Redox Active α-Lipoic Acid Differentially Improves Mitochondrial Dysfunction in a Cellular Model of Alzheimer and Its Control Cells
Abstract
:1. Introduction
2. Results
2.1. Effect of Alpha Lipoic Acid on Adenosine Triphosphate Levels
2.2. Mitochondrial Membrane Potential
2.3. Respiration
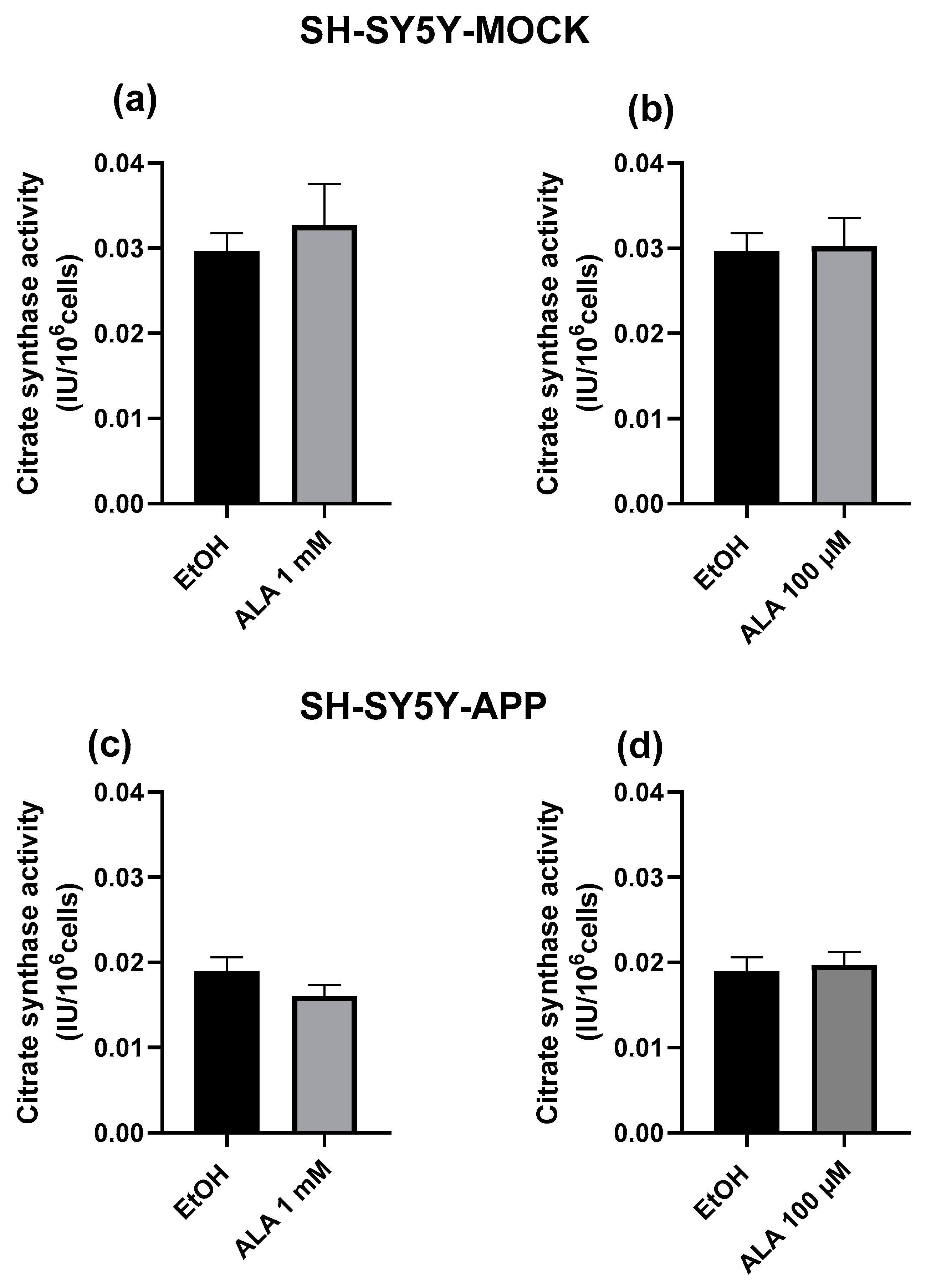
2.4. Citrate Synthase Activity
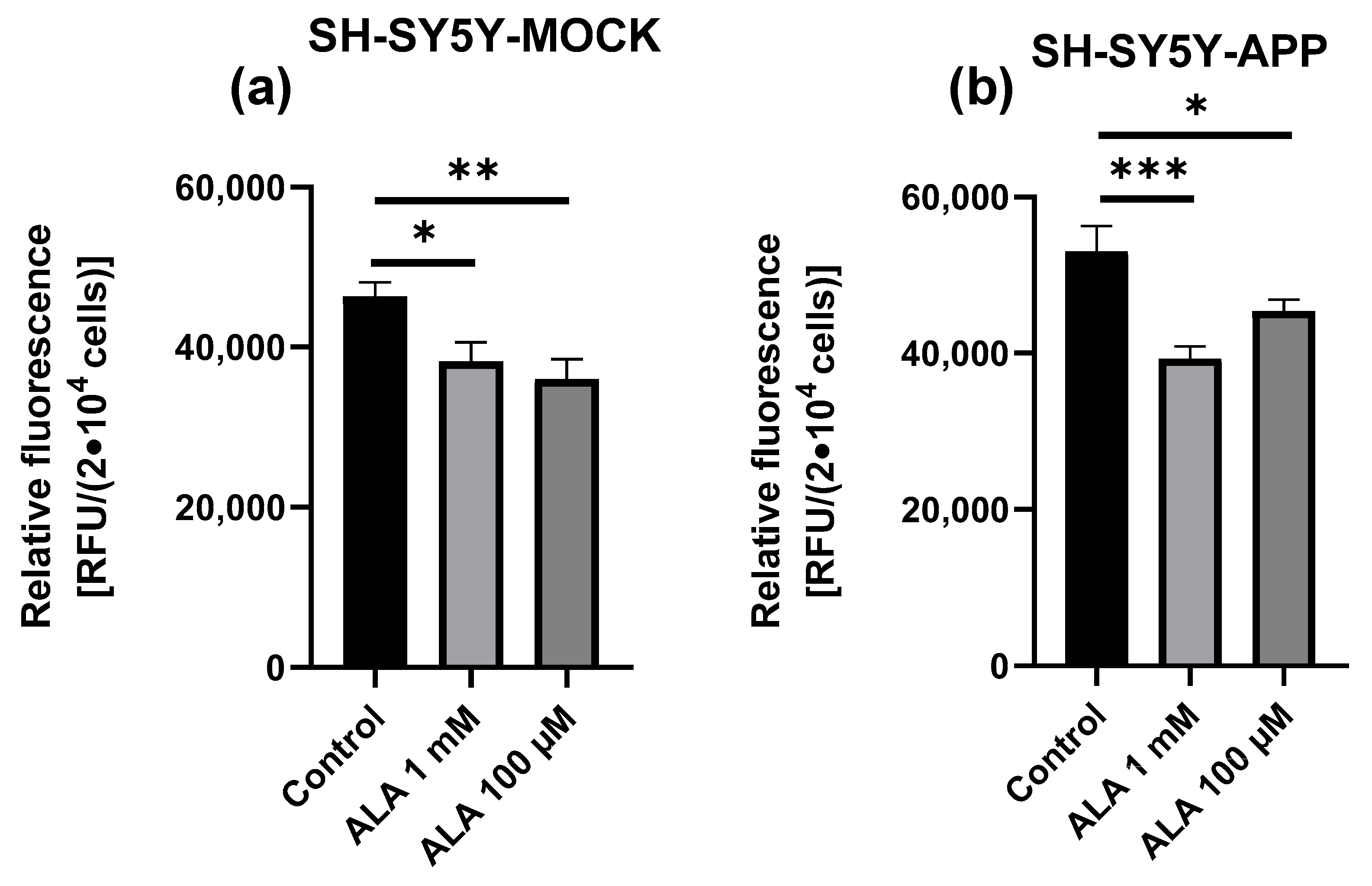
2.5. ROS Measurement
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Cell Lines
4.3. Measurement of ATP Concentrations
4.4. Measurement of Mitochondrial Membrane Potential (MMP)
4.5. High-Resolution Respirometry
4.6. Citrate Synthase Activity
4.7. Protein Content
4.8. ROS Measurement
4.9. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mathys, H.; Davila-Velderrain, J.; Peng, Z.; Gao, F.; Mohammadi, S.; Young, J.Z.; Menon, M.; He, L.; Abdurrob, F.; Jiang, X.; et al. Single-cell transcriptomic analysis of Alzheimer’s disease. Nature 2019, 570, 332–337. [Google Scholar] [CrossRef]
- Hardy, J.; Selkoe, D.J. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef] [PubMed]
- World Alzheimer Report 2019-Summary. 1 September 2019. Available online: https://www.alzint.org/u/WorldAlzheimerReport2019.pdf (accessed on 2 July 2022).
