Dual Inhibition of EGFR and IGF-1R Signaling Leads to Enhanced Antitumor Efficacy against Esophageal Squamous Cancer
Abstract
:1. Introduction
2. Results
2.1. Lapatinib or Gefitinib in Combination with Linsitinib Is Synergistic in the Growth Inhibition of ESCC Cells
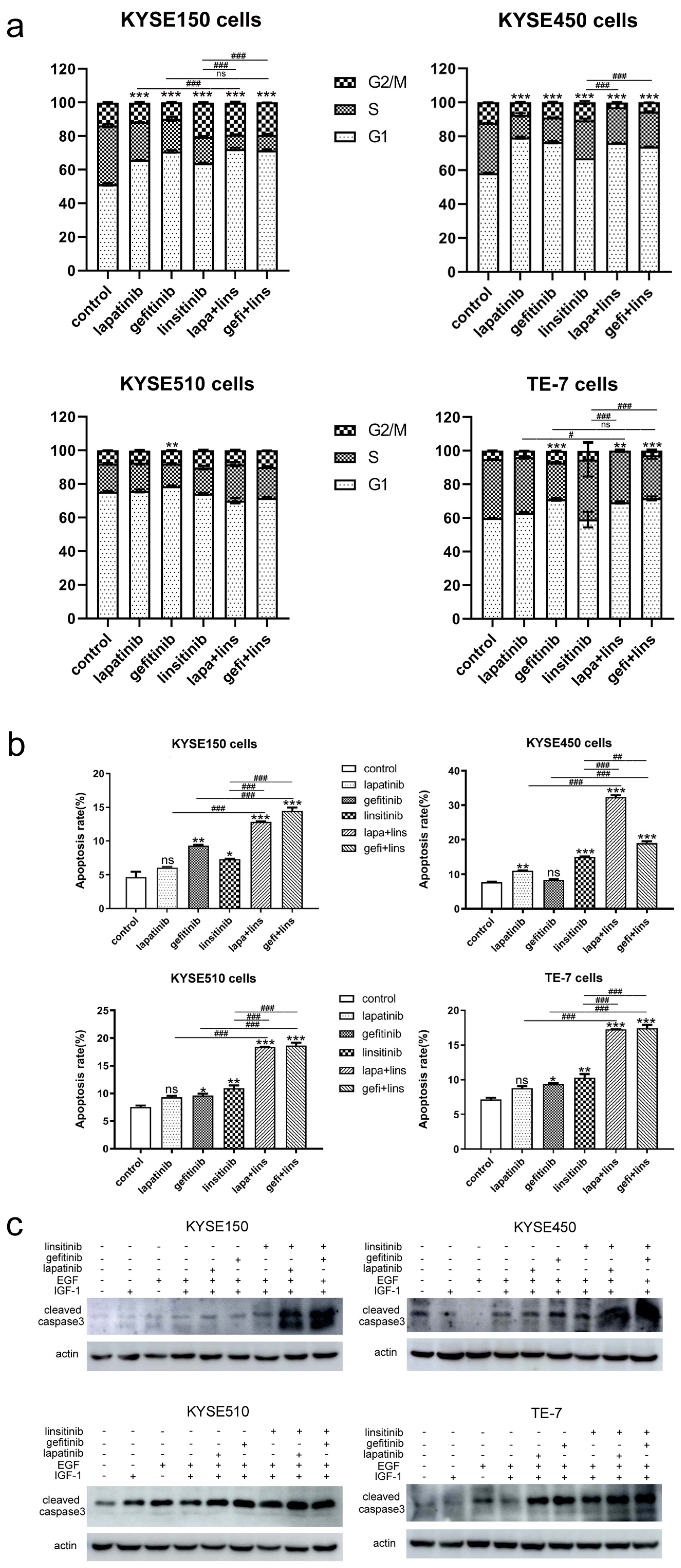
2.2. Lapatinib or Gefitinib in Combination with Linsitinib Induces Enhanced G1 Arrest and Cell Apoptosis in ESCC Cells
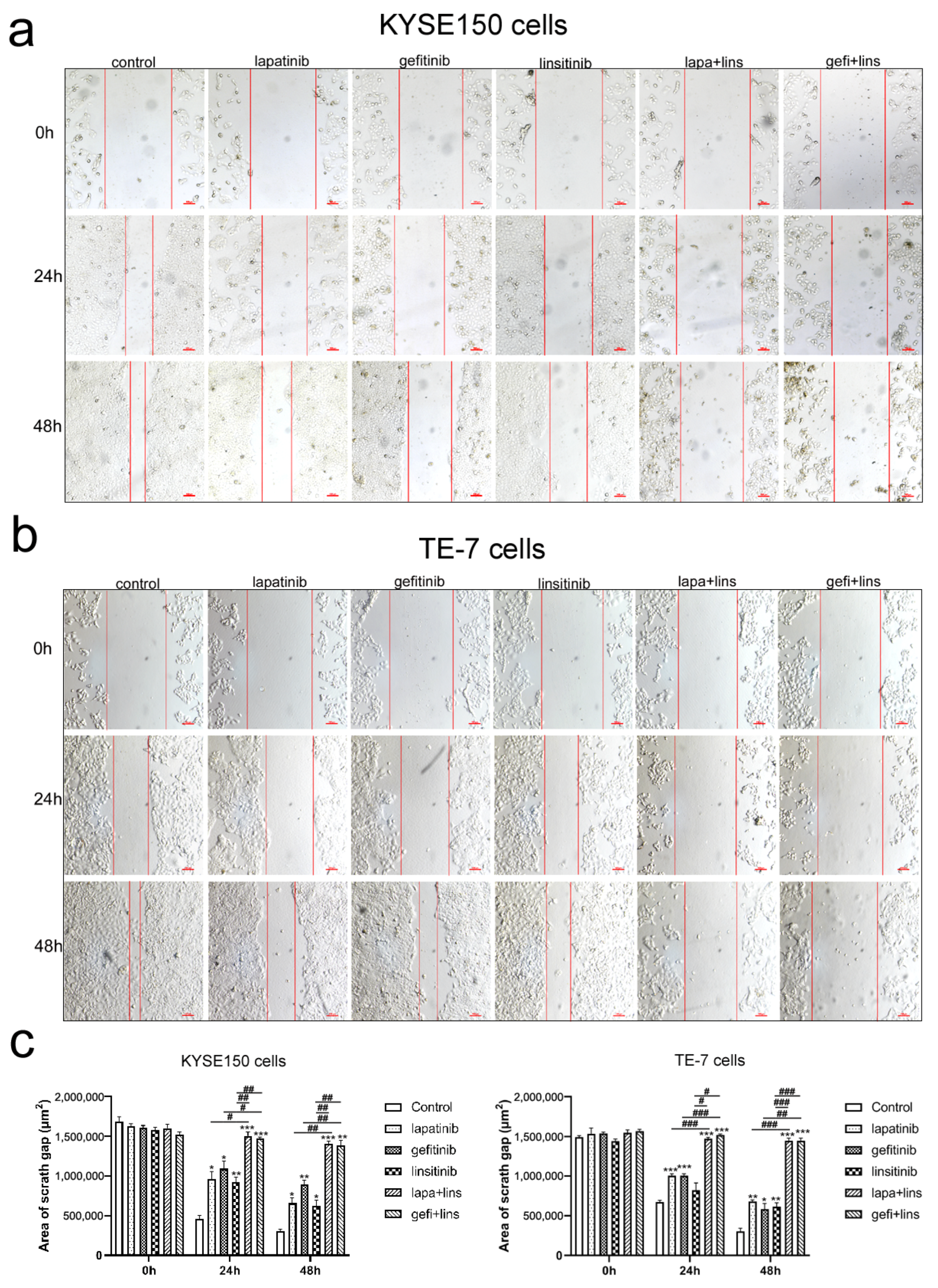
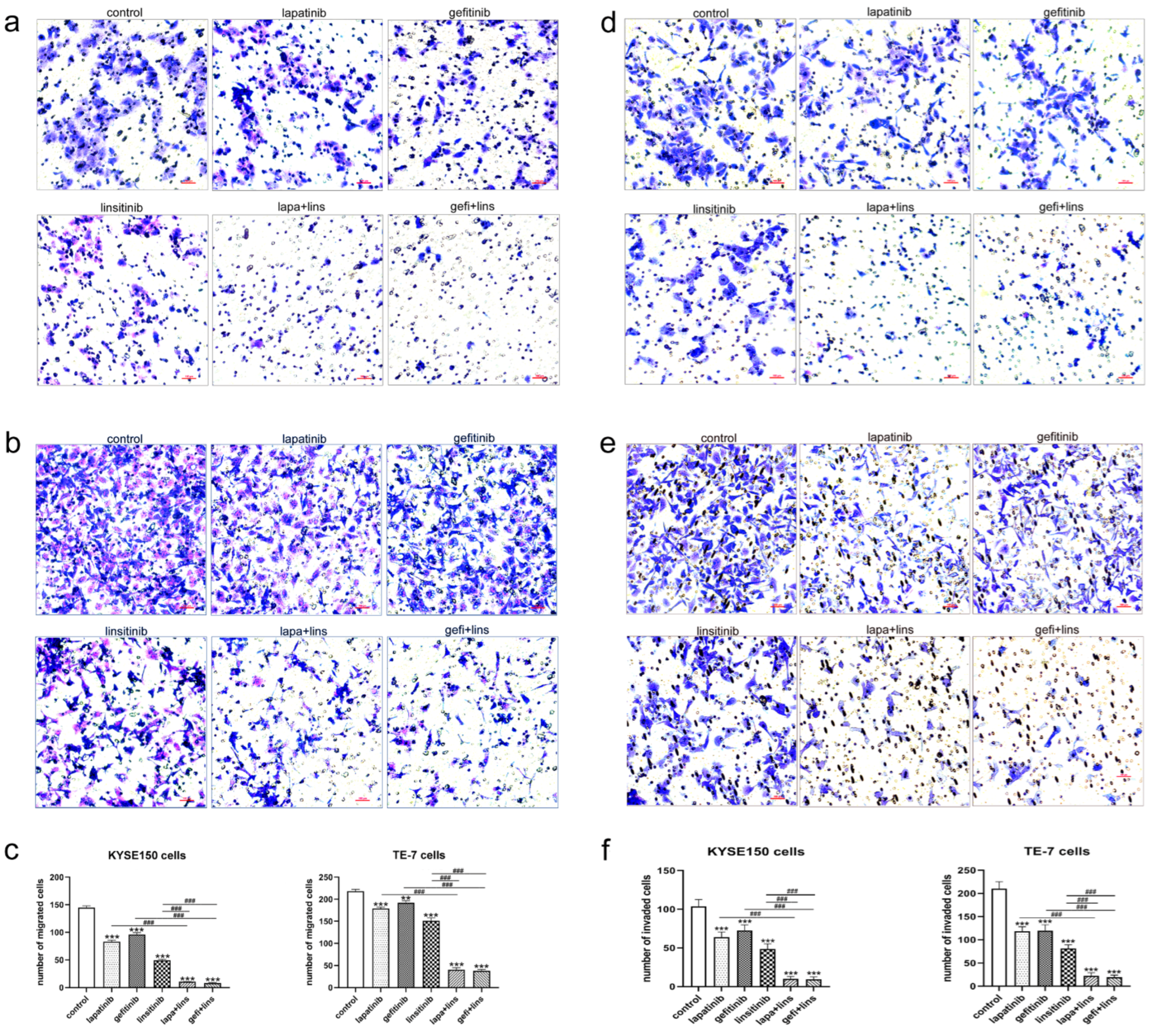
2.3. Lapatinib or Gefitinib in Combination with Linsitinib More Potently Inhibited the Invasion and Migration of ESCC Cells
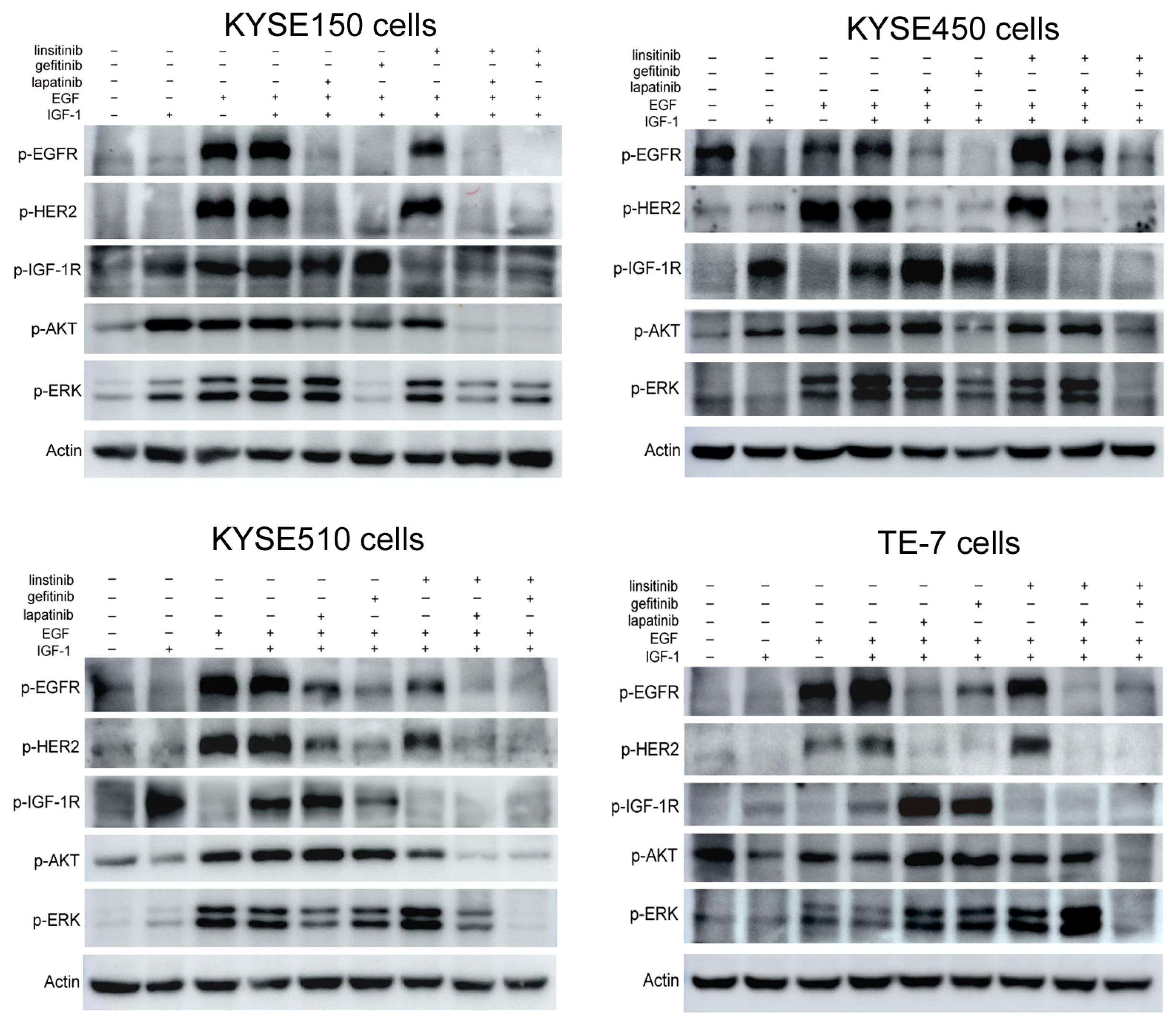
2.4. Lapatinib or Gefitinib in Combination with Linsitinib Synergistically Inhibited the EGFR/HER2 and IGF-1R Signaling Activation
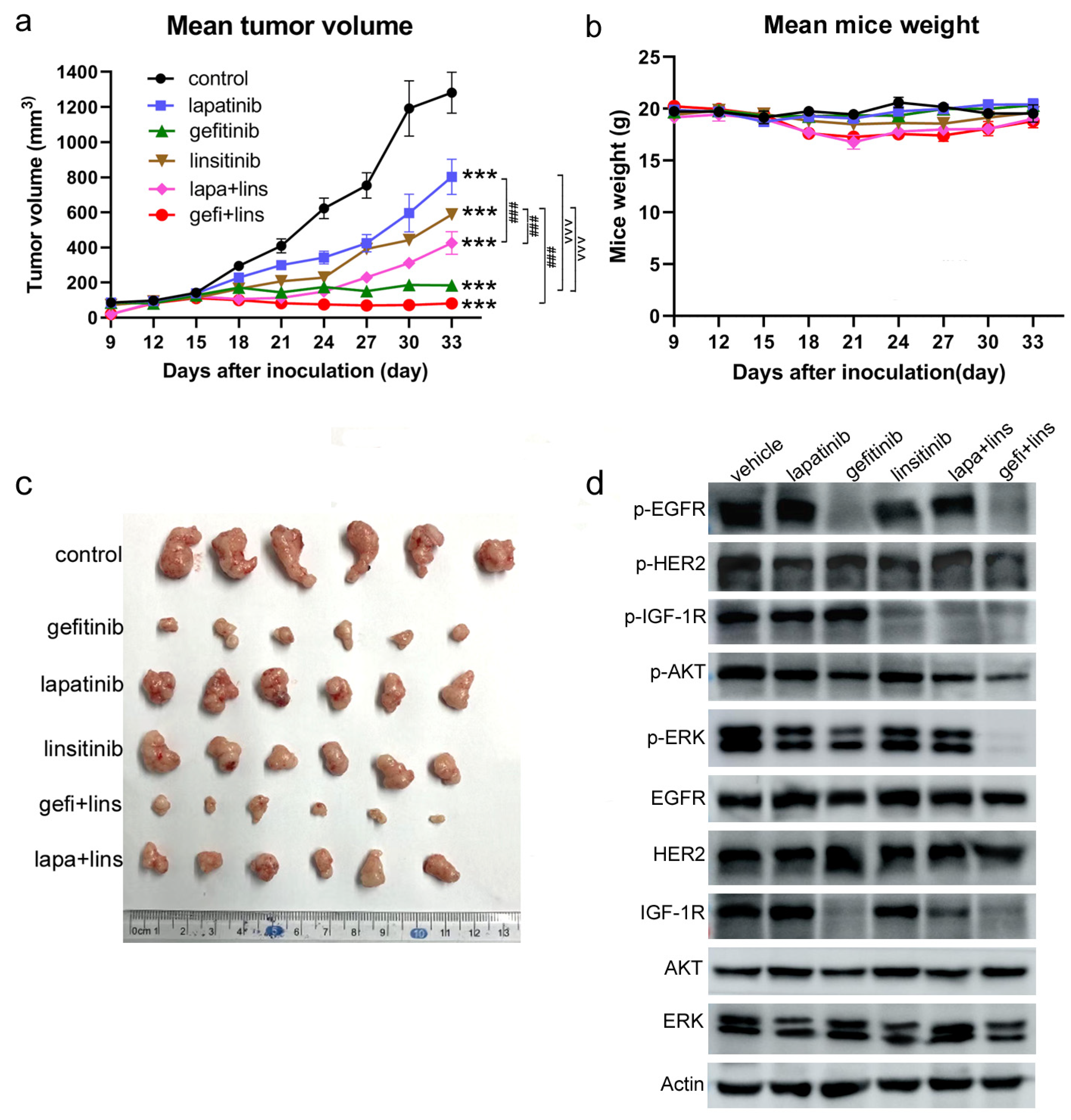
2.5. Lapatinib or Gefitinib Combined with Linsitinib More Potently Inhibited the Tumor Growth In Vivo
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Culture
4.2. Reagents and Antibodies
4.3. Cell Viability Assay
4.4. CI Value Calculation
4.5. Cell Wound Scratch Assay
4.6. Transwell Migration and Invasion Assay
4.7. Cell Apoptosis Assay
4.8. Cell Cycle Analysis
4.9. Western Blot Assay
4.10. In Vivo Efficacy Assay
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EGFR | Epidermal Growth Factor Receptor |
| IGF-1R | Insulin-like Growth Factor 1 Receptor |
| HER2 | Human Epidermal Growth Factor Receptor 2 |
| VEGFR | Vascular Endothelial Growth Factor Receptor |
| ESCC | Esophageal squamous cell carcinoma |
| EAC | Esophageal Adenocarcinoma |
| RTK | Receptor Tyrosine Kinase |
| TKI | Tyrosine Kinase Inhibitor |
| lapa | Lapatinib |
| gefi | Gefitinib |
| lins | Linsitinib |
| IC50 | Half maximal inhibitory concentration |
