Abstract
Plants produce a variety of high-value chemicals (e.g., secondary metabolites) which have a plethora of biological activities, which may be utilised in many facets of industry (e.g., agrisciences, cosmetics, drugs, neutraceuticals, household products, etc.). Exposure to various different environments, as well as their treatment (e.g., exposure to chemicals), can influence the chemical makeup of these plants and, in turn, which chemicals will be prevalent within them. Essential oils (EOs) usually have complex compositions (>300 organic compounds, e.g., alkaloids, flavonoids, phenolic acids, saponins and terpenes) and are obtained from botanically defined plant raw materials by dry/steam distillation or a suitable mechanical process (without heating). In certain cases, an antioxidant may be added to the EO (EOs are produced by more than 17,500 species of plants, but only ca. 250 EOs are commercially available). The interesting bioactivity of the chemicals produced by plants renders them high in value, motivating investment in their production, extraction and analysis. Traditional methods for effectively extracting plant-derived biomolecules include cold pressing and hydro/steam distillation; newer methods include solvent/Soxhlet extractions and sustainable processes that reduce waste, decrease processing times and deliver competitive yields, examples of which include microwave-assisted extraction (MAE), ultrasound-assisted extraction (UAE), subcritical water extraction (SWE) and supercritical CO2 extraction (scCO2). Once extracted, analytical techniques such as chromatography and mass spectrometry may be used to analyse the contents of the high-value extracts within a given feedstock. The bioactive components, which can be used in a variety of formulations and products (e.g., displaying anti-aging, antibacterial, anticancer, anti-depressive, antifungal, anti-inflammatory, antioxidant, antiparasitic, antiviral and anti-stress properties), are biorenewable high-value chemicals.
1. Introduction
The strive for sustainability in industry motivates the consideration of plants as a source of biorenewable feedstocks, due to their abundance, the range of molecules they produce and our ability to employ engineering biology to generate new/valuable biomolecules [1,2,3]. Plants produce primary metabolites, which are common to most organisms (such as fats, proteins and sugars), and the more diverse secondary metabolites (species-specific, expressing various bioactives [4,5], and potentially therapeutic [6], used for the treatment of cancer, heart disease, circulatory disease and viral infection [7]).
Biomolecules extracted from plant material may display bioactivity [8] (e.g., as natural preservatives for food [9], perfumes [10], etc.), resulting in their incorporation in real-world products. Natural variation in the molecular makeup of plant biomass has necessitated studies seeking to understand the factors influencing variations in the molecular composition/yield after employing a specific extraction method (species, location/environment, harvest time, plant maturity, genetic and physiological factors [10,11,12,13,14]) to facilitate the harvest of a rich and diverse portfolio of molecules [11]. The majority of these molecules may be classified into several sub-groups of chemical species, including alkaloids, monoterpenes, sesqueterpenes, triterpenes, saponins, steroids, flavonoids, polyacetylenes and polyketides. Some representative structures are illustrated in Figure 1.
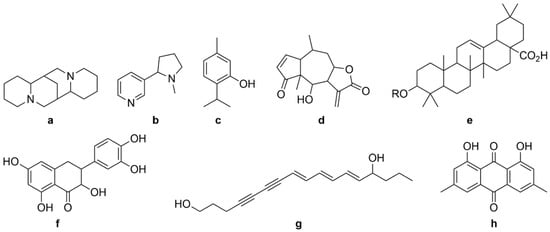
Figure 1.
General structures of different categories of plant bioactive compounds: alkaloids (a,b), monoterpenes (c), sesqueterpenes (d), triterpenes, saponins, steroids (e), flavonoids (f), polyacetylenes (g), polyketides (h).
Terpenes (terpenoids) are constituents of plant biomass and are known to be responsible for many of the medicinal and pharmacological applications of chemicals extracted from plants [15,16], resulting from their antimicrobial, antibacterial, anticancer, anti-malaria and anti-inflammatory properties [11,17]. Alkaloids, flavonoids, phenolic acids and saponins are also observed to have pharmacological benefits, including anti-inflammatory, antioxidant, anti-microbial, anti-diabetic, anti-mutagenic, anti-spasmodic, hepato-protective and immune-stimulant properties [18,19]. A huge variety of biomolecules have been reported, including >25,000 terpenes, >12,000 alkaloids and >8,000 phenolic compounds, the structures of which govern their physicochemical properties and pharmacological behaviour [4]. The typical characteristic of terpene molecules is the presence of a five-carbon isoprene (2-methyl-1,3-butadiene) base unit, which may be considered the building block of terpenes [20]. Several permutations of terpenes exist, depending on the number of isoprene units they contain. Monoterpenes contain two isoprenes; sesquiterpenes contain three isoprenes; diterpenoids contain four isoprenes; sesterpenes contain five isoprenes; triterpenes contain six isoprenes; and meroterpenes are a broad class of compounds with partial terpenoid skeletons [21]. Alkaloids are identified by the presence of one or more nitrogen atoms as amines/amides/heterocycles [22]. Flavonoids are polyphenolic compounds containing benzo-gamma-pyrone structures [23], wherein the number and position of hydroxyl groups influences the antioxidant potential of the compound [24]. Saponins are glycosides containing sugar chains attached to triterpenes or sapogenins, rendering them amphiphilic and enabling their utilisation in detergents and wetting, emulsifying and foaming agents [25].
Secondary metabolites are not of direct use for a plant’s growth/reproduction but play important roles in an organism’s interaction with its environment, functioning as chemical defences that mitigate the impacts of biotic and abiotic stresses [26,27]. This is fundamental for understanding the nature of these compounds and their commercialisation, and this field of study (allelopathic activity) is defined as “The science that studies any process involving secondary metabolites produced by plants, algae, bacteria and fungi that influences the growth and development of agricultural and biological systems” [11]. These molecules, known as allelochemicals, may have both detrimental and beneficial effects on a target organism [28]. It is important to consider this, as the default effects of these secondary metabolites may have differing results when introduced into an unintended organism. For instance, caffeine, a purine alkaloid, is originally synthesised by plants for its antimicrobial activity and as a natural insecticide. However, when ingested by people, it results in both analgesic and stimulatory responses by improving alertness, vigilance, attention, reaction time and attention [29,30], which are in direct contrast to its natural use. Likewise, untested and unidentified compounds [31] within a plant feedstock may also have unpredictable effects on users.
Plants produce aromatic compounds that may have attractive scents designed to attract pollinators and facilitate seed dispersion [31], which can be extracted from specimens, including flowers, barks, roots, fruits, leaves and other parts of plants. These include many volatile chemicals, which are often unsaturated hydrocarbons, alcohol, aldehydes, esters, ethers, ketones, phenols and terpenes [32]. The diverse nature of these compounds, coupled with interspecies and intraspecies variation, results in a large portfolio of possible applications of compounds extracted from plants (e.g., see Table 1). The full economic, health and societal benefits of these chemicals necessitates efficient extraction methods and the analysis of the constituent compounds. Conventional methods employed for the extraction of biomolecules typically utilise distillation for volatile components or cold pressing. Greener alternatives have been developed (extraction methods employing solvents of various types, including organic/aqueous solvent systems, microwaves, ultrasound and supercritical CO2, as outlined below) [33,34], that subsequently employ analytical techniques, such as gas chromatography (GC) or high-performance liquid chromatography (HPLC) coupled with an appropriate mass spectrometry (MS) system (i.e., GC-MS and HPLC-MS), in order to identify the compounds isolated.

Table 1.
Essential oils for common medical problems. Adapted from the literature [32] (open access, CC BY-NC-ND 4.0).
Molecules extracted from plant material are industrially interesting because of their therapeutic, medicinal and biochemical properties [35,36,37], resulting in their potential applications as antiseptics, anti-inflammatories, cosmetics, flavourings, fragrances, preservatives and sedatives [8], enabling the treatment of numerous diseases and ailments [8,38,39], which explains their popularity in healthcare formulations [31]. The planet’s rich biodiversity means that bioactive molecules may already be identified or, indeed, as yet unidentified.
A promising mode of application of the biomolecules produced by plants is the fight against pathogens displaying antimicrobial resistance, such as alkaloids, cyanogenic glucosides, phenolics, terpenoids, steroids, etc., which have a bioactivity that results in degradative physiological changes to the pathogens [40]. The fact that many plant extracts are relatively unexplored/under-investigated highlights their potential for other high-value applications (e.g., as antimicrobials/antivirals [41]). While herpes simplex virus is typically treated with acyclovir and other synthetic drugs, issues related to their efficacy and side effects have led to the successful exploration of plant-derived biomolecules as a means of combating such infections [42].
The utilisation of plant-derived biomolecules to combat diseases such as cancer is motivated by a desire to minimise treatments including surgery, radiotherapy, chemotherapy and immunotherapy, and their concomitant side effects on patients [43]. It is also important to note that, although plant-derived biomolecules create an opportunity for their use in cancer therapy, some biomolecules (e.g., some of those extracted from Salvia sclarea and Melaleuca quinquenerviaxi) have been shown to induce oestrogen-dependent cancers. Indeed, certain molecules present in plants, such as cyanine, flavins, porphyrins and psoralen, may be carcinogenic [8]. Plant-derived biomolecules may be used for their anticancer effects, which are derived from their ability to regulate the production of reactive oxygen species (ROS), which are associated with inflammation, oxidative stresses and signalling pathways that may lead to cancer and tumour development. Alternatively, plant-derived biomolecules may also be utilised for inducing apoptosis as a means of coping with cancer [8,31,44]. Plant-derived biomolecules may also find application in methods for coping with the indirect factors of diseases such cancer on a patient’s health, such as anxiety and insomnia, and their use has been shown to be effective for treating both of these [45], as exemplified by lavender-derived biomolecules, which have been shown to improve sleep quality and reduce anxiety [46,47].
Plant-derived biomolecules may be utilised for the treatment of numerous other mental ailments, further to their use in anxiety and insomnia. Their use has potential for the treatment of Alzheimer’s disease (AD), a neurodegenerative disease associated with memory and cognition [48]. A major therapeutic strategy for AD is the inhibition of the enzyme acetylcholinesterase. Aromatherapy (exploiting the volatile components of plants) also provides an effective non-pharmacological therapy that can be used against neurodegenerative diseases; for instance, volatiles from rosemary/lemon and lavender/orange proved effective for addressing symptoms and cognition [48,49]. Nervonic acid, a chemical constituent of plant seeds, enhances brain health through biosynthesis and the maintenance of nerve cell myelin. It therefore aids in the repair of nerve pathways, providing an effective treatment against several mental ailments, including schizophrenia, psychosis and alcoholism [50]. The Acer genus has been identified as a promising raw material used to produce nervonic acid [50], but several other species have also been identified, which may prove to be useful resources in various environments, including, but not limited to, Lunaria annua seed oils, which have ~25% nervonic acid, and Cardamine graeca, which have ~45% [51]. Other notable species include Tropaeolum speciosum, Borago officinalis and Cannabis sativa, all of which contain nervonic acid in their seed oils and may therefore contribute to the treatment of neurological disorders and, in general, pharmacological and nutraceutical applications resulting from their bio-functionality [52].
Plant-derived biomolecules have also shown promising signs in the area of dental care. Commercial brands such as LISTERINE® have incorporated them into mouthwashes for domestic use, which offers strong evidence to support the notion that plant-derived biomolecules have anti-plaque and anti-gingivitis properties [53,54]. It is also in competition with chlorhexidine, first investigated over 50 years ago, and is one of the most widely used oral antiseptics. Although both may stain teeth, chlorhexidine may also stain the tongue and gingiva and result in bitter and salty taste reductions [53]. A potential benefit may then be argued for the use of plant-derived biomolecules as a better alternative. Plant-derived biomolecules integrated into herbal toothpastes may exhibit antibacterial properties, which aid in tackling Streptococcus mutans, a bacterium known to result in the initial formation of tooth decay [55]. This activity may be the result of phytochemical biomolecules, such as menthol and eugenol, which influence the overall biological activity of their admixtures [56]. A study also concluded that the use of mouthwash containing plant-derived biomolecules alongside curcumin aided in supplementary treatments for reducing rheumatoid arthritis and chronic periodontitis activity [57], suggesting that plant-derived biomolecules, within oral/dental care, may be used as supplementary therapeutic agents for fighting against other diseases, in addition to their biological activities, which act as agents in dental care.
As early as 4500 BCE, in ancient Egypt there, was documentary evidence of the use of plant-derived biomolecules in cosmetics and ointments [31], and between 3000 to 2000 BCE, there were reports of the use of plants and their constituents for medicinal purposes, as well as fragrances in ancient India [37,58]. While various methods have been used to extract plant-derived biomolecules, only a handful are competitive on the industrial scale today.
Hydro-distillation (HD) involves the boiling of an aqueous suspension of plant material within an alembic, followed by the distillation of the resulting steam and plant-derived vapor, the collection of the condensate and the separation of the volatiles from the aqueous phase [59,60]. The closely related steam distillation utilises steam to volatilise plant-derived biomolecules that are of interest [34,59,60]. Solvent extractions involve the suspension of plant materials within organic solvents, followed by heating, filtration and solvent evaporation to isolate a filtrate, commonly in the form of a resin (resinoid) or a mixture of wax, fragrance and oils [59,60]. Cold pressing involves the mechanical compression of plant biomass to release components in the form of a watery emulsion, which is centrifuged to separate any hydrophobic oils [60,61]. Soxhlet extractions are a popular method, using a solid–liquid contact medium to remove compounds from a solid/gel matrix through their dissolution into a refluxing liquid phase [34].
