The Role of Thymoquinone in Inflammatory Response in Chronic Diseases
Abstract
:1. Introduction
2. TQ Effect on Inflammation
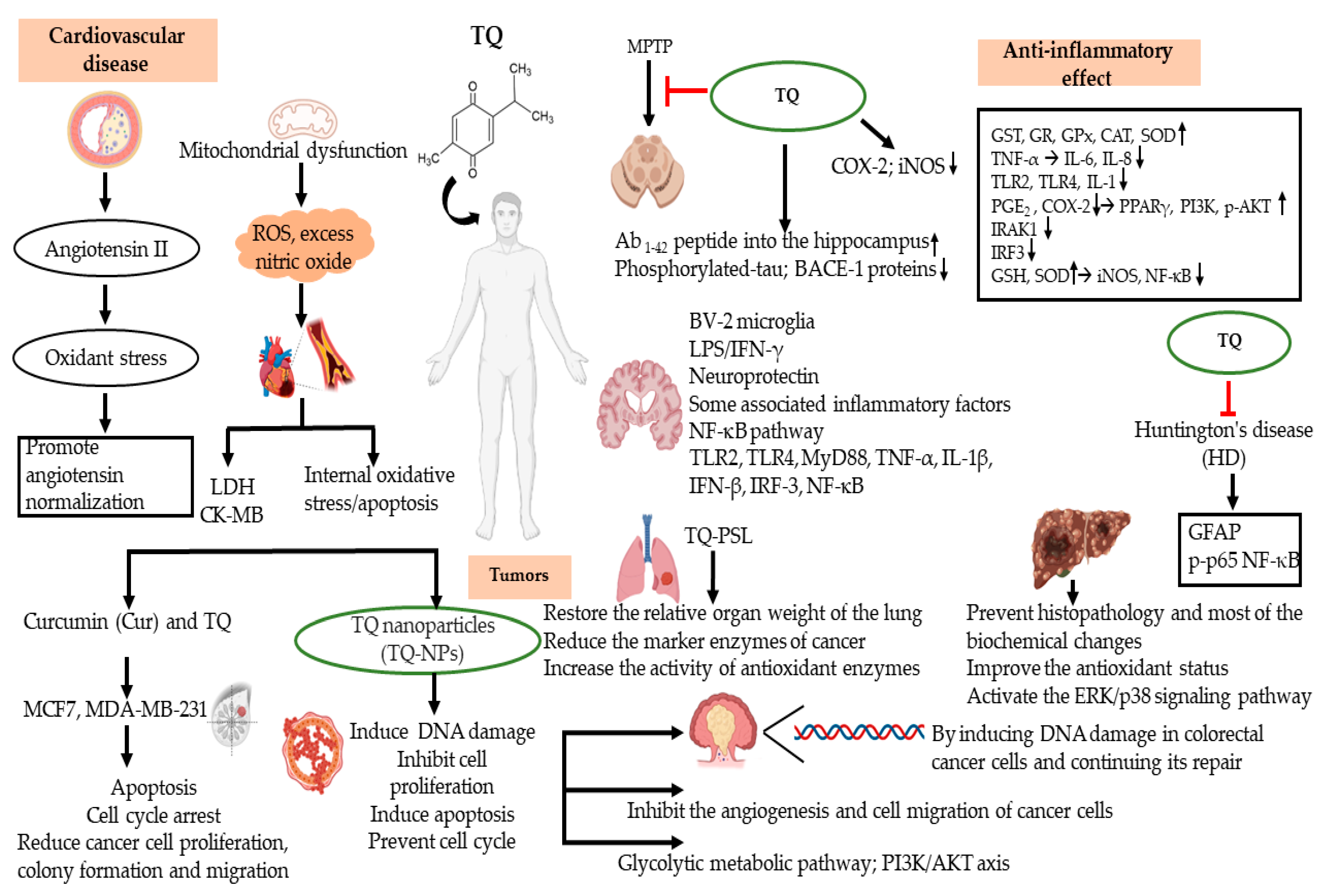
3. Anti-Inflammatory Effects of TQ in Cardiovascular Disease (CVDs)
3.1. Atherosclerosis (AS)
3.2. Myocardial Infarction (MI)
4. Anti-Inflammatory Effects of TQ in Tumors
4.1. Breast Cancer
4.2. Colorectal Cancer (CRC)
4.3. Lung Cancer
4.4. Hepatocellular Carcinoma (HCC)
4.5. Other Cancers
5. Anti-Inflammatory Effects of TQ in Neurodegenerative Disorders
5.1. Parkinson’s Disease (PD)
5.2. Alzheimer’s Disease (AD)
5.3. Huntington’s Disease (HD)
6. Anti-Inflammatory Effect of TQ in Other Diseases
7. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TQ | Thymoquinone, 2-isopropyl-5-methylbenzo-1,4-quinone |
| GST | Glutathione S-transferase |
| GR | Glutathione reductase |
| GPx | Glutathione peroxidase |
| CAT | Catalase |
| EAE | Experimental autoimmune encephalomyelitis |
| MS | Multiple sclerosis |
| GSH | Glutathione |
| GSTA3 | Glutathione-S-transferase enzyme alpha-3 |
| MPO | Myeloperoxidase |
| DAI | Disease activity index |
| TPA | 12-O-tetradecanoylphorbol-13-acetate |
| AS | Atherosclerosis |
| MI | Myocardial Infarction |
| CRC | Colorectal Cancer |
| HCC | Hepatocellular carcinoma |
| AD | Alzheimer’s disease |
| PD | Parkinson’s disease |
| NO | Nitric oxide |
| CCL12 | Chemokine C-C motif ligand 12 |
| MCP-5 | Monocyte chemotactic protein 5 |
| GCSF | Granulocyte colony-stimulating factor |
| mPGES-1 | Microsomal prostaglandin E synthase-1 |
| COX-2 | Cyclooxygenase-2 |
| IL-1β | Interleukin 1 β |
| IL-6 | Interleukin 6 |
| IFN-γ | Interferon gamma |
| TNF-α | Tumor necrosis factor alpha |
| PGE2 | Prostaglandin E2 |
| LPS | Lipopolysaccharides |
| ARE | Antioxidant responsive element |
| NFE2L2/Nrf2 | Nuclear factor (erythroid-derived 2) -like 2 |
| PI3K | Phosphoinositide 3-kinase |
| PKB | Protein kinase B |
| MPTP | 1-methyl-4-phenyl 1,2,3,6 tetrahydropyridine |
| iNOS | Nitric oxide synthase |
| Aβ1-42 | The amyloid beta 1-42 |
References
- Raskov, H.; Orhan, A.; Salanti, A.; Gaggar, S.; Gogenur, I. Natural killer cells in cancer and cancer immunotherapy. Cancer Lett. 2021, 520, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Hung, A.C.; Lo, S.; Yuan, S.F. Adipocytokines visfatin and resistin in breast cancer: Clinical relevance, biological mechanisms, and therapeutic potential. Cancer Lett. 2021, 498, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Kohandel, Z.; Farkhondeh, T.; Aschner, M.; Samarghandian, S. Anti-inflammatory effects of thymoquinone and its protective effects against several diseases. Biomed. Pharmacother. 2021, 138, 111492. [Google Scholar] [CrossRef]
- Hannan, M.A.; Zahan, M.S.; Sarker, P.P.; Moni, A.; Ha, H.; Uddin, M.J. Protective effects of black cumin (Nigella sativa) and its bioactive constituent, thymoquinone against kidney injury: An aspect on pharmacological insights. Int. J. Mol. Sci. 2021, 22, 9078. [Google Scholar] [CrossRef] [PubMed]
- Gholamnezhad, Z.; Havakhah, S.; Boskabady, M.H. Preclinical and clinical effects of Nigella sativa and its constituent, thymoquinone: A review. J. Ethnopharmacol. 2016, 190, 372–386. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.; Singh, A.; Negi, P.; Kapoor, V.K. Thymoquinone: A small molecule from nature with high therapeutic potential. Drug Discov. Today 2021, 26, 2716–2725. [Google Scholar] [CrossRef]
- Rathod, S.; Agrawal, Y.; Sherikar, A.; Nakhate, K.T.; Patil, C.R.; Nagoor Meeran, M.F.; Ojha, S.; Goyal, S.N. Thymoquinone produces cardioprotective effect in beta-receptor stimulated myocardial infarcted rats via subsiding oxidative stress and inflammation. Nutrients 2022, 14, 2742. [Google Scholar] [CrossRef]
