Th17, Th22, and Myeloid-Derived Suppressor Cell Population Dynamics and Response to IL-6 in 4T1 Mammary Carcinoma
Abstract
1. Introduction
2. Results
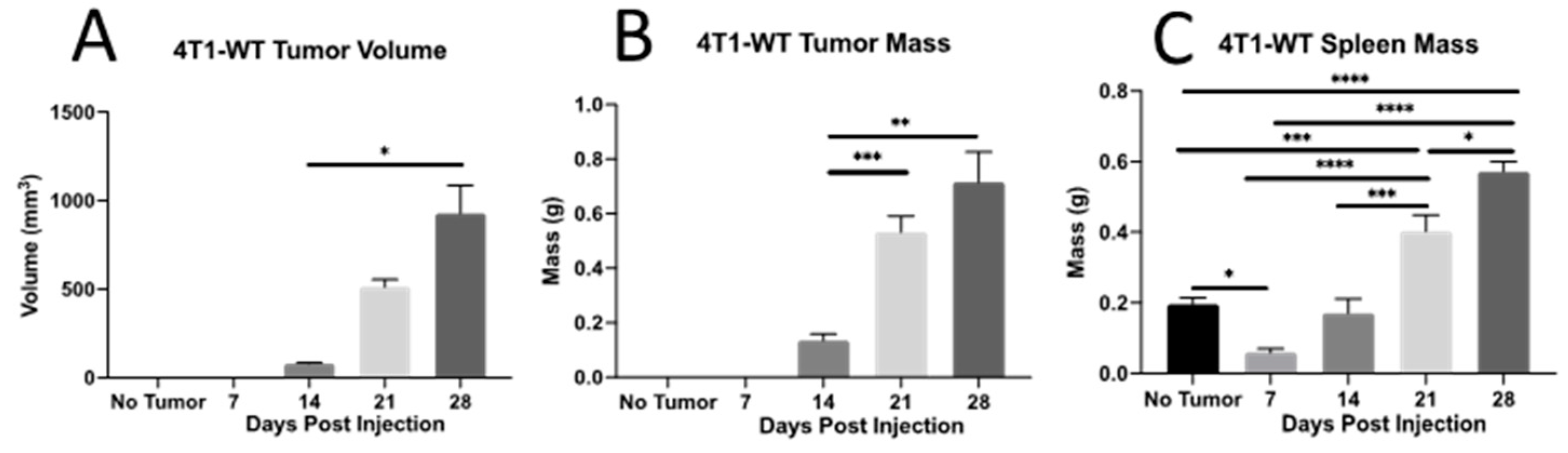
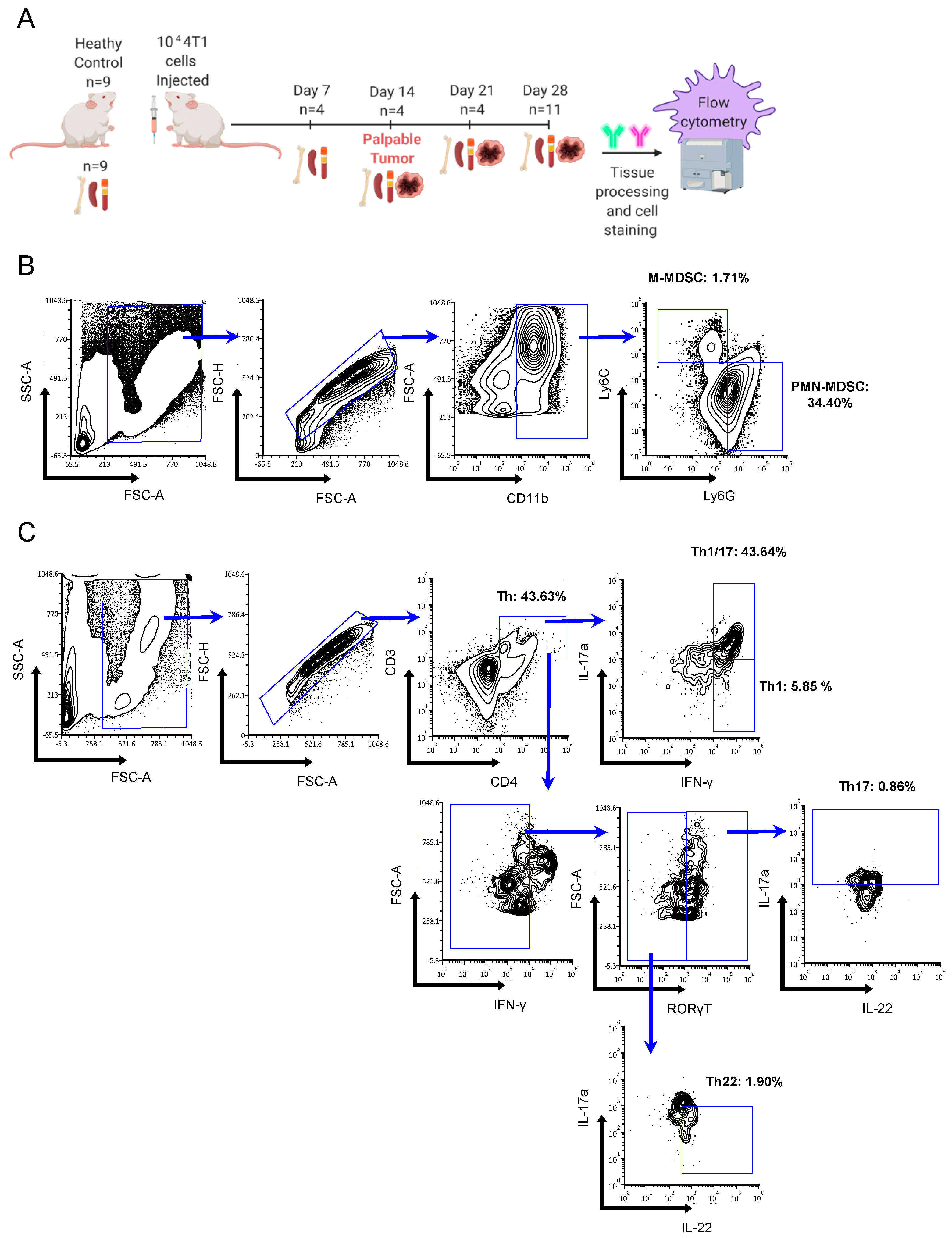
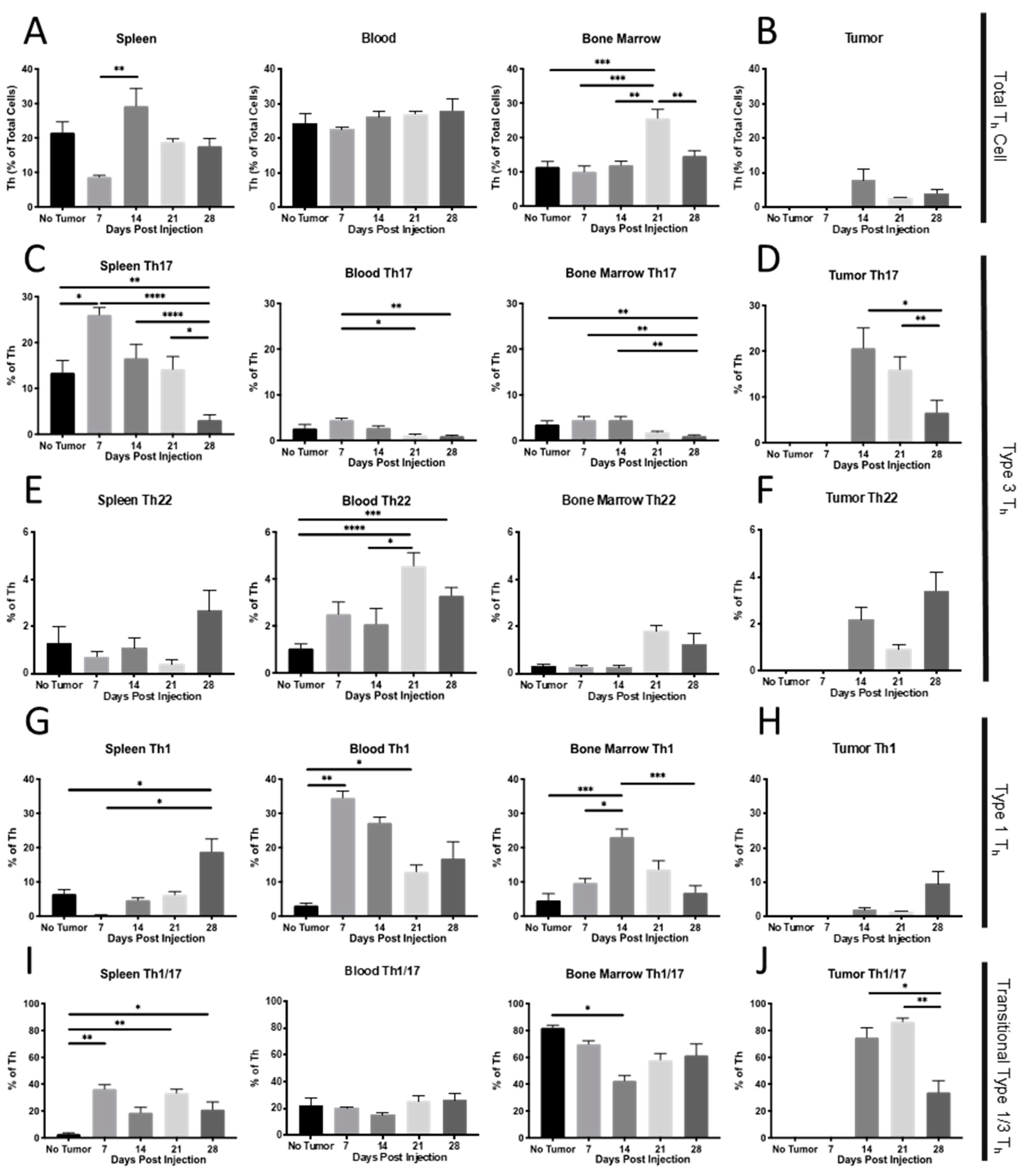
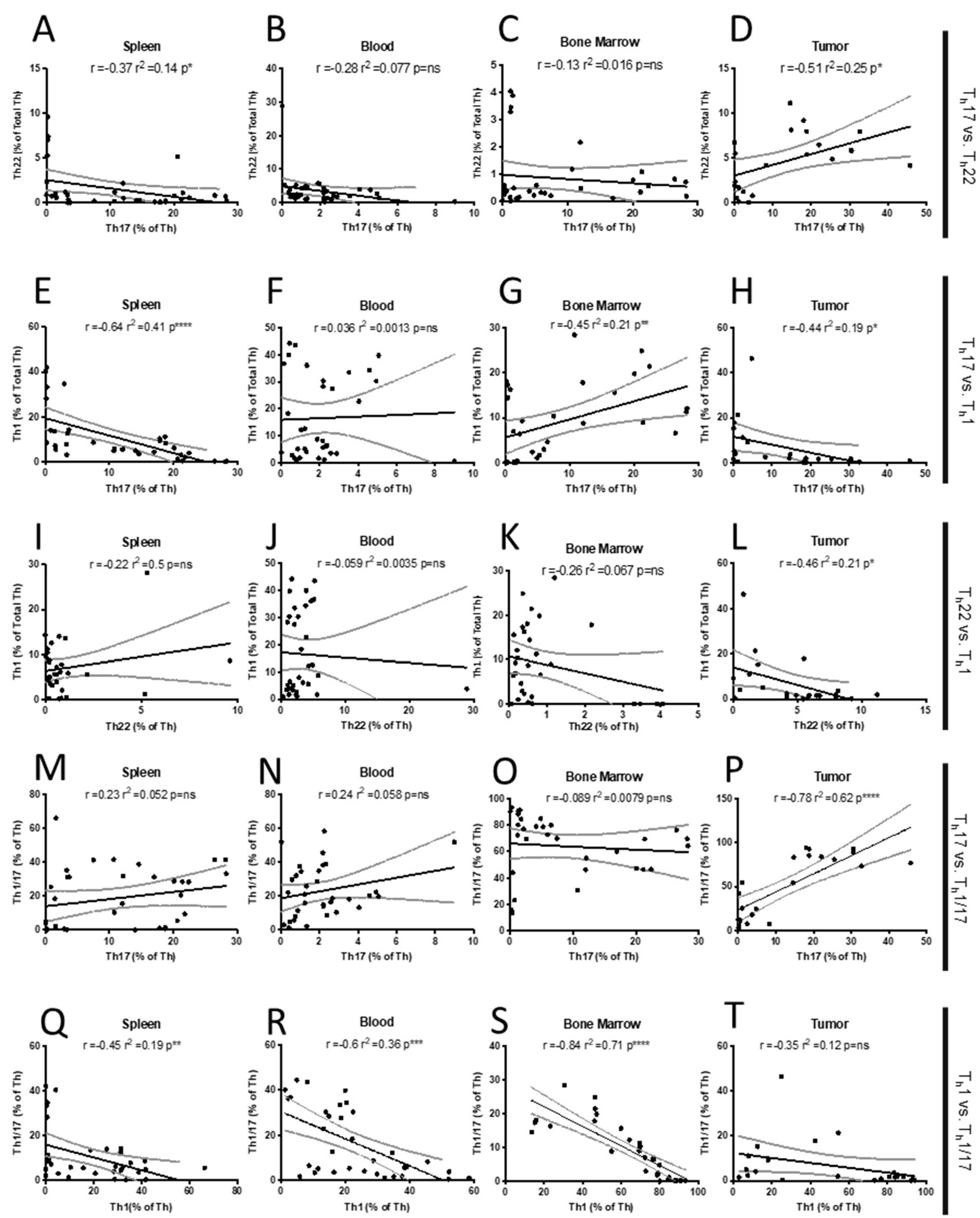
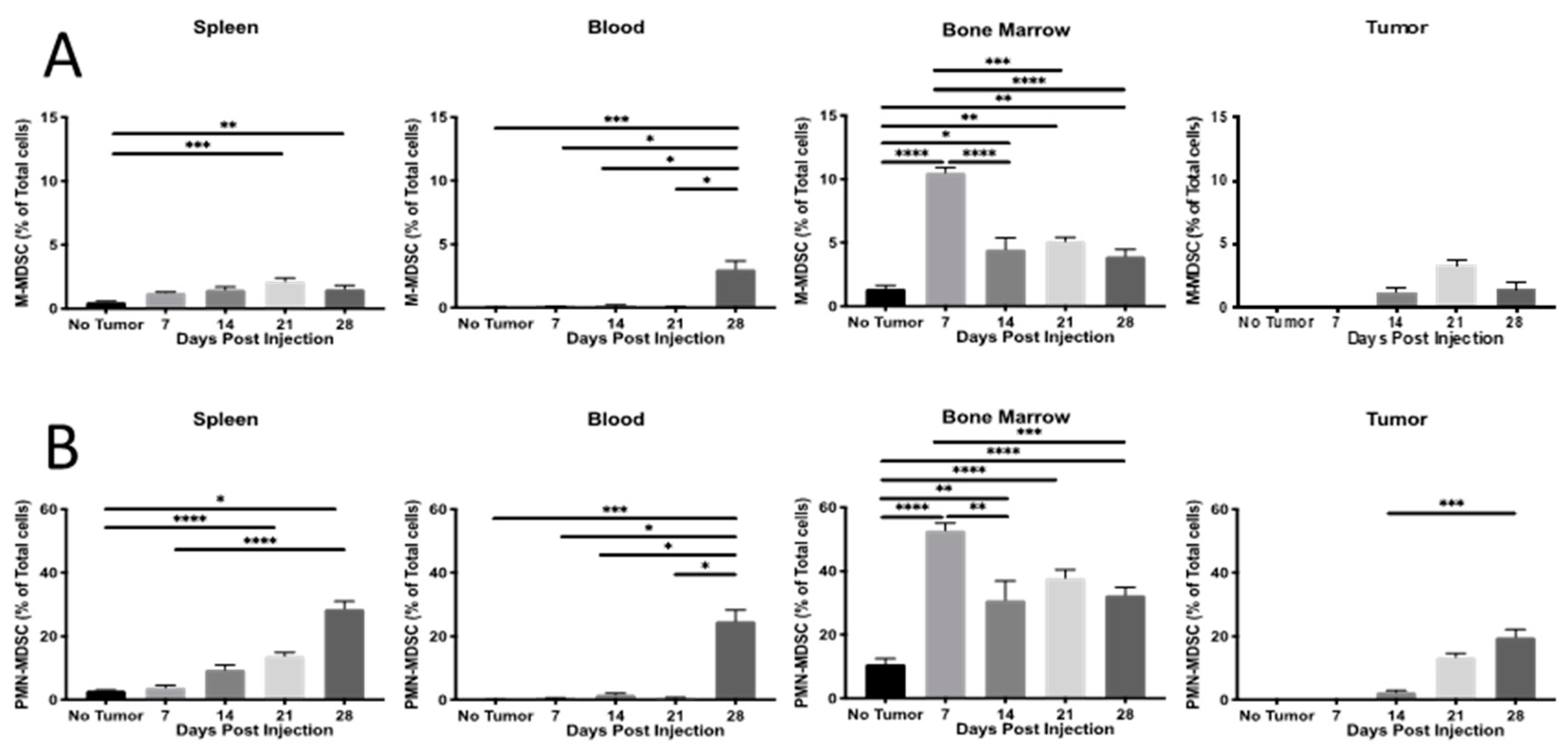
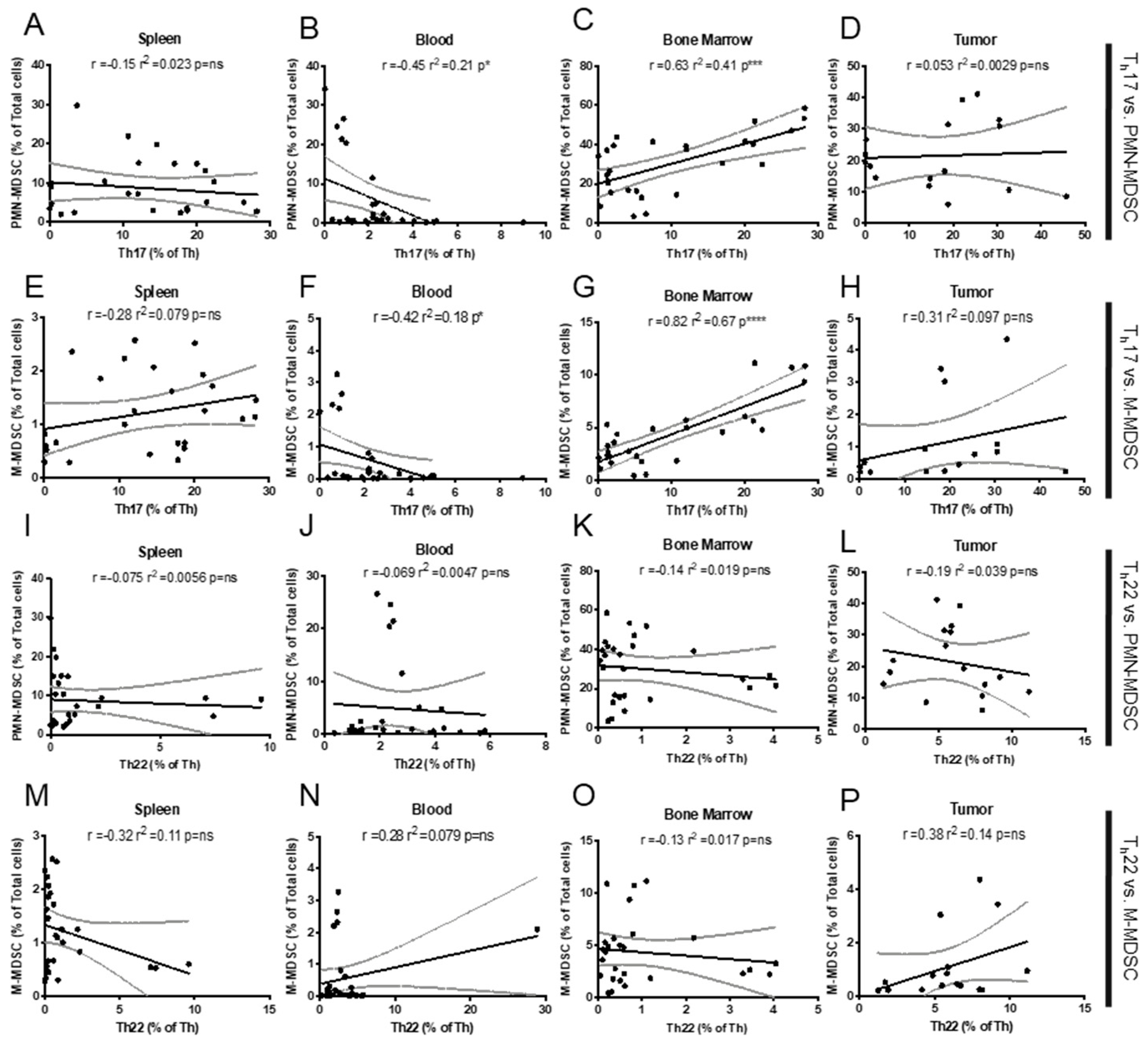
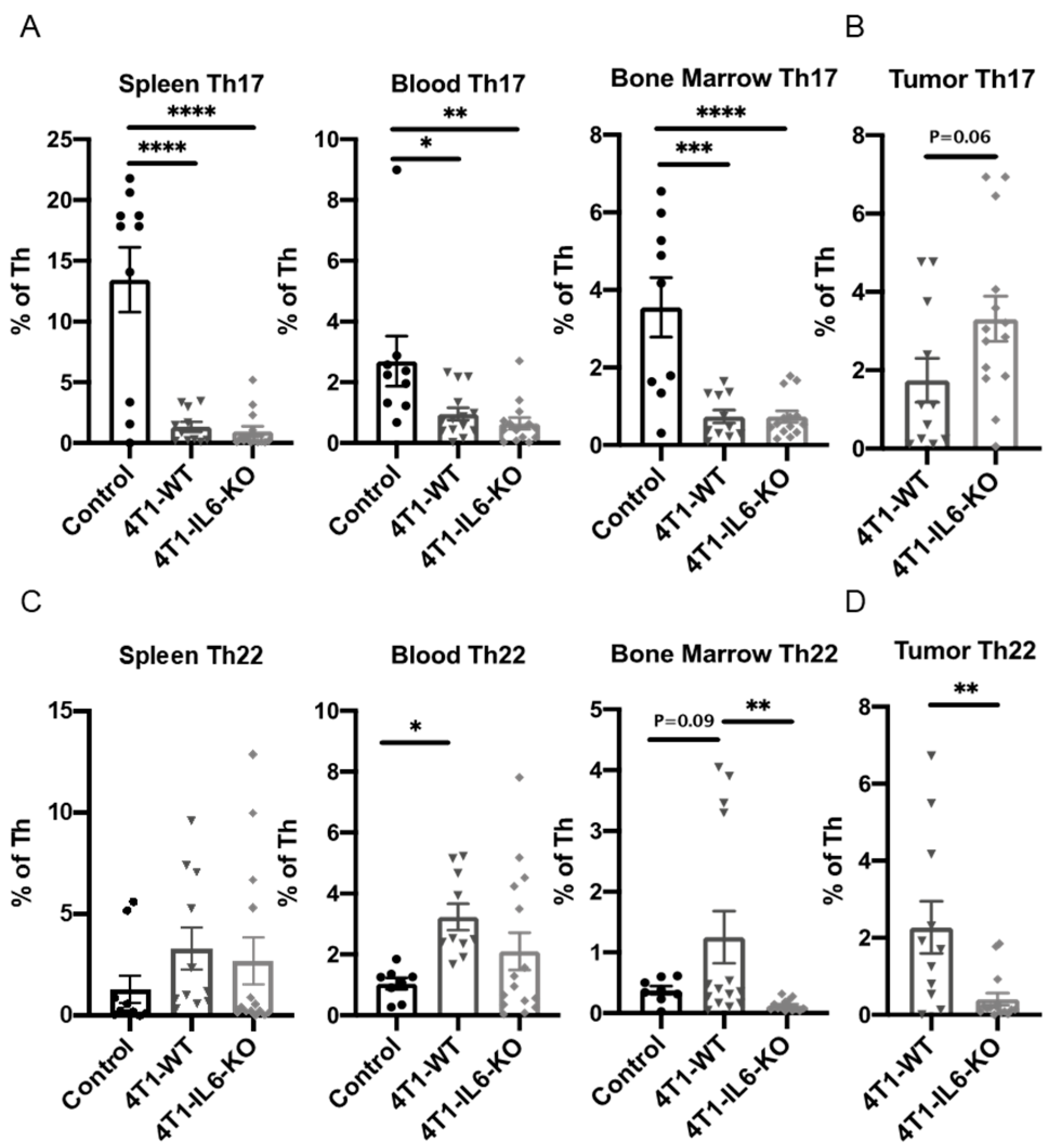
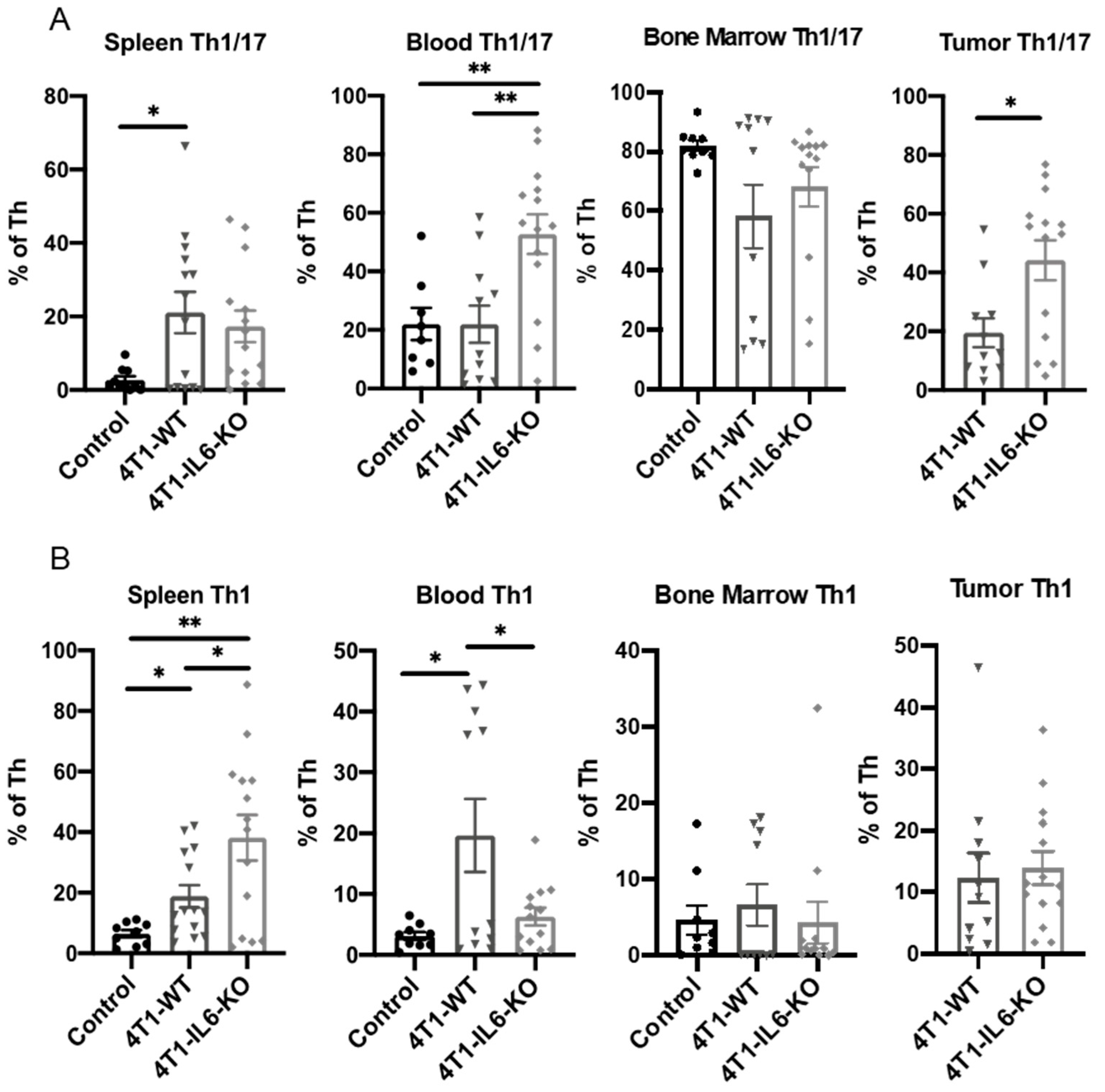
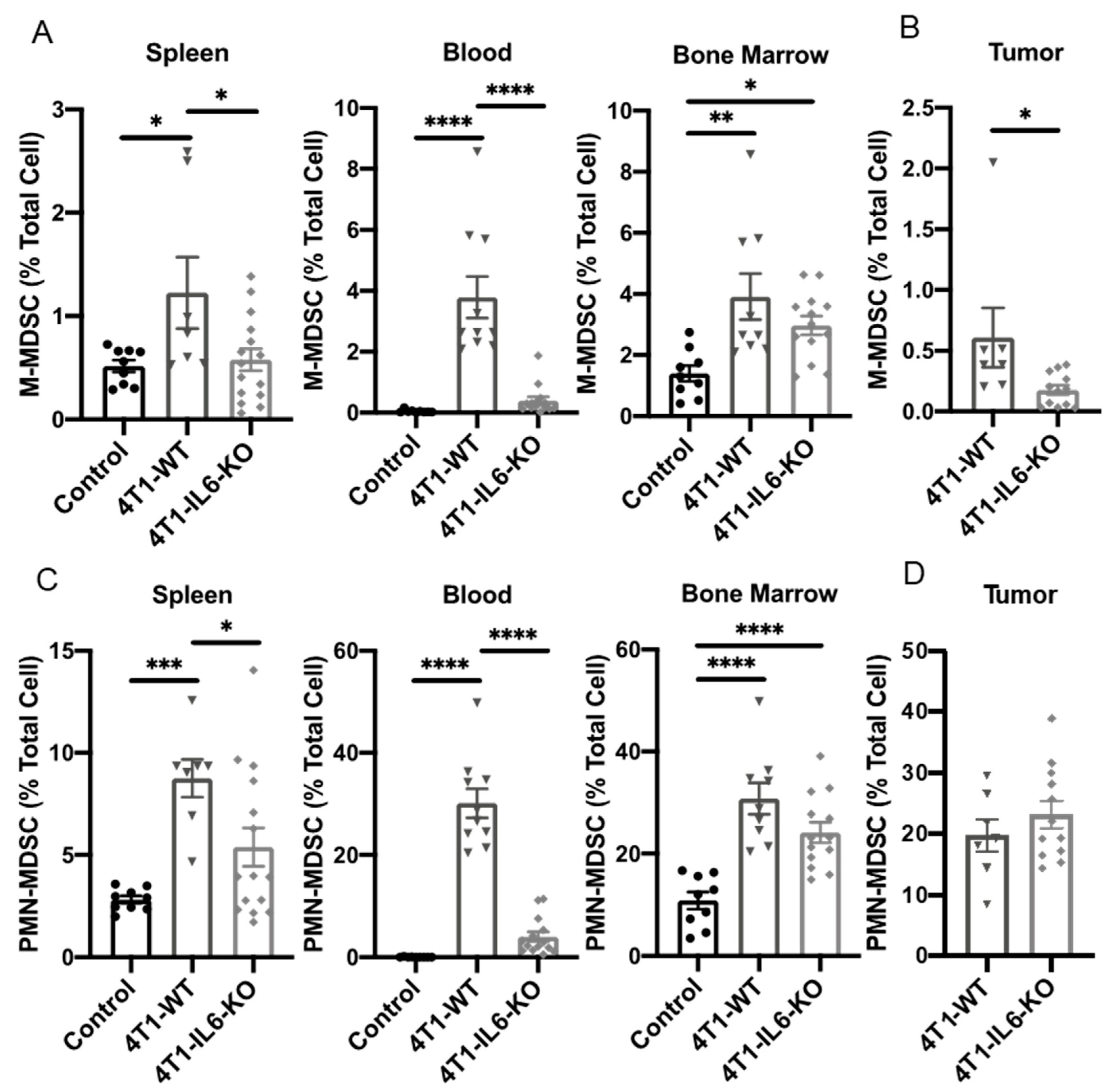
2.1. Th Populations in Response to 4T1-WT Tumors
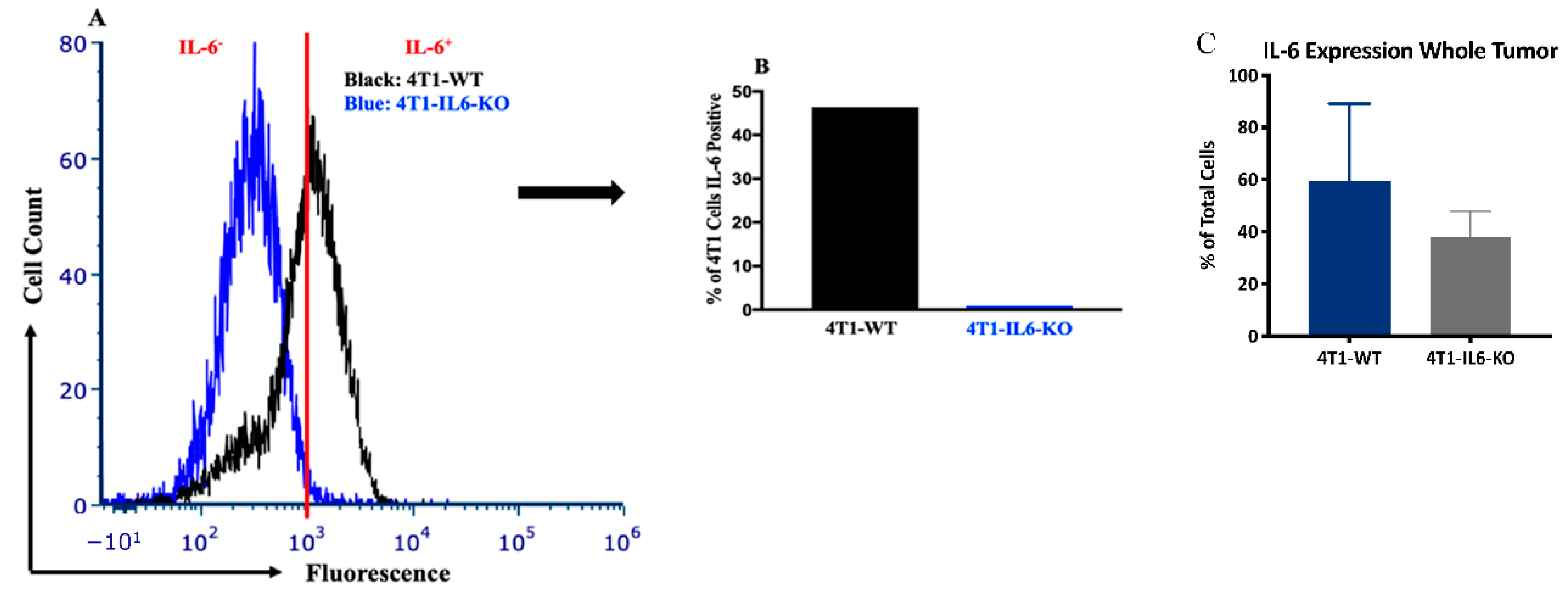
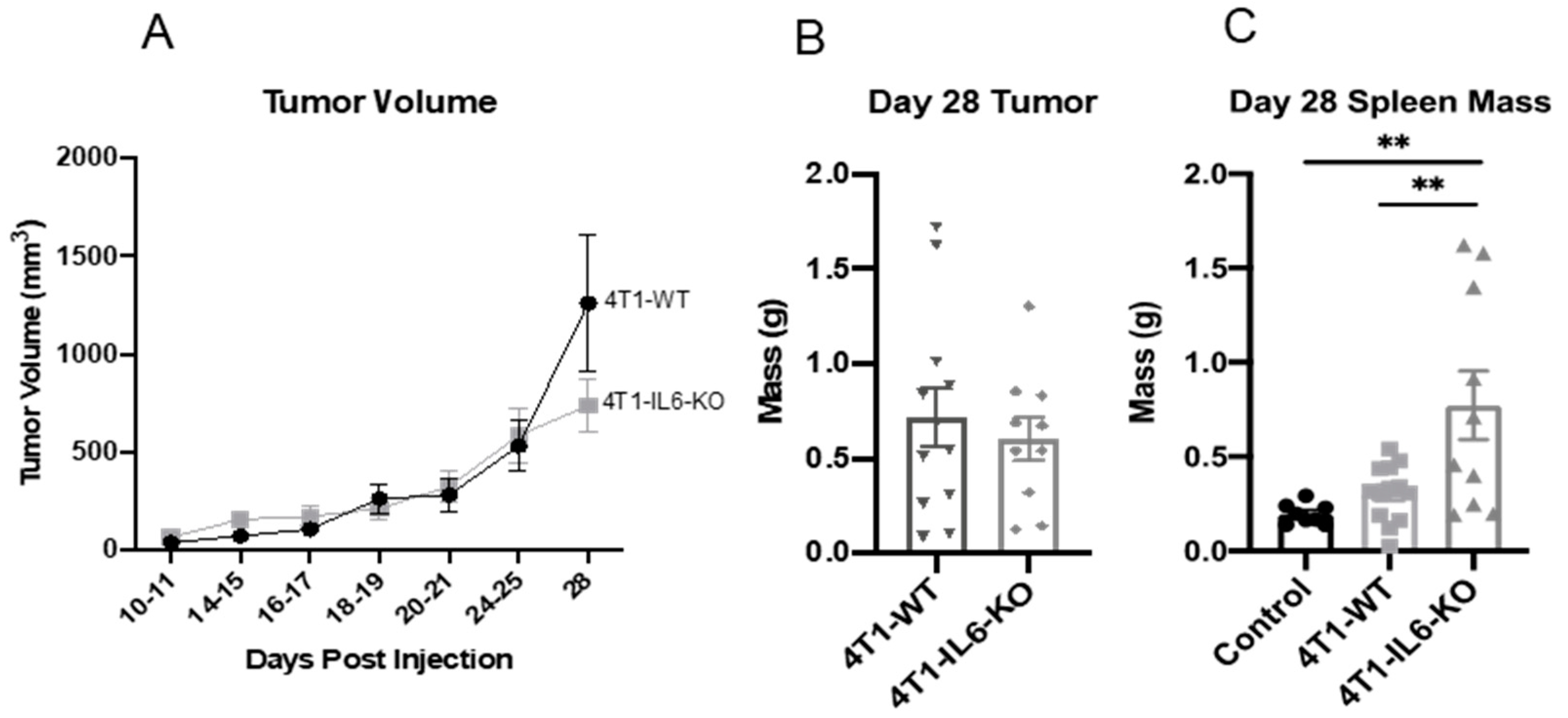
2.2. Tumor IL-6 Knock Out Effects on Type 3 Th Cell Recruitment
3. Discussion
4. Methods and Materials
4.1. Mice and 4T1 Mammary Tumors
4.2. il6 Gene Knock-Out in 4T1 Cells
4.3. Tissue Processing
4.4. Flow Cytometry and Antibodies
4.5. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CTLA-4 | cytotoxic T-lymphocyte-associated protein 4 |
| G-CSF | granulocyte colony-stimulating factor |
| IFN | interferon |
| IL | interleukin |
| M-CSF | macrophage colony-stimulating factor |
| MDSC | myeloid-derived suppressor cell |
| MHS | major histocompatibility complex |
| M-MDSC | mononuclear MDSC |
| PD-1/PD-L1 | programmed cell death protein 1/programmed death ligand 1 |
| PMN-MDSC | polymorphonuclear MDSC |
| TGF | transforming growth factor |
| Th | T helper lymphocyte |
| TIL | Tumor-infiltrating lymphocyte |
References
- Ansell, S.M.; Lesokhin, A.M.; Borrello, I.; Halwani, A.; Scott, E.C.; Gutierrez, M.; Schuster, S.J.; Millenson, M.M.; Cattry, D.; Freeman, G.J.; et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N. Engl. J. Med. 2015, 372, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S.; Soria, J.-C.; Kowanetz, M.; Fine, G.D.; Hamid, O.; Gordon, M.S.; Sosman, J.A.; McDermott, D.F.; Powderly, J.D.; Gettinger, S.N.; et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014, 515, 563–567. [Google Scholar] [CrossRef]
- Topalian, S.L.; Taube, J.M.; Anders, R.A.; Pardoll, D.M. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat. Cancer 2016, 16, 275–287. [Google Scholar] [CrossRef]
- Hamid, O.; Robert, C.; Daud, A.; Hodi, F.S.; Hwu, W.J.; Kefford, R.; Wolchok, J.D.; Hersey, P.; Joseph, R.; Weber, J.S.; et al. Five-year survival outcomes for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2019, 30, 582–588. [Google Scholar] [CrossRef]