| ERK | Extracellular signal regulated kinase |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Harada, K.; Rogers, J.E.; Iwatsuki, M.; Yamashita, K.; Baba, H.; Ajani, J.A. Recent advances in treating oesophageal cancer. F1000Resarch 2020, 9, F1000 Faculty Rev-1189. [Google Scholar] [CrossRef]
- Li, S.; Chen, H.; Man, J.; Zhang, T.; Yin, X.; He, Q.; Yang, X.; Lu, M. Changing trends in the disease burden of esophageal cancer in China from 1990 to 2017 and its predicted level in 25 years. Cancer Med. 2021, 10, 1889–1899. [Google Scholar] [CrossRef] [PubMed]
- Hirano, H.; Kato, K. Systemic treatment of advanced esophageal squamous cell carcinoma: Chemotherapy, molecular-targeting therapy and immunotherapy. Jpn. J. Clin. Oncol. 2019, 49, 412–420. [Google Scholar] [CrossRef]
- Watanabe, M.; Otake, R.; Kozuki, R.; Toihata, T.; Takahashi, K.; Okamura, A.; Imamura, Y. Recent progress in multidisciplinary treatment for patients with esophageal cancer. Surg. Today 2020, 50, 12–20. [Google Scholar] [CrossRef]
- Yang, Y.M.; Hong, P.; Xu, W.W.; He, Q.Y.; Li, B. Advances in targeted therapy for esophageal cancer. Signal Transduct. Target Ther. 2020, 5, 229. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.; Han, Y.; Brain, L. Epidermal growth factor receptor: An important target in esophageal cancer. Expert Opin. Ther. Targets 2013, 17, 1179–1185. [Google Scholar] [CrossRef]
- Denduluri, S.; Idowu, O.; Wang, Z.; Liao, Z.; Yan, Z.; Mohammed, M.K.; Ye, J.; Wei, Q.; Wang, J.; Zhao, L.; et al. Insulin-like growth factor (IGF) signaling in tumorigenesis and the development of cancer drug resistance. Genes Dis. 2015, 2, 13–25. [Google Scholar] [CrossRef]
- Simpson, A.; Petnga, W.; Macaulay, V.M.; Weyer-Czernilofsky, U.; Bogenrieder, T. Insulin-like growth factor (IGF) pathway targeting in cancer: Role of the IGF axis and opportunities for future combination studies. Target Oncol. 2017, 12, 571–597. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Sun, X.; Shen, B. Molecular imaging of IGF-1R in cancer. Mol. Imaging 2017, 16, 1536012117736648. [Google Scholar] [CrossRef] [Green Version]