- Xue, Q.; He, N.; Wang, Z.; Fu, X.; Aung, L.H.H.; Liu, Y.; Li, M.; Cho, J.Y.; Yang, Y.; Yu, T. Functional roles and mechanisms of ginsenosides from Panax ginseng in atherosclerosis. J. Ginseng Res. 2021, 45, 22–31. [Google Scholar] [CrossRef]
- Kim, J.H.; Yi, Y.S.; Kim, M.Y.; Cho, J.Y. Role of ginsenosides, the main active components of Panax ginseng, in inflammatory responses and diseases. J. Ginseng Res. 2017, 41, 435–443. [Google Scholar] [CrossRef]
- Yang, J.; Li, Y.; Sun, Z.; Zhan, H. Macrophages in pancreatic cancer: An immunometabolic perspective. Cancer Lett. 2021, 498, 188–200. [Google Scholar] [CrossRef]
- Chen, Y.; Ho, L.; Tergaonkar, V. sORF-Encoded MicroPeptides: New players in inflammation, metabolism, and precision medicine. Cancer Lett. 2021, 500, 263–270. [Google Scholar] [CrossRef]
- Kim, J.K.; Shin, K.K.; Kim, H.; Hong, Y.H.; Choi, W.; Kwak, Y.S.; Han, C.K.; Hyun, S.H.; Cho, J.Y. Korean Red Ginseng exerts anti-inflammatory and autophagy-promoting activities in aged mice. J. Ginseng Res. 2021, 45, 717–725. [Google Scholar] [CrossRef]
- Wan, Y.; Wang, J.; Xu, J.F.; Tang, F.; Chen, L.; Tan, Y.Z.; Rao, C.L.; Ao, H.; Peng, C. Panax ginseng and its ginsenosides: Potential candidates for the prevention and treatment of chemotherapy-induced side effects. J. Ginseng Res. 2021, 45, 617–630. [Google Scholar] [CrossRef]
- Abad, E.; Samino, S.; Grodzicki, R.L.; Pagano, G.; Trifuoggi, M.; Graifer, D.; Potesil, D.; Zdrahal, Z.; Yanes, O.; Lyakhovich, A. Identification of metabolic changes leading to cancer susceptibility in Fanconi anemia cells. Cancer Lett. 2021, 503, 185–196. [Google Scholar] [CrossRef]
- Lu, W.; Li, X.; Luo, Y. FGF21 in obesity and cancer: New insights. Cancer Lett. 2021, 499, 5–13. [Google Scholar] [CrossRef]
- Chen, H.; Zhou, X.H.; Li, J.R.; Zheng, T.H.; Yao, F.B.; Gao, B.; Xue, T.C. Neutrophils: Driving inflammation during the development of hepatocellular carcinoma. Cancer Lett. 2021, 522, 22–31. [Google Scholar] [CrossRef]
- Tan, Y.N.; Li, Y.P.; Huang, J.D.; Luo, M.; Li, S.S.; Lee, A.W.; Hu, F.Q.; Guan, X.Y. Thermal-sensitive lipid nanoparticles potentiate anti-PD therapy through enhancing drug penetration and T lymphocytes infiltration in metastatic tumor. Cancer Lett. 2021, 522, 238–254. [Google Scholar] [CrossRef]
- Gao, Y.; Yuan, D.; Gai, L.; Wu, X.; Shi, Y.; He, Y.; Liu, C.; Zhang, C.; Zhou, G.; Yuan, C. Saponins from Panax japonicus ameliorate age-related renal fibrosis by inhibition of inflammation mediated by NF-kappaB and TGF-beta1/Smad signaling and suppression of oxidative stress via activation of Nrf2-ARE signaling. J. Ginseng Res. 2021, 45, 408–419. [Google Scholar] [CrossRef]
- Lee, M.J.; Choi, J.H.; Oh, J.; Lee, Y.H.; In, J.G.; Chang, B.J.; Nah, S.Y.; Cho, I.H. Rg3-enriched Korean Red Ginseng extract inhibits blood-brain barrier disruption in an animal model of multiple sclerosis by modulating expression of NADPH oxidase 2 and 4. J. Ginseng Res. 2021, 45, 433–441. [Google Scholar] [CrossRef]
- Mohamed, A.; Waris, H.M.; Ramadan, H.; Quereshi, M.; Kalra, J. Amelioration of chronic relapsing experimental autoimmune encephalomyelitis (cr-eae) using thymoquinone - biomed 2009. Biomed. Sci. Instrum. 2009, 45, 274–279. [Google Scholar]
- Mohamed, A.; Shoker, A.; Bendjelloul, F.; Mare, A.; Alzrigh, M.; Benghuzzi, H.; Desin, T. Improvement of experimental allergic encephalomyelitis (EAE) by thymoquinone. An oxidative stress inhibitor. Biomed. Sci. Instrum. 2003, 39, 440–445. [Google Scholar] [PubMed]