- Tan, D.S.W. Changing the natural history of non–small-cell lung cancer through upfront programmed death protein 1/programmed death-ligand 1 blockade. J. Clin. Oncol. 2017, 35, 2735–2736. [Google Scholar] [CrossRef]
- Kanjanapan, Y.; Day, D.; Wang, L.; Al-Sawaihey, H.; Abbas, E.; Namini, A.; Siu, L.L.; Hansen, A.; Razak, A.A.; Spreafico, A.; et al. Hyperprogressive disease in early-phase immunotherapy trials: Clinical predictors and association with immune-related toxicities. Cancer 2019, 125, 1341–1349. [Google Scholar] [CrossRef]
- Frelaut, M.; Le Tourneau, C.; Borcoman, E. Hyperprogression under immunotherapy. Int. J. Mol. Sci. 2019, 20, 2674. [Google Scholar] [CrossRef] [PubMed]
- Sama, S.; Hadfield, M.J.; Lia, N.L.; Vredenburgh, J. Hyperprogression in PDL1 expressive, recurrent gastroesophageal-junction adenocarcinoma after pembrolizumab. Cureus 2019, 11, e4862. [Google Scholar] [CrossRef]
- Champiat, S.; Dercle, L.; Ammari, S.; Massard, C.; Hollebecque, A.; Postel-Vinay, S.; Chaput, N.; Eggermont, A.; Marabelle, A.; Soria, J.C.; et al. Hyperprogressive disease is a new pattern of progression in cancer patients treated by anti-PD-1/PD-L1. Clin. Cancer Res. 2017, 23, 1920–1928. [Google Scholar] [PubMed]
- Saâda-Bouzid, E.; Defaucheux, C.; Karabajakian, A.; Coloma, V.P.; Servois, V.; Paoletti, X.; Even, C.; Fayette, J.; Guigay, J.; Loirat, D.; et al. Hyperprogression during anti-PD-1/PD-L1 therapy in patients with recurrent and/or metastatic head and neck squamous cell carcinoma. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2017, 28, 1605–1611. [Google Scholar] [CrossRef] [PubMed]
- Swoboda, A.; Nanda, R. Immune checkpoint blockade for breast cancer. Cancer Treat. Res. 2018, 173, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.; Hellstrom, I.; Yip, Y.Y.; Sjögren, H.O.; Hellstrom, K.E. Tumor regression and cure depends on sustained Th1 responses. J. Immunother. 2018, 41, 369–378. [Google Scholar] [CrossRef]
- Castro, F.; Cardoso, A.P.; Gonçalves, R.M.; Serre, K.; Oliveira, M.J. Interferon-gamma at the crossroads of tumor immune surveillance or evasion. Front. Immunol. 2018, 9, 847. [Google Scholar] [CrossRef]
- Coffelt, S.B.; de Visser, K.E. Immune-mediated mechanisms influencing the efficacy of anticancer therapies. Trends Immunol. 2015, 36, 198–216. [Google Scholar]
- Capasso, A.; Lang, J.; Pitts, T.M.; Jordan, K.R.; Lieu, C.H.; Davis, S.L.; Diamond, J.R.; Kopetz, S.; Barbee, J.; Peterson, J.; et al. Characterization of immune responses to anti-PD-1 mono and combination immunotherapy in hematopoietic humanized mice implanted with tumor xenografts. J. Immunother. Cancer 2019, 7, 37. [Google Scholar] [CrossRef] [PubMed]
