E.K.; Baggs, R.B.; Tank, A.W.; Di Monte, D.A.; Cory-Slechta, D.A. Age-Related irreversible progressive nigrostriatal dopaminergic neurotoxicity in the paraquat and maneb model of the Parkinson’s disease phenotype. Eur. J. Neurosci. 2003, 18, 589–600. [Google Scholar] [CrossRef]
- Laden, F.; Neas, L.M.; Spiegelman, D.; Hankinson, S.E.; Willett, W.C.; Ireland, K.; Wolff, M.S.; Hunter, D.J. Predictors of plasma concentrations of DDE and PCBs in a group of U.S. women. Env. Health Perspect 1999, 107, 75–81. [Google Scholar] [CrossRef]
- Whitmore, R.W.; Immerman, F.W.; Camann, D.E.; Bond, A.E.; Lewis, R.G.; Schaum, J.L. Non-occupational exposures to pesticides for residents of two U.S. cities. Arch. Environ. Contam. Toxicol. 1994, 26, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Hjelmborg, P.S.; Andreassen, T.K.; Bonefeld-Jørgensen, E.C. Cellular uptake of lipoproteins and persistent organic compounds—An update and new data. Environ. Res. 2008, 108, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Scarselli, M.; Fasciani, I.; Marampon, F.; Maggio, R.; Pietrantoni, I. Dichlorodiphenyltrichloroethane, an old pesticide with a new mechanism of toxicity. Curr. Top. Pharmacol. 2018, 22, 69–77. [Google Scholar]
- Costa, L.G. The neurotoxicity of organochlorine and pyrethroid pesticides. Handb. Clin. Neurol. 2015, 131, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.; Roy, A.; Shalat, S.L.; Von Stein, R.T.; Hossain, M.M.; Buckley, B.; Gearing, M.; Levey, A.I.; German, D.C. Elevated Serum Pesticide Levels and Risk for Alzheimer Disease. JAMA Neurol. 2014, 71, 284–290. [Google Scholar] [CrossRef]
- World Health Organization. Public Health Impact of Pesticides Used in Agriculture; World Health Organization: Geneva, Switerland, 1990.
- de Jong, G. A study of exposure, health effects and mortality of workers engaged in the manufacture and formulation of the insecticides aldrin and dieldrin. Toxicol. Lett. 1991, 56, iii-206. [Google Scholar] [CrossRef]
- Cavender, F.L.; Cook, B.T.; Page, N.P. Carcinogenicity Assessment of Aldrin and Dieldrin; US Environmental Protection Agency, Office of Health and Environmental Assessment: Washington, DC, USA, 1988.
- National Academy of Sciences. Committee on Toxicology, an Assessment of the Health Risks of Seven Pesticides Used for Termite Control; National Academy of Sciences: Washington, DC, USA, 1982. [Google Scholar]
- Jorgenson, J.L. Aldrin and dieldrin: A review of research on their production, environmental deposition and fate, bioaccumulation, toxicology, and epidemiology in the United States. Environ. Health Perspect. 2001, 109 (Suppl. S1), 113–139. [Google Scholar]
- Rodan, B.D.; Pennington, D.W.; Eckley, N.; Boethling, R.S. Screening for Persistent Organic Pollutants: Techniques To Provide a Scientific Basis for POPs Criteria in International Negotiations. Environ. Sci. Technol. 1999, 33, 3482–3488. [Google Scholar] [CrossRef]
- ATSDR. Toxicological Profile for Aldrin/Dieldrin; Agency for Toxic Substances and Disease Registry, US Department of Health: Washington, DC, USA, 2002.
- Zhao, X.; Salgado, V.L.; Yeh, J.Z.; Narahashi, T. Differential Actions of Fipronil and Dieldrin Insecticides on GABA-Gated Chloride Channels in Cockroach Neurons. J. Pharmacol. Exp. Ther. 2003, 306, 914–924. [Google Scholar] [CrossRef]
- Fleming, L.; Mann, J.B.; Bean, J.; Briggle, T.; Sanchez-Ramos, J.R. Parkinson’s disease and brain levels of organochlorine pesticides. Ann. Neurol. 1994, 36, 100–103. [Google Scholar] [CrossRef]
- Corrigan, F.M.; French, M.; Murray, L. Organochlorine compounds in human brain. Hum. Exp. Toxicol. 1996, 15, 262–264. [Google Scholar] [CrossRef] [PubMed]
- Corrigan, F.; Murray, L.; Wyatt, C.; Shore, R. Diorthosubstituted polychlorinated biphenyls in caudate nucleus in Parkinson’s disease. Exp. Neurol. 1998, 150, 339–342. [Google Scholar] [CrossRef]
- Sanchez-Ramos, J.; Facca, A.; Basit, A.; Song, S. Toxicity of Dieldrin for Dopaminergic Neurons in Mesencephalic Cultures. Exp. Neurol. 1998, 150, 263–271. [Google Scholar] [CrossRef]
- Chhillar, N.; Singh, N.K.; Banerjee, B.D.; Bala, K.; Mustafa; Sharma, D.; Chhillar, M. Organochlorine Pesticide Levels and Risk of Parkinson’s Disease in North Indian Population. ISRN Neurol. 2013, 2013, 371034. [Google Scholar] [CrossRef] [PubMed]
- Weisskopf, M.G.; Knekt, P.; O’Reilly, E.J.; Lyytinen, J.; Reunanen, A.; Laden, F.; Altshul, L.; Ascherio, A. Persistent organochlorine pesticides in serum and risk of Parkinson disease. Neurology 2010, 74, 1055–1061. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.R.; Caudle, W.M.; Wang, M.; Dean, E.D.; Pennell, K.D.; Miller, G.W. Developmental exposure to the pesticide dieldrin alters the dopamine system and increases neurotoxicity in an animal model of Parkinson’s disease. Faseb. J. 2006, 20, 1695–1697. [Google Scholar] [CrossRef] [PubMed]
- Gezer, A.O.; Kochmanski, J.; VanOeveren, S.E.; Cole-Strauss, A.; Kemp, C.J.; Patterson, J.R.; Miller, K.M.; Kuhn, N.C.; Herman, D.E.; McIntire, A.; et al. Developmental exposure to the organochlorine pesticide dieldrin causes male-specific exacerbation of α-synuclein-preformed fibril-induced toxicity and motor deficits. Neurobiol. Dis. 2020, 141, 104947. [Google Scholar] [CrossRef]
