Abstract
A convincing deal of evidence supports the fact that severe psoriasis is associated with cardiovascular diseases. However, the precise underlying mechanisms linking psoriasis and cardiovascular diseases are not well defined. Psoriasis shares common pathophysiologic mechanisms with atherosclerosis and cardiovascular (CV) risk factors. In particular, polymorphism in the IL-23R and IL-23 genes, as well as other genes involved in lipid and fatty-acid metabolism, renin–angiotensin system and endothelial function, have been described in patients with psoriasis and with cardiovascular risk factors. Moreover, systemic inflammation in patients with psoriasis, including elevated serum proinflammatory cytokines (e.g., TNF-α, IL-17, and IL-23) may contribute to an increased risk of atherosclerosis, hypertension, alteration of serum lipid composition, and insulin resistance. The nonlinear and intricate interplay among various factors, impacting the molecular pathways in different cell types, probably contributes to the development of psoriasis and cardiovascular disease (CVD). Future research should, therefore, aim to fully unravel shared and differential molecular pathways underpinning the association between psoriasis and CVD.
Keywords:
psoriasis; cardiovascular; MACE; myocardial infarction; atherosclerosis; hypertension; hyperlipidemia; diabetes; obesity 1. Introduction
Psoriasis is a chronic skin immune-mediated inflammatory disease with a global prevalence of 1–3%; it presents as raised, erythematous, and scaly plaques [1]. Psoriasis-related inflammation may extend beyond the skin [2]. Patients with psoriasis have an increased risk for cardiovascular (CV) disease (CVD), a common cause of morbidity and mortality in psoriasis, and CV risk factors [3,4,5]. In particular, an independent association between psoriasis and an increased risk of myocardial infarction (MI), chronic heart failure (CHF), and cardiac arrythmia has been demonstrated [6,7,8,9]. In this paper, we focused our attention on ischemic heart disease (IHD). IHD describes a group of clinical syndromes characterized by myocardial ischemia, i.e., an imbalance between myocardial blood supply and demand [2]. Several mechanisms may lead to IHD. The most common is atherosclerotic coronary artery disease. However, a relevant proportion of patients may have no significant epicardial disease and often have microvascular disease as the underlying pathophysiology.
Notably, the increased ischemic CV risk in psoriasis patients seems to be associated with a higher activity of psoriasis. Indeed, severe psoriasis confers the highest CV risk (compared with control subjects), including up to threefold increased risk of MI, 60% increased risk of stroke, and 40% increased risk of CV-related deaths [6]. This association could only be partially explained by a higher prevalence of traditional modifiable CV risk factors [10,11,12,13,14,15,16,17] including hypertension, diabetes, hyperlipidemia, obesity, nonalcoholic fatty liver disease (NAFLD), and smoking, along with metabolic syndrome. These conditions are often neglected in patients with severe psoriasis [18]. In addition to this, chronic systemic inflammation associated with psoriasis is likely to independently contribute to the increased risk of IHD.
Here, we review current knowledge on the associations linking psoriasis, CV risk factors, and CV events, with an emphasis on the common molecular and genetic underpinnings.
2. Psoriasis as an Independent CV Risk Factor
2.1. Shared Genetic and Molecular Pathways between Psoriasis and Atherosclerosis
Several studies, involving large sample sizes and complex multivariate models, have consistently suggested that severe psoriasis may be an independent risk factor of CVD [4,19,20,21,22,23].
Cardiologists have, therefore, included it in the European and American guidelines on cardiovascular disease prevention as a 1.5 risk factor multiplier for CV risk [24], as well as a risk-enhancing factor for the start of statin treatment in nondiabetic adults with a CV intermediate risk [25].
Chronic psoriatic disease has a negative impact on vascular inflammation and major adverse cardiovascular events (MACE), suggesting that exposure to low-grade chronic inflammation may accelerate vascular disease development and MACE [26]. Vascular inflammation by 18-FDG PET/CT is significantly associated with disease duration (β = 0.171, p = 0.002). Moreover, the duration of psoriasis has a strong relationship with MACE even after accounting for age, sex, socioeconomic status, CVD history, smoking, alcohol abuse, diabetes, hypertension, and use of statins (an increase of 1.0% for each additional year of PSO, HR 1.010; 95% CI, 1.007 to 1.013).
Despite the epidemiological association between psoriasis and CVD, the precise underlying mechanisms linking psoriasis and cardiovascular diseases are not well defined. It is unknown whether localized, chronic cutaneous inflammation directly promotes vascular inflammation and atherosclerotic plaque formation.
Figure 1 illustrates pathogenesis of psoriasis, associated comorbidities and involved molecular pathways.
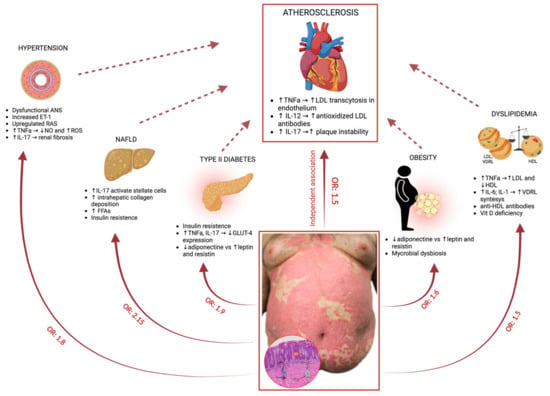
Figure 1.
Molecular pathways involved in the link between psoriasis and its comorbidities. Psoriasis per se is considered an independent risk factor for atherosclerosis. Moreover, psoriasis is also associated with a high prevalence of traditional modifiable CV risk factors, such as hypertension, diabetes, hyperlipidemia, obesity, and nonalcoholic fatty liver disease (NAFLD) [27,28,29]. Aberrantly activated molecular pathways involved in the link between psoriasis and its comorbidities are indicated.