- Jiao, S.; Subudhi, S.K.; Aparicio, A.; Ge, Z.; Guan, B.; Miura, Y.; Sharma, P. Differences in tumor microenvironment dictate t helper lineage polarization and response to immune checkpoint therapy. Cell 2019, 179, 1177–1190.e13. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Zhang, X.; Liu, Z.; Xu, H.; Wang, T.; He, L.; Zhao, A. Granulocytic myeloid-derived suppressor cells maintain feto-maternal tolerance by inducing Foxp3 expression in CD4 + CD25 − T cells by activation of the TGF-β/β-catenin pathway. Mol. Hum. Reprod. 2016, 22, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Alspach, E.; Lussier, D.M.; Miceli, A.P.; Kizhvatov, I.; DuPage, M.; Luoma, A.M.; Meng, W.; Lichti, C.F.; Esaulova, E.; Vomund, A.N.; et al. MHC-II neoantigens shape tumour immunity and response to immunotherapy. Nature 2019, 574, 696–701. [Google Scholar] [CrossRef] [PubMed]
- Bianco, T.M.; Abdalla, D.R.; Desidério, C.S.; Thys, S.; Simoens, C.; Bogers, J.-P.; Murta, E.F.; Michelin, M.A. The influence of physical activity in the anti-tumor immune response in experimental breast tumor. Immunol. Lett. 2017, 190, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Qin, J.; Zhong, L.; Gong, L.; Zhang, B.; Zhang, Y.; Gao, W.-Q. CCL5-Mediated Th2 immune polarization promotes metastasis in luminal breast cancer. Cancer Res. 2015, 75, 4312–4321. [Google Scholar] [CrossRef]
- Kidd, P. Th1/Th2 balance: The hypothesis, its limitations, and implications for health and disease. Altern. Med. Rev. 2003, 8, 223–246. [Google Scholar] [PubMed]
- Wu, H.; Zhen, Y.; Ma, Z.; Li, H.; Yu, J.; Xu, Z.-G.; Wang, X.-Y.; Yi, H.; Yang, Y.-G. Arginase-1–dependent promotion of Th17 differentiation and disease progression by MDSCs in systemic lupus erythematosus. Sci. Transl. Med. 2016, 8, 331ra40. [Google Scholar] [CrossRef] [PubMed]
- Gillentine, M.A.; Berry, L.N.; Goin-Kochel, R.P.; Ali, M.A.; Ge, J.; Guffey, D.; Rosenfeld, J.A.; Hannig, V.; Bader, P.; Proud, M.; et al. Myeloid-derived suppressor cells have a proinflammatory role in the pathogenesis of autoimmune arthritis. J. Autism. Dev. Disord. 2017, 47, 549–562. [Google Scholar] [CrossRef]
- Glenn, J.D.; Liu, C.; Whartenby, K.A. Induction of experimental autoimmune encephalomyelitis mobilizes Th17-promoting myeloid derived suppressor cells to the lung. J. Leukoc. Biol. 2019, 105, 829–841. [Google Scholar]
- Lim, C.; Savan, R. The role of the IL-22/IL-22R1 axis in cancer. Cytokine Growth Factor Rev. 2014, 25, 257–271. [Google Scholar] [PubMed]
