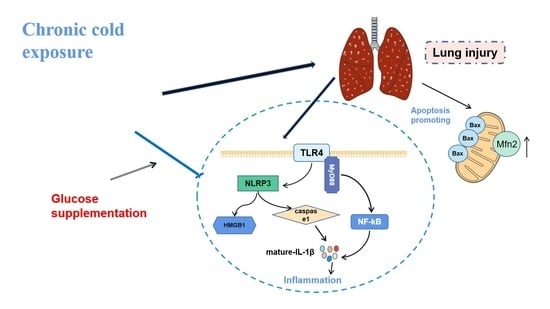
Activation of Inflammatory Networks in the Lungs Caused by Chronic Cold Stress Is Moderately Attenuated by Glucose Supplementation
Abstract
:1. Introduction
2. Results
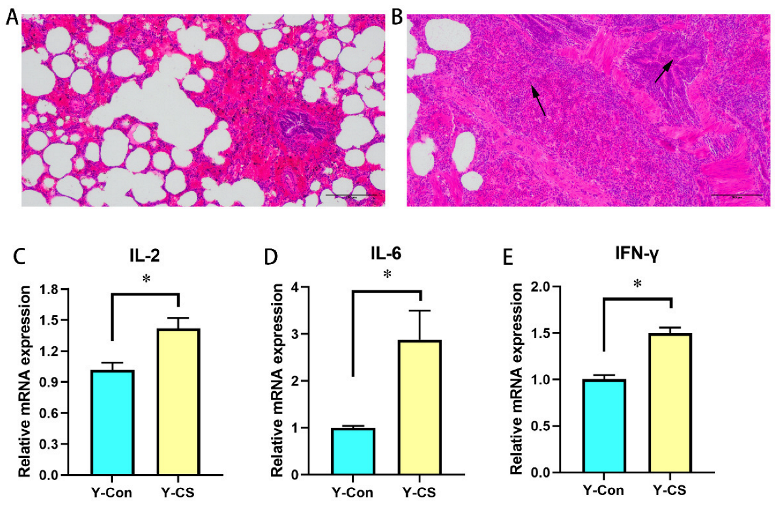
2.1. Chronic Cold Stress Induces Lung Injury and Enhances Inflammatory Factors Expression in Lungs
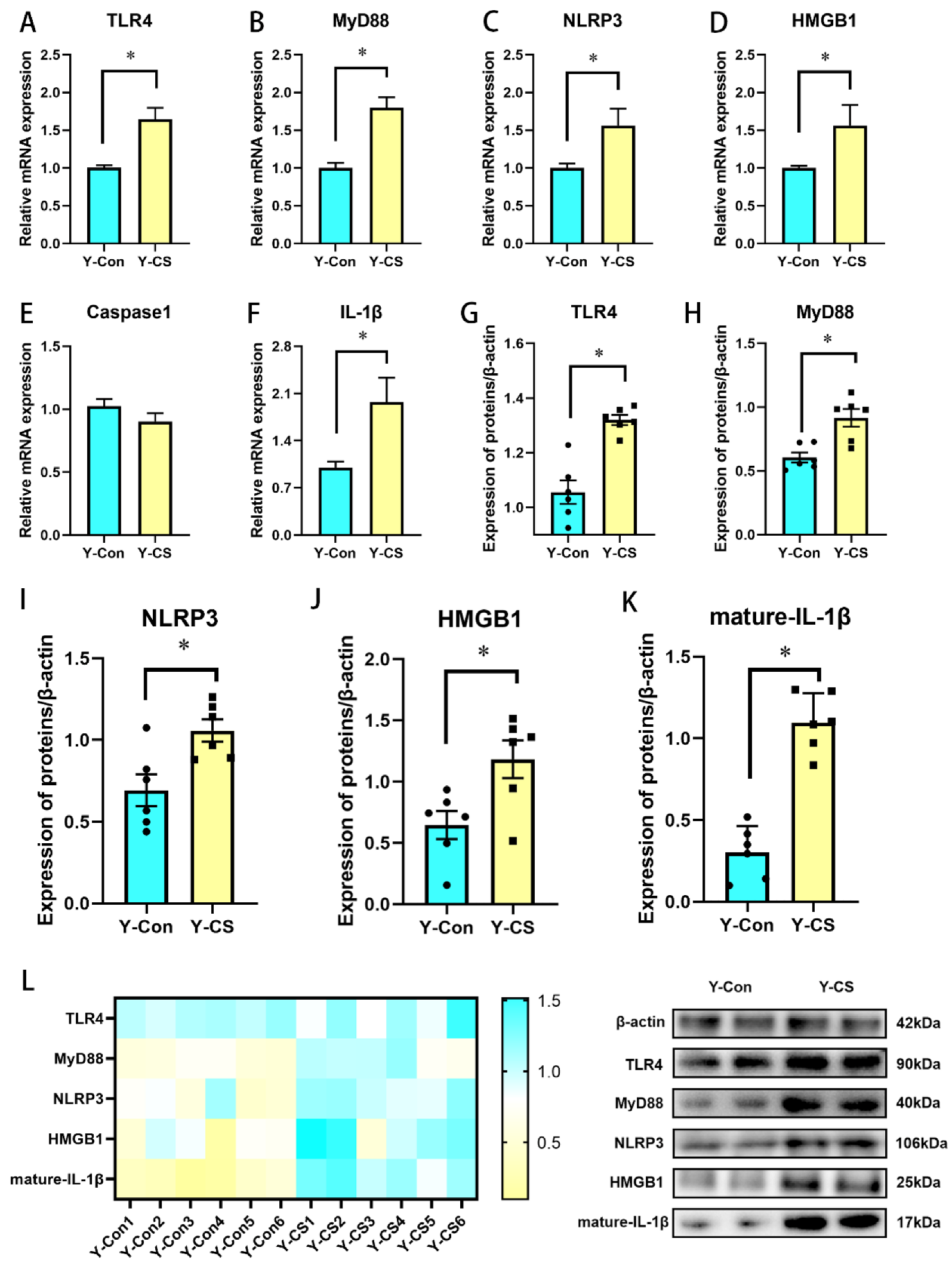
2.2. Chronic Cold Stress Activates the TLR4/MyD88 Pathway and Pyroptosis
2.3. Chronic Cold Stress Promotes Apoptosis and Inhibits the Expression of Brain-Derived Nutritional Factors in Lungs
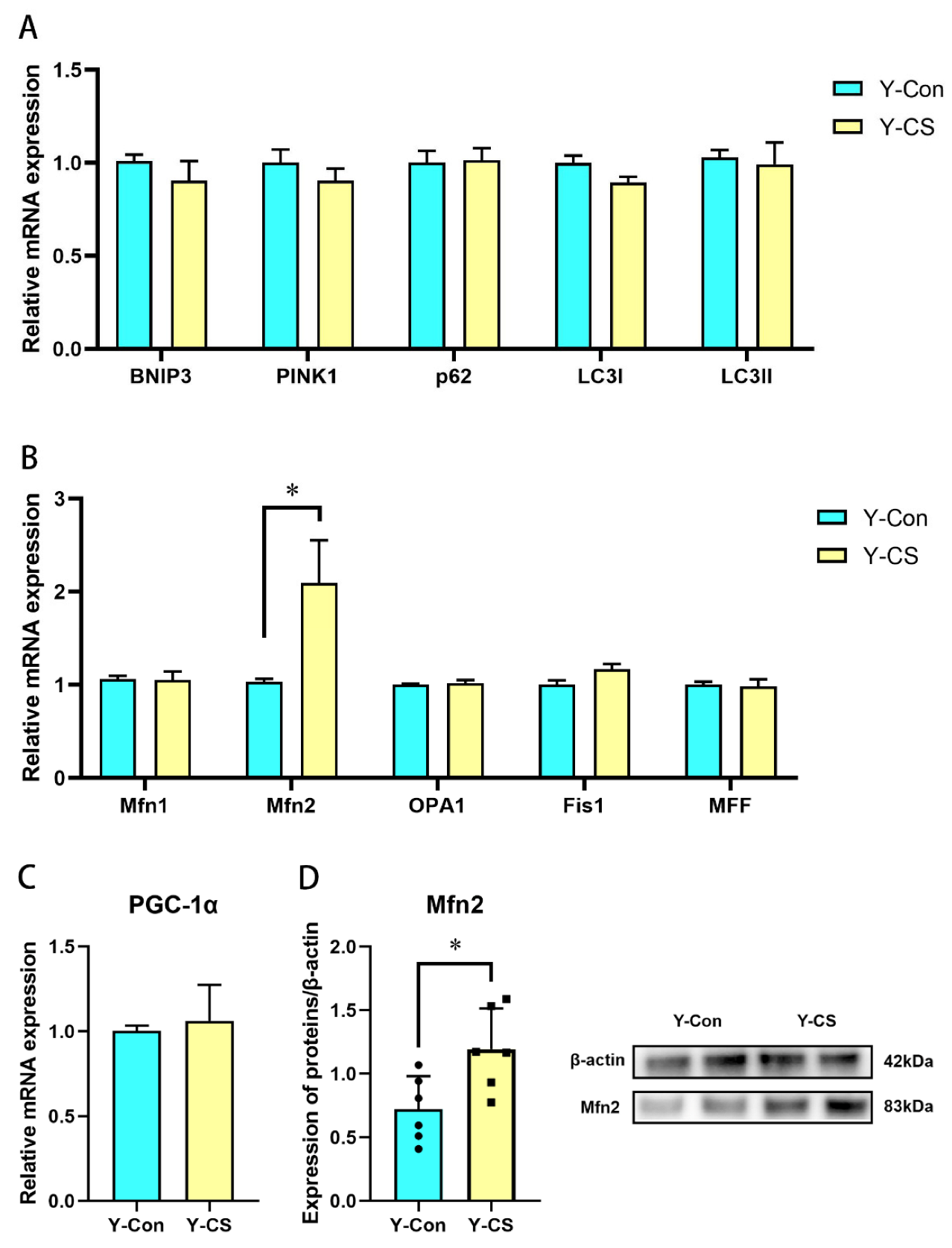
2.4. Chronic Cold Stress Promotes Mitochondrial Fusion in Lungs
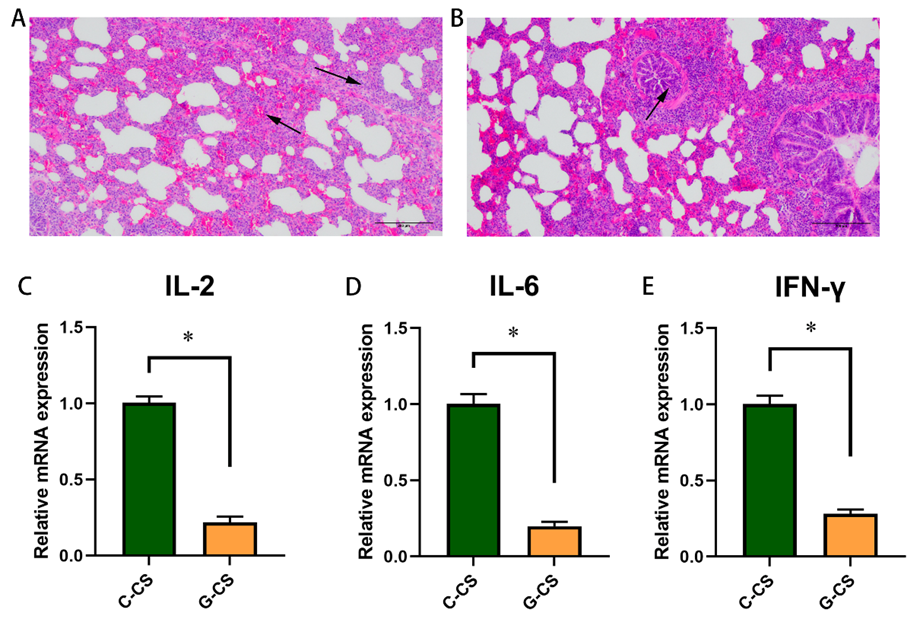
2.5. Glucose Supplementation Alleviates Lung Injury Induced by Chronic Cold Stress to a Certain Extent
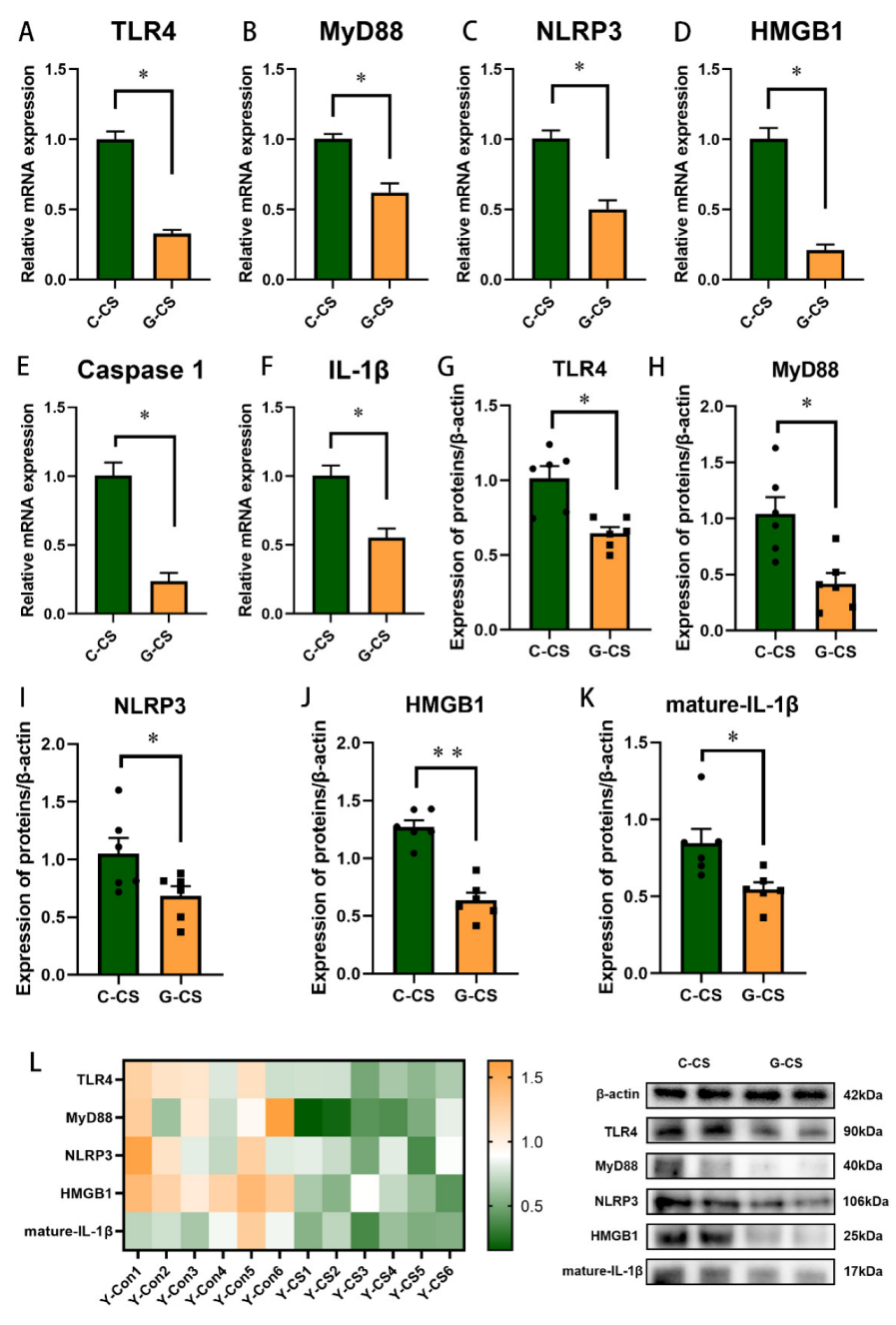
2.6. Glucose Supplementation Inhibits TLR4/MyD88 Pathways and Pyroptosis in Lungs during Chronic Cold Stress
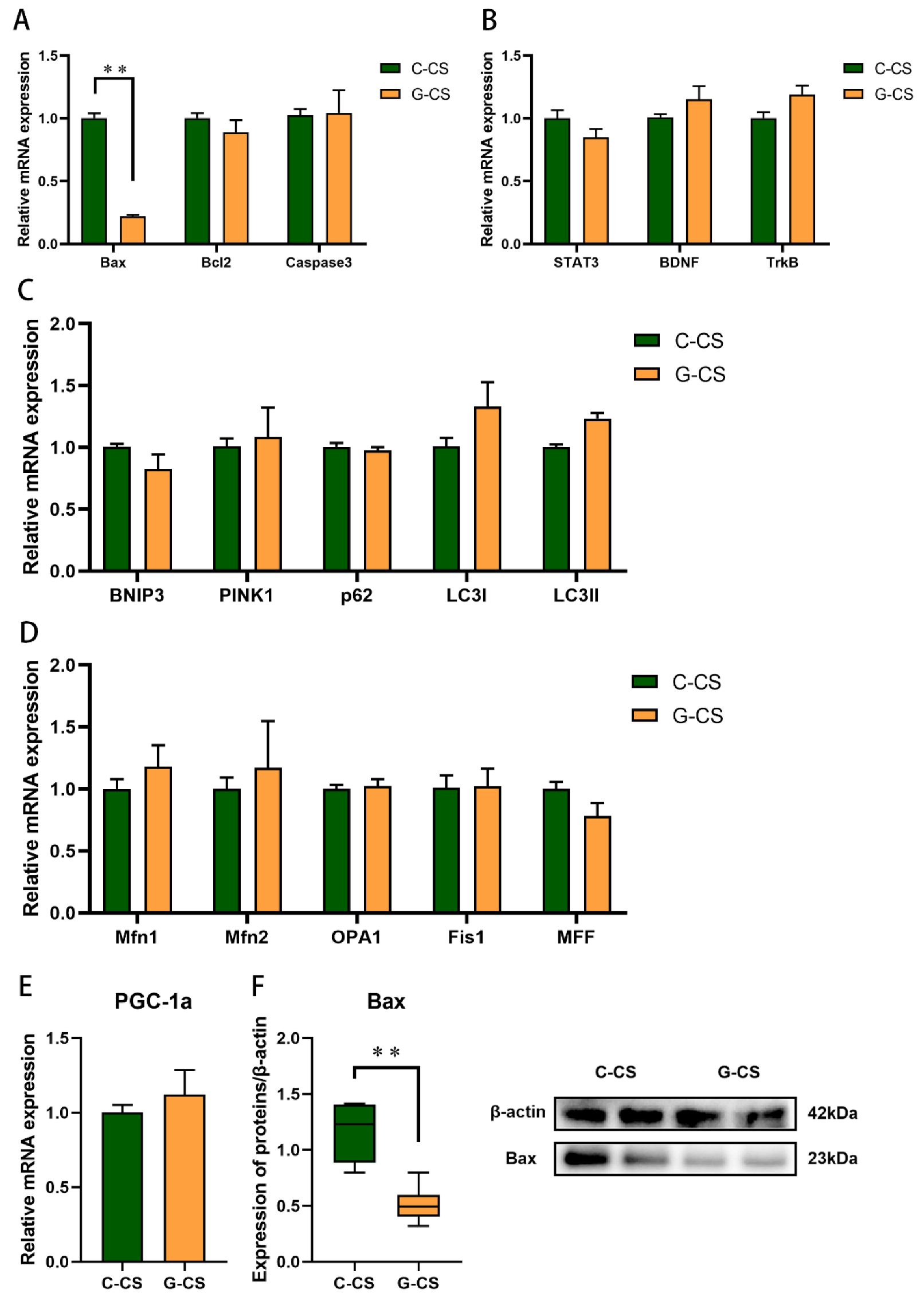
2.7. Glucose Supplementation Inhibits Apoptosis Induced by Chronic Cold Stress but Don’t Modulate Mitochondrial Function in Lungs
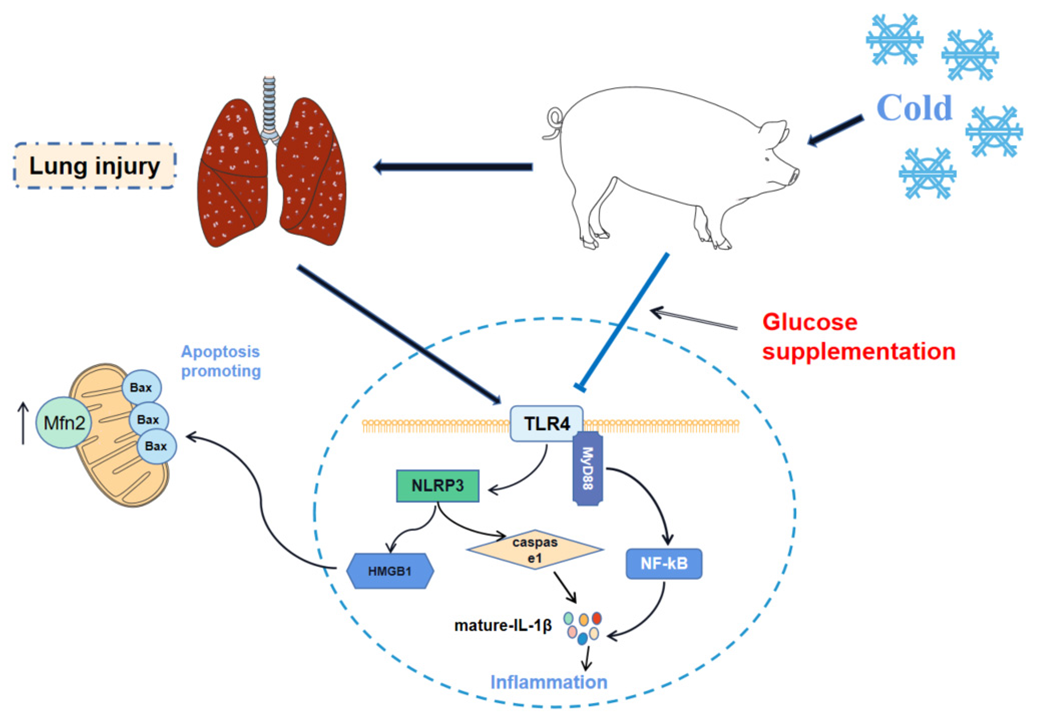
3. Discussion
4. Materials and Methods
4.1. Animals, Administrations, and Procedures
4.2. Sample Collection
4.3. Hematoxylin and Eosin (HE) Staining
4.4. Total RNA Extraction, Reverse Transcription, and Relative Quantitative Real-Time PCR
4.5. Western Blot Analysis
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Cheung, S.S.; Lee, J.K.W.; Oksa, J. Thermal stress, human performance, and physical employment standards. Appl. Physiol. Nutr. Metab. 2016, 41, S148–S164. [Google Scholar] [CrossRef] [PubMed]
- Tirloni, A.S.; dos Reis, D.C.; Dias, N.F.; Moro, A.R.P. The Use of Personal Protective Equipment: Finger Temperatures and Thermal Sensation of Workers’ Exposure to Cold Environment. Int. J. Environ. Res. Public Health 2018, 15, 2583. [Google Scholar] [CrossRef] [PubMed]
