Hypoxia Promotes Angiogenic Effect in Extracranial Arteriovenous Malformation Endothelial Cells
Abstract
1. Introduction
2. Results
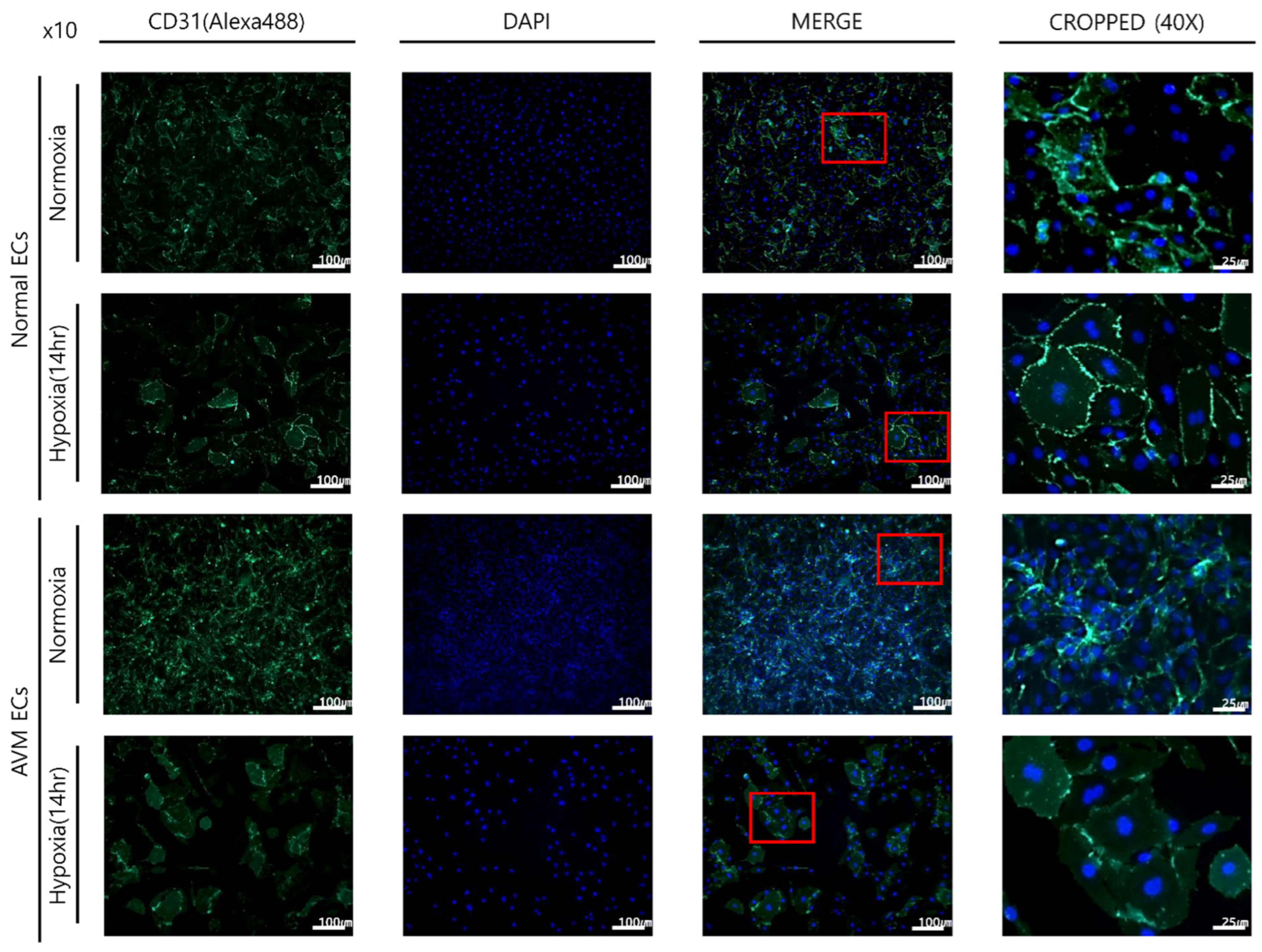
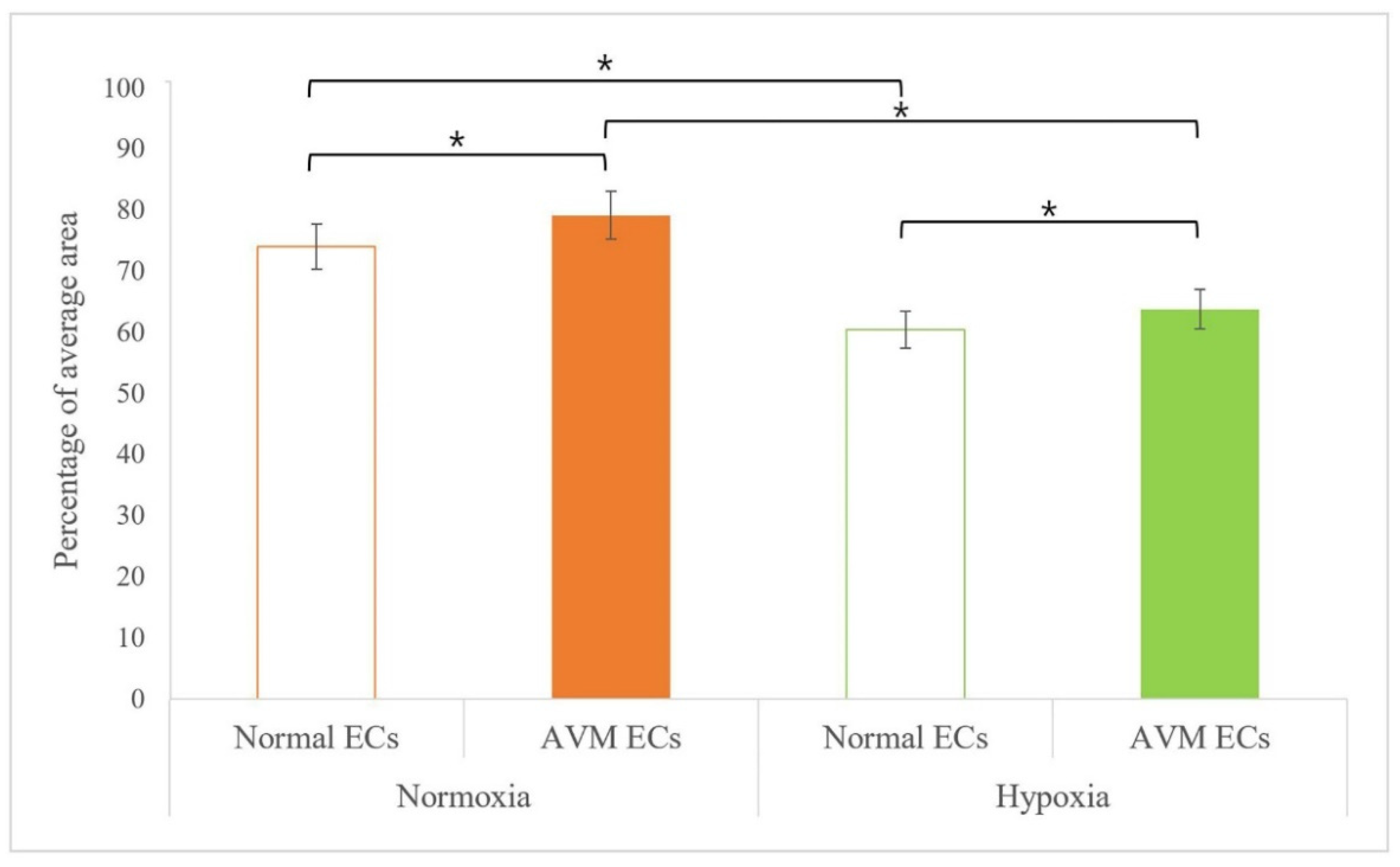
2.1. Immunofluorescence
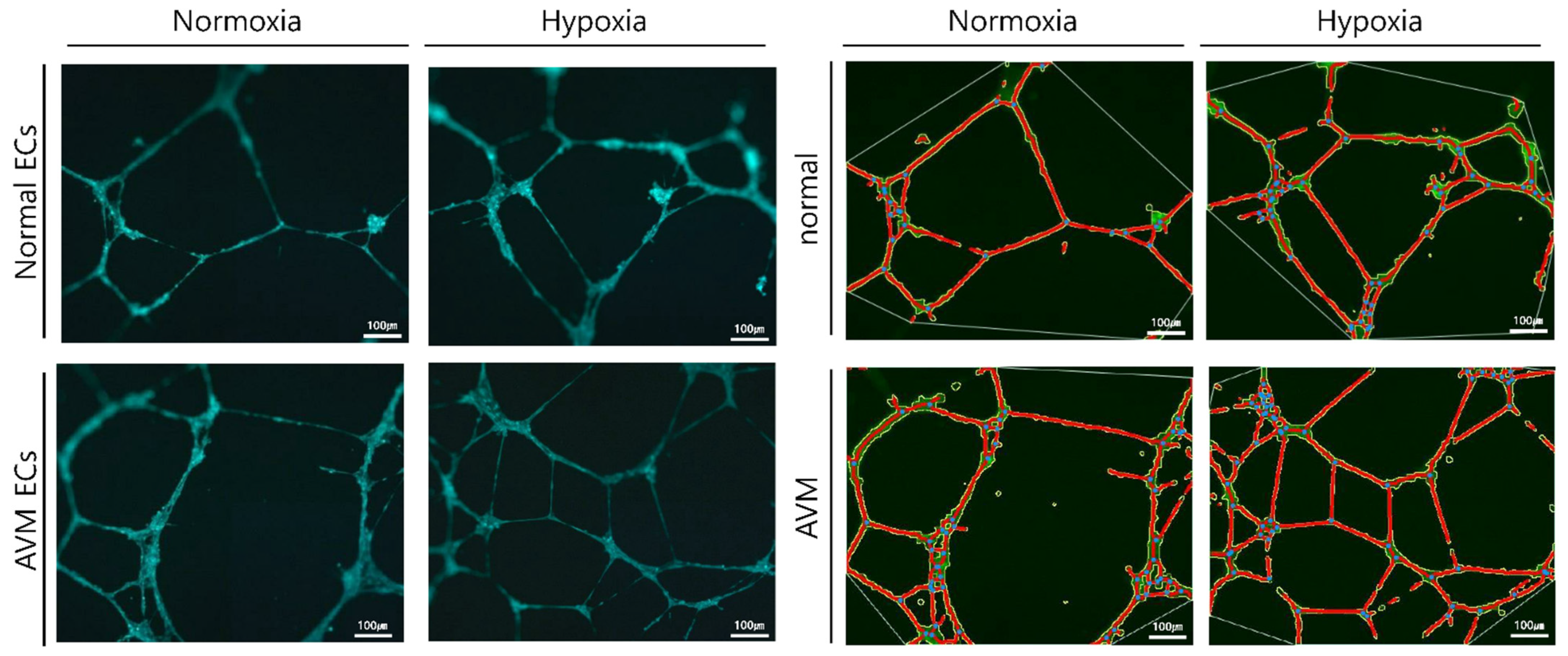
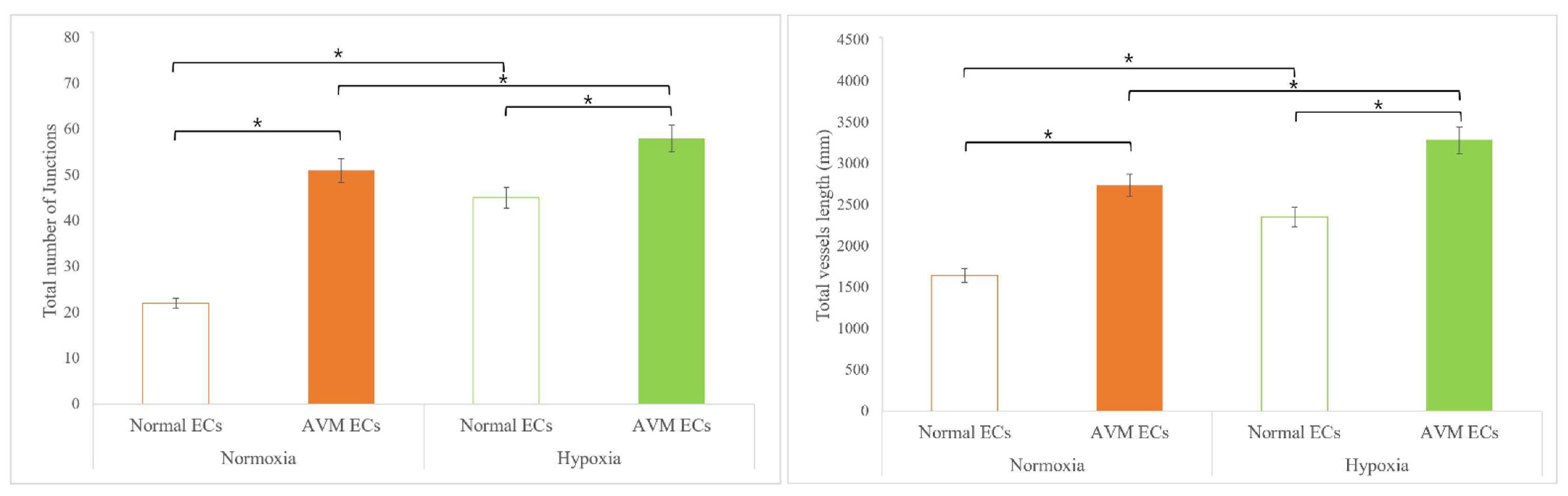
2.2. Tube Formation Assay
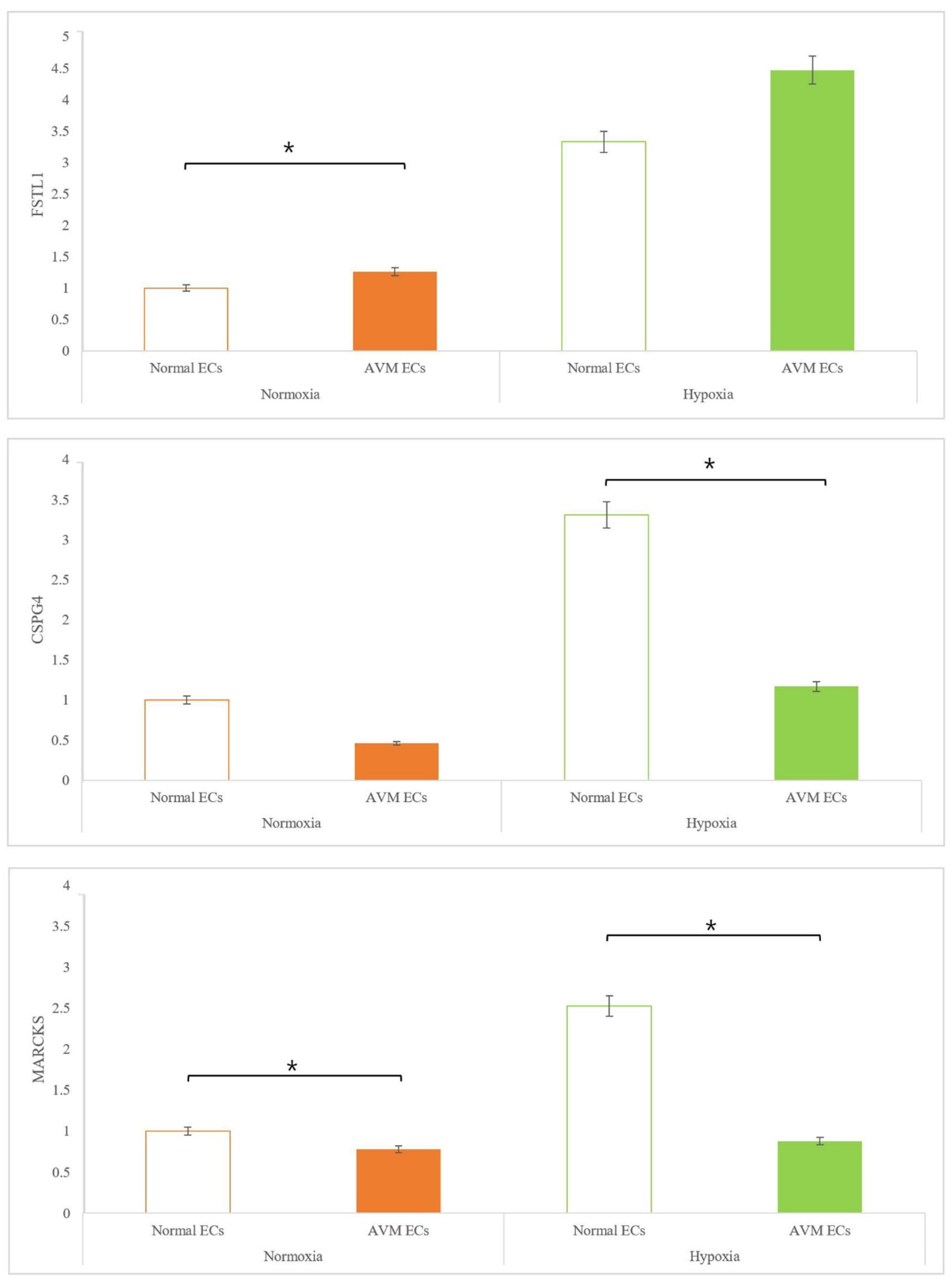
2.3. Real-Time PCR
3. Discussion
4. Materials and Methods
4.1. Isolation and Culture of ECs
4.2. Hypoxic Conditions
4.3. Immunofluorescence Imaging
4.4. Tube Formation Assay
4.5. Real-Time PCR
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adams, D.M. Practical genetic and biologic therapeutic considerations in vascular anomalies. Tech. Vasc. Interv. Radiol. 2019, 22, 100629. [Google Scholar] [CrossRef]
- Halim, A.X.; Johnston, S.C.; Singh, V.; McCulloch, C.E.; Bennett, J.P.; Achrol, A.S.; Sidney, S.; Young, W.L. Longitudinal risk of intracranial hemorrhage in patients with arteriovenous malformation of the brain within a defined population. Stroke 2004, 35, 1697–1702. [Google Scholar] [CrossRef] [PubMed]
- Shovlin, C.L.; Jackson, J.E.; Bamford, K.B.; Jenkins, I.H.; Benjamin, A.R.; Ramadan, H.; Kulinskaya, E. Primary determinants of ischaemic stroke/brain abscess risks are independent of severity of pulmonary arteriovenous malformations in hereditary haemorrhagic telangiectasia. Thorax 