The desire for innovation has resulted in the development of methods with reduced energy demands and CO2 emissions [62]. Microwave-assisted extraction (MAE) uses a microwave oven to irradiate and promote heat generation within the plant material, so that the energy load, CO2 emissions, costs and overall process time are reduced [34,63,64,65]. The applicability of MAE is quite broad relative to other methods, giving rise to various permutations of MAE, including compressed air microwave distillation (CAMD), vacuum microwave hydro distillation (VMHD), microwave hydro distillation (MWHD), solvent-free microwave extraction (SFME) and microwave-accelerated steam distillation (MASD) [63,65]. Similar to MAE, ultrasound-assisted extraction (UAE) is another option. UAE uses high frequency pulses that are applied to the sample to induce the cavitation phenomenon, which subsequently increases the rate of the mass transfer of molecules into the solvent [66,67,68]. Subcritical water extraction (SWE) operates by raising the temperature of the water to between 100–374 °C and applying a pressure high enough to maintain a liquid state. As the temperature is raised, there is an increase in the diffusion rate, as well as a decrease in the surface tension and viscosity. This may be optimised for the solubility of polar molecules, which is higher at lower temperatures, and less polar molecules, which have a higher solubility at higher temperatures [69,70]. Supercritical CO2 (SC-CO2) extraction operates by raising the temperature and pressure of CO2 above its critical points, 31.2 °C and 7.38 MPa, in order to attain a supercritical state. SC-CO2 may then be used as a solvent for molecule extraction, and by varying the temperature and pressure, the selectivity of these molecules may also be optimised. SC-CO2 passes through the feedstock to load bioactive molecules of the plant. The resulting discharge is then decompressed, enabling the separation of extracts with no remaining solvent residue, as the CO2 is allowed to evaporate [59,60,71].
Each method comes with it its own series of pros and cons in terms of what material may be optimised for a specific method, the sensitivity to the extraction method, yield, purity, cost and toxicity, as well as the specificity to various compounds within the feedstock, as outlined below. However, owing to the breadth of the literature, it is impossible to offer a comprehensive guide to the advantages and disadvantages of the extraction techniques discussed herein, as these are clearly compound-specific. However, the avid reader is directed towards some interesting literature, mentioned in the specific sections below, and some excellent reviews [66,72,73,74,75,76].
2. Extraction Methods for High-Value Chemicals
2.1. Hydrodistillation
Hydrodistillation is commonly applied in industry. However, due to the effects of temperature and pH within the distillation process, it has been found to result in potential alterations to the chemical composition of plant-derived biomolecules. This results from hydrolysis or the solubilisation of sensitive molecules [77,78]. However, typically, HD employs lower temperatures in order to reduce the risk of chemical decomposition. This is also advantageous, as it enables extraction from delicate flowers that would not survive typical distillation at temperatures above 100 °C. HD is also a method for easily extracting low-volatility and non-water-soluble molecules [30,59,60]. The outcomes of HD are feedstock specific, with HD typically being used for hydrophobic molecules with high boiling points within wood and plant material. The process is outlined in Figure 2 [59,60]. In some cases, HD extraction may damage and alter the chemical composition of the feedstock, but in others, the extract composition will be unaffected by the process conditions, enabling successful extraction.
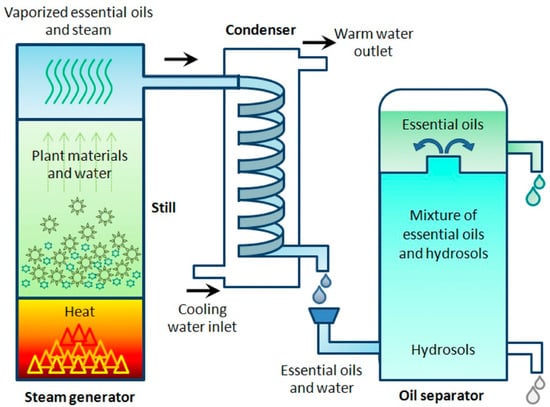
Figure 2.
Diagrammatic illustration of the hydrodistillation method. Reproduced from [60] with permission.
Process times can vary depending on the feedstock and extraction specificity, with increasing distillation time resulting in increasing yields (process times typically vary between 5 and 240 min); however, this will be context-specific, based on the apparatus/feedstocks [79,80,81,82,83]. An important benefit of HD is the lack of toxic residues left on the products after extraction due to the use of water for the extraction process. HD also offers the benefit of its relatively inexpensive capital costs, resulting from the lack of steam required compared to steam distillation. The capital costs would include the construction of industrial stills, varying from 1000–2000 L capacities, which are typically made of copper, tinned on the inside and surrounded by insulating brick [84]. The large capacity enables the offset of the low yield provided by HD, equating to 1–2% by weight, in the case of fresh aromatic plants, with values as low as a 0.015% yield for the distillation of roses [85]. One key drawback of HD is the heat/energy requirements and subsequent CO2 emissions, which have led to innovative technologies aiming to reduce the energy consumption and costs and to increase the quality [86]. Whereas conventional methods can result in more than 70% of the total process energy being spent on the extraction process, methods such as MAHD seek to accelerate extraction while offering similar extraction compositions, with more effective heating, reductions in thermal gradients, higher yields and a more efficient process [87,88].
2.2. Steam Distillation
Steam distillation is a method similar to that of hydro-distillation, but, in this case, the feedstock is treated directly with steam, which breaks down the cellular structures within the plant feedstocks, opens cavities containing the volatile components and enables them to volatilize for subsequent condensation and collection. The process is depicted in Figure 3 [60,89,90]. Steam distillation is also noted for its ability to be used for extractions of leaves and flowers [91], rendering it very popular in comparison with other methods of extraction [92]. The popularity of this method is indicative of its applicability to the distillation of polar, acidic and basic organic compounds of reasonable volatility [93]. However, polar compounds may be lost in the aqueous distillate and to the water within the still, which may require recovery through the redistillation of the water via cohobation, with the cost of added energy consumption. A subsequent consideration is the non-selective nature of steam distillation, leading to undesired extractions and the potential hydrolysis of active components resulting from elevated temperatures [94,95].
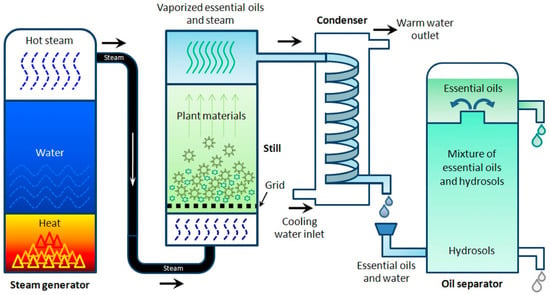
Figure 3.
Diagrammatic illustration of the steam distillation method. Reproduced from [60] with permission.
In summary, the advantages and disadvantages of steam distillation are as follows. The advantages include: solvent-free products, a lack of requirement for subsequent separation, a potential for industrial scale processing, inexpensive equipment and technological maturity. By comparison, the disadvantages include: the thermal degradation/hydrolysis of sensitive compounds, lengthy extraction times (1–5 h) and high energy consumption levels [95,96]. However, the extraction time requirements are context-dependent, with the multi-stage steam distillation extraction of volatiles from Rosemarinuse officinialis L. potentially being <1 h, although longer times have led to greater yields [97]. An investigation of the influence of steam distillation extraction times on the chemical composition of Patchouli oil concluded that increasing the extraction time only increases the quality of the resulting oil to an extent [98]. There is no simple rule regarding appropriate steam distillation extraction times due to variations in feedstocks, etc. [99].
2.3. Cold Pressing
Cold pressing (illustrated in Figure 4) has been noted to be an effective method for the extraction of plant-derived biomolecules from various feedstocks, with a notable focus on the extraction of molecules from citrus-based peels and seeds [34,61,90,91]. Resulting from this extraction method’s reliance on mechanical action, no external chemical input is required; hence, the resulting oil is 100% pure, retaining all its original properties [34,100]. Another commendable attribute of cold pressing and a likely reason for its adoption within industry is the health, safety, economic and environmental benefits resulting from the lack of chemical involvement [101]. Cold pressing has also been demonstrated to favour polyphenolic and antioxidant activity [101,102]. This may result from the lower operating temperatures (<40 °C), preventing the degradation of polyphenols and resulting in higher polyphenolic contents and antioxidant activity [103].
Cold pressing for the purpose of extraction may appear to be a simple approach to feedstock extraction; however, the pre-treatment methods necessary for some plant-derived biomolecules dictate the yield and extraction quality. Pre-treatment methods may include peeling, drying and the solvent or enzymatic treatment of raw materials [104], the specifics of which vary between species and target biomolecules [105]. Although several forms of presses exist, screw presses are the most common, offering a majority extraction in a single pass (typically leaving only 20% of the oil content within the output meal, while a second pass will leave 5–7% of the oil left in the resulting cake) [106]. An industrial-scale mechanical pressing plant has a typical throughput of 60–100 tons/day yet, while the throughput is high, the yield of mechanical pressing is typically relatively low. Consequently, a well-practiced strategy is the sequential use of solvent extraction with mechanical pressing (leaving only 1–2% of the oil content left in the final meal) [106,107,108].
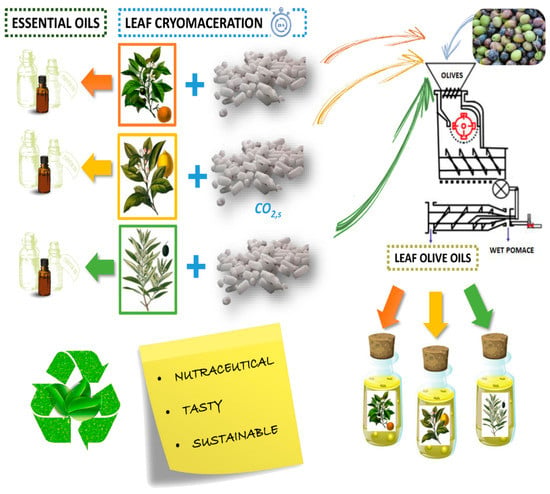
Figure 4.
Schematic of the cold pressing setup. Reproduced from [109] with permission (open access, Attribution 4.0 International (CC BY 4.0)).
2.4. Solvent Extraction
Solvent extraction offers a selective approach to extracting bioactives, with the variation of the solvent controlling the yield/composition of the molecules isolated. Solvents with a polarity similar to a given solute often result in better extractions, and there is a trend towards the use of green solvents from renewable resources (examples of which are depicted in Figure 5) [110]. Various solvents, when used sequentially, may also be utilised to extract molecules with differing polarities [76,111,112]. Another benefit of solvent extractions is the opportunity for their implementation in extractions from fragile/delicate flowers, which may not be robust enough to tolerate the potentially degradative effects of heat intensive methods, such as steam distillation [60]. However, potential complications include the particle size of the raw materials, solvent to solid ratios, the extraction temperature and the duration [76].
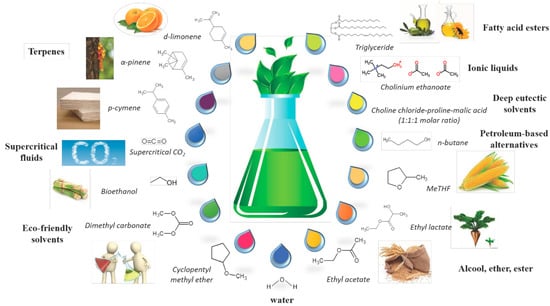
Figure 5.
Examples of solvents used for extraction setups. Reproduced from [110] with permission (open access, Attribution 4.0 International (CC BY 4.0)).
For appropriate extraction, solvents require distillation or treatment prior to extraction in order to remove any impurities (e.g., stabilisers) that they contain, thereby minimising the prospects of complications. Moreover, solvents should be easily removable, inert, non-toxic and lacking flammability in order to enable simple processing [113] and minimise the potential for issues downstream (e.g., the presence of toxic residual solvents in the products) [114,115]. Consequently, techniques such as UAE are becoming ever more attractive [116,117].
A widely noted benefit of the greener methods, further to the reduction in solvent usage, is the emphasis on reducing the energy required to maintain the process as compared with organic solvent extraction methods [115,117,118,119]. Solvent extraction, particularly extraction through maceration, may require long extraction times and high solvent usage [76,119,120]; however, longer extraction times lead to higher costs [120].
2.5. Soxhlet Extraction
Soxhlet extraction (depicted in Figure 6) is a method which was originally designed for lipid extraction from a solid matrix (e.g., leaves) [34] and is particularly useful for biological and environmental samples (e.g., soils, sediments, animal and plant material) [121,122]. Soxhlet extraction operates by using solvents such as dichloromethane, which may also be mixed with acetone or hexane, whilst non-polar solvents are typically not used [122]. While this technology is mature/established [123,124] and acts as a useful reference point for comparison against other extraction techniques [125,126], a disadvantage of this method is that the solvent utilised is the only parameter in the process allowing for selectivity, and subsequent concentration/purification may be required [127]. Potential drawbacks of Soxhlet extraction are its high energy/solvent consumption [127,128], long extraction times (with a typical minimum extraction time of approximately 8 h [122]) and reduced sample throughput [129]. Process optimisation for the extraction of crop oil from seed kernels can reduce extraction times to ca. 4.5 h [130], while in the case of pesticides, the extractions may be longer (6 to 24 h) [131]. Such differences in extraction times highlight the context dependence of each extraction method.
The prevalence of Soxhlet extraction is testament to the established advantages of the method. Soxhlet extraction is able to produce a higher yield of Eucalyptus oil from Eucalyptus leaves than HD (36.3% via Soxhlet extraction instead of 3.8% via HD). In addition to the higher yield, Soxhlet extraction enables the extraction of volatile components and high-molecular-weight molecules, as opposed to only volatiles in the use of HD [132]. Soxhlet extraction was also acknowledged to be better suited for the extraction of sterols, a type of lipid, from tobacco when compared with accelerated solvent extraction [133].
Similar to other extraction methods, given the parameters and context-driven nature of the investigation, the yields are variable. A study of the extraction of spent coffee grounds found that the oil yields obtained via Soxhlet extraction varied from 7 to 30% (dry weight); however, the authors noted that this may be due to variations in the feedstocks of spent coffee beans that were used [125]. The combination of innovative techniques, such as MAE/UAE, with Soxhlet extraction may prove capable of overcoming the challenges of using Soxhlet extraction alone [126].
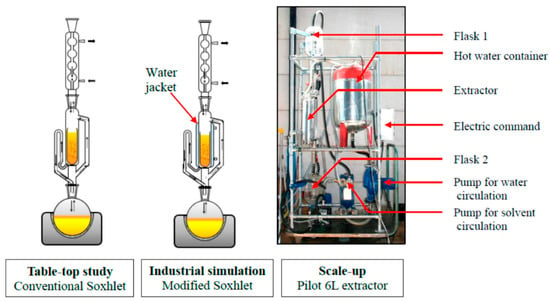
Figure 6.
Schematic of Soxhlet extraction setups. Reproduced from [134] with permission (open access, Attribution 4.0 International (CC BY 4.0)).
