Ameliorative Effect of Dabigatran on CFA-Induced Rheumatoid Arthritis via Modulating Kallikrein-Kinin System in Rats
Abstract
1. Introduction
2. Results
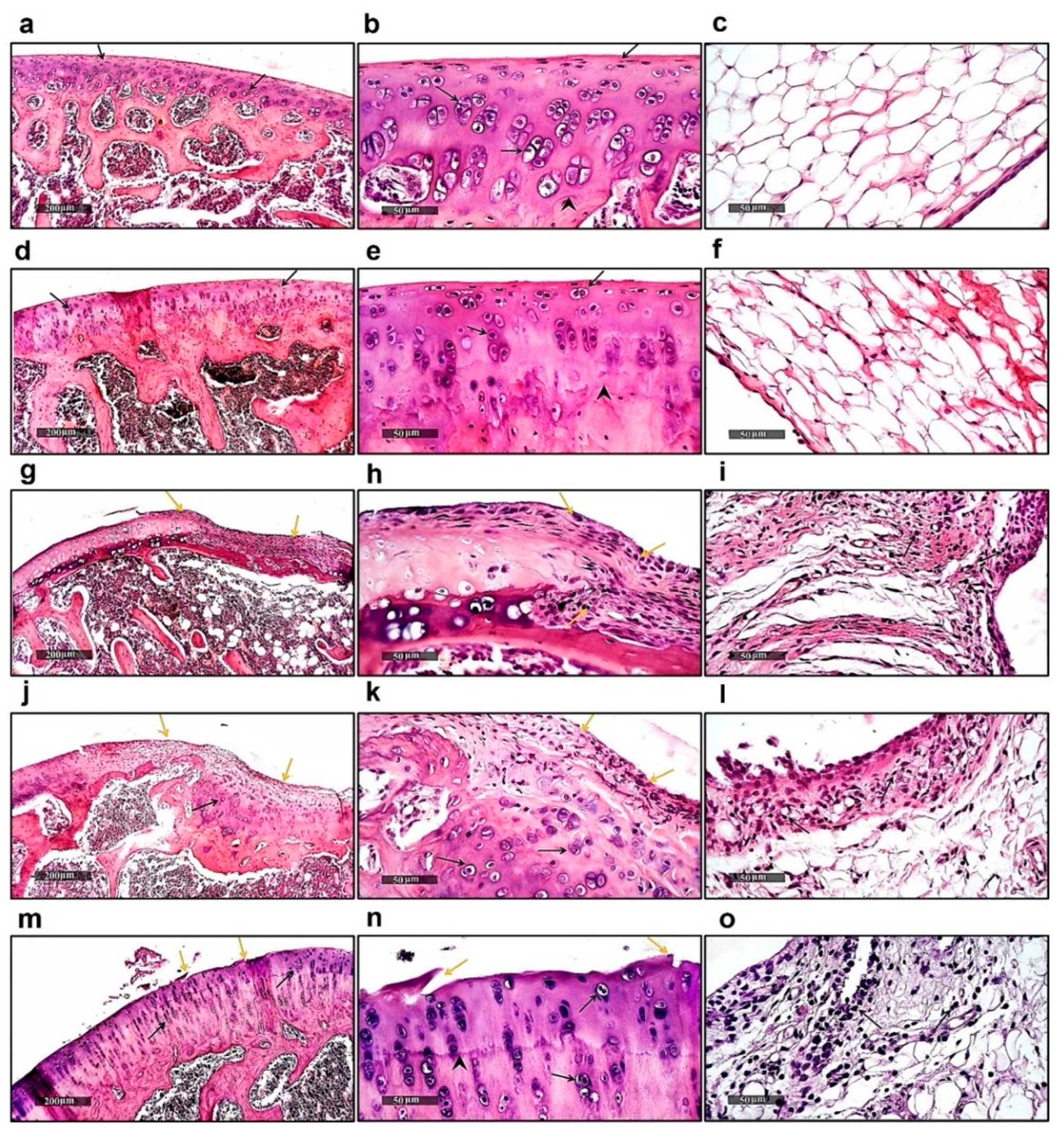
2.1. Histological Examination
2.2. Effect on ACPA and MDA
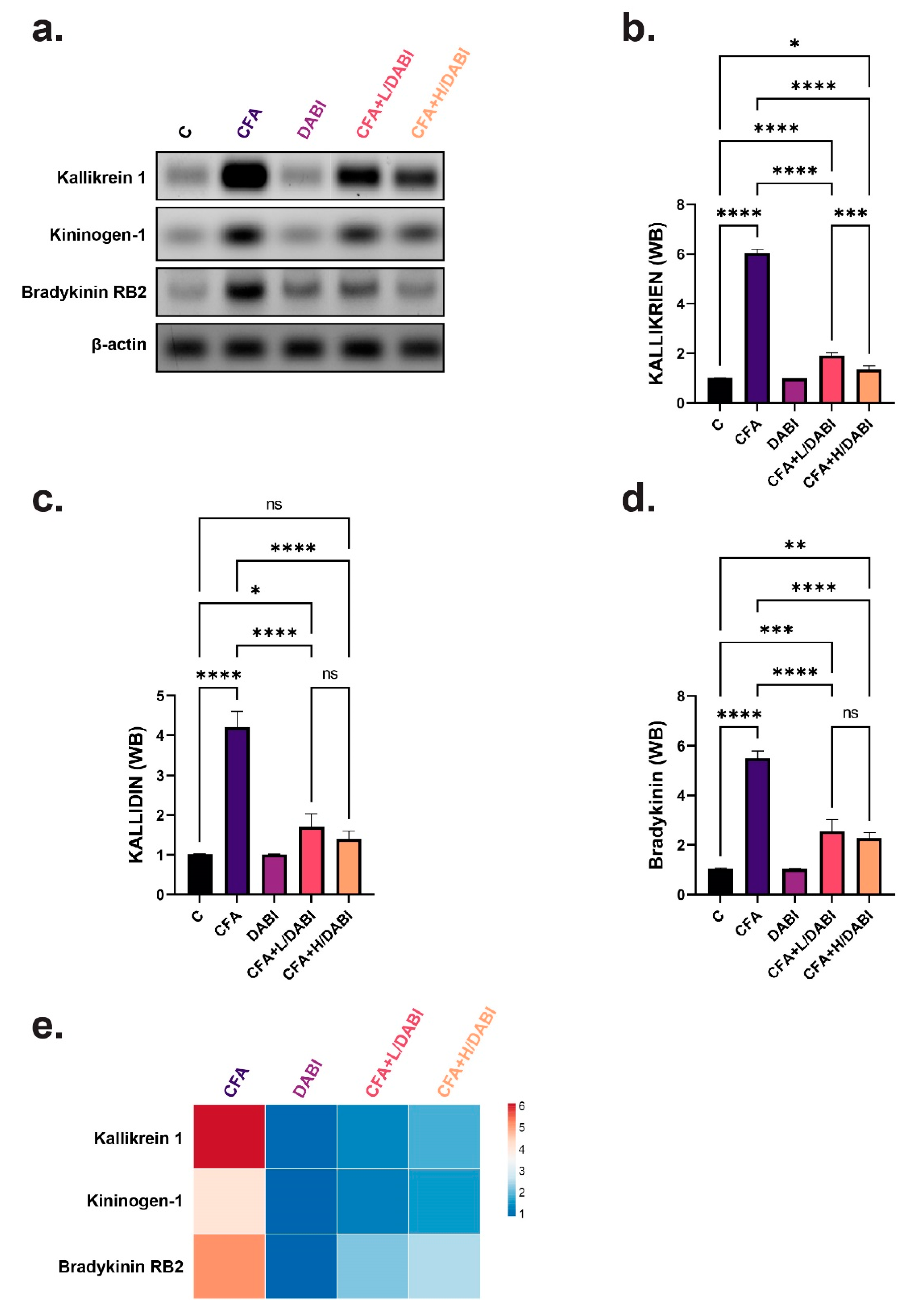
2.3. Effect on Kallikrein, Kallidin, and Bradykinin
2.4. Effect on TLR4 and RANKL
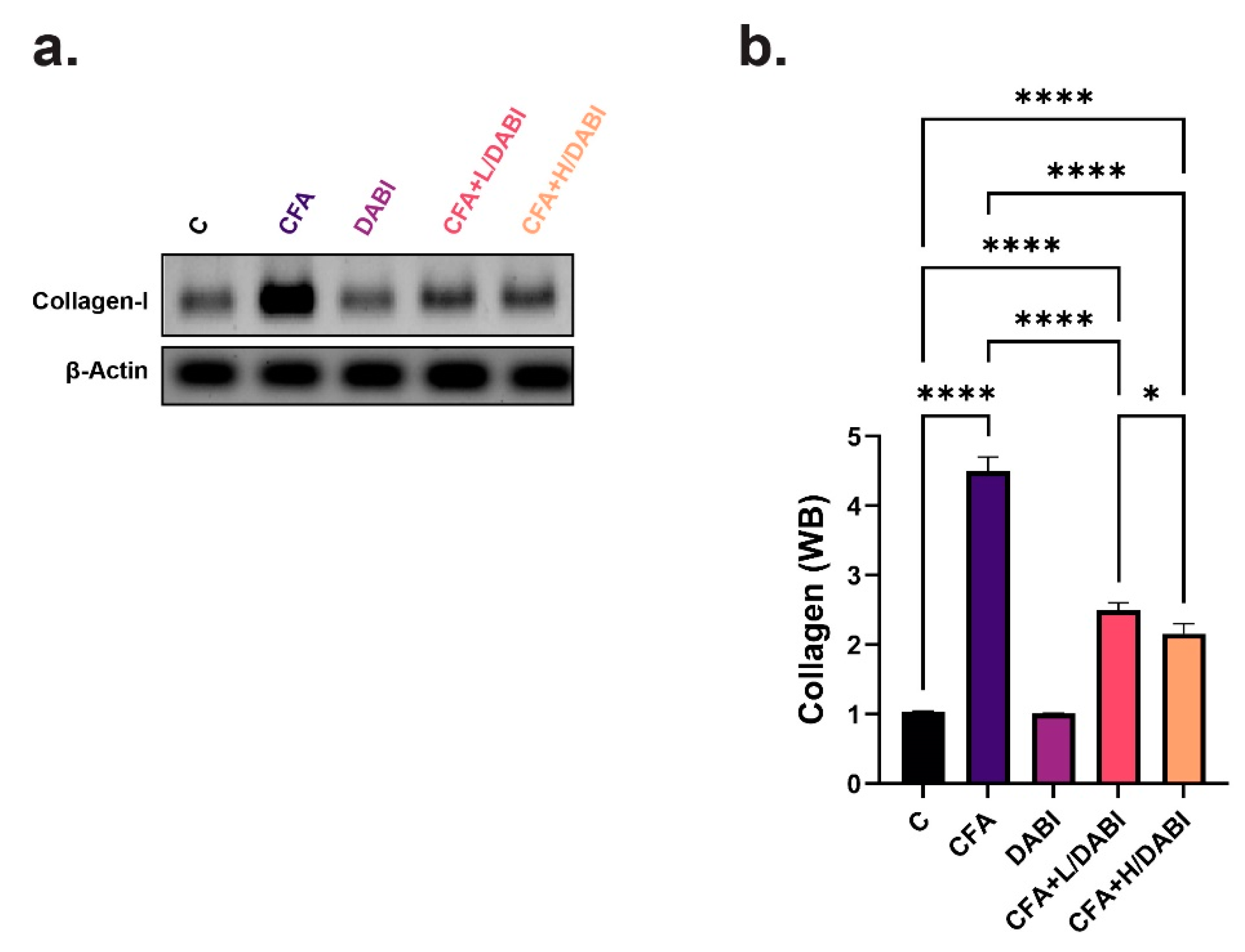
2.5. Effect on Collagen I
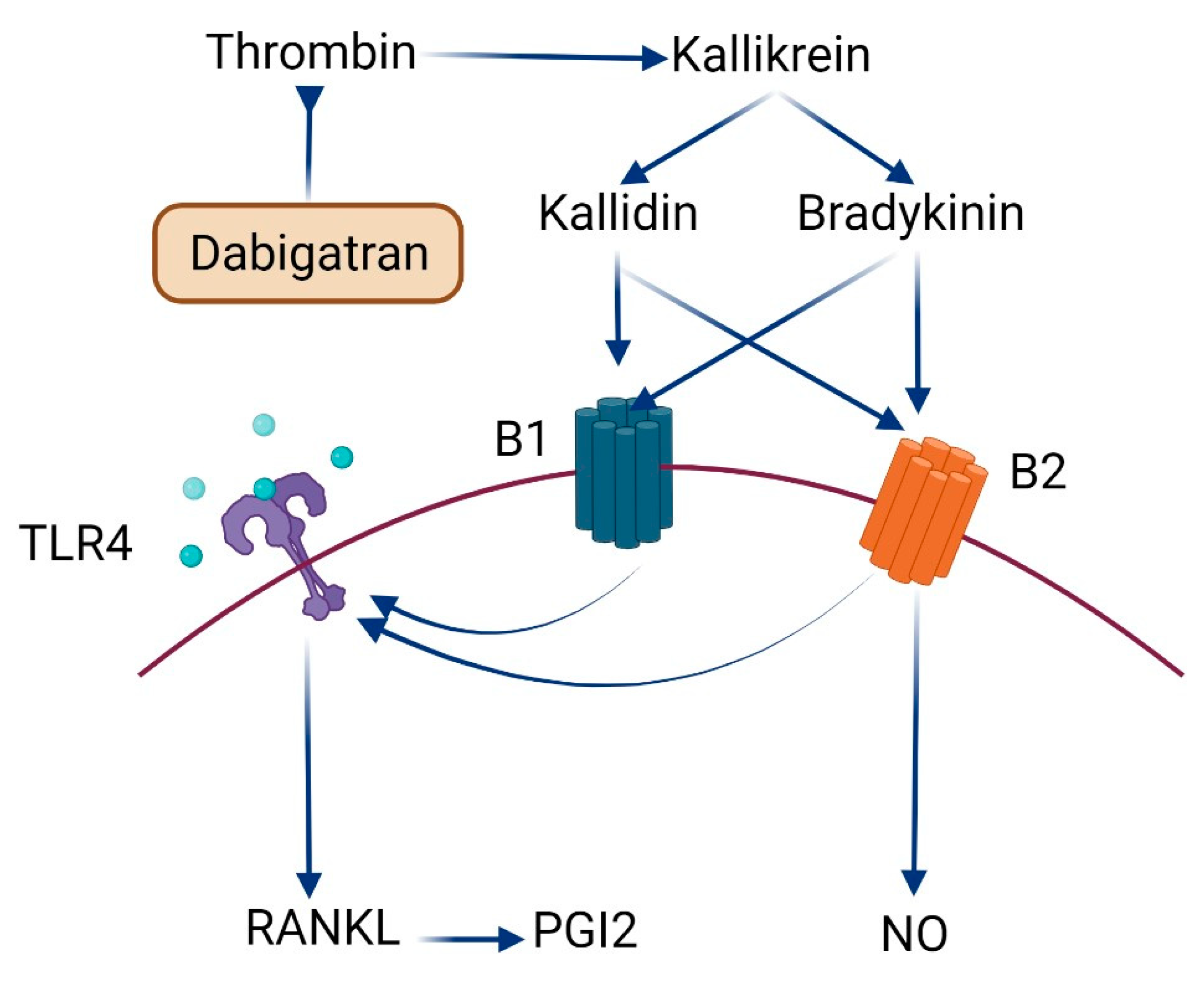
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Chemicals, Antibodies, and Reagent Kits
4.3. Experimental Approach
4.4. Induction of Arthritis
4.5. Serum Sampling
4.6. Tissue Sampling
4.7. Analysis of Serum Biomarkers
4.8. Immunoblot Analysis of Tissue Biomarkers
4.9. Histopathology
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Youssef, M.E.; Abd El-Fattah, E.E.; Abdelhamid, A.M.; Eissa, H.; El-Ahwany, E.; Amin, N.A.; Hetta, H.F.; Mahmoud, M.H.; El-Saber Batiha, G.; Gobba, N.; et al. Interference With the AMPKα/mTOR/NLRP3 Signaling and the IL-23/IL-17 Axis Effectively Protects Against the Dextran Sulfate Sodium Intoxication in Rats: A New Paradigm in Empagliflozin and Metformin Reprofiling for the Management of Ulcerative Colitis. Front. Pharmacol. 2021, 12, 719984. [Google Scholar] [CrossRef] [PubMed]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Aletaha, D.; Smolen, J.S. Diagnosis and management of rheumatoid arthritis: A review. JAMA 2018, 320, 1360–1372. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Zhao, Y.; Li, C.; Luo, Y.; Hao, W.; Zhang, W. Case report: Successful treatment of refractory SAPHO syndrome with the JAK inhibitor tofacitinib. Medicine 2018, 97, e11149. [Google Scholar] [CrossRef]
- Köhler, B.M.; Günther, J.; Kaudewitz, D.; Lorenz, H.-M. Current therapeutic options in the treatment of rheumatoid arthritis. J. Clin. Med. 2019, 8, 938. [Google Scholar] [CrossRef] [PubMed]
- Steinmeyer, J.; Bock, F.; Stöve, J.; Jerosch, J.; Flechtenmacher, J. Pharmacological treatment of knee osteoarthritis: Special considerations of the new German guideline. Orthop. Rev. 2018, 10, 7782. [Google Scholar] [CrossRef]