- Ates, M.B.; Ortatatli, M. The effects of Nigella sativa seeds and thymoquinone on aflatoxin phase-2 detoxification through glutathione and glutathione-S-transferase alpha-3, and the relationship between aflatoxin B1-DNA adducts in broilers. Toxicon. 2021, 193, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.O.; Kim, J.H.; Kim, S.; Kim, M.Y.; Hong, Y.H.; Kim, H.G.; Cho, J.Y. Gastroprotective effects of the nonsaponin fraction of Korean Red Ginseng through cyclooxygenase-1 upregulation. J. Ginseng Res. 2020, 44, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, R.M.; Delgobo, M.; Agnes, J.P.; das Neves, R.N.; Falchetti, M.; Casagrande, T.; Garcia, A.P.V.; Vieira, T.C.; Somensi, N.; Bruxel, M.A.; et al. COX-2 promotes mammary adipose tissue inflammation, local estrogen biosynthesis, and carcinogenesis in high-sugar/fat diet treated mice. Cancer Lett. 2021, 502, 44–57. [Google Scholar] [CrossRef]
- Chen, E.P.; Smyth, E.M. COX-2 and PGE2-dependent immunomodulation in breast cancer. Prostaglandins Other Lipid Mediat. 2011, 96, 14–20. [Google Scholar] [CrossRef]
- Al Wafai, R.J. Nigella sativa and thymoquinone suppress cyclooxygenase-2 and oxidative stress in pancreatic tissue of streptozotocin-induced diabetic rats. Pancreas 2013, 42, 841–849. [Google Scholar] [CrossRef]
- Venkataraman, B.; Almarzooqi, S.; Raj, V.; Alhassani, A.T.; Alhassani, A.S.; Ahmed, K.J.; Subramanian, V.S.; Ojha, S.K.; Attoub, S.; Adrian, T.E.; et al. Thymoquinone, a dietary bioactive compound, exerts anti-inflammatory effects in colitis by stimulating expression of the colonic epithelial PPAR-gamma transcription factor. Nutrients 2021, 13, 1343. [Google Scholar] [CrossRef]
- Kundu, J.K.; Liu, L.; Shin, J.W.; Surh, Y.J. Thymoquinone inhibits phorbol ester-induced activation of NF-kappaB and expression of COX-2, and induces expression of cytoprotective enzymes in mouse skin in vivo. Biochem. Biophys. Res. Commun. 2013, 438, 721–727. [Google Scholar] [CrossRef]
- Umar, S.; Zargan, J.; Umar, K.; Ahmad, S.; Katiyar, C.K.; Khan, H.A. Modulation of the oxidative stress and inflammatory cytokine response by thymoquinone in the collagen induced arthritis in Wistar rats. Chem. Biol. Interact. 2012, 197, 40–46. [Google Scholar] [CrossRef]
- Ahmad, A.; Raish, M.; Alkharfy, K.M. The potential role of thymoquinone in preventing the cardiovascular complications of COVID-19. Vascul. Pharmacol. 2021, 141, 106899. [Google Scholar] [CrossRef]
- Jang, Y.J.; Aravinthan, A.; Hossain, M.A.; Kopalli, S.R.; Kim, B.; Kim, N.S.; Kang, C.W.; Kim, J.H. Effect of Korean Red Ginseng through comparative analysis of cardiac gene expression in db/db mice. J. Ginseng Res. 2021, 45, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Idris-Khodja, N.; Schini-Kerth, V. Thymoquinone improves aging-related endothelial dysfunction in the rat mesenteric artery. Naunyn Schmiedebergs Arch. Pharmacol. 2012, 385, 749–758. [Google Scholar] [CrossRef]
- Nader, M.A.; el-Agamy, D.S.; Suddek, G.M. Protective effects of propolis and thymoquinone on development of atherosclerosis in cholesterol-fed rabbits. Arch. Pharm. Res. 2010, 33, 637–643. [Google Scholar] [CrossRef]
- Xiao, J.; Ke, Z.P.; Shi, Y.; Zeng, Q.; Cao, Z. The cardioprotective effect of thymoquinone on ischemia-reperfusion injury in isolated rat heart via regulation of apoptosis and autophagy. J. Cell Biochem. 2018, 119, 7212–7217. [Google Scholar] [CrossRef]