2.6. Microwave-Assisted Extraction (MAE)
MAE (in Figure 7) is used in conjunction with other forms of extraction techniques for the provision of auxiliary energy in order to increase energy savings, reduce solvent usage, decrease extraction times by up to 9 times and increase the product yield and quality, in accordance with the principles of ‘green’ chemistry [63,65]. MAE has a wide range of applications in extraction processes [135,136] and works in three stages. The energy provided through microwave irradiation increases the temperature and pressure in the extraction process, resulting in solute separation. The solute is released into the solvent from the sample matrix, and then the solvent becomes diffused across the sample matrix [137]. The efficiency of MAE, however, is still dependent on the solvent, sample and components under extraction, with the dielectric constant being an important parameter [135]. The dielectric constant, ε, is the ratio of the electric permeability of the material to the electric permeability of the free space [138], where the higher the constant is, the stronger the absorption will be. Thus, molecules reach the operational temperature quicker, and a closed vessel is preferred, allowing the solvent to be heated above its boiling point [139]. When the ε of the solvent is low, an open vessel is used, so that the sample components with relatively higher ε values will move into the surrounding cold solvent [140].
The rapid extraction of analytes coupled with the rapid heating of the sample–solvent mixture facilitates the extraction of thermally unstable compounds [135]. A comparison of the Soxhlet extraction method (6 h using hexanes) with MAE (20 s using hexanes) for the extraction of fresh peppermint oils showed higher yields obtained through the Soxhlet extraction; however, MAE produced a better quality extract, without the need for subsequent purification [64]. Interestingly, permutations of MAE, MAHD and SFME have led to reduced extraction times compared to HD in the case of citrus extractions, resulting in microwaves rupturing the biomass, without significant differences in the refractive index, specific gravity, visual appearance, colour or composition of extract. The significant reduction in CO2 emissions makes the MAHD and SFE methods appealing compared to HD, particularly on the large scale [141]. However, non-polar solvents are typically avoided in MAE due to their low absorption of microwave heating, although it is possible to add other chemicals in order to increase the microwave absorption or to carry out a pre-treatment with a polar solvent when non-polar solvents are a necessity [65]. There is potential for uneven heating and overheating due to variations in ε within the sample, reducing the extraction efficiency and leading to potential thermal degradation. There is also a requirement for downstream filtration steps in order to appropriately separate the residue from the liquid extract, and cooling times within the process must be considered for an optimal system to be designed [67,142]. These additional steps, as well as a clean-up step, may also lead to extract being lost through downstream procedures [143].
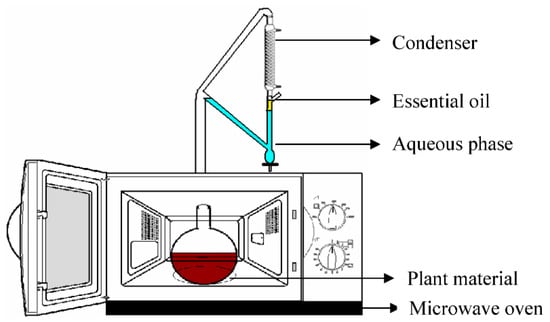
Figure 7.
Schematic of the microwave-assisted extraction setup. Reproduced from [144] with permission (open access, Attribution 4.0 International (CC BY 4.0)).
2.7. Ultrasound-Assisted Extraction (UAE)
The ease with which ultrasound may be coupled with other extraction techniques and the ability to operate at room temperature minimizes the problems associated with the oxidation/decomposition of target products, successfully enabling the isolation of phenolic compounds, antioxidants and cyanine [145], which are important for producing cosmetics, nutraceutics and pharmaceutics [66]. UAE can be applied on both the microscale and macroscale to extract compounds derived from various forms of life [146], where the application of UAE is energy/solvent/time efficient, potentially offering high yields of delicate compounds [66,145,147]. The mechanical effects of ultrasound have wider implications than extraction alone, and by varying the power and frequencies, it can find applications both upstream and downstream, with a simple and inexpensive commercial integration [148] and optimisation [149]. A significant benefit of UAE is the simple equipment involved, as sonicating water-baths are widely available (as depicted in Figure 8). UAE is a less aggressive stimulus than MAE, which is therefore preferential for the extraction of unstable compounds (particularly in solvent extraction and maceration) [68]; however, UAE offers fewer modes of utilisation within extraction processes. UAE can be employed in both solvent extraction from the biomass and the evaporation process in order to enable solvent removal at lower temperatures, resulting in a greener process [150]. UAE may be faster/safer in the case of acid digestion, as it is possible to work at lower pressures/temperatures when using this method, and simpler procedures lead to lower contamination risks. However, the requirements of the particle size distribution of the feedstocks necessitates important pre-treatment steps, and the robustness of the probe surface may also be an issue [151]. In the case of extractions from grape seeds, UAE for 30 min gave similar results to Soxhlet extraction conducted for 6 h, with no significant differences in the fatty acid content. UAE used prior to maceration also allows for a higher polyphenol content [152]. The use of UAE has also proved beneficial in the extraction of anthraquinones, an aromatic compound, from Heterophyllaea pustulata, where the efficiency was >10 times more efficient with the use of UAE after 2 h compared to 16 h using the Soxhlet extraction method. Moreover, the efficiency and process time were improved further when UAE was coupled with subsequent MAE [153]. Comparing the phenolic compounds extracted from Salvia officinalis L. via UAE or water/solvent extraction, UAE resulted in a higher polyphenol extraction and lower solvent usage (under optimal UAE conditions, this equates to a 20% higher polyphenol extraction and up to a ~3-fold reduction in the process time) [154]. The aforementioned studies highlight the potential for utilising UAE for extractions, potentially coupled with MAE to further improve the extraction efficacy.
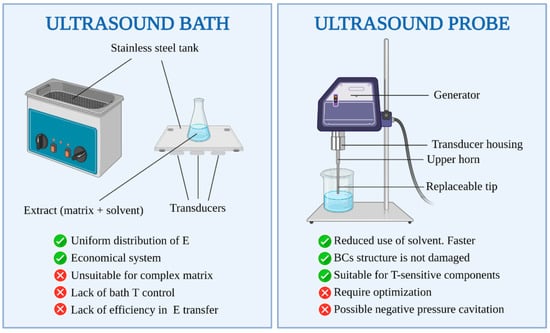
Figure 8.
Schematics of ultrasound-assisted extraction setups. Reproduced from [155] with permission (open access, Attribution 4.0 International (CC BY 4.0)).
2.8. Subcritical Water Extraction (SWE)
SWE is a method that utilises only water in the extraction of compounds (including, but not limited to, antioxidants, carbohydrates, flavonoids, phenolics, proteins, etc.) from feedstocks (for example, Chlorella vulgaris, Orostachys japonicus, Zataria multiflora, etc.) [69,70,156]. The utilisation of subcritical water for extraction (as depicted in Figure 9) is flexible, offering users the ability to vary the temperature, hence altering the dielectric constant and, in turn, controlling the ability to solvate organic compounds with varying polarities [157]. Temperature is the most frequently varied parameter because of its effect on the dielectric constants, with room temperature enabling the solvation of polar compounds, and higher temperatures, in a subcritical state, enabling the solvation of low/intermediate-polarity molecules. Viscosity, surface tension and interactions with the matrix itself also play important roles in the SWE process [158]. Pressure within SWE plays a lesser role in the extraction process, as it does not have a dominant effect on the solvent characteristics, selectivity and efficiency, whereas temperature is the parameter with a dominant influence on the solvent characteristics through changing the dielectric constant [69,157,158]. SWE is an inexpensive and environmentally friendly mode of extraction (particularly due to its complete elimination of the use of potentially toxic organic) [159,160]. Moreover, it can be performed in either a dynamic or static state, or a state in between the two. However, a static state runs the risks of low recovery, low solubility or highly concentrated solutes, because water is not continually supplied, meaning that the extraction is limited, irrespective of the retention time, temperature or pressure. By comparison, a dynamic process may combat this issue, but the energy efficiency and volumes of the solvents used must still be considered [161]. Working at higher pressures facilitates extraction from samples, as the pressure forces water into the pores of the matrix, which is not typically subject to interactions [162]. However, a limitation of this method is that the high temperatures involved in this process may lead to the risk of thermal degradation of the molecules [163], potentially limiting the products to more thermally robust molecules and requiring the systematic optimisation in the case of a respective class of compounds in order to ensure the efficacy of the process [161,162]. A good complement to pilot experiments are solubility models and theoretical approximations, which can aid in predicting the solvent properties and/or the solubility of compounds within a solvent [164].
In a comparison between the continuous SWE of fennel with HD and solvent extraction, several benefits were identified. SWE facilitated a shorter extraction time of 50 min, compared to 4 h for HD and 24 h for solvent extraction. Costs were reduced via reduced energy requirements necessary to meet the SWE conditions, and, finally, the ability to alter the composition by changing the parameters of the temperature, flowrate and static extraction time was noted [165]. Similar benefits were identified for the extraction of Lavandula stoechas by SWE when compared with HD, but more important benefits were identified when this method was compared with UAE-solvent extraction. The aroma produced by SWE was also found to be more concentrated and powerful than that isolated by either HD or UAE-solvent extractions [166]. The former of these observations shows that SWE is competitive in relation with UAE and capable of producing value-added attributes, such as a stronger scent. Similarly, SWE, compared with the Soxhlet extraction (ethanol) and MAE-solvent (ethanol) extraction of Chaya, also showed promising results. Here, 10 min of SWE led to no significant differences in the global extraction yield compared to 6 h of Soxhlet extraction at similar temperatures, whereas 10 min of MAE produced approximately half the global extraction yield of Soxhlet (albeit that it yielded a product with greater antioxidant activity than that obtained by SWE or Soxhlet extraction) [167]. However, such considerations should be contemplated on a case by case basis, and some advantages and disadvantages of SWE have been discussed in a concise review [168].
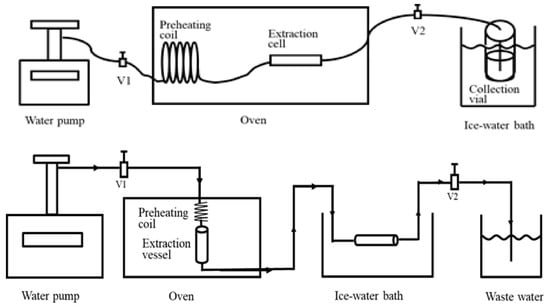
Figure 9.
Subcritical water extraction system without solid trapping (top) and with solid trapping (bottom). Reproduced from [169] with permission (open access, Attribution 4.0 International (CC BY 4.0)).
2.9. Supercritical CO2 Extraction (scCO2)
Supercritical fluid extraction has been employed to extract valuable components from numerous feedstocks [71,170,171,172]. The broad applicability of scCO2 (depicted in Figure 10) can be attributed to the fact that the selectivity and solvation characteristics may be optimised by the manipulation of the temperature and pressure [173]. Supercritical fluids can facilitate much higher diffusion coefficients of lipids and waxes than other liquids, allowing for quicker extractions. The lack of surface tension within supercritical fluids, coupled with lower viscosities, enables a greater penetration into the pores of a matrix compared with other liquids [174]. Extractions using scCO2 simplify the downstream processing and handling, particularly because CO2 is inflammable, relatively inexpensive and, when handled carefully, non-toxic. The separation of molecules from plant matter is also relatively easy as, once adjusted to ambient conditions, CO2 readily evaporates, requiring less downstream processing, and the low critical temperatures required for CO2 reduce the potential for problems related to the thermal degradation of the molecules [175]. Importantly, scCO2 is regarded as a green technology [174,176]. While the equipment necessary for scCO2 processing is not cheap, it is a process in which the oxidation of the target molecules is minimal [177]. Due to the fact that scCO2 is a non-polar solvent, it is optimal for the extraction of non-polar and weakly polar compounds [178,179]. However, when coupled with polar organic solvents, such as methanol or ethanol, as co-solvents, the extraction efficiency of the polar compounds may be increased [179,180] (and the integration of (bio)ethanol is considered to be environmentally benign and relatively safe for human health). Interestingly, the use of scCO2 may facilitate the extraction of a wider portfolio of compounds, whereas conventional methods such as HD and solvent extraction are optimal for compounds that are volatile or high in molecular weight [181].
A study of the isolation of tocochromanols from cereals demonstrated that scCO2 was comparable to Soxhlet extraction in terms of the yield (~85%), but the benefits of scCO2 processing included its low solvent usage and ease of analysis [182]. It was reported that scCO2 gave a slightly lower yield of isoflavones from soybeans than conventional extraction using 80% ethanol solvent, but this was counterbalanced by improvements in the processing due to the use of scCO2 (i.e., the lack of use of organic solvents) and its ease of clean-up and analysis [183]. Moreover, it was noted that the integration of ethanol into the scCO2 extraction process (92.5% CO2 and 7.5% ethanol) improved the yield of isoflavones [183]. The investigation of the extraction of secondary metabolites from Syzygium campanulatum using scCO2 with added ethanol resulted in a higher recovery (of 25–85%) compared with conventional solvent extraction, with values ranging from 0.9–66% [184], highlighting the potential benefit of adding an organic solvent to the extraction process (some advantages and disadvantages of scCO2 extraction are discussed in a concise review [168]).
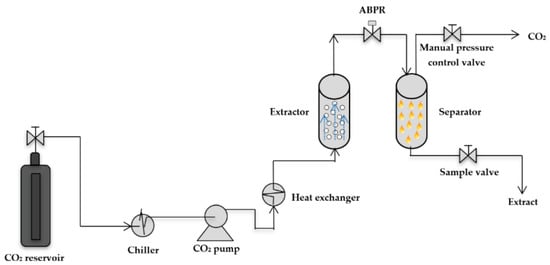
Figure 10.
Schematic of the supercritical CO2 extraction setup. Reproduced from [185] with permission (open access, Attribution 4.0 International (CC BY 4.0)).
