Anti-Inflammatory Effect of Topiramate in a Chronic Model of TNBS-Induced Colitis
Abstract
:1. Introduction
2. Results
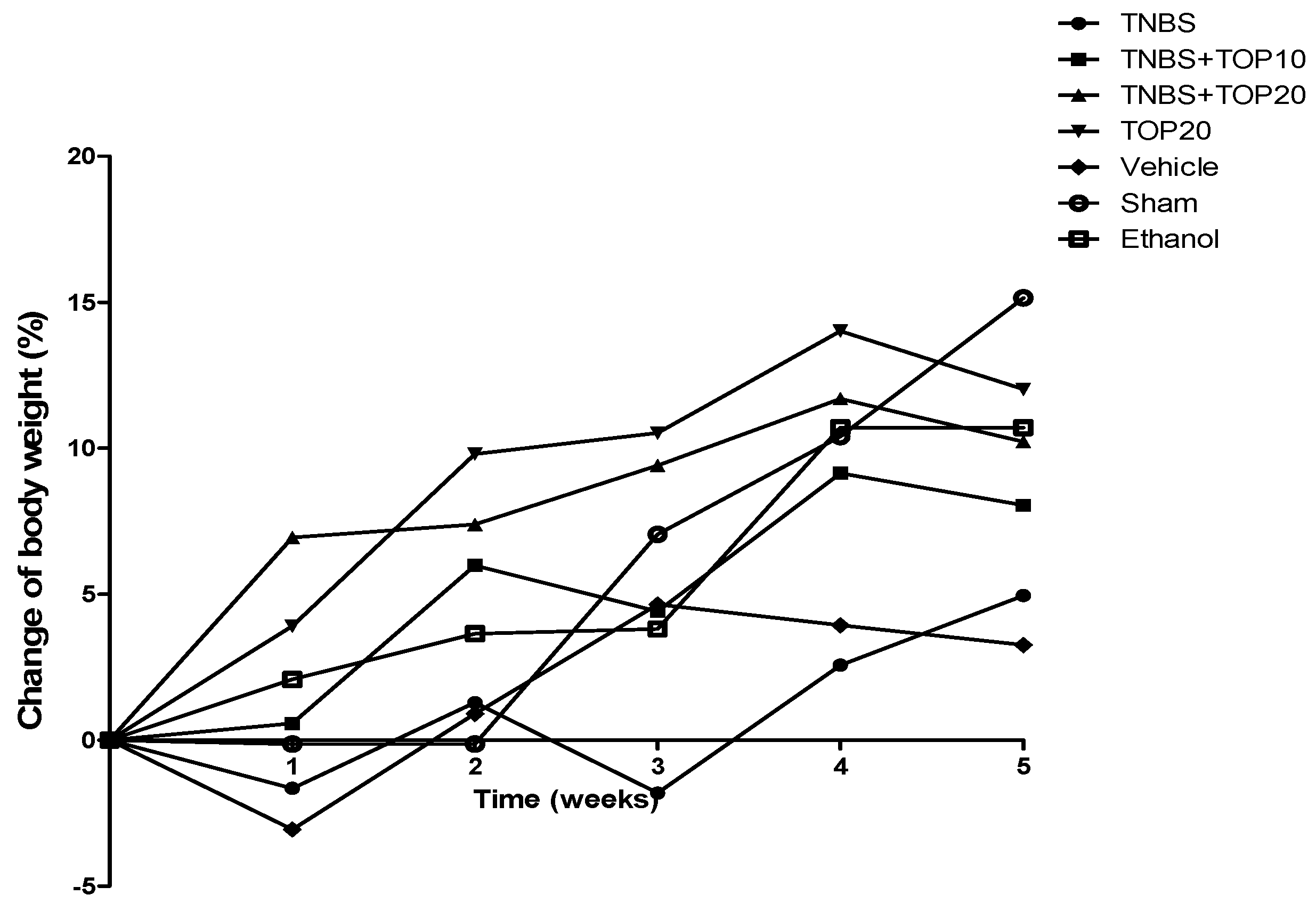
2.1. Clinical Signs
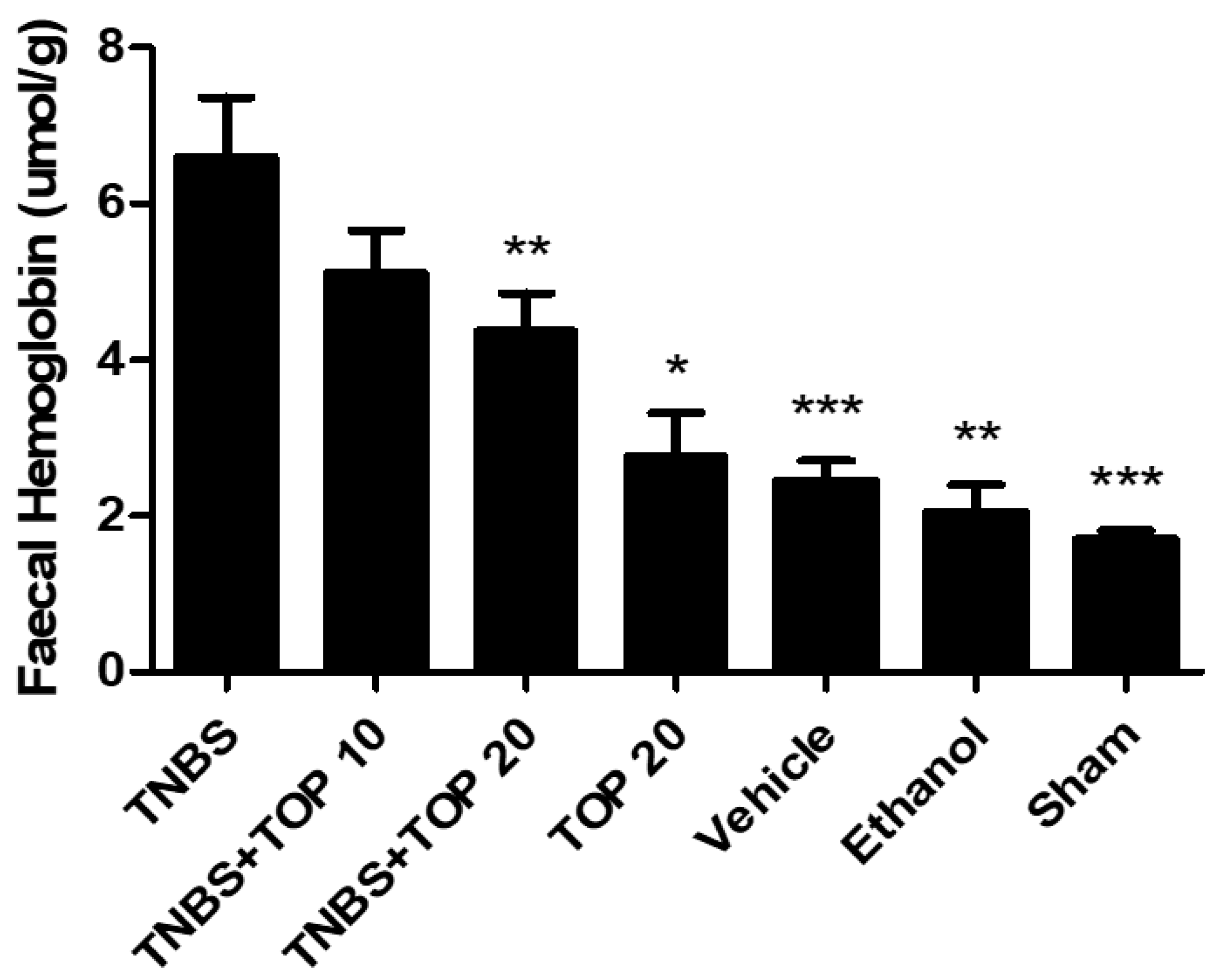
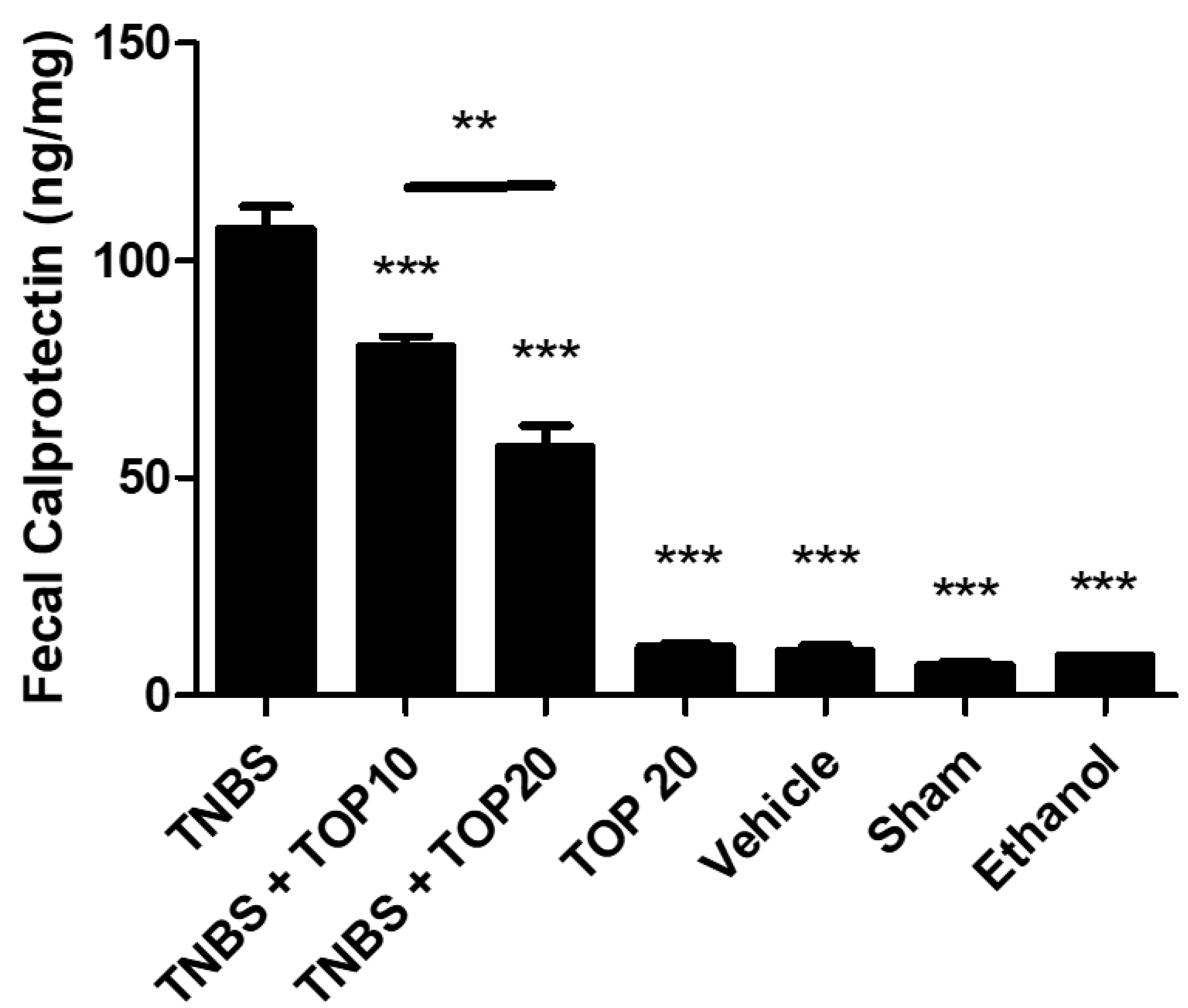
2.2. Biochemical Markers
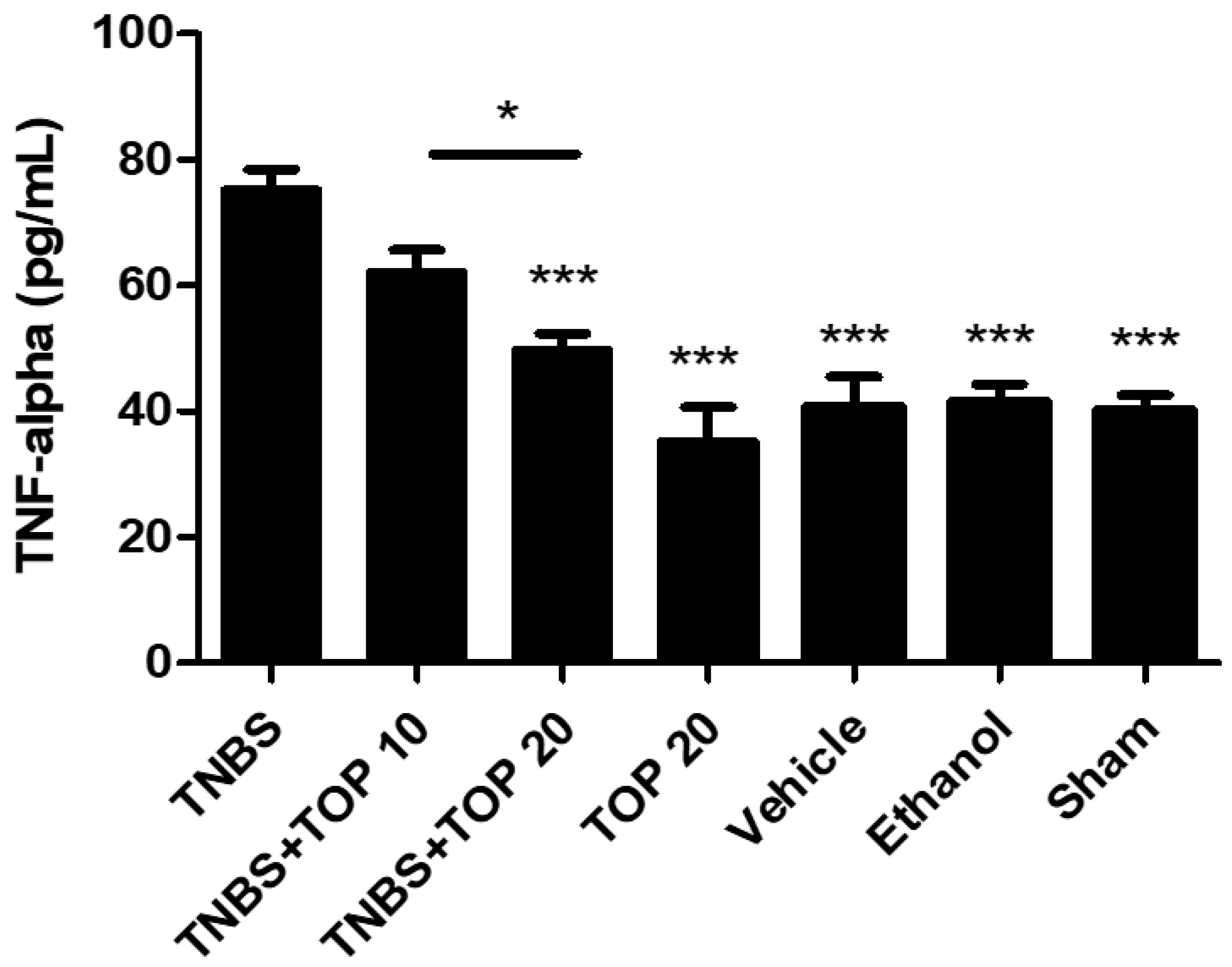
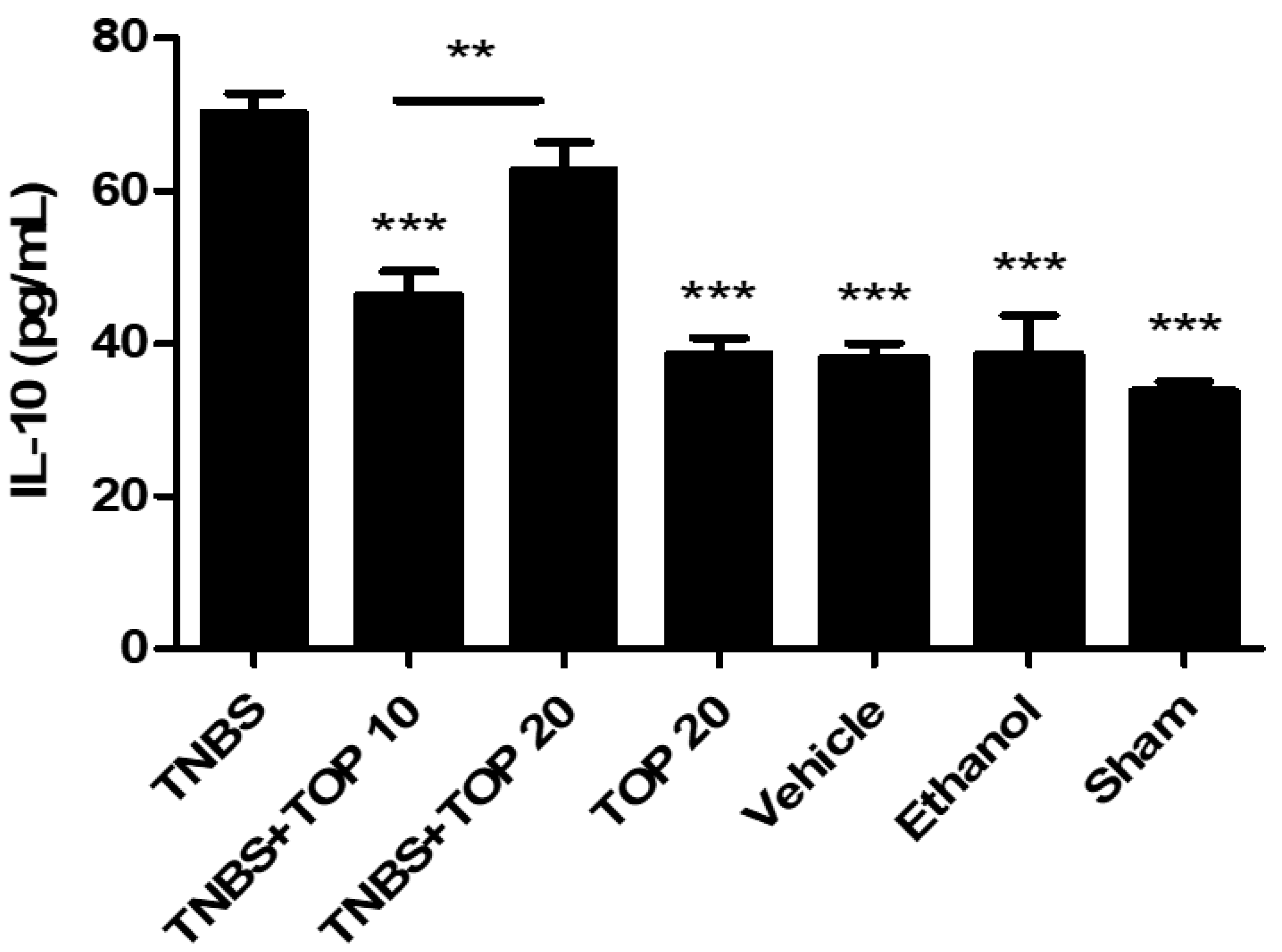
2.3. Measurement of Cytokines
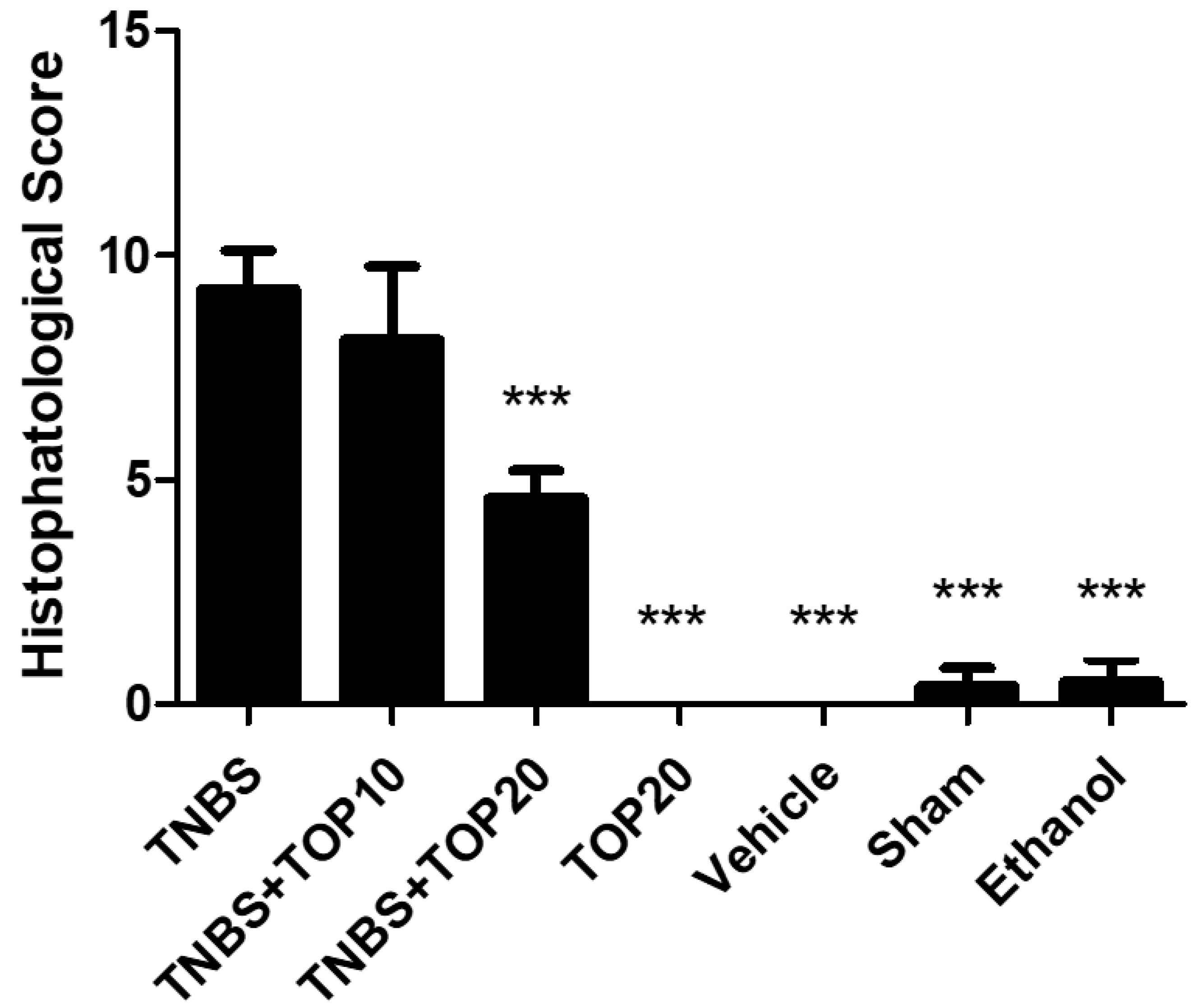
2.4. Microscopial Assessment of Colitis Severity
3. Discussion
4. Materials and Methods
4.1. Drugs and Chemicals
4.2. Animals
4.3. Induction of TNBS-Induced Colitis and Administration Protocol of Topiramate
4.4. Experimental Groups
4.5. Monitoring of Clinical Signs
4.6. Biochemical Markers and Cytokines
4.7. Microscopical Evaluation
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.-Z.; Li, Y.-Y. Inflammatory Bowel Disease: Pathogenesis. World J. Gastroenterol. 2014, 20, 91–99. [Google Scholar] [CrossRef]