2008, 63, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Schimmel, K.; Ali, M.K.; Tan, S.Y.; Teng, J.; Do, H.M.; Steinberg, G.K.; Stevenson, D.A.; Spiekerkoetter, E. Arteriovenous Malformations-Current Understanding of the Pathogenesis with Implications for Treatment. Int. J. Mol. Sci. 2021, 22, 9037. [Google Scholar] [CrossRef] [PubMed]
- Uebelhoer, M.; Boon, L.M.; Vikkula, M. Vascular anomalies: From genetics toward models for therapeutic trials. Cold Spring Harb. Perspect. Med. 2012, 2, a009688. [Google Scholar] [CrossRef][Green Version]
- Soulez, G.; Gilbert, M.D.; Frcpc, P.; Giroux, M.D.; Frcpc, M.F.; Racicot, M.D.; Frcpc, J.N.; Dubois, J. Interventional management of arteriovenous malformations. Tech. Vasc. Interv. Radiol. 2019, 22, 100633. [Google Scholar] [CrossRef]
- Nicholson, C.L.; Flanagan, S.; Murati, M.; Boull, C.; McGough, E.; Ameduri, R.; Weigel, B.; Maguiness, S. Successful management of an arteriovenous malformation with trametinib in a patient with capillary-malformation arteriovenous malformation syndrome and cardiac compromise. Pediatr. Dermatol. 2022, 39, 316–319. [Google Scholar] [CrossRef]
- Timbang, M.R.; Richter, G.T. Update on extracranial arteriovenous malformations: A staged multidisciplinary approach. Semin. Pediatr. Surg. 2020, 29, 150965. [Google Scholar] [CrossRef]