- Zhang, W.; Tian, X.; Mumtahana, F.; Jiao, J.; Zhang, T.; Della Croce, K.; Ma, D.; Kong, B.; Cui, B. The existence of Th22, pure Th17 and Th1 cells in CIN and Cervical Cancer along with their frequency variation in different stages of cervical cancer. BMC Cancer 2015, 15, 717. [Google Scholar] [CrossRef] [PubMed]
- Housseau, F.; Geis, A.; Thiele-Orberg, E.; Llosa, N. Interleukin-17 and type 17 helper T cells in cancer management and research. ImmunoTargets Ther. 2014, 3, 39–54. [Google Scholar] [CrossRef][Green Version]
- Zhang, Y.; Liu, C.; Gao, J.; Shao, S.; Cui, Y.; Yin, S.; Pan, B. IL-22 promotes tumor growth of breast cancer cells in mice. Aging 2020, 12, 13354–13364. [Google Scholar] [CrossRef]
- Zhuang, Y.; Cheng, P.; Liu, X.-F.; Peng, L.-S.; Li, B.-S.; Wang, T.-T.; Chen, N.; Li, W.-H.; Shi, Y.; Chen, W.; et al. A pro-inflammatory role for Th22 cells in Helicobacter pylori-associated gastritis. Gut 2014, 64, 1368–1378. [Google Scholar] [CrossRef]
- Wegner, A.; Verhagen, J.; Wraith, D.C. Myeloid-derived suppressor cells mediate tolerance induction in autoimmune disease. Immunology 2017, 151, 26–42. [Google Scholar] [CrossRef]
- Jayakumar, A.; Bothwell, A.L.M. Functional diversity of myeloid-derived suppressor cells: The multitasking hydra of cancer. J. Immunol. 2019, 203, 1095–1103. [Google Scholar] [CrossRef]
- Veglia, F.; Perego, M.; Gabrilovich, D. Myeloid-derived suppressor cells coming of age review-article. Nat. Immunol. 2018, 19, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Plank, M.W.; Kaiko, G.E.; Maltby, S.; Weaver, J.; Tay, H.L.; Shen, W.; Wilson, M.S.; Durum, S.K.; Foster, P.S. Th22 Cells form a distinct TH lineage from th17 cells in vitro with unique transcriptional properties and TBET-dependent th1 plasticity. J. Immunol. 2017, 198, 2182–2190. [Google Scholar] [CrossRef]
- Eyerich, K.; DiMartino, V.; Cavani, A. IL-17 and IL-22 in immunity: Driving protection and pathology. Eur. J. Immunol. 2017, 47, 607–614. [Google Scholar] [CrossRef]
- Zenewicz, L.A. IL-22: There Is a Gap in Our Knowledge. ImmunoHorizons 2018, 2, 198–207. [Google Scholar] [CrossRef]
- Vitiello, G.A.; Miller, G. Targeting the interleukin-17 immune axis for cancer immunotherapy. J. Exp. Med. 2019, 217, e20190456. [Google Scholar] [CrossRef]
