Erythrocyte-Derived Nanoparticles with Folate Functionalization for Near Infrared Pulsed Laser-Mediated Photo-Chemotherapy of Tumors
Abstract
:1. Introduction
2. Results
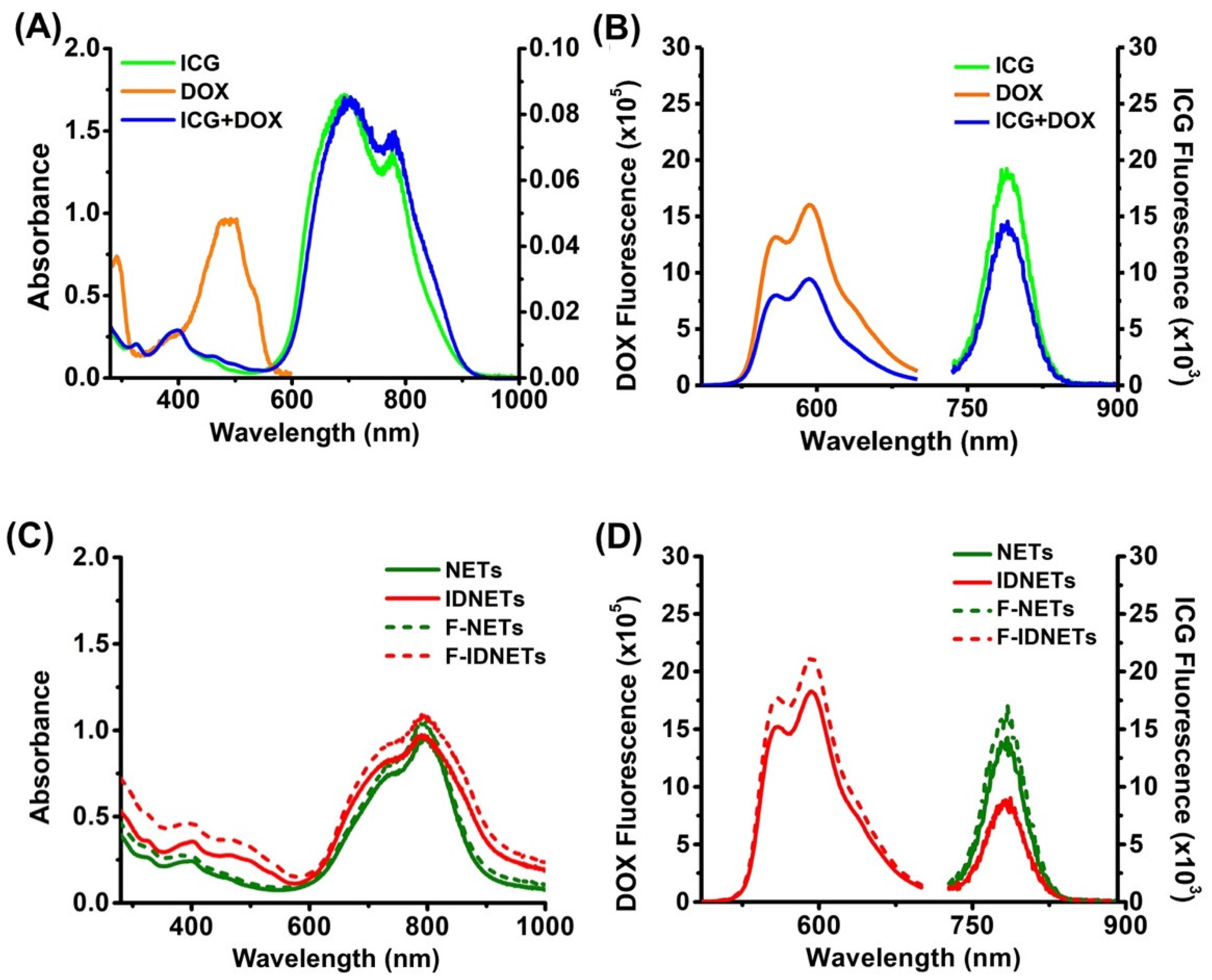
2.1. Physical and Optical Characteristics
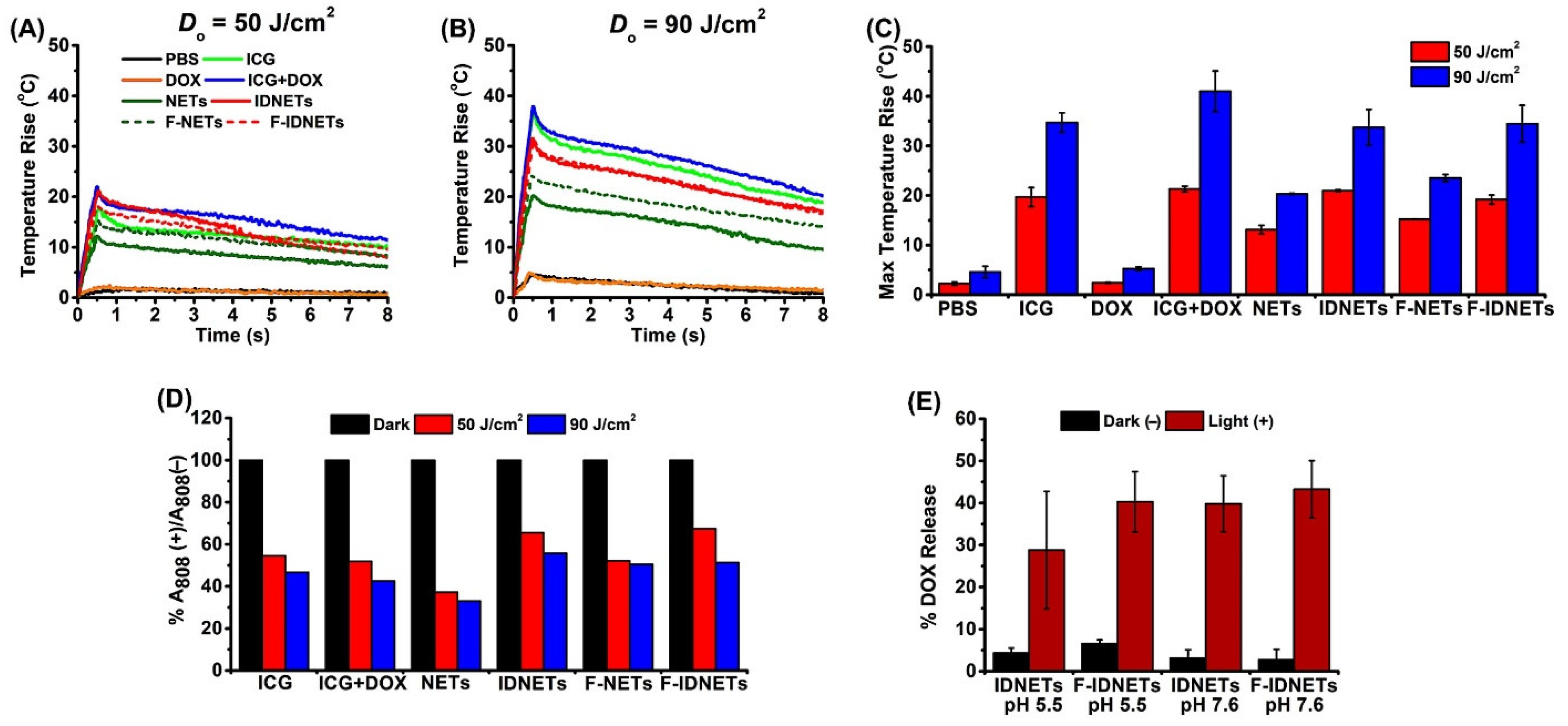
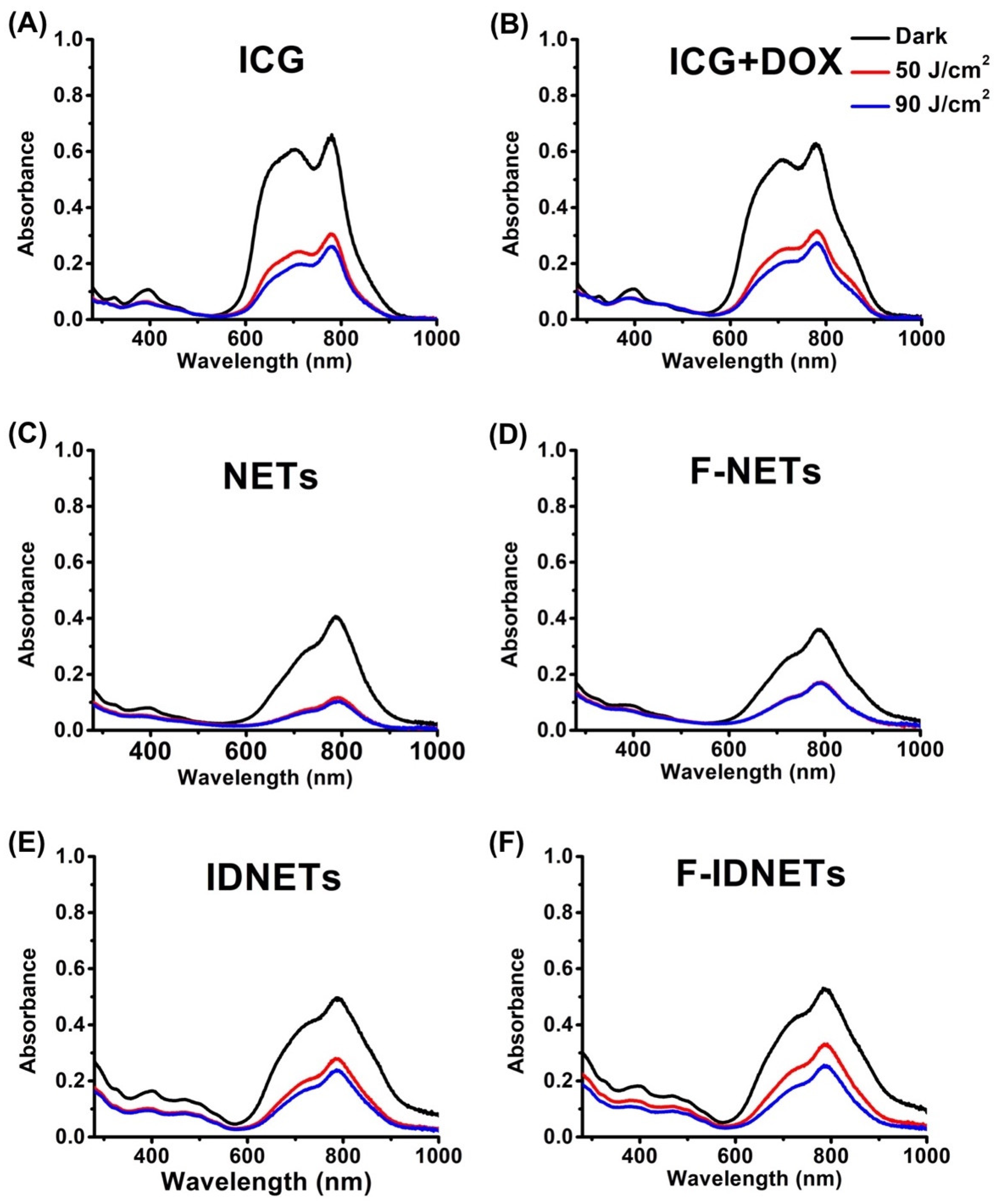
2.2. Photothermal Response and Photostability of Nanoparticles
2.3. In Vitro Photothermal-Chemo Effects
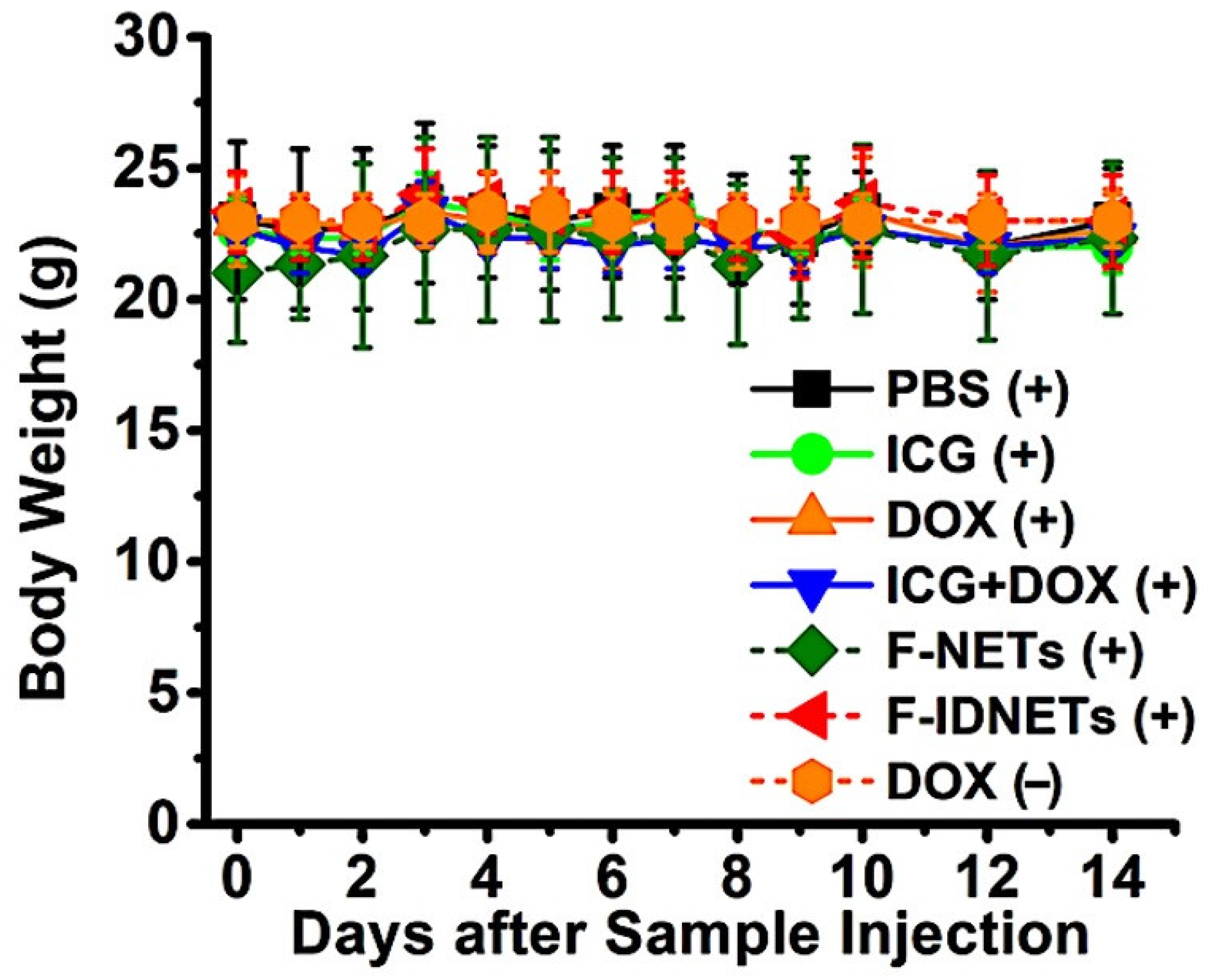
2.4. In Vivo Photothermal-Chemo Effects
3. Discussion
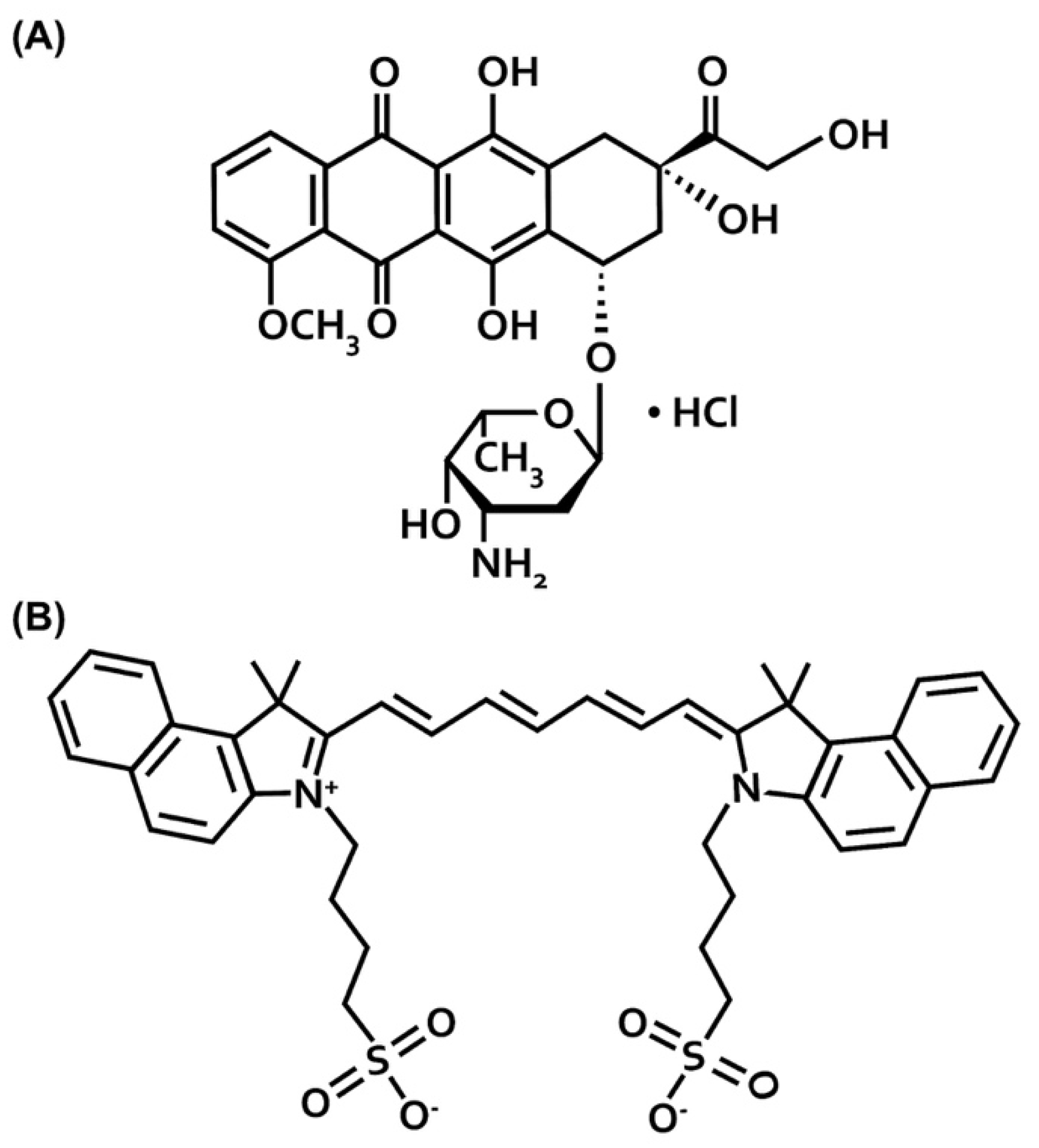
4. Materials and Methods
4.1. Fabrication of Nanoparticles
4.2. Physical and Optical Characterizations
4.3. Radiometric Temperature Measurements
4.4. Pulsed Laser Irradiation
4.5. Photothermal Response and Photostability of Nanoparticles
4.6. Effect of pH and Pulsed Laser Irradiation on DOX Release
4.7. Cell Culture
4.8. Fluorescence Imaging of Cancer Cells
4.9. Cell Viability Assay
4.10. Animal Studies
4.11. Caspase-3 Staining
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviation | Definition |
| A808 | Absorbance at 808 nm |
| Al | Aluminum |
| CW | Continuous wave |
| DAPI | 4′,6-diamidino-2-phenylindole |
| DLS | Dynamic light scattering |
| DOX | Doxorubicin hydrochloride |
| DSPE | 1,2-distearoyl-sn-glycero-3-phosphoethanolamine |
| EM-CCD | Electron multiplying charged-coupled device |
| F-IDNETs | Folate-functionalized ICG + DOX containing NETs |
| F-NETs | Folate-functionalized NETs |
| FBS | Fetal bovine serum |
| FDA | Food and Drug Administration |
| FITC | Fluorescein isothiocyanate |
| FR-α | Folate receptor-α |
| ICG | Indocyanine green |
| i.v. | Intravenous |
| IDNETs | ICG + DOX containing NETs |
| LD50 | Lethal dose that causes death in 50% of animals |
| nEGs | nano-sized erythrocyte ghosts |