- Lekwuttikarn, R.; Lim, Y.H.; Admani, S.; Choate, K.A.; Teng, J.M.C. Genotype-Guided Medical Treatment of an Arteriovenous Malformation in a Child. JAMA Dermatol. 2019, 155, 256–257. [Google Scholar] [CrossRef]
- Triana, P.; Dore, M.; Cerezo, V.N.; Cervantes, M.; Sánchez, A.V.; Ferrero, M.M.; González, M.D.; Lopez-Gutierrez, J.C.; Triana, P. Sirolimus in the Treatment of Vascular Anomalies. Eur. J. Pediatr. Surg. 2017, 27, 86–90. [Google Scholar] [CrossRef]
- Govindarajan, V.; Burks, J.D.; Luther, E.M.; Thompson, J.W.; Starke, R.M. Medical Adjuvants in the Treatment of Surgically Refractory Arteriovenous Malformations of the Head and Face: Case Report and Review of Literature. Cerebrovasc. Dis. 2021, 50, 493–499. [Google Scholar] [CrossRef]
- Mack, J.M.; Verkamp, B.; Richter, G.T.; Nicholas, R.; Stewart, K.; Crary, S.E. Effect of sirolimus on coagulopathy of slow-flow vascular malformations. Pediatr. Blood Cancer 2019, 66, e27896. [Google Scholar] [CrossRef]
- Marqués, L.; Núñez-Córdoba, J.M.; Aguado, L.; Pretel, M.; Boixeda, P.; Nagore, E.; Baselga, E.; Redondo, P. Topical rapamycin combined with pulsed dye laser in the treatment of capillary vascular malformations in Sturge-Weber syndrome: Phase II, randomized, double-blind, intraindividual placebo-controlled clinical trial. J. Am. Acad. Dermatol. 2015, 72, 151–158.e1. [Google Scholar] [CrossRef]