3. Analytical Methods
A variety of analytical techniques can be utilised when extracting high-value chemicals from plants. Chromatographic separation enables the isolation of individual compounds from mixed feedstocks and their subsequent analysis via crystallography, spectrometry, spectroscopy, etc. [186]. Modern approaches to this technique involve combinations of methods for the separation and analysis, where a chromatographic technique is coupled with spectrometry, giving rise to methods such as gas chromatography–mass spectrometry (GC-MS) or high-performance liquid chromatography–mass spectrometry (HPLC-MS) [187,188], both of which are suitable for low-molecular-weight compounds [189]. Gel permeation chromatography (GPC)–matrix-assisted laser desorption ionization (MALDI) mass spectrometry, on the other hand, is more suitable for high-molecular-weight compounds. GC-MS and HPLC-MS can produce valuable results, even when the yield of the extraction process may be low (potentially too low for other methods that require significant amounts of the sample, e.g., nuclear magnetic resonance spectroscopy) [190]. Examples of natural products analysed by variants of GC-MS and HPLC-MS are depicted in Figure 11 [191].
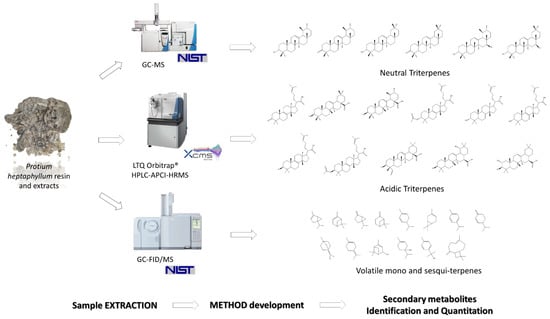
Figure 11.
Examples of natural products analysed by variants of GC-MS and HPLC-MS. Reproduced from [191] with permission (open access, Attribution 4.0 International (CC BY 4.0)).
Antioxidant potential and oxidative stability are used to examine the bioactivity of the compounds extracted from feedstocks. Oxidative processes have been noted to reduce the quality and pharmacological activity of plant-derived chemicals [192] and, since oxidative stability is synonymous with the shelf-life of the extract [193,194], it is a useful determinant for predicting its longevity. Factors influencing oxidative stability include the composition of the extract itself and its respective antioxidant capabilities, as well as the pro-oxidant effects of the environment on the extract during processing and storage [195], which include heat, light, oxygen availability and the presence of trace metals [196,197,198]. Antioxidant potential (which is associated with oxidative stability [195]) correlates with the shelf-life of a product and is important in the food industry for maintaining nutritional quality [199,200].
3.1. Gas Chromatography Mass Spectrometry (GC-MS)
GC-MS is an analytical technique routinely used to analyse volatile, thermally stable compounds [201,202]. Certain species may be too polar or large to be sufficiently volatile in order to pass from the liquid phase to the mobile gas phase prior to analysis via MS [203], and this limitation can be overcome by derivatization (i.e., chemical modification, ion-pairing techniques, photochemistry, electrochemistry, complexation and metal chelation) [204], which alters the physical or chemical characteristics to improve the sensitivity or selectivity within the analysis [205]. Derivatization should create sufficiently volatile and thermally stable compounds, which increases the sensitivity, selectivity or specificity within the analysis [206,207], thereby increasing the scope of the analytes that GC-MS may be used for [203,208,209]. GC-MS offers a high resolution and reproducibility within chromatographic analysis [210,211]. The complexity of the sample preparation limits the size and type of the molecule under analysis [209], and whilst this can be mitigated to a certain extent by derivatisation, derivatization can be complicated by the selectivity of functional group modification and other issues [212].
The use of GC-MS in plant extract analysis is popular because of its ability to identify a plethora of bioactive phytochemicals from a given feedstock employing large spectral libraries [213]. The analysis of an ethanolic extract of Evolvulus alsinoides (L.) identified its potential chemo-preventive, anti-cancer, anti-microbial, antioxidant and anti-diabetic activity due to the presence of secondary metabolites in the extract [214]. The phytochemical analysis of the ethyl acetate and methanolic extract of Amomum nilgiricum via GC-MS identified 25 phytochemicals with potential antibacterial, antifungal, antiviral, antioxidant and antidiabetic properties [215]. The GC-MS analysis of an ethanolic leaf extract of Phyllowdium pulchellum L. identified 10 phytochemical compounds with anti-inflammatory, antioxidant, anti-microbial, anti-malaria, anti-fungal, cytotoxic and hypoglycaemic activity [216]. Numerous studies have also presented similar findings in the case of plant extracts whilst using GC-MS as a tool of analysis [217,218,219,220], which can be cross-referenced against a database for compound identification and biological property analysis/prediction [217]. The aforementioned studies highlight the power of GC-MS as an analytical tool for identifying commercially viable, bioactive and therapeutic molecules from plant extracts. For the avid reader, we recommend the state-of-the-art of the literature on GC-MS [221,222,223].
3.2. High-Performance Liquid Chromatography Mass Spectrometry (HPLC-MS)
HPLC-MS is a potent technique for separation via HPLC followed by mass analysis via MS [224,225]. The benefit of HPLC-MS, compared to GC-MS, is the fact that the analytes do not need to be volatile in order to be analysed [226], enabling the broad applicability of HPLC-MS for the separation, identification and quantification of small molecules with a high sensitivity/selectivity in trace multicomponent and complex mixture analysis [227,228]. Similar to GC-MS, HPLC-MS may employ derivatization in order to improve the detection characteristics, compound stabilization and binding to HPLC columns, thus facilitating easy compound separation [229]. LC-MS offers versatility in the mode of LC operation with reverse-phase liquid chromatography (RPLC), normal-phase liquid chromatography (NPLC), hydrophilic interaction liquid chromatography (HILC), ion exchange chromatography (IELC) and size exclusion chromatography (SELC) [230,231]. RPLC is popular in the analysis of metabolomics [232,233], whilst HILC is used for polar molecules to increase the range of the compounds that may be investigated by LC [232,234], with IELC expanding the scope of LC-MS even further for the analysis of highly polar molecules [232,235]. The versatility of LC-MS facilitates the analysis of a wide range of molecules and, importantly, mixed-mode chromatography may also be utilised, which combines two or more of the aforementioned techniques in order to offer superior separations than those of the individual methods alone [236]. This underpins the popularity of HPLC-MS as a tool for the analysis of a plethora of analyte families. For the avid reader, we recommend the literature on the various state-of-the-art chromatographic techniques [237] that are coupled with MS: LC [238], HILC-MS [239], HPLC-MS [240], IELC-MS [241,242] and SELC-MS [243,244].
4. Conclusions
Chemicals produced by plants (e.g., alkaloids, flavonoids, phenolic acids, saponins and terpenes) have a variety of high-value applications in industry. Extraction techniques used to isolate mixtures containing these compounds have been developed, which employ complex combinations of chemical/engineering methods used to extract crude oils. The desire to find more sustainable/circular solutions has motivated the development of innovative industrially scalable extraction methods (e.g., the provision of auxiliary energy via microwaves and ultrasound, which enables quicker/greener processing via reductions in the organic solvent requirements, energy loads and waste production, thereby increasing the efficiency). Subcritical water and supercritical CO2 have been shown to be effective greener solvents, with reduced heating requirements and waste. Such extraction techniques are typically coupled with analytical techniques, such as GC-MS and HPLC-MS, to identify and potentially quantify the valuable compounds. While there are context-specific variations in the yields of high-value chemicals from plants (including the extraction methodology and feedstock), the application of the techniques outlined herein offers the potential for significant economic, environmental and societal impacts in the future.
Author Contributions
Conceptualization, P.K., J.C., M.R.R. and J.G.H.; methodology, P.K., M.R.R. and J.G.H.; formal analysis, P.K., A.S.N., J.C., M.R.R. and J.G.H.; investigation, P.K. and J.C.; writing—original draft preparation, P.K., A.S.N., J.C., M.R.R. and J.G.H.; writing—review and editing, P.K., A.S.N., J.C., M.R.R. and J.G.H.; supervision, J.C., M.R.R. and J.G.H.; project administration, J.C., M.R.R. and J.G.H.; funding acquisition, J.C., M.R.R. and J.G.H. All authors have read and agreed to the published version of the manuscript.
Funding
This project was supported by the European Regional Development Funds (project ref: 03R19P03809).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We acknowledge the administrative support of Carolyn Hayes and Andy Pickard and thank David Townsend for his technical support during the early stages of the project.
Conflicts of Interest
J.C. is Director of CO2 Extraction Ltd. All other authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Kose, M.D.; Hardy, J.G.; Sheridan, E.; Bozoğlan, F.; Bayraktar, O. Research Trends in Plant-Derived Oligomers for Health Applications. Curr. Nutraceuticals 2020, 2, 3–13. [Google Scholar] [CrossRef]
- Lange, L.; Connor, K.O.; Arason, S.; Bundgård-Jørgensen, U.; Canalis, A.; Carrez, D.; Gallagher, J.; Gøtke, N.; Huyghe, C.; Jarry, B.; et al. Developing a Sustainable and Circular Bio-Based Economy in EU: By Partnering Across Sectors, Upscaling and Using New Knowledge Faster, and For the Benefit of Climate, Environment & Biodiversity, and People & Business. Front. Bioeng. Biotechnol. 2021, 8, 619066. [Google Scholar] [PubMed]
- Serna-Loaiza, S.; Miltner, A.; Miltner, M.; Friedl, A. A Review on the Feedstocks for the Sustainable Production of Bioactive Compounds in Biorefineries. Sustainability 2019, 11, 6765. [Google Scholar] [CrossRef]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for Extraction of Bioactive Compounds from Plant Materials: A Review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Wink, M. Plant Secondary Metabolites Modulate Insect Behavior-Steps toward Addiction? Front. Physiol. 2018, 9, 364. [Google Scholar] [CrossRef] [PubMed]
- Poiroux-Gonord, F.; Bidel, L.P.R.; Fanciullino, A.L.; Gautier, H.; Lauri-Lopez, F.; Urban, L. Health Benefits of Vitamins and Secondary Metabolites of Fruits and Vegetables and Prospects to Increase Their Concentrations by Agronomic Approaches. J. Agric. Food Chem. 2010, 58, 12065–12082. [Google Scholar] [CrossRef]
- Makkar, H.; Siddhuraju, P.; Becker, K. Plant Secondary Metabolites. In Methods in Molecular BiologyTM; Springer: Berlin, Germany, 2007. [Google Scholar]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological Effects of Essential Oils—A Review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar]
- Fernández-López, J.; Viuda-Martos, M. Introduction to the Special Issue: Application of Essential Oils in Food Systems. Foods 2018, 7, 56. [Google Scholar] [CrossRef] [PubMed]
- Sarkic, A.; Stappen, I. Essential Oils and Their Single Compounds in Cosmetics—A Critical Review. Cosmetics 2018, 5, 11. [Google Scholar] [CrossRef]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential Oils’ Chemical Characterization and Investigation of Some Biological Activities: A Critical Review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef] [PubMed]
- Hmaied, M.; Bouafif, H.; Magdouli, S.; Braghiroli, F.L.; Koubaa, A. Effect of Forest Biomass Pretreatment on Essential Oil Yield and Properties. Forests 2019, 10, 1042. [Google Scholar] [CrossRef] [Green Version]
- Said-Al Ahl, H.A.H.; Sabra, A.S.; Alataway, A.; Astatkie, T.; Mahmoud, A.A.; Bloem, E. Biomass Production and Essential Oil Composition of Thymus vulgaris in Response to Water Stress and Harvest Time. J. Essent. Oil Res. 2019, 31, 63–68. [Google Scholar] [CrossRef]
- Mossi, A.J.; Pauletti, G.F.; Rota, L.; Echeverrigaray, S.; Barros, I.B.I.; Oliveira, J.V.; Paroul, N.; Cansian, R.L. Effect of Different Liming Levels on the Biomass Production and Essential Oil Extraction Yield of Cunila galioides Benth. Braz. J. Biol. 2012, 72, 787–793. [Google Scholar] [CrossRef]