- Nam, J.-S.; Terabe, M.; Kang, M.-J.; Chae, H.; Voong, N.; Yang, Y.-A.; Laurence, A.; Michalowska, A.; Mamura, M.; Lonning, S.; et al. Transforming growth factor β subverts the immune system into directly promoting tumor growth through interleukin-17. Cancer Res. 2008, 68, 3915–3923. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Chen, J.; Zhang, W.; Zhang, R.; Ye, Y.; Liu, P.; Yu, W.; Wei, F.; Ren, X.; Yu, J. Interleukin-6 trans-signaling pathway promotes immunosuppressive myeloid-derived suppressor cells via suppression of suppressor of cytokine signaling 3 in breast cancer. Front. Immunol. 2017, 8, 1840. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Daenthanasanmak, A.; Chakraborty, P.; Wyatt, M.; Dhar, P.; Selvam, S.P.; Fu, J.; Zhang, J.; Nguyen, H.; Kang, I.; et al. CD38-NAD+Axis regulates immunotherapeutic anti-tumor T cell response. Cell Metab. 2018, 27, 85–100.e8. [Google Scholar] [CrossRef]
- Grasselly, C.; Denis, M.; Bourguignon, A.; Talhi, N.; Mathe, D.; Tourette, A.; Serre, L.; Jordheim, L.P.; Matera, E.L.; Dumontet, C. The antitumor activity of combinations of cytotoxic chemotherapy and immune checkpoint inhibitors is model-dependent. Front. Immunol. 2018, 9, 2100. [Google Scholar] [CrossRef]
- Castle, J.C.; Loewer, M.; Boegel, S.; Tadmor, A.D.; Boisguerin, V.; De Graaf, J.; Paret, C.; Diken, M.; Kreiter, S.; Türeci, Ö.; et al. Mutated tumor alleles are expressed according to their DNA frequency. Sci. Rep. 2014, 4, 4743. [Google Scholar] [CrossRef]
- Bunt, S.K.; Yang, L.; Sinha, P.; Clements, V.K.; Leips, J.; Ostrand-Rosenberg, S. Reduced inflammation in the tumor microenvironment delays the accumulation of myeloid-derived suppressor cells and limits tumor progression. Cancer Res. 2007, 67, 10019–10026. [Google Scholar] [CrossRef]
- Talmadge, J.E.; Gabrilovich, D.I. History of myeloid-derived suppressor cells. Nat. Cancer 2013, 13, 739–752. [Google Scholar] [CrossRef] [PubMed]
- Dupre’, S.A.; Hunter, K.W. Murine mammary carcinoma 4T1 induces a leukemoid reaction with splenomegaly: Association with tumor-derived growth factors. Exp. Mol. Pathol. 2007, 82, 12–24. [Google Scholar] [CrossRef]
- Harbour, S.N.; Maynard, C.L.; Zindl, C.L.; Schoeb, T.R.; Weaver, C.T. Th17 cells give rise to Th1 cells that are required for the pathogenesis of colitis. Proc. Natl. Acad. Sci. USA 2015, 112, 7061–7066. [Google Scholar] [CrossRef]
- Bosiljcic, M.; Cederberg, R.A.; Hamilton, M.J.; LePard, N.E.; Harbourne, B.T.; Collier, J.L.; Halvorsen, E.C.; Shi, R.; Franks, S.E.; Kim, A.Y.; et al. Targeting myeloid-derived suppressor cells in combination with primary mammary tumor resection reduces metastatic growth in the lungs. Breast Cancer Res. 2019, 21, 103. [Google Scholar] [CrossRef] [PubMed]