- Altieri, P.; Bertolotto, M.; Fabbi, P.; Sportelli, E.; Balbi, M.; Santini, F.; Brunelli, C.; Canepa, M.; Montecucco, F.; Ameri, P. Thrombin induces protease-activated receptor 1 signaling and activation of human atrial fibroblasts and dabigatran prevents these effects. Int. J. Cardiol. 2018, 271, 219–227. [Google Scholar] [CrossRef]
- Connolly, S.J.; Ezekowitz, M.D.; Yusuf, S.; Eikelboom, J.; Oldgren, J.; Parekh, A.; Pogue, J.; Reilly, P.A.; Themeles, E.; Varrone, J.; et al. Dabigatran versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2009, 361, 1139–1151. [Google Scholar] [CrossRef]
- Loke, Y.K.; Kwok, C.S. Dabigatran and rivaroxaban for prevention of venous thromboembolism–systematic review and adjusted indirect comparison. J. Clin. Pharm. Ther. 2011, 36, 111–124. [Google Scholar] [CrossRef]
- Paar, V.; Jirak, P.; Gruber, S.; Prodinger, C.; Cadamuro, J.; Wernly, B.; Motloch, L.J.; Haschke-Becher, E.; Hoppe, U.C.; Lichtenauer, M. Influence of dabigatran on pro-inflammatory cytokines, growth factors and chemokines—Slowing the vicious circle of coagulation and inflammation. Life Sci. 2020, 262, 118474. [Google Scholar] [CrossRef] [PubMed]
- Rahadian, A.; Fukuda, D.; Salim, H.M.; Yagi, S.; Kusunose, K.; Yamada, H.; Soeki, T.; Shimabukuro, M.; Sata, M. Thrombin inhibition by dabigatran attenuates endothelial dysfunction in diabetic mice. Vasc. Pharmacol. 2020, 124, 106632. [Google Scholar] [CrossRef]
- Ellinghaus, P.; Perzborn, E.; Hauenschild, P.; Gerdes, C.; Heitmeier, S.; Visser, M.; Summer, H.; Laux, V. Expression of pro-inflammatory genes in human endothelial cells: Comparison of rivaroxaban and dabigatran. Thromb. Res. 2016, 142, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Feldmann, K.; Grandoch, M.; Kohlmorgen, C.; Valentin, B.; Gerfer, S.; Nagy, N.; Hartwig, S.; Lehr, S.; Fender, A.C.; Fischer, J.W. Decreased M1 macrophage polarization in dabigatran-treated Ldlr-deficient mice: Implications for atherosclerosis and adipose tissue inflammation. Atherosclerosis 2019, 287, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Bryant, J.; Shariat-Madar, Z. Human plasma kallikrein-kinin system: Physiological and biochemical parameters. Cardiovasc. Hematol. Agents Med. Chem. 2009, 7, 234–250. [Google Scholar] [CrossRef] [PubMed]
- Schmaier, A.H. The contact activation and kallikrein/kinin systems: Pathophysiologic and physiologic activities. J. Thromb. Haemost. 2016, 14, 28–39. [Google Scholar] [CrossRef]