- Noriega, P. Terpenes in Essential Oils: Bioactivity and Applications. In Terpenes and Terpenoids—Recent Advances; IntechOpen: London, UK, 2021. [Google Scholar]
- Masyita, A.; Sari, R.M.; Astuti, A.D.; Yasir, B.; Rumata, N.R.; Emran, T.B.; Nainu, F.; Simal-Gandara, J. Terpenes and Terpenoids as Main Bioactive Compounds of Essential Oils, Their Roles in Human Health and Potential Application as Natural Food Preservatives. Food Chem. X 2022, 13, 100217. [Google Scholar] [CrossRef]
- Cox-Georgian, D.; Ramadoss, N.; Dona, C.; Basu, C. Therapeutic and Medicinal Uses of Terpenes. In Medicinal Plants: From Farm to Pharmacy; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Segneanu, A.; Velciov, S.M.; Olariu, S.; Cziple, F.; Damian, D.; Grozescu, I. Bioactive Molecules Profile from Natural Compounds. In Amino Acid—New Insights and Roles in Plant and Animal; IntechOpen: London, UK, 2017. [Google Scholar]
- Hohtola, A. Bioactive Compounds from Northern Plants. Adv. Exp. Med. Biol. 2010, 698, 99–109. [Google Scholar] [CrossRef]
- Yang, W.; Chen, X.; Li, Y.; Guo, S.; Wang, Z.; Yu, X. Advances in Pharmacological Activities of Terpenoids. Nat. Prod. Commun. 2020, 15, 1934578X20903555. [Google Scholar] [CrossRef]
- Perveen, S. Introductory Chapter: Terpenes and Terpenoids. In Terpenes and Terpenoids; BoD–Books on Demand: Norderstedt, Germany, 2018. [Google Scholar]
- Kurek, J. Introductory Chapter: Alkaloids-Their Importance in Nature and Human Life; BoD–Books on Demand: Norderstedt, Germany, 2019. [Google Scholar]
- Kumar, S.; Pandey, A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Goel, N. Phenolic Acids: Natural Versatile Molecules with Promising Therapeutic Applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar]
- Guclu-Ustundag, Ö.; Mazza, G. Saponins: Properties, Applications and Processing. Crit. Rev. Food Sci. Nutr. 2007, 47, 231–258. [Google Scholar] [CrossRef]
- Pagare, S.; Bhatia, M.; Tripathi, N.; Pagare, S.; Bansal, Y.K. Secondary Metabolites of Plants and Their Role: Overview. Curr. Trends Biotechnol. Pharm. 2015, 9, 293–304. [Google Scholar]
- Isah, T. Stress and Defense Responses in Plant Secondary Metabolites Production. Biol. Res. 2019, 52, 39. [Google Scholar] [PubMed]
- Cheng, F.; Cheng, Z. Research Progress on the Use of Plant Allelopathy in Agriculture and the Physiological and Ecological Mechanisms of Allelopathy. Front. Plant Sci 2015, 6, 1020. [Google Scholar] [CrossRef]
- Pech-Kú, R.; Muñoz-Sánchez, J.A.; Monforte-González, M.; Vázquez-Flota, F.; Rodas-Junco, B.A.; Hernández-Sotomayor, S.M.T. Caffeine Extraction, Enzymatic Activity and Gene Expression of Caffeine Synthase from Plant Cell Suspensions. J. Vis. Exp. 2018, 140, e58166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McLellan, T.M.; Caldwell, J.A.; Lieberman, H.R. A Review of Caffeine’s Effects on Cognitive, Physical and Occupational Performance. Neurosci. Biobehav. Rev. 2016, 71, 294–312. [Google Scholar] [PubMed]
- Sharifi-Rad, J.; Sureda, A.; Tenore, G.C.; Daglia, M.; Sharifi-Rad, M.; Valussi, M.; Tundis, R.; Sharifi-Rad, M.; Loizzo, M.R.; Oluwaseun Ademiluyi, A.; et al. Biological Activities of Essential Oils: From Plant Chemoecology to Traditional Healing Systems. Molecules 2017, 22, 70. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.; Al-Wabel, N.A.; Shams, S.; Ahamad, A.; Khan, S.A.; Anwar, F. Essential Oils Used in Aromatherapy: A Systemic Review. Asian Pac. J. Trop. Biomed. 2015, 5, 601–611. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green Extraction of Natural Products: Concept and Principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef]
- Rassem, H.H.; Nour, A.H.; Yunus, R.M. Techniques for Extraction of Essential Oils from Plants: A Review. Aust. J. Basic Appl. Sci. 2016, 10, 117–127. [Google Scholar]
- Feyaerts, A.F.; Luyten, W.; van Dijck, P. Striking Essential Oil: Tapping into a Largely Unexplored Source for Drug Discovery. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Reddy, D.N. Essential Oils Extracted from Medicinal Plants and Their Applications. In Natural Bio-Active Compounds: Volume 1: Production and Applications; Springer: Singapore, 2019. [Google Scholar]
- Elshafie, H.S.; Camele, I. An Overview of the Biological Effects of Some Mediterranean Essential Oils on Human Health. Biomed. Res. Int. 2017, 2017, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Sakkas, H.; Papadopoulou, C. Antimicrobial Activity of Basil, Oregano, and Thyme Essential Oils. J. Microbiol. Biotechnol. 2017, 27, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Koyama, S.; Heinbockel, T. The Effects of Essential Oils and Terpenes in Relation to Their Routes of Intake and Application. Int. J. Mol. Sci. 2020, 21, 1558. [Google Scholar] [CrossRef]
- Zaynab, M.; Fatima, M.; Abbas, S.; Sharif, Y.; Umair, M.; Zafar, M.H.; Bahadar, K. Role of Secondary Metabolites in Plant Defense against Pathogens. Microb. Pathog. 2018, 124, 198–202. [Google Scholar] [CrossRef]
- Wińska, K.; Mączka, W.; Łyczko, J.; Grabarczyk, M.; Czubaszek, A.; Szumny, A. Essential Oils as Antimicrobial Agents—Myth or Real Alternative? Molecules 2019, 24, 2130. [Google Scholar] [CrossRef] [Green Version]
- Schnitzler, P. Essential Oils for the Treatment of Herpes Simplex Virus Infections. Chemotherapy 2019, 64, 1–7. [Google Scholar] [CrossRef]
- Shin, S.A.; Moon, S.Y.; Kim, W.Y.; Paek, S.M.; Park, H.H.; Lee, C.S. Structure-Based Classification and Anti-Cancer Effects of Plant Metabolites. Int. J. Mol. Sci. 2018, 19, 2651. [Google Scholar] [CrossRef]
- Leyva-López, N.; Gutiérrez-Grijalva, E.P.; Vazquez-Olivo, G.; Heredia, J.B. Essential Oils of Oregano: Biological Activity beyond Their Antimicrobial Properties. Molecules 2017, 22, 989. [Google Scholar] [CrossRef]
- Price, S. Using Essential Oils in Professional Practice. Complement. Ther. Nurs. Midwifery 1998, 4, 144–147. [Google Scholar] [CrossRef]
- Malcolm, B.J.; Tallian, K. Essential Oil of Lavender in Anxiety Disorders: Ready for Prime Time? Ment. Health Clin. 2017, 7, 147–155. [Google Scholar] [CrossRef]
- Lillehei, A.S.; Halcón, L.L.; Savik, K.; Reis, R. Effect of Inhaled Lavender and Sleep Hygiene on Self-Reported Sleep Issues: A Randomized Controlled Trial. J. Altern. Complement. Med. 2015, 21, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Dey, A.; Bhattacharya, R.; Mukherjee, A.; Pandey, D.K. Natural Products against Alzheimer’s Disease: Pharmaco-Therapeutics and Biotechnological Interventions. Biotechnol. Adv. 2017, 35, 178–216. [Google Scholar] [PubMed]
- Wojtunik-Kulesza, K.A.; Kasprzak, K.; Oniszczuk, T.; Oniszczuk, A. Natural Monoterpenes: Much More than Only a Scent. Chem. Biodivers. 2019, 16, e1900434. [Google Scholar] [CrossRef]
- Qiao, Q.; Wang, X.; Ren, H.; An, K.; Feng, Z.; Cheng, T.; Sun, Z. Oil Content and Nervonic Acid Content of Acer Truncatum Seeds from 14 Regions in China. Hortic. Plant J. 2019, 5, 24–30. [Google Scholar] [CrossRef]
- Marillia, E.F.; Francis, T.; Falk, K.C.; Smith, M.; Taylor, D.C. Palliser’s Promise: Brassica Carinata, An Emerging Western Canadian Crop for Delivery of New Bio-Industrial Oil Feedstocks. Biocatal. Agric. Biotechnol. 2014, 3, 65–74. [Google Scholar] [CrossRef]
- Li, Q.; Chen, J.; Yu, X.; Gao, J.M. A Mini Review of Nervonic Acid: Source, Production, and Biological Functions. Food Chem. 2019, 301, 125286. [Google Scholar] [CrossRef] [PubMed]
- Alshehri, F.A. The Use of Mouthwash Containing Essential Oils (LISTERINE®) to Improve Oral Health: A Systematic Review. Saudi Dent. J. 2018, 30, 2–6. [Google Scholar] [CrossRef]
- Lynch, M.C.; Cortelli, S.C.; McGuire, J.A.; Zhang, J.; Ricci-Nittel, D.; Mordas, C.J.; Aquino, D.R.; Cortelli, J.R. The Effects of Essential Oil Mouthrinses with or without Alcohol on Plaque and Gingivitis: A Randomized Controlled Clinical Study. BMC Oral Health 2018, 18, 6. [Google Scholar] [CrossRef] [PubMed]
- Karadağlıoğlu, Ö.İ.; Ulusoy, N.; Başer, K.H.C.; Hanoğlu, A.; Şık, İ. Antibacterial Activities of Herbal Toothpastes Combined with Essential Oils against Streptococcus mutans. Pathogens 2019, 8, 20. [Google Scholar] [CrossRef]
- Freires, I.A.; Denny, C.; Benso, B.; de Alencar, S.M.; Rosalen, P.L. Antibacterial Activity of Essential Oils and Their Isolated Constituents against Cariogenic Bacteria: A Systematic Review. Molecules 2015, 20, 7329–7358. [Google Scholar] [CrossRef]
- Anusha, D.; Chaly, P.; Junaid, M.; Nijesh, J.; Shivashankar, K.; Sivasamy, S. Efficacy of a Mouthwash Containing Essential Oils and Curcumin as an Adjunct to Nonsurgical Periodontal Therapy among Rheumatoid Arthritis Patients with Chronic Periodontitis: A Randomized Controlled Trial. Indian J. Dent. Res. 2019, 30, 506–511. [Google Scholar] [CrossRef]
- Rao, B.; Sastry, K. Major Essential Oils of South Lndia-A Perspective. Fafai J. 2003, 5, 19–24. [Google Scholar]
- Aziz, Z.A.A.; Ahmad, A.; Setapar, S.H.M.; Karakucuk, A.; Azim, M.M.; Lokhat, D.; Rafatullah, M.; Ganash, M.; Kamal, M.A.; Ashraf, G.M. Essential Oils: Extraction Techniques, Pharmaceutical and Therapeutic Potential—A Review. Curr. Drug Metab. 2018, 19, 1100–1110. [Google Scholar] [CrossRef]
- Tongnuanchan, P.; Benjakul, S. Essential Oils: Extraction, Bioactivities, and Their Uses for Food Preservation. J. Food Sci. 2014, 79, R1231–R1249. [Google Scholar] [CrossRef] [PubMed]
- Ferhat, M.A.; Meklati, B.Y.; Chemat, F. Comparison of Different Isolation Methods of Essential Oil from Citrus Fruits: Cold Pressing, Hydrodistillation and Microwave “dry” Distillation. Flavour Fragr. J. 2007, 22, 494–504. [Google Scholar] [CrossRef]
- Bousbia, N.; Vian, M.A.; Ferhat, M.A.; Meklati, B.Y.; Chemat, F. A New Process for Extraction of Essential Oil from Citrus Peels: Microwave Hydrodiffusion and Gravity. J. Food Eng. 2009, 90, 409–413. [Google Scholar] [CrossRef]
- Cardoso-Ugarte, G.A.; Juárez-Becerra, G.P.; Sosa-Morales, M.E.; López-Malo, A. Microwave-Assisted Extraction of Essential Oils from Herbs. J. Microw. Power Electromagn. Energy 2013, 47, 63–72. [Google Scholar] [CrossRef]
- De Castro, M.L.; Jiménez-Carmona, M.M.; Fernandez-Perez, V. Towards More Rational Techniques for the Isolation of Valuable Essential Oils from Plants. TrAC-Trends Anal. Chem. 1999, 18, 708–716. [Google Scholar] [CrossRef]
- Kokolakis, A.K.; Golfinopoulos, S.K. Microwave-Assisted Techniques (MATs); a Quick Way to Extract a Fragrance: A Review. Nat. Prod. Commun. 2013, 8, 1493–1504. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.G.; Meullemiestre, A.; Fabiano-Tixier, A.S.; Abert-Vian, M. Ultrasound Assisted Extraction of Food and Natural Products. Mechanisms, Techniques, Combinations, Protocols and Applications. A Review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef] [PubMed]
- Al Jitan, S.; Alkhoori, S.A.; Yousef, L.F. Chapter 13-Phenolic Acids from Plants: Extraction and Application to Human Health; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Pacheco-Fernández, I.; Pino, V. Extraction with Ionic Liquids-Organic Compounds. In Liquid-Phase Extraction; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Haghighi, A.; Khajenoori, M. Mass Transfer-Advances in Sustainable Energy and Environment Oriented Numerical Modeling; BoD–Books on Demand: Norderstedt, Germany, 2013. [Google Scholar]
- Ko, M.J.; Nam, H.H.; Chung, M.S. Subcritical Water Extraction of Bioactive Compounds from Orostachys japonicus A. Berger (Crassulaceae). Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Hrncic, M.K.; Cör, D.; Verboten, M.T.; Knez, Z. Application of Supercritical and Subcritical Fluids in Food Processing. Food Qual. Saf. 2018, 2, 59–67. [Google Scholar] [CrossRef]
- Mejri, J.; Aydi, A.; Abderpabba, M.; Mejri, M. Emerging Extraction Processes of Essential Oils: A Review. Asian J. Green Chem. 2018, 2, 246–267. [Google Scholar]
- Azmin, S.N.H.M.; Manan, Z.A.; Alwi, S.R.W.; Chua, L.S.; Mustaffa, A.A.; Yunus, N.A. Herbal Processing and Extraction Technologies. Sep. Purif. Rev. 2016, 45, 305–320. [Google Scholar] [CrossRef]
- Geow, C.H.; Tan, M.C.; Yeap, S.P.; Chin, N.L. A Review on Extraction Techniques and Its Future Applications in Industry. Eur. J. Lipid Sci. Technol. 2021, 123, 2000302. [Google Scholar] [CrossRef]