- Cao, H.Y.; Guo, X.F.; Zhu, X.F.; Li, S.S.; Zhen, Y.S. A ligand-based and enediyne-energized bispecific fusion protein targeting epidermal growth factor receptor and insulin-like growth factor-1 receptor shows potent antitumor efficacy against esophageal cancer. Oncol. Rep. 2017, 37, 3329–3340. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Alex, J.M.; Bast, F. Insulin receptor (IR) and insulin-like growth factor receptor 1 (IGF-1R) signaling systems: Novel treatment strategies for cancer. Med. Oncol. 2014, 31, 805. [Google Scholar] [CrossRef] [PubMed]
- Kindler, H.L.; Richards, D.A.; Garbo, L.E.; Garon, E.B.; Stephenson Jr, J.J.; Rocha-Lima, C.M.; Safran, H.; Chan, D.; Kocs, D.M.; Galimi, F.; et al. A randomized, placebo-controlled phase 2 study of ganitumab (AMG 479) or conatumumab (AMG 655) in combination with gemcitabine in patients with metastatic pancreatic cancer. Ann. Oncol. 2012, 23, 2834–2842. [Google Scholar] [CrossRef]
- Pappo, A.S.; Patel, S.R.; Crowley, J.; Reinke, D.K.; Kuenkele, K.P.; Chawla, S.P.; Toner, G.C.; Maki, R.G.; Meyers, P.A.; Chugh, R.; et al. R1507, a monoclonal antibody to the insulin-like growth factor 1 receptor, in patients with recurrent or refractory Ewing sarcoma family of tumors: Results of a phase II Sarcoma Alliance for Research through Collaboration study. J. Clin. Oncol. 2011, 29, 4541–4547. [Google Scholar] [CrossRef] [PubMed]
- Langer, C.J.; Novello, S.; Park, K.; Krzakowski, M.; Karp, D.D.; Mok, T.; Benner, R.J.; Scranton, J.R.; Olszanski, A.J.; Jassem, J. Randomized, phase III trial of first-line figitumumab in combination with paclitaxel and carboplatin versus paclitaxel and carboplatin alone in patients with advanced non-small-cell lung cancer. J. Clin. Oncol. 2014, 32, 2059–2066. [Google Scholar] [CrossRef] [PubMed]