- Swerdlow, R.H. The mitochondrial hypothesis: Dysfunction, bioenergetic defects, and the metabolic link to Alzheimer’s disease. Int. Rev. Neurobiol. 2020, 154, 207–233. [Google Scholar] [PubMed]
- Weller, J.; Budson, A. Current understanding of Alzheimer’s disease diagnosis and treatment. F1000Research 2018, 7, 1161. [Google Scholar] [CrossRef] [PubMed]
- Stockburger, C.; Eckert, S.; Eckert, G.P.; Friedland, K.; Müller, W.E. Mitochondrial Function, Dynamics, and Permeability Transition: A Complex Love Triangle as A Possible Target for the Treatment of Brain Aging and Alzheimer’s Disease. J. Alzheimer’s Dis. 2018, 64 (Suppl. S1), S455–S467. [Google Scholar] [CrossRef] [PubMed]
- Friedland-Leuner, K.; Stockburger, C.; Denzer, I.; Eckert, G.P.; Müller, W.E. Mitochondrial dysfunction: Cause and consequence of Alzheimer’s disease. Prog. Mol. Biol. Transl. Sci. 2014, 127, 183–210. [Google Scholar]
- Parker, W.D.; Parks, J.; Filley, C.M.; Kleinschmidt-DeMasters, B.K. Electron transport chain defects in Alzheimer’s disease brain. Neurology 1994, 44, 1090–1096. [Google Scholar] [CrossRef]
- Lin, M.T.; Beal, M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 2006, 443, 787–795. [Google Scholar] [CrossRef]
- Swerdlow, R.H. Mitochondria and cell bioenergetics: Increasingly recognized components and a possible etiologic cause of Alzheimer’s disease. Antioxid. Redox Signal. 2012, 16, 1434–1455. [Google Scholar] [CrossRef]
- Bittner, F.; Murchison, C.; Koop, D.; Bourdette, D.; Spain, R. Lipoic Acid Pharmacokinetics at Baseline and 1 year in Secondary Progressive MS. Neurol. Neuroimmunol. Neuroinflamm. 2017, 4, e380. [Google Scholar] [CrossRef]
- Ikuta, N.; Chikamoto, K.; Asano, Y.; Yasui, Y.; Yokokawa, H.; Terao, K.; Rimbach, G.; Matsugo, S. Time Course Effect of R-Alpha-Lipoic Acid on Cellular Metabolomics in Cultured Hepatoma Cells. J. Med. Food 2017, 20, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Maldonado, A.; Pastoriza, S.; Rufián-Henares, J.Á. Assessing the antioxidant and metabolic effect of an alpha-lipoic acid and acetyl-L-carnitine nutraceutical. Curr. Res. Food Sci. 2021, 4, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, S.M.; Romeiro, C.F.R.; Rodrigues, C.A.; Cerqueira, A.R.L.; Monteiro, M.C. Mitochondrial Dysfunction and Alpha-Lipoic Acid: Beneficial or Harmful in Alzheimer’s Disease? Oxidative Med. Cell. Longev. 2019, 2019, 8409329. [Google Scholar] [CrossRef] [PubMed]
- Gilgun-Sherki, Y.; Melamed, E.; Offen, D. Oxidative stress induced-neurodegenerative diseases: The need for antioxidants that penetrate the blood brain barrier. Neuropharmacology 2001, 40, 959–975. [Google Scholar] [CrossRef]
- Tibullo, D.; Li, V.G.; Giallongo, C.; Grasso, S.; Tomassoni, D.; Anfuso, C.D.; Lupo, G.; Amenta, F.; Avola, R.; Bramanti, V. Biochemical and clinical relevance of alpha lipoic acid: Antioxidant and anti-inflammatory activity, molecular pathways and therapeutic potential. Inflamm. Res. Off. J. Eur. Histamine Res. Soc. 2017, 66, 947–959. [Google Scholar] [CrossRef] [PubMed]
