Progesterone Receptor Modulates Extraembryonic Mesoderm and Cardiac Progenitor Specification during Mouse Gastrulation
Abstract
1. Introduction
2. Results
2.1. The Nuclear Receptor PGR Is Expressed in Primed Pluripotent Cells
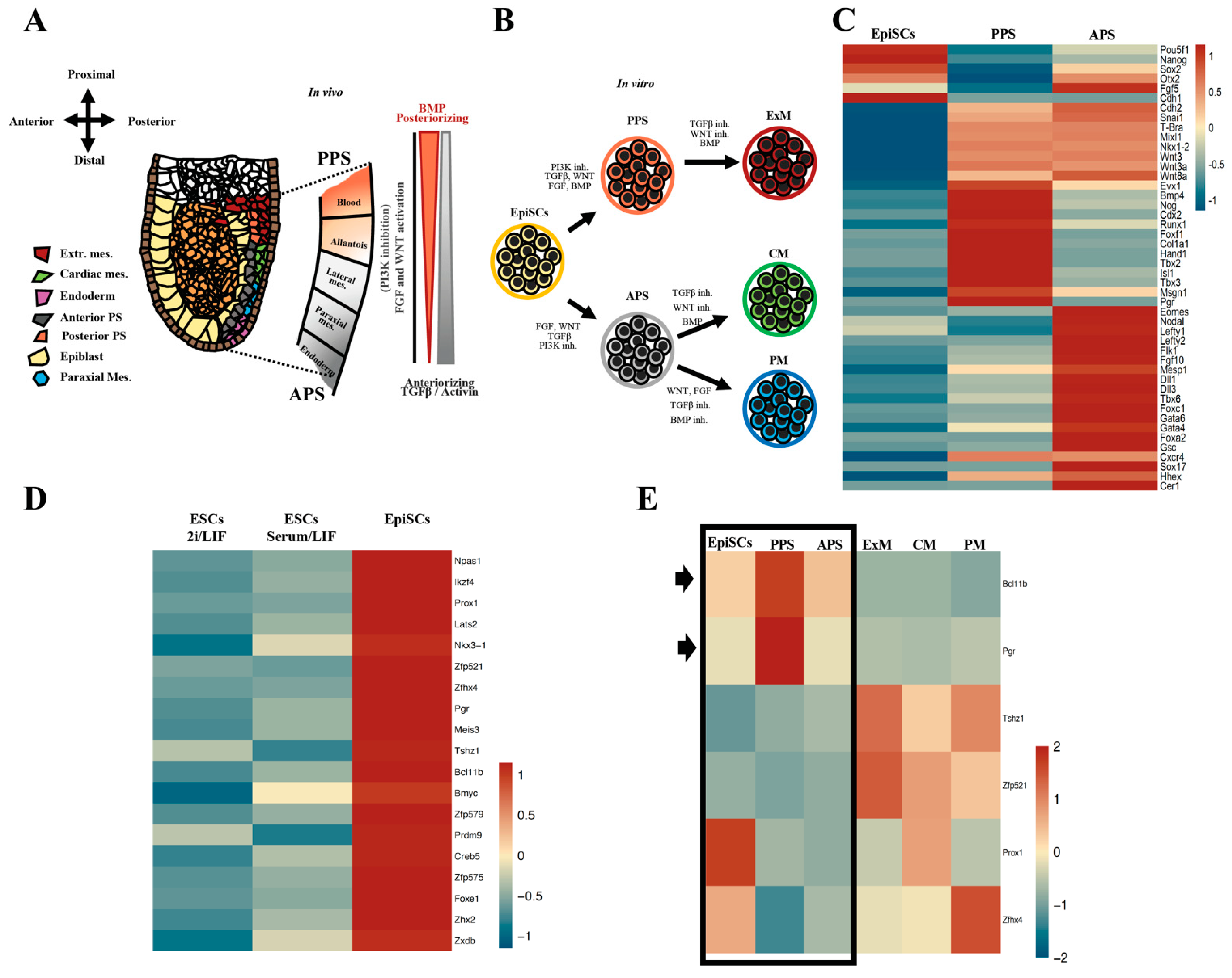
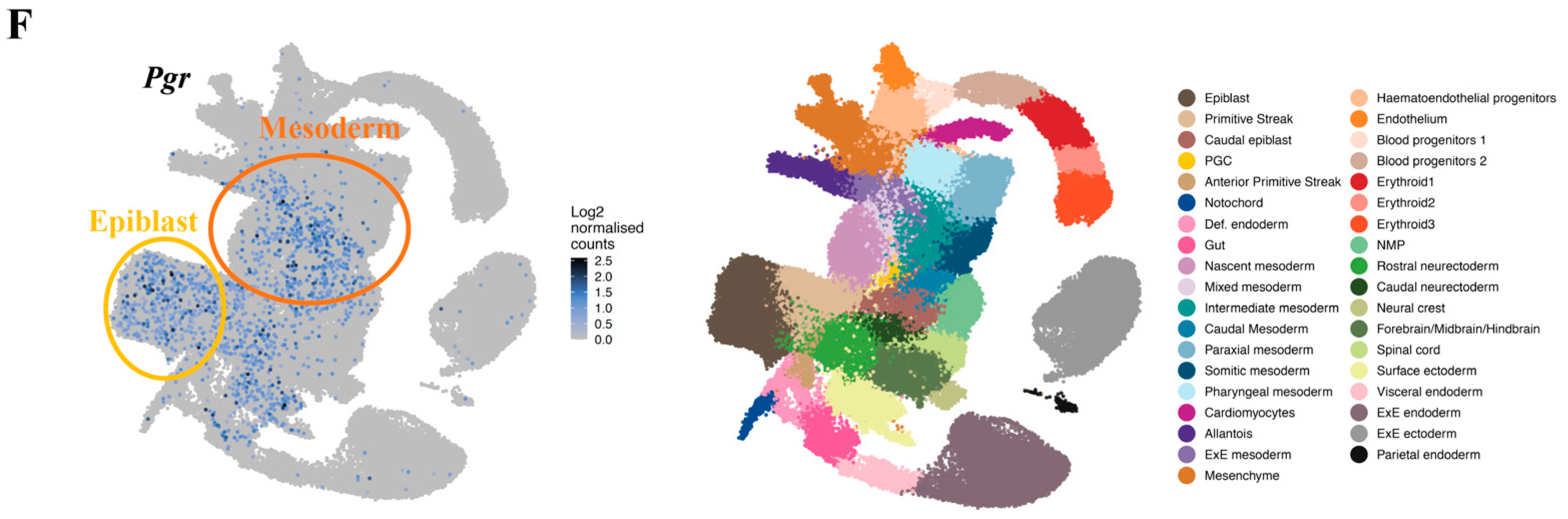
2.2. PGR Is Expressed in the Primitive Streak with a Posterior-to-Anterior Gradient
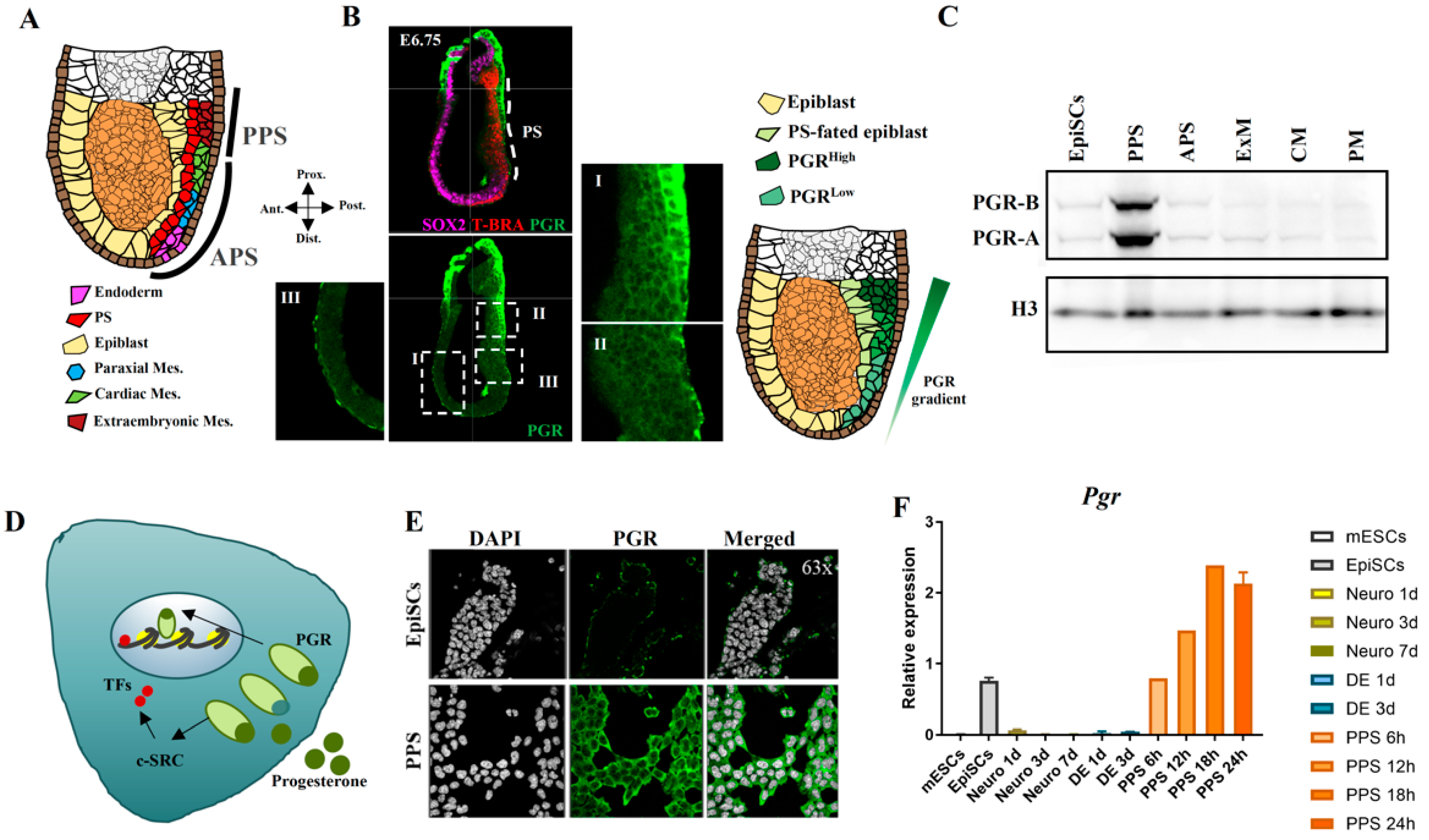
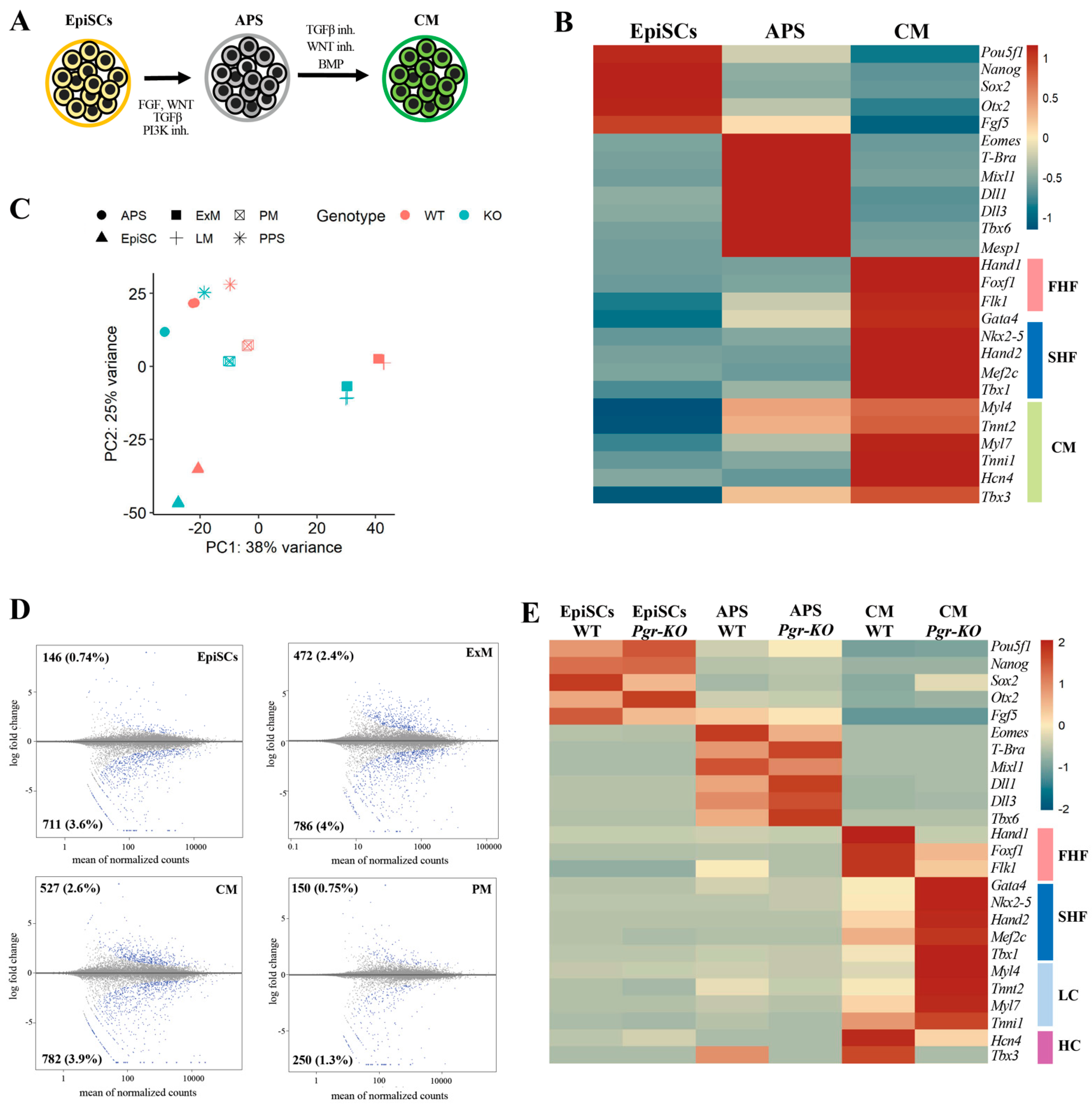
2.3. PGR Modulates the Differentiation of the Posterior Primitive Streak and Extraembryonic Mesoderm
2.4. PGR Influences the Acquisition of the First Heart Field Fate
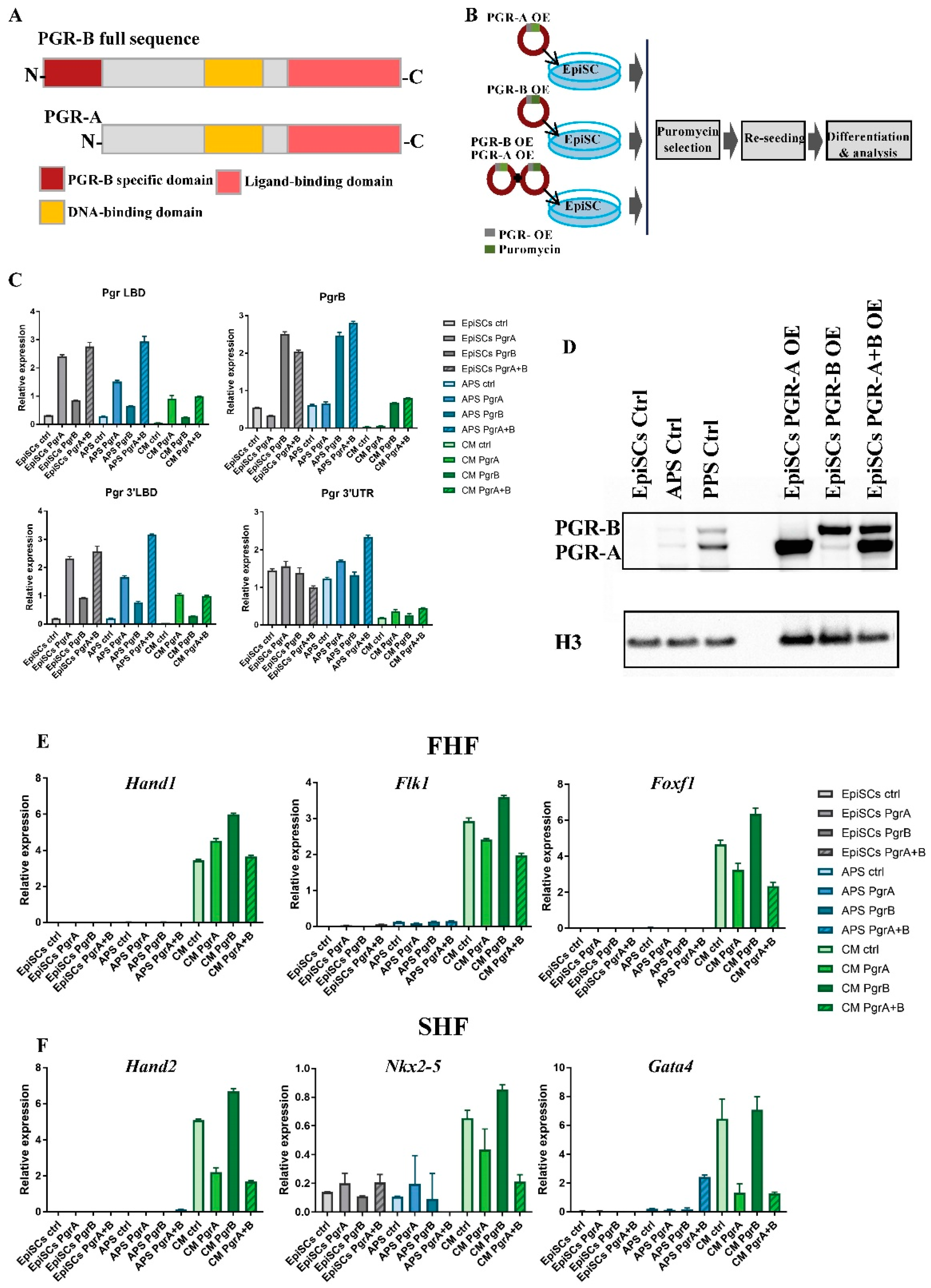
2.5. PGR Modulates Mesoderm Differentiation in an Isoform-Dependent Manner
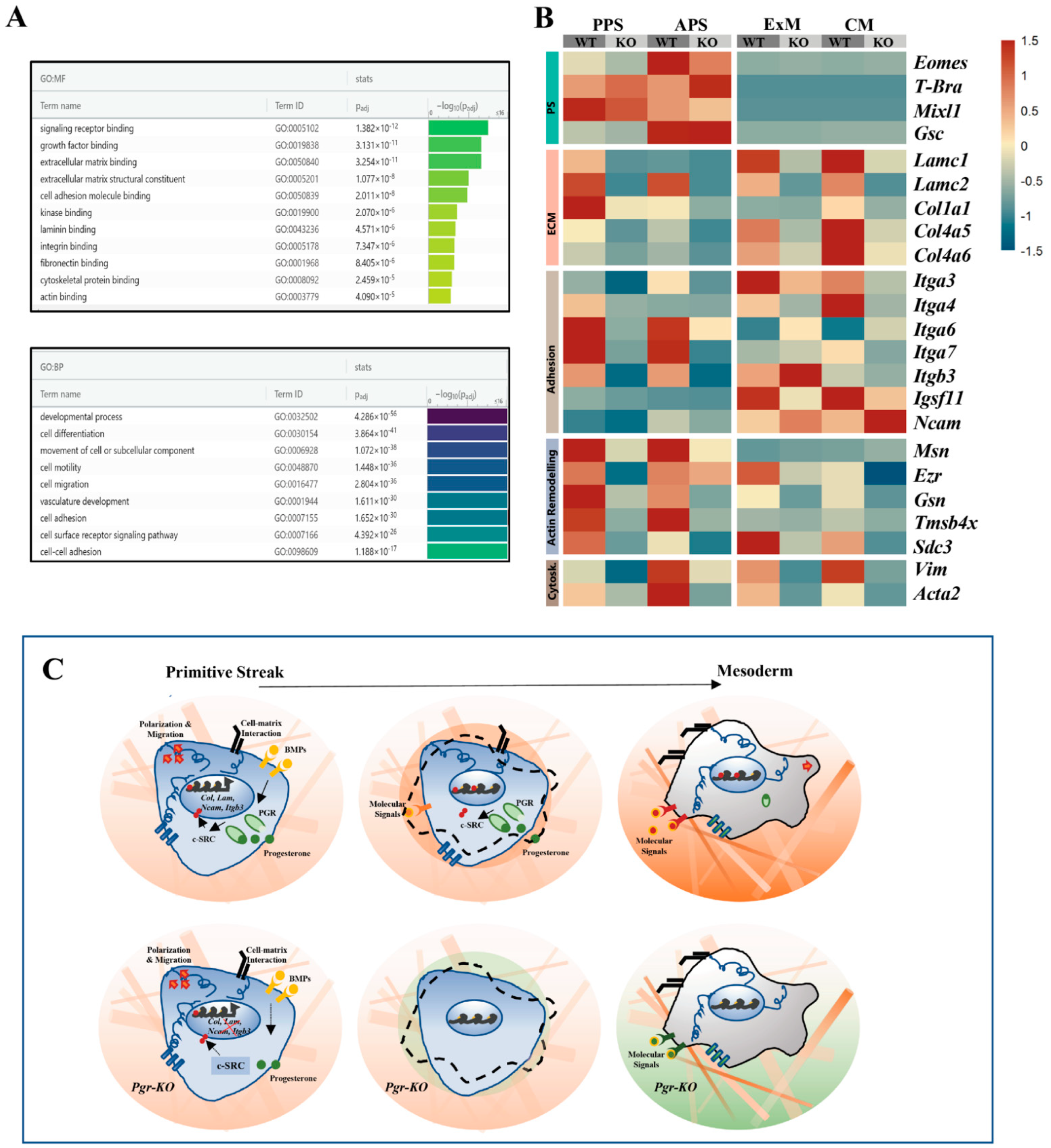
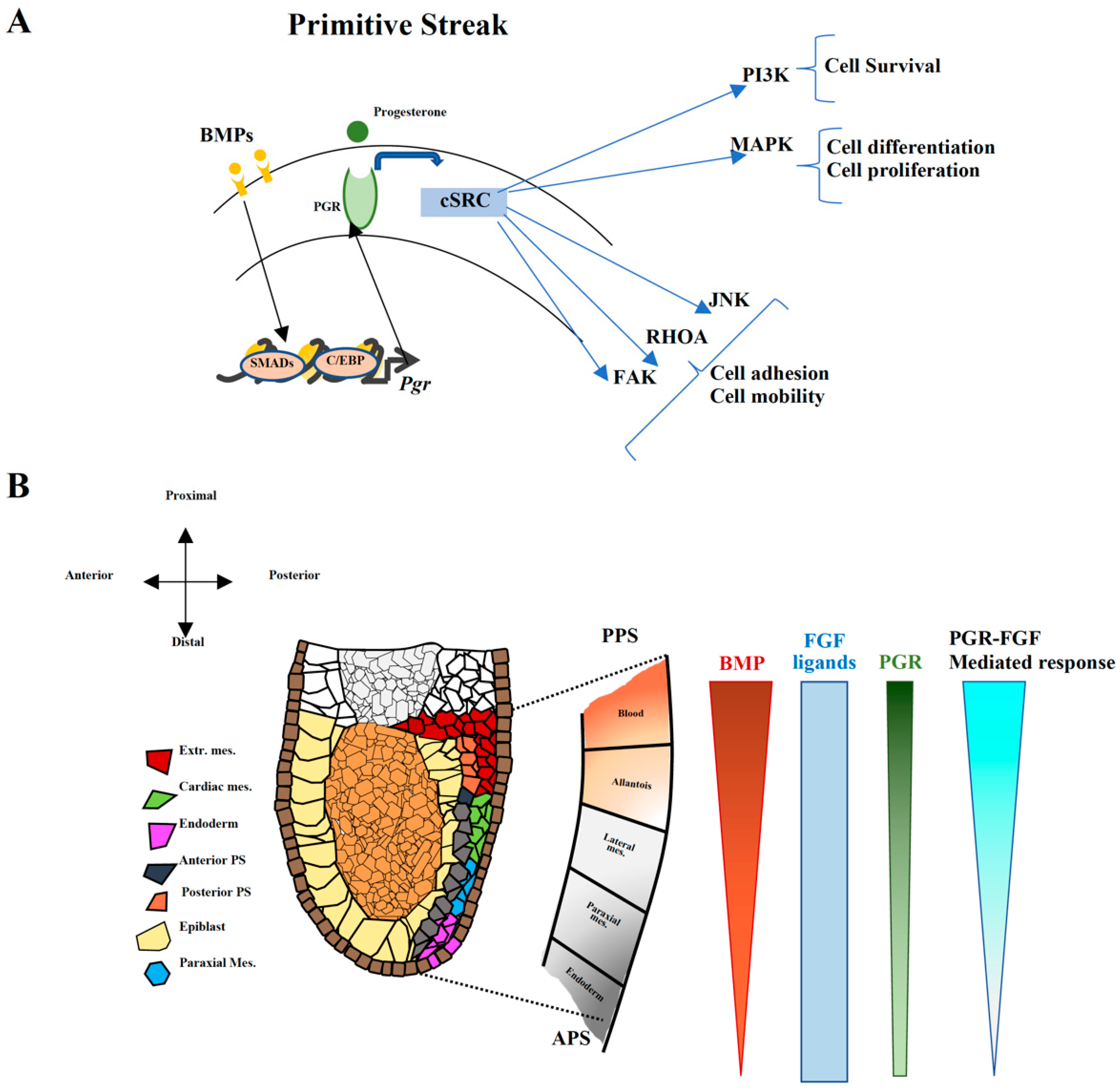
2.6. PGR Influences Cell–ECM Interaction and Cytoskeletal Remodeling
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. EpiSC Differentiation to PS and Mesoderm Progenitors
4.3. RT-qPCR
4.4. Western Blot
4.5. Immunofluorescence and Imaging
4.6. scRNA-Seq
4.7. Bulk RNA-Seq
4.8. Cloning of the Overexpression Vectors and Lentiviral Packaging
4.9. Isoform-Specific PGR Overexpression during Differentiation
4.10. Cell Lines Genotyping and Karyotyping
4.11. Mice
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, R.; Wang, X.; Huang, Z.; Balaji, J.; Kim, T.H.; Wang, T.; Zhou, L.; Deleon, A.; Cook, M.E.; Marbrey, M.W.; et al. The role of epithelial progesterone receptor isoforms in embryo implantation. iScience 2021, 24, 103487. [Google Scholar] [CrossRef] [PubMed]
- Graham, J.D.; Clarke, C.L. Physiological Action of Progesterone in Target Tissues. Endocr. Rev. 1997, 18, 502–519. [Google Scholar] [CrossRef]
- Olbrich, L.; Wessel, L.; Balakrishnan-Renuka, A.; Böing, M.; Brand-Saberi, B.; Theiss, C. Rapid Impact of Progesterone on the Neuronal Growth Cone. Endocrinology 2013, 154, 3784–3795. [Google Scholar] [CrossRef] [PubMed]
- Sim, C.B.; Phipson, B.; Ziemann, M.; Rafehi, H.; Mills, R.J.; Watt, K.I.; Abu-Bonsrah, K.D.; Kalathur, R.K.; Voges, H.K.; Dinh, D.T.; et al. Sex-Specific Control of Human Heart Maturation by the Progesterone Receptor. Circulation 2021, 143, 1614–1628. [Google Scholar] [CrossRef] [PubMed]