- McDowell, C.; Farooq, U.; Haseeb, M. Inflammatory Bowel Disease. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Penate Medina, T.; Pan, J.; Damoah, C.; Humbert, J.; Köpnick, A.-L.; Will, O.; Sebens, S.; Penate Medina, O. Utilizing Sphingomyelinase Sensitizing Liposomes in Imaging Intestinal Inflammation in Dextran Sulfate Sodium-Induced Murine Colitis. Biomedicines 2022, 10, 413. [Google Scholar] [CrossRef] [PubMed]
- Abraham, C.; Cho, J.H. Inflammatory Bowel Disease. N. Engl. J. Med. 2009, 361, 2066–2078. [Google Scholar] [CrossRef]
- Lange, K.M.; Barrett, J.C. Understanding Inflammatory Bowel Disease via Immunogenetics. J. Autoimmun. 2015, 64, 91–100. [Google Scholar] [CrossRef]
- Mak, W.Y.; Zhao, M.; Ng, S.C.; Burisch, J. The Epidemiology of Inflammatory Bowel Disease: East Meets West. J. Gastroenterol. Hepatol. 2020, 35, 380–389. [Google Scholar] [CrossRef]
- Yadav, V.; Varum, F.; Bravo, R.; Furrer, E.; Bojic, D.; Basit, A.W. Inflammatory Bowel Disease: Exploring Gut Pathophysiology for Novel Therapeutic Targets. Transl. Res. 2016, 176, 38–68. [Google Scholar] [CrossRef]