- Medhet, M.; El-Bakly, W.M.; Badr, A.M.; Awad, A.; El-Demerdash, E. Thymoquinone attenuates isoproterenol-induced myocardial infarction by inhibiting cytochrome C and matrix metalloproteinase-9 expression. Clin. Exp. Pharmacol. Physiol. 2022, 49, 391–405. [Google Scholar] [CrossRef] [PubMed]
- El-Far, A.H.; Saddiq, A.A.; Mohamed, S.A.; Almaghrabi, O.A.; Mousa, S.A. Curcumin and thymoquinone combination attenuates breast cancer cell lines’ progression. Integr. Cancer Ther. 2022, 21, 15347354221099537. [Google Scholar] [CrossRef] [PubMed]
- Sjs, B.; Sumathi, S. Modulation of gene expression by thymoquinone conjugated Zinc Oxide nanoparticles arrested cell cycle, DNA damage and increased apoptosis in triple negative breast cancer cell line MDA-MB-231. Drug Dev. Ind. Pharm. 2022, 1–19. [Google Scholar] [CrossRef]
- Al Bitar, S.; Ballout, F.; Monzer, A.; Kanso, M.; Saheb, N.; Mukherji, D.; Faraj, W.; Tawil, A.; Doughan, S.; Hussein, M.; et al. Thymoquinone radiosensitizes human colorectal cancer cells in 2D and 3D culture models. Cancers 2022, 14, 1363. [Google Scholar] [CrossRef]
- Karim, S.; Burzangi, A.S.; Ahmad, A.; Siddiqui, N.A.; Ibrahim, I.M.; Sharma, P.; Abualsunun, W.A.; Gabr, G.A. PI3K-AKT pathway modulation by thymoquinone limits tumor growth and glycolytic metabolism in colorectal cancer. Int. J. Mol. Sci. 2022, 23, 2305. [Google Scholar] [CrossRef]
- Asfour, H.Z.; Fahmy, U.A.; Alharbi, W.S.; Almehmady, A.M.; Alamoudi, A.J.; Tima, S.; Mansouri, R.A.; Omar, U.M.; Ahmed, O.A.A.; Zakai, S.A.; et al. Phyto-phospholipid conjugated Scorpion venom nanovesicles as promising carrier that improves efficacy of thymoquinone against adenocarcinoma human alveolar basal epithelial cells. Pharmaceutics 2021, 13, 2144. [Google Scholar] [CrossRef]
- Upadhyay, P.; Ghosh, A.; Basu, A.; Pranati, P.A.; Gupta, P.; Das, S.; Sarker, S.; Bhattacharjee, M.; Bhattacharya, S.; Ghosh, S.; et al. Delivery of gefitinib in synergism with thymoquinone via transferrin-conjugated nanoparticle sensitizes gefitinib-resistant non-small cell lung carcinoma to control metastasis and stemness. Biomater. Sci. 2021, 9, 8285–8312. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.; Fahim, S.A.; Tadros, S.A.; Badary, O.A. Suppressive effects of thymoquinone on the initiation stage of diethylnitrosamine hepatocarcinogenesis in rats. J. Biochem. Mol. Toxicol. 2022, 36, e23078. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Ting, W.J.; Gao, J.; Kang, Z.F.; Huang, C.Y.; Weng, Y.J. Erk phosphorylation reduces the thymoquinone toxicity in human hepatocarcinoma. Environ. Toxicol. 2021, 36, 1990–1998. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, P.; Farghadani, R.; Nyamathulla, S.; Rajarajeswaran, J.; Thirugnanasampandan, R.; Bhuwaneswari, G. Natural quinones induce ROS-mediated apoptosis and inhibit cell migration in PANC-1 human pancreatic cancer cell line. J. Biochem. Mol. Toxicol. 2022, 36, e23008. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Du, H.; Wang, L.; Yue, Y.; Zhang, P.; Huang, Z.; Lv, W.; Ma, J.; Shao, Q.; Ma, M.; et al. Thymoquinone suppresses invasion and metastasis in bladder cancer cells by reversing EMT through the Wnt/beta-catenin signaling pathway. Chem. Biol. Interact. 2020, 320, 109022. [Google Scholar] [CrossRef] [PubMed]
- Ardah, M.T.; Merghani, M.M.; Haque, M.E. Thymoquinone prevents neurodegeneration against MPTP in vivo and modulates alpha-synuclein aggregation in vitro. Neurochem. Int. 2019, 128, 115–126. [Google Scholar] [CrossRef]
- Elibol, B.; Beker, M.; Terzioglu-Usak, S.; Dalli, T.; Kilic, U. Thymoquinone administration ameliorates Alzheimer’s disease-like phenotype by promoting cell survival in the hippocampus of amyloid beta1-42 infused rat model. Phytomedicine 2020, 79, 153324. [Google Scholar] [CrossRef]
