Senescence Markers in Peripheral Blood Mononuclear Cells in Amnestic Mild Cognitive Impairment and Alzheimer’s Disease
Abstract
1. Introduction
2. Results
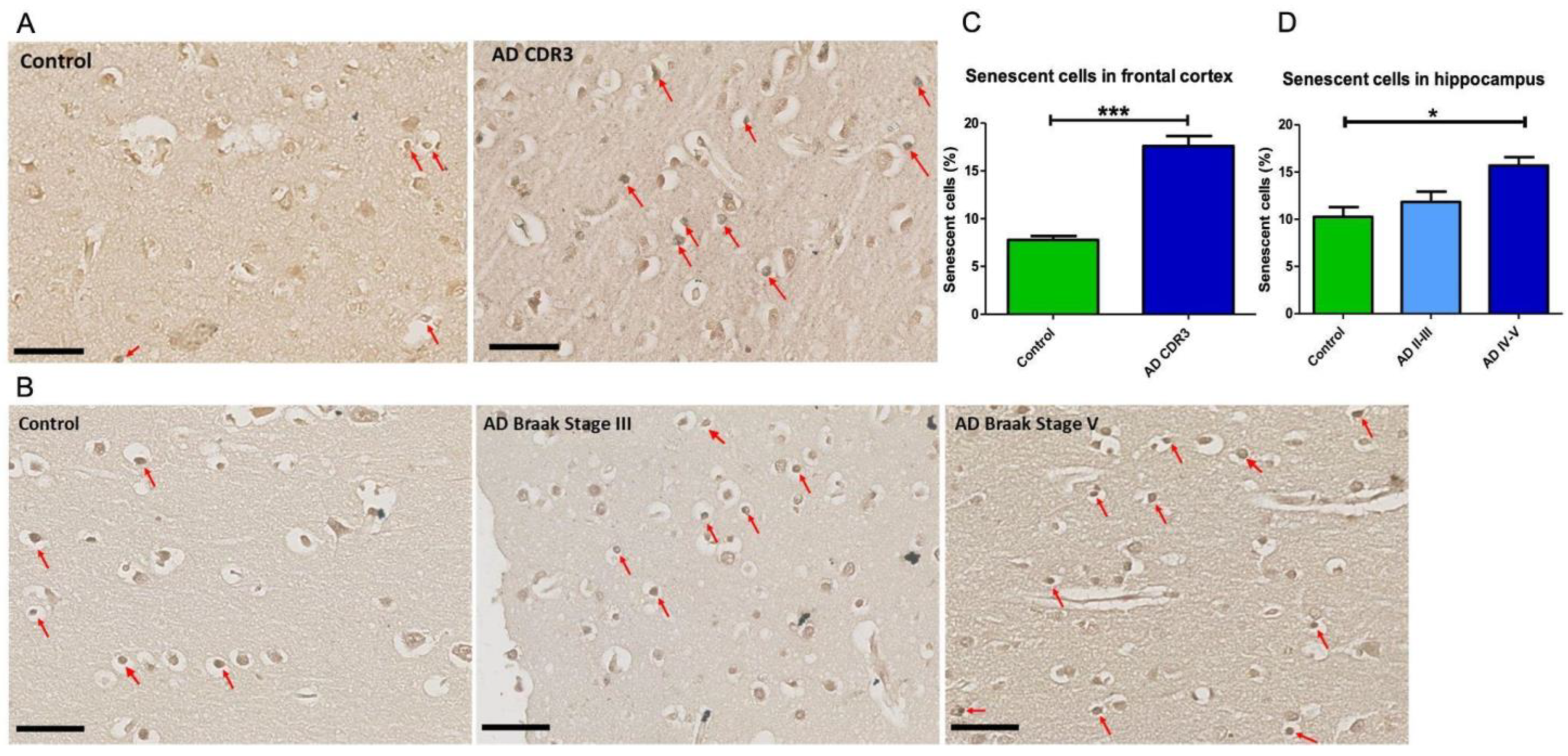
2.1. Senescent Mark in Frontal and Hippocampal Cells of AD Patients
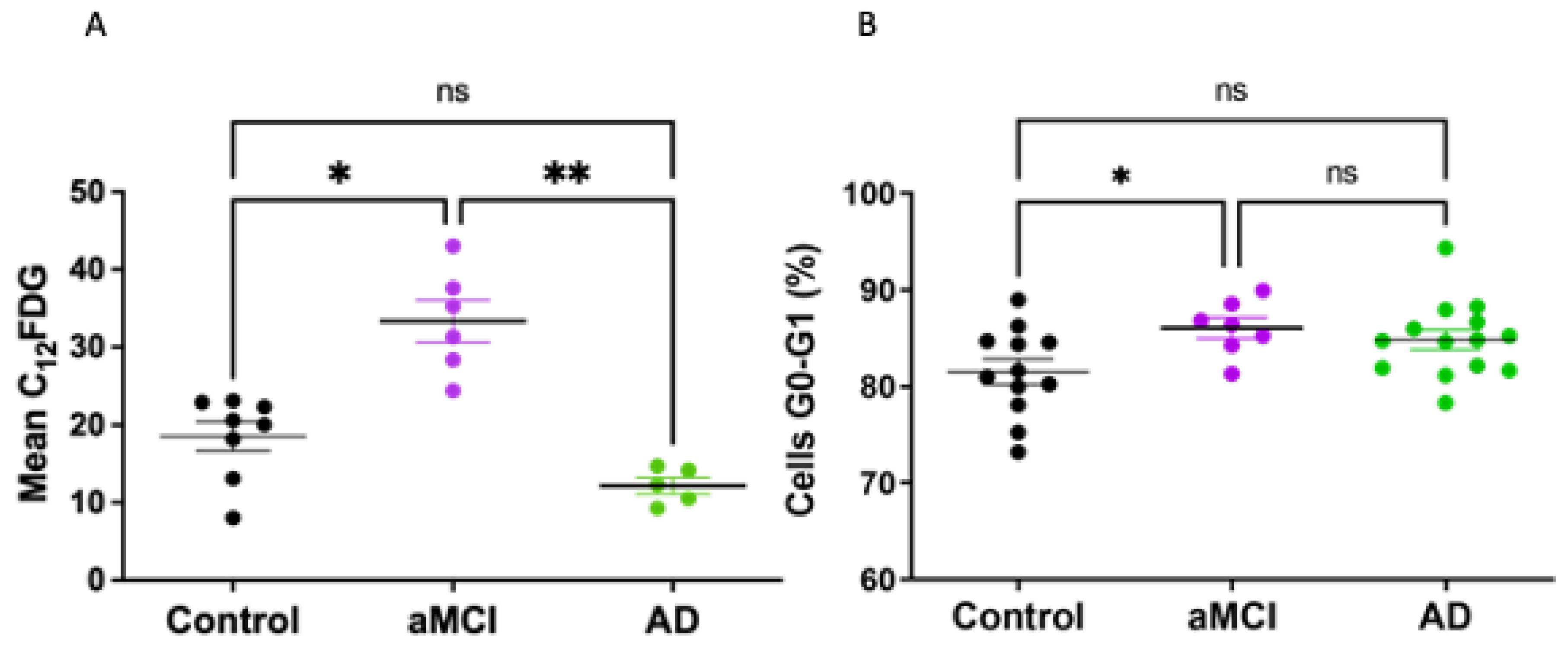
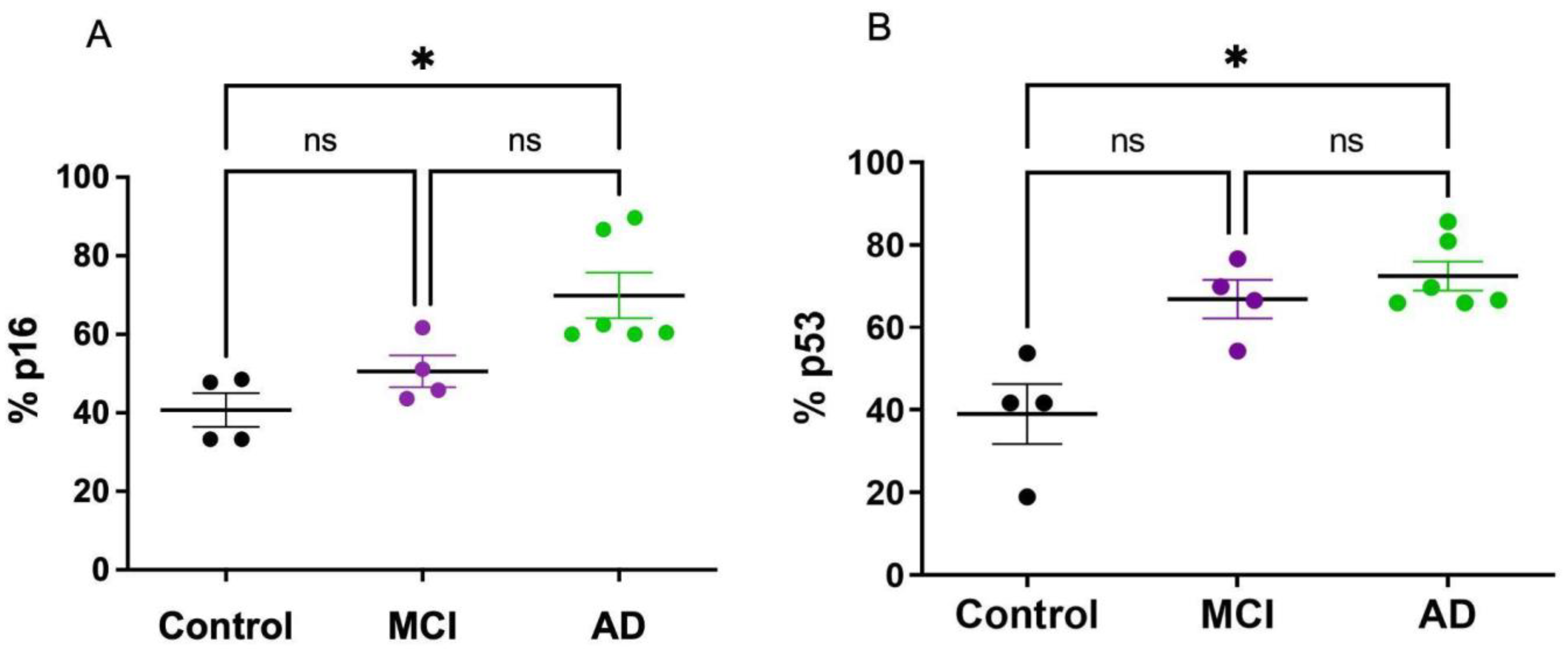
2.2. Senescent Mark in Peripheral Blood Mononuclear Cells
3. Discussion
4. Materials and Methods
4.1. Subjects
4.2. Neuropathological Samples
4.3. Staining of Lipofuscin
4.4. Peripheral Blood Mononuclear Cells (PBMCs) Isolation
4.5. β-Galactosidase Activity
4.6. G0-G1 Phase Cell-Cycle Arrest
4.7. p16 and p53 Expression
4.8. RNA Isolation and PCR Analysis
4.9. ELISA
4.10. γH2AX Immunocytochemistry
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Alzheimer’s Disease Fact Sheet|National Institute on Aging. Available online: https://www.nia.nih.gov/health/alzheimers-disease-fact-sheet (accessed on 5 August 2022).
- Cummings, J.; Reiber, C.; Kumar, P. The Price of Progress: Funding and Financing Alzheimer’s Disease Drug Development. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2018, 4, 330–343. [Google Scholar] [CrossRef] [PubMed]
- Saez-Atienzar, S.; Masliah, E. Cellular Senescence and Alzheimer Disease: The Egg and the Chicken Scenario. Nat. Rev. Neurosci. 2020, 21, 433–444. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef]
- Gorgoulis, V.; Adams, P.D.; Alimonti, A.; Bennett, D.C.; Bischof, O.; Bishop, C.; Campisi, J.; Collado, M.; Evangelou, K.; Ferbeyre, G.; et al. Cellular Senescence: Defining a Path Forward. Cell 2019, 179, 813–827. [Google Scholar] [CrossRef]
- Cuollo, L.; Antonangeli, F.; Santoni, A.; Soriani, A. The Senescence-Associated Secretory Phenotype (SASP) in the Challenging Future of Cancer Therapy and Age-Related Diseases. Biology 2020, 9, 485. [Google Scholar] [CrossRef]
- Baker, D.J.; Wijshake, T.; Tchkonia, T.; Lebrasseur, N.K.; Childs, B.G.; Van De Sluis, B.; Kirkland, J.L.; Van Deursen, J.M. Clearance of P16 Ink4a-Positive Senescent Cells Delays Ageing-Associated Disorders. Nature 2011, 479, 232–236. [Google Scholar] [CrossRef]