- Fariba, K.A.; Saadabadi, A. Topiramate; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Thiry, A.; Dogné, J.-M.; Supuran, C.T.; Masereel, B. Anticonvulsant Sulfonamides/Sulfamates/Sulfamides with Carbonic Anhydrase Inhibitory Activity: Drug Design and Mechanism of Action. Curr. Pharm. Des. 2008, 14, 661–671. [Google Scholar] [CrossRef]
- De Winter, B.Y.; De Man, J.G. Interplay between Inflammation, Immune System and Neuronal Pathways: Effect on Gastrointestinal Motility. World J. Gastroenterol. 2010, 16, 5523–5535. [Google Scholar] [CrossRef]
- Kaminski, R.M.; Banerjee, M.; Rogawski, M.A. Topiramate Selectively Protects against Seizures Induced by ATPA, a GluR5 Kainate Receptor Agonist. Neuropharmacology 2004, 46, 1097–1104. [Google Scholar] [CrossRef]
- Tan, T.; Barry, P.; Reken, S.; Baker, M.; Guideline Development Group. Pharmacological Management of Neuropathic Pain in Non-Specialist Settings: Summary of NICE Guidance. BMJ 2010, 340, c1079. [Google Scholar] [CrossRef]
- Estaki, M.; DeCoffe, D.; Gibson, D.L. Interplay between Intestinal Alkaline Phosphatase, Diet, Gut Microbes and Immunity. World J. Gastroenterol. 2014, 20, 15650–15656. [Google Scholar] [CrossRef]