- Salinthone, S.; Yadav, V.; Bourdette, D.N.; Carr, D.W. Lipoic acid: A novel therapeutic approach for multiple sclerosis and other chronic inflammatory diseases of the CNS. Endocr. Metab. Immune Disord. Drug Targets 2008, 8, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Golbidi, S.; Badran, M.; Laher, I. Diabetes and alpha lipoic Acid. Front. Pharmacol. 2011, 2, 69. [Google Scholar] [CrossRef]
- Vallianou, N.; Evangelopoulos, A.; Koutalas, P. Alpha-Lipoic Acid and Diabetic Neuropathy. Rev. Diabet. Stud. RDS 2010, 6, 230–236. [Google Scholar] [CrossRef]
- Kamenova, P. Improvement of insulin sensitivity in patients with type 2 diabetes mellitus after oral administration of alpha-lipoic acid. Hormones 2006, 5, 251–258. [Google Scholar] [CrossRef]
- Islam, M.T. Antioxidant activities of dithiol alpha-lipoic acid. Bangladesh J. Med. Sci. 2009, 8, 46–51. [Google Scholar] [CrossRef]
- May, J.M.; Qu, Z.C.; Mendiratta, S. Protection and recycling of alpha-tocopherol in human erythrocytes by intracellular ascorbic acid. Arch. Biochem. Biophys. 1998, 349, 281–289. [Google Scholar] [CrossRef]
- Kaur, D.; Behl, T.; Sehgal, A.; Singh, S.; Sharma, N.; Chigurupati, S.; Alhowail, A.; Abdeen, A.; Ibrahim, S.F.; Vargas-De-La-Cruz, C.; et al. Decrypting the potential role of α-lipoic acid in Alzheimer’s disease. Life Sci. 2021, 284, 119899. [Google Scholar] [CrossRef] [PubMed]
- Farr, S.A.; Price, T.O.; Banks, W.A.; Ercal, N.; Morley, J.E. Effect of alpha-lipoic acid on memory, oxidation, and lifespan in SAMP8 mice. J. Alzheimer’s Dis. JAD 2012, 32, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Head, E.; Gharib, A.M.; Yuan, W.; Ingersoll, R.T.; Hagen, T.M.; Cotman, C.W.; Ames, B.N. Memory loss in old rats is associated with brain mitochondrial decay and RNA/DNA oxidation: Partial reversal by feeding acetyl-L-carnitine and/or R-alpha -lipoic acid. Proc. Natl. Acad. Sci. USA 2002, 99, 2356–2361. [Google Scholar] [CrossRef]
- Xing, Z.G.; Yu, G.D.; Qin, L.; Jiang, F.; Zhao, W.H. Effects and mechanism of lipoic acid on beta-amyloid-intoxicated C6 glioma cells. Genet. Mol. Res. 2015, 14, 13880–13888. [Google Scholar] [CrossRef]
- Shinto, L.; Quinn, J.; Montine, T.; Dodge, H.H.; Woodward, W.; Baldauf-Wagner, S.; Waichunas, D.; Bumgarner, L.; Bourdette, D.; Silbert, L.; et al. A randomized placebo-controlled pilot trial of omega-3 fatty acids and alpha lipoic acid in Alzheimer’s disease. J. Alzheimer’s Dis. JAD 2014, 38, 111–120. [Google Scholar] [CrossRef]
- Molz, P.; Schröder, N. Potential Therapeutic Effects of Lipoic Acid on Memory Deficits Related to Aging and Neurodegeneration. Front. Pharmacol. 2017, 8, 849. [Google Scholar] [CrossRef] [PubMed]
- Hager, K.; Kenklies, M.; McAfoose, J.; Engel, J.; Münch, G. α-Lipoic acid as a new treatment option for Alzheimer’s disease—A 48 months follow-up analysis. In Neuropsychiatric Disorders An Integrative Approach; Springer: Vienna, Austria, 2007; pp. 189–193. [Google Scholar]