- Wu, Y. Contact pathway of coagulation and inflammation. Thromb. J. 2015, 13, 17. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, A.P.; Joseph, K.; Silverberg, M. Pathways for bradykinin formation and inflammatory disease. J. Allergy Clin. Immunol. 2002, 109, 195–209. [Google Scholar] [CrossRef]
- Dray, A.; Perkins, M. Bradykinin and inflammatory pain. Trends Neurosci. 1993, 16, 99–104. [Google Scholar] [CrossRef]
- Lopatko Fagerstrom, I.; Stahl, A.-L.; Mossberg, M.; Tati, R.; Kristoffersson, A.-C.; Kahn, R.; Bascands, J.-L.; Klein, J.; Schanstra, J.P.; Segelmark, M.; et al. Blockade of the kallikrein-kinin system reduces endothelial complement activation in vascular inflammation. eBioMedicine 2019, 47, 319–328. [Google Scholar] [CrossRef]
- De Falco, L.; Fioravanti, A.; Galeazzi, M.; Tenti, S. Bradykinin and its role in osteoarthritis. Reumatismo 2013, 65, 97–104. [Google Scholar] [CrossRef][Green Version]
- Choudhary, N.; Bhatt, L.K.; Prabhavalkar, K.S. Experimental animal models for rheumatoid arthritis. Immunopharmacol. Immunotoxicol. 2018, 40, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Uknis, A.B.; DeLa Cadena, R.A.; Janardham, R.; Sartor, R.B.; Whalley, E.T.; Colman, R.W. Bradykinin receptor antagonists type 2 attenuate the inflammatory changes in peptidoglycan-induced acute arthritis in the Lewis rat. Inflamm. Res. 2001, 50, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wang, H.; Chen, J.; Wei, W. Inhibition of plasma kallikrein–kinin system to alleviate renal injury and arthritis symptoms in rats with adjuvant-induced arthritis. Immunopharmacol. Immunotoxicol. 2018, 40, 134–148. [Google Scholar] [CrossRef]
- Meini, S.; Maggi, C. Knee osteoarthritis: A role for bradykinin? Inflamm. Res. 2008, 57, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Bullock, J.; Rizvi, S.A.A.; Saleh, A.M.; Ahmed, S.S.; Do, D.P.; Ansari, R.A.; Ahmed, J. Rheumatoid arthritis: A brief overview of the treatment. Med. Princ. Pract. 2018, 27, 501–507. [Google Scholar] [CrossRef]
- Billiau, A.; Matthys, P. Modes of action of Freund’s adjuvants in experimental models of autoimmune diseases. J. Leukoc. Biol. 2001, 70, 849–860. [Google Scholar]