- Mateus, V.; Rocha, J.; Mota-Filipe, H.; Sepodes, B.; Pinto, R. Hemin Reduces Inflammation Associated with TNBS-Induced Colitis. Clin. Exp. Gastroenterol. 2018, 11, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Stremmel, W.; Staffer, S.; Schneider, M.J.; Gan-Schreier, H.; Wannhoff, A.; Stuhrmann, N.; Gauss, A.; Wolburg, H.; Mahringer, A.; Swidsinski, A.; et al. Genetic Mouse Models with Intestinal-Specific Tight Junction Deletion Resemble an Ulcerative Colitis Phenotype. J. Crohn’s Colitis 2017, 11, 1247–1257. [Google Scholar] [CrossRef] [PubMed]
- Rennick, D.; Berg, D.; Holland, G. Interleukin 10: An Overview. Prog. Growth Factor Res. 1992, 4, 207–227. [Google Scholar] [CrossRef]
- Dudley, J.T.; Sirota, M.; Shenoy, M.; Pai, R.K.; Roedder, S.; Chiang, A.P.; Morgan, A.A.; Sarwal, M.M.; Pasricha, P.J.; Butte, A.J. Computational Repositioning of the Anticonvulsant Topiramate for Inflammatory Bowel Disease. Sci. Transl. Med. 2011, 3, 96ra76. [Google Scholar] [CrossRef] [PubMed]
- Mateus, V.; Rocha, J.; Alves, P.; Mota-Filipe, H.; Sepodes, B.; Pinto, R.M.A. Anti-Inflammatory Effect of Erythropoietin in the TNBS-Induced Colitis. Basic Clin. Pharmacol. Toxicol. 2017, 120, 138–145. [Google Scholar] [CrossRef]