- Stockburger, C.; Gold, V.A.M.; Pallas, T.; Kolesova, N.; Miano, D.; Leuner, K.; Müller, W.E. A cell model for the initial phase of sporadic Alzheimer’s disease. J. Alzheimer’s Dis. 2014, 42, 395–411. [Google Scholar] [CrossRef]
- Larsen, S.; Nielsen, J.; Hansen, C.N.; Nielsen, L.B.; Wibrand, F.; Stride, N.; Schroder, H.D.; Boushel, R.; Helge, J.W.; Dela, F.; et al. Biomarkers of mitochondrial content in skeletal muscle of healthy young human subjects. J. Physiol. 2012, 590, 3349–3360. [Google Scholar] [CrossRef]
- Swerdlow, R.H.; Burns, J.M.; Khan, S.M. The Alzheimer’s disease mitochondrial cascade hypothesis: Progress and perspectives. Biochim. Et Biophys. Acta 2014, 1842, 1219–1231. [Google Scholar] [CrossRef]
- Swerdlow, R.H. Mitochondria and Mitochondrial Cascades in Alzheimer’s Disease. J. Alzheimer’s Dis. JAD 2018, 62, 1403–1416. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Sobenin, I.A.; Revin, V.V.; Orekhov, A.N.; Bobryshev, Y.V. Mitochondrial aging and age-related dysfunction of mitochondria. BioMed Res. Int. 2014, 2014, 238463. [Google Scholar] [CrossRef]
- Paula, V.D.J.R.D.; Guimarães, F.M.; Diniz, B.S.; Forlenza, O.V. Neurobiological pathways to Alzheimer’s disease: Amyloid-beta, TAU protein or both? Dement. Neuropsychol. 2009, 3, 188–194. [Google Scholar] [CrossRef]
- Esselun, C.; Theyssen, E.; Eckert, G.P. Effects of Urolithin A on Mitochondrial Parameters in a Cellular Model of Early Alzheimer Disease. Int. J. Mol. Sci. 2021, 22, 8333. [Google Scholar] [CrossRef] [PubMed]
- Arrozi, A.P.; Shukri, S.N.S.; Wan Ngah, W.Z.; Mohd Yusof, Y.A.; Ahmad Damanhuri, M.H.; Jaafar, F.; Makpol, S. Comparative Effects of Alpha- and Gamma-Tocopherol on Mitochondrial Functions in Alzheimer’s Disease In Vitro Model. Sci. Rep. 2020, 10, 8962. [Google Scholar] [CrossRef]
- Babylon, L.; Grewal, R.; Stahr, P.-L.; Eckert, R.W.; Keck, C.M.; Eckert, G.P. Hesperetin Nanocrystals Improve Mitochondrial Function in a Cell Model of Early Alzheimer Disease. Antioxidants 2021, 10, 1003. [Google Scholar] [CrossRef]
- Grewal, R.; Reutzel, M.; Dilberger, B.; Hein, H.; Zotzel, J.; Marx, S.; Tretzel, J.; Sarafeddinov, A.; Fuchs, C.; Eckert, G. Purified oleocanthal and ligstroside protect against mitochondrial dysfunction in models of early Alzheimer’s disease and brain ageing. Exp. Neurol. 2020, 328, 113248. [Google Scholar] [CrossRef]
- Poirier, Y.; Grimm, A.; Schmitt, K.; Eckert, A. Link between the unfolded protein response and dysregulation of mitochondrial bioenergetics in Alzheimer’s disease. Cell. Mol. Life Sci. CMLS 2019, 76, 1419–1431. [Google Scholar] [CrossRef]
- Palaniappan, A.R.; Dai, A. Mitochondrial Ageing and the Beneficial Role of α-Lipoic Acid. Neurochem. Res. 2007, 32, 1552–1558. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhao, X.; Liu, L.; Zhang, H.; Xuan, M.; Guo, Z.; Wang, H.; Liu, C. Neurochemical effects of the R form of α-lipoic acid and its neuroprotective mechanism in cellular models of Parkinson’s disease. Int. J. Biochem. Cell Biol. 2017, 87, 86–94. [Google Scholar] [CrossRef]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Mailloux, R.J. An Update on Mitochondrial Reactive Oxygen Species Production. Antioxidants 2020, 9, 472. [Google Scholar] [CrossRef] [PubMed]