- Kitazawa, M.; Anantharam, V.; Kanthasamy, A.G. Dieldrin-induced oxidative stress and neurochemical changes contribute to apoptopic cell death in dopaminergic cells. Free Radic. Biol. Med. 2001, 31, 1473–1485. [Google Scholar] [CrossRef]
- Kitazawa, M.; Anantharam, V.; Kanthasamy, A.; Kanthasamy, A.G. Dieldrin Promotes Proteolytic Cleavage of Poly(ADP-Ribose) Polymerase and Apoptosis in Dopaminergic Cells: Protective Effect of Mitochondrial Anti-Apoptotic Protein Bcl-2. NeuroToxicology 2004, 25, 589–598. [Google Scholar] [CrossRef]
- Sharma, H.; Zhang, P.; Barber, D.S.; Liu, B. Organochlorine pesticides dieldrin and lindane induce cooperative toxicity in dopaminergic neurons: Role of oxidative stress. NeuroToxicology 2010, 31, 215–222. [Google Scholar] [CrossRef]
- Schmidt, J.T.; Rushin, A.; Boyda, J.; Souders, C.L.; Martyniuk, C.J. Dieldrin-induced neurotoxicity involves impaired mitochondrial bioenergetics and an endoplasmic reticulum stress response in rat dopaminergic cells. NeuroToxicology 2017, 63, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kochmanski, J.; VanOeveren, S.E.; Patterson, J.R.; Bernstein, A. Developmental Dieldrin Exposure Alters DNA Methylation at Genes Related to Dopaminergic Neuron Development and Parkinson’s Disease in Mouse Midbrain. Toxicol. Sci. 2019, 169, 593–607. [Google Scholar] [CrossRef] [PubMed]
- Horning, K.J.; Caito, S.W.; Tipps, K.G.; Bowman, A.B.; Aschner, M. Manganese Is Essential for Neuronal Health. Annu. Rev. Nutr. 2015, 35, 71–108. [Google Scholar] [CrossRef] [PubMed]
- Greger, J.L. Nutrition versus toxicology of manganese in humans: Evaluation of potential biomarkers. NeuroToxicology 1999, 20, 205–212. [Google Scholar]
- Blanc, P.D. The early history of manganese and the recognition of its neurotoxicity, 1837–1936. NeuroToxicology 2018, 64, 5–11. [Google Scholar] [CrossRef]
- O’Neal, S.L.; Zheng, W. Manganese Toxicity upon Overexposure: A Decade in Review. Curr. Environ. Health Rep. 2015, 2, 315–328. [Google Scholar] [CrossRef]
- Tuschl, K.; Mills, P.B.; Clayton, P.T. Manganese and the Brain. Int. Rev. Neurobiol. 2013, 110, 277–312. [Google Scholar] [CrossRef]
- Walter, E.; Alsaffar, S.; Livingstone, C.; Ashley, S.L. Manganese toxicity in critical care: Case report, literature review and recommendations for practice. J. Intensiv. Care Soc. 2016, 17, 252–257. [Google Scholar] [CrossRef]
- Tanner, C.M.; Ross, G.W.; Jewell, S.A.; Hauser, R.A.; Jankovic, J.; Factor, S.A.; Bressman, S.; Deligtisch, A.; Marras, C.; Lyons, K.E.; et al. Occupation and risk of parkinsonism: A multicenter case-control study. Arch. Neurol. 2009, 66, 1106–1113. [Google Scholar] [CrossRef]
- Pal, P.K.; Samii, A.; Calne, D.B. Manganese neurotoxicity: A review of clinical features, imaging and pathology. NeuroToxicology 1999, 20, 227–238. [Google Scholar]
- Zota, A.R.; Riederer, A.M.; Ettinger, A.S.; Schaider, L.A.; Shine, J.P.; Amarasiriwardena, C.J.; Wright, R.; Spengler, J.D. Associations between metals in residential environmental media and exposure biomarkers over time in infants living near a mining-impacted site. J. Expo. Sci. Environ. Epidemiol. 2015, 26, 510–519. [Google Scholar] [CrossRef] [PubMed]
- Zota, A.R.; Schaider, L.A.; Ettinger, A.S.; Wright, R.O.; Shine, J.P.; Spengler, J.D. Metal sources and exposures in the homes of young children living near a mining-impacted Superfund site. J. Expo. Sci. Environ. Epidemiol. 2011, 21, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Menezes-Filho, J.A.; Novaes, C.D.O.; Moreira, J.C.; Sarcinelli, P.N.; Mergler, D. Elevated manganese and cognitive performance in school-aged children and their mothers. Environ. Res. 2011, 111, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Haynes, E.N.; Sucharew, H.; Hilbert, T.J.; Kuhnell, P.; Spencer, A.; Newman, N.C.; Burns, R.; Wright, R.; Parsons, P.J.; Dietrich, K.N. Impact of air manganese on child neurodevelopment in East Liverpool, Ohio. NeuroToxicology 2017, 64, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Flynn, M.R.; Susi, P. Neurological risks associated with manganese exposure from welding operations—A literature review. Int. J. Hyg. Environ. Health 2009, 212, 459–469. [Google Scholar] [CrossRef]
- Stepens, A.; Logina, I.; Liguts, V.; Aldiņš, P.; Ekšteina, I.; Platkājis, A.; Mārtiņsone, I.; Tērauds, E.; Rozentāle, B.; Donaghy, M. A Parkinsonian Syndrome in Methcathinone Users and the Role of Manganese. N. Engl. J. Med. 2008, 358, 1009–1017. [Google Scholar] [CrossRef]
- Varlibas, F.; Delipoyraz, I.; Yuksel, G.; Filiz, G.; Tireli, H.; Gecim, N.O. Neurotoxicity following chronic intravenous use of “Russian cocktail”. Clin. Toxicol. 2009, 47, 157–160. [Google Scholar] [CrossRef]
- Crossgrove, J.; Zheng, W. Manganese toxicity upon overexposure. NMR Biomed. 2004, 17, 544–553. [Google Scholar] [CrossRef]
- Lucchini, R.; Bergamaschi, E.; Smargiassi, A.; Festa, D.; Apostoli, P. Motor Function, Olfactory Threshold, and Hematological Indices in Manganese-Exposed Ferroalloy Workers. Environ. Res. 1997, 73, 175–180. [Google Scholar] [CrossRef]