- Kot, A.; Zhong, Z.A.; Zhang, H.L.; Lay, Y.-A.E.; ELane, N.; Yao, W. Sex dimorphic regulation of osteoprogenitor progesterone in bone stromal cells. J. Mol. Endocrinol. 2017, 59, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Murphy, B.D. Progesterone is critical for the development of mouse embryos. Endocrine 2014, 46, 615–623. [Google Scholar] [CrossRef]
- Arnold, S.J.; Robertson, E.J. Making a commitment: Cell lineage allocation and axis patterning in the early mouse embryo. Nat. Rev. Mol. Cell Biol. 2009, 10, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, E.; Hadjantonakis, A.-K. Mesoderm specification and diversification: From single cells to emergent tissues. Curr. Opin. Cell Biol. 2019, 61, 110–116. [Google Scholar] [CrossRef]
- Kinder, S.; Tsang, T.; Quinlan, G.; Hadjantonakis, A.; Nagy, A.; Tam, P. The orderly allocation of mesodermal cells to the extraembryonic structures and the anteroposterior axis during gastrulation of the mouse embryo. Development 1999, 126, 4691–4701. [Google Scholar] [CrossRef] [PubMed]
- Brons, I.G.M.; Smithers, L.E.; Trotter, M.W.B.; Rugg-Gunn, P.; Sun, B.; de Sousa Lopes, S.M.C.; Howlett, S.K.; Clarkson, A.; Ahrlund-Richter, L.; Pedersen, R.A.; et al. Derivation of pluripotent epiblast stem cells from mammalian embryos. Nat. Cell Biol. 2007, 448, 191–195. [Google Scholar] [CrossRef]
- Tesar, P.J.; Chenoweth, J.G.; Brook, F.A.; Davies, T.J.; Evans, E.P.; Mack, D.L.; Gardner, R.L.; McKay, R.D.G. New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature 2007, 448, 196–199. [Google Scholar] [CrossRef] [PubMed]
- Pfister, S.; Steiner, K.A.; Tam, P.P. Gene expression pattern and progression of embryogenesis in the immediate post-implantation period of mouse development. Gene Expr. Patterns 2007, 7, 558–573. [Google Scholar] [CrossRef] [PubMed]
- Najm, F.J.; Chenoweth, J.G.; Anderson, P.D.; Nadeau, J.H.; Redline, R.W.; McKay, R.D.; Tesar, P.J. Isolation of Epiblast Stem Cells from Preimplantation Mouse Embryos. Cell Stem Cell 2011, 8, 318–325. [Google Scholar] [CrossRef]
- Morgani, S.; Nichols, J.; Hadjantonakis, A.-K. The many faces of Pluripotency: In vitro adaptations of a continuum of in vivo states. BMC Dev. Biol. 2017, 17, 7. [Google Scholar] [CrossRef] [PubMed]
- Osteil, P.; Studdert, J.B.; Goh, H.N.; Wilkie, E.E.; Fan, X.; Khoo, P.-L.; Peng, G.; Salehin, N.; Knowles, H.; Han, J.-D.J.; et al. Dynamics of Wnt activity on the acquisition of ectoderm potency in epiblast stem cells. Development 2019, 146, dev172858. [Google Scholar] [CrossRef] [PubMed]