| NETs | NIR erythrocyte-derived transducers |
| PBS | Phosphate buffer saline |
| PTT | Photothermal therapy |
| PVD | Photovoltaic detector |
| RBCs | Red blood cells |
| RPMI | Rosewell Park Memorial Institute |
| SD | Standard deviation |
| UV-Vis | Ultraviolet-Visible |
| µEGs | Micro-sized erythrocyte ghosts |
References
- Chabner, B.A.; Roberts, T.G., Jr. Timeline: Chemotherapy and the war on cancer. Nat. Rev. Cancer 2005, 5, 65–72. [Google Scholar] [CrossRef]
- Biemar, F.; Fot, M. Global progress against cancer-challenges and opportunities. Cancer Biol. Med. 2013, 10, 183–186. [Google Scholar]
- Green, A.E.; Rose, P.G. Pegylated liposomal doxorubicin in ovarian cancer. Int. J. Nanomed. 2006, 1, 229–239. [Google Scholar]
- Staropoli, N.; Ciliberto, D.; Botta, C.; Fiorillo, L.; Grimaldi, A.; Lama, S.; Caraglia, M.; Salvino, A.; Tassone, P.; Tagliaferri, P. Pegylated liposomal doxorubicin in the management of ovarian cancer: A systematic review and metaanalysis of randomized trials. Cancer Biol. Ther. 2014, 15, 707–720. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic: An update. Bioeng. Transl. Med. 2019, 4, e10143. [Google Scholar] [CrossRef]
- Bulten, B.F.; Sollini, M.; Boni, R.; Massri, K.; de Geus-Oei, L.-F.; van Laarhoven, H.W.M.; Slart, R.H.J.A.; Erba, P. Cardiac molecular pathways influenced by doxorubicin treatment in mice. Sci. Rep. 2019, 9, 2514. [Google Scholar] [CrossRef] [PubMed]
- Tangpong, J.; Miriyala, S.; Noel, T.; Sinthupibulyakit, C.; Jungsuwadee, P.; St. Clair, D.K. Doxorubicin-induced central nervous system toxicity and protection by xanthone derivative of Garcinia mangostana. Neuorscience 2011, 175, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Zhang, J.; Liu, M.; Iwahata, H.; Rogers, H.B.; Woodruff, T.K. Doxorubicin Has Dose-Dependent Toxicity on Mouse Ovarian Follicle Development, Hormone Secretion, and Oocyte Maturation. Toxicol. Sci. 2017, 157, 329. [Google Scholar] [CrossRef]