- Baker, D.J.; Childs, B.G.; Durik, M.; Wijers, M.E.; Sieben, C.J.; Zhong, J.; Saltness, R.A.; Jeganathan, K.B.; Verzosa, G.C.; Pezeshki, A.; et al. Naturally Occurring P16 Ink4a-Positive Cells Shorten Healthy Lifespan. Nature 2016, 530, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Jurk, D.; Wang, C.; Miwa, S.; Maddick, M.; Korolchuk, V.; Tsolou, A.; Gonos, E.S.; Thrasivoulou, C.; Jill Saffrey, M.; Cameron, K.; et al. Postmitotic Neurons Develop a P21-Dependent Senescence-like Phenotype Driven by a DNA Damage Response. Aging Cell 2012, 11, 996. [Google Scholar] [CrossRef] [PubMed]
- Musi, N.; Valentine, J.M.; Sickora, K.R.; Baeuerle, E.; Thompson, C.S.; Shen, Q.; Orr, M.E. Tau Protein Aggregation Is Associated with Cellular Senescence in the Brain. Aging Cell 2018, 17, e12840. [Google Scholar] [CrossRef]
- Flanary, B. The Role of Microglial Cellular Senescence in the Aging and Alzheimer Diseased Brain. Rejuvenation Res. 2005, 8, 82–85. [Google Scholar] [CrossRef]
- Zhang, P.; Kishimoto, Y.; Grammatikakis, I.; Gottimukkala, K.; Cutler, R.G.; Zhang, S.; Abdelmohsen, K.; Bohr, V.A.; Misra Sen, J.; Gorospe, M.; et al. Senolytic Therapy Alleviates Aβ-Associated Oligodendrocyte Progenitor Cell Senescence and Cognitive Deficits in an Alzheimer’s Disease Model. Nat. Neurosci. 2019, 22, 719–728. [Google Scholar] [CrossRef] [PubMed]
- He, N.; Jin, W.L.; Lok, K.H.; Wang, Y.; Yin, M.; Wang, Z.J. Amyloid-β(1-42) Oligomer Accelerates Senescence in Adult Hippocampal Neural Stem/Progenitor Cells via Formylpeptide Receptor 2. Cell Death Dis. 2013, 4, e924. [Google Scholar] [CrossRef] [PubMed]
- Bussian, T.J.; Aziz, A.; Meyer, C.F.; Swenson, B.L.; van Deursen, J.M.; Baker, D.J. Clearance of Senescent Glial Cells Prevents Tau-Dependent Pathology and Cognitive Decline. Nature 2018, 562, 578–582. [Google Scholar] [CrossRef] [PubMed]
- Ogrodnik, M.; Evans, S.A.; Fielder, E.; Victorelli, S.; Kruger, P.; Salmonowicz, H.; Weigand, B.M.; Patel, A.D.; Pirtskhalava, T.; Inman, C.L.; et al. Whole-Body Senescent Cell Clearance Alleviates Age-Related Brain Inflammation and Cognitive Impairment in Mice. Aging Cell 2021, 20, e13296. [Google Scholar] [CrossRef]
- Gasek, N.S.; Kuchel, G.A.; Kirkland, J.L.; Xu, M. Strategies for Targeting Senescent Cells in Human Disease. Nat. Aging 2021, 1, 870–879. [Google Scholar] [CrossRef]