- Thetkathuek, A.; Yingratanasuk, T.; Jaidee, W.; Ekburanawat, W. Cold exposure and health effects among frozen food processing workers in eastern Thailand. Saf. Health Work 2015, 6, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Ikaheimo, T.M. Cardiovascular diseases, cold exposure and exercise. Temperature 2018, 5, 123–146. [Google Scholar] [CrossRef]
- Castellani, J.W.; Young, A.J. Human physiological responses to cold exposure: Acute responses and acclimatization to prolonged exposure. Auton. Neurosci.-Basic Clin. 2016, 196, 63–74. [Google Scholar] [CrossRef]
- Raatikka, V.P.; Rytkonen, M.; Nayha, S.; Hassi, J. Prevalence of cold-related complaints, symptoms and injuries in the general population: The FINRISK 2002 cold substudy. Int. J. Biometeorol. 2007, 51, 441–448. [Google Scholar] [CrossRef]
- Makinen, T.M.; Hassi, J. Health Problems in Cold Work. Ind. Health 2009, 47, 207–220. [Google Scholar] [CrossRef]
- Koskela, H.O. Cold air-provoked respiratory symptoms: The mechanisms and management. Int. J. Circumpolar Health 2007, 66, 91–100. [Google Scholar] [CrossRef]
- Luo, B.; Shi, H.X.; Zhang, K.; Wei, Q.Z.; Niu, J.P.; Wang, J.L.; Hammond, S.K.; Liu, S. Cold stress provokes lung injury in rats co-exposed to fine particulate matter and lipopolysaccharide. Ecotoxicol. Environ. Saf. 2019, 168, 9–16. [Google Scholar] [CrossRef]
- Peri, F.; Calabrese, V. Toll-like Receptor 4 (TLR4) Modulation by Synthetic and Natural Compounds: An Update. J. Med. Chem. 2014, 57, 3612–3622. [Google Scholar] [CrossRef]
- Doz, E.; Noulin, N.; Boichbt, E.; Guenon, I.; Fick, L.; Le Bert, M.; Lagente, V.; Ryffel, B.; Schnyder, B.; Quesniaux, V.F.J.; et al. Cigarette smoke-induced pulmonary inflammation is TLR4/MyD88 and IL-1R1/MyD88 signaling dependent. J. Immunol. 2008, 180, 1169–1178. [Google Scholar] [CrossRef]
- Murakami, K.; Kamimura, D.; Hasebe, R.; Uchida, M.; Abe, N.; Yamamoto, R.; Jiang, J.J.; Hidaka, Y.; Nakanishi, Y.; Fujita, S.; et al. Rhodobacter azotoformans LPS (RAP99-LPS) Is a TLR4 Agonist That Inhibits Lung Metastasis and Enhances TLR3-Mediated Chemokine Expression. Front. Immunol. 2021, 12, 675909. [Google Scholar] [CrossRef]
- Tschopp, J.; Schroder, K. NLRP3 inflammasome activation: The convergence of multiple signalling pathways on ROS production? Nat. Rev. Immunol. 2010, 10, 210–215. [Google Scholar] [CrossRef]