- Lim, E.; Kim, T.; Paik, S.; Haam, S.; Huh, Y.; Lee, K. Nanomaterials for theranostics: Recent advances and future challenges. Chem. Rev. 2015, 115, 327–394. [Google Scholar] [CrossRef]
- Liu, M.; Du, H.; Zhang, W.; Zhai, G. Internal stimuli-responsive nanocarriers for drug delivery: Design strategies and applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 71, 1267–1280. [Google Scholar] [CrossRef]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef]
- Chen, T.; Tam, N.; Mao, Y.; Sun, C.; Wang, Z.; Hou, Y.; Xia, W.; Yu, J.; Wu, L. A multi-hit therapeutic nanoplatform for hepatocellular carcinoma: Dual stimuli-responsive drug release, dual-modal imaging, and in situ oxygen supply to enhance synergistic therapy. Mater. Today Bio 2022, 16, 100338. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Xie, S.; Zhou, C.; Chen, Y.; Li, H.; Liu, P.; Jian, R.; Hang, L.; Jiang, G. All-in-one second near-infrared light-responsive drug delivery system for synergistic chemo-photothermal therapy. ACS Appl. Bio Mater. 2022. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Guo, L.; Rao, Y.; Zheng, W.; Gao, D.; Zhang, J.; Luo, L.; Kuang, X.; Sukumar, S.; Tu, Y.; et al. In situ injection of pH- and temperature-sensitive nanomaterials increases chemo-photothermal efficacy by alleviating the tumorimmunosuppressive microenvironment. Int. J. Nanomed. 2022, 17, 2661–2678. [Google Scholar] [CrossRef]
- Klein, A.; Szeimies, R.M.; Baumler, W.; Zeman, F.; Schreml, S.; Hohenleutner, U.; Landthaler, M.; Koller, M.; Babilas, P. Indocyanine green-augmented diode laser treatment of port-wine stains: Clinical and histological evidence for a new treatment option from a randomized controlled trial. Br. J. Dermatol. 2012, 167, 333–342. [Google Scholar] [CrossRef]