- Bolon, B.; Stolina, M.; King, C.; Middleton, S.; Gasser, J.; Zack, D.; Feige, U. Rodent preclinical models for developing novel antiarthritic molecules: Comparative biology and preferred methods for evaluating efficacy. J. Biomed. Biotechnol. 2010, 2011, 569068. [Google Scholar] [CrossRef]
- Loredo-Pérez, A.A.; Montalvo-Blanco, C.E.; Hernández-González, L.I.; Anaya-Reyes, M.; Fernández del Valle-Laisequilla, C.; Reyes-García, J.G.; Acosta-González, R.I.; Martínez-Martínez, A.; Villarreal-Salcido, J.C.; Vargas-Muñoz, V.M.; et al. High-fat diet exacerbates pain-like behaviors and periarticular bone loss in mice with CFA-induced knee arthritis. Obesity 2016, 24, 1106–1115. [Google Scholar] [CrossRef]
- Ratiu, C.; Brocks, M.; Costea, T.; Moldovan, L.; Cavalu, S. PRGF-modified collagen membranes for guided bone regeneration: Spectroscopic, microscopic and nano-mechanical investigations. Appl. Sci. 2019, 9, 1035. [Google Scholar] [CrossRef]
- Loeser, R.F.; Goldring, S.R.; Scanzello, C.R.; Goldring, M.B. Osteoarthritis: A disease of the joint as an organ. Arthritis Rheum. 2012, 64, 1697. [Google Scholar] [CrossRef] [PubMed]
- Robinson, W.H.; Lepus, C.M.; Wang, Q.; Raghu, H.; Mao, R.; Lindstrom, T.M.; Sokolove, J. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Kurowska, W.; Kuca-Warnawin, E.H.; Radzikowska, A.; Maśliński, W. The role of anti-citrullinated protein antibodies (ACPA) in the pathogenesis of rheumatoid arthritis. Cent. Eur. J. Immunol. 2017, 42, 390. [Google Scholar] [CrossRef]
- Thiele, G.M.; Duryee, M.J.; Anderson, D.R.; Klassen, L.W.; Mohring, S.M.; Young, K.A.; Benissan-Messan, D.; Sayles, H.; Dusad, A.; Hunter, C.D.; et al. Malondialdehyde-acetaldehyde adducts and anti–malondialdehyde-acetaldehyde antibodies in rheumatoid arthritis. Arthritis Rheumatol. 2015, 67, 645–655. [Google Scholar] [CrossRef]
- Grönwall, C.; Amara, K.; Hardt, U.; Krishnamurthy, A.; Steen, J.; Engström, M.; Sun, M.; Ytterberg, A.J.; Zubarev, R.A.; Scheel-Toellner, D.; et al. Autoreactivity to malondialdehyde-modifications in rheumatoid arthritis is linked to disease activity and synovial pathogenesis. J. Autoimmun. 2017, 84, 29–45. [Google Scholar] [CrossRef]
- Böckmann, S.; Paegelow, I. Kinins and kinin receptors: Importance for the activation of leukocytes. J. Leukoc. Biol. 2000, 68, 587–592. [Google Scholar]