- McKee, C.M.; Coll, R.C. NLRP3 inflammasome priming: A riddle wrapped in a mystery inside an enigma. J. Leukoc. Biol. 2020, 108, 937–952. [Google Scholar] [CrossRef]
- Swanson, K.V.; Deng, M.; Ting, J.P.Y. The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef]
- Lamkanfi, M.; Sarkar, A.; Vande Walle, L.; Vitari, A.C.; Amer, A.O.; Wewers, M.D.; Tracey, K.J.; Kanneganti, T.D.; Dixit, V.M. Inflammasome-Dependent Release of the Alarmin HMGB1 in Endotoxemia. J. Immunol. 2010, 185, 4385–4392. [Google Scholar] [CrossRef]
- Song, B.; Sun, Y.; Chu, Y.F.; Wang, J.; Zheng, H.W.; Liu, L.L.; Cai, W.; Zhang, H.Q. Ginsenoside Rb1 Alleviated High-Fat-Diet-Induced Hepatocytic Apoptosis via Peroxisome Proliferator-Activated Receptor gamma. BioMed Res. Int. 2020, 2020, 2315230. [Google Scholar] [CrossRef]
- Brady, H.J.; Gil-Gomez, G. Bax. The pro-apoptotic Bcl-2 family member, Bax. Int. J. Biochem. Cell Biol. 1998, 30, 647–650. [Google Scholar] [CrossRef]
- Fraser, A.G. Programmed cell death in C. elegans. Cancer Metastasis Rev. 1999, 18, 285–294. [Google Scholar] [CrossRef]
- Ocobock, C. Human energy expenditure, allocation, and interactions in natural temperate, hot, and cold environments. Am. J. Phys. Anthropol. 2016, 161, 667–675. [Google Scholar] [CrossRef]
- Sanderson, T.H.; Raghunayakula, S.; Kumar, R. Release of mitochondrial Opa1 following oxidative stress in HT22 cells. Mol. Cell. Neurosci. 2015, 64, 116–122. [Google Scholar] [CrossRef]
- Sun, G.; Song, X.; Zou, Y.; Teng, T.; Jiang, L.; Shi, B. Dietary Glucose Ameliorates Impaired Intestinal Development and Immune Homeostasis Disorders Induced by Chronic Cold Stress in Pig Model. Int. J. Mol. Sci. 2022, 23, 7730. [Google Scholar] [CrossRef]
- Wang, M.; Donovan, S.M. Human Microbiota-Associated Swine: Current Progress and Future Opportunities. Ilar J. 2015, 56, 63–73. [Google Scholar] [CrossRef]
- Powell, E.J.; Cunnick, J.E.; Tuggle, C.K. SCID pigs: An emerging large animal NK model. J. Rare Dis. Res. Treat. 2017, 2, 1–6. [Google Scholar]