- Morris, J.K.; Honea, R.A.; Vidoni, E.D.; Swerdlow, R.H.; Burns, J.M. Is Alzheimer’s Disease a Systemic Disease? Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2014, 1842, 1340–1349. [Google Scholar] [CrossRef]
- Wang, J.; Gu, B.J.; Masters, C.L.; Wang, Y.J. A Systemic View of Alzheimer Disease-Insights from Amyloid-β Metabolism beyond the Brain. Nat. Rev. Neurol. 2017, 13, 612–623. [Google Scholar] [CrossRef]
- Wang, X.; Wang, D.; Su, F.; Li, C.; Chen, M. Immune Abnormalities and Differential Gene Expression in the Hippocampus and Peripheral Blood of Patients with Alzheimer’s Disease. Ann. Transl. Med. 2022, 10, 29. [Google Scholar] [CrossRef]
- Garfias, S.; Tamaya Domínguez, B.; Toledo Rojas, A.; Arroyo, M.; Rodríguez, U.; Boll, C.; Sosa, A.L.; Sciutto, E.; Adalid-Peralta, L.; Martinez López, Y.; et al. Peripheral Blood Lymphocyte Phenotypes in Alzheimer and Parkinson’s Diseases. Neurología 2022, 37, 110–121. [Google Scholar] [CrossRef]
- Cao, M.; Liu, J.; Zhang, X.; Yang, T.; Wang, Y.; Hou, Y.; Song, Q.; Cui, Y.; Wang, Y.; Wang, P. ABI3 Is a Novel Early Biomarker of Alzheimer’s Disease. J. Alzheimer’s Dis. 2022, 87, 335–344. [Google Scholar] [CrossRef]
- Udin, M.S.; Kabir, M.T.; Tewari, D.; Mamun, A.A.; Mathew, B.; Aleya, L.; Barreto, G.E.; Bin-Jumah, M.N.; Abdel-Daim, M.M.; Ashraf, G.M. Revisiting the role of brain and peripheral Aβ in the pathogenesis of Alzheimer’s disease. J. Neurol. Sci. 2020, 416, 116974. [Google Scholar] [CrossRef] [PubMed]
- Trushina, E. Alzheimer’s Disease Mechanisms in Peripheral Cells: Promises and Challenges. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2019, 5, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Leuner, K.; Schulz, K.; Schütt, T.; Pantel, J.; Prvulovic, D.; Rhein, V.; Savaskan, E.; Czech, C.; Eckert, A.; Müller, W.E. Peripheral Mitochondrial Dysfunction in Alzheimer’s Disease: Focus on Lymphocytes. Mol. Neurobiol. 2012, 46, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Behrens, M.I.; Silva, M.; Salech, F.; Ponce, D.P.; Merino, D.; Sinning, M.; Xiong, C.; Roe, C.M.; Quest, A.F.G. Inverse Susceptibility to Oxidative Death of Lymphocytes Obtained from Alzheimer’s Patients and Skin Cancer Survivors: Increased Apoptosis in Alzheimer’s and Reduced Necrosis in Cancer. J. Gerontol.-Ser. A Biol. Sci. Med. Sci. 2012, 67, 1036–1040. [Google Scholar] [CrossRef]