- Cobourne-Duval, M.K.; Taka, E.; Mendonca, P.; Soliman, K.F.A. Thymoquinone increases the expression of neuroprotective proteins while decreasing the expression of pro-inflammatory cytokines and the gene expression NFkappaB pathway signaling targets in LPS/IFNgamma-activated BV-2 microglia cells. J. Neuroimmunol. 2018, 320, 87–97. [Google Scholar] [CrossRef]
- Abulfadl, Y.S.; El-Maraghy, N.N.; Ahmed, A.E.; Nofal, S.; Abdel-Mottaleb, Y.; Badary, O.A. Thymoquinone alleviates the experimentally induced Alzheimer’s disease inflammation by modulation of TLRs signaling. Hum. Exp. Toxicol. 2018, 37, 1092–1104. [Google Scholar] [CrossRef]
- Ramachandran, S.; Thangarajan, S. Thymoquinone loaded solid lipid nanoparticles counteracts 3-Nitropropionic acid induced motor impairments and neuroinflammation in rat model of Huntington’s disease. Metab. Brain Dis. 2018, 33, 1459–1470. [Google Scholar] [CrossRef]
- Xu, J.; Zhu, L.; Liu, H.; Li, M.; Liu, Y.; Yang, F.; Pei, Z. Thymoquinone reduces cardiac damage caused by hypercholesterolemia in apolipoprotein E-deficient mice. Lipids Health Dis. 2018, 17, 173. [Google Scholar] [CrossRef] [Green Version]
- Huang, Q.; Gao, S.; Zhao, D.; Li, X. Review of ginsenosides targeting mitochondrial function to treat multiple disorders: Current status and perspectives. J. Ginseng Res. 2021, 45, 371–379. [Google Scholar] [CrossRef]
- Farag, M.M.; Khalifa, A.A.; Elhadidy, W.F.; Rashad, R.M. Thymoquinone dose-dependently attenuates myocardial injury induced by isoproterenol in rats via integrated modulations of oxidative stress, inflammation, apoptosis, autophagy, and fibrosis. Naunyn Schmiedebergs Arch. Pharmacol. 2021, 394, 1787–1801. [Google Scholar] [CrossRef]
- Shi, W.; Yang, X.; Xie, S.; Zhong, D.; Lin, X.; Ding, Z.; Duan, S.; Mo, F.; Liu, A.; Yin, S.; et al. A new PD-1-specific nanobody enhances the antitumor activity of T-cells in synergy with dendritic cell vaccine. Cancer Lett. 2021, 522, 184–197. [Google Scholar] [CrossRef]
- Huang, C.P.; Liu, L.C.; Chang, C.C.; Wu, C.C.; Shyr, C.R. Intratumoral xenogeneic tissue-specific cell immunotherapy inhibits tumor growth by increasing antitumor immunity in murine triple negative breast and pancreatic tumor models. Cancer Lett. 2021. [Google Scholar] [CrossRef]
- Murphy, E.M.; Centner, C.S.; Bates, P.J.; Malik, M.T.; Kopechek, J.A. Delivery of thymoquinone to cancer cells with as1411-conjugated nanodroplets. PLoS One 2020, 15, e0233466. [Google Scholar] [CrossRef]
- Salama, B.; Alzahrani, K.J.; Alghamdi, K.S.; Al-Amer, O.; Hassan, K.E.; Elhefny, M.A.; Albarakati, A.J.A.; Alharthi, F.; Althagafi, H.A.; Al Sberi, H.; et al. Silver nanoparticles enhance oxidative stress, inflammation, and apoptosis in liver and kidney tissues: Potential protective role of thymoquinone. Biol. Trace Elem. Res. 2022. [Google Scholar] [CrossRef]
- Li, N.; Lu, B.; Luo, C.; Cai, J.; Lu, M.; Zhang, Y.; Chen, H.; Dai, M. Incidence, mortality, survival, risk factor and screening of colorectal cancer: A comparison among China, Europe, and northern America. Cancer Lett. 2021, 522, 255–268. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, Y.; Wang, J.; Yang, Y.; Zhang, Y.; Qu, X.; Peng, S.; Yao, Z.; Zhao, S.; He, B.; et al. FoxO3 reverses 5-fluorouracil resistance in human colorectal cancer cells by inhibiting the Nrf2/TR1 signaling pathway. Cancer Lett. 2020, 470, 29–42. [Google Scholar] [CrossRef]