- Suzuki, T.; Kawamoto, S.; Nakagawa, A.; Endo, T.; Tominaga, T.; Akiyama, M.; Adachi, O.; Kumagai, K.; Saiki, Y. Application of actuator-driven pulsed water jet for coronary artery bypass grafting: Assessment in a swine model. J. Artif. Organs 2018, 21, 247–253. [Google Scholar] [CrossRef]
- Mourtzoukou, E.G.; Falagas, M.E. Exposure to cold and respiratory tract infections. Int. J. Tuberc. Lung Dis. 2007, 11, 938–943. [Google Scholar]
- Larsson, K.; Tornling, G.; Gavhed, D.; Muller-Suur, C.; Palmberg, L. Inhalation of cold air increases the number of inflammatory cells in the lungs in healthy subjects. Eur. Respir. J. 1998, 12, 825–830. [Google Scholar] [CrossRef]
- Zhou, L.; Chong, M.M.W.; Littman, D.R. Plasticity of CD4(+) T Cell Lineage Differentiation. Immunity 2009, 30, 646–655. [Google Scholar] [CrossRef]
- Nielsen, C.M.; Wolf, A.S.; Goodier, M.R.; Riley, E.M. Synergy between Common gamma Chain Family Cytokines and IL-18 Potentiates Innate and Adaptive Pathways of NK Cell Activation. Front. Immunol. 2016, 7, 101. [Google Scholar] [CrossRef]
- Hunter, C.A.; Jones, S.A. IL-6 as a keystone cytokine in health and disease. Nat. Immunol. 2015, 16, 448–457. [Google Scholar] [CrossRef]
- Beutler, B. Toll-like receptors: How they work and what they do. Curr. Opin. Hematol. 2002, 9, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.C.; Lo, Y.C.; Wu, H. Helical assembly in the MyD88-IRAK4-IRAK2 complex in TLR/IL-1R signalling. Nature 2010, 465, 885–890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Layoun, A.; Huang, H.; Calve, A.; Santos, M.M. Toll-Like Receptor Signal Adaptor Protein MyD88 Is Required for Sustained Endotoxin-Induced Acute Hypoferremic Response in Mice. Am. J. Pathol. 2012, 180, 2340–2350. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Bryant, C.E.; Symmons, M.; Gay, N.J. Toll-like receptor signalling through macromolecular protein complexes. Mol. Immunol. 2015, 63, 162–165. [Google Scholar] [CrossRef] [PubMed]
- Lv, T.F.; Shen, X.K.; Shi, Y.; Song, Y. TLR4 is Essential in Acute Lung Injury Induced by Unresuscitated Hemorrhagic Shock. J. Trauma-Inj. Infect. Crit. Care 2009, 66, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Schroder, K.; Tschopp, J. The Inflammasomes. Cell 2010, 140, 821–832. [Google Scholar] [CrossRef]