- Ponce, D.P.; Salech, F.; Sanmartin, C.D.; Silva, M.; Xiong, C.; Roe, C.M.; Henriquez, M.; Quest, A.F.; Behrens, M.I. Increased Susceptibility to Oxidative Death of Lymphocytes from Alzheimer Patients Correlates with Dementia Severity. Curr. Alzheimer Res. 2014, 11, 892–898. [Google Scholar] [CrossRef][Green Version]
- Salech, F.; Ponce, D.P.; SanMartín, C.D.; Rogers, N.K.; Chacón, C.; Henríquez, M.; Behrens, M.I. PARP-1 and P53 Regulate the Increased Susceptibility to Oxidative Death of Lymphocytes from MCI and AD Patients. Front. Aging Neurosci. 2017, 9, 310. [Google Scholar] [CrossRef] [PubMed]
- Evangelou, K.; Lougiakis, N.; Rizou, S.V.; Kotsinas, A.; Kletsas, D.; Muñoz-Espín, D.; Kastrinakis, N.G.; Pouli, N.; Marakos, P.; Townsend, P.; et al. Robust, Universal Biomarker Assay to Detect Senescent Cells in Biological Specimens. Aging Cell 2017, 16, 192–197. [Google Scholar] [CrossRef]
- Debacq-Chainiaux, F.; Erusalimsky, J.D.; Campisi, J.; Toussaint, O. Protocols to Detect Senescence-Associated Beta-Galactosidase (SA-Βgal) Activity, a Biomarker of Senescent Cells in Culture and In Vivo. Nat. Protoc. 2009, 4, 1798–1806. [Google Scholar] [CrossRef]
- Kennedy, B.K.; Berger, S.L.; Brunet, A.; Campisi, J.; Cuervo, A.M.; Epel, E.S.; Franceschi, C.; Lithgow, G.J.; Morimoto, R.I.; Pessin, J.E.; et al. Geroscience: Linking Aging to Chronic Disease. Cell 2014, 159, 709–713. [Google Scholar] [CrossRef]
- Hou, Y.; Dan, X.; Babbar, M.; Wei, Y.; Hasselbalch, S.G.; Croteau, D.L.; Bohr, V.A. Ageing as a Risk Factor for Neurodegenerative Disease. Nat. Rev. Neurol. 2019, 15, 565–581. [Google Scholar] [CrossRef]
- He, S.; Sharpless, N.E. Senescence in Health and Disease. Cell 2017, 169, 1000. [Google Scholar] [CrossRef] [PubMed]
- Childs, B.G.; Durik, M.; Baker, D.J.; Van Deursen, J.M. Cellular Senescence in Aging and Age-Related Disease: From Mechanisms to Therapy. Nat. Med. 2015, 21, 1424–1435. [Google Scholar] [CrossRef]
- Xu, M.; Pirtskhalava, T.; Farr, J.N.; Weigand, B.M.; Palmer, A.K.; Weivoda, M.M.; Inman, C.L.; Ogrodnik, M.B.; Hachfeld, C.M.; Fraser, D.G.; et al. Senolytics Improve Physical Function and Increase Lifespan in Old Age. Nat. Med. 2018, 24, 1246–1256. [Google Scholar] [CrossRef]