- Scheiffele, F.; Fuss, I.J. Induction of TNBS Colitis in Mice. Curr. Protoc. Immunol. 2002, 49, 15.19.1–15.19.14. [Google Scholar] [CrossRef]
- Motavallian-Naeini, A.; Andalib, S.; Rabbani, M.; Mahzouni, P.; Afsharipour, M.; Minaiyan, M. Validation and Optimization of Experimental Colitis Induction in Rats Using 2, 4, 6-Trinitrobenzene Sulfonic Acid. Res. Pharm. Sci. 2012, 7, 159–169. [Google Scholar]
- Majewska-Szczepanik, M.; Góralska, M.; Marcińska, K.; Zemelka-Wiącek, M.; Strzępa, A.; Dorożyńska, I.; Szczepanik, M. Epicutaneous Immunization with Protein Antigen TNP-Ig Alleviates TNBS-Induced Colitis in Mice. Pharmacol. Rep. 2012, 64, 1497–1504. [Google Scholar] [CrossRef]
- Keates, A.C.; Castagliuolo, I.; Cruickshank, W.W.; Qiu, B.; Arseneau, K.O.; Brazer, W.; Kelly, C.P. Interleukin 16 Is Up-Regulated in Crohn’s Disease and Participates in TNBS Colitis in Mice. Gastroenterology 2000, 119, 972–982. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.-S.; Kim, Y.-H.; Kwon, O.