In psoriatic plaques, activation of plasmacytoid dendritic cells (pDCs) promotes the maturation of myeloid dendritic cells (mDCs) and the production of TNF-α, IL-12, and IL-23. This leads to the activation of T helper 1 and 17 (Th1 and Th17) cells and subsequent secretion of inflammatory cytokines, such as TNF-α, IL-17, IL-21, and IL-22 [30]. Keratinocytes are then activated by these cytokines, particularly IL-17, and they produce antimicrobial peptides, cytokines, and chemokines, contributing to the amplification of inflammation.
Interestingly, the pathophysiology of atherosclerosis and psoriasis shares common mechanisms.
Indeed, atherosclerosis and coronary artery disease are both chronic inflammatory disorders, involving the immune cells, including Th1 and Th17 cells, with inflammatory factor infiltration observed at all stages of the disease [2].
2.2. Genetic Background
No association between IL-12 or IL-17 gene polymorphism and CV diseases has been reported so far [31,32]. Instead, genetic studies have shown that polymorphisms of the IL-23 gene are associated with the occurrence or progression of coronary artery disease [32].
In particular, the IL-23R rs6682925T/C polymorphism appears to be linked to the development of coronary artery disease [33,34]. Moreover, two small studies have revealed an association between IL-23R and CVD. The functional single-nucleotide polymorphism (SNP) rs11209026 G/A in IL-23R, which is associated with reduced IL-23 receptor signal transmission, is described to be protective against atherosclerosis progression [35]. Moreover, the IL-23 gene rs2066808 polymorphism, already associated with susceptibility to psoriasis [36,37] and psoriatic arthritis [38], is reported to increase the genetic risk of premature coronary artery disease (CAD) [39]. Likewise, a large-scale genome-wide association study (GWAS) for psoriasis [40] showed that the variants of IL-23R are significantly associated with psoriasis susceptibility [41]. A TNF-α 238 G/A polymorphism was found to be significantly associated with a reduced risk of psoriasis in the general population [42].
On the contrary, TNF-α 238 G/A locus A has a significant association with CAD susceptibility in Europeans and North Asians [43]. Interestingly, transcriptional activity in people with the TNF-α-238A allele is significantly reduced, and monocytes carrying the TNF-α-238A allele in the peripheral blood, when stimulated, produce significantly less TNF-α [44].
2.3. Shared Molecular Pathways
Despite the high discrepancies between results obtained from different models, TNF-α has been recognized as one of the most potent proatherogenic cytokines [45], by increasing LDL transcytosis in endothelial cells [46,47] and by regulating the activity of macrophage scavenger receptor and foam cell formation [48]. Moreover, the blockade of TNF-α at the time of myocardial infarction improves cardiac functions, reduces infarct size, and promotes cardiomyocytes apoptosis [48].
TNF-α is also known to increase reactive oxygen species (ROS) levels and decrease nitric oxide production in blood vessels, which can lead to endothelial dysfunction, the initial step in the development of atherogenesis [49,50]. Similarly, several studies have consistently reported that the serum levels of TNF-α are significantly increased in patients with psoriasis compared to healthy controls [51].
Surprisingly, despite the central role of IL-12/23p40 and IL-17 in the pathogenesis of psoriasis, no significant difference in the serum levels of these molecules between the psoriasis and control groups was observed in most studies [51]. Indeed, serum cytokine levels do not necessarily reflect disease-specific activity, since their concentrations may be affected by several processes such as deposition and diffusion in other tissues. Nevertheless, IL-12 and IL-17 are produced in high amounts in psoriatic plaques, and several studies have reported a significant correlation between IL-17 and the psoriasis area and severity index (PASI) score [52,53,54]. Therefore, in patients with greater disease severity, skin-derived IL-12 and IL-17 may have a systemic impact.
Preclinical studies have suggested a possible role of IL-12 in the progression of atherosclerosis. Elevated serum IL-12 levels have been observed in ApoE-deficient mice, and they are associated with the progression of atherosclerosis [55]. Preclinical reports have also demonstrated that treatment with exogenous recombinant murine IL-12 significantly aggravated the progression of atherosclerosis, and it increased aortic atherosclerotic plaque areas in both ApoE-deficient mice and low-density lipoprotein receptor-deficient mice, whereas abrogating IL-12 levels significantly diminished such effects [56,57,58].
Plasma IL-12 concentrations have been demonstrated to be significantly higher in atherosclerosis and atherosclerotic cardiovascular disease [59], including stable angina pectoris, non-ST segment elevation MI, ST-elevation MI, and acute MI (reviewed in [32]). Most of these studies had small sample sizes and did not adjust for the multiplicity of confounding factors. Moreover, some studies excluded patients with autoimmune disorders. However, the consistency of these findings points toward the potential role of IL-12 in CV diseases.
IL-17 appears to play a less important role in atherosclerotic plaque development compared to Th1 cytokines (TNF-α and IL-12), and IL-17 confers both proatherogenic and protective effects in atherosclerosis, thus having controversial roles [60,61,62] (reviewed in [63]).
Th17 cells and IL-17 are present in murine and human atherosclerotic lesions [64]. IL-17 is increasingly expressed in symptomatic carotid plaques as opposed to asymptomatic plaques, and IL-17 mRNA expression appears higher in complex, unstable, and lipid-rich lesions [65]. In a murine model, IL-17A inhibition decreased the atheroma dimensions and the vessel stenosis, seemingly inducing a stabilization of the plaque, as shown by the increase in intravascular smooth muscle cells and in the collagen of the fibrous cap, the downregulation of metalloproteinases, and the decreased apoptosis in the lesion [66].