- Zhao, P.; Zheng, M.; Luo, Z.; Gong, P.; Gao, G.; Sheng, Z.; Zheng, C.; Ma, Y.; Cai, L. NIR-driven smart theranostic nanomedicine for on-demand drug release and synergistic antitumour therapy. Sci. Rep. 2015, 5, 14258. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Yue, C.; Ma, Y.; Gong, P.; Zhao, P.; Zheng, C.; Sheng, Z.; Zhang, P.; Wang, Z.; Cai, L. Single-step assembly of DOX/ICG loaded lipid—polymer nanoparticles for highly effective chemo-photothermal combination therapy. ACS Nano 2013, 7, 2056–2067. [Google Scholar] [CrossRef]
- Cheung, A.; Bax, H.J.; Josephs, D.H.; Ilieva, K.M.; Pellizzari, G.; Opzoomer, J.; Bloomfield, J.; Fittall, M.; Grigoriadis, A.; Figini, M.; et al. Targeting folate receptor alpha for cancer treatment. Oncotarget 2016, 7, 52553–52574. [Google Scholar] [CrossRef] [PubMed]
- Corbin, I.R.; Ng, K.K.; Ding, L.; Jurisicova, A.; Zheng, G. Near-infrared fluorescent imaging of metastatic ovarian cancer using folate receptor-targeted high-density lipoprotein nanocarriers. Nanomedicine 2013, 8, 875–890. [Google Scholar] [CrossRef]
- Kalli, K.; Oberg, A.L.; Keeney, G.L.; Christianson, T.J.H.; Low, P.S.; Knutson, K.L.; Hartmann, L.C. Folate receptor alpha as a tumor target in epithelial ovarian cancer. Gynecol. Oncol. 2008, 108, 619–626. [Google Scholar] [CrossRef]
- van Dam, G.M.; Themelis, G.; Crane, L.M.; Harlaar, N.J.; Pleijhuis, R.G.; Kelder, W.; Sarantopoulos, A.; de Jong, J.S.; Arts, H.J.; van der Zee, A.G.; et al. Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-α targeting: First in-human results. Nat. Med. 2011, 17, 1315–1319. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.-M.J.; Zhang, L.; Aryal, S.; Cheung, C.; Fang, R.H.; Zhang, L. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc. Natl. Acad. Sci. USA 2011, 108, 10980–10985. [Google Scholar] [CrossRef] [Green Version]