- Cassim, B.; Mody, G.; Bhoola, K.D. Kallikrein cascade and cytokines in inflamed joints. Pharmacol. Ther. 2002, 94, 1–34. [Google Scholar] [CrossRef]
- Campbell, D.J. The kallikrein–kinin system in humans. Clin. Exp. Pharmacol. Physiol. 2001, 28, 1060–1065. [Google Scholar] [CrossRef]
- Pinheiro, A.S.; Silbak, S.; Schmaier, A.H. Bradykinin—An elusive peptide in measuring and understanding. Res. Pract. Thromb. Haemost. 2022, 6, e12673. [Google Scholar] [CrossRef]
- Dellalibera-Joviliano, R.; Bestetti, R.B.; Lopes, G.S.; Furlan-Daniel, R.; Lopes, K.C.; Faria-Junior, M.; Junior, N.I. Kinins and nitric oxide in patients with chronic chagas disease and systemic arterial hypertension. Cardiovasc. Pathol. 2020, 49, 107257. [Google Scholar] [CrossRef]
- Leonidou, A.; Lepetsos, P.; Mintzas, M.; Kenanidis, E.; Macheras, G.; Tzetis, M.; Potoupnis, M.; Tsiridis, E. Inducible nitric oxide synthase as a target for osteoarthritis treatment. Expert Opin. Ther. Targets 2018, 22, 299–318. [Google Scholar] [CrossRef]
- Jiménez-Dalmaroni, M.J.; Gerswhin, M.E.; Adamopoulos, I.E. The critical role of toll-like receptors—from microbial recognition to autoimmunity: A comprehensive review. Autoimmun. Rev. 2016, 15, 1–8. [Google Scholar] [CrossRef]
- Souza, P.P.C.; Lundberg, P.; Lundgren, I.; Magalhães, F.A.C.; Costa-Neto, C.M.; Lerner, U.H. Activation of Toll-like receptor 2 induces B1 and B2 kinin receptors in human gingival fibroblasts and in mouse gingiva. Sci. Rep. 2019, 9, 2973. [Google Scholar] [CrossRef]
- Gaur, U.; Aggarwal, B.B. Regulation of proliferation, survival and apoptosis by members of the TNF superfamily. Biochem. Pharmacol. 2003, 66, 1403–1408. [Google Scholar] [CrossRef]
- Infante, M.; Fabi, A.; Cognetti, F.; Gorini, S.; Caprio, M.; Fabbri, A. RANKL/RANK/OPG system beyond bone remodeling: Involvement in breast cancer and clinical perspectives. J. Exp. Clin. Cancer Res. 2019, 38, 12. [Google Scholar] [CrossRef]
- Abdel-Ghany, R.; Rabia, I.; El-Ahwany, E.; Saber, S.; Gamal, R.; Nagy, F.; Mahmoud, O.; Hamad, R.S.; Barakat, W. Blockade of PGE2, PGD2 Receptors Confers Protection against Prepatent Schistosomiasis Mansoni in Mice. J. Egypt. Soc. Parasitol. 2015, 45, 511–520. [Google Scholar]