- Kouroupi, M.; Sivridis, E.; Papazoglou, D.; Koukourakis, M.I.; Giatromanolaki, A. Hypoxia inducible factor expression and angiogenesis—Analysis in the pituitary gland and patterns of death. Vivo 2018, 32, 185–190. [Google Scholar] [CrossRef]
- Liu, J.; Kang, H.; Lu, J.; Dai, Y.; Wang, F. Experimental study of the effects of hypoxia simulator on osteointegration of titanium prosthesis in osteoporotic rats. BMC Musculoskelet. Disord. 2021, 22, 944. [Google Scholar] [CrossRef]
- O’Reilly, M.S.; Holmgren, L.; Shing, Y.; Chen, C.; Rosenthal, R.A.; Moses, M.; Lane, W.S.; Cao, Y.; Saeg, E.H.; Folkman, J. Angiostatin: A novel angiogenesis inhibitor that mediates the suppression of metastasis by a Lewis lung carcinoma. Cell 1994, 79, 315–328. [Google Scholar] [CrossRef]
- Folkman, J. Is tissue mass regulated by vascular endothelial cells? Prostate as the first evidence. Endocrinology 1998, 139, 441–442. [Google Scholar] [CrossRef]
- Rupnick, M.A.; Panigrahy, D.; Zhang, C.Y.; Dallabrida, S.M.; Lowell, B.B.; Langer, R.; Folkman, M.J. Adipose tissue mass can be regulated through the vasculature. Proc. Natl. Acad. Sci. USA 2002, 99, 10730–10735. [Google Scholar] [CrossRef]
- Greene, A.K.; Wiener, S.; Puder, M.; Yoshida, A.; Shi, B.; Perez-Atayade, A.R.; Efstathiou, J.A.; Holmgren, L.; Adamis, A.P.; Rupnick, M.; et al. Endothelial directed hepatic regeneration after partial hepatectomy. Ann. Surg. 2003, 237, 530–535. [Google Scholar] [CrossRef]