- Haisa, M. The type 1 insulin-like growth factor receptor signalling system and targeted tyrosine kinase inhibition in cancer. J. Int. Med. Res. 2013, 41, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Chiappori, A.A.; Otterson, G.A.; Dowlati, A.; Traynor, A.M.; Horn, L.; Owonikoko, T.K.; Ross, H.J.; Hann, C.L.; Hejleh, T.A.; Nieva, J.; et al. A randomized phase II study of linsitinib (OSI-906) versus topotecan in patients with relapsed small-cell lung cancer. Oncologist 2016, 21, 1163–1164. [Google Scholar] [CrossRef] [PubMed]
- Haluska, P.; Menefee, M.; Plimack, E.R.; Rosenberg, J.; Northfelt, D.; LaVallee, T.; Shi, L.; Yu, X.Q.; Burke, P.; Huang, J.; et al. Phase I dose-escalation study of MEDI-573, a bispecific, antiligand monoclonal antibody against IGFI and IGFII, in patients with advanced solid tumors. Clin. Cancer Res. 2014, 20, 4747–4757. [Google Scholar] [CrossRef]
- Friedbichler, K.; Hofmann, M.H.; Kroez, M.; Ostermann, E.; Lamche, H.R.; Koessl, C.; Borges, E.; Pollak, M.N.; Adolf, G.; Adam, P.J. Pharmacodynamic and antineoplastic activity of BI 836845, a fully human IGF ligand-neutralizing antibody, and mechanistic rationale for combination with rapamycin. Mol. Cancer Ther. 2014, 13, 399–409. [Google Scholar] [CrossRef]
- Shitara, K.; Bang, Y.J.; Iwasa, S.; Sugimoto, N.; Ryn, M.H.; Sakai, D.; Chung, H.C.; Kawakami, H.; Yabusaki, H.; Lee, J.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Gastric Cancer. N. Engl. J. Med. 2020, 382, 2419–2430. [Google Scholar] [CrossRef]
- Suntharalingam, M.; Winter, K.; Ilson, D.; Dicker, A.P.; Kachnic, L.; Konski, A.; Chakravarthy, A.B.; Anker, C.J.; Thakrar, H.; Horiba, N.; et al. Effect of the Addition of Cetuximab to Paclitaxel, Cisplatin, and Radiation Therapy for Patients With Esophageal Cancer: The NRG Oncology RTOG 0436 Phase 3 Randomized Clinical Trial. JAMA Oncol. 2017, 3, 1520–1528. [Google Scholar] [CrossRef] [PubMed]