- Shay, K.P.; Moreau, R.F.; Smith, E.J.; Hagen, T.M. Is α-lipoic acid a scavenger of reactive oxygen species in vivo? Evidence for its initiation of stress signaling pathways that promote endogenous antioxidant capacity. IUBMB Life 2008, 60, 362–367. [Google Scholar] [CrossRef]
- de Arriba, S.G.; Loske, C.; Meiners, I.; Fleischer, G.; Lobisch, M.; Wessel, K.; Tritschler, H.; Schinzel, R.; Münch, G. Advanced Glycation Endproducts Induce Changes in Glucose Consumption, Lactate Production, and ATP Levels in SH-SY5Y Neuroblastoma Cells by a Redox-Sensitive Mechanism. J. Cereb. Blood Flow Metab. 2003, 23, 1307–1313. [Google Scholar] [CrossRef] [PubMed]
- Pang, L.; Deng, P.; Liang, Y.-D.; Qian, J.-Y.; Wu, L.-C.; Yang, L.-L.; Yu, Z.-P.; Zhou, Z. Lipoic acid antagonizes paraquat-induced vascular endothelial dysfunction by suppressing mitochondrial reactive oxidative stress. Toxicol. Res. 2019, 8, 918–927. [Google Scholar] [CrossRef] [PubMed]
- Packer, L.; Witt, E.H.; Tritschler, H.J. Alpha-lipoic acid as a biological antioxidant. Free. Radic. Biol. Med. 1995, 19, 227–250. [Google Scholar] [CrossRef]
- Moini, H.; Packer, L.; Saris, N.-E.L. Antioxidant and Prooxidant Activities of α-Lipoic Acid and Dihydrolipoic Acid. Toxicol. Appl. Pharmacol. 2002, 182, 84–90. [Google Scholar] [CrossRef]
- Fu, B.; Zhang, J.; Zhang, X.; Zhang, C.; Li, Y.; Zhang, Y.; He, T.; Li, P.; Zhu, X.; Zhao, Y.; et al. Alpha-lipoic acid upregulates SIRT1-dependent PGC-1α expression and protects mouse brain against focal ischemia. Neuroscience 2014, 281, 251–257. [Google Scholar] [CrossRef]
- Rodgers, J.T.; Lerin, C.; Gerhart-Hines, Z.; Puigserver, P. Metabolic adaptations through the PGC-1 alpha and SIRT1 pathways. FEBS Lett. 2008, 582, 46–53. [Google Scholar] [CrossRef]
- Singh, H.P.P.; Bowman, R.H. Effect of DL-α-lipoic acid on the citrate concentration and phosphofructokinase activity of perfused hearts from normal and diabetic rats. Biochem. Biophys. Res. Commun. 1970, 41, 555–561. [Google Scholar] [CrossRef]
- Zorova, L.D.; Popkov, V.A.; Plotnikov, E.Y.; Silachev, D.N.; Pevzner, I.B.; Jankauskas, S.S.; Babenko, V.A.; Zorov, S.D.; Balakireva, A.V.; Juhaszova, M.; et al. Mitochondrial membrane potential. Anal. Biochem. 2018, 552, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, W.; Ijaz, B.; Shabbiri, K.; Ahmed, F.; Rehman, S. Oxidative toxicity in diabetes and Alzheimer’s disease: Mechanisms behind ROS/RNS generation. J. Biomed. Sci. 2017, 24, 76. [Google Scholar] [CrossRef] [PubMed]
- Onyango, I.G.; Dennis, J.; Khan, S.M. Mitochondrial Dysfunction in Alzheimer’s Disease and the Rationale for Bioenergetics Based Therapies. Aging Dis. 2016, 7, 201–214. [Google Scholar] [CrossRef]