- Qu, J.; Tao, X.Y.; Teng, P.; Zhang, Y.; Guo, C.L.; Hu, L.; Qian, Y.N.; Jiang, C.Y.; Liu, W.T. Blocking ATP-sensitive potassium channel alleviates morphine tolerance by inhibiting HSP70-TLR4-NLRP3-mediated neuroinflammation. J. Neuroinflamm. 2017, 14, 228. [Google Scholar] [CrossRef]
- Chi, W.; Chen, H.R.; Li, F.; Zhu, Y.T.; Yin, W.; Zhuo, Y.H. HMGB1 promotes the activation of NLRP3 and caspase-8 inflammasomes via NF-kappa B pathway in acute glaucoma. J. Neuroinflamm. 2015, 12, 137. [Google Scholar] [CrossRef]
- Kim, E.J.; Park, S.Y.; Baek, S.E.; Jang, M.A.; Lee, W.S.; Bae, S.S.; Kim, K.; Kim, C.D. HMGB1 Increases IL-1 beta Production in Vascular Smooth Muscle Cells via NLRP3 Inflammasome. Front. Physiol. 2018, 9, 313. [Google Scholar] [CrossRef]
- Palsson-McDermott, E.M.; Curtis, A.M.; Goel, G.; Lauterbach, M.A.R.; Sheedy, F.J.; Gleeson, L.E.; van den Bosch, M.W.M.; Quinn, S.R.; Domingo-Fernandez, R.; Johnson, D.G.W.; et al. Pyruvate Kinase M2 Regulates Hif-1 alpha Activity and IL-1 beta Induction and Is a Critical Determinant of the Warburg Effect in LPS-Activated Macrophages. Cell Metab. 2015, 21, 65–80. [Google Scholar] [CrossRef]
- Mills, E.L.; Kelly, B.; Logan, A.; Costa, A.S.H.; Varma, M.; Bryant, C.E.; Tourlomousis, P.; Dabritz, J.H.M.; Gottlieb, E.; Latorre, I.; et al. Succinate Dehydrogenase Supports Metabolic Repurposing of Mitochondria to Drive Inflammatory Macrophages. Cell 2016, 167, 457–470. [Google Scholar] [CrossRef] [Green Version]
- Jha, A.K.; Huang, S.C.C.; Sergushichev, A.; Lampropoulou, V.; Ivanova, Y.; Loginicheva, E.; Chmielewski, K.; Stewart, K.M.; Ashall, J.; Everts, B.; et al. Network Integration of Parallel Metabolic and Transcriptional Data Reveals Metabolic Modules that Regulate Macrophage Polarization. Immunity 2015, 42, 419–430. [Google Scholar] [CrossRef]
- Oltvai, Z.N.; Milliman, C.L.; Korsmeyer, S.J. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell 1993, 74, 609–619. [Google Scholar] [CrossRef]