- Chemat, F.; Abert Vian, M.; Fabiano-Tixier, A.S.; Nutrizio, M.; Režek Jambrak, A.; Munekata, P.E.S.; Lorenzo, J.M.; Barba, F.J.; Binello, A.; Cravotto, G. A Review of Sustainable and Intensified Techniques for Extraction of Food and Natural Products. Green Chem. 2020, 22, 2325–2353. [Google Scholar] [CrossRef]
- Zhang, Q.W.; Lin, L.G.; Ye, W.C. Techniques for Extraction and Isolation of Natural Products: A Comprehensive Review. Chin. Med. 2018, 13, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Presti, M.L.; Ragusa, S.; Trozzi, A.; Dugo, P.; Visinoni, F.; Fazio, A.; Dugo, G.; Mondello, L. A Comparison between Different Techniques for the Isolation of Rosemary Essential Oil. J. Sep. Sci. 2005, 28, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Temelli, F.; Saldaña, M.D.A.; Comin, L. Application of Supercritical Fluid Extraction in Food Processing. In Comprehensive Sampling and Sample Preparation; Elsevier: Amsterdam, The Netherlands, 2012; Volume 4. [Google Scholar]
- Zheljazkov, V.D.; Astatkie, T.; Schlegel, V. Hydrodistillation Extraction Time Effect on Essential Oil Yield, Composition, and Bioactivity of Coriander Oil. J. Oleo Sci. 2014, 63, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Abidin, Z.; Ahmad, B.; Yusoff, Z.B.; Fikri, A.; Awang, B.; Fifadli, A.; Mohd, B.; Rudin, N.; Saiful, M.; Bin, H.; et al. Hydro-Distillation Process in Extracting of Agarwood Essential Oil; Politeknik Kuching: Kuching, Malaysia, 2009. [Google Scholar]
- Huzar, E.; Dziecioł, M.; Wodnicka, A.; Orün, H.; Icoz, A.; Cicek, E. Influence of Hydrodistillation Conditions on Yield and Composition of Coriander (Coriandrum Sativum L.) Essential Oil. Pol. J. Food Nutr. Sci. 2018, 68, 243–249. [Google Scholar] [CrossRef]
- Semerdjieva, I.B.; Shiwakoti, S.; Cantrell, C.L.; Zheljazkov, V.D.; Astatkie, T.; Schlegel, V.; Radoukova, T. Hydrodistillation Extraction Kinetics Regression Models for Essential Oil Yield and Composition in Juniperus Virginiana, J. Excelsa, and J. Sabina. Molecules 2019, 24, 986. [Google Scholar] [CrossRef] [PubMed]
- Sintim, H.Y.; Burkhardt, A.; Gawde, A.; Cantrell, C.L.; Astatkie, T.; Obour, A.E.; Zheljazkov, V.D.; Schlegel, V. Hydrodistillation Time Affects Dill Seed Essential Oil Yield, Composition, and Bioactivity. Ind. Crops Prod. 2015, 63, 190–196. [Google Scholar] [CrossRef]
- Collin, H.A. Secondary Metabolites|Extraction and Industrial Processes. In Encyclopedia of Rose Science; Elsevier: Amsterdam, The Netherlands, 2003; pp. 726–735. [Google Scholar]
- Tisserand, R.; Young, R. Essential Oil Composition. In Essential Oil Safety; Elsevier: Amsterdam, The Netherlands, 2013; pp. 5–22. [Google Scholar]
- Périno-Issartier, S.; Ginies, C.; Cravotto, G.; Chemat, F. A Comparison of Essential Oils Obtained from Lavandin via Different Extraction Processes: Ultrasound, Microwave, Turbohydrodistillation, Steam and Hydrodistillation. J. Chromatogr. A 2013, 1305, 41–47. [Google Scholar] [CrossRef]
- Pingret, D.; Fabiano-Tixier, A.S.; Chemat, F. Accelerated Methods for Sample Preparation in Food. In Comprehensive Sampling and Sample Preparation; Elsevier: Amsterdam, The Netherlands, 2012; Volume 4. [Google Scholar]
- Beoletto, V.G.; de las Mercedes Oliva, M.; Marioli, J.M.; Carezzano, M.E.; Demo, M.S. Antimicrobial Natural Products Against Bacterial Biofilms. In Antibiotic Resistance: Mechanisms and New Antimicrobial Approaches; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Scott, R. Encyclopedia of Analytical Science. In Reference Reviews, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2005; Volume 19, pp. 554–561. [Google Scholar]
- Peter, K.V. Handbook of Herbs and Spices, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2012; Volume 1, ISBN 9780857095671. [Google Scholar]
- El-Toumy, S.A.; Hussein, A.A. Cold Pressed Yuzu (Citrus junos Sieb. Ex Tanaka) Oil. In Cold Pressed Oils; Academic Press: Cambridge, MA, USA, 2020. [Google Scholar]
- Masango, P. Cleaner Production of Essential Oils by Steam Distillation. J. Clean Prod. 2005, 13, 833–839. [Google Scholar] [CrossRef]
- Richard, J.J.; Junk, G.A. Steam Distillation, Solvent Extraction, and Ion Exchange for Determining Polar Organics in Shale Process Waters. Anal. Chem. 1984, 56, 1625–1628. [Google Scholar] [CrossRef]
- Irmak, S.; Erbatur, O. Additives for Environmentally Compatible Active Food Packaging. In Environmentally Compatible Food Packaging; Woodhead Publishing: Cambridge, MA, USA, 2008. [Google Scholar]
- Prado, J.M.; Vardanega, R.; Debien, I.C.; Meireles, M.A.A.; Gerschenson, L.N.; Sowbhagya, H.B.; Chemat, S. Chapter 6-Conventional Extraction. In Food Waste Recovery; Academic Press: Cambridge, MA, USA, 2015. [Google Scholar]
- Vidic, D.; Čopra-Janićijević, A.; Miloš, M.; Maksimović, M. Effects of Different Methods of Isolation on Volatile Composition of Artemisia Annua L. Int. J. Anal. Chem. 2018, 2018, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Malekydozzadeh, M.; Khadiv-Parsi, P.; Rezazadeh, S.; Abolghasemi, H.; Salehi, Z.; Li, Q. Application of Multistage Steam Distillation Column for Extraction of Essential Oil of Rosemarinuse Officinialis L. Iran. J. Chem. Eng. 2012, 9, 55. [Google Scholar]
- Yahya, A.; Yunus, R.M. Influence of Sample Preparation and Extraction Time on Chemical Composition of Steam Distillation Derived Patchouli Oil. Procedia Eng. 2013, 53, 1–6. [Google Scholar] [CrossRef]
- Božović, M.; Navarra, A.; Garzoli, S.; Pepi, F.; Ragno, R. Esential Oils Extraction: A 24-Hour Steam Distillation Systematic Methodology. Nat. Prod. Res. 2017, 31, 2387–2396. [Google Scholar] [CrossRef] [PubMed]
- Geramitcioski, T.; Mitrevski, V.; Mijakovski, V. Design of a Small Press for Extracting Essential Oil According VDI 2221. IOP Conf. Ser. Mater. Sci. Eng. 2018, 393, 012131. [Google Scholar] [CrossRef]
- Faugno, S.; Piccolella, S.; Sannino, M.; Principio, L.; Crescente, G.; Baldi, G.M.; Fiorentino, N.; Pacifico, S. Can Agronomic Practices and Cold-Pressing Extraction Parameters Affect Phenols and Polyphenols Content in Hempseed Oils? Ind Crops Prod. 2019, 130, 511–519. [Google Scholar] [CrossRef]
- Al Juhaimi, F.; Özcan, M.M.; Ghafoor, K.; Babiker, E.E.; Hussain, S. Comparison of Cold-Pressing and Soxhlet Extraction Systems for Bioactive Compounds, Antioxidant Properties, Polyphenols, Fatty Acids and Tocopherols in Eight Nut Oils. J. Food Sci. Technol. 2018, 55, 3163–3173. [Google Scholar] [CrossRef]
- Kostadinovic-Velickovska, S.; Mitrev, S. Characterization of Fatty Acid Profile, Polyphenolic Content and Antioxidant Activity of Cold Pressed and Refined Edible Oils from Macedonia. J. Food Chem. Nutr. 2013, 1, 16–21. [Google Scholar]
- Çakaloğlu, B.; Özyurt, V.H.; Ötleş, S. Cold Press in Oil Extraction. A Review. Ukr. Food J. 2018, 7, 640–654. [Google Scholar] [CrossRef]
- Savoire, R.; Lanoisellé, J.L.; Vorobiev, E. Mechanical Continuous Oil Expression from Oilseeds: A Review. Food Bioproc. Tech. 2013, 6, 1–16. [Google Scholar] [CrossRef]
- Kristoferson, L.; Bolkalders, V. Chapter 11: Production of Biomass Engine Fuels. In Renewable Energy Technologies: Their Applications in Developing Countries; Elsevier: Amsterdam, The Netherlands, 1986; pp. 131–149. [Google Scholar]
- El-Haggar, S.M. Sustainable Industrial Design and Waste Management: Cradle-to-cradle for Sustainable Development; Academic Press: Cambridge, MA, USA, 2007; pp. 21–84. [Google Scholar] [CrossRef]
- Balaman, Ş.Y. Biomass-Based Production Systems. In Decision-Making for Biomass-Based Production Chains; Academic Press: Cambridge, MA, USA, 2019. [Google Scholar]
- Sanmartin, C.; Taglieri, I.; Macaluso, M.; Sgherri, C.; Ascrizzi, R.; Flamini, G.; Venturi, F.; Quartacci, M.F.; Luro, F.; Curk, F.; et al. Cold-Pressing Olive Oil in the Presence of Cryomacerated Leaves of Olea or Citrus: Nutraceutical and Sensorial Features. Molecules 2019, 24, 2625. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Vian, M.A.; Ravi, H.K.; Khadhraoui, B.; Hilali, S.; Perino, S.; Tixier, A.S.F. Review of Alternative Solvents for Green Extraction of Food and Natural Products: Panorama, Principles, Applications and Prospects. Molecules 2019, 24, 3007. [Google Scholar] [CrossRef] [PubMed]
- Felhi, S.; Daoud, A.; Hajlaoui, H.; Mnafgui, K.; Gharsallah, N.; Kadri, A. Solvent Extraction Effects on Phytochemical Constituents Profiles, Antioxidant and Antimicrobial Activities and Functional Group Analysis of Ecballium Elaterium Seeds and Peels Fruits. Food Sci. Technol. 2017, 37, 483–492. [Google Scholar] [CrossRef] [Green Version]
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.G.; Lightfoot, D.A. Phytochemicals: Extraction, Isolation, and Identification of Bioactive Compounds from Plant Extracts. Plants 2017, 6, 42. [Google Scholar] [CrossRef]
- Silva, G.L.; Lee, I.-S.; Kinghorn, A.D. Special Problems with the Extraction of Plants. In Natural Products Isolation.; Humana Press: Totowa, NJ, USA, 1998; pp. 343–363. [Google Scholar]
- Monteiro, G.A.; Cabral, J.M.S.; Prazeres, D.M.F. Appendix 1. Essential Guides for Isolation/Purification of Nucleic Acids. In Encyclopedia of Separation Science; Elsevier: Amsterdam, The Netherlands, 2000. [Google Scholar]
- Ferhat, M.A.; Tigrine-Kordjani, N.; Chemat, S.; Meklati, B.Y.; Chemat, F. Rapid Extraction of Volatile Compounds Using a New Simultaneous Microwave Distillation: Solvent Extraction Device. Chromatographia 2007, 65, 217–222. [Google Scholar] [CrossRef]
- Rajaei, A.; Barzegar, M.; Yamini, Y. Supercritical Fluid Extraction of Tea Seed Oil and Its Comparison with Solvent Extraction. Eur. Food Res. Technol. 2005, 220, 401–405. [Google Scholar] [CrossRef]
- Tabaraki, R.; Nateghi, A. Optimization of Ultrasonic-Assisted Extraction of Natural Antioxidants from Rice Bran Using Response Surface Methodology. Ultrason. Sonochem. 2011, 18, 1279–1286. [Google Scholar] [CrossRef]
- Kothari, V.; Gupta, A.; Naraniwal, M. Modern Extraction Methods for Preparation of Bioactive Plant Extra-cts. Int. J. Appl. Nat. Sci. 2012, 1, 8–26. [Google Scholar]
- Fierascu, R.C.; Fierascu, I.; Ortan, A.; Georgiev, M.I.; Sieniawska, E. Innovative Approaches for Recovery of Phytoconstituents from Medicinal/Aromatic Plants and Biotechnological Production. Molecules 2020, 25, 309. [Google Scholar] [CrossRef] [PubMed]
- Tambunan, A.P.; Bahtiar, A.; Tjandrawinata, R.R. Influence of Extraction Parameters on the Yield, Phytochemical, Tlc-Densitometric Quantification of Quercetin, and LC-MS Profile, and How to Standardize Different Batches for Long Term from Ageratum conyoides L. Leaves. Pharmacogn. J. 2014, 9, 767–774. [Google Scholar] [CrossRef]
- Zygler, A.; Słomińska, M.; Namieśnik, J. Soxhlet Extraction and New Developments Such as Soxtec. In Comprehensive Sampling and Sample Preparation; Elsevier: Amsterdam, The Netherlands, 2012; Volume 2. [Google Scholar]
- Worsfold, P.; Townshend, A.; Poole, C.; Miró, M. Encyclopedia of Analytical Science; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Kim, J.; Choi, K.; Chung, D.S. Sample Preparation for Capillary Electrophoretic Applications. In Comprehensive Sampling and Sample Preparation; Elsevier: Amsterdam, The Netherlands, 2012; Volume 3. [Google Scholar]
- Gopalasatheeskumar, K. Significant Role of Soxhlet Extraction Process in Phytochemical Research. Mintage J. Pharm. Med. Sci. 2018, 7, 43–47. [Google Scholar]
- Efthymiopoulos, I.; Hellier, P.; Ladommatos, N.; Russo-Profili, A.; Eveleigh, A.; Aliev, A.; Kay, A.; Mills-Lamptey, B. Influence of Solvent Selection and Extraction Temperature on Yield and Composition of Lipids Extracted from Spent Coffee Grounds. Ind. Crops Prod. 2018, 119, 49–56. [Google Scholar] [CrossRef]
- De Castro, M.L.; Priego-Capote, F. Soxhlet Extraction: Past and Present Panacea. J. Chromatogr. A 2010, 1217, 2383–2389. [Google Scholar] [CrossRef]
- Ridgway, K. Sample Preparation for Food Contaminant Analysis. LCGC Eur. 2012, 25, 60–71. [Google Scholar]
- De Castro, M.L.; Garcıa-Ayuso, L.E. Soxhlet Extraction of Solid Materials: An Outdated Technique with a Promising Innovative Future. Anal. Chim. Acta 1998, 369, 1–10. [Google Scholar] [CrossRef]
- Mussatto, S.I. Generating Biomedical Polyphenolic Compounds from Spent Coffee or Silverskin. In Coffee in Health and Disease Prevention; Academic Press: Cambridge, MA, USA, 2015. [Google Scholar]