- Hagan, C.R.; Lange, C.A. Molecular determinants of context-dependent progesterone receptor action in breast cancer. BMC Med. 2014, 12, 32. [Google Scholar] [CrossRef] [PubMed]
- Werner, L.R.; Gibson, K.A.; Goodman, M.L.; Helm, D.E.; Walter, K.R.; Holloran, S.M.; Trinca, G.M.; Hastings, R.C.; Yang, H.H.; Hu, Y.; et al. Progesterone promotes immunomodulation and tumor development in the murine mammary gland. J. Immunother. Cancer 2021, 9, e001710. [Google Scholar] [CrossRef]
- Kim, J.J.; Kurita, T.; Bulun, S.E. Progesterone Action in Endometrial Cancer, Endometriosis, Uterine Fibroids, and Breast Cancer. Endocr. Rev. 2013, 34, 130–162. [Google Scholar] [CrossRef] [PubMed]
- Marquardt, R.M.; Kim, T.H.; Shin, J.-H.; Jeong, J.-W. Progesterone and Estrogen Signaling in the Endometrium: What Goes Wrong in Endometriosis? Int. J. Mol. Sci. 2019, 20, 3822. [Google Scholar] [CrossRef] [PubMed]
- Gebril, M.; Hirota, Y.; Aikawa, S.; Fukui, Y.; Kaku, T.; Matsuo, M.; Hirata, T.; Akaeda, S.; Hiraoka, T.; Shimizu-Hirota, R.; et al. Uterine Epithelial Progesterone Receptor Governs Uterine Receptivity Through Epithelial Cell Differentiation. Endocrinology 2020, 161, bqaa195. [Google Scholar] [CrossRef] [PubMed]
- Pijuan-Sala, B.; Griffiths, J.; Guibentif, C.; Hiscock, T.; Jawaid, W.; Calero-Nieto, F.; Mulas, C.; Ibarra-Soria, X.; Tyser, R.C.V.; Ho, D.L.L.; et al. A single-cell molecular map of mouse gastrulation and early organogenesis. Nature 2019, 566, 490–495. [Google Scholar] [CrossRef]
- Wilkinson, D.; Bhatt, S.; Herrmann, B.G. Expression pattern of the mouse T gene and its role in mesoderm formation. Nature 1990, 343, 657–659. [Google Scholar] [CrossRef]
- Perrot-Applanat, M.; Lescop, P.; Milgrom, E. The cytoskeleton and the cellular traffic of the progesterone receptor. J. Cell Biol. 1992, 119, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Boonyaratanakornkit, V.; Scott, M.P.; Ribon, V.; Sherman, L.; Anderson, S.M.; Maller, J.L.; Miller, W.; Edwards, D.P. Progesterone Receptor Contains a Proline-Rich Motif that Directly Interacts with SH3 Domains and Activates c-Src Family Tyrosine Kinases. Mol. Cell 2001, 8, 269–280. [Google Scholar] [CrossRef]