-J.; Lee, I.-G. Effects of Topiramate on Body Weight and Serum Levels of Insulin and Leptin in Young Rats Fed a High Fat Diet. J. Korean Child Neurol. Soc. 2010, 18, 292–299. [Google Scholar]
- Elson, C.O.; Beagley, K.W.; Sharmanov, A.T.; Fujihashi, K.; Kiyono, H.; Tennyson, G.S.; Cong, Y.; Black, C.A.; Ridwan, B.W.; McGhee, J.R. Hapten-Induced Model of Murine Inflammatory Bowel Disease: Mucosa Immune Responses and Protection by Tolerance. J. Immunol. 1996, 157, 2174–2185. [Google Scholar]
- Seibel, J.; Molzberger, A.F.; Hertrampf, T.; Laudenbach-Leschowski, U.; Diel, P. Oral Treatment with Genistein Reduces the Expression of Molecular and Biochemical Markers of Inflammation in a Rat Model of Chronic TNBS-Induced Colitis. Eur. J. Nutr. 2009, 48, 213–220. [Google Scholar] [CrossRef]
- Hirata, I.; Hoshimoto, M.; Saito, O.; Kayazawa, M.; Nishikawa, T.; Murano, M.; Toshina, K.; Wang, F.-Y.; Matsuse, R. Usefulness of Faecal Lactoferrin and Hemoglobin in Diagnosis of Colorectal Diseases. World J. Gastroenterol. 2007, 13, 1569–1574. [Google Scholar] [CrossRef]