- Hanley, T.; Vankayala, R.; Lee, C.-H.; Tang, J.C.; Burns, J.M.; Anvari, B. Phototheranostics using erythrocyte-based particles. Biomolecules 2021, 11, 729. [Google Scholar] [CrossRef]
- Jia, W.; Burns, J.M.; Villantay, B.; Tang, J.C.; Vankayala, R.; Lertsakdadet, B.; Choi, B.; Nelson, J.S.; Anvari, B. Intravital vascular phototheranostics and real-time circulation dynamics of micro- and nanosized erythrocyte-derived carriers. ACS Appl. Mater. Interfaces 2020, 12, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Villa, C.H.; Anselmo, A.C.; Mitragotri, S.; Muzykantov, V. Red blood cells: Supercarriers for drugs, biologicals, and nanoparticles and inspiration for advanced delivery systems. Adv. Drug Deliv. Rev. 2016, 106 Pt A, 88–103. [Google Scholar] [CrossRef]
- Antonelli, A.; Pacifico, S.; Sfara, C.; Tamma, M.; Magnani, M. Ferucarbotran-loaded red blood cells as long circulating MRI contrast agents: First in vivo results in mice. Nanomedicine 2018, 13, 675–687. [Google Scholar] [CrossRef] [PubMed]
- Bahmani, B.; Bacon, D.; Anvari, B. Erythrocyte-derived photo-theranostic agents: Hybrid nano-vesicles containing indocyanine green for near infrared imaging and therapeutic applications. Sci. Rep. 2013, 3, 2180. [Google Scholar] [CrossRef]
- Huang, Y.X.; Wu, Z.J.; Mehrishi, J.; Hunag, B.T.; Chen, X.Y.; Zheng, X.J.; Liu, W.J.; Luo, M. Human red blood cell aging: Correlative changes in surface charge and cell properties. J. Cell Mol. Med. 2011, 15, 2634–2642. [Google Scholar] [CrossRef] [PubMed]
- Jung, B.; Vullev, V.; Anvari, B. Revisiting indocyanine green: Effects of serum and physiological temperature on absorption and fluorescence characteristics. IEEE J. Sel. Top. Quantum Electron. 2014, 20, 7000409. [Google Scholar]