- Raffaele, M.; Phd, V.; Vinciguerra, M.; Raffaele, M. The Costs and Benefits of Senotherapeutics for Human Health. Lancet Health Longev. 2022, 3, e67–e77. [Google Scholar] [CrossRef]
- Tuttle, C.S.L.; Luesken, S.W.M.; Waaijer, M.E.C.; Maier, A.B. Senescence in Tissue Samples of Humans with Age-Related Diseases: A Systematic Review. Ageing Res. Rev. 2021, 68, 101334. [Google Scholar] [CrossRef]
- Liu, J.Y.; Souroullas, G.P.; Diekman, B.O.; Krishnamurthy, J.; Hall, B.M.; Sorrentino, J.A.; Parker, J.S.; Sessions, G.A.; Gudkov, A.V.; Sharpless, N.E. Cells Exhibiting Strong P16 INK4a Promoter Activation in Vivo Display Features of Senescence. Proc. Natl. Acad. Sci. USA 2019, 116, 2603–2611. [Google Scholar] [CrossRef]
- Sharpless, N.E.; Sherr, C.J. Forging a Signature of in vivo Senescence. Nat. Rev. Cancer 2015, 15, 397–408. [Google Scholar] [CrossRef]
- Hernandez-Segura, A.; de Jong, T.V.; Melov, S.; Guryev, V.; Campisi, J.; Demaria, M. Unmasking Transcriptional Heterogeneity in Senescent Cells. Curr. Biol. 2017, 27, 2652–2660.e4. [Google Scholar] [CrossRef]
- Sapieha, P.; Mallette, F.A. Cellular Senescence in Postmitotic Cells: Beyond Growth Arrest. Trends Cell Biol. 2018, 28, 595–607. [Google Scholar] [CrossRef]
- Kohli, J.; Veenstra, I.; Demaria, M. The Struggle of a Good Friend Getting Old: Cellular Senescence in Viral Responses and Therapy. EMBO Rep. 2021, 22, e52243. [Google Scholar] [CrossRef]
- Dehkordi, S.K.; Walker, J.; Sah, E.; Bennett, E.; Atrian, F.; Frost, B.; Woost, B.; Bennett, R.E.; Orr, T.C.; Zhou, Y.; et al. Profiling Senescent Cells in Human Brains Reveals Neurons with CDKN2D/P19 and Tau Neuropathology. Nat. Aging 2021, 1, 1107–1116. [Google Scholar] [CrossRef]
- Roher, A.E.; Esh, C.L.; Kokjohn, T.A.; Castaño, E.M.; Van Vickle, G.D.; Kalback, W.M.; Patton, R.L.; Luehrs, D.C.; Daugs, I.D.; Kuo, Y.M.; et al. Amyloid Beta Peptides in Human Plasma and Tissues and Their Significance for Alzheimer’s Disease. Alzheimer’s Dement. 2009, 5, 18–29. [Google Scholar] [CrossRef]
- Myung, N.H.; Zhu, X.; Kruman, I.I.; Castellani, R.J.; Petersen, R.B.; Siedlak, S.L.; Perry, G.; Smith, M.A.; Lee, H.G. Evidence of DNA Damage in Alzheimer Disease: Phosphorylation of Histone H2AX in Astrocytes. Age 2008, 30, 209–215. [Google Scholar] [CrossRef]