- Bumb, A.; Diederich, N.; Beyenburg, S. Adding Topiramate to Valproate Therapy May Cause Reversible Hepatic Failure. Epileptic Disord. Int. Epilepsy J. Videotape 2003, 5, 157–159. [Google Scholar]
- Mumolo, M.G.; Bertani, L.; Ceccarelli, L.; Laino, G.; Di Fluri, G.; Albano, E.; Tapete, G.; Costa, F. From bench to bedside: Fecal calprotectin in inflammatory bowel diseases clinical setting. World J. Gastroenterol. 2018, 24, 3681–3694. [Google Scholar] [CrossRef]
- Silva, I.; Solas, J.; Pinto, R.; Mateus, V. Chronic Experimental Model of TNBS-Induced Colitis to Study Inflammatory Bowel Disease. Int. J. Mol. Sci. 2022, 23, 4739. [Google Scholar] [CrossRef]
- Corazza, N.; Eichenberger, S.; Eugster, H.-P.; Mueller, C. Nonlymphocyte-Derived Tumor Necrosis Factor Is Required for Induction of Colitis in Recombination Activating Gene (Rag)2−/− Mice upon Transfer of Cd4+Cd45rbhi T Cells. J. Exp. Med. 1999, 190, 1479–1492. [Google Scholar] [CrossRef] [PubMed]