- Zhao, R.-Z.; Jiang, S.; Zhang, L.; Yu, Z.-B. Mitochondrial electron transport chain, ROS generation and uncoupling (Review). Int. J. Mol. Med. 2019, 44, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Terzi, R.; Saruhan, G.N.; Güven, F.G.; Kadioglu, A. Alpha lipoic acid treatment induces the antioxidant system and ameliorates lipid peroxidation in maize seedlings under osmotic stress. Arch. Biol. Sci. 2018, 70, 503–511. [Google Scholar] [CrossRef]
- McCarty, M.F.; Barroso-Aranda, J.; Contreras, F. The “rejuvenatory” impact of lipoic acid on mitochondrial function in aging rats may reflect induction and activation of PPAR-gamma coactivator-1alpha. Med. Hypotheses 2009, 72, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Ames, B.N.; Liu, J. Delaying the Mitochondrial Decay of Aging with Acetylcarnitine. Ann. N. Y. Acad. Sci. 2004, 1033, 108–116. [Google Scholar] [CrossRef]
- Deng, Y.-N.; Shi, J.; Liu, J.; Qu, Q.-M. Celastrol protects human neuroblastoma SH-SY5Y cells from rotenone-induced injury through induction of autophagy. Neurochem. Int. 2013, 63, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Leuner, K.; Schulz, K.; Schütt, T.; Pantel, J.; Prvulovic, D.; Rhein, V.; Savaskan, E.; Czech, C.; Eckert, A.; Müller, W.E. Peripheral Mitochondrial Dysfunction in Alzheimer’s Disease: Focus on Lymphocytes. Mol. Neurobiol. 2012, 46, 194–204. [Google Scholar] [CrossRef]
- Holper, L.; Ben-Shachar, D.; Mann, J.J. Multivariate meta-analyses of mitochondrial complex I and IV in major depressive disorder, bipolar disorder, schizophrenia, Alzheimer disease, and Parkinson disease. Neuropsychopharmacology 2019, 44, 837–849. [Google Scholar] [CrossRef]
- Giachin, G.; Bouverot, R.; Acajjaoui, S.; Pantalone, S.; Soler-López, M. Dynamics of Human Mitochondrial Complex I Assembly: Implications for Neurodegenerative Diseases. Front. Mol. Biosci. 2016, 3, 43. [Google Scholar] [CrossRef] [PubMed]
- Sudheesh, N.P.; Ajith, T.A.; Janardhanan, K.K.; Krishnan, C.V. Palladium α-lipoic acid complex formulation enhances activities of Krebs cycle dehydrogenases and respiratory complexes I–IV in the heart of aged rats. Food Chem. Toxicol. 2009, 47, 2124–2128. [Google Scholar] [CrossRef] [PubMed]
- Nickel, A.; Kohlhaas, M.; Maack, C. Mitochondrial reactive oxygen species production and elimination. J. Mol. Cell. Cardiol. 2014, 73, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Liu, Z.; Wang, L.; Shi, R.; Chu, C.; Xiang, M.; Tian, Q.; Liu, X. Protective effects of lipoic acid against acrylamide-induced neurotoxicity: Involvement of mitochondrial energy metabolism and autophagy. Food Funct. 2017, 8, 4657–4667. [Google Scholar] [CrossRef]
- Lei, L.; Zhu, Y.; Gao, W.; Du, X.; Zhang, M.; Peng, Z.; Fu, S.; Li, X.; Zhe, W.; Li, X.; et al. Alpha-lipoic acid attenuates endoplasmic reticulum stress-induced insulin resistance by improving mitochondrial function in HepG2 cells. Cell. Signal. 2016, 28, 1441–1450. [Google Scholar] [CrossRef]