- Wojas-Krawczyk, K.; Krawczyk, P.; Gil, M.; Strzemski, M. Two complementarity immunotherapeutics in non-small-cell lung cancer patients-Mechanism of action and future concepts. Cancers 2021, 13, 2836. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, Y.; Wang, K.; Li, T.; Yang, M.; Wang, R.; Chen, Y.; Cao, M.; Hu, R. Flumethasone enhances the efficacy of chemotherapeutic drugs in lung cancer by inhibiting Nrf2 signaling pathway. Cancer Lett. 2020, 474, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.L.; Chou, H.Y.; Chiu, Y.C.; Cheng, A.N.; Fan, C.C.; Chang, Y.N.; Chen, C.H.; Jiang, S.S.; Chen, N.J.; Lee, A.Y. Mitochondrial oxidative stress by Lon-PYCR1 maintains an immunosuppressive tumor microenvironment that promotes cancer progression and metastasis. Cancer Lett. 2020, 474, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Alsahli, M.A.; Aljasir, M.A.; Maswadeh, H.; Mobark, M.A.; Azam, F.; Allemailem, K.S.; Alrumaihi, F.; Alhumaydhi, F.A.; Almatroudi, A.A.; et al. Experimental and theoretical insights on chemopreventive effect of the liposomal thymoquinone against benzo[a]pyrene-induced lung cancer in Swiss albino mice. J. Inflamm. Res. 2022, 15, 2263–2280. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, S.; Zarrabi, A.; Hashemi, F.; Zabolian, A.; Saleki, H.; Ranjbar, A.; Seyed Saleh, S.H.; Bagherian, M.; Sharifzadeh, S.O.; Hushmandi, K.; et al. Regulation of nuclear factor-kappaB (NF-kappaB) signaling pathway by non-coding RNAs in cancer: Inhibiting or promoting carcinogenesis? Cancer Lett. 2021, 509, 63–80. [Google Scholar] [CrossRef]
- Zhang, R.; Wu, T.; Zheng, P.; Liu, M.; Xu, G.; Xi, M.; Yu, J. Thymoquinone sensitizes human hepatocarcinoma cells to TRAIL-induced apoptosis via oxidative DNA damage. DNA Repair 2021, 103, 103117. [Google Scholar] [CrossRef]
- Lin, K.; Sze, S.C.; Liu, B.; Zhang, Z.; Zhang, Z.; Zhu, P.; Wang, Y.; Deng, Q.; Yung, K.K.; Zhang, S. 20(S)-protopanaxadiol and oleanolic acid ameliorate cognitive deficits in APP/PS1 transgenic mice by enhancing hippocampal neurogenesis. J. Ginseng Res. 2021, 45, 325–333. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, M.; Chen, R.; Sun, X.; Sun, G.; Sun, X. Gypenoside XVII protects against myocardial ischemia and reperfusion injury by inhibiting ER stress-induced mitochondrial injury. J. Ginseng Res. 2021, 45, 642–653. [Google Scholar] [CrossRef]
- Pottoo, F.H.; Ibrahim, A.M.; Alammar, A.; Alsinan, R.; Aleid, M.; Alshehhi, A.; Alshehri, M.; Mishra, S.; Alhajri, N. Thymoquinone: Review of its potential in the treatment of neurological diseases. Pharmaceuticals 2022, 15, 408. [Google Scholar] [CrossRef]
- Abbas, F.; Eladl, M.A.; El-Sherbiny, M.; Abozied, N.; Nabil, A.; Mahmoud, S.M.; Mokhtar, H.I.; Zaitone, S.A.; Ibrahim, D. Celastrol and thymoquinone alleviate aluminum chloride-induced neurotoxicity: Behavioral psychomotor performance, neurotransmitter level, oxidative-inflammatory markers, and BDNF expression in rat brain. Biomed. Pharmacother. 2022, 151, 113072. [Google Scholar] [CrossRef]
- Behl, T.; Madaan, P.; Sehgal, A.; Singh, S.; Makeen, H.A.; Albratty, M.; Alhazmi, H.A.; Meraya, A.M.; Bungau, S. Demystifying the neuroprotective role of neuropeptides in Parkinson’s Disease: A newfangled and eloquent therapeutic perspective. Int. J. Mol. Sci. 2022, 23, 4565. [Google Scholar] [CrossRef]
- Gonzales, M.M.; Garbarino, V.R.; Pollet, E.; Palavicini, J.P.; Kellogg, D.L., Jr.; Kraig, E.; Orr, M.E. Biological aging processes underlying cognitive decline and neurodegenerative disease. J. Clin. Invest. 2022, 132. [Google Scholar] [CrossRef]