These findings are similar to those published in other studies in which recombinant IL-17A induced proatherogenic changes and increased plaque instability, whilst IL-17A inhibition reduced the development of atherosclerosis [64,67,68,69]. In another mouse model (K14-IL-17Aind/+) investigating IL-17A overexpression in keratinocytes inducing psoriasis-like lesions, evidence of vascular dysfunction and arterial hypertension, along with large aortic wall cellular infiltrates and reduced survival compared to the controls, was observed [70]. Likewise, Wang and al. showed that higher blood levels of IL-17 and TNF-α induced by cutaneous inflammation were associated with aortic inflammation and thrombosis [71]. These data sustain the view, at least in mouse models, that skin psoriasis-related inflammation promotes vascular inflammation and increases the risk of CVD.
Another contribution of IL-17 to the formation of atherosclerotic plaque seems to involve the induction of maturation and differentiation of macrophages; these cells, which are precursor of the foam cells, would then be activated by oxidized low-density lipoprotein (oxLDL), thereby starting the process of atherogenesis [72].
On the contrary, in another mouse model, a significant reduction in atherosclerotic lesion development was found in mice supplemented with rIL-17 [73]. A potential role of the IL-17-mediated mechanism of plaque stabilization has been suggested [69,74]. These contradicting observations may reflect the limitations of the experimental animal models of atherosclerosis used by the respective investigators. Overall, the majority of animal/preclinical data available to date suggest the role of IL-17 in the development of atherosclerosis or CVD.
In humans, higher IL-17 plasma levels were found in patients with coronary artery and carotid artery disease [75]. Moreover, high serum levels of IL-17A are associated with increased CV mortality [76]. This observation might, however, be interpreted both as a cause and as a result of compensatory mechanisms to ameliorate CV damaging effects.
In summary, the published evidence, while not definite, underpins the proatherogenic role of IL-17 and its involvement in the development of CV events. Taken together, these findings provide insight into skin-specific inflammatory contributions that support the epidemiological evidence indicating that patients with severe psoriasis have an increased risk of CVD and cardiovascular mortality.
3. Higher Prevalence of Traditional CV Risk Factors in Psoriasis Patients
Patients with psoriasis have an increased chance of traditional modifiable CV risk factors including hypertension, diabetes, hyperlipidemia, and obesity, all of which are combined and defined as metabolic syndrome [10,11,12,13,14,15,16,17]. Indeed, a “dose effect” of psoriasis on the metabolic syndrome and its components has been demonstrated [17]. Below, we discuss the genetic and molecular relationship between each component of metabolic syndrome and psoriasis. Table 1 summarizes the most relevant pathways shared between psoriasis and atherosclerosis or traditional CV risk factors.

Table 1.
List of the shared genetic and molecular pathways between psoriasis and CV events or CV risk factors.
3.1. Hypertension
The prevalence of hypertension in patients with psoriasis is estimated to be 38.8% [77], whereas it is about 31.1% in the general population [78]. Indeed, the risk of hypertension in patients with psoriasis was 1.7 times that of a control group [79], and the severity of psoriasis positively correlated with the risk of hypertension [80]. Although the association between psoriasis and hypertension has been documented, the mechanism underlying this link has not yet been unequivocally defined.
3.1.1. Genetic Background
Numerous studies have been carried out to evaluate the genetic predisposition of patients with psoriasis to develop hypertension. Genetic studies, especially GWAS, found that there are some common genes/loci between psoriasis and its comorbidities.
Ogretmen et al. [81] explored genetic mutations of the eNOS gene coding for the endothelial nitric oxide enzyme in patients with psoriasis. This enzyme is involved in the production of nitric oxide at the endothelial level and, therefore, in the regulation of vascular smooth muscle tone. eNOS gene mutations are risk factors for coronary artery disease, myocardial infarction, and hypertension [82]. In particular, the researchers noticed a significantly increased frequency of the T allele in eNOS Glu298Asp in patients with psoriasis as compared to normotensive non-psoriatic healthy volunteers. The T allele frequency was also found to be greater in hypertensive psoriatic patients than in normotensive psoriatic patients. In conclusion, the results indicated that the Glu298Asp polymorphism of the eNOS gene appears to be an independent risk factor for psoriasis and is a potential genetic susceptibility for hypertension [81].
Cheng et al. [83] analyzed polymorphisms of the gene LNPEP, also named insulin-responsive aminopeptidase, a crucial component in the renin–angiotensin system pathway, and they identified it as an angiotensin IV receptor [84]. LNPEP and its genetic variants have been implicated in hypertension and diabetes [85,86] because of their biological effects on vasopressin clearance, serum sodium regulation [87], and glucose uptake via the interaction of insulin receptor signaling with the insulin-responsive glucose transporter GLUT4 [88]. In particular, researchers identified a missense LNPEP A763T mutation in patients with psoriasis leading to the disruption of peptide function, which was downregulated in the psoriatic skin compared with the control and uninvolved patient skin. Therefore, this raises the speculation that LNPEP may have a role in the pathogenesis of both psoriasis and metabolic conditions, such as hypertension and diabetes [83].
Interestingly, the angiotensin-converting enzyme (ACE) gene, which is also a key component in the renin–angiotensin system, was widely reported to be associated with psoriasis [89,90,91,92,93,94], further supporting the involvement of the renin–angiotensin system in the development of psoriasis and its related hypertension. In particular, different polymorphisms in the angiotensinogen gene (AGT) have been reported to influence the rate of AGT transcription [95], and the D allele is associated with higher concentrations of ACE mRNA in cells and increased ACE concentration and activity in plasma and serum [91]. Weger et al. demonstrated that ACE insertion/deletion(I/D) polymorphism may affect susceptibility to early-onset psoriasis [90]. Moreover, Veletza et al. showed that patients with psoriasis vulgaris or early-onset psoriasis have a statistically significantly higher frequency of I/D heterozygosity, as well as the presence of allele D compared to the controls [91].