- Takayanagi, H. Inflammatory bone destruction and osteoimmunology. J. Periodontal Res. 2005, 40, 287–293. [Google Scholar] [CrossRef]
- Nakashima, T.T.; Penninger, J.M. RANKL and RANK as novel therapeutic targets for arthritis. Curr. Opin. Rheumatol. 2003, 15, 280–287. [Google Scholar] [CrossRef]
- Harifi, G.; Sibilia, J. Pathogenic role of platelets in rheumatoid arthritis and systemic autoimmune diseases: Perspectives and therapeutic aspects. Saudi Med. J. 2016, 37, 354. [Google Scholar] [CrossRef]
- Pankratz, S.; Bittner, S.; Kehrel, B.E.; Langer, H.F.; Kleinschnitz, C.; Meuth, S.G.; Göbel, K. The inflammatory role of platelets: Translational insights from experimental studies of autoimmune disorders. Int. J. Mol. Sci. 2016, 17, 1723. [Google Scholar] [CrossRef]
- Nurden, A.T. Platelets, inflammation and tissue regeneration. Thromb. Haemost. 2011, 105, S13–S33. [Google Scholar] [CrossRef]
- Costa-Neto, C.M.; Duarte, D.A.; Lima, V.; Maria, A.G.; Prando, E.C.; Rodriguez, D.Y.; Santos, G.A.; Souza, P.P.C.; Parreiras-e-Silva, L.T. Non-canonical signalling and roles of the vasoactive peptides angiotensins and kinins. Clin. Sci. 2014, 126, 753–774. [Google Scholar] [CrossRef]
- Gustafson, G.T.; Lerner, U. Thrombin, a stimulator of bone resorption. Biosci. Rep. 1983, 3, 255–261. [Google Scholar] [CrossRef]
- Song, J.; Jiang, N.; Gan, X.; Zhi, W.; Zhu, Z. Thrombin inhibitor argatroban modulates bone marrow stromal cells behaviors and promotes osteogenesis through canonical Wnt signaling. Life Sci. 2021, 269, 119073. [Google Scholar] [CrossRef]
- Lerner, U.H. Regulation of bone metabolism by the kallikrein-kinin system, the coagulation cascade, and the acute-phase reactants. Oral Surg. Oral Med. Oral Pathol. 1994, 78, 481–493. [Google Scholar] [CrossRef]
- Stern, P.H.; Stathopoulos, V.M.; Shankar, G.; Fenton, J.W. Second messengers in thrombin-stimulated bone resorption. J. Bone Miner. Res. 1990, 5, 443–449. [Google Scholar] [CrossRef]
- Muñoz-Rodríguez, C.; Fernández, S.; Osorio, J.M.; Olivares, F.; Anfossi, R.; Bolivar, S.; Humeres, C.; Boza, P.; Vivar, R.; Pardo-Jimenez, V.; et al. Expression and function of TLR4-induced B1R bradykinin receptor on cardiac fibroblasts. Toxicol. Appl. Pharmacol. 2018, 351, 46–56. [Google Scholar] [CrossRef]