- Meyer-Baron, M.; Schäper, M.; Knapp, G.; Lucchini, R.; Zoni, S.; Bast-Pettersen, R.; Ellingsen, D.G.; Thomassen, Y.; He, S.; Yuan, H.; et al. The neurobehavioral impact of manganese: Results and challenges obtained by a meta-analysis of individual participant data. NeuroToxicology 2013, 36, 1–9. [Google Scholar] [CrossRef]
- Roth, J.A.; Garrick, M. Iron interactions and other biological reactions mediating the physiological and toxic actions of manganese. Biochem. Pharmacol. 2003, 66, 1–13. [Google Scholar] [CrossRef]
- Peres, T.V.; Eyng, H.; Lopes, S.C.; Colle, D.; Gonçalves, F.M.; Venske, D.K.R.; Lopes, M.W.; Ben, J.; Bornhorst, J.; Schwerdtle, T.; et al. Developmental exposure to manganese induces lasting motor and cognitive impairment in rats. NeuroToxicology 2015, 50, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, J.; Ahmed, S.S.S.J.; Ng, H.K.T.; Naiya, T.; Ghosh, E.; Banerjee, T.K.; Lakshmi, J.; Guha, G.; Rao, V.R. Metallomic Biomarkers in Cerebrospinal fluid and Serum in patients with Parkinson’s disease in Indian population. Sci. Rep. 2016, 6, 35097. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, T.; Tan, X.; Luo, Y.; Kanda, H. Relationship between Blood Levels of Heavy Metals and Parkinson’s Disease in China. Neuroepidemiology 2009, 34, 18–24. [Google Scholar] [CrossRef]
- Brenneman, K.A.; Wong, B.A.; Buccellato, M.A.; Costa, E.R.; Gross, E.A.; Dorman, D.C. Direct Olfactory Transport of Inhaled Manganese (54MnCl2) to the Rat Brain: Toxicokinetic Investigations in a Unilateral Nasal Occlusion Model. Toxicol. Appl. Pharmacol. 2000, 169, 238–248. [Google Scholar] [CrossRef]
- Frisbie, S.H.; Mitchell, E.J.; Roudeau, S.; Domart, F.; Carmona, A.; Ortega, R. Manganese levels in infant formula and young child nutritional beverages in the United States and France: Comparison to breast milk and regulations. PLoS ONE 2019, 14, e0223636. [Google Scholar] [CrossRef]
- Chen, P.; Bornhorst, J.; Aschner, M. Manganese metabolism in humans. Front Biosci. 2018, 23, 1655–1679. [Google Scholar] [CrossRef]
- Liu, C.; Jursa, T.; Aschner, M.; Smith, D.R.; Mukhopadhyay, S. Up-regulation of the manganese transporter SLC30A10 by hypoxia-inducible factors defines a homeostatic response to manganese toxicity. Proc. Natl. Acad. Sci. USA 2021, 118, 35. [Google Scholar] [CrossRef]
- Omokhodion, F.O.; Howard, J.M. Trace elements in the sweat of acclimatized persons. Clin. Chim. Acta 1994, 231, 23–28. [Google Scholar] [CrossRef]
- O’Neal, S.L.; Hong, L.; Fu, S.; Jiang, W.; Jones, A.; Nie, L.H.; Zheng, W. Manganese accumulation in bone following chronic exposure in rats: Steady-state concentration and half-life in bone. Toxicol. Lett. 2014, 229, 93–100. [Google Scholar] [CrossRef]
- Dierschke, K.; Isaxon, C.; Andersson, U.B.K.; Assarsson, E.; Axmon, A.; Stockfelt, L.; Gudmundsson, A.; Jönsson, B.A.G.; Kåredal, M.; Londahl, J.; et al. Acute respiratory effects and biomarkers of inflammation due to welding-derived nanoparticle aggregates. Int. Arch. Occup. Environ. Health 2017, 90, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Tarale, P.; Chakrabarti, T.; Sivanesan, S.; Naoghare, P.; Bafana, A.; Krishnamurthi, K. Potential Role of Epigenetic Mechanism in Manganese Induced Neurotoxicity. BioMed Res. Int. 2016, 2016, 2548792. [Google Scholar] [CrossRef] [PubMed]
- Cantone, L.; Nordio, F.; Hou, L.; Apostoli, P.; Bonzini, M.; Tarantini, L.; Angelici, L.; Bollati, V.; Zanobetti, A.; Schwartz, J.; et al. Inhalable Metal-Rich Air Particles and Histone H3K4 Dimethylation and H3K9 Acetylation in a Cross-sectional Study of Steel Workers. Environ. Health Perspect. 2011, 119, 964–969. [Google Scholar] [CrossRef] [PubMed]
- Bollati, V.; Marinelli, B.; Apostoli, P.; Bonzini, M.; Nordio, F.; Hoxha, M.; Pegoraro, V.; Motta, V.; Tarantini, L.; Cantone, L.; et al. Exposure to Metal-Rich Particulate Matter Modifies the Expression of Candidate MicroRNAs in Peripheral Blood Leukocytes. Environ. Health Perspect. 2010, 118, 763–768. [Google Scholar] [CrossRef]
- Harischandra, D.S.; Ghaisas, S.; Rokad, D.; Zamanian, M.; Jin, H.; Anantharam, V.; Kimber, M.; Kanthasamy, A.; Kanthasamy, A.G. Environmental neurotoxicant manganese regulates exosome-mediated extracellular miRNAs in cell culture model of Parkinson’s disease: Relevance to α-synuclein misfolding in metal neurotoxicity. Neurotoxicology 2018, 64, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Castillo, S.; Muñoz, P.; Behrens, M.I.; Diaz-Grez, F.; Segura-Aguilar, J. On the Role of Mining Exposure in Epigenetic Effects in Parkinson’s Disease. Neurotox. Res. 2017, 32, 172–174. [Google Scholar] [CrossRef]
- Nwanaji-Enwerem, J.C.; Colicino, E.; Specht, A.J.; Gao, X.; Wang, C.; Vokonas, P.; Weisskopf, M.G.; Boyer, E.W.; Baccarelli, A.A.; Schwartz, J. Individual species and cumulative mixture relationships of 24-hour urine metal concentrations with DNA methylation age variables in older men. Environ. Res. 2020, 186, 109573. [Google Scholar] [CrossRef]
- Fu, S.; O’Neal, S.; Hong, L.; Jiang, W.; Zheng, W. Elevated Adult Neurogenesis in Brain Subventricular Zone Following In vivo Manganese Exposure: Roles of Copper and DMT1. Toxicol. Sci. 2015, 143, 482–498. [Google Scholar] [CrossRef]