3.1.2. Molecular Pathways
Many pathophysiological and molecular pathways have been explored to shed light on the link between psoriasis and hypertension. Among these, the role of the autonomous nervous system (ANS), involved in the regulation of blood pressure and heart rate, has been explored, and evidence indicates that ANS is affected in patients with psoriasis [96,97]. Of note, Mastrolonardo et al. investigated the experimental stress-induced cardiovascular system response in patients with psoriasis. Post-stress systolic/diastolic blood pressure and hemodynamic responses of patients with psoriasis and those of the control group were compared, revealing statistically significantly increased values in patients with psoriasis.
Other evidence suggests that endothelin-1 may play a role in the development of hypertension among patients with psoriasis. Endothelin-1 is a mediator of vasoconstriction and increases blood pressure. It is synthetized by several cell types including keratinocytes, and its expression seems to be modified in psoriasis patients [98]. Compared to non-psoriatic controls, endothelin-1 expression appears to be higher in lesional skin and the blood of patients with psoriasis. Furthermore, endothelin-1 levels increase with psoriasis disease severity [98]. Higher endothelin-1 levels may, thus, contribute to the development of hypertension, due to an increased vasoconstrictive action on the blood vessels [99].
The enhancement of the renin–angiotensin signaling pathway may also induce hypertension less responsive to treatment in patients with psoriasis [80]. Renin is involved in the release of aldosterone, vasoconstriction, and increased blood pressure. Moreover, patients with psoriasis have an enhanced renin activity and increased urinary aldosterone excretion [100]. Interestingly, Suarez et al. performed a transcriptome analysis and observed increased expression of the renin gene in lesional skin of patients with moderate to severe psoriasis compared with matched non-lesional skin, suggesting that products of the psoriatic plaque have a hormone-like action and influence the biology of distal sites [101].
TNF-α contributes to an increased risk of hypertension and CHD through various mechanisms [102]. In particular, TNF-α blocks the activation of endothelial nitric oxide synthase (eNOS), degrades eNOS mRNA, alters vasomotor function by acting on vascular smooth muscle cells, and induces oxidative stress by increasing the production of ROS, leading to an unbalanced microvascular dilation/constriction [103,104,105]. Moreover, it has been hypothesized that crosstalk between the renin–angiotensin system and proinflammatory molecules, such as TNF-α, may regulate cardiovascular functions. Indeed, Sriramula et al. demonstrated the implications of TNF-α in mediating chronic angiotensin II-induced effects on increasing salt-appetite and blood pressure, possibly via its role in upregulating AT1 receptors, as well as enhancing NF-κB activity [106].
There is growing interest in the role of IL-17, which has been demonstrated to be overexpressed in patients with hypertension [107]. In a preclinical model of psoriasis, the dermal overexpression of IL-17A induces vascular oxidative stress and arterial hypertension [70]. Moreover, IL-17A also promotes endothelial dysfunction and angiotensin II-induced hypertension [108]. IL-17 may cause hypertension by inducing vessel inflammation and stiffness by inducing oxidative stress, as well as cardiac and renal fibrosis [109,110,111]. In line with the latter hypothesis, the administration of anti-IL-17 antibodies in a mouse model of hypertension reduced renal transforming growth factor-beta (TGF-β) levels, a well-known marker of fibrosis, compared to the control group. Inhibition of IL-17 signaling also ameliorated the progression of albuminuria and attenuated renal and vascular lymphocyte infiltration, thus suggesting that the inhibition of IL-17 may be a useful adjunct treatment for hypertension and the associated end-organ dysfunction [112].
3.2. Diabetes
Epidemiological studies strongly support an association between psoriasis and type 2 diabetes (T2D) [13,113,114,115]. Indeed, T2D is reported in 10–20% of patients with psoriasis [116] compared to a prevalence of 6.7% in the general population [117]. Of note, patients with psoriasis with normal blood glucose levels demonstrate insulin resistance or impaired insulin sensitivity, which may potentially result in the development of diabetes mellitus [118].
3.2.1. Genetic Background
Genetic studies have suggested a shared mechanism between psoriasis and diabetes. Several genetic psoriasis susceptibility loci (PSORS) have been identified, and PSORS2, PSORS3, and PSORS4 have been proposed as susceptibility loci of metabolic diseases, including T2D [113]. SNPs of the CDKAL1 gene, which can reduce the glucose sensitivity of pancreatic beta-cells and, therefore, predispose individuals to the onset of diabetes, are also associated with a higher incidence of psoriasis [119,120]. Furthermore, some genetic polymorphisms in JAZF1 and ST6GAL1, recently identified as causal risk genes of T2D in the general population [95], have been demonstrated to influence not only the risk for psoriasis but also disease severity [121]. Lastly, common DNA variants of IL-12B, IL-23R, and IL-23A were associated with increased susceptibility to psoriasis and contributed to an increased risk of developing T2D in a Spanish population [122].