- Rivera-Pérez, J.A.; Magnuson, T. Primitive streak formation in mice is preceded by localized activation of Brachyury and Wnt3. Dev. Biol. 2005, 288, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Chazaud, C.; Yamanaka, Y. Lineage specification in the mouse preimplantation embryo. Development 2016, 143, 1063–1074. [Google Scholar] [CrossRef]
- Morikawa, Y.; Cserjesi, P. Extra-embryonic vasculature development is regulated by the transcription factor HAND1. Development 2004, 131, 2195–2204. [Google Scholar] [CrossRef]
- Mahlapuu, M.; Ormestad, M.; Enerback, S.; Carlsson, P. The forkhead transcription factor Foxf1 is required for differentiation of extra-embryonic and lateral plate mesoderm. Development 2001, 128, 155–166. [Google Scholar] [CrossRef]
- Ghimire, S.; Van Der Jeught, M.; Neupane, J.; Roost, M.S.; Anckaert, J.; Popovic, M.; Van Nieuwerburgh, F.; Mestdagh, P.; Vandesompele, J.; Deforce, D.; et al. Comparative analysis of naive, primed and ground state pluripotency in mouse embryonic stem cells originating from the same genetic background. Sci. Rep. 2018, 8, 5884. [Google Scholar] [CrossRef]
- Barnes, R.M.; Firulli, B.A.; Conway, S.J.; Vincentz, J.W.; Firulli, A.B. Analysis of the Hand1 cell lineage reveals novel contributions to cardiovascular, neural crest, extra-embryonic, and lateral mesoderm derivatives. Dev. Dyn. 2010, 239, 3086–3097. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Cao, J.; Martin, B.K.; Li, T.; Welsh, I.C.; Srivatsan, S.; Huang, X.; Calderon, D.; Noble, W.S.; Disteche, C.M.; et al. Systematic reconstruction of cellular trajectories across mouse embryogenesis. Nat. Genet. 2022, 54, 328–341. [Google Scholar] [CrossRef]
- Xiong, J.-W. Molecular and developmental biology of the hemangioblast. Dev. Dyn. 2008, 237, 1218–1231. [Google Scholar] [CrossRef] [PubMed]
- Winnier, G.; Blessing, M.; Labosky, P.A.; Hogan, B.L. Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes Dev. 1995, 9, 2105–2116. [Google Scholar] [CrossRef]
- Fujiwara, T.; Dunn, N.R.; Hogan, B.L.M. Bone morphogenetic protein 4 in the extraembryonic mesoderm is required for allantois development and the localization and survival of primordial germ cells in the mouse. Proc. Natl. Acad. Sci. USA 2001, 98, 13739–13744. [Google Scholar] [CrossRef]