- Fu, S.; Jiang, W.; Gao, X.; Zeng, A.; Cholger, D.; Cannon, J.; Chen, J.; Zheng, W. Aberrant Adult Neurogenesis in the Subventricular Zone-Rostral Migratory Stream-Olfactory Bulb System Following Subchronic Manganese Exposure. Toxicol. Sci. 2016, 150, 347–368. [Google Scholar] [CrossRef]
- Horgusluoglu, E.; Nudelman, K.; Nho, K.; Saykin, A.J. Adult neurogenesis and neurodegenerative diseases: A systems biology perspective. Am. J. Med Genet. Part B Neuropsychiatr. Genet. 2016, 174, 93–112. [Google Scholar] [CrossRef]
- Lim, D.A.; Alvarez-Buylla, A. The Adult Ventricular–Subventricular Zone (V-SVZ) and Olfactory Bulb (OB) Neurogenesis. Cold Spring Harb. Perspect. Biol. 2016, 8, a018820. [Google Scholar] [CrossRef] [PubMed]
- Marxreiter, F.; Regensburger, M.; Winkler, J. Adult neurogenesis in Parkinson’s disease. Cell Mol. Life Sci. 2012, 70, 459–473. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.L.; Li, J.-D.; Cheng, M.Y.; Leslie, F.M.; Lee, A.G.; Zhou, Q.-Y. Dependence of Olfactory Bulb Neurogenesis on Prokineticin 2 Signaling. Science 2005, 308, 1923–1927. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ohishi, T.; Shiraki, A.; Morita, R.; Akane, H.; Ikarashi, Y.; Mitsumori, K.; Shibutani, M. Developmental Exposure to Manganese Chloride Induces Sustained Aberration of Neurogenesis in the Hippocampal Dentate Gyrus of Mice. Toxicol. Sci. 2012, 127, 508–521. [Google Scholar] [CrossRef]
- Cheng, M.; Leslie, F.M.; Zhou, Q.-Y. Expression of prokineticins and their receptors in the adult mouse brain. J. Comp. Neurol. 2006, 498, 796–809. [Google Scholar] [CrossRef]
- Zoni, S.; Bonetti, G.; Lucchini, R. Olfactory functions at the intersection between environmental exposure to manganese and Parkinsonism. J. Trace Elem. Med. Biol. Organ Soc. Miner. Trace Elem. (GMS) 2012, 26, 179–182. [Google Scholar] [CrossRef]
- Mogi, M.; Harada, M.; Kondo, T.; Riederer, P.; Inagaki, H.; Minami, M.; Nagatsu, T. Interleukin-1 beta, interleukin-6, epidermal growth factor and transforming growth factor-alpha are elevated in the brain from parkinsonian patients. Neurosci. Lett. 1994, 180, 147–150. [Google Scholar] [CrossRef]
- Freeman, L.C.; Ting, J.P.-Y. The pathogenic role of the inflammasome in neurodegenerative diseases. J. Neurochem. 2015, 136, 29–38. [Google Scholar] [CrossRef]
- Pajarillo, E.; Johnson, J.; Rizor, A.; Nyarko-Danquah, I.; Adinew, G.; Bornhorst, J.; Stiboller, M.; Schwerdtle, T.; Son, D.-S.; Aschner, M.; et al. Astrocyte-specific deletion of the transcription factor Yin Yang 1 in murine substantia nigra mitigates manganese-induced dopaminergic neurotoxicity. J. Biol. Chem. 2020, 295, 15662–15676. [Google Scholar] [CrossRef]
- Huang, Y.; Wen, Q.; Huang, J.; Luo, M.; Xiao, Y.; Mo, R.; Wang, J. Manganese (II) chloride leads to dopaminergic neurotoxicity by promoting mitophagy through BNIP3-mediated oxidative stress in SH-SY5Y cells. Cell. Mol. Biol. Lett. 2021, 26, 23. [Google Scholar] [CrossRef]
- Zhang, D.; Kanthasamy, A.; Anantharam, V.; Kanthasamy, A. Effects of manganese on tyrosine hydroxylase (TH) activity and TH-phosphorylation in a dopaminergic neural cell line. Toxicol. Appl. Pharmacol. 2011, 254, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Liu, K.; Li, X.R.; Wang, C.; Liu, C.; Yan, D.Y.; Deng, Y.; Liu, W.; Xu, B. Alpha-synuclein is involved in manganese-induced spatial memory and synaptic plasticity impairments via TrkB/Akt/Fyn-mediated phosphorylation of NMDA receptors. Cell Death Dis. 2020, 11, 834. [Google Scholar] [CrossRef]
- Choi, C.J.; Anantharam, V.; Martin, D.P.; Nicholson, E.M.; Richt, J.A.; Kanthasamy, A.; Kanthasamy, A.G. Manganese Upregulates Cellular Prion Protein and Contributes to Altered Stabilization and Proteolysis: Relevance to Role of Metals in Pathogenesis of Prion Disease. Toxicol. Sci. 2010, 115, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Ohishi, T.; Wang, L.; Akane, H.; Shiraki, A.; Goto, K.; Ikarashi, Y.; Suzuki, K.; Mitsumori, K.; Shibutani, M. Reversible aberration of neurogenesis affecting late-stage differentiation in the hippocampal dentate gyrus of rat offspring after maternal exposure to manganese chloride. Reprod. Toxicol. 2012, 34, 408–419. [Google Scholar] [CrossRef] [PubMed]
- Haikal, C.; Chen, Q.Q.; Li, J.Y. Microbiome changes: An indicator of Parkinson’s disease? Transl. Neurodegener. 2019, 8, 38. [Google Scholar] [CrossRef]
- Yang, D.; Zhao, D.; Ali Shah, S.Z.; Wu, W.; Lai, M.; Zhang, X.; Li, J.; Guan, Z.; Zhao, H.; Li, W.; et al. The Role of the Gut Microbiota in the Pathogenesis of Parkinson’s Disease. Front. Neurol. 2019, 10, 1155. [Google Scholar] [CrossRef]
- Koller, W.C.; Lyons, K.E.; Truly, W. Effect of levodopa treatment for parkinsonism in welders: A double-blind study. Neurology 2004, 62, 730–733. [Google Scholar] [CrossRef]
- Jiang, Y.-M.; Mo, X.-A.; Du, F.-Q.; Fu, X.; Zhu, X.-Y.; Gao, H.-Y.; Xie, J.-L.; Liao, F.-L.; Pira, E.; Zheng, W. Effective Treatment of Manganese-Induced Occupational Parkinsonism with p-Aminosalicylic Acid: A Case of 17-Year Follow-Up Study. J. Occup. Environ. Med. 2006, 48, 644–649. [Google Scholar] [CrossRef]