- Asahara, T.; Masuda, H.; Takahashi, T.; Pastore, C.; Silver, M.; Kearne, M.; Magner, M.; Ishner, J.M. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ. Res. 1999, 85, 221–228. [Google Scholar] [CrossRef]
- Takahashi, T.; Kalka, C.; Masuda, H.; Chen, D.; Silver, M.; Kearney, M.; Magner, M.; Isner, J.M.; Asahara, T. Ischemia-and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat. Med. 1999, 5, 434–438. [Google Scholar] [CrossRef]
- Peichev, M.; Naiyer, A.J.; Pereira, D.; Lane, W.J.; Williams, M.; Oz, M.C.; Hicklin, D.J.; Witte, L.; Moore, M.A.; Rafii, S. Expression of VEGFR-2 and AC133 by circulating human CD34() cells identifies a population of functional endothelial precursors. Blood 2000, 95, 952–958. [Google Scholar] [CrossRef]
- Tepper, O.M.; Capla, J.M.; Galiano, R.D.; Ceradini, D.J.; Callaghan, M.J.; Kleinman, M.E.; Gurtner, G.C. Adult vasculogenesis occurs through in situ recruitment, proliferation, and tubulization of circulating bone marrow-derived cells. Blood 2005, 105, 1068–1077. [Google Scholar] [CrossRef]
- Lu, L.; Bischoff, J.; Mulliken, J.B.; Bielenberg, D.R.; Fishman, S.J.; Greene, A.K. Increased endothelial progenitor cells and vasculogenic factors in higher-staged arteriovenous malformations. Plast. Reconstr. Surg. 2011, 128, 260e–269e. [Google Scholar] [CrossRef]