3.2.2. Molecular Pathways
Systemic inflammation in patients with psoriasis is increasingly thought to be the basis of the increased risk of T2D and insulin resistance [123]. Consistently, a “dose effect” of psoriasis severity on the risk of T2D has been reported [13,114], and insulin resistance significantly correlates with the PASI score [124]. TNF-α and IL-23/IL-17, as well as adipokines, have been proven to affect the regulation of insulin sensitivity by acting on the signaling pathways linking insulin receptors, cytokines, and adipocytokines [125,126]. In particular, TNF-α is known to impair insulin signaling via serine phosphorylation of IRS-1, thus leading to the reduction in GLUT-4 expression and a subsequent decrease in glucose entry into cells [127]. In line with this, in preclinical models, the exogenous administration of TNF-α induced insulin resistance, whereas animals deficient in TNF-α receptors were protected against insulin resistance [128,129,130]. Of note, treatment with the anti-TNF-α drugs etanercept and adalimumab, commonly used in the treatment of psoriasis, was shown to exert positive effects on insulin sensitivity [131,132]. Interestingly, patients with T2D, particularly female patients, show significantly higher IL-17 serum levels that correlate with age, insulin resistance, fasting blood sugar, BMI, and waist circumference [133]. Moreover, a reduction in glycated hemoglobin concentration (HbA1c) upon treatment in patients newly diagnosed with T2D has been associated with a decrease in IL-17 levels [134]. In animal models, it has been demonstrated that IL-17 deficiency is related to increased glucose tolerance and insulin sensitivity. Of note, IL-17 has also been associated with a reduction in serum insulin and is indicative of improved insulin sensitivity [135]. Intriguingly, in KK-Ay diabetic mice, the inhibition of IL-17 ameliorated insulin resistance and enhanced glucose uptake by muscle tissue, but not by fat tissue [136]. From a molecular point of view, it is reasonable to believe that IL-17 may be able to induce insulin resistance by acting on insulin signaling pathways [63]. In particular, it might inhibit the phosphorylation of IRS-1 via the direct activation of the IkB kinase (IKK)/NFkB pathway and via the indirect activation of JNK, through the induction of the expression of IL-6 [63,137,138].
Adipokines are a large family of cytokines including adiponectin, leptin, and resistin, which are generated and released by adipocytes and are involved in obesity and insulin resistance [139]. Adiponectin is inversely related to body mass index (BMI); hence, its expression and release decline with increasing obesity [140]. It enhances insulin sensitivity through activation of adenosine monophosphate protein kinase [141], thereby inhibiting acetyl-CoA carboxylase. As a result, it leads to a declined lipogenesis and is associated with enhanced fatty-acid beta-oxidation, and an increased internalization of glucose, through the expression of GLUT 1 and GLUT4. On the contrary, leptin, and resistin are considered insulin-antagonizing adipokines, as they inhibit insulin signaling pathways [142]. The adipokine milieu in patients with psoriasis is similar to that of prediabetic subjects [143], who present with lower levels of adiponectin [51,144,145] and increased levels of leptin and resistin compared to healthy controls [124,146]. Interestingly, it has been reported that both TNF-α and IL-17 may impact the production of adiponectin, resistin, and leptin by adipocytes [135,147,148].
3.3. Hyperlipidemia
The association between psoriasis and changes in blood lipid profile has been established by several studies [149], and dyslipidemia was found to be a risk factor for psoriasis development [150]. This association could be due to shared genetic variants, aberrantly activated molecular pathways, or lifestyle modifications [151].
3.3.1. Genetic Background
The relevance of genetic predisposition for dyslipidemia in psoriasis was established by Mallbris et al., who demonstrated that lipid profile is altered at the initial stages of psoriasis development, suggesting that inflammatory actions follow the metabolic ones, and not vice versa [152].
In particular, novel SNPs in the HLA gene region, which is traditionally associated with immune-mediated conditions including psoriasis, correlate with increased risk for dyslipidemia [151].
ApoE polymorphisms, especially ε2 and ε4 in the European population, are associated with psoriasis onset and/or increased severity, while, on the other hand, the allele ε3 shows a protective effect [153]. At the same time, ApoE alleles, especially ε4, are associated with dyslipidemia [153].
Paraoxonase 1 (PON1) is an apolipoprotein-associated enzyme that protects lipoproteins from oxidative damage. Dysfunction of PON1 is implicated in metabolic disorders, and the PON1 55 M allele is a risk factor for psoriasis with an odds ratio of 1.57 for heterozygotes and 1.97 for homozygotes [154].
In psoriatic lesions, the expression levels of genes involved in lipid and fatty-acid metabolism are substantially altered; however, most of these local modifications do not have systemic consequences [155]. Conversely, a broader effect seems to be played by the genes LXR-α and PPAR-α, implicated in the metabolism of lipids. Notably, LXR-α regulates the transport of cholesterol from atherosclerotic lesions to the liver, while PPAR-α is a key lipid metabolism regulator, influencing Apo-A1 and HDL serum levels; thus, it is used as a therapeutic target in the treatment of dyslipidemia [156]. Both genes are downregulated in psoriatic skin, and the downregulation is proportional to skin inflammation. The downregulation of these genes also has systemic consequences due to the corresponding decline in Apo-A1 and HDL levels in blood [156].
3.3.2. Molecular Pathways
The alteration of serum lipid composition is, at least partly, a consequence of chronic systemic inflammation. TNF-α is elevated in psoriasis and increases proatherogenic small dense LDL and ox-LDL levels while lowering HDL concentrations. Concomitantly, IL-6 and IL-1β induce VLDL synthesis and lower triglyceride clearance, further contributing to the production of small dense LDL and oxLDL [157]. Moreover, HDL isolated from psoriatic patients is less efficient in stimulating cholesterol efflux from macrophages, thus increasing cardiovascular risk despite normal HDL levels [158,159].
Furthermore, in several inflammatory disorders including psoriasis, the formation of anti-HDL antibodies has been reported. This correlates with increased (CV) risk, and the autoantibody titers in psoriasis seem to correlate with the clinical severity of the disease, providing a possible additional explanation for the increased CV risk reported in severe psoriasis [160].