- Dutton, S.J.; Ferry, D.R.; Blazeby, J.M.; Abbas, H.; Dahle-Smith, A.; Mansoor, W.; Thompson, J.; Harrison, M.; Chatterjee, A.; Falk, S.; et al. Gefitinib for oesophageal cancer progressing after chemotherapy (COG): A phase 3, multicentre, double-blind, placebo-controlled randomised trial. Lancet Oncol. 2014, 15, 894–904. [Google Scholar] [CrossRef]
- Lorenzen, S.; Knorrenschild, J.R.; Haag, G.M.; Pohl, M.; Thuss-Patience, P.; Bassermann, F.; Helbig, U.; Weibinger, F.; Schnoy, E.; Becker, K.; et al. Lapatinib versus lapatinib plus capecitabine as second-line treatment in human epidermal growth factor receptor 2-amplified metastatic gastro-oesophageal cancer: A randomised phase II trial of the Arbeitsgemeinschaft Internistische Onkologie. Eur. J. Cancer 2015, 51, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Hecht, J.R.; Bang, Y.J.; Qin, S.K.; Chung, H.C.; Xu, J.M.; Park, J.O.; Jeziorski, K.; Shparyk, Y.; Hoff, P.M.; Sobrero, A.; et al. Lapatinib in Combination with Capecitabine Plus Oxaliplatin in Human Epidermal Growth Factor Receptor 2-Positive Advanced or Metastatic Gastric, Esophageal, or Gastroesophageal Adenocarcinoma: TRIO-013/LOGiC—A Randomized Phase III Trial. J. Clin. Oncol. 2016, 34, 443–451. [Google Scholar] [CrossRef]
- Van der Veeken, J.; Oliveira, S.; Schiffelers, R.M.; Storm, G.; van Bergen En Henegouwen, P.M.; Roovers, R.C. Crosstalk between epidermal growth factor receptor- and insulin-like growth factor-1 receptor signaling: Implications for cancer therapy. Curr. Cancer Drug Targets 2009, 9, 748–760. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, J.; Ji, D.; Wang, C.; Liu, R.; Wu, Z.; Liu, L.; Zhu, D.; Chang, J.; Geng, R.; et al. Functional genetic approach identifies MET, HER3, IGF1R, INSR pathways as determinants of lapatinib unresponsiveness in HER2-positive gastric cancer. Clin. Cancer Res. 2014, 20, 4559–4573. [Google Scholar] [CrossRef]