- Simpson, J.E.; Ince, P.G.; Haynes, L.J.; Theaker, R.; Gelsthorpe, C.; Baxter, L.; Forster, G.; Lace, G.L.; Shaw, P.J.; Matthews, F.E.; et al. Population Variation in Oxidative Stress and Astrocyte DNA Damage in Relation to Alzheimer-Type Pathology in the Ageing Brain. Neuropathol. Appl. Neurobiol. 2010, 36, 25–40. [Google Scholar] [CrossRef]
- Siddiqui, M.S.; Francois, M.; Hecker, J.; Faunt, J.; Fenech, M.F.; Leifert, W.R. ΓH2AX Is Increased in Peripheral Blood Lymphocytes of Alzheimer’s Disease Patients in the South Australian Neurodegeneration, Nutrition and DNA Damage (SAND) Study of Aging. Mutat. Res. Toxicol. Environ. Mutagen. 2018, 829–830, 6–18. [Google Scholar] [CrossRef]
- Siddiqui, M.S.; Francois, M.; Rainey-Smith, S.; Martins, R.; Masters, C.L.; Ames, D.; Rowe, C.C.; Macaulay, L.S.; Fenech, M.F.; Leifert, W.R. Evaluation of GammaH2AX in Buccal Cells as a Molecular Biomarker of DNA Damage in Alzheimer’s Disease in the AIBL Study of Ageing. Life 2020, 10, 141. [Google Scholar] [CrossRef]
- Mariotti, L.G.; Pirovano, G.; Savage, K.I.; Ghita, M.; Ottolenghi, A.; Prise, K.M.; Schettino, G. Use of the γ-H2AX Assay to Investigate DNA Repair Dynamics Following Multiple Radiation Exposures. PLoS ONE 2013, 8, e79541. [Google Scholar] [CrossRef]
- Bernadotte, A.; Mikhelson, V.M.; Spivak, I.M. Markers of Cellular Senescence. Telomere Shortening as a Marker of Cellular Senescence. Aging 2016, 8, 3–11. [Google Scholar] [CrossRef]
- Magaki, S.; Mueller, C.; Dickson, C.; Kirsch, W. Increased Production of Inflammatory Cytokines in Mild Cognitive Impairment. Exp. Gerontol. 2007, 42, 233–240. [Google Scholar] [CrossRef]
- Vicencio, J.M.; Galluzzi, L.; Tajeddine, N.; Ortiz, C.; Criollo, A.; Tasdemir, E.; Morselli, E.; Ben Younes, A.; Maiuri, M.C.; Lavandero, S.; et al. Senescence, Apoptosis or Autophagy? When a Damaged Cell Must Decide Its Path—A Mini-Review. Gerontology 2008, 54, 92–99. [Google Scholar] [CrossRef]
- Minami, R.; Muta, K.; Umemura, T.; Motomura, S.; Abe, Y.; Nishimura, J.; Nawata, H. P16INK4a Induces Differentiation and Apoptosis in Erythroid Lineage Cells. Exp. Hematol. 2003, 31, 355–362. [Google Scholar] [CrossRef]