- Zhang, H.; Su, Y.; Sun, Z.; Chen, M.; Han, Y.; Li, Y.; Dong, X.; Ding, S.; Fang, Z.; Li, W.; et al. Ginsenoside Rg1 alleviates Abeta deposition by inhibiting NADPH oxidase 2 activation in APP/PS1 mice. J. Ginseng Res. 2021, 45, 665–675. [Google Scholar] [CrossRef]
- Kim, M.; Mok, H.; Yeo, W.S.; Ahn, J.H.; Choi, Y.K. Role of ginseng in the neurovascular unit of neuroinflammatory diseases focused on the blood-brain barrier. J. Ginseng Res. 2021, 45, 599–609. [Google Scholar] [CrossRef]
- Yardim, A.; Kandemir, F.M.; Ozdemir, S.; Kucukler, S.; Comakli, S.; Gur, C.; Celik, H. Quercetin provides protection against the peripheral nerve damage caused by vincristine in rats by suppressing caspase 3, NF-kappaB, ATF-6 pathways and activating Nrf2, Akt pathways. Neurotoxicology 2020, 81, 137–146. [Google Scholar] [CrossRef]
- Hua, K.F.; Chao, A.C.; Lin, T.Y.; Chen, W.T.; Lee, Y.C.; Hsu, W.H.; Lee, S.L.; Wang, H.M.; Yang, D.I.; Ju, T.C. Ginsenoside compound K reduces the progression of Huntington’s disease via the inhibition of oxidative stress and overactivation of the ATM/AMPK pathway. J. Ginseng Res. 2022, 46, 572–584. [Google Scholar] [CrossRef]
- Ghosh, S.; Manchala, S.; Raghunath, M.; Sharma, G.; Singh, A.K.; Sinha, J.K. Role of phytomolecules in the treatment of obesity: Targets, mechanisms and limitations. Curr. Top. Med. Chem. 2021, 21, 863–877. [Google Scholar] [CrossRef]
- Seitz, K.P.; Qian, E.T.; Semler, M.W. Intravenous fluid therapy in sepsis. Nutr. Clin. Pract. 2022. [Google Scholar] [CrossRef]
- Zielinska, M.; Deren, K.; Polak-Szczybylo, E.; Stepien, A.E. The role of bioactive compounds of Nigella sativa in rheumatoid arthritis therapy-Current reports. Nutrients 2021, 13, 3369. [Google Scholar] [CrossRef]
- Ahmad, A.; Rehman, M.U.; Ahmad, P.; Alkharfy, K.M. Covid-19 and thymoquinone: Connecting the dots. Phytother. Res. 2020, 34, 2786–2789. [Google Scholar] [CrossRef]
- Xu, H.; Liu, B.; Xiao, Z.; Zhou, M.; Ge, L.; Jia, F.; Liu, Y.; Jin, H.; Zhu, X.; Gao, J.; et al. Computational and experimental studies reveal that thymoquinone blocks the entry of Coronaviruses into in vitro cells. Infect. Dis. Ther. 2021, 10, 483–494. [Google Scholar] [CrossRef]
- Fang, F.; Ni, Y.; Yu, H.; Yin, H.; Yang, F.; Li, C.; Sun, D.; Pei, T.; Ma, J.; Deng, L.; et al. Inflammatory endothelium-targeted and cathepsin responsive nanoparticles are effective against atherosclerosis. Theranostics 2022, 12, 4200–4220. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahim, M.; Esmail, A.; Al Saadi, N.; Zsigmond, E.; Al Najjar, E.; Bugazia, D.; Al-Rawi, H.; Alsaadi, A.; Kaseb, A.O. Thymoquinone’s antiviral effects: It is time to be proven in the Covid-19 pandemic era and its Omicron variant surge. Front. Pharmacol. 2022, 13, 848676. [Google Scholar] [CrossRef] [PubMed]
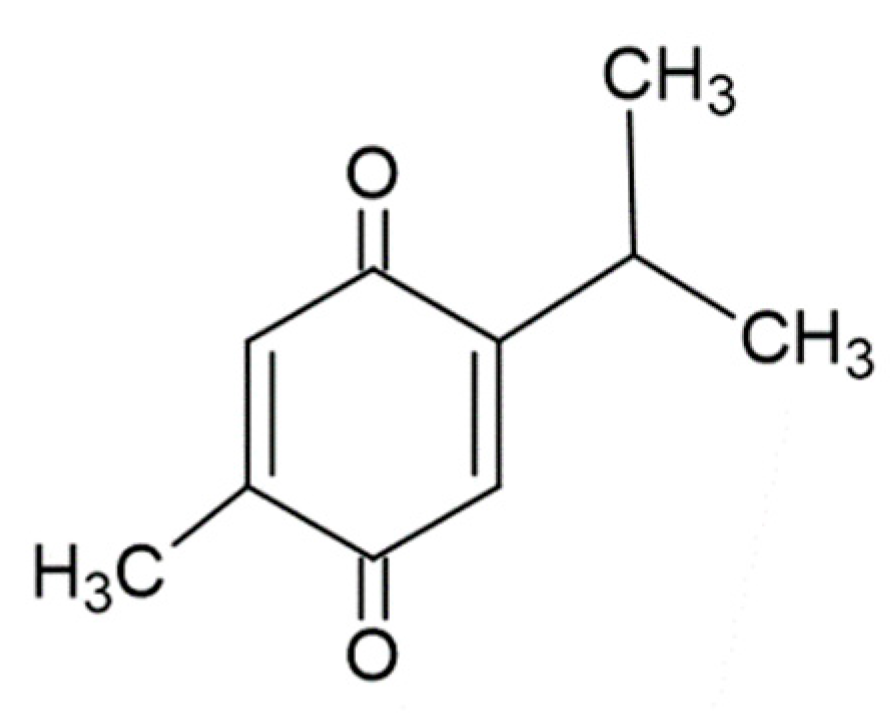

| Disease Type | Disease | Mechanisms | Control Type | Test Type | References |
|---|---|---|---|---|---|
| Cardiovascular | Atherosclerosis (AS) | TQ decreases angiotensin II expression, inhibits oxidative stress, and promotes angiotensin normalization. TQ reduces lipid accumulation and enhances antioxidant capacity and renal function. | 3% ethanol; EEP (75 mg/kg) | In vivo and in vitro | [32,33] |
| Cardiovascular Tumors | Myocardial Infarction (MI) | TQ improves heart function, reduces infarct size, reduces cardiac LDH and CK-MB, and inhibits internal oxidative stress and apoptosis. TQ antiapoptotic activity and inhibitory modulation of MMP-9 expression contribute to the TQ protective effect in MI. | Vehicle (100 μL/kg); Saline | In vivo and in vitro; In vivo | [34,35] |