- Radzikinas, K.; Aven, L.; Jiang, Z.H.; Tran, T.; Paez-Cortez, J.; Boppidi, K.; Lu, J.N.; Fine, A.; Ai, X.B. A Shh/miR-206/BDNF Cascade Coordinates Innervation and Formation of Airway Smooth Muscle. J. Neurosci. 2011, 31, 15407–15415. [Google Scholar] [CrossRef]
- Paris, A.J.; Hayer, K.E.; Oved, J.H.; Avgousti, D.C.; Toulmin, S.A.; Zepp, J.A.; Zacharias, W.J.; Katzen, J.B.; Basil, M.C.; Kremp, M.M.; et al. STAT3-BDNF-TrkB signalling promotes alveolar epithelial regeneration after lung injury. Nat. Cell Biol. 2020, 22, 1197–1210. [Google Scholar] [CrossRef]
- Chan, P.H. Mitochondria and neuronal death/survival signaling pathways in cerebral ischemia. Neurochem. Res. 2004, 29, 1943–1949. [Google Scholar] [CrossRef]
- Anzell, A.R.; Maizy, R.; Przyklenk, K.; Sanderson, T.H. Mitochondrial Quality Control and Disease: Insights into Ischemia-Reperfusion Injury. Mol. Neurobiol. 2018, 55, 2547–2564. [Google Scholar] [CrossRef]
- Chen, H.C.; Detmer, S.A.; Ewald, A.J.; Griffin, E.E.; Fraser, S.E.; Chan, D.C. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J. Cell Biol. 2003, 160, 189–200. [Google Scholar]
- Cipolat, S.; de Brito, O.M.; Dal Zilio, B.; Scorrano, L. OPA1 requires mitofusin 1 to promote mitochondrial fusion. Proc. Natl. Acad. Sci. USA 2004, 101, 15927–15932. [Google Scholar] [CrossRef]
- Zheng, J.H.; Chen, M.H.; Fu, Z.Y.; Li, N.; Xie, L. PD98059 Protects Cerebral Cortex Mitochondria! Structure and Function at 48 h Post-Resuscitation in a Rat Model of Cardiac Arrest. Drug Des. Dev. Ther. 2020, 14, 1107–1115. [Google Scholar] [CrossRef]
- Vasquez-Trincado, C.; Garcia-Carvajal, I.; Pennanen, C.; Parra, V.; Hill, J.A.; Rothermel, B.A.; Lavandero, S. Mitochondrial dynamics, mitophagy and cardiovascular disease. J. Physiol.-Lond. 2016, 594, 509–525. [Google Scholar] [CrossRef]

| Basic Diet Ingredients | Content (%) |
|---|---|
| Corn | 73.00 |
| Soybean meal, de-hulled | 15.30 |
| Full-fat soybean meal, puffed | 5.00 |
| Fish meal | 2.00 |
| Soybean oil | 1.00 |
| L-Lysine | 0.39 |
| DL-Methionine | 0.04 |
| L-Threonine | 0.12 |
| L-Tryptophan | 0.02 |
| Calcium hydrogen phosphate | 1.19 |
| Limestone | 0.66 |
| Salt | 0.28 |
| Premix A | 1.00 |
| Chemical levels B | |
| Net energy (Mcal/kg) | 2.50 |
| Crude protein | 16.05 |