- Renn, T.-Y.; Huang, Y.-K.; Feng, S.-W.; Wang, H.-W.; Lee, W.-F.; Lin, C.-T.; Burnouf, T.; Chen, L.-Y.; Kao, P.-F.; Chang, H.-M. Prophylactic supplement with melatonin successfully suppresses the pathogenesis of periodontitis through normalizing RANKL/OPG ratio and depressing the TLR 4/MyD88 signaling pathway. J. Pineal Res. 2018, 64, e12464. [Google Scholar] [CrossRef]
- Gaafar, A.G.A.; Messiha, B.A.S.; Abdelkafy, A.M.L. Nicorandil and theophylline can protect experimental rats against complete Freund’s adjuvant-induced rheumatoid arthritis through modulation of JAK/STAT/RANKL signaling pathway. Eur. J. Pharmacol. 2018, 822, 177–185. [Google Scholar] [CrossRef]
- Eisert, W.G.; Hauel, N.; Stangier, J.; Wienen, W.; Clemens, A.; van Ryn, J. Dabigatran: An oral novel potent reversible nonpeptide inhibitor of thrombin. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1885–1889. [Google Scholar] [CrossRef]
- Holmdahl, R. Female preponderance for development of arthritis in rats is influenced by both sex chromosomes and sex steroids. Scand. J. Immunol. 1995, 42, 104–109. [Google Scholar] [CrossRef]
- Ro, J.Y.; Zhang, Y.; Tricou, C.; Yang, D.; da Silva, J.T.; Zhang, R. Age and Sex Differences in Acute and Osteoarthritis-Like Pain Responses in Rats. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, 1465–1472. [Google Scholar] [CrossRef]
- Engvall, E. Quantitative enzyme immunoassay (ELISA) in microbiology. Med. Biol. 1977, 55, 193–200. [Google Scholar] [PubMed]
- Burnette, W.N. “Western blotting”: Electrophoretic transfer of proteins from sodium dodecyl sulfate-polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal. Biochem. 1981, 112, 195–203. [Google Scholar] [CrossRef]
- Amponsah, P.S.; Yahya, G.; Zimmermann, J.; Mai, M.; Mergel, S.; Mühlhaus, T.; Storchova, Z.; Morgan, B. Peroxiredoxins couple metabolism and cell division in an ultradian cycle. Nat. Chem. Biol. 2021, 17, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Parisi, E.; Yahya, G.; Flores, A.; Aldea, M. Cdc48/p97 segregase is modulated by cyclin-dependent kinase to determine cyclin fate during G1 progression. EMBO J. 2018, 37, e98724. [Google Scholar] [CrossRef]