- Ouchi, N.; Oshima, Y.; Ohashi, K.; Higuchi, A.; Ikegami, C.; Izumiya, Y.; Walsh, K. Follistatin-like 1, a secreted muscle protein, promotes endothelial cell function and revascularization in ischemic tissue through a nitric-oxide synthase-dependent mechanism. J. Biol. Chem. 2008, 283, 32802–32811. [Google Scholar] [CrossRef]
- Miyabe, M.; Ohashi, K.; Shibata, R.; Uemura, Y.; Ogura, Y.; Yuasa, D.; Kambara, T.; Kataoka, Y.; Yamamoto, T.; Matsuo, K.; et al. Muscle-derived follistatin-like 1 functions to reduce neointimal formation after vascular injury. Cardiovasc. Res. 2014, 103, 111–120. [Google Scholar] [CrossRef]
- Yang, Y.; Mu, T.; Li, T.; Xie, S.; Zhou, J.; Liu, M.; Li, D. Effects of FSTL1 on the proliferation and motility of breast cancer cells and vascular endothelial cells. Thorac. Cancer 2017, 8, 606–612. [Google Scholar] [CrossRef]
- Murfee, W.L.; Skalak, T.C.; Peirce, S.M. Differential arterial/venous expression of NG2 proteoglycan in perivascular cells along microvessels: Identifying a venule-specific phenotype. Microcirculation 2005, 12, 151–160. [Google Scholar] [CrossRef]
- Murfee, W.L.; Rehorn, M.R.; Peirce, S.M.; Skalak, T.C. Perivascular cells along venules upregulate NG2 expression during microvascular remodeling. Microcirculation 2006, 13, 261–273. [Google Scholar] [CrossRef]
- Ampofo, E.; Schmitt, B.M.; Menger, M.D.; Laschke, M.W. The regulatory mechanisms of NG2/CSPG4 expression. Cell. Mol. Biol. Lett. 2017, 22, 4. [Google Scholar] [CrossRef]