- Suwari; Kotta, H.Z.; Buang, Y. Optimization of Soxhlet Extraction and Physicochemical Analysis of Crop Oil from Seed Kernel of Feun Kase (Thevetia peruviana). AIP Conf. Proc. 2017, 1911, 020005. [Google Scholar]
- Picó, Y. Recent Advances in Sample Preparation for Pesticide Analysis. In Comprehensive Sampling and Sample Preparation; Academic Press: Cambridge, MA, USA, 2012; Volume 3, pp. 569–590. ISBN 9780123813749. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, D. Supercritical CO2 Extraction of Eucalyptus Leaves Oil and Comparison with Soxhlet Extraction and Hydro-Distillation Methods. Sep. Purif. Technol. 2014, 133, 443–451. [Google Scholar] [CrossRef]
- Shen, J.; Shao, X. A Comparison of Accelerated Solvent Extraction, Soxhlet Extraction, and Ultrasonic-Assisted Extraction for Analysis of Terpenoids and Sterols in Tobacco. Anal. Bioanal. Chem. 2005, 383, 1003–1008. [Google Scholar] [CrossRef] [PubMed]
- Sicaire, A.G.; Vian, M.; Fine, F.; Joffre, F.; Carré, P.; Tostain, S.; Chemat, F. Alternative Bio-Based Solvents for Extraction of Fat and Oils: Solubility Prediction, Global Yield, Extraction Kinetics, Chemical Composition and Cost of Manufacturing. Int. J. Mol. Sci. 2015, 16, 8430–8453. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, H. Pharmaceutical Analysis Sample Preparation. In Encyclopedia of Analytical Science, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 231–255. [Google Scholar]
- Llompart, M.; Garcia-Jares, C.; Celeiro, M.; Dagnac, T. Microwave-Assisted Extraction. In Encyclopedia of Analytical Science, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 67–77. [Google Scholar]
- Rehman, M.U.; Abdullah; Khan, F.; Niaz, K. Introduction to Natural Products Analysis. In Recent Advances in Natural Products Analysis; Elsevier: Amsterdam, The Netherlands, 2020; ISBN 9780128164556. [Google Scholar]
- Bogdal, D. Chapter 1-Interaction of Microwaves with Differnt Materials. Tetrahedron Org. Chem. Ser. 2005, 25, 1–11. [Google Scholar]
- de la Guardia, M.; Armenta, S. Greening Sample Treatments. Compr. Anal. Chem. 2011, 57, 87–120. [Google Scholar] [CrossRef]
- Costa, R. The Chemistry of Mushrooms: A Survey of Novel Extraction Techniques Targeted to Chromatographic and Spectroscopic Screening. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2016; Volume 49, pp. 279–306. [Google Scholar]
- Golmakani, M.T.; Moayyedi, M. Comparison of Heat and Mass Transfer of Different Microwave-Assisted Extraction Methods of Essential Oil from Citrus limon (Lisbon Variety) Peel. Food Sci. Nutr. 2015, 3, 506–518. [Google Scholar] [CrossRef] [PubMed]
- Letellier, M.; Budzinski, H. Microwave Assisted Extraction of Organic Compounds. Analusis 1999, 27, 259–270. [Google Scholar] [CrossRef]
- Chan, C.H.; Yusoff, R.; Ngoh, G.C.; Kung, F.W.L. Microwave-Assisted Extractions of Active Ingredients from Plants. J. Chromatogr. A 2011, 1218, 6213–6225. [Google Scholar] [CrossRef] [PubMed]
- Phutdhawong, W.; Kawaree, R.; Sanjaiya, S.; Sengpracha, W.; Buddhasukh, D. Microwave-Assisted Isolation of Essential Oil of Cinnamomum Iners Reinw. ex Bl.: Comparison with Conventional Hydrodistillation. Molecules 2007, 12, 868–877. [Google Scholar] [CrossRef]
- Louie, K.B.; Kosina, S.M.; Hu, Y.; Otani, H.; de Raad, M.; Kuftin, A.N.; Mouncey, N.J.; Bowen, B.P.; Northen, T.R. Mass Spectrometry for Natural Product Discovery. In Comprehensive Natural Products III; Elsevier: Amsterdam, The Netherlands, 2020; ISBN 9780081026908. [Google Scholar]
- Roohinejad, S.; Nikmaram, N.; Brahim, M.; Koubaa, M.; Khelfa, A.; Greiner, R. Potential of Novel Technologies for Aqueous Extraction of Plant Bioactives. In Water Extraction of Bioactive Compounds: From Plants to Drug Development; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Zhou, J. Applications and Prospects of Ultrasound-Assisted Extraction in Chinese Herbal Medicine. Open Access J. Biomed. Sci. 2019, 1, 5–15. [Google Scholar] [CrossRef]
- Chahardoli, A.; Jalilian, F.; Farzaei, H.; Shokoohinia, Y. Chapter 26-Analysis of Organic Acids. In Recent Advances in Natural Products Analysis; Elsevier: Amsterdam, The Netherlands, 2020; pp. 767–823. [Google Scholar]
- Picó, Y. Ultrasound-Assisted Extraction for Food and Environmental Samples. TrAC-Trends Anal. Chem. 2013, 43, 84–99. [Google Scholar] [CrossRef]
- Vinatoru, M.; Mason, T.J.; Calinescu, I. Ultrasonically Assisted Extraction (UAE) and Microwave Assisted Extraction (MAE) of Functional Compounds from Plant Materials. TrAC-Trends Anal. Chem. 2017, 97, 159–178. [Google Scholar] [CrossRef]
- Luque de Castro, M.D.; Priego Capote, F. Analytical Uses of Ultrasounds. In Techniques and Instrumentation in Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2006; Volume 26. [Google Scholar]
- Da Porto, C.; Porretto, E.; Decorti, D. Comparison of Ultrasound-Assisted Extraction with Conventional Extraction Methods of Oil and Polyphenols from Grape (Vitis vinifera L.) Seeds. Ultrason. Sonochem. 2013, 20, 1076–1080. [Google Scholar] [CrossRef]
- Barrera Vázquez, M.F.; Comini, L.R.; Martini, R.E.; Núñez Montoya, S.C.; Bottini, S.; Cabrera, J.L. Comparisons between Conventional, Ultrasound-Assisted and Microwave-Assisted Methods for Extraction of Anthraquinones from Heterophyllaea pustulata Hook f. (Rubiaceae). Ultrason. Sonochem. 2014, 21, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Dent, M.; Dragovic-Uzelac, V.; Garofulic, I.E.; Bosiljkov, T.; Ježek, D.; Brncic, M. Comparison of Conventional and Ultrasound-Assisted Extraction Techniques on Mass Fraction of Phenolic Compounds from Sage (Salvia Officinalis L.). Chem. Biochem. Eng. Q. 2015, 29, 475–484. [Google Scholar] [CrossRef]
- Carreira-Casais, A.; Otero, P.; Garcia-Perez, P.; Garcia-Oliveira, P.; Pereira, A.G.; Carpena, M.; Soria-Lopez, A.; Simal-Gandara, J.; Prieto, M.A. Benefits and Drawbacks of Ultrasound-Assisted Extraction for the Recovery of Bioactive Compounds from Marine Algae. Int. J. Environ. Res. Public Health 2021, 18, 9153. [Google Scholar] [CrossRef] [PubMed]
- Awaluddin, S.A.; Thiruvenkadam, S.; Izhar, S.; Hiroyuki, Y.; Danquah, M.K.; Harun, R. Subcritical Water Technology for Enhanced Extraction of Biochemical Compounds from Chlorella vulgaris. Biomed. Res. Int. 2016, 2016, 1–10. [Google Scholar] [CrossRef]
- Luong, D.; Sephton, M.A.; Watson, J.S. Subcritical Water Extraction of Organic Matter from Sedimentary Rocks. Anal. Chim. Acta 2015, 879, 48–57. [Google Scholar] [CrossRef]
- Nastić, N.; Švarc-Gajić, J.; Delerue-Matos, C.; Barroso, M.F.; Soares, C.; Moreira, M.M.; Morais, S.; Mašković, P.; Gaurina Srček, V.; Slivac, I.; et al. Subcritical Water Extraction as an Environmentally-Friendly Technique to Recover Bioactive Compounds from Traditional Serbian Medicinal Plants. Ind. Crops Prod. 2018, 111, 579–589. [Google Scholar] [CrossRef]
- Liang, X.; Fan, Q. Application of Sub-Critical Water Extraction in Pharmaceutical Industry. J. Mater. Sci. Chem. Eng. 2013, 1, 1–6. [Google Scholar] [CrossRef]
- Soto Ayala, R.; De Castro, M.L. Continuous Subcritical Water Extraction as a Useful Tool for Isolation of Edible Essential Oils. Food Chem. 2001, 75, 109–113. [Google Scholar] [CrossRef]
- Gbashi, S.; Madala, N.E.; Adebo, O.A.; Piater, L.; Phoku, J.Z.; Njobeh, P.B. Subcritical Water Extraction and Its Prospects for Aflatoxins Extraction in Biological Materials. In Aflatoxin-Control, Analysis, Detection and Health Risks; IntechOpen: London, UK, 2017. [Google Scholar]
- Teo, C.C.; Tan, S.N.; Yong, J.W.H.; Hew, C.S.; Ong, E.S. Pressurized Hot Water Extraction (PHWE). J. Chromatogr. A 2010, 1217, 2484–2494. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, S.M.; Kamal, S.M.M. Subcritical Water Extraction of Bioactive Compounds from Plants and Algae: Applications in Pharmaceutical and Food Ingredients. Food Eng. Rev. 2016, 8, 23–34. [Google Scholar] [CrossRef]
- Plaza, M.; Turner, C. Pressurized Hot Water Extraction of Bioactives. TrAC-Trends Anal. Chem. 2015, 71, 39–54. [Google Scholar] [CrossRef]
- Gámiz-Gracia, L.; De Castro, M.L. Continuous Subcritical Water Extraction of Medicinal Plant Essential Oil: Comparison with Conventional Techniques. Talanta 2000, 51, 1179–1185. [Google Scholar] [CrossRef]
- Giray, E.S.; Kirici, S.; Kaya, D.A.; Türk, M.; Sönmez, Ö.; Inan, M. Comparing the Effect of Sub-Critical Water Extraction with Conventional Extraction Methods on the Chemical Composition of Lavandula stoechas. Talanta 2008, 74, 930–935. [Google Scholar] [CrossRef]
- Rodrigues, L.G.G.; Mazzutti, S.; Siddique, I.; da Silva, M.; Vitali, L.; Ferreira, S.R.S. Subcritical Water Extraction and Microwave-Assisted Extraction Applied for the Recovery of Bioactive Components from Chaya (Cnidoscolus aconitifolius Mill.). J. Supercrit. Fluids 2020, 165, 104976. [Google Scholar] [CrossRef]
- Cvetanović, A. Extractions Without Organic Solvents: Advantages and Disadvantages. Chem. Afr. 2019, 2, 343–349. [Google Scholar] [CrossRef]
- Cheng, Y.; Xue, F.; Yu, S.; Du, S.; Yang, Y. Subcritical Water Extraction of Natural Products. Molecules 2021, 26, 4004. [Google Scholar] [CrossRef]
- Khosravi-Darani, K.; Vasheghani-Farahani, E. Application of Supercritical Fluid Extraction in Biotechnology. Crit. Rev. Biotechnol. 2005, 25, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Simandi, B.; Kery, A.; Lemberkovics, E.; Oszagyan, M.; Ronyai, E.; Mathe, I.; Fekete, J.; Hethelyi, E. Supercritical Fluid Extraction of Medicinal Plants. In Process Technology Proceedings; Elsevier: Amsterdam, The Netherlands, 1996; pp. 357–362. [Google Scholar]
- Radcliffe, C.; Maguire, K.; Lockwood, B. Applications of Supercritical Fluid Extraction and Chromatography in Forensic Science. J. Biochem Biophys Methods 2000, 43, 261–272. [Google Scholar] [CrossRef]
- Týskiewicz, K.; Konkol, M.; Rój, E. The Application of Supercritical Fluid Extraction in Phenolic Compounds Isolation from Natural Plant Materials. Molecules 2018, 23, 2625. [Google Scholar] [CrossRef]
- Yang, Y.; Hu, B. Bio-Based Chemicals from Biorefining: Lipid and Wax Conversion and Utilization. In Advances in Biorefineries: Biomass and Waste Supply Chain Exploitation; Keith, W., Ed.; Woodhead Publishing: Cambridge, UK, 2014. [Google Scholar]
- Moradi-kheibari, N.; Ahmadzadeh, H.; Talebi, A.F.; Hosseini, M.; Murry, M.A. Recent Advances in Lipid Extraction for Biodiesel Production. In Advances in Feedstock Conversion Technologies for Alternative Fuels and Bioproducts: New Technologies, Challenges and Opportunities; Woodhead Publishing: Duxford, UK, 2019. [Google Scholar]
- Baskar, G.; Kalavathy, G.; Aiswarya, R.; Abarnaebenezer Selvakumari, I. Advances in Bio-Oil Extraction from Nonedible Oil Seeds and Algal Biomass. In Advances in Eco-Fuels for a Sustainable Environment; Woodhead Publishing: Duxford, UK, 2019. [Google Scholar]
- Pieczykolan, A.; Pietrzak, W.; Rój, E.; Nowak, R. Effects of Supercritical Carbon Dioxide Extraction (SC-CO2) on the Content of Tiliroside in the Extracts from Tilia L. Flowers. Open Chem. 2019, 17, 187–210. [Google Scholar] [CrossRef]
- Wang, Q.; Shi, A.; Liu, H.; Liu, L.; Zhang, Y.; Li, N.; Gong, K.; Yu, M.; Zheng, L. Peanut By-Products Utilization Technology. In Peanuts: Processing Technology and Product Development; Academic Press: Cambridge, MA, USA, 2016. [Google Scholar]
- Abhari, K.; Mousavi Khaneghah, A. Alternative Extraction Techniques to Obtain, Isolate and Purify Proteins and Bioactive from Aquaculture and by-Products. In Advances in Food and Nutrition Research; Academic Press: Cambridge, MA, USA, 2020; Volume 92. [Google Scholar]
- Tarafder, A. Metamorphosis of Supercritical Fluid Chromatography to SFC: An Overview. TrAC-Trends Anal. Chem. 2016, 81, 3–10. [Google Scholar] [CrossRef]
- Capuzzo, A.; Maffei, M.E.; Occhipinti, A. Supercritical Fluid Extraction of Plant Flavors and Fragrances. Molecules 2013, 18, 7194–7238. [Google Scholar] [CrossRef] [PubMed]
- Fratianni, A.; Caboni, M.F.; Irano, M.; Panfili, G. A Critical Comparison between Traditional Methods and Supercritical Carbon Dioxide Extraction for the Determination of Tocochromanols in Cereals. Eur. Food Res. Technol. 2002, 215, 353–358. [Google Scholar] [CrossRef]
- Pyo, D.; Yoo, J.; Surh, J. Comparison of Supercritical Fluid Extraction and Solvent Extraction of Isoflavones from Soybeans. J. Liq. Chromatogr. Relat. Technol. 2009, 32, 923–932. [Google Scholar] [CrossRef]