- Chang, Q.; Bournazou, E.; Sansone, P.; Berishaj, M.; Gao, S.P.; Daly, L.; Wels, J.; Theilen, T.; Granitto, S.; Zhang, X.; et al. The IL-6/JAK/Stat3 Feed-forward loop drives tumorigenesis and metastasis. Neoplasia 2013, 15, 848–862, IN40–IN45. [Google Scholar] [CrossRef]
- Mühl, H.; Bachmann, M.; Pfeilschifter, J. Inducible NO synthase and antibacterial host defence in times of Th17/Th22/T22 immunity. Cell. Microbiol. 2011, 13, 340–348. [Google Scholar] [CrossRef]
- De Henau, O.; Rausch, M.; Winkler, D.; Campesato, L.F.; Liu, C.; Cymerman, D.H.; Budhu, S.; Ghosh, A.; Pink, M.; Tchaicha, J.; et al. Overcoming resistance to checkpoint blockade therapy by targeting PI3Kγ in myeloid cells. Nature 2016, 539, 443–447. [Google Scholar] [CrossRef]
- Mandal, P.K.; Biswas, S.; Mandal, G.; Purohit, S.; Gupta, A.; (Giri), A.M.; Chowdhury, S.R.; Bhattacharyya, A. CCL2 conditionally determines CCL22-dependent Th2-accumulation during TGF-β-induced breast cancer progression. Immunobiology 2018, 223, 151–161. [Google Scholar] [CrossRef]
- Annunziato, F.; Romagnani, C.; Romagnani, S. The 3 major types of innate and adaptive cell-mediated effector immunity. J. Allergy Clin. Immunol. 2015, 135, 626–635. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhang, Z.; Xing, H.; Wang, L.; Zhang, G.; Yu, N.; Wang, J.; Guo, W.; Jiang, J. Elevated Th22 cells and related cytokines in patients with epithelial ovarian cancer. Medicine 2017, 96, e8359. [Google Scholar] [CrossRef]
- Wang, S.; Yao, Y.; Yao, M.; Fu, P.; Wang, W. Interleukin-22 promotes triple negative breast cancer cells migration and paclitaxel resistance through JAK-STAT3/MAPKs/AKT signaling pathways. Biochem. Biophys. Res. Commun. 2018, 503, 1605–1609. [Google Scholar] [CrossRef] [PubMed]
- Arnold, K.M.; Opdenaker, L.M.; Flynn, D.; Sims-Mourtada, J. Wound healing and cancer stem cells: Inflammation as a driver of treatment resistance in breast cancer. Cancer Growth Metastasis 2015, 8, CGM.S11286-13. [Google Scholar] [CrossRef]
- Bronte, V.; Brandau, S.; Chen, S.-H.; Colombo, M.P.; Frey, A.B.; Greten, T.F.; Mandruzzato, S.; Murray, P.J.; Ochoa, A.; Ostrand-Rosenberg, S.; et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat. Commun. 2016, 7, 12150. [Google Scholar] [CrossRef]
- Ostrand-Rosenberg, S.; Sinha, P.; Figley, C.; Long, R.; Park, D.; Carter, D.; Clements, V.K. Frontline science: Myeloid-derived suppressor cells (MDSCs) facilitate maternal–fetal tolerance in mice. J. Leukoc. Biol. 2016, 101, 1091–1101. [Google Scholar] [CrossRef]