- Chen, C.H.; Fong, L.W.R.; Yu, E.; Wu, R.; Trott, J.F.; Weiss, R.H. Upregulation of MARCKS in kidney cancer and its potential as a therapeutic target. Oncogene 2017, 36, 3588–3598. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Neltner, B.S.; Davis, H.W. Role of MARCKS in regulating endothelial cell proliferation. Am. J. Physiol. Cell. Physiol. 2000, 279, C1611–C1620. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.Y.; Kim, Y.H.; Lee, J.S.; Lee, J.W.; Oh, E.J.; Kim, H.M.; Lee, S.J.; Lee, J.; Lee, S.Y.; Huh, S.; et al. Oscillatory shear stress promotes angiogenic effects in arteriovenous malformations endothelial cells. Mol. Med. 2021, 31, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Carpentier, G.; Berndt, S.; Ferratge, S.; Rasband, W.; Cuendet, M.; Uzan, G.; Albanese, P. Angiogenesis Analyzer for ImageJ—A comparative morphometric analysis of “Endothelial Tube Formation Assay” and “Fibrin Bead Assay”. Sci. Rep. 2020, 10, 11568. [Google Scholar] [CrossRef]
| Gene | Primer Sequence (5′-3′) | |
|---|---|---|
| GAPDH | Forward Sequence | GGAAGGTGAAGGTCGGAGTCA |
| Reverse Sequence | GTCATTGATGGCAACAATATCCACT | |
| FSTL1 | Forward Sequence | TCGCATCATCCAGTGGCTGGAA |
| Reverse Sequence | TCACTGGAGTCCAGGCGAGAAT | |
| MARCKS | Forward Sequence | CTCCTCGACTTCTTCGCCCAAG |
| Reverse Sequence | TCTTGAAGGAGAAGCCGCTCAG | |
| CSPG4 | Forward Sequence | GTCCTGCCTGTCAATGACCAAC |