| Breast cancer | TQ induces apoptosis and cell cycle arrest; reduces cancer cell proliferation, colony formation, and migration; and demonstrates promising anticancer benefits in breast cancer. TQ induces DNA damage, inhibits cell proliferation, induces apoptosis, and prevents cell cycle progression. | DMSO | In vitro | [36,37] | |
| Tumors Neurodegenerative disorders | Colorectal Cancer (CRC) | TQ inhibits the angiogenesis and cell migration of cancer cells. TQ inhibits the carcinogenesis and development of colorectal RC by regulating the glycolytic metabolic pathway and the PI3/AKT axis. | Methanol; 3-bromopyruvate | In vitro | [38,39] |
| Lung cancer | TQ inhibits tumor cell proliferation by causing lung cancer cell apoptosis to significantly arrest the S phase cell cycle and significantly reduce the activity of TNF-a and NF-κB to achieve anticancer effects. TQ regulates the STAT3/PTEN/AKT/miR-21 axis. | PLGA nanoparticles; Normal saline (1 mL/kg/day) | In vitro; In vivo and in vitro | [40,41] | |
| Hepatocellular carcinoma (HCC) | TQ pretreatment in rats can prevent histopathology and most of the biochemical changes and improve the antioxidant status. TQ induces HCC cell death and activates the Erk and p38 signaling pathway. TQ exhibits excellent anti-HCC potential under suitable p-Erk inhibition conditions. TQ cooperates with TRAIL to induce apoptosis in cancer cells, mediated through DNA damage. | DMSO | In vivo and in vitro | [42,43] | |
| Pancreatic cancer | TQ significantly increases the level of ROS production in human pancreatic cancer cells and inhibits cancer cell proliferation and migration. | DMSO | In vivo | [44] | |
| Bladder cancer | TQ inhibits the fusion of autophagosomes and lysosomes, leading to apoptosis in cancer cells. TQ initiates the miR-877-5p and PD-L1 signaling pathways, inhibiting the migration and EMT of bladder cancer cells. | DMSO | In vivo and in vitro | [45] | |
| Alzheimer’s disease (AD) | TQ reduces activity of superoxide dismutase and catalase and the expression of COX-2 and iNOS. TQ enhances the Aβ1-42 peptides injected into the hippocampus and improves the memory ability of rats. TQ significantly reduced the expression of Aβ, phosphorylated-tau, and BACE-1 proteins. | 0.9% NaCl saline solution | In vitro | [46,47] | |
| Neurodegenerative disorders | Parkinson’s disease (PD) | TQ inhibits activation of the NF-κB pathway. TQ reduces the expression of TLR-2, TLR-4, MyD88, TNF-α, IL-1β, IFN-β, IRF-3, and NF-κB. | Normal saline and corn oil; 0.9% saline | In vivo | [48,49] |
| Huntington’s disease (HD) | TQ attenuates the expression of GFAP, proinflammatory cytokines, and p-p65 NF-κB nuclear translocation. | 0.9% saline | In vivo | [50] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Huang, L.; Kim, M.-Y.; Cho, J.Y. The Role of Thymoquinone in Inflammatory Response in Chronic Diseases. Int. J. Mol. Sci. 2022, 23, 10246. https://doi.org/10.3390/ijms231810246
Liu Y, Huang L, Kim M-Y, Cho JY. The Role of Thymoquinone in Inflammatory Response in Chronic Diseases. International Journal of Molecular Sciences. 2022; 23(18):10246. https://doi.org/10.3390/ijms231810246
Chicago/Turabian StyleLiu, Yan, Lei Huang, Mi-Yeon Kim, and Jae Youl Cho. 2022. "The Role of Thymoquinone in Inflammatory Response in Chronic Diseases" International Journal of Molecular Sciences 23, no. 18: 10246. https://doi.org/10.3390/ijms231810246
APA StyleLiu, Y., Huang, L., Kim, M.-Y., & Cho, J. Y. (2022). The Role of Thymoquinone in Inflammatory Response in Chronic Diseases. International Journal of Molecular Sciences, 23(18), 10246. https://doi.org/10.3390/ijms231810246