- Gabrilovich, D.I. Myeloid-derived suppressor cells. Cancer Immunol. Res. 2017, 5, 3–8. [Google Scholar] [CrossRef]
- Ortiz, M.L.; Kumar, V.; Martner, A.; Mony, S.; Donthireddy, L.; Condamine, T.; Seykora, J.; Knight, S.C.; Malietzis, G.; Lee, G.H.; et al. Immature myeloid cells directly contribute to skin tumor development by recruiting IL-17–producing CD4+ T cells. J. Exp. Med. 2015, 212, 351–367. [Google Scholar] [CrossRef] [PubMed]
- Garritson, J.; Krynski, L.; Haverbeck, L.; Haughian, J.M.; Pullen, N.A.; Hayward, R. Physical activity delays accumulation of immunosuppressive myeloid-derived suppressor cells. PLoS ONE 2020, 15, e0234548. [Google Scholar] [CrossRef] [PubMed]
- Guéry, L.; Hugues, S. Th17 cell plasticity and functions in cancer immunity. BioMed Res. Int. 2015, 2015, 314620. [Google Scholar] [CrossRef]
- Fisher, D.T.; Appenheimer, M.M.; Evans, S.S. The two faces of IL-6 in the tumor microenvironment. Semin. Immunol. 2014, 26, 38–47. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, M.; Chen, J.; Zhang, R.; Ye, Y.; Liu, P.; Yu, W.; Yu, J. SOCS3 suppression promoted the recruitment of CD11b+Gr-1-F4/80-MHCII- early-stage myeloid-derived suppressor cells and accelerated interleukin-6-related tumor invasion via affecting myeloid differentiation in breast cancer. Front. Immunol. 2018, 9, 1699. [Google Scholar] [CrossRef] [PubMed]
- Actemra (Tocilizumab) for Intravenous Infusion. Genentech. January 2010. Available online: http://www.gene.com/gene/products/information/actemra/pdf/pi.pdf (accessed on 1 July 2022).
- Sitenga, J.; Aird, G.; Ahmed, A.; Silberstein, P.T. Impact of siltuximab on patient-related outcomes in multicentric Castleman’s disease. Patient Relat. Outcome Meas. 2018, 9, 35–41. [Google Scholar] [CrossRef]
- Zhuang, Y.; Peng, L.-S.; Zhao, Y.-L.; Shi, Y.; Mao, X.-H.; Guo, G.; Chen, W.; Liu, X.-F.; Zhang, J.-Y.; Liu, T.; et al. Increased intratumoral IL-22-producing CD4+ T cells and Th22 cells correlate with gastric cancer progression and predict poor patient survival. Cancer Immunol. Immunother. 2012, 61, 1965–1975. [Google Scholar] [CrossRef]
- Wu, X.; Tian, J.; Wang, S. Insight into non-pathogenic Th17 Cells in autoimmune diseases. Front. Immunol. 2018, 9, 1112. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Sun, X.; Oh, S.F.; Wu, M.; Zhang, Y.; Zheng, W.; Geva-Zatorsky, N.; Jupp, R.; Mathis, D.; Benoist, C.; et al. Microbial bile acid metabolites modulate gut RORγ+ regulatory T cell homeostasis. Nature 2020, 577, 410–415. [Google Scholar] [CrossRef]