- Nguyen, T.N.; Nguyen, T.T.; Nghiem, T.H.L.; Nguyen, D.T.; Tran, T.T.H.; Vu, D.; Nguyen, T.B.N.; Nguyen, T.M.H.; Nguyen, V.T.; Nguyen, M.H. Optical properties of doxorubicin hydrochloride load and release on silica nanoparticle platform. Molecules 2021, 26, 3968. [Google Scholar] [CrossRef]
- Kasha, M.; Rawls, H.R.; Ashraf El-Bayoumi, M. The exciton model in molecular spectroscopy. Pure Appl. Chem. 1965, 11, 371–392. [Google Scholar]
- Justus, C.R.; Dong, L.; Yang, L.V. Acidic tumor microenvironment and pH-sensing G protein-coupled receptors. Front. Physiol. 2013, 4, 354. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Yaseen, M.A.; Anvari, B.; Wong, M.S. Synthesis of near-infrared-absorbing nanoparticle-assembled capsules. Chem. Mater. 2007, 19, 1277–1284. [Google Scholar] [CrossRef]
- Burns, J.M.; Vankayala, R.; Mac, J.T.; Anvari, B. Erythrocyte-derived theranostic nanoplatforms for near infrared fluorescence imaging and photodustruction of tumors. ACS. Appl. Mater. Interfaces 2018, 10, 27621–27630. [Google Scholar] [CrossRef]
- Li, S.S.; Zhang, M.; Wang, J.H.; Yang, F.; Kang, B.; Xu, J.J.; Chen, H.Y. Monitoring the changes of pH in lysosomes during autophagy and ppoptosis by plasmon enhanced raman imaging. Anal. Chem. 2019, 91, 8398–8405. [Google Scholar] [CrossRef]
- Crane, L.M.; van Oosten, M.; Pleijhuis, R.G.; Motekallemi, A.; Dowdy, S.C.; Cliby, W.A.; van der Zee, A.G.; van Dam, G.M. Intraoperative imaging in ovarian cancer: Fact or fiction. Mol. Imaging 2011, 10, 248–257. [Google Scholar] [CrossRef]
- Mac, J.T.; Nunez, V.; Burns, M.J.; Guerrero, Y.A.; Vullev, I.V.; Anvari, B. Erythrocyte-derived nano-probes functionalized with antibodies for targeted near infrared fluorescence imaging of cancer cells. Biomed. Opt. Express 2016, 7, 1311–1322. [Google Scholar] [CrossRef]