- Doki, Y.; Ajani, J.A.; Kato, K.; Xu, J.; Wyrwicz, L.; Motoyama, S.; Ogata, T.; Kawakami, H.; Hsu, C.H.; Adenis, A.; et al. Nivolumab combination therapy in advanced esophageal squamous-cell carcinoma. N. Engl. J. Med. 2022, 386, 449–462. [Google Scholar] [CrossRef]
- Sun, J.M.; Shen, L.; Shah, M.A.; Enzinger, P.; Adenis, A.; Doi, T.; Kojima, T.; Metges, J.P.; Li, Z.; Kim, S.B.; et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): A randomised, placebo-controlled, phase 3 study. Lancet 2021, 398, 759–771. [Google Scholar] [CrossRef]
- Huang, X.; Gao, L.; Wang, S.; McManaman, J.L.; Thor, A.D.; Yang, X.; Esteva, F.J.; Liu, B. Heterotrimerization of the growth factor receptors erbB2, erbB3, and insulin-like growth factor-i receptor in breast cancer cells resistant to herceptin. Cancer Res. 2010, 70, 1204–1214. [Google Scholar] [CrossRef]
- Wheeler, D.L.; Dunn, E.F.; Harari, P.M. Understanding resistance to EGFR inhibitors-impact on future treatment strategies. Nat. Rev. Clin. Oncol. 2010, 7, 493–507. [Google Scholar] [CrossRef] [Green Version]
- Tandon, R.; Kapoor, S.; Vali, S.; Senthil, V.; Nithya, D.; Venkataramanan, R.; Sharma, A.; Talwadkar, A.; Ray, A.; Bhatnagar, P.K.; et al. Dual epidermal growth factor receptor (EGFR)/insulin-like growth factor-1 receptor (IGF-1R) inhibitor: A novel approach for overcoming resistance in anticancer treatment. Eur. J. Pharmacol. 2011, 667, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Clayburgh, D.R.; Gross, N.D.; Proby, C.; Koide, J.; Wong, M.H. Effects of epidermal growth factor receptor and insulin-like growth factor 1 receptor inhibition on proliferation and intracellular signaling in cutaneous SCCHN: Potential for dual inhibition as a