- Zohny, M.H.; Cavalu, S.; Youssef, M.E.; Kaddah, M.M.Y.; Mourad, A.A.E.; Gaafar, A.G.A.; El-Ahwany, E.; Amin, N.A.; Arakeep, H.M.; Shata, A.; et al. Coomassie brilliant blue G-250 dye attenuates bleomycin-induced lung fibrosis by regulating the NF-κB and NLRP3 crosstalk: A novel approach for filling an unmet medical need. Biomed. Pharmacother. 2022, 148, 112723. [Google Scholar] [CrossRef]
- Youssef, M.E.; El-Azab, M.F.; Abdel-Dayem, M.A.; Yahya, G.; Alanazi, I.S.; Saber, S. Electrocardiographic and histopathological characterizations of diabetic cardiomyopathy in rats. Environ. Sci. Pollut. Res. 2022, 29, 25723–25732. [Google Scholar] [CrossRef]
- Nasr, M.; Teiama, M.; Ismail, A.; Ebada, A.; Saber, S. In vitro and in vivo evaluation of cubosomal nanoparticles as an ocular delivery system for fluconazole in treatment of keratomycosis. Drug Deliv. Transl. Res. 2020, 10, 1841–1852. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Youssef, M.E.; Abdel-Reheim, M.A.; Morsy, M.A.; El-Daly, M.; Atwa, G.M.K.; Yahya, G.; Cavalu, S.; Saber, S.; Ahmed Gaafar, A.G. Ameliorative Effect of Dabigatran on CFA-Induced Rheumatoid Arthritis via Modulating Kallikrein-Kinin System in Rats. Int. J. Mol. Sci. 2022, 23, 10297. https://doi.org/10.3390/ijms231810297
Youssef ME, Abdel-Reheim MA, Morsy MA, El-Daly M, Atwa GMK, Yahya G, Cavalu S, Saber S, Ahmed Gaafar AG. Ameliorative Effect of Dabigatran on CFA-Induced Rheumatoid Arthritis via Modulating Kallikrein-Kinin System in Rats. International Journal of Molecular Sciences. 2022; 23(18):10297. https://doi.org/10.3390/ijms231810297
Chicago/Turabian StyleYoussef, Mahmoud E., Mustafa A. Abdel-Reheim, Mohamed A. Morsy, Mahmoud El-Daly, Gamal M. K. Atwa, Galal Yahya, Simona Cavalu, Sameh Saber, and Ahmed Gaafar Ahmed Gaafar. 2022. "Ameliorative Effect of Dabigatran on CFA-Induced Rheumatoid Arthritis via Modulating Kallikrein-Kinin System in Rats" International Journal of Molecular Sciences 23, no. 18: 10297. https://doi.org/10.3390/ijms231810297
APA StyleYoussef, M. E., Abdel-Reheim, M. A., Morsy, M. A., El-Daly, M., Atwa, G. M. K., Yahya, G., Cavalu, S., Saber, S., & Ahmed Gaafar, A. G. (2022). Ameliorative Effect of Dabigatran on CFA-Induced Rheumatoid Arthritis via Modulating Kallikrein-Kinin System in Rats. International Journal of Molecular Sciences, 23(18), 10297. https://doi.org/10.3390/ijms231810297