| Reverse Sequence | CGATGGTGTAGACCAGATCCTC | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.S.; Cho, H.G.; Ryu, J.Y.; Oh, E.J.; Kim, H.M.; Kwak, S.; Lee, S.-J.; Lee, J.; Lee, S.Y.; Huh, S.; et al. Hypoxia Promotes Angiogenic Effect in Extracranial Arteriovenous Malformation Endothelial Cells. Int. J. Mol. Sci. 2022, 23, 9109. https://doi.org/10.3390/ijms23169109
Lee JS, Cho HG, Ryu JY, Oh EJ, Kim HM, Kwak S, Lee S-J, Lee J, Lee SY, Huh S, et al. Hypoxia Promotes Angiogenic Effect in Extracranial Arteriovenous Malformation Endothelial Cells. International Journal of Molecular Sciences. 2022; 23(16):9109. https://doi.org/10.3390/ijms23169109
Chicago/Turabian StyleLee, Joon Seok, Hyun Geun Cho, Jeong Yeop Ryu, Eun Jung Oh, Hyun Mi Kim, Suin Kwak, Seok-Jong Lee, Jongmin Lee, Sang Yub Lee, Seung Huh, and et al. 2022. "Hypoxia Promotes Angiogenic Effect in Extracranial Arteriovenous Malformation Endothelial Cells" International Journal of Molecular Sciences 23, no. 16: 9109. https://doi.org/10.3390/ijms23169109
APA StyleLee, J. S., Cho, H. G., Ryu, J. Y., Oh, E. J., Kim, H. M., Kwak, S., Lee, S.-J., Lee, J., Lee, S. Y., Huh, S., Kim, J. Y., & Chung, H. Y. (2022). Hypoxia Promotes Angiogenic Effect in Extracranial Arteriovenous Malformation Endothelial Cells. International Journal of Molecular Sciences, 23(16), 9109. https://doi.org/10.3390/ijms23169109






