Biophysical Characterization of LTX-315 Anticancer Peptide Interactions with Model Membrane Platforms: Effect of Membrane Surface Charge
Abstract
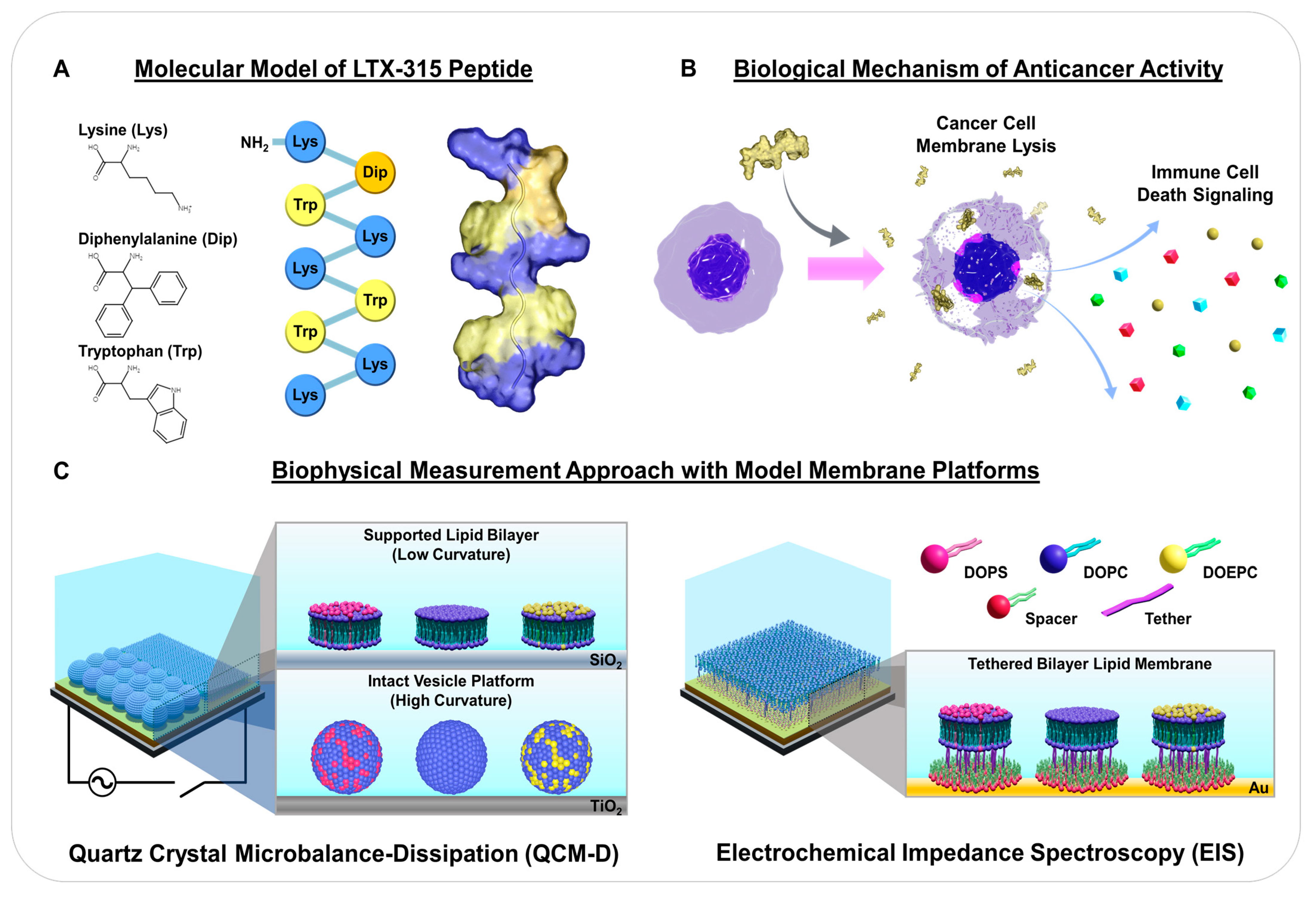
1. Introduction
2. Results and Discussion
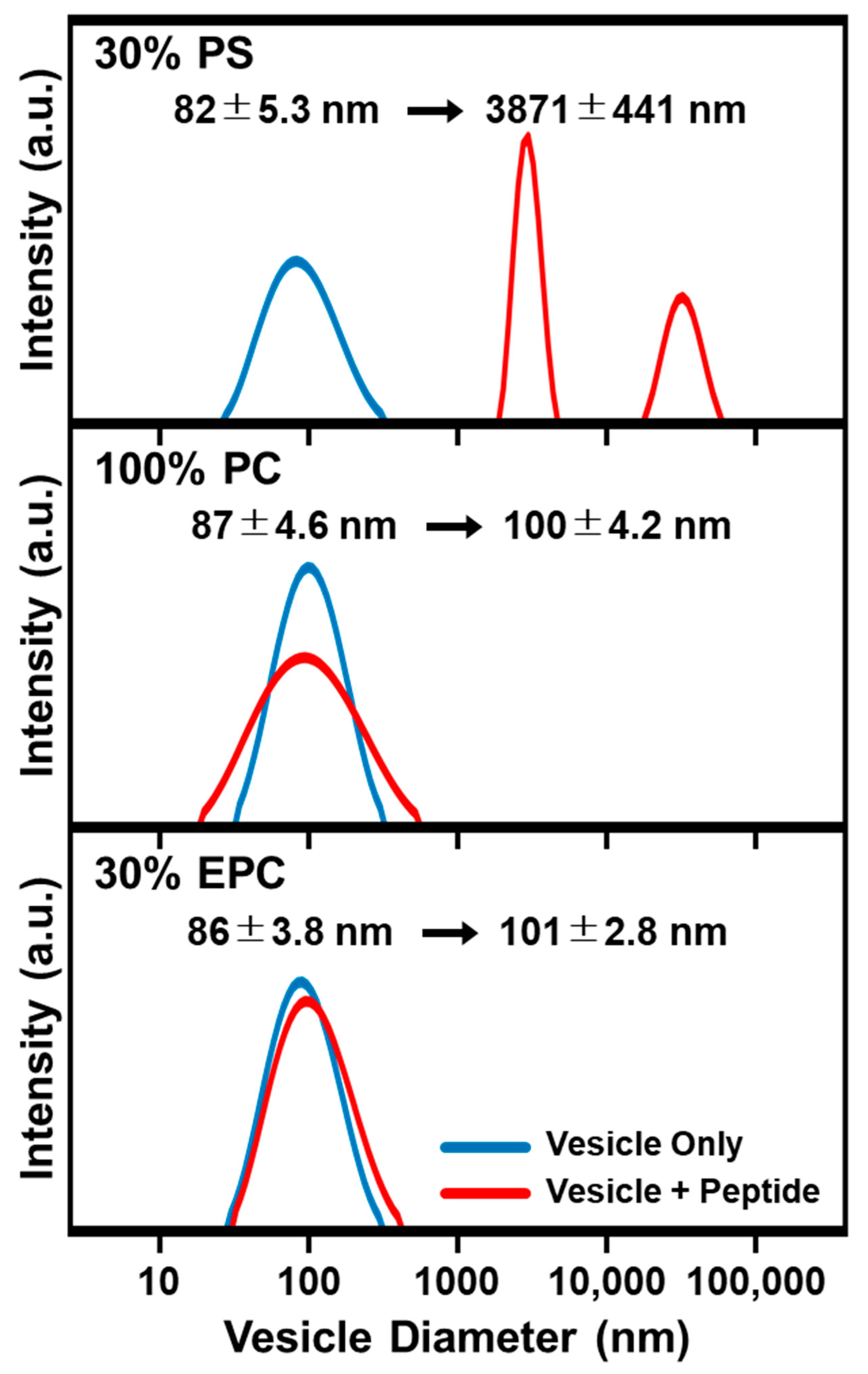
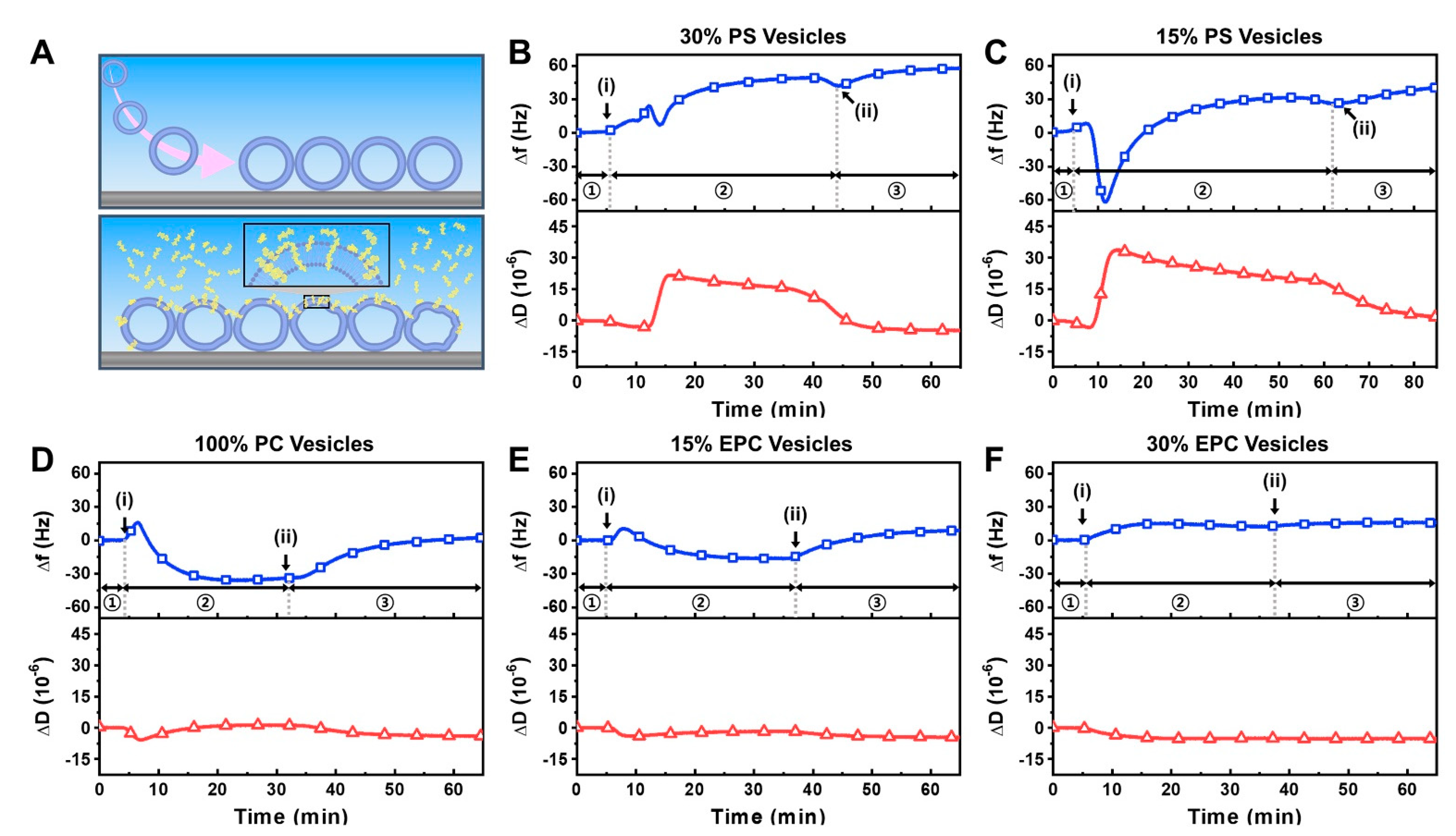
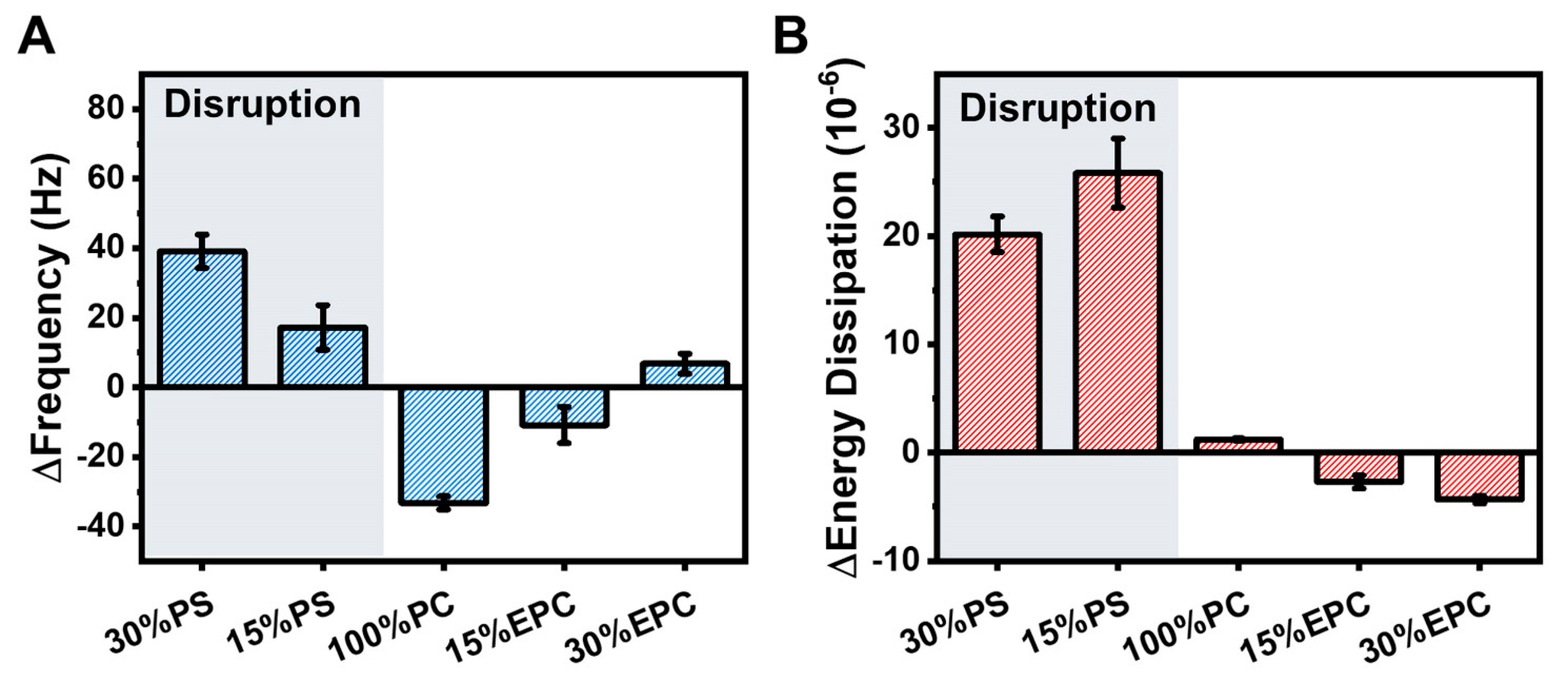
2.1. Intact Vesicle Platform
2.2. Supported Lipid Bilayer Platform
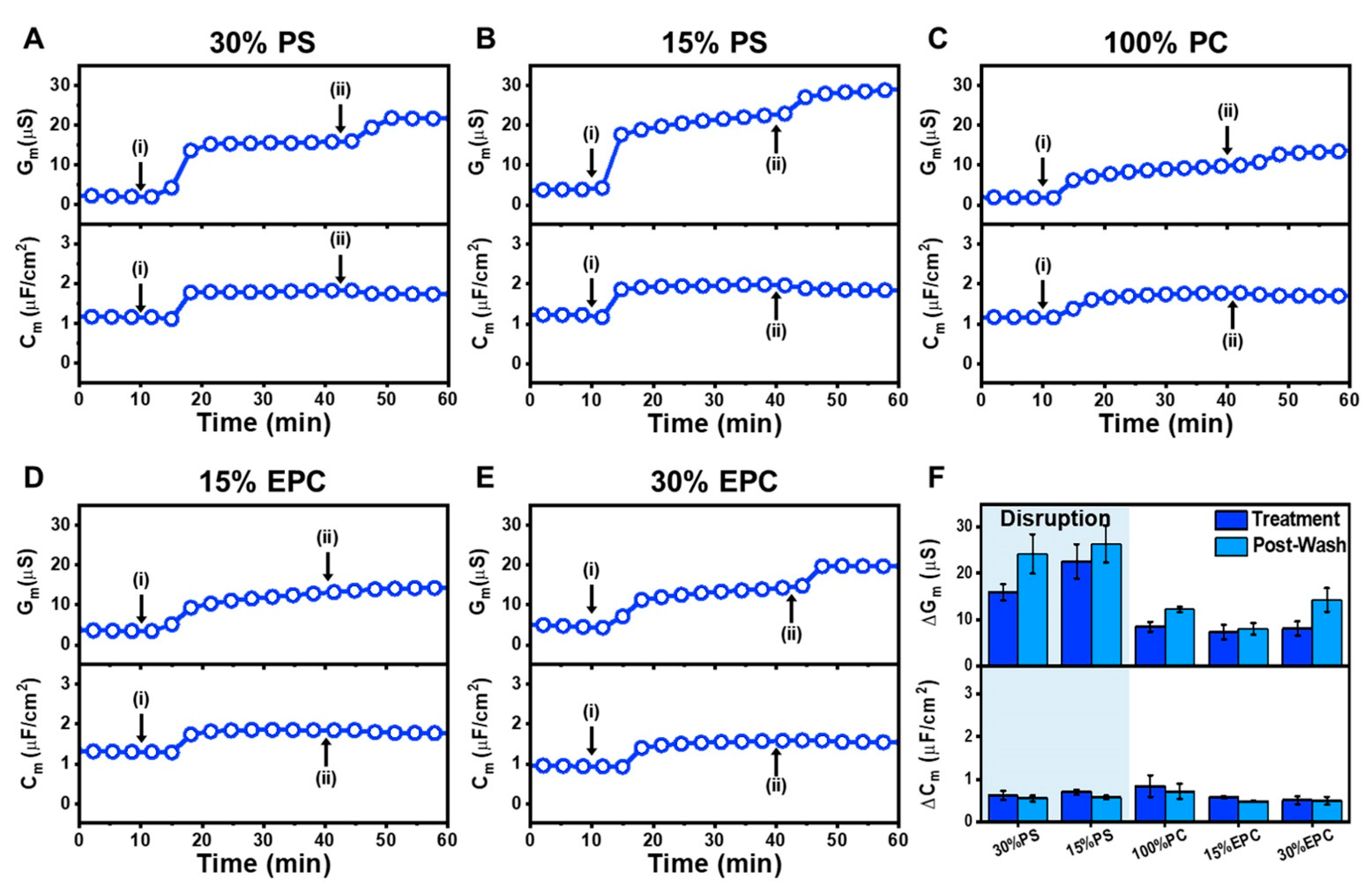
2.3. Tethered Bilayer Lipid Membrane Platform
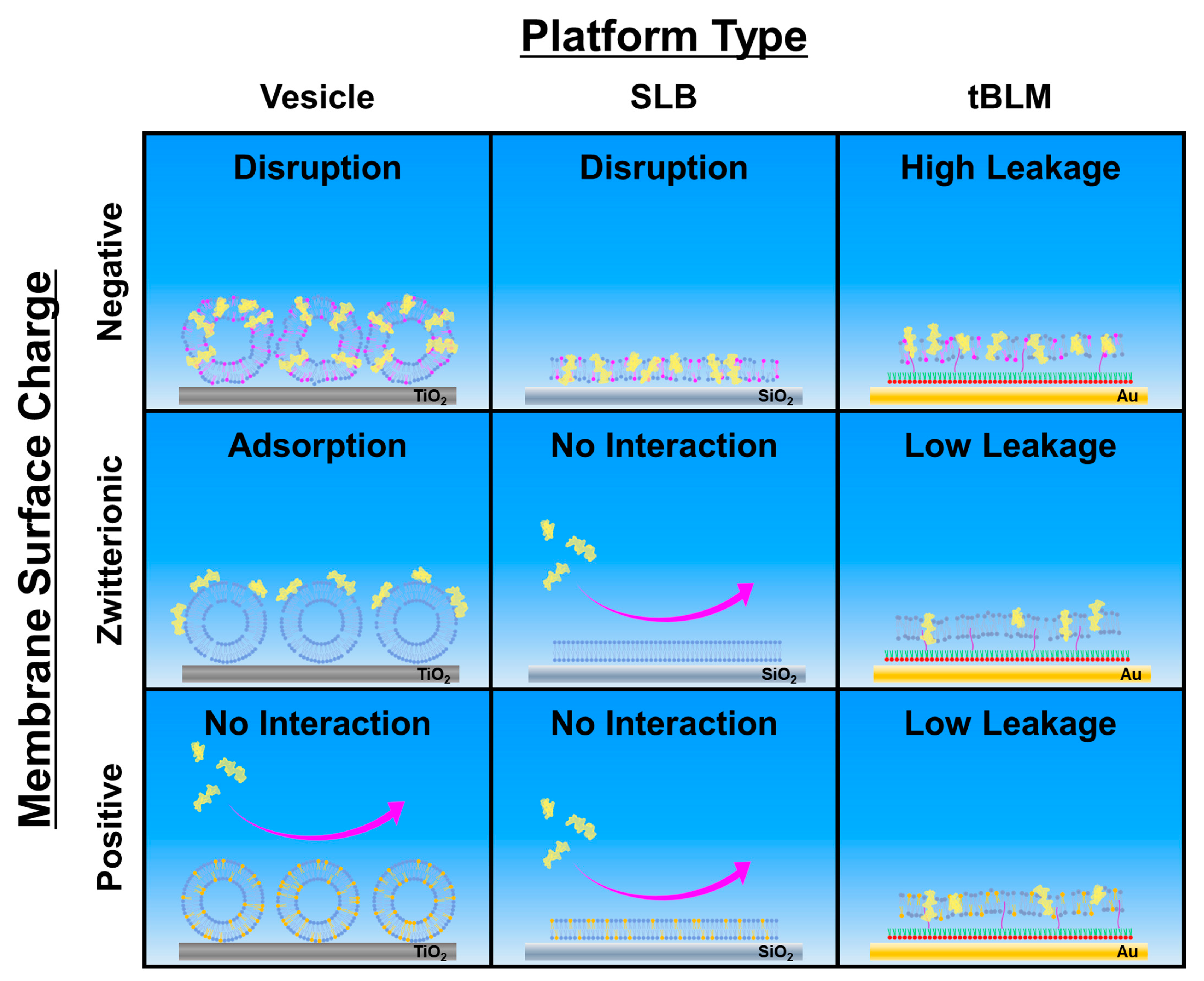
2.4. Mechanistic Analysis of Membrane-Peptide Interactions
3. Materials and Methods
3.1. Peptide
3.2. Vesicle Preparation
3.3. Circular Dichroism (CD) Spectroscopy
3.4. Dynamic Light Scattering (DLS)
3.5. Quartz crystal Microbalance-Dissipation (QCM-D)
3.6. Electrochemical Impedance Spectroscopy (EIS)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACP | Anticancer peptide |
| AMP | Antimicrobial peptide |
| CD | Circular dichroism |
| DLS | Dynamic light scattering |
| DOPC | 1,2-dioleoyl-sn-glycero-3-phosphocholine |
| DOPS | 1,2-dioleoyl-sn-glycero-3-phospho-L-serine |
| DOEPC | 1,2-dioleoyl-sn-glycero-3-ethylphosphocholine |
| EIS | Electrochemical impedance spectroscopy |
| EPC | Ethylphosphatidylcholine |
| PC | Phosphatidylcholine |
| PS | Phosphatidylserine |
| QCM-D | Quartz crystal microbalance-dissipation |
| SLB | Supported lipid bilayer |
| tBLM | Tethered bilayer lipid membrane |
References
- Gabernet, G.; Müller, A.T.; Hiss, J.A.; Schneider, G. Membranolytic anticancer peptides. MedChemComm 2016, 7, 2232–2245. [Google Scholar] [CrossRef]
- Kardani, K.; Bolhassani, A. Antimicrobial/anticancer peptides: Bioactive molecules and therapeutic agents. Immunotherapy 2021, 13, 669–684. [Google Scholar] [CrossRef] [PubMed]
- Karpiński, T.M.; Adamczak, A. Anticancer activity of bacterial proteins and peptides. Pharmaceutics 2018, 10, 54. [Google Scholar] [CrossRef] [PubMed]
- Deslouches, B.; Di, Y.P. Antimicrobial peptides with selective antitumor mechanisms: Prospect for anticancer applications. Oncotarget 2017, 8, 46635. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, A.; Tuknait, A.; Anand, P.; Gupta, S.; Sharma, M.; Mathur, D.; Joshi, A.; Singh, S.; Gautam, A.; Raghava, G.P. CancerPPD: A database of anticancer peptides and proteins. Nucleic Acids Res. 2015, 43, D837–D843. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, D.; Veiga, A.S.; Castanho, M.A. From antimicrobial to anticancer peptides. A review. Front. Microbiol. 2013, 4, 294. [Google Scholar] [CrossRef]
- Haug, B.E.; Camilio, K.A.; Eliassen, L.T.; Stensen, W.; Svendsen, J.S.; Berg, K.; Mortensen, B.; Serin, G.; Mirjolet, J.-F.; Bichat, F. Discovery of a 9-mer cationic peptide (LTX-315) as a potential first in class oncolytic peptide. J. Med. Chem. 2016, 59, 2918–2927. [Google Scholar] [CrossRef]
- Gaspar, D.; Veiga, A.S.; Sinthuvanich, C.; Schneider, J.P.; Castanho, M.A. Anticancer peptide SVS-1: Efficacy precedes membrane neutralization. Biochemistry 2012, 51, 6263–6265. [Google Scholar] [CrossRef][Green Version]
- Hoskin, D.W.; Ramamoorthy, A. Studies on anticancer activities of antimicrobial peptides. Biochim. Biophys. Acta Biomembr. 2008, 1778, 357–375. [Google Scholar] [CrossRef]
- Kurrikoff, K.; Aphkhazava, D.; Langel, Ü. The future of peptides in cancer treatment. Curr. Opin. Pharmacol. 2019, 47, 27–32. [Google Scholar] [CrossRef]
- Felício, M.R.; Silva, O.N.; Gonçalves, S.; Santos, N.C.; Franco, O.L. Peptides with dual antimicrobial and anticancer activities. Front. Chem. 2017, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Thundimadathil, J. Cancer treatment using peptides: Current therapies and future prospects. J. Amino Acids 2012, 2012, 967347. [Google Scholar] [CrossRef] [PubMed]
- Schweizer, F. Cationic amphiphilic peptides with cancer-selective toxicity. Eur. J. Pharmacol. 2009, 625, 190–194. [Google Scholar] [CrossRef]
- Agrawal, P.; Bhagat, D.; Mahalwal, M.; Sharma, N.; Raghava, G.P. AntiCP 2.0: An updated model for predicting anticancer peptides. Briefings Bioinf. 2021, 22, bbaa153. [Google Scholar] [CrossRef]
- Connor, J.; Bucana, C.; Fidler, I.J.; Schroit, A.J. Differentiation-dependent expression of phosphatidylserine in mammalian plasma membranes: Quantitative assessment of outer-leaflet lipid by prothrombinase complex formation. Proc. Natl. Acad. Sci. USA 1989, 86, 3184–3188. [Google Scholar] [CrossRef] [PubMed]
- Nishino, M.; Matsuzaki, I.; Musangile, F.Y.; Takahashi, Y.; Iwahashi, Y.; Warigaya, K.; Kinoshita, Y.; Kojima, F.; Murata, S.-i. Measurement and visualization of cell membrane surface charge in fixed cultured cells related with cell morphology. PLoS ONE 2020, 15, e0236373. [Google Scholar]
- Huang, Y.-b.; Wang, X.-f.; Wang, H.-y.; Liu, Y.; Chen, Y. Studies on mechanism of action of anticancer peptides by modulation of hydrophobicity within a defined structural framework. Mol. Cancer Ther. 2011, 10, 416–426. [Google Scholar] [CrossRef] [PubMed]
- Chiangjong, W.; Chutipongtanate, S.; Hongeng, S. Anticancer peptide: Physicochemical property, functional aspect and trend in clinical application. Int. J. Oncol. 2020, 57, 678–696. [Google Scholar] [CrossRef]
- Hu, C.; Huang, Y.; Chen, Y. Targeted modification of the cationic anticancer peptide HPRP-A1 with iRGD to improve specificity, penetration, and tumor-tissue accumulation. Mol. Pharm. 2018, 16, 561–572. [Google Scholar] [CrossRef]
- Fosgerau, K.; Hoffmann, T. Peptide therapeutics: Current status and future directions. Drug Discov. Today 2015, 20, 122–128. [Google Scholar] [CrossRef]
- Sveinbjørnsson, B.; Camilio, K.A.; Haug, B.E.; Rekdal, Ø. LTX-315: A first-in-class oncolytic peptide that reprograms the tumor microenvironment. Future Med. Chem. 2017, 9, 1339–1344. [Google Scholar] [CrossRef] [PubMed]
- Camilio, K.A.; Berge, G.; Ravuri, C.S.; Rekdal, Ø.; Sveinbjørnsson, B. Complete regression and systemic protective immune responses obtained in B16 melanomas after treatment with LTX-315. Cancer Immunol. Immunother. 2014, 63, 601–613. [Google Scholar] [CrossRef] [PubMed]
- Camilio, K.A.; Rekdal, Ø.; Sveinbjörnsson, B. LTX-315 (Oncopore™) a short synthetic anticancer peptide and novel immunotherapeutic agent. Oncoimmunology 2014, 3, e29181. [Google Scholar] [CrossRef] [PubMed]
- Spicer, J.; Marabelle, A.; Baurain, J.-F.; Jebsen, N.L.; Jøssang, D.E.; Awada, A.; Kristeleit, R.; Loirat, D.; Lazaridis, G.; Jungels, C. Safety, antitumor activity, and T-cell responses in a dose-ranging phase I trial of the oncolytic peptide LTX-315 in patients with solid tumors. Clin. Cancer Res. 2021, 27, 2755–2763. [Google Scholar] [CrossRef]
- Zhou, H.; Forveille, S.; Sauvat, A.; Yamazaki, T.; Senovilla, L.; Ma, Y.; Liu, P.; Yang, H.; Bezu, L.; Müller, K. The oncolytic peptide LTX-315 triggers immunogenic cell death. Cell Death Dis. 2016, 7, e2134. [Google Scholar] [CrossRef]
- Camilio, K.A.; Wang, M.-Y.; Mauseth, B.; Waagene, S.; Kvalheim, G.; Rekdal, Ø.; Sveinbjørnsson, B.; Mælandsmo, G.M. Combining the oncolytic peptide LTX-315 with doxorubicin demonstrates therapeutic potential in a triple-negative breast cancer model. Breast Cancer Res. 2019, 21, 9. [Google Scholar] [CrossRef]
- Zhou, H.; Forveille, S.; Sauvat, A.; Sica, V.; Izzo, V.; Durand, S.; Müller, K.; Liu, P.; Zitvogel, L.; Rekdal, Ø. The oncolytic peptide LTX-315 kills cancer cells through Bax/Bak-regulated mitochondrial membrane permeabilization. Oncotarget 2015, 6, 26599. [Google Scholar] [CrossRef]
- Vitale, I.; Yamazaki, T.; Wennerberg, E.; Sveinbjørnsson, B.; Rekdal, Ø.; Demaria, S.; Galluzzi, L. Targeting cancer heterogeneity with immune responses driven by oncolytic peptides. Trends Cancer 2021, 7, 557–572. [Google Scholar] [CrossRef]
- Lai, Y.; Gallo, R.L. AMPed up immunity: How antimicrobial peptides have multiple roles in immune defense. Trends Immunol. 2009, 30, 131–141. [Google Scholar] [CrossRef]
- Jackman, J.A.; Cho, N.-J. Model membrane platforms for biomedicine: Case study on antiviral drug development. Biointerphases 2012, 7, 18. [Google Scholar] [CrossRef]
- Jackman, J.A.; Goh, H.Z.; Zhdanov, V.P.; Knoll, W.; Cho, N.-J. Deciphering how pore formation causes strain-induced membrane lysis of lipid vesicles. J. Am. Chem. Soc. 2016, 138, 1406–1413. [Google Scholar] [CrossRef] [PubMed]
- Andrä, J.; Böhling, A.; Gronewold, T.M.; Schlecht, U.; Perpeet, M.; Gutsmann, T. Surface acoustic wave biosensor as a tool to study the interaction of antimicrobial peptides with phospholipid and lipopolysaccharide model membranes. Langmuir 2008, 24, 9148–9153. [Google Scholar] [CrossRef] [PubMed]
- Mozsolits, H.; Wirth, H.-J.; Werkmeister, J.; Aguilar, M.-I. Analysis of antimicrobial peptide interactions with hybrid bilayer membrane systems using surface plasmon resonance. Biochim. Biophys. Acta Biomembr. 2001, 1512, 64–76. [Google Scholar] [CrossRef]
- Höök, F.; Kasemo, B. The QCM-D technique for probing biomacromolecular recognition reactions. In Piezoelectric Sensors; Janshoff, A., Steinem, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 425–447. [Google Scholar]
- Kanazawa, K.; Cho, N.-J. Quartz crystal microbalance as a sensor to characterize macromolecular assembly dynamics. J. Sens. 2009, 2009, 824947. [Google Scholar] [CrossRef]
- Dixon, M.C. Quartz crystal microbalance with dissipation monitoring: Enabling real-time characterization of biological materials and their interactions. J. Biomol. Tech. 2008, 19, 151. [Google Scholar]
- Cho, N.-J.; Frank, C.W.; Kasemo, B.; Höök, F. Quartz crystal microbalance with dissipation monitoring of supported lipid bilayers on various substrates. Nat. Protoc. 2010, 5, 1096–1106. [Google Scholar] [CrossRef]
- Alghalayini, A.; Garcia, A.; Berry, T.; Cranfield, C.G. The use of tethered bilayer lipid membranes to identify the mechanisms of antimicrobial peptide interactions with lipid bilayers. Antibiotics 2019, 8, 12. [Google Scholar] [CrossRef]
- Berry, T.; Dutta, D.; Chen, R.; Leong, A.; Wang, H.; Donald, W.A.; Parviz, M.; Cornell, B.; Willcox, M.; Kumar, N. Lipid membrane interactions of the cationic antimicrobial peptide chimeras melimine and cys-melimine. Langmuir 2018, 34, 11586–11592. [Google Scholar] [CrossRef]
- Cranfield, C.G.; Berry, T.; Holt, S.A.; Hossain, K.R.; Le Brun, A.P.; Carne, S.; Al Khamici, H.; Coster, H.; Valenzuela, S.M.; Cornell, B. Evidence of the key role of H3O+ in phospholipid membrane morphology. Langmuir 2016, 32, 10725–10734. [Google Scholar]
- Cranfield, C.G.; Cornell, B.A.; Grage, S.L.; Duckworth, P.; Carne, S.; Ulrich, A.S.; Martinac, B. Transient potential gradients and impedance measures of tethered bilayer lipid membranes: Pore-forming peptide insertion and the effect of electroporation. Biophys. J. 2014, 106, 182–189. [Google Scholar] [CrossRef]
- Sen, T.Z.; Jernigan, R.L.; Garnier, J.; Kloczkowski, A. GOR V server for protein secondary structure prediction. Bioinformatics 2005, 21, 2787–2788. [Google Scholar] [CrossRef] [PubMed]
- Milner-White, E.J.; Russell, M.J. Predicting the conformations of peptides and proteins in early evolution. Biol. Direct 2008, 3, 3. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Sun, L.; Huang, S.; Zhu, C.; Li, P.; He, J.; Mackey, V.; Coy, D.H.; He, Q. The antimicrobial peptides and their potential clinical applications. Am. J. Transl. Res. 2019, 11, 3919. [Google Scholar]
- Zan, G.H.; Jackman, J.A.; Cho, N.-J. AH peptide-mediated formation of charged planar lipid bilayers. J. Phys. Chem. B 2014, 118, 3616–3621. [Google Scholar] [CrossRef]
- Reimhult, E.; Höök, F.; Kasemo, B. Vesicle adsorption on SiO2 and TiO2: Dependence on vesicle size. J. Chem. Phys. 2002, 117, 7401–7404. [Google Scholar] [CrossRef]
- Reviakine, I.; Rossetti, F.F.; Morozov, A.N.; Textor, M. Investigating the properties of supported vesicular layers on titanium dioxide by quartz crystal microbalance with dissipation measurements. J. Chem. Phys. 2005, 122, 204711. [Google Scholar] [CrossRef]
- Mechler, A.; Praporski, S.; Atmuri, K.; Boland, M.; Separovic, F.; Martin, L.L. Specific and selective peptide-membrane interactions revealed using quartz crystal microbalance. Biophys. J. 2007, 93, 3907–3916. [Google Scholar] [CrossRef]
- John, T.; Abel, B.; Martin, L.L. The quartz crystal microbalance with dissipation monitoring (QCM-D) technique applied to the study of membrane-active peptides. Aust. J. Chem. 2018, 71, 543–546. [Google Scholar] [CrossRef]
- Martin, L.L.; Kubeil, C.; Piantavigna, S.; Tikkoo, T.; Gray, N.P.; John, T.; Calabrese, A.N.; Liu, Y.; Hong, Y.; Hossain, M.A. Amyloid aggregation and membrane activity of the antimicrobial peptide uperin 3.5. Pept. Sci. 2018, 110, e24052. [Google Scholar] [CrossRef]
- Losada-Pérez, P.; Khorshid, M.; Hermans, C.; Robijns, T.; Peeters, M.; Jiménez-Monroy, K.; Truong, L.; Wagner, P. Melittin disruption of raft and non-raft-forming biomimetic membranes: A study by quartz crystal microbalance with dissipation monitoring. Colloids Surf. B 2014, 123, 938–944. [Google Scholar] [CrossRef]
- Khorshid, M.; Losada-Pérez, P.; Wackers, G.; Yongabi, D.; Renner, F.U.; Thoelen, R.; Wagner, P. Real-time monitoring of interactions between Ebola fusion peptide and solid-supported phospholipid membranes: Effect of peptide concentration and layer geometry. Phys. Med. Biol. 2017, 4, 1–7. [Google Scholar]
- Liu, J.; Xiao, S.; Li, J.; Yuan, B.; Yang, K.; Ma, Y. Molecular details on the intermediate states of melittin action on a cell membrane. Biochim. Biophys. Acta Biomembr. 2018, 1860, 2234–2241. [Google Scholar] [CrossRef]
- Lu, N.; Yang, K.; Yuan, B.; Ma, Y. Molecular response and cooperative behavior during the interactions of melittin with a membrane: Dissipative quartz crystal microbalance experiments and simulations. J. Phys. Chem. B 2012, 116, 9432–9438. [Google Scholar] [CrossRef] [PubMed]
- Ferhan, A.R.; Ma, G.J.; Jackman, J.A.; Sut, T.N.; Park, J.H.; Cho, N.-J. Probing the interaction of dielectric nanoparticles with supported lipid membrane coatings on nanoplasmonic arrays. Sensors 2017, 17, 1484. [Google Scholar] [CrossRef] [PubMed]
- McCubbin, G.A.; Praporski, S.; Piantavigna, S.; Knappe, D.; Hoffmann, R.; Bowie, J.H.; Separovic, F.; Martin, L.L. QCM-D fingerprinting of membrane-active peptides. Eur. Biophys. J. 2011, 40, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Jackman, J.A.; Cho, N.-J. Comparing the membrane-interaction profiles of two antiviral peptides: Insights into structure–function relationship. Langmuir 2019, 35, 9934–9943. [Google Scholar] [CrossRef]
- Tan, S.W.; Jeon, W.-Y.; Yoon, B.K.; Jackman, J.A. Mechanistic Evaluation of Antimicrobial Lipid Interactions with Tethered Lipid Bilayers by Electrochemical Impedance Spectroscopy. Sensors 2022, 22, 3712. [Google Scholar] [CrossRef]
- Cranfield, C.G.; Henriques, S.T.; Martinac, B.; Duckworth, P.; Craik, D.J.; Cornell, B. Kalata b1 and kalata b2 have a surfactant-like activity in phosphatidylethanolomine-containing lipid membranes. Langmuir 2017, 33, 6630–6637. [Google Scholar] [CrossRef]
- Hatzakis, N.S.; Bhatia, V.K.; Larsen, J.; Madsen, K.L.; Bolinger, P.-Y.; Kunding, A.H.; Castillo, J.; Gether, U.; Hedegård, P.; Stamou, D. How curved membranes recruit amphipathic helices and protein anchoring motifs. Nat. Chem. Biol. 2009, 5, 835–841. [Google Scholar]
- Jackman, J.A.; Zan, G.H.; Zhdanov, V.P.; Cho, N.-J. Rupture of lipid vesicles by a broad-spectrum antiviral peptide: Influence of vesicle size. J. Phys. Chem. B 2013, 117, 16117–16128. [Google Scholar] [CrossRef]
- Shai, Y.; Oren, Z. From “carpet” mechanism to de-novo designed diastereomeric cell-selective antimicrobial peptides. Peptides 2001, 22, 1629–1641. [Google Scholar] [CrossRef]
- Park, S.; Avsar, S.Y.; Cornell, B.; Ferhan, A.R.; Jeon, W.-Y.; Chung, M.; Cho, N.-J. Probing the influence of tether density on tethered bilayer lipid membrane (tBLM)-peptide interactions. Appl. Mater. Today 2020, 18, 100527. [Google Scholar] [CrossRef]
- Kuroda, K.; Okumura, K.; Isogai, H.; Isogai, E. The human cathelicidin antimicrobial peptide LL-37 and mimics are potential anticancer drugs. Front. Oncol. 2015, 5, 144. [Google Scholar] [CrossRef] [PubMed]
- Nizalapur, S.; Ho, K.K.; Kimyon, Ö.; Yee, E.; Berry, T.; Manefield, M.; Cranfield, C.G.; Willcox, M.; Black, D.S.; Kumar, N. Synthesis and biological evaluation of N-naphthoyl-phenylglyoxamide-based small molecular antimicrobial peptide mimics as novel antimicrobial agents and biofilm inhibitors. Org. Biomol. Chem. 2016, 14, 3623–3637. [Google Scholar] [CrossRef]
- Oren, Z.; Lerman, J.C.; Gudmundsson, G.H.; Agerberth, B.; Shai, Y. Structure and organization of the human antimicrobial peptide LL-37 in phospholipid membranes: Relevance to the molecular basis for its non-cell-selective activity. Biochem. J. 1999, 341, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Shahmiri, M.; Cornell, B.; Mechler, A. Phenylalanine residues act as membrane anchors in the antimicrobial action of Aurein 1.2. Biointerphases 2017, 12, 05G605. [Google Scholar] [CrossRef]
- Yu, T.T.; Nizalapur, S.; Ho, K.K.; Yee, E.; Berry, T.; Cranfield, C.G.; Willcox, M.; Black, D.S.; Kumar, N. Design, Synthesis and Biological Evaluation of N-Sulfonylphenyl glyoxamide-Based Antimicrobial Peptide Mimics as Novel Antimicrobial Agents. ChemistrySelect 2017, 2, 3452–3461. [Google Scholar] [CrossRef]
- Aroui, S.; Kenani, A. Cell-penetrating peptides: A challenge for drug delivery. In Cheminformatics and Its Applications; Stefaniu, A., Rasul, A., Hussain, G., Eds.; IntechOpen: London, UK, 2020. [Google Scholar]
- Afonin, S.; Frey, A.; Bayerl, S.; Fischer, D.; Wadhwani, P.; Weinkauf, S.; Ulrich, A.S. The cell-penetrating peptide TAT (48–60) induces a non-lamellar phase in DMPC membranes. ChemPhysChem 2006, 7, 2134–2142. [Google Scholar] [CrossRef]
- Troeira Henriques, S.; Quintas, A.; Bagatolli, L.A.; Homblé, F.; Castanho, M.A. Energy-independent translocation of cell-penetrating peptides occurs without formation of pores. A biophysical study with pep-1. Mol. Membr. Biol. 2007, 24, 282–293. [Google Scholar] [CrossRef]
- Bobone, S.; Piazzon, A.; Orioni, B.; Pedersen, J.Z.; Nan, Y.H.; Hahm, K.S.; Shin, S.Y.; Stella, L. The thin line between cell-penetrating and antimicrobial peptides: The case of Pep-1 and Pep-1-K. J. Pept. Sci. 2011, 17, 335–341. [Google Scholar] [CrossRef]
- Hadianamrei, R.; Tomeh, M.A.; Brown, S.; Wang, J.; Zhao, X. Rationally designed short cationic α-helical peptides with selective anticancer activity. J. Colloid Interface Sci. 2022, 607, 488–501. [Google Scholar] [CrossRef] [PubMed]
- Vermeer, L.S.; Lan, Y.; Abbate, V.; Ruh, E.; Bui, T.T.; Wilkinson, L.J.; Kanno, T.; Jumagulova, E.; Kozlowska, J.; Patel, J. Conformational flexibility determines selectivity and antibacterial, antiplasmodial, and anticancer potency of cationic α-helical peptides. J. Biol. Chem. 2012, 287, 34120–34133. [Google Scholar] [CrossRef] [PubMed]
- Hadianamrei, R.; Tomeh, M.A.; Brown, S.; Wang, J.; Zhao, X. Correlation between the secondary structure and surface activity of β-sheet forming cationic amphiphilic peptides and their anticancer activity. Colloids Surf. B 2022, 209, 112165. [Google Scholar] [CrossRef] [PubMed]
- Szlasa, W.; Zendran, I.; Zalesińska, A.; Tarek, M.; Kulbacka, J. Lipid composition of the cancer cell membrane. J. Bioenerg. Biomembr. 2020, 52, 321–342. [Google Scholar] [PubMed]
- Utsugi, T.; Schroit, A.J.; Connor, J.; Bucana, C.D.; Fidler, I.J. Elevated expression of phosphatidylserine in the outer membrane leaflet of human tumor cells and recognition by activated human blood monocytes. Cancer Res. 1991, 51, 3062–3066. [Google Scholar] [PubMed]
- van der Veen, J.N.; Kennelly, J.P.; Wan, S.; Vance, J.E.; Vance, D.E.; Jacobs, R.L. The critical role of phosphatidylcholine and phosphatidylethanolamine metabolism in health and disease. Biochim. Biophys. Acta Biomembr. 2017, 1859, 1558–1572. [Google Scholar] [CrossRef] [PubMed]
- Beaven, G.H.; Holiday, E. Ultraviolet absorption spectra of proteins and amino acids. Adv. Protein Chem. 1952, 7, 319–386. [Google Scholar]
- Grimsley, G.R.; Pace, C.N. Spectrophotometric determination of protein concentration. Curr. Protoc. Protein Sci. 2003, 33, 3.1.1–3.1.9. [Google Scholar] [CrossRef]
- Hazra, C.; Samanta, T.; Mahalingam, V. A resonance energy transfer approach for the selective detection of aromatic amino acids. J. Mater. Chem. C 2014, 2, 10157–10163. [Google Scholar] [CrossRef]
- Phung, C.D.; Nguyen, B.L.; Jeong, J.H.; Chang, J.H.; Jin, S.G.; Choi, H.G.; Ku, S.K.; Kim, J.O. Shaping the “hot” immunogenic tumor microenvironment by nanoparticles co-delivering oncolytic peptide and TGF-β1 siRNA for boosting checkpoint blockade therapy. Bioeng. Transl. Med. 2022, e10392. [Google Scholar] [CrossRef]
- Jackman, J.A.; Zhao, Z.; Zhdanov, V.P.; Frank, C.W.; Cho, N.-J. Vesicle adhesion and rupture on silicon oxide: Influence of freeze–thaw pretreatment. Langmuir 2014, 30, 2152–2160. [Google Scholar] [CrossRef] [PubMed]
- Tabaei, S.R.; Jackman, J.A.; Kim, M.; Yorulmaz, S.; Vafaei, S.; Cho, N.-J. Biomembrane fabrication by the solvent-assisted lipid bilayer (SALB) method. J. Vis. Exp. 2015, 106, e53073. [Google Scholar] [CrossRef]
- Sut, T.N.; Yoon, B.K.; Jeon, W.-Y.; Jackman, J.A.; Cho, N.-J. Supported lipid bilayer coatings: Fabrication, bioconjugation, and diagnostic applications. Appl. Mater. Today 2021, 25, 101183. [Google Scholar] [CrossRef]
- Jackman, J.A.; Saravanan, R.; Zhang, Y.; Tabaei, S.R.; Cho, N.J. Correlation between membrane partitioning and functional activity in a single lipid vesicle assay establishes design guidelines for antiviral peptides. Small 2015, 11, 2372–2379. [Google Scholar] [CrossRef] [PubMed]
- Greenfield, N.J.; Fasman, G.D. Computed circular dichroism spectra for the evaluation of protein conformation. Biochemistry 1969, 8, 4108–4116. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.J.; Ferhan, A.R.; Jackman, J.A.; Cho, N.-J. Conformational flexibility of fatty acid-free bovine serum albumin proteins enables superior antifouling coatings. Commun. Mater. 2020, 1, 45. [Google Scholar] [CrossRef]
- Cranfield, C.; Carne, S.; Martinac, B.; Cornell, B. The assembly and use of tethered bilayer lipid membranes (tBLMs). In Methods in Membrane Lipids; Springer: Berlin/Heidelberg, Germany, 2015; pp. 45–53. [Google Scholar]
- Hoiles, W.; Gupta, R.; Cornell, B.; Cranfield, C.; Krishnamurthy, V. The effect of tethers on artificial cell membranes: A coarse-grained molecular dynamics study. PLoS ONE 2016, 11, e0162790. [Google Scholar]
- Alghalayini, A.; Jiang, L.; Gu, X.; Yeoh, G.H.; Cranfield, C.G.; Timchenko, V.; Cornell, B.A.; Valenzuela, S.M. Real-time monitoring of heat transfer between gold nanoparticles and tethered bilayer lipid membranes. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183334. [Google Scholar] [CrossRef]
- Alghalayini, A.; Jiang, L.; Gu, X.; Yeoh, G.H.; Cranfield, C.G.; Timchenko, V.; Cornell, B.A.; Valenzuela, S.M. Tethered bilayer lipid membranes to monitor heat transfer between gold nanoparticles and lipid membranes. J. Vis. Exp. 2020, 166, e61851. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koo, D.J.; Sut, T.N.; Tan, S.W.; Yoon, B.K.; Jackman, J.A. Biophysical Characterization of LTX-315 Anticancer Peptide Interactions with Model Membrane Platforms: Effect of Membrane Surface Charge. Int. J. Mol. Sci. 2022, 23, 10558. https://doi.org/10.3390/ijms231810558
Koo DJ, Sut TN, Tan SW, Yoon BK, Jackman JA. Biophysical Characterization of LTX-315 Anticancer Peptide Interactions with Model Membrane Platforms: Effect of Membrane Surface Charge. International Journal of Molecular Sciences. 2022; 23(18):10558. https://doi.org/10.3390/ijms231810558
Chicago/Turabian StyleKoo, Dong Jun, Tun Naw Sut, Sue Woon Tan, Bo Kyeong Yoon, and Joshua A. Jackman. 2022. "Biophysical Characterization of LTX-315 Anticancer Peptide Interactions with Model Membrane Platforms: Effect of Membrane Surface Charge" International Journal of Molecular Sciences 23, no. 18: 10558. https://doi.org/10.3390/ijms231810558
APA StyleKoo, D. J., Sut, T. N., Tan, S. W., Yoon, B. K., & Jackman, J. A. (2022). Biophysical Characterization of LTX-315 Anticancer Peptide Interactions with Model Membrane Platforms: Effect of Membrane Surface Charge. International Journal of Molecular Sciences, 23(18), 10558. https://doi.org/10.3390/ijms231810558