- Zheng, W.; Jiang, Y.-M.; Zhang, Y.; Jiang, W.; Wang, X.; Cowan, D.M. Chelation therapy of manganese intoxication with para-aminosalicylic acid (PAS) in Sprague–Dawley rats. NeuroToxicology 2009, 30, 240–248. [Google Scholar] [CrossRef]
- Ky, S.Q.; Deng, H.S.; Xie, P.Y.; Hu, W. A report of two cases of chronic serious manganese poisoning treated with sodium para-aminosalicylic acid. Occup. Environ. Med. 1992, 49, 66–69. [Google Scholar] [CrossRef]
- Ahmadi, N.; Ghanbarinejad, V.; Ommati, M.M.; Jamshidzadeh, A.; Heidari, R. Taurine prevents mitochondrial membrane permeabilization and swelling upon interaction with manganese: Implication in the treatment of cirrhosis-associated central nervous system complications. J. Biochem. Mol. Toxicol. 2018, 32, e22216. [Google Scholar] [CrossRef] [PubMed]
- Ommati, M.M.; Heidari, R.; Ghanbarinejad, V.; Abdoli, N.; Niknahad, H. Taurine Treatment Provides Neuroprotection in a Mouse Model of Manganism. Biol. Trace Elem. Res. 2018, 190, 384–395. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.-L.; Tang, S.; Meng, Z.-J.; He, Y.-Y.; Song, L.-Y.; Liu, Y.-P.; Ma, N.; Li, X.-Y.; Guo, S.-C. Taurine improves the spatial learning and memory ability impaired by sub-chronic manganese exposure. J. Biomed. Sci. 2014, 21, 51. [Google Scholar] [CrossRef] [PubMed]
- Gitler, A.D.; Chesi, A.; Geddie, M.L.; Strathearn, K.E.; Hamamichi, S.; Hill, K.J.; Caldwell, K.A.; Caldwell, G.A.; Cooper, A.A.; Rochet, J.C.; et al. Alpha-Synuclein is part of a diverse and highly conserved interaction network that includes PARK9 and manganese toxicity. Nat. Genet. 2009, 41, 308–315. [Google Scholar] [CrossRef]
- Tan, J.; Zhang, T.; Jiang, L.; Chi, J.; Hu, D.; Pan, Q.; Wang, D.; Zhang, Z. Regulation of Intracellular Manganese Homeostasis by Kufor-Rakeb Syndrome-associated ATP13A2 Protein. J. Biol. Chem. 2011, 286, 29654–29662. [Google Scholar] [CrossRef] [PubMed]
- Harischandra, D.S.; Jin, H.; Anantharam, V.; Kanthasamy, A.; Kanthasamy, A.G. α-Synuclein Protects Against Manganese Neurotoxic Insult During the Early Stages of Exposure in a Dopaminergic Cell Model of Parkinson’s Disease. Toxicol. Sci. 2014, 143, 454–468. [Google Scholar] [CrossRef]
- Pessoa, J.C.; Etcheverry, S.; Gambino, D. Vanadium compounds in medicine. Coord. Chem. Rev. 2015, 301–302, 24–48. [Google Scholar] [CrossRef]
- Rehder, D. Bioinorganic Vanadium Chemistry; John Wiley & Sons: Chichester, UK; Hoboken, NJ, USA, 2008; 213p. [Google Scholar]
- Rehder, D. The potentiality of vanadium in medicinal applications. Future Med. Chem. 2012, 4, 1823–1837. [Google Scholar] [CrossRef]
- Barceloux, D.G. Vanadium. J. Toxicol. Clin. Toxicol. 1999, 37, 265–278. [Google Scholar] [CrossRef]
- Amorim, F.A.C.; Welz, B.; Costa, A.C.S.; Lepri, F.G.; Vale, M.G.R.; Ferreira, S.L.C. Determination of vanadium in petroleum and petroleum products using atomic spectrometric techniques. Talanta 2007, 72, 349–359. [Google Scholar] [CrossRef]
- Pyrzyńska, K.; Wierzbicki, T. Determination of vanadium species in environmental samples. Talanta 2004, 64, 823–829. [Google Scholar] [CrossRef] [PubMed]
- McNeilly, J.D.; Heal, M.R.; Beverland, I.J.; Howe, A.; Gibson, M.D.; Hibbs, L.R.; MacNee, W.; Donaldson, K. Soluble transition metals cause the pro-inflammatory effects of welding fumes in vitro. Toxicol. Appl. Pharmacol. 2004, 196, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Schlesinger, W.H.; Klein, E.M.; Vengosh, A. Global biogeochemical cycle of vanadium. Proc. Natl. Acad. Sci. USA 2017, 114, E11092–E11100. [Google Scholar] [CrossRef]
- Ścibior, A.; Pietrzyk, .; Plewa, Z.; Skiba, A. Vanadium: Risks and possible benefits in the light of a comprehensive overview of its pharmacotoxicological mechanisms and multi-applications with a summary of further research trends. J. Trace Elem. Med. Biol. 2020, 61, 126508. [Google Scholar] [CrossRef] [PubMed]
- Thompson, K.H.; Lichter, J.; LeBel, C.; Scaife, M.C.; McNeill, J.H.; Orvig, C. Vanadium treatment of type 2 diabetes: A view to the future. J. Inorg. Biochem. 2009, 103, 554–558. [Google Scholar] [CrossRef]
- Korbecki, J.; Baranowska-Bosiacka, I.; Gutowska, I.; Chlubek, D. Biochemical and medical importance of vanadium compounds. Acta Biochim. Pol. 2012, 59, 195–200. [Google Scholar] [CrossRef]
- Shechter, Y.; Shisheva, A. Vanadium salts and the future treatment of diabetes. Endeavour 1993, 17, 27–31. [Google Scholar] [CrossRef]
- Bishayee, A.; Waghray, A.; Patel, M.A.; Chatterjee, M. Vanadium in the detection, prevention and treatment of cancer: The in vivo evidence. Cancer Lett. 2010, 294, 1–12. [Google Scholar] [CrossRef]
- Myron, D.R.; Givand, S.H.; Nielsen, F.H. Vanadium content of selected foods as determined by flameless atomic absorption spectroscopy. J. Agric. Food Chem. 1977, 25, 297–300. [Google Scholar] [CrossRef]
- Byrne, A.; Kosta, L. Vanadium in foods and in human body fluids and tissues. Sci. Total Environ. 1978, 10, 17–30. [Google Scholar] [CrossRef]