- McKhann, G.M.; Knopman, D.S.; Chertkow, H.; Hyman, B.T.; Jack, C.R.; Kawas, C.H.; Klunk, W.E.; Koroshetz, W.J.; Manly, J.J.; Mayeux, R.; et al. The Diagnosis of Dementia Due to Alzheimer’s Disease: Recommendations from the National Institute on Aging-Alzheimer’s Association Workgroups on Diagnostic Guidelines for Alzheimer’s Disease. Alzheimers. Dement. 2011, 7, 263–269. [Google Scholar] [CrossRef]
- Morris, J.C. The Clinical Dementia Rating (CDR): Current Version and Scoring Rules. Neurology 1993, 43, 2412–2414. [Google Scholar] [CrossRef]
- Berg, L.; Miller, J.P.; Storandt, M.; Duchek, J.; Morris, J.C.; Rubin, E.H.; Burke, W.J.; Coben, L.A. Mild Senile Dementia of the Alzheimer Type: 2. Longitudinal Assessment. Ann. Neurol. 1988, 23, 477–484. [Google Scholar] [CrossRef]
- Galvin, J.E.; Roe, C.M.; Powlishta, K.K.; Coats, M.A.; Muich, S.J.; Grant, E.; Miller, J.P.; Storandt, M.; Morris, J.C. The AD8: A Brief Informant Interview to Detect Dementia. Neurology 2005, 65, 559–564. [Google Scholar] [CrossRef]
- Muñoz, C.; Núñez, J.; Flores, P.; Behrens, M.I.P.; Slachevsky, A. Usefulness of a Brief Informant Interview to Detect Dementia, Translated into Spanish (AD8-Ch). Rev. Med. Chil. 2010, 138, 1063–1065. [Google Scholar] [CrossRef][Green Version]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A Brief Screening Tool For Mild Cognitive Impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Delgado, C.; Araneda, A.; Behrens, M.I. Validation of the Spanish-Language Version of the Montreal Cognitive Assessment Test in Adults Older than 60 Years. Neurologia 2017, 34, 376–385. [Google Scholar] [CrossRef]
- SanMartín, C.D.; Henriquez, M.; Chacon, C.; Ponce, D.P.; Salech, F.; Rogers, N.K.; Behrens, M.I. Vitamin D Increases Aβ140 Plasma Levels and Protects Lymphocytes from Oxidative Death in Mild Cognitive Impairment Patients. Curr. Alzheimer Res. 2018, 15, 561–569. [Google Scholar] [CrossRef]


| Healthy Controls n = 26 | aMCI n = 22 | AD n = 12 | p | |
|---|---|---|---|---|
| Age, years, mean ± SE (range) | 75.6 ± 1.8 (65–85) | 77.2 ± 1.5 (63–93) | 77.5 ± 1.9 (72–89) | =0.590 |
| Female sex, (%) | 17 (68.0) | 16 (72.7) | 11 (91.7) | =0.232 |
| Education (years) | 12.5 ± 1.0 | 10.1 ± 1.1 | 10.3 ± 1.5 | =0.220 |
| MoCA test score (mean ± SE) | 28.2 ± 0.4 | 19.8 ± 0.9 | 11.8 ± 1.6 | p< 0.0001 (Ctr vs. aMCI and Ctr vs. AD); p = 0.065 (aMCI vs. AD) |