- Firulli, A.B.; McFadden, D.G.; Lin, Q.; Srivastava, D.; Olson, E.N. Heart and extra-embryonic mesodermal defects in mouse embryos lacking the bHLH transcription factor Hand1. Nat. Genet. 1998, 18, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Kelly, R.G.; Buckingham, M.E.; Moorman, A.F. Heart Fields and Cardiac Morphogenesis. Cold Spring Harb. Perspect. Med. 2014, 4, a015750. [Google Scholar] [CrossRef]
- Rossi, G.; Broguiere, N.; Miyamoto, M.; Boni, A.; Guiet, R.; Girgin, M.; Kelly, R.G.; Kwon, C.; Lutolf, M.P. Capturing Cardiogenesis in Gastruloids. Cell Stem Cell 2021, 28, 230–240.e6. [Google Scholar] [CrossRef]
- Herrmann, F.; Bundschu, K.; Kühl, S.J.; Kühl, M. Tbx5 overexpression favors a first heart field lineage in murine embryonic stem cells and in Xenopus laevis embryos. Dev. Dyn. 2011, 240, 2634–2645. [Google Scholar] [CrossRef]
- Später, D.; Abramczuk, M.K.; Buac, K.; Zangi, L.; Stachel, M.W.; Clarke, J.; Sahara, M.; Ludwig, A.; Chien, K.R. A HCN4+ cardiomyogenic progenitor derived from the first heart field and human pluripotent stem cells. Nat. Cell Biol. 2013, 15, 1098–1106. [Google Scholar] [CrossRef] [PubMed]
- Bakker, M.L.; Boukens, B.J.; Mommersteeg, M.T.; Brons, J.F.; Wakker, V.; Moorman, A.F.; Christoffels, V.M. Transcription Factor Tbx3 Is Required for the Specification of the Atrioventricular Conduction System. Circ. Res. 2008, 102, 1340–1349. [Google Scholar] [CrossRef] [PubMed]
- Kastner, P.; Krust, A.; Turcotte, B.; Stropp, U.; Tora, L.; Gronemeyer, H.; Chambon, P. Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B. EMBO J. 1990, 9, 1603–1614. [Google Scholar] [CrossRef]
- Sartorius, C.A.; Melville, M.Y.; Hovland, A.R.; Takimoto, G.S.; Horwitz, K.B.; Tung, L. A third transactivation function (AF3) of human progesterone receptors located in the unique N-terminal segment of the B-isoform. Mol. Endocrinol. 1994, 8, 1347–1360. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Pérez, J.A.; Hadjantonakis, A.-K. The Dynamics of Morphogenesis in the Early Mouse Embryo. Cold Spring Harb. Perspect. Biol. 2014, 7, a015867. [Google Scholar] [CrossRef]
- Cheng, L.; Pricolo, V.; Biancani, P.; Behar, J. Overexpression of progesterone receptor B increases sensitivity of human colon muscle cells to progesterone. Am. J. Physiol. Liver Physiol. 2008, 295, G493–G502. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Piccolo, S.; Sladitschek-Martens, H.L.; Cordenonsi, M. Mechanosignaling in vertebrate development. Dev. Biol. 2022, 488, 54–67. [Google Scholar] [CrossRef] [PubMed]