- Hansen, T.V.; Aaseth, J.; Alexander, J. The effect of chelating agents on vanadium distribution in the rat body and on uptake by human erythrocytes. Arch. Toxicol. 1982, 50, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Mussali-Galante, P.; Rodríguez-Lara, V.; Hernández-Tellez, B.; Avila-Costa, M.R.; Colín-Barenque, L.; Bizarro-Nevarez, P.; Martínez-Levy, G.; Rojas-Lemus, M.; Piñón-Zarate, G.; Saldivar-Osorio, L.; et al. Inhaled vanadium pentoxide decrease gamma-tubulin of mouse testes at different exposure times. Toxicol. Ind. Health 2005, 21, 215–222. [Google Scholar] [CrossRef]
- Kiss, T.; Kiss, E.; Garribba, E.; Sakurai, H. Speciation of insulin-mimetic VO(IV)-containing drugs in blood serum. J. Inorg. Biochem. 2000, 80, 65–73. [Google Scholar] [CrossRef]
- Fatola, O.I.; Olaolorun, F.A.; Olopade, F.E.; Olopade, J.O. Trends in vanadium neurotoxicity. Brain Res. Bull. 2019, 145, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Garcia, G.B.; Biancardi, M.E.; Quiroga, A.D. Vanadium (V)-Induced Neurotoxicity in the Rat Central Nervous System: A Histo-Immunohistochemical Study. Drug Chem. Toxicol. 2005, 28, 329–344. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.P.; Coulombe, R.A.; Srisuchart, B. Effects of dietary vanadium exposure on levels of regional brain neurotransmitters and their metabolites. Biochem. Pharmacol. 1986, 35, 461–465. [Google Scholar] [CrossRef]
- Sanchez, D.J.; Colomina, M.T.; Domingo, J.L. Effects of Vanadium on Activity and Learning in Rats. Physiol. Behav. 1998, 63, 345–350. [Google Scholar] [CrossRef]
- Folarin, O.; Olopade, F.; Onwuka, S.; Olopade, J. Memory Deficit Recovery after Chronic Vanadium Exposure in Mice. Oxidative Med. Cell. Longev. 2016, 2016, 4860582. [Google Scholar] [CrossRef]
- Soazo, M.; Garcia, G.B. Vanadium exposure through lactation produces behavioral alterations and CNS myelin deficit in neonatal rats. Neurotoxicol. Teratol. 2007, 29, 503–510. [Google Scholar] [CrossRef]
- Mustapha, O.A.; Oke, B.; Offen, N.; Sirén, A.-L.; Olopade, J. Neurobehavioral and cytotoxic effects of vanadium during oligodendrocyte maturation: A protective role for erythropoietin. Environ. Toxicol. Pharmacol. 2014, 38, 98–111. [Google Scholar] [CrossRef]
- Ohiomokhare, S.; Olaolorun, F.; Ladagu, A.; Olopade, F.; Howes, M.-J.; Okello, E.; Olopade, J.; Chazot, P. The Pathopharmacological Interplay between Vanadium and Iron in Parkinson’s Disease Models. Int. J. Mol. Sci. 2020, 21, 6719. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.-L.; Feng, C.-Y.; Lan, Y.-J.; Wang, Z.-M.; Huang, S.; Wang, M.-Z.; Zhu, T. Paired-control study on the effect of vanadium on neurobehavioral functions. Sichuan Da Xue Xue Bao. Yi Xue Ban J. Sichuan Univ. Med Sci. Ed. 2007, 38, 468–470. [Google Scholar]
- Li, H.; Zhou, D.; Zhang, Q.; Feng, C.; Zheng, W.; He, K.; Lan, Y. Vanadium exposure-induced neurobehavioral alterations among Chinese workers. NeuroToxicology 2013, 36, 49–54. [Google Scholar] [CrossRef]
- Schlake, H.-P.; Bertram, H.P.; Husstedt, I.W.; Schuierer, G. Acute systemic vanadate poisoning presenting as cerebrovascular ischemia with prolonged reversible neurological deficits (PRIND). Clin. Neurol. Neurosurg. 1994, 96, 92–95. [Google Scholar] [CrossRef]
- Bonner, J.C.; Rice, A.B.; Moomaw, C.R.; Morgan, D.L. Airway fibrosis in rats induced by vanadium pentoxide. Am. J. Physiol. Cell. Mol. Physiol. 2000, 278, L209–L216. [Google Scholar] [CrossRef] [PubMed]
- Woodin, M.A.; Liu, Y.; Neuberg, D.; Hauser, R.; Smith, T.J.; Christiani, D.C. Acute respiratory symptoms in workers exposed to vanadium-rich fuel-oil ash. Am. J. Ind. Med. 2000, 37, 353–363. [Google Scholar] [CrossRef]
- Irsigler, G.B.; Visser, P.J.; Spangenberg, P.A. Asthma and chemical bronchitis in vanadium plant workers. Am. J. Ind. Med. 1999, 35, 366–374. [Google Scholar] [CrossRef]
- Fortoul, T.I.; Piñón-Zárate, G.; Diaz-Bech, M.E.; Gonzalez-Villalva, A.; Mussali-Galante, P.; Rodriguez-Lara, V.; Colin-Barenque, L.; Martinez-Pedraza, M.; Montaño, L.F. Spleen and bone marrow megakaryocytes as targets for inhaled vanadium. Histol. Histopathol. 2008, 23, 1321–1326. [Google Scholar]
- González-Villalva, A.E.; Falcon-Rodriguez, C.I.; Der Goes, T.I.F.-V. Signaling pathways involved in megakaryopoiesis. Gac. Med. Mex 2010, 146, 136–143. [Google Scholar]
- Gonzalez-Villalva, A.; Fortoul, T.I.; Avila-Costa, M.R.; Piñón-Zárate, G.; Rodriguez-Laraa, V.; Martínez-Levy, G.A.; Rojas-Lemus, M.; Bizarro-Nevarez, P.; Díaz-Bech, P.; Mussali-Galante, P.; et al. Thrombocytosis induced in mice after subacute and subchronic V2O5 inhalation. Toxicol. Ind. Health 2006, 22, 113–116. [Google Scholar] [CrossRef]
- Al-Bayati, M.A.; Giri, S.N.; Raabe, O.G.; Rosenblatt, L.S.; Shifrine, M. Time and dose-response study of the effects of vanadate on rats: Morphological and biochemical changes in organs. J. Environ. Pathol. Toxicol. Oncol. 1989, 9, 435–455. [Google Scholar]
- Lahav, M.; Rennert, H.; Barzilai, D. Inhibition by vanadate of cyclic AMP production in rat corpora lutea incubated in vitro. Life Sci. 1986, 39, 2557–2564. [Google Scholar] [CrossRef]
- Fortoul, T.; Bizarronevares, P.; Acevedonava, S.; Pinonzarate, G.; Rodriguezlara, V.; Colinbarenque, L.; Mussaligalante, P.; Avilacasado, M.; Avilacosta, M.; Saldivarosorio, L. Ultrastructural findings in murine seminiferous tubules as a consequence of subchronic vanadium pentoxide inhalation. Reprod. Toxicol. 2007, 23, 588–592. [Google Scholar] [CrossRef] [PubMed]