| Lysine | 0.98 |
| Methionine | 0.29 |
| Threonine | 0.60 |
| Leucine | 0.17 |
| Calcium | 0.66 |
| Total phosphorus | 0.56 |
| Available phosphorus | 0.33 |
| Sodium | 0.14 |
| Chlorine | 0.19 |
| Basic Diet Ingredients | Content (%) |
|---|---|
| Corn | 60.68 |
| Soybean meal, de-hulled | 17.53 |
| Full-fat soybean meal, puffed | 5.00 |
| Fish meal | 2.00 |
| Glucose | 10.00 |
| Soybean oil | 1.00 |
| L-Lysine | 0.35 |
| DL-Methionine | 0.05 |
| L-Threonine | 0.11 |
| L-Tryptophan | 0.01 |
| Calcium hydrogen phosphate | 1.25 |
| Limestone | 0.62 |
| Salt | 0.40 |
| Premix A | 1.00 |
| Chemical levels B | |
| Net energy (Mcal/kg) | 2.63 |
| Crude protein | 16.05 |
| Lysine | 0.98 |
| Methionine | 0.29 |
| Threonine | 0.60 |
| Leucine | 0.17 |
| Calcium | 0.66 |
| Total phosphorus | 0.56 |
| Available phosphorus | 0.34 |
| Sodium | 0.19 |
| Chlorine | 0.26 |
| Gene | GenBank ID | Primer Sequences (5’ to 3’) |
|---|---|---|
| β-actin | AY550069 | F: ATGCTTCTAGGCGGACTGT |
| R: CCATCCAACCGACTGCT | ||
| Bax | XM_005664710 | F: ATGGAGCTGCAGAGGATGAT |
| R: AAAGTAGAAAAGCGCGACCA | ||
| Bcl2 | NM_001164511.2 | F: ACTTCTGCGAAAGCGAATTGCC |
| R: AGCCTCCGTTTTGCCTTATCC | ||
| Caspase3 | NM_214131 | F: CGGACAGTGGGACTGAAGTA |
| R: GATCCGTCCTTTGAATTTCG | ||
| NLRP3 | NM_001256770.2 | F: CTGGGACTCTGACTAGGGCT |
| R: TTTTTCTGTCTGGCCCCGAG | ||
| HMGB1 | NM_001004034.1 | F: GAGGAAACTTGAGACCCACCA |
| R: GTGTCCTTCCTTCCCTCATGT | ||
| Caspase1 | NM_214162.1 | F: TACAAGAATCCCAGGCGGTG |
| R: CCTTTGGGCTATGTCTGGGG | ||
| TLR4 | NM_001113039.2 | F: CAGTCAAGATACTGGACCTGAGC |
| R: GGCTCCCAGGGCTAAAACTCT | ||
| MyD88 | NM_001099923.1 | F: CCATTCGAGATGACCCCCTG |
| R: TAGCAATGGACCAGACGCAG | ||
| IL-1β | NM_2140551.1 | F: GCCAACGTGCAGTCTATGGAGTG |
| R: GGTGGAGAGCCTTCAGCATGTG | ||
| PGC-1α | NM_213963.2 | F: ATGGAGCAATAAAGCGAAGAGCATTTG |
| R: GAGGAGGGTCATCATTTGTGGTCAG | ||
| Mfn1 | NM_001315732.1 | F: TGGACTTTATCCGAAACCAGATGAACC |
| R: AACCTTATTTGCCACCTCCTCTGTAAC | ||
| Mfn2 | XM_021095369.1 | F: CCACACCACCAACTGCTTCCTG |
| R: TCTTGACGCTCCTCTTCTCCTCTG | ||
| MFF | NM_001244126.1 | F: CAGGTTCCAGAGAGAATTGTCGTAGC |
| R: TTAGTGCCAGAGGTTTAAAGGGAGTTG | ||