- Reiners, J.J., Jr.; Caruso, J.A.; Mathieu, P.; Chelladurai, B.; Yin, X.M.; Kessel, D. Release of cytochrome c and activation of pro-caspase-9 following lysosomal photodamage involves Bid cleavage. Cell Death Differ. 2002, 9, 934–944. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Konorev, E.A.; Kotamraju, S.; Joseph, J.; Kalivendi, S.; Kalyanaraman, B. Doxorubicin induces apoptosis in normal and tumor cells via distinctly different mechanisms. intermediacy of H2O2- and p53-dependent pathways. J. Biol. Chem. 2004, 279, 25535–25543. [Google Scholar] [CrossRef] [PubMed]
- Altshuler, G.B.; Anderson, R.R.; Manstein, D.; Zenzie, H.H.; Smirnov, M.Z. Extended theory of selective photothermolysis. Lasers Surg. Med. 2001, 29, 416–432. [Google Scholar] [CrossRef] [PubMed]
- Gurney, H. How to calculate the dose of chemotherapy. Br. J. Cancer 2002, 86, 1297–1302. [Google Scholar] [CrossRef]
- Tobis, S.; Knopf, J.; Silvers, C.; Yao, J.; Rashid, H.; Wu, G.; Golijanin, D. Near infrared fluorescence imaging with robotic assisted laparoscopic partial nephrectomy: Initial clinical experience for renal cortical tumors. J. Urol. 2011, 186, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.; Baumler, H.; Buschmann, M.; Landthaler, M.; Babilas, P. A randomized controlled trial to optimize indocyanine green-augmented diode laser therapy of capillary malformations. Lasers Surg. Med. 2013, 45, 216–224. [Google Scholar] [CrossRef]
- Ebert, B.; Riefke, B.; Sukowski, U.; Licha, K. Cyanine dyes as contrast agents for near-infrared imaging in vivo: Acute tolerance, pharmacokinetics, and fluorescence imaging. J. Biomed. Opt. 2011, 16, 0660003. [Google Scholar] [CrossRef]