- Sjoberg, S.G. Vanadium pentoxide Dust. A clinical and experimental investigation on its effect after inhalation. Acta Med. Scand. Suppl. 1950, 238, 1–188. [Google Scholar]
- Valko, M.; Morris, H.; Cronin, M.T. Metals, toxicity and oxidative stress. Curr. Med. Chem. 2005, 12, 1161–1208. [Google Scholar] [CrossRef] [PubMed]
- Todorich, B.; Olopade, J.O.; Surguladze, N.; Zhang, X.; Neely, E.; Connor, J.R. The Mechanism of Vanadium-Mediated Developmental Hypomyelination Is Related to Destruction of Oligodendrocyte Progenitors Through a Relationship with Ferritin and Iron. Neurotox. Res. 2010, 19, 361–373. [Google Scholar] [CrossRef]
- Ding, M.; Gannett, P.; Rojanasakul, Y.; Liu, K.; Shi, X. One-electron reduction of vanadate by ascorbate and related free radical generation at physiological pH. J. Inorg. Biochem. 1994, 55, 101–112. [Google Scholar] [CrossRef]
- Shi, X.; Dalal, N.S. Hydroxyl Radical Generation in the Nadh/Microsomal Reduction of vanadate. Free Radic. Res. Commun. 1992, 17, 369–376. [Google Scholar] [CrossRef]
- Shi, X.; Dalal, N. Vanadate-Mediated Hydroxyl Radical Generation from Superoxide Radical in the Presence of NADH: Haber-Weiss vs Fenton Mechanism. Arch. Biochem. Biophys. 1993, 307, 336–341. [Google Scholar] [CrossRef]
- Capella, L.S.; Gefé, M.R.; Silva, E.F.; Affonso-Mitidieri, O.; Lopes, A.G.; Rumjanek, V.M.; Capella, M.A. Mechanisms of vanadate-induced cellular toxicity: Role of cellular glutathione and NADPH. Arch. Biochem. Biophys. 2002, 406, 65–72. [Google Scholar] [CrossRef]
- Crans, D.C.; Zhang, B.; Gaidamauskas, E.; Keramidas, A.D.; Willsky, G.R.; Roberts, C.R. Is vanadate reduced by thiols under biological conditions? Changing the redox potential of V(V)/V(IV) by complexation in aqueous solution. Inorg. Chem. 2010, 49, 4245–4256. [Google Scholar] [CrossRef] [PubMed]
- Huyer, G.; Liu, S.; Kelly, J.; Moffat, J.; Payette, P.; Kennedy, B.; Tsaprailis, G.; Gresser, M.J.; Ramachandran, C. Mechanism of Inhibition of Protein-tyrosine Phosphatases by Vanadate and Pervanadate. J. Biol. Chem. 1997, 272, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.-G.; Zhang, Z.-Y. Redox regulation of protein tyrosine phosphatase activity by hydroxyl radical. Biochim. Biophys. Acta (BBA) Proteins Proteom. 2012, 1834, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Sturla, L.-M.; Amorino, G.; Alexander, M.S.; Mikkelsen, R.B.; Valerie, K.; Schmidt-Ullrichr, R.K. Requirement of Tyr-992 and Tyr-1173 in Phosphorylation of the Epidermal Growth Factor Receptor by Ionizing Radiation and Modulation by SHP2. J. Biol. Chem. 2005, 280, 14597–14604. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Esselman, W.J. Inhibition of PTPs by H2O2 regulates the activation of distinct MAPK pathways. Free Radic. Biol. Med. 2002, 33, 1121–1132. [Google Scholar] [CrossRef]
- Zhao, Z.; Tan, Z.; Diltz, C.D.; You, M.; Fischer, E.H.; Zaia, J.; Boynton, R.E.; McIntosh, A.; Marshak, D.R.; Olsson, H.; et al. Activation of Mitogen-activated Protein (MAP) Kinase Pathway by Pervanadate, a Potent Inhibitor of Tyrosine Phosphatases. J. Biol. Chem. 1996, 271, 22251–22255. [Google Scholar] [CrossRef] [PubMed]
- Chien, P.-S.; Mak, O.-T.; Huang, H.-J. Induction of COX-2 protein expression by vanadate in A549 human lung carcinoma cell line through EGF receptor and p38 MAPK-mediated pathway. Biochem. Biophys. Res. Commun. 2006, 339, 562–568. [Google Scholar] [CrossRef]
- Migliore, L.; Coppedè, F. Environmental-induced oxidative stress in neurodegenerative disorders and aging. Mutat. Res. Toxicol. Environ. Mutagen. 2009, 674, 73–84. [Google Scholar] [CrossRef]
- Brown, T.P.; Rumsby, P.C.; Capleton, A.C.; Rushton, L.; Levy, L.S. Pesticides and Parkinson’s disease--is there a link? Environ. Health Perspect. 2006, 114, 156–164. [Google Scholar] [CrossRef]
- Suntres, Z.E. Role of antioxidants in paraquat toxicity. Toxicology 2002, 180, 65–77. [Google Scholar] [CrossRef]
- Uversky, V.N. Neurotoxicant-induced animal models of Parkinson’s disease: Understanding the role of rotenone, maneb and paraquat in neurodegeneration. Cell Tissue Res. 2004, 318, 225–241. [Google Scholar] [CrossRef]
- Costa, C.; Teodoro, M.; Rugolo, C.A.; Alibrando, C.; Giambò, F.; Briguglio, G.; Fenga, C. MicroRNAs alteration as early biomarkers for cancer and neurodegenerative diseases: New challenges in pesticides exposure. Toxicol. Rep. 2020, 7, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Aloizou, A.M.; Siokas, V.; Sapouni, E.M.; Sita, N.; Liampas, I.; Brotis, A.G.; Rakitskii, V.N.; Burykina, T.I.; Aschner, M.; Bogdanos, D.P.; et al. Parkinson’s disease and pesticides: Are microRNAs the missing link? Sci. Total Environ. 2020, 744, 140591. [Google Scholar] [CrossRef] [PubMed]
- Caggiu, E.; Paulus, K.; Mameli, G.; Arru, G.; Sechi, G.P.; Sechi, L.A. Differential expression of miRNA 155 and miRNA 146a in Parkinson’s disease patients. eNeurologicalSci 2018, 13, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Goh, S.Y.; Chao, Y.X.; Dheen, S.T.; Tan, E.-K.; Tay, S.S.-W. Role of MicroRNAs in Parkinson’s Disease. Int. J. Mol. Sci. 2019, 20, 5649. [Google Scholar] [CrossRef]