- Burroughs, C. Distribution of the neural cell adhesion molecule (NCAM) during heart development. J. Mol. Cell. Cardiol. 1991, 23, 1411–1422. [Google Scholar] [CrossRef]
- Guennoun, R. Progesterone in the Brain: Hormone, Neurosteroid and Neuroprotectant. Int. J. Mol. Sci. 2020, 21, 5271. [Google Scholar] [CrossRef] [PubMed]
- Bishop, C.V.; Hennebold, J.D.; Kahl, C.A.; Stouffer, R.L. Knockdown of Progesterone Receptor (PGR) in Macaque Granulosa Cells Disrupts Ovulation and Progesterone Production. Biol. Reprod. 2016, 94, 109. [Google Scholar] [CrossRef]
- Sánchez-Iranzo, H.; Galardi-Castilla, M.; Minguillón, C.; Sanz-Morejón, A.; González-Rosa, J.M.; Felker, A.; Ernst, A.; Guzmán-Martínez, G.; Mosimann, C.; Mercader, N. Tbx5a lineage tracing shows cardiomyocyte plasticity during zebrafish heart regeneration. Nat. Commun. 2018, 9, 428. [Google Scholar] [CrossRef] [PubMed]
- Clementi, C.; Tripurani, S.K.; Large, M.J.; Edson, M.A.; Creighton, C.J.; Hawkins, S.M.; Kovanci, E.; Kaartinen, V.; Lydon, J.P.; Pangas, S.A.; et al. Activin-Like Kinase 2 Functions in Peri-implantation Uterine Signaling in Mice and Humans. PLoS Genet. 2013, 9, e1003863. [Google Scholar] [CrossRef] [PubMed]
- Klaus, A.; Saga, Y.; Taketo, M.M.; Tzahor, E.; Birchmeier, W. Distinct roles of Wnt/β-catenin and Bmp signaling during early cardiogenesis. Proc. Natl. Acad. Sci. USA 2007, 104, 18531–18536. [Google Scholar] [CrossRef] [PubMed]
- Klaus, A.; Müller, M.; Schulz, H.; Saga, Y.; Martin, J.F.; Birchmeier, W. Wnt/β-catenin and Bmp signals control distinct sets of transcription factors in cardiac progenitor cells. Proc. Natl. Acad. Sci. USA 2012, 109, 10921–10926. [Google Scholar] [CrossRef]
- Satoh, K.; Hovey, R.C.; Malewski, T.; Warri, A.; Goldhar, A.S.; Ginsburg, E.; Saito, K.; Lydon, J.P.; Vonderhaar, B.K. Progesterone enhances branching morphogenesis in the mouse mammary gland by increased expression of Msx2. Oncogene 2007, 26, 7526–7534. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-C.; Lee, W.-S. Progesterone-Induced Migration Inhibition in Male Rat Aortic Smooth Muscle Cells Through the cSrc/AKT/ERK 2/p38 Pathway-Mediated Up-Regulation of p27. Endocrinology 2014, 155, 1428–1435. [Google Scholar] [CrossRef] [PubMed]