- Hedtmann, U.; Fehlhaber, H.W.; Sukastasch, D.A.; Weber, W.; Hoffmann, D.; Kraemer, H.P. The new cytotoxic antibiotic cytorhodin X, an unusual anthracyclinone-9 alpha-glycoside. J. Antibiot. 1992, 45, 1373–1375. [Google Scholar] [CrossRef]




| Group Number | Administered Agent (Dose) | Laser Irradiation (Yes/No) |
|---|---|---|
| Group 1 | PBS | Yes |
| Group 2 | Free ICG (2.67 mg/kg) | Yes |
| Group 3 | Free DOX (0.76 mg/kg) | No |
| Group 4 | Free ICG (2.67 mg/kg) + Free DOX (0.76 mg/kg) | Yes |
| Group 5 | F-NETs (2.67 mg ICG/kg) | Yes |
| Group 6 | F-IDNETs (2.67 mg ICG/kg + 0.76 mg DOX/kg) | Yes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mac, J.T.; Vankayala, R.; Lee, C.-H.; Anvari, B. Erythrocyte-Derived Nanoparticles with Folate Functionalization for Near Infrared Pulsed Laser-Mediated Photo-Chemotherapy of Tumors. Int. J. Mol. Sci. 2022, 23, 10295. https://doi.org/10.3390/ijms231810295
Mac JT, Vankayala R, Lee C-H, Anvari B. Erythrocyte-Derived Nanoparticles with Folate Functionalization for Near Infrared Pulsed Laser-Mediated Photo-Chemotherapy of Tumors. International Journal of Molecular Sciences. 2022; 23(18):10295. https://doi.org/10.3390/ijms231810295
Chicago/Turabian StyleMac, Jenny T., Raviraj Vankayala, Chi-Hua Lee, and Bahman Anvari. 2022. "Erythrocyte-Derived Nanoparticles with Folate Functionalization for Near Infrared Pulsed Laser-Mediated Photo-Chemotherapy of Tumors" International Journal of Molecular Sciences 23, no. 18: 10295. https://doi.org/10.3390/ijms231810295
APA StyleMac, J. T., Vankayala, R., Lee, C.-H., & Anvari, B. (2022). Erythrocyte-Derived Nanoparticles with Folate Functionalization for Near Infrared Pulsed Laser-Mediated Photo-Chemotherapy of Tumors. International Journal of Molecular Sciences, 23(18), 10295. https://doi.org/10.3390/ijms231810295