- Lee, B.W.; Kwon, S.J.; Chae, H.Y.; Kang, J.G.; Kim, C.S.; Lee, S.J.; Yoo, H.J.; Kim, J.H.; Park, K.S.; Ihm, S.-H. Dose-related cytoprotective effect of alpha-lipoic acid on hydrogen peroxide-induced oxidative stress to pancreatic beta cells. Free. Radic. Res. 2009, 43, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Lovell, M.A.; Xie, C.; Xiong, S.; Markesbery, W.R. Protection against amyloid beta peptide and iron/hydrogen peroxide toxicity by alpha lipoic acid. J. Alzheimer’s Dis. 2003, 5, 229–239. [Google Scholar] [CrossRef]
- Ono, K.; Hirohata, M.; Yamada, M. Alpha-lipoic acid exhibits anti-amyloidogenicity for beta-amyloid fibrils in vitro. Biochem. Biophys. Res. Commun. 2006, 341, 1046–1052. [Google Scholar] [CrossRef]
- Rhein, V.; Baysang, G.; Rao, S.; Meier, F.; Bonert, A.; Müller-Spahn, F.; Eckert, A. Amyloid-beta Leads to Impaired Cellular Respiration, Energy Production and Mitochondrial Electron Chain Complex Activities in Human Neuroblastoma Cells. Cell Mol. Neurobiol. 2009, 29, 1063–1071. [Google Scholar] [CrossRef]
- Mitochondrial Pathways and Respiratory Control. An Introduction to OXPHOS Analysis. Mitochondr Physiol Network 19.12; 2014. Available online: https://wiki.oroboros.at/images/f/fc/Gnaiger_2014_Mitochondr_Physiol_Network_MitoPathways.pdf (accessed on 25 July 2022).
- Stadlmann, S.; Renner, K.; Pollheimer, J.; Moser, P.L.; Zeimet, A.G.; Offner, F.A.; Gnaiger, E. Preserved coupling of oxidative phosphorylation but decreased mitochondrial respiratory capacity in IL-1beta-treated human peritoneal mesothelial cells. Cell Biochem. Biophys. 2006, 44, 179–186. [Google Scholar] [CrossRef]
- Hagl, S.; Grewal, R.; Ciobanu, I.; Helal, A.; Khayyal, M.T.; Muller, W.E.; Eckert, G.P. Rice bran extract compensates mitochondrial dysfunction in a cellular model of early Alzheimer’s disease. J. Alzheimer’s Dis. JAD 2015, 43, 927–938. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dieter, F.; Esselun, C.; Eckert, G.P. Redox Active α-Lipoic Acid Differentially Improves Mitochondrial Dysfunction in a Cellular Model of Alzheimer and Its Control Cells. Int. J. Mol. Sci. 2022, 23, 9186. https://doi.org/10.3390/ijms23169186
Dieter F, Esselun C, Eckert GP. Redox Active α-Lipoic Acid Differentially Improves Mitochondrial Dysfunction in a Cellular Model of Alzheimer and Its Control Cells. International Journal of Molecular Sciences. 2022; 23(16):9186. https://doi.org/10.3390/ijms23169186
Chicago/Turabian StyleDieter, Fabian, Carsten Esselun, and Gunter P. Eckert. 2022. "Redox Active α-Lipoic Acid Differentially Improves Mitochondrial Dysfunction in a Cellular Model of Alzheimer and Its Control Cells" International Journal of Molecular Sciences 23, no. 16: 9186. https://doi.org/10.3390/ijms23169186
APA StyleDieter, F., Esselun, C., & Eckert, G. P. (2022). Redox Active α-Lipoic Acid Differentially Improves Mitochondrial Dysfunction in a Cellular Model of Alzheimer and Its Control Cells. International Journal of Molecular Sciences, 23(16), 9186. https://doi.org/10.3390/ijms23169186