- Seamons, A.; Treuting, P.M.; Brabb, T.; Maggio-Price, L. Characterization of Dextran Sodium Sulfate-Induced Inflammation and Colonic Tumorigenesis in Smad3−/− Mice with Dysregulated TGFβ. PLoS ONE 2013, 8, E79182. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, I.; Mendes, P.; Guerra, S.; Pinto, R.; Mateus, V. Anti-Inflammatory Effect of Topiramate in a Chronic Model of TNBS-Induced Colitis. Int. J. Mol. Sci. 2022, 23, 9127. https://doi.org/10.3390/ijms23169127
Silva I, Mendes P, Guerra S, Pinto R, Mateus V. Anti-Inflammatory Effect of Topiramate in a Chronic Model of TNBS-Induced Colitis. International Journal of Molecular Sciences. 2022; 23(16):9127. https://doi.org/10.3390/ijms23169127
Chicago/Turabian StyleSilva, Inês, Priscila Mendes, Sofia Guerra, Rui Pinto, and Vanessa Mateus. 2022. "Anti-Inflammatory Effect of Topiramate in a Chronic Model of TNBS-Induced Colitis" International Journal of Molecular Sciences 23, no. 16: 9127. https://doi.org/10.3390/ijms23169127
APA StyleSilva, I., Mendes, P., Guerra, S., Pinto, R., & Mateus, V. (2022). Anti-Inflammatory Effect of Topiramate in a Chronic Model of TNBS-Induced Colitis. International Journal of Molecular Sciences, 23(16), 9127. https://doi.org/10.3390/ijms23169127