- Memon, A.H.; Hamil, M.S.R.; Laghari, M.; Rithwan, F.; Zhari, S.; Saeed, M.A.A.; Ismail, Z.; Majid, A.M.S.A. A Comparative Study of Conventional and Supercritical Fluid Extraction Methods for the Recovery of Secondary Metabolites from Syzygium campanulatum Korth. J. Zhejiang Univ. Sci. B 2016, 17, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Cerón-Martínez, L.J.; Hurtado-Benavides, A.M.; Ayala-Aponte, A.; Serna-Cock, L.; Tirado, D.F. A Pilot-Scale Supercritical Carbon Dioxide Extraction to Valorize Colombian Mango Seed Kernel. Molecules 2021, 26, 2279. [Google Scholar] [CrossRef]
- Bertoli, A.; Ruffoni, B.; Pistelli, L.; Pistelli, L. Analytical Methods for the Extraction and Identification of Secondary Metabolite Production in “in Vitro” Plant Cell Cultures. Adv. Exp. Med. Biol. 2010, 698, 250–266. [Google Scholar] [CrossRef] [PubMed]
- Thirumal, Y.; Laavu, S. HPLC Profile of Medicinal Plant Extracts and Its Application in Aquaculture. J. Aquac. Res. Dev. 2017, 8, 2–6. [Google Scholar] [CrossRef]
- Boligon, A.A.; Athayde, M.L. Importance of HPLC in Analysis of Plants Extracts. Austin Chromatogr. 2014, 1, 2. [Google Scholar]
- Salam, A.M.; Lyles, J.T.; Quave, C.L. Methods in the Extraction and Chemical Analysis of Medicinal Plants. In Methods and Techniques in Ethnobiology and Ethnoecology; Humana Press: New York, NY, USA, 2019. [Google Scholar]
- Feng, W.; Li, M.; Hao, Z.; Zhang, J. Analytical Methods of Isolation and Identification. In Phytochemicals in Human Health; IntechOpen: London, UK, 2020. [Google Scholar]
- Asteggiano, A.; Occhipinti, A.; Capuzzo, A.; Mecarelli, E.; Aigotti, R.; Medana, C. Quali-Quantitative Characterization of Volatile and Non-Volatile Compounds in Protium heptaphyllum (Aubl.) Marchand Resin by GC-MS Validated Method, GC-FID and HPLC-HRMS2. Molecules 2021, 26, 1447. [Google Scholar] [CrossRef]
- Turek, C.; Stintzing, F.C. Stability of Essential Oils: A Review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 40–53. [Google Scholar] [CrossRef]
- Choe, E.; Min, D.B. Mechanisms and Factors for Edible Oil Oxidation. Compr. Rev. Food Sci. Food Saf. 2006, 5, 169–186. [Google Scholar] [CrossRef]
- Nkengurutse, J.; Mansouri, F.; Bekkouch, O.; ben Moumen, A.; Masharabu, T.; Gahungu, G.; Serghini, H.C.; Khalid, A. Chemical Composition and Oral Toxicity Assessment of Anisophyllea boehmii Kernel Oil: Potential Source of New Edible Oil with High Tocopherol Content. Food Chem. 2019, 278, 795–804. [Google Scholar] [CrossRef]
- Melo, E.; Michels, F.; Arakaki, D.; Lima, N.; Gonçalves, D.; Cavalheiro, L.; Oliveira, L.; Caires, A.; Hiane, P.; Nascimento, V. First Study on the Oxidative Stability and Elemental Analysis of Babassu (Attalea speciosa) Edible Oil Produced in Brazil Using a Domestic Extraction Machine. Molecules 2019, 24, 4235. [Google Scholar] [CrossRef] [PubMed]
- Grosshagauer, S.; Steinschaden, R.; Pignitter, M. Strategies to Increase the Oxidative Stability of Cold Pressed Oils. LWT 2019, 106, 72–77. [Google Scholar] [CrossRef]
- Llorent-Martínez, E.J.; Ortega-Barrales, P.; Fernández-De Córdova, M.L.; Domínguez-Vidal, A.; Ruiz-Medina, A. Investigation by ICP-MS of Trace Element Levels in Vegetable Edible Oils Produced in Spain. Food Chem. 2011, 127, 1257–1262. [Google Scholar] [CrossRef]
- Zhu, F.; Fan, W.; Wang, X.; Qu, L.; Yao, S. Health Risk Assessment of Eight Heavy Metals in Nine Varieties of Edible Vegetable Oils Consumed in China. Food Chem. Toxicol. 2011, 49, 3081–3085. [Google Scholar] [CrossRef]
- Kumar, V.; Mathela, C.S.; Kumar, M.; Tewari, G. Antioxidant Potential of Essential Oils from Some Himalayan Asteraceae and Lamiaceae Species. Med. Drug Discov. 2019, 1, 100004. [Google Scholar] [CrossRef]
- Amorati, R.; Foti, M.C.; Valgimigli, L. Antioxidant Activity of Essential Oils. J. Agric. Food Chem. 2013, 61, 10835–10847. [Google Scholar] [CrossRef]
- Sparkman, O.; Penton, Z.; Kitson, F. Introduction and History. In Gas Chromatography and Mass Spectrometry: A Practical Guide; Academic Press: Cambridge, MA, USA, 2011; pp. 2–13. [Google Scholar]
- He, P.; Aga, D.S. Comparison of GC-MS/MS and LC-MS/MS for the Analysis of Hormones and Pesticides in Surface Waters: Advantages and Pitfalls. Anal. Methods 2019, 11, 1436–1448. [Google Scholar] [CrossRef]
- Rockwood, A.; Kushnir, M.; Clarke, N. Mass Spectrometry. In Principles and Applications of Clinical Mass Spectrometry; Elsevier: Amsterdam, The Netherlands, 2018; pp. 33–65. ISBN 9780128160633. [Google Scholar]
- Niwa, M. Derivatization and Labeling Techniques. In Encyclopedia of Analytical Science; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Lawrence, J.F. Advantages and limitations of chemical derivatization for trace analysis by liquid chromatography. J. Chromatogr. Sci. 1985, 23, 484–487. [Google Scholar] [CrossRef]
- Lei, Z.; Huhman, D.V.; Sumner, L.W. Mass Spectrometry Strategies in Metabolomics. J. Biol. Chem. 2011, 286, 25435–25442. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, S.; Hu, Y.; Li, P.; Wan, J.B. Current State of the Art of Mass Spectrometry-Based Metabolomics Studies—A Review Focusing on Wide Coverage, High Throughput and Easy Identification. RSC Adv. 2015, 5, 78728–78737. [Google Scholar] [CrossRef]
- Lynch, K.L. Toxicology: Liquid Chromatography Mass Spectrometry. In Mass Spectrometry for the Clinical Laboratory; Academic Press: Cambridge, MA, USA, 2017. [Google Scholar]
- Kaluarachchi, M.; Lewis, M.R.; Lindon, J.C. Standardized Protocols for MS-Based Metabolic Phenotyping. In Encyclopedia of Spectroscopy and Spectrometry; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Chan, E.C.Y.; Pasikanti, K.K.; Hong, Y.; Ho, P.C.; Mahendran, R.; Raman Nee Mani, L.; Chiong, E.; Esuvaranathan, K. Metabonomic Profiling of Bladder Cancer. J. Proteome Res. 2015, 14, 587–602. [Google Scholar] [CrossRef]
- Pasikanti, K.K.; Ho, P.C.; Chan, E.C.Y. Gas Chromatography/Mass Spectrometry in Metabolic Profiling of Biological Fluids. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2008, 871, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Perez, H.L.; Evans, C.A. Chemical Derivatization in Bioanalysis. Bioanalysis 2015, 7, 2435–2437. [Google Scholar] [CrossRef]
- Savolainen, O.I.; Sandberg, A.S.; Ross, A.B. A Simultaneous Metabolic Profiling and Quantitative Multimetabolite Metabolomic Method for Human Plasma Using Gas-Chromatography Tandem Mass Spectrometry. J. Proteome Res. 2016, 15, 259–265. [Google Scholar] [CrossRef]
- Gomathi, D.; Kalaiselvi, M.; Ravikumar, G.; Devaki, K.; Uma, C. GC-MS Analysis of Bioactive Compounds from the Whole Plant Ethanolic Extract of Evolvulus alsinoides (L.) L. J. Food Sci. Technol. 2015, 52, 1212–1217. [Google Scholar] [CrossRef] [PubMed]
- Konappa, N.; Udayashankar, A.C.; Krishnamurthy, S.; Pradeep, C.K.; Chowdappa, S.; Jogaiah, S. GC–MS Analysis of Phytoconstituents from Amomum nilgiricum and Molecular Docking Interactions of Bioactive Serverogenin Acetate with Target Proteins. Sci. Rep. 2020, 10, 1–23. [Google Scholar] [CrossRef]
- Velmurugan, G.; Anand, S.P. GC-MS Analysis of Bioactive Compounds on Ethanolic Leaf Extract of Phyllodium pulchellum L. Desv. Int. J. Pharmacogn. Phytochem. Res. 2017, 9, 114–118. [Google Scholar] [CrossRef]
- Rukshana, M.; Doss, A.; Kumari Pushpa Rani, T. Phytochemical Screening and GC-MS Analysis of Leaf Extract of Pergularia daemia (Forssk) Chiov. Asian J. Plant Sci. Res. 2017, 1, 9–15. [Google Scholar]
- Swamy, M.K.; Sinniah, U.R.; Akhtar, M.S. In Vitro Pharmacological Activities and GC-Ms Analysis of Different Solvent Extracts of Lantana camara Leaves Collected from Tropical Region of Malaysia. Evid.-Based Complement. Altern. Med. 2015, 2015, 1–9. [Google Scholar] [CrossRef]
- Jalal Ali, M.; Makky, E.A.; Zareen, S.; Yusoff, M.M. Identification of Bioactive Phytochemicals Using GC-Mass and TLC to the Estimation of Antimicrobial Susceptibility of Plant Extracts. J. Phys. Conf. Ser. 2019, 1294, 062013. [Google Scholar] [CrossRef]
- Asha, K.R.; Priyanga, S.; Hemmalakshmi, S.; Devaki, K. GC-MS Analysis of the Ethanolic Extract of the Whole Plant Drosera Indica L. Int. J. Pharmacogn. Phytochem. Res. 2017, 9, 685–688. [Google Scholar] [CrossRef]
- Zaikin, V.G.; Borisov, R.S. Mass Spectrometry as a Crucial Analytical Basis for Omics Sciences. J. Anal. Chem. 2021, 76, 1567–1587. [Google Scholar] [CrossRef]
- Wiley Registry of Mass Spectral Data, 12th ed.; Wiley: Hoboken, NJ, USA, 2020.
- Hübschmann, H.-J. Handbook of GC-MS: Fundamentals and Applications, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Tilvi, S.; Majik, M.S.; Singh, K.S. Mass Spectrometry for Determination of Bioactive Compounds. In Comprehensive Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2014; Volume 65. [Google Scholar]
- Pang, B.; Zhu, Y.; Lu, L.; Gu, F.; Chen, H. The Applications and Features of Liquid Chromatography-Mass Spectrometry in the Analysis of Traditional Chinese Medicine. Evid.-Based Complement. Altern. Med. 2016, 2016, 3837270. [Google Scholar] [CrossRef] [PubMed]
- Grebe, S.K.G.; Singh, R.J. LC-MS/MS in the Clinical Laboratory-Where to from Here? Clin. Biochem. Rev. 2011, 32, 5. [Google Scholar] [PubMed]
- Mukherjee, P.K. LC–MS: A Rapid Technique for Understanding the Plant Metabolite Analysis. In Quality Control and Evaluation of Herbal Drugs; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Zhang, Z.; Hu, X.; Li, P. Chromatography: Combined Chromatography and Mass Spectrometry. In Encyclopedia of Food and Health; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Aretz, I.; Meierhofer, D. Advantages and Pitfalls of Mass Spectrometry Based Metabolome Profiling in Systems Biology. Int. J. Mol. Sci. 2016, 17, 632. [Google Scholar] [CrossRef] [PubMed]
- McDonald, J.; Ivanova, P.; Brown, A. Chapter 2- Approaches to Lipid Analysis. In Biochemistry of Lipids, Lipoproteins and Membranes, 6th ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 41–72. [Google Scholar]
- Malviya, R.; Bansal, V.; Pal, O.P.; Sharma, P.K. High Performance Liquid Chromatography: A Short Review. J. Glob. Pharma Technol. 2010, 2, 22–26. [Google Scholar]
- Gika, H.G.; Wilson, I.D.; Theodoridis, G.A. Omics|Metabolomics: An Analytical Perspective. In Encyclopedia of Analytical Science; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Zisi, C.; Nikitas, P.; Pappa-Louisi, A. Computer-Aided Separation Optimization in Reversed-Phase Liquid Chromatography. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Buszewski, B.; Noga, S. Hydrophilic Interaction Liquid Chromatography (HILIC)—A Powerful Separation Technique. Anal. Bioanal. Chem. 2012, 402, 231–247. [Google Scholar] [CrossRef] [PubMed]
- Smoluch, M.; Mielczarek, P.; Drabik, A.; Silberring, J. Online and Offline Sample Fractionation. In Proteomic Profiling and Analytical Chemistry: The Crossroads, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Zhang, K.; Liu, X. Mixed-Mode Chromatography in Pharmaceutical and Biopharmaceutical Applications. J. Pharm. Biomed. Anal. 2016, 128, 73–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vitha, M.F. Chromatography: Principles and Instrumentation; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Fanali, C.; Haddad, P.R.; Poole, C.F.; Riekkola, M.L. Liquid Chromatography: Fundamentals and Instrumentation, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2017; Volume 1. [Google Scholar]
- Olsen, B.A.; Pack, B.W. Hydrophilic Interaction Chromatography: A Guide for Practitioners; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Eugster, P.J.; Wolfender, J.-L. UHPLC in Life Sciences; Royal Society of Chemistry: London, UK, 2012; Volume 44. [Google Scholar]
- Weiss, J. Handbook of Ion Chromatography; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Bahadir Acikara, O. Ion-Exchange Chromatography and Its Applications. In Column Chromatography; 2013; Available online: https://www.intechopen.com/chapters/44033 (accessed on 4 September 2022).
- Podzimek, S. Light Scattering, Size Exclusion Chromatography and Asymmetric Flow Field Flow Fractionation: Powerful Tools for the Characterization of Polymers, Proteins and Nanoparticles; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Striegel, A.M.; Yau, W.W.; Kirkland, J.J.; Bly, D.D. Modern Size-Exclusion Liquid Chromatography: Practice of Gel Permeation and Gel Filtration Chromatography, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).