| AD8 | 0.4 ± 0.1 | 4.3 ± 0.5 | 6.4 ± 0.4 | p< 0.0001 (Ctr vs. aMCI and Ctr vs. AD); p = 0.186 (aMCI vs. AD) |
| CDR-SOB | 0.1 ± 0.04 | 2.0 ± 0.2 | 7.1 ± 1.1 | p< 0.0001 (Ctr vs. aMCI and Ctr vs. AD); p = 0.017 (aMCI vs. AD) |
| CDR 0 (number of patients) | 26 | 0 | 0 | p< 0.0001 CDR0 vs. CDR > 0 |
| CDR 0.5 (number of patients) | 0 | 22 | 0 | |
| CDR 1 (number of patients) | 0 | 0 | 6 | |
| CDR 2 (number of patients) | 0 | 0 | 3 | |
| CDR 3 (number of patients) | 0 | 0 | 3 | |
| Diabetes/Insulin Resistance, n (%) | 6 (22.2) | 5 (25.0) | 3 (25.0) | =0.988 |
| Hypertension, n (%) | 19 (72.2) | 9 (45.0) | 4 (33.3) | =0.025 (ctr vs. MCI and Ctr vs. AD) |
| Hypercholesterolemia, n (%) | 7 (27.8) | 9 (47.4) | 6 (50.0) | =0.340 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salech, F.; SanMartín, C.D.; Concha-Cerda, J.; Romero-Hernández, E.; Ponce, D.P.; Liabeuf, G.; Rogers, N.K.; Murgas, P.; Bruna, B.; More, J.; et al. Senescence Markers in Peripheral Blood Mononuclear Cells in Amnestic Mild Cognitive Impairment and Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 9387. https://doi.org/10.3390/ijms23169387
Salech F, SanMartín CD, Concha-Cerda J, Romero-Hernández E, Ponce DP, Liabeuf G, Rogers NK, Murgas P, Bruna B, More J, et al. Senescence Markers in Peripheral Blood Mononuclear Cells in Amnestic Mild Cognitive Impairment and Alzheimer’s Disease. International Journal of Molecular Sciences. 2022; 23(16):9387. https://doi.org/10.3390/ijms23169387
Chicago/Turabian StyleSalech, Felipe, Carol D. SanMartín, Jorge Concha-Cerda, Esteban Romero-Hernández, Daniela P. Ponce, Gianella Liabeuf, Nicole K. Rogers, Paola Murgas, Bárbara Bruna, Jamileth More, and et al. 2022. "Senescence Markers in Peripheral Blood Mononuclear Cells in Amnestic Mild Cognitive Impairment and Alzheimer’s Disease" International Journal of Molecular Sciences 23, no. 16: 9387. https://doi.org/10.3390/ijms23169387
APA StyleSalech, F., SanMartín, C. D., Concha-Cerda, J., Romero-Hernández, E., Ponce, D. P., Liabeuf, G., Rogers, N. K., Murgas, P., Bruna, B., More, J., & Behrens, M. I. (2022). Senescence Markers in Peripheral Blood Mononuclear Cells in Amnestic Mild Cognitive Impairment and Alzheimer’s Disease. International Journal of Molecular Sciences, 23(16), 9387. https://doi.org/10.3390/ijms23169387