- Anani, W.; Shurin, M.R. Targeting myeloid-derived suppressor cells in cancer. In Advances in Experimental Medicine and Biology; Springer New York LLC.: New York, NY, USA, 2017; Volume 1036, pp. 105–128. [Google Scholar]
- Luker, A.J.; Graham, L.J.; Smith, T.M.; Camarena, C.; Zellner, M.P.; Gilmer, J.-J.S.; Damle, S.R.; Conrad, D.H.; Bear, H.D.; Martin, R.K. The DNA methyltransferase inhibitor, guadecitabine, targets tumor-induced myelopoiesis and recovers T cell activity to slow tumor growth in combination with adoptive immunotherapy in a mouse model of breast cancer. BMC Immunol. 2020, 21, 8. [Google Scholar] [CrossRef]
- Faustino-Rocha, A.; Oliveira, P.A.; Pinho-Oliveira, J.; Teixeira-Guedes, C.; Soares-Maia, R.; da Costa, R.G.; Colaço, B.; Pires, M.J.; Colaço, J.; Ferreira, R.; et al. Estimation of rat mammary tumor volume using caliper and ultrasonography measurements. Lab. Anim. 2013, 42, 217–224. [Google Scholar] [CrossRef]
- Cong, L.; Ran, F.A.; Cox, D.; Lin, S.; Barretto, R.; Habib, N.; Hsu, P.D.; Wu, X.; Jiang, W.; Marraffini, L.A.; et al. Multiplex genome engineering using CRISPR/Cas systems. Science 2013, 339, 819–823. [Google Scholar] [CrossRef]
- Kasuya, E. On the use of r and r squared in correlation and regression. Ecol. Res. 2019, 34, 235–236. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rasé, V.J.; Hayward, R.; Haughian, J.M.; Pullen, N.A. Th17, Th22, and Myeloid-Derived Suppressor Cell Population Dynamics and Response to IL-6 in 4T1 Mammary Carcinoma. Int. J. Mol. Sci. 2022, 23, 10299. https://doi.org/10.3390/ijms231810299
Rasé VJ, Hayward R, Haughian JM, Pullen NA. Th17, Th22, and Myeloid-Derived Suppressor Cell Population Dynamics and Response to IL-6 in 4T1 Mammary Carcinoma. International Journal of Molecular Sciences. 2022; 23(18):10299. https://doi.org/10.3390/ijms231810299
Chicago/Turabian StyleRasé, Viva J., Reid Hayward, James M. Haughian, and Nicholas A. Pullen. 2022. "Th17, Th22, and Myeloid-Derived Suppressor Cell Population Dynamics and Response to IL-6 in 4T1 Mammary Carcinoma" International Journal of Molecular Sciences 23, no. 18: 10299. https://doi.org/10.3390/ijms231810299
APA StyleRasé, V. J., Hayward, R., Haughian, J. M., & Pullen, N. A. (2022). Th17, Th22, and Myeloid-Derived Suppressor Cell Population Dynamics and Response to IL-6 in 4T1 Mammary Carcinoma. International Journal of Molecular Sciences, 23(18), 10299. https://doi.org/10.3390/ijms231810299