| Fis1 | XM_021086263.1 | F: CAGACAGAGCCACAGAACAACCAG |
| R: CAAGTCCAATGAGTCCAGCCAGTC | ||
| OPA1 | XM_021070063.1 | F: ACAGAGGATGGTGCTTGTTGACTTAC |
| R: ACACAGTATGATGGCGTTGGGATTC | ||
| STAT3 | NM_001044580.1 | F: CAGCGGTAAGACCCAGATCC |
| R: AGGGTAGAGGTAGACCAGCG | ||
| BDNF | NM_214259.2 | F: CAGAGCAGCTGCCTTGATGTT |
| R: CTTTCATGGGGGCAGCCTTC | ||
| TrkB | XM_021064650.1 | F: TCTCGGTCTACGCTGTGGTA |
| R: GCAGCATCAACCAACAAGCA | ||
| BNIP3 | XM_003359404.4 | F: GAGGAGGATTACATGGAGAGGAGGAG |
| R: TCGGGTGCTTGAAGAGGAGGAAC | ||
| PINK1 | XM_021095478.1 | F: GGCGGTGATTGACTACAGCAAGG |
| R: TGGTAACTGCGGCTTTCAAGGTG | ||
| p62 | NM_001244307.1 | F: CTGCCTGAAGACTATTACACGAGACC |
| R: GAAGATGCTTGTGCCGAGGATAGAG | ||
| LC3Ⅰ | NM_001170827.1 | F: GCCTTCTTCCTGCTGGTGAACC |
| R: GGGAGGCGTAGACCATGTAGAGG | ||
| LC3Ⅱ | NM_001190290.1 | F: TTCTTCCTGTTAGTGAACGGACATAGC |
| R: ATCCATCTTCATCCTTCTCGCTTTCG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teng, T.; Yang, H.; Xu, T.; Sun, G.; Song, X.; Bai, G.; Shi, B. Activation of Inflammatory Networks in the Lungs Caused by Chronic Cold Stress Is Moderately Attenuated by Glucose Supplementation. Int. J. Mol. Sci. 2022, 23, 10697. https://doi.org/10.3390/ijms231810697
Teng T, Yang H, Xu T, Sun G, Song X, Bai G, Shi B. Activation of Inflammatory Networks in the Lungs Caused by Chronic Cold Stress Is Moderately Attenuated by Glucose Supplementation. International Journal of Molecular Sciences. 2022; 23(18):10697. https://doi.org/10.3390/ijms231810697
Chicago/Turabian StyleTeng, Teng, Hao Yang, Tianqi Xu, Guodong Sun, Xin Song, Guangdong Bai, and Baoming Shi. 2022. "Activation of Inflammatory Networks in the Lungs Caused by Chronic Cold Stress Is Moderately Attenuated by Glucose Supplementation" International Journal of Molecular Sciences 23, no. 18: 10697. https://doi.org/10.3390/ijms231810697
APA StyleTeng, T., Yang, H., Xu, T., Sun, G., Song, X., Bai, G., & Shi, B. (2022). Activation of Inflammatory Networks in the Lungs Caused by Chronic Cold Stress Is Moderately Attenuated by Glucose Supplementation. International Journal of Molecular Sciences, 23(18), 10697. https://doi.org/10.3390/ijms231810697