However, dysfunctional HDL levels might be associated with immune dysregulation. In fact, by influencing the lipid structure of cell membranes, they interfere with immune cell physiology, modulate the maturation of DC cells, alter the expression of MHC molecules, play a role in lymphocyte activation, regulate cytokine production, and interfere with the complement system [161,162]. This might provide an alternative explanation to the observation that dyslipidemia occurs at an early stage of psoriasis development [152]. In addition to a possible common genetic background, a dysfunctional lipid profile could even contribute to the immune dysregulation underlying psoriasis.
Vitamin D deficiency is another common mechanism that could contribute to both psoriasis and dyslipidemia. Indeed, vitamin D contributes to the integrity of the skin barrier and modulates the functioning of the cutaneous immune system. Notably, it stimulates T regulatory cells, downregulates the expression of proinflammatory cytokines, and suppresses dendritic cell activation [163]. In keeping with this observation, vitamin D levels are lower in both psoriasis and psoriatic arthritis, compared to controls, and are inversely correlated with inflammatory markers and disease severity [164,165,166]. On the other hand, low levels of vitamin D have been associated with increased triglyceride levels in large epidemiologic studies [167,168]. Interventional studies showed that supplementation with vitamin D or its derivates results in a significant decrease in LDL and total cholesterol [169], as well as triglyceride levels [170,171,172]. It has, thus, been hypothesized that vitamin D might influence both intrahepatic metabolism and peripheral clearance of lipids through calcium and parathormone-mediated mechanisms [168], as well as modulate their synthesis by directly acting on gene transcription [169].
3.4. Obesity
Psoriasis and obesity are deeply intertwined conditions, as demonstrated by several epidemiologic studies. In fact, not only is psoriasis more common and more severe in patients with obesity, but obesity is also more prevalent in patients with psoriasis [12,17,173], including children [174]. The two conditions are linked by common lifestyle modifications such as unhealthy diet, social isolation, and reduction in physical activity, as well as by dysregulated metabolic and inflammatory pathways [175,176].
3.4.1. Genetic Background
A common genetic basis for psoriasis and obesity was suggested by a study that found a significant correlation between the two conditions in twins [177]. As both diseases have a multifactorial etiology with a polygenic component, several genes have been hypothesized to play a role.
The FTO gene encodes a protein implicated in the regulation of the body adipose mass, and its polymorphisms carry a higher risk for obesity and have been associated with a higher PASI in patients with psoriasis [95,178]. Polymorphisms of the MC4R gene have been linked to obesity both in the general population and in patients with psoriasis with an increased risk for onset of psoriatic arthritis in the latter [95,179]. As far as genetic variants of adipokines are concerned, no association has been reported between alleles of the leptin [180,181], leptin receptor, adiponectin [181], and omentin [182,183] genes and psoriasis onset or severity.
3.4.2. Molecular Pathways
The adipose tissue is responsible for the active secretion of several bioactive molecules whose effects can spread systemically and play a relevant role in the pathways implicated in psoriatic inflammation [184].
For instance, free fatty acids, which are abundantly released from the adipose tissue of patients with obesity, have been shown to exert a proinflammatory role and to worsen psoriasis in murine models, at least partly via activation of keratinocytes [184]. Moreover, adipokines, cytokines secreted by the adipose tissue, have been demonstrated to interfere with the function of several cell types. Leptin, whose circulating levels are higher in patients with obesity, acts on immune cells, leading to the stimulation of the production of proinflammatory cytokines and reactive oxygen species, chemotaxis, phagocytosis, and proliferation, as well as the polarization of the Th1/Th17 axis in lymphocytes [184,185]. Furthermore, it also stimulates skin resident cells, namely, keratinocytes, fibroblasts, and endothelial cells, further promoting the establishment of a psoriatic milieu [186]. Coherently, murine models genetically deficient for leptin showed attenuation of psoriasis [187], while studies conducted on patients with psoriasis demonstrated higher levels of this hormone compared to healthy controls, even in nonobese subjects [146,188].
Conversely, adiponectin is an adipokine with anti-inflammatory effects whose levels are lower in patients with obesity. In addition to a global suppression of inflammatory cytokine production, adiponectin suppresses IL-17 production in the skin [184,189]. Accordingly, its levels have been demonstrated to be lower in patients with psoriasis [188].
A similar trend has been observed for other adipokines in psoriasis, with an increase in those with proinflammatory effects, including resistin [188,190], chemerin [191], and visfatin [192], and a decrease in those with anti-inflammatory effects, such as omentin [182,183]. Even more significantly, healing of psoriasis following effective therapy results in the normalization of the aberrant levels of these molecules [185,191].
Furthermore, the adipose tissue itself can produce proinflammatory cytokines, such as IL-6, IL-1, and TNF, partly due to a direct secretion from adipocytes and partly from immune cells infiltrating the fat septa [184]. In fat tissues of patients with obesity, the macrophages in the immune cell infiltration show an abnormal polarization toward the M1 phenotype, and lymphocytes show a Th1 shift, both contributing to the enhanced secretion of proinflammatory cytokines [193]. Therefore, in patients with obesity, higher levels of circulating IL-6 [194,195], TNF [194,196], and IL-1 [197] are observed. This abnormal cytokine secretion induces a pathological polarization toward the Th17 axis in obese subjects, as shown by the higher number of IL-17-producing lymphocytes found in both lymphoid and nonlymphoid tissues [198].