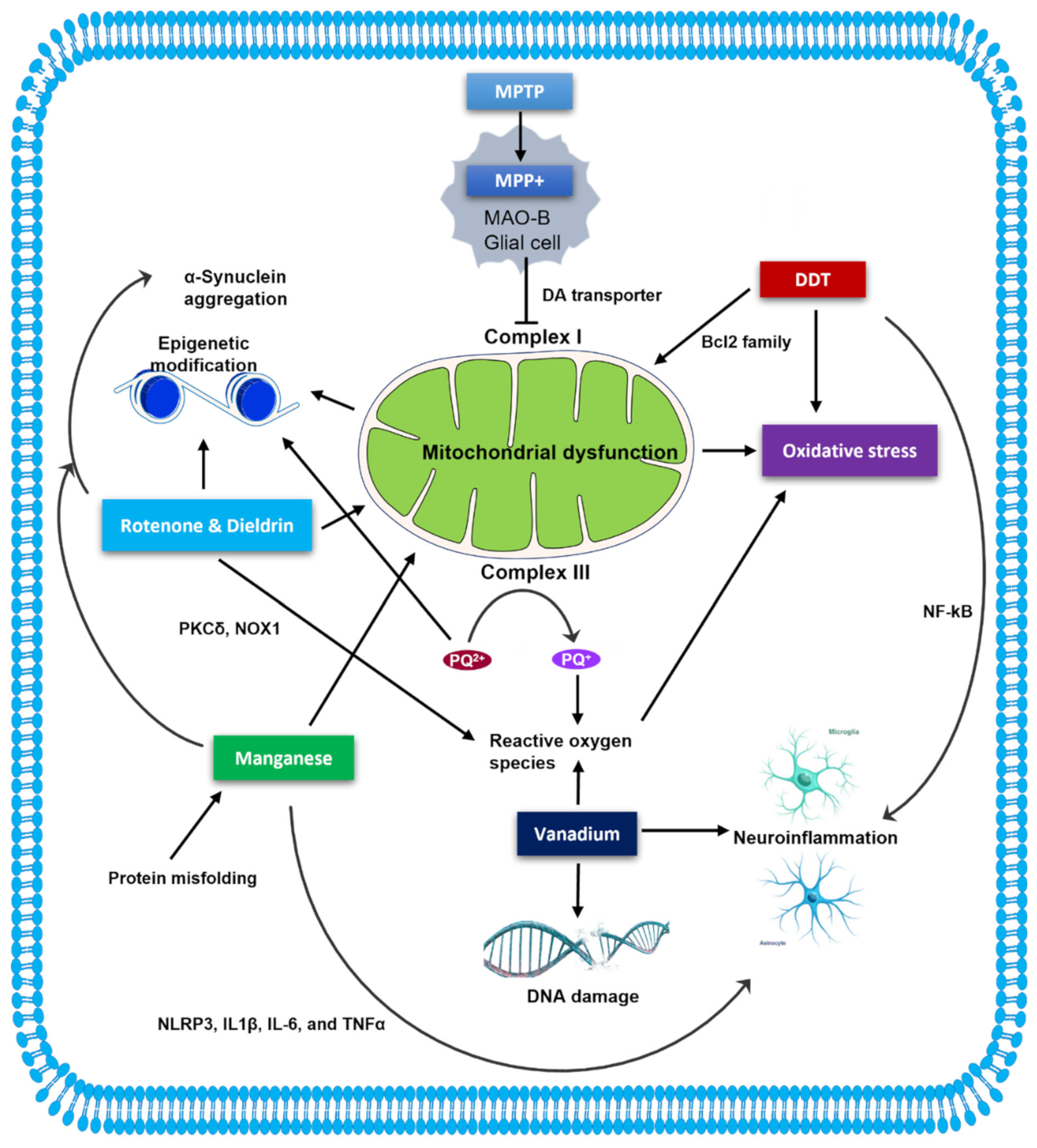
| Neurotoxins | Pathogenesis | Mechanism of Toxicity | Molecular and Cellular Alterations | References |
|---|---|---|---|---|
| MPTP | - DAergic neurons in SNPc ↓ - Striatal DA ↓ - Neurodegeneration in locus coeruleus - α-synuclein ↑ - Astrogliosis and microglial activation | - Crosses BBB and metabolized into the toxic cation MPP+ - Taken up by DA neurons via DAT - Concentrates in mitochondria and causes complex I defect - Reactive oxygen species ↑ | - Mitochondrial fragmentation and mitophagy ↑ - Mitochondrial biogenesis ↓ - Intracellular Ca2+ ions ↑ - NFκB- dependent transactivation of iNOS, ↑ JNK and Bax, cytochrome c release, and caspase-3 and -9 activation leading to apoptosis | [13,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49] |
| Rotenone | - DAergic neurodegeneration - α-synuclein rich LB-like inclusions - TH ↓ - Microglial activation and augmented neuroinflammation | - Crosses BBB - Mitochondrial complex I inhibition - Reactive oxygen species ↑ - Microtubule destabilizing activity | - ATP synthesis ↓ - Mitochondrial fragmentation ↑ and altered mitochondrial fission/fusion and biogenesis - Intracellular Ca2+ ions ↑ - Altered PI3K/Akt/GSK-3β/CREB signaling pathway - Mitochondrial impairment by Parkin ↓ and PINK1 ↑ - Caspase-3 and -9 activation leading to apoptosis | [50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72] |
| Paraquat | - DAergic neurons in SN ↓ - α-synuclein accumulation and aggregation | - Enters DAergic neurons via DAT - Generates oxygen-free radicals - Produces H202 via mitochondrial complex III | - Oxidative stress, cytochrome c release, caspase-3 and -9 activation, mitophagy and apoptosis - Ca2+ dyshomeostasis - Altered DA signaling pathway - Dysregulation of histone acetylation | [27,73,74,75,76,77,78,79] |
| DDT | Inconclusive evidence | - CNS excitation by sustained depolarization of nerve membrane - Mitochondrial complex II and V inhibition | - Cytosolic Ca2+ release and activation of apoptotic factors - Bcl2 ↓ and apoptosis induction through caspase-3 and -9 and GSK-3β - p53, NFκB and caspase-3 ↑ - Inhibition of DAT and VMAT | [18,80,81,82,83,84] |
| Dieldrin | - DAergic neurodegeneration | - Crosses BBB - May impair mitochondrial electron transport chain - Exacerbates MPTP and α-synuclein pre-formed fibril-mediated toxicity | - Mitochondrial dysfunction and oxidative stress - Caspase-3 activity ↑ and Fyn-mediated PKCδ activation followed by apoptosis - Ubiquitin-proteasome system dysfunction - Histone 3 and 4 acetylation ↑ | [19,20,85,86,87,88,89,90] |
| Manganese | - DAergic neurons in SNPc ↓ - Striatal DA ↓ - Olfactory dysfunction - Microglial activation - Impaired neurogenesis | - Reactive oxygen species ↑ | - Mitochondrial dysfunction and Ca2+ homeostasis disruption - Altered mitochondrial fission/fusion and biogenesis - Apoptosis-associated activation of PKCδ - Release of proinflammatory cytokines IL-1β, IL-6 and TNFα - Epigenetic dysregulation | [34,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114] |
| Vanadium | - DAergic neurotransmission in olfactory bulb ↓ - Hippocampal CA1 pyramidal and cerebellar Purkinje cells ↓ - Changes in catecholaminergic levels - Astrogliosis and microgliosis | - Crosses BBB - Reactive oxygen species ↑ | - Mitochondrial oxidative stress - Cytochrome c release, caspase-3 and -9 activation, and PKCδ activation leading to apoptosis | [115,116,117,118,119,120,121,122,123] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, M.; Bargues-Carot, A.; Riaz, Z.; Wickham, H.; Zenitsky, G.; Jin, H.; Anantharam, V.; Kanthasamy, A.; Kanthasamy, A.G. Impact of Environmental Risk Factors on Mitochondrial Dysfunction, Neuroinflammation, Protein Misfolding, and Oxidative Stress in the Etiopathogenesis of Parkinson’s Disease. Int. J. Mol. Sci. 2022, 23, 10808. https://doi.org/10.3390/ijms231810808
Huang M, Bargues-Carot A, Riaz Z, Wickham H, Zenitsky G, Jin H, Anantharam V, Kanthasamy A, Kanthasamy AG. Impact of Environmental Risk Factors on Mitochondrial Dysfunction, Neuroinflammation, Protein Misfolding, and Oxidative Stress in the Etiopathogenesis of Parkinson’s Disease. International Journal of Molecular Sciences. 2022; 23(18):10808. https://doi.org/10.3390/ijms231810808
Chicago/Turabian StyleHuang, Minhong, Alejandra Bargues-Carot, Zainab Riaz, Hannah Wickham, Gary Zenitsky, Huajun Jin, Vellareddy Anantharam, Arthi Kanthasamy, and Anumantha G. Kanthasamy. 2022. "Impact of Environmental Risk Factors on Mitochondrial Dysfunction, Neuroinflammation, Protein Misfolding, and Oxidative Stress in the Etiopathogenesis of Parkinson’s Disease" International Journal of Molecular Sciences 23, no. 18: 10808. https://doi.org/10.3390/ijms231810808
APA StyleHuang, M., Bargues-Carot, A., Riaz, Z., Wickham, H., Zenitsky, G., Jin, H., Anantharam, V., Kanthasamy, A., & Kanthasamy, A. G. (2022). Impact of Environmental Risk Factors on Mitochondrial Dysfunction, Neuroinflammation, Protein Misfolding, and Oxidative Stress in the Etiopathogenesis of Parkinson’s Disease. International Journal of Molecular Sciences, 23(18), 10808. https://doi.org/10.3390/ijms231810808