- Sawada, H.; Rateri, D.L.; Moorleghen, J.J.; Majesky, M.W.; Daugherty, A. Smooth Muscle Cells Derived From Second Heart Field and Cardiac Neural Crest Reside in Spatially Distinct Domains in the Media of the Ascending Aorta—Brief Report. Arter. Thromb. Vasc. Biol. 2017, 37, 1722–1726. [Google Scholar] [CrossRef]
- Lan, C.; Cao, N.; Chen, C.; Qu, S.; Fan, C.; Luo, H.; Zeng, A.; Yu, C.; Xue, Y.; Ren, H.; et al. Progesterone, via yes-associated protein, promotes cardiomyocyte proliferation and cardiac repair. Cell Prolif. 2020, 53, e12910. [Google Scholar] [CrossRef]
- Saykali, B.; Mathiah, N.; Nahaboo, W.; Racu, M.-L.; Hammou, L.; Defrance, M.; Migeotte, I. Distinct mesoderm migration phenotypes in extra-embryonic and embryonic regions of the early mouse embryo. eLife 2019, 8, e42434. [Google Scholar] [CrossRef] [PubMed]
- Hynes, R.O. Integrins: Bidirectional, Allosteric Signaling Machines. Cell 2002, 110, 673–687. [Google Scholar] [CrossRef]
- Zhu, C.; Kong, Z.; Wang, B.; Cheng, W.; Wu, A.; Meng, X. ITGB3/CD61: A hub modulator and target in the tumor microenvironment. Am. J. Transl. Res. 2019, 11, 7195–7208. [Google Scholar]
- Huang, K.; Gao, J.; Du, J.; Ma, N.; Zhu, Y.; Wu, P.; Zhang, T.; Wang, W.; Li, Y.; Chen, Q.; et al. Generation and Analysis of GATA2 w/eGFP Human ESCs Reveal ITGB3/CD61 as a Reliable Marker for Defining Hemogenic Endothelial Cells during Hematopoiesis. Stem Cell Rep. 2016, 7, 854–868. [Google Scholar] [CrossRef]
- Eckerle, S.; Ringler, M.; Lecaudey, V.; Nitschke, R.; Driever, W. Progesterone modulates microtubule dynamics and epiboly progression during zebrafish gastrulation. Dev. Biol. 2017, 434, 249–266. [Google Scholar] [CrossRef] [PubMed]
- Peavey, M.C.; Wu, S.-P.; Li, R.; Liu, J.; Emery, O.M.; Wang, T.; Zhou, L.; Wetendorf, M.; Yallampalli, C.; Gibbons, W.E.; et al. Progesterone receptor isoform B regulates the Oxtr-Plcl2-Trpc3 pathway to suppress uterine contractility. Proc. Natl. Acad. Sci. USA 2021, 118, e2011643118. [Google Scholar] [CrossRef]
- Coomarasamy, A.; Gallos, I.D.; Papadopoulou, A.; Dhillon-Smith, R.K.; Al-Memar, M.; Brewin, J.; Christiansen, O.B.; Stephenson, M.D.; Oladapo, O.T.; Wijeyaratne, C.N.; et al. Sporadic miscarriage: Evidence to provide effective care. Lancet 2021, 397, 1668–1674. [Google Scholar] [CrossRef]