Another possible mechanism shared by obesity and psoriasis derives from disturbances in the gut microbiota. Microbial dysbiosis and altered production of bacterial metabolites appear to have strong similarities in the two conditions [199]. Consistently, a study on the composition of human gut microbiota in patients with psoriasis observed significant alterations compared to healthy controls. However, no differences were observed when comparing psoriatic patients with obesity with healthy patients with obesity [200].
Notably, an increased Firmicutes/Bacterioidetes ratio has been associated with both psoriasis and obesity [200]. This imbalance could represent a shared pathogenic mechanism due to the altered production of short-chain fatty acids by these bacterial phyla, which play an important role in metabolic and anti-inflammatory processes [199] Additionally, a decrease in Akkermansia species abundance has been reported in both psoriatic patients and patients with obesity, and it may be relevant in light of its role in modulating inflammatory pathways including that of nuclear factor kappa B [200]
3.5. NAFLD
An increasing deal of evidence shows that NAFLD is associated with an increased risk of CV events independent of traditional CV risk factors [201]. A 2015 systematic review and meta-analysis indicated that patients with psoriasis have a twofold increased risk of NAFLD compared to controls [29].
In addition to the important role of adipose tissue in mediating the interplay between skin and liver, severe psoriasis may have a direct impact on NAFLD, possibly via mechanisms beyond obesity [202]. Indeed, NAFLD in the general population can also occur among individuals who are not obese and have a normal body mass index (BMI). These individuals are labeled as “lean NAFLD” [203]. Interestingly, subjects with “lean” NAFLD compared to healthy subjects show higher mean serum C-reactive protein levels, suggesting that systemic inflammation might be one of the pathogenic factors of “lean” NAFLD.
Indeed, IL-6, IL-17, and TNF-α produced by the liver (hepatokines) and inflamed skin might have synergistic effects on these organs. This inflammatory-related relationship between psoriasis and NAFLD has been defined as the “hepato-dermal axis” [202]. IL-17 can induce the activation of hepatic stellate cells and subsequent collagen production [204]. By doing so, IL-17 facilitates the progression from simple liver steatosis to steatohepatitis [202,204]. NAFLD to (nonalcoholic steatohepatitis) NASH progression correlates with a higher frequency of IL-17(+) cells among liver CD4(+) T cells and higher Th17/resting Treg and Th2/resting Treg ratios in the blood [205]. Accordingly, IL-17 blockade restores insulin resistance and prevents NAFLD inflammation in mouse models [206]. The genetic ablation of IL-17RA also alleviates NAFLD [206].
4. Conclusions
Patients with psoriasis have an increased risk of developing CVD and traditional CV risk factors including hypertension, diabetes, hyperlipidemia, obesity, and NAFLD. The mechanism underlying this association has not been unequivocally defined so far and could be due to shared genetic variants, aberrantly activated molecular pathways, or lifestyle modifications. The literature suggests that psoriasis shares genetic and molecular associations with atherosclerosis and CV risk factors. The nonlinear and intricate interplay among various factors, impacting the molecular pathways in different cell types, probably contributes to the development of psoriasis and CVD. Future research should, therefore, aim to fully unravel shared and differential molecular pathways underpinning the association between psoriasis and CVD. This could enable physicians to customize therapeutic interventions on the basis of individual molecular and clinical profiles.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nestle, F.O.; Kaplan, D.H.; Barker, J. Psoriasis. N. Engl. J. Med. 2009, 361, 496–509. [Google Scholar] [CrossRef] [PubMed]
- Garshick, M.S.; Ward, N.L.; Krueger, J.G.; Berger, J.S. Cardiovascular Risk in Patients with Psoriasis: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2021, 77, 1670–1680. [Google Scholar] [CrossRef] [PubMed]
- Gelfand, J.M.; Troxel, A.B.; Lewis, J.D.; Kurd, S.K.; Shin, D.B.; Wang, X.; Margolis, D.J.; Strom, B.L. The risk of mortality in patients with psoriasis: Results from a population-based study. Arch Dermatol. 2007, 143, 1493–1499. [Google Scholar] [CrossRef]
- Ogdie, A.; Yu, Y.; Haynes, K.; Love, T.J.; Maliha, S.; Jiang, Y.; Troxel, A.B.; Hennessy, S.; Kimmel, S.R.; Margolis, D.J.; et al. Risk of major cardiovascular events in patients with psoriatic arthritis, psoriasis and rheumatoid arthritis: A population-based cohort study. Ann. Rheum. Dis. 2015, 74, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Boehncke, W.-H.; Boehncke, S.; Tobin, A.-M.; Kirby, B. The “psoriatic march”: A concept of how severe psoriasis may drive cardiovascular comorbidity. Exp. Dermatol. 2011, 20, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Samarasekera, E.J.; Neilson, J.M.; Warren, R.B.; Parnham, J.; Smith, C.H. Incidence of Cardiovascular Disease in Individuals with Psoriasis: A Systematic Review and Meta-Analysis. J. Investig. Dermatol. 2013, 133, 2340–2346. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, E.J.; Harskamp, C.T.; Armstrong, A.W. Psoriasis and major adverse cardiovascular events: A systematic review and meta-analysis of observational studies. J Am Heart Assoc 2013, 2, e000062. [Google Scholar] [CrossRef] [PubMed]