therapeutic modality. Head Neck 2013, 35, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Sereno, A.; Aivazian, D.; Langley, E.; Miller, B.R.; Snyder, W.B.; Chan, E.; Cantele, M.; Morena, R.; Joseph, I.B.; et al. A stable IgG-like bispecific antibody targeting the epidermal growth factor receptor and the type I insulin-like growth factor receptor demonstrates superior anti-tumor activity. MAbs 2011, 3, 273–288. [Google Scholar] [CrossRef] [PubMed]
- Kwak, E.K.; Jankowski, J.; Thayer, S.P.; Lauwers, G.Y.; Brannigan, B.W.; Harris, P.L.; Okimoto, R.A.; Haserlat, S.M.; Driscoll, D.R.; Ferry, D.; et al. Epidermal growth factor receptor kinase domain mutations in esophageal and pancreatic adenocarcinomas. Clin. Cancer Res. 2006, 12 Pt 1, 4283–4287. [Google Scholar] [CrossRef] [PubMed]
- Van Erp, N.P.; Gelderblom, H.; Guchelaar, H.J. Clinical pharmacokinetics of tyrosine kinase inhibitors. Cancer Treat Rev. 2009, 35, 692–706. [Google Scholar] [CrossRef]
- McKillop, D.; Partridge, E.A.; Kemp, J.V.; Spence, M.P.; Kendrew, J.; Barnett, S.; Wood, P.G.; Giles, P.B.; Patterson, A.B.; Bichat, F.; et al. Tumor penetration of gefitinib, an epidermal growth factor receptor tyrosine kinase inhibitor. Mol. Cancer Ther. 2005, 4, 641–649. [Google Scholar] [CrossRef]
- Camblin, A.J.; Tan, G.; Curley, M.D.; Yannatos, I.; Iadevaia, S.; Rimkunas, V.; Mino-Kenudson, M.; Bloom, T.; Schoeberl, B.; Drummond, D.C.; et al. Dual targeting of IGF-1R and ErbB3 as a potential therapeutic regimen for ovarian cancer. Sci. Rep. 2019, 9, 16832. [Google Scholar] [CrossRef]
- Camblin, A.J.; Pace, E.A.; Adams, S.; Curley, M.D.; Rimkunas, V.; Nie, L.; Tan, G.; Bloom, T.; Iadevaia, S.; Baum, J.; et al. Dual Inhibition of IGF-1R and ErbB3 Enhances the Activity of Gemcitabine and Nab-Paclitaxel in Preclinical Models of Pancreatic Cancer. Clin. Cancer Res. 2018, 24, 2873–2885. [Google Scholar] [CrossRef] [Green Version]
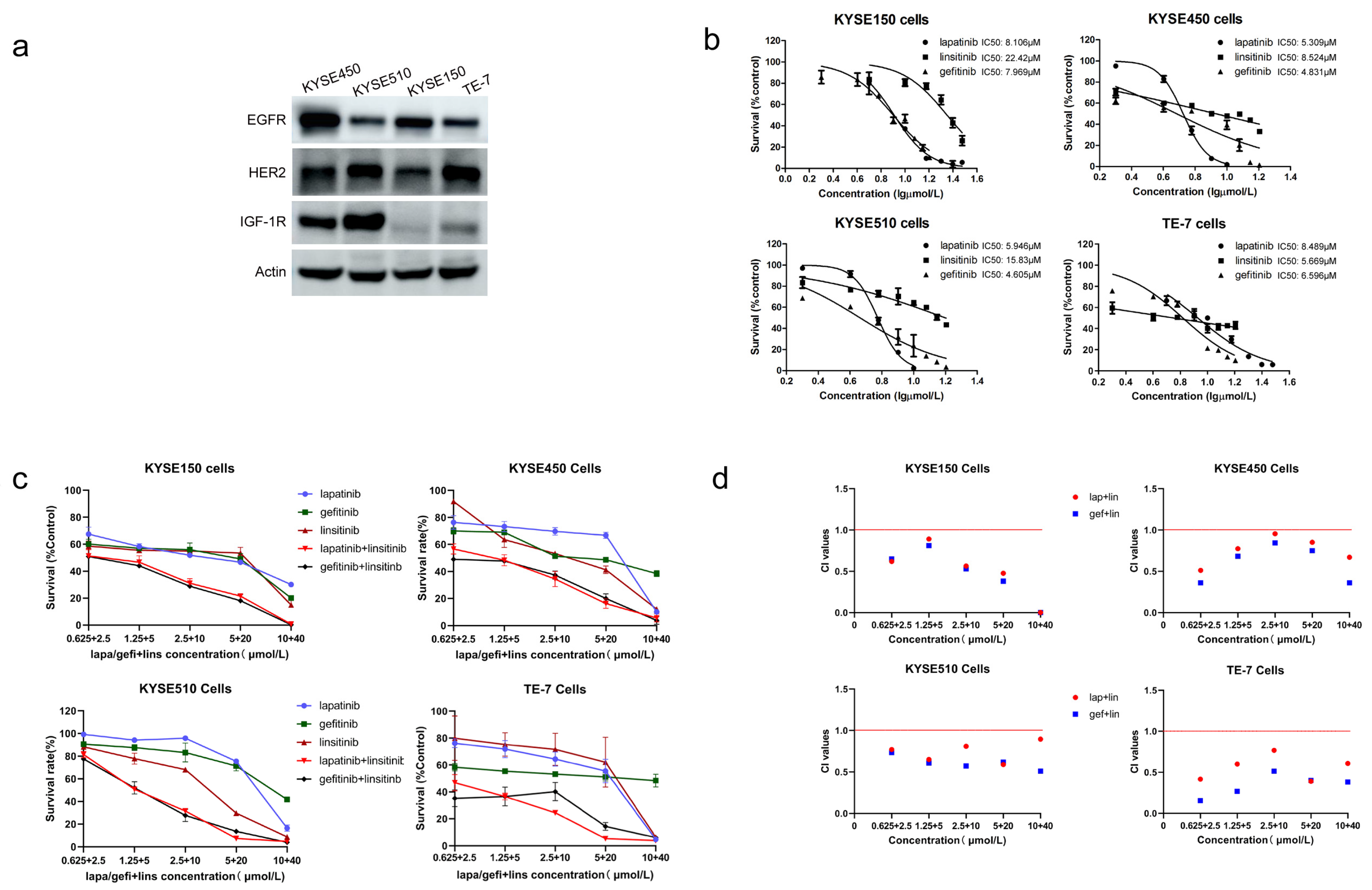
| Cell Lines | Lapatinib (μmol/L) | Gefitinib (μmol/L) | Linsitinib (μmol/L) |
|---|---|---|---|
| KYSE150 | 8.106 | 7.969 | 22.42 |
| KYSE450 | 5.309 | 4.831 | 8.524 |
| KYSE510 | 5.946 | 4.605 | 15.83 |
| TE-7 | 8.489 | 6.596 | 5.669 |
| Cell Lines | Combination Index | ||
|---|---|---|---|
| ED50 | ED75 | ED90 | |
| lapatinib | |||
| KYSE150 | 0.93 | 0.27 | 0.08 |
| KYSE450 | 0.68 | 0.74 | 0.81 |
| KYSE510 | 0.72 | 0.73 | 0.75 |
| TE-7 | 0.51 | 0.53 | 0.55 |
| gefitinib | |||
| KYSE150 | 0.99 | 0.28 | 0.08 |
| KYSE450 | 0.59 | 0.50 | 0.55 |
| KYSE510 | 0.63 | 0.58 | 0.55 |
| TE-7 | 0.29 | 0.30 | 0.46 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, J.; Guo, Z.; Zhang, H.; Guo, R.; Zhu, X.; Guo, X. Dual Inhibition of EGFR and IGF-1R Signaling Leads to Enhanced Antitumor Efficacy against Esophageal Squamous Cancer. Int. J. Mol. Sci. 2022, 23, 10382. https://doi.org/10.3390/ijms231810382
Kang J, Guo Z, Zhang H, Guo R, Zhu X, Guo X. Dual Inhibition of EGFR and IGF-1R Signaling Leads to Enhanced Antitumor Efficacy against Esophageal Squamous Cancer. International Journal of Molecular Sciences. 2022; 23(18):10382. https://doi.org/10.3390/ijms231810382
Chicago/Turabian StyleKang, Jia, Zanzan Guo, Haoqi Zhang, Rongqi Guo, Xiaofei Zhu, and Xiaofang Guo. 2022. "Dual Inhibition of EGFR and IGF-1R Signaling Leads to Enhanced Antitumor Efficacy against Esophageal Squamous Cancer" International Journal of Molecular Sciences 23, no. 18: 10382. https://doi.org/10.3390/ijms231810382
APA StyleKang, J., Guo, Z., Zhang, H., Guo, R., Zhu, X., & Guo, X. (2022). Dual Inhibition of EGFR and IGF-1R Signaling Leads to Enhanced Antitumor Efficacy against Esophageal Squamous Cancer. International Journal of Molecular Sciences, 23(18), 10382. https://doi.org/10.3390/ijms231810382