- Kim, S.W.; Woo, H.-J.; Kim, E.H.; Kim, H.S.; Na Suh, H.; Kim, S.-H.; Song, J.-J.; Wulansari, N.; Kang, M.; Choi, S.-Y.; et al. Neural stem cells derived from human midbrain organoids as a stable source for treating Parkinson’s disease: Midbrain organoid-NSCs (Og-NSC) as a stable source for PD treatment. Prog. Neurobiol. 2021, 204, 102086. [Google Scholar] [CrossRef]
- Siehler, J.; Blöchinger, A.K.; Meier, M.; Lickert, H. Engineering islets from stem cells for advanced therapies of diabetes. Nat. Rev. Drug Discov. 2021, 20, 920–940. [Google Scholar] [CrossRef] [PubMed]
- Ai, X.; Yan, B.; Witman, N.; Gong, Y.; Yang, L.; Tan, Y.; Chen, Y.; Liu, M.; Lu, T.; Luo, R.; et al. Transient secretion of VEGF protein from transplanted hiPSC-CMs enhances engraftment and improves rat heart function post MI. Mol. Ther. 2022, in press. [Google Scholar] [CrossRef]
- Sugimoto, M.; Kondo, M.; Koga, Y.; Shiura, H.; Ikeda, R.; Hirose, M.; Ogura, A.; Murakami, A.; Yoshiki, A.; Chuva de Sousa Lopes, S.M.; et al. A simple and robust method for establishing homogeneous mouse epiblast stem cell lines by wnt inhibition. Stem Cell Rep. 2015, 4, 744–757. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drozd, A.M.; Mariani, L.; Guo, X.; Goitea, V.; Menezes, N.A.; Ferretti, E. Progesterone Receptor Modulates Extraembryonic Mesoderm and Cardiac Progenitor Specification during Mouse Gastrulation. Int. J. Mol. Sci. 2022, 23, 10307. https://doi.org/10.3390/ijms231810307
Drozd AM, Mariani L, Guo X, Goitea V, Menezes NA, Ferretti E. Progesterone Receptor Modulates Extraembryonic Mesoderm and Cardiac Progenitor Specification during Mouse Gastrulation. International Journal of Molecular Sciences. 2022; 23(18):10307. https://doi.org/10.3390/ijms231810307
Chicago/Turabian StyleDrozd, Anna Maria, Luca Mariani, Xiaogang Guo, Victor Goitea, Niels Alvaro Menezes, and Elisabetta Ferretti. 2022. "Progesterone Receptor Modulates Extraembryonic Mesoderm and Cardiac Progenitor Specification during Mouse Gastrulation" International Journal of Molecular Sciences 23, no. 18: 10307. https://doi.org/10.3390/ijms231810307
APA StyleDrozd, A. M., Mariani, L., Guo, X., Goitea, V., Menezes, N. A., & Ferretti, E. (2022). Progesterone Receptor Modulates Extraembryonic Mesoderm and Cardiac Progenitor Specification during Mouse Gastrulation. International Journal of Molecular Sciences, 23(18), 10307. https://doi.org/10.3390/ijms231810307