- Khalid, U.; Ahlehoff, O.; Gislason, G.H.; Kristensen, S.L.; Skov, L.; Torp-Pedersen, C.; Hansen, P.R. Psoriasis and risk of heart failure: A nationwide cohort study. Eur. J. Heart Fail. 2014, 16, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Upala, S.; Shahnawaz, A.; Sanguankeo, A. Psoriasis increases risk of new-onset atrial fibrillation: A systematic review and meta-analysis of prospective observational studies. J. Dermatol. Treat. 2017, 28, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Love, T.J.; Qureshi, A.A.; Karlson, E.W.; Gelfand, J.M.; Choi, H.K. Prevalence of the metabolic syndrome in psoriasis: Results from the National Health and Nutrition Examination Survey, 2003–2006. Arch Dermatol. 2011, 147, 419–424. [Google Scholar] [CrossRef]
- Herron, M.D.; Hinckley, M.; Hoffman, M.S.; Papenfuss, J.; Hansen, C.B.; Callis, K.P.; Krueger, G.G. Impact of obesity and smoking on psoriasis presentation and management. Arch Dermatol. 2005, 141, 1527–1534. [Google Scholar] [CrossRef] [PubMed]
- Neimann, A.L.; Shin, D.B.; Wang, X.; Margolis, D.J.; Troxel, A.B.; Gelfand, J.M. Prevalence of cardiovascular risk factors in patients with psoriasis. J. Am. Acad. Dermatol. 2006, 55, 829–835. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.W.; Harskamp, C.T.; Armstrong, E.J. Psoriasis and the risk of diabetes mellitus: A systematic review and meta-analysis. JAMA Dermatol. 2013, 149, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.W.; Harskamp, C.T.; Armstrong, E.J. The association between psoriasis and hypertension: A systematic review and meta-analysis of observational studies. J. Hypertens 2013, 31, 433. [Google Scholar] [CrossRef] [PubMed]
- Dreiher, J.; Weitzman, D.; Davidovici, B.; Shapiro, J.; Cohen, A.D. Psoriasis and dyslipidaemia: A population-based study. Acta Derm. Venereol. 2008, 88, 561–565. [Google Scholar] [CrossRef] [PubMed]
- Praveenkumar, U.; Ganguly, S.; Ray, L.; Nanda, S.K.; Kuruvila, S. Prevalence of Metabolic Syndrome in Psoriasis Patients and its Relation to Disease Duration: A Hospital Based Case-Control Study. J. Clin. Diagn. Res. 2016, 10, WC01–WC05. [Google Scholar] [CrossRef] [PubMed]
- Langan, S.M.; Seminara, N.M.; Shin, D.B.; Troxel, A.B.; Kimmel, S.E.; Mehta, N.N.; Margolis, D.J.; Gelfand, J.M. Prevalence of metabolic syndrome in patients with psoriasis: A population-based study in the United Kingdom. J. Invest. Dermatol. 2012, 132, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Kimball, A.B.; Szapary, P.; Mrowietz, U.; Reich, K.; Langley, R.G.; You, Y.; Hsu, M.C.; Yeilding, N.; Rader, D.J.; Mehta, N.N. Underdiagnosis and undertreatment of cardiovascular risk factors in patients with moderate to severe psoriasis. J. Am. Acad. Dermatol. 2012, 67, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Tinggaard, A.B.; Hjuler, K.F.; Andersen, I.T.; Winther, S.; Iversen, L.; Bøttcher, M. Prevalence and severity of coronary artery disease linked to prognosis in psoriasis and psoriatic arthritis patients: A multi-centre cohort study. J. Intern. Med. 2021, 290, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Mehta, N.N.; Yu, Y.; Pinnelas, R.; Krishnamoorthy, P.; Shin, D.B.; Troxel, A.B.; Gelfand, J.M. Attributable risk estimate of severe psoriasis on major cardiovascular events. Am. J. Med. 2011, 124, 775.e1–775.e6. [Google Scholar] [CrossRef]
- Abuabara, K.; Azfar, R.S.; Shin, D.B.; Neimann, A.L.; Troxel, A.B.; Gelfand, J.M. Cause-specific mortality in patients with severe psoriasis: A population-based cohort study in the UK. Br. J. Dermatol. 2010, 163, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Prodanovich, S.; Kirsner, R.S.; Kravetz, J.D.; Ma, F.; Martinez, L.; Federman, D.G. Association of psoriasis with coronary artery, cerebrovascular, and peripheral vascular diseases and mortality. Arch Dermatol. 2009, 145, 700–703. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-Q.; Han, J.-L.; Manson, J.E.; Rimm, E.B.; Rexrode, K.M.; Curhan, G.C.; Qureshi, A.A. Psoriasis and risk of nonfatal cardiovascular disease in, U.S. women: A cohort study. Br. J. Dermatol. 2012, 166, 811–818. [Google Scholar] [CrossRef]
- Piepoli, M.F.; Hoes, A.W.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A.L.; Cooney, M.T.; Corrà, U.; Cosyns, B.; Deaton, C.; et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur. Heart J. 2016, 37, 2315–2381. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; de Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 139, E1082–E1143. [Google Scholar] [CrossRef]
- Egeberg, A.; Skov, L.; Joshi, A.A.; Mallbris, L.; Gislason, G.H.; Wu, J.J.; Rodante, J.; Lerman, J.B.; Ahlman, M.A.; Gelfand, J.M.; et al. The relationship between duration of psoriasis, vascular inflammation, and cardiovascular events. J. Am. Acad. Dermatol. 2017, 77, 650–656. [Google Scholar] [CrossRef]
- Miller, I.M.; Ellervik, C.; Yazdanyar, S.; Jemec, G.B.E. Meta-analysis of psoriasis, cardiovascular disease, and associated risk factors. J. Am. Acad. Dermatol. 2013, 69, 1014–1024. [Google Scholar] [CrossRef]
- Armstrong, A.W.; Harskamp, C.T.; Armstrong, E.J. Psoriasis and metabolic syndrome: A systematic review and meta-analysis of observational studies. J. Am. Acad. Dermatol. 2013, 68, 654–662. [Google Scholar] [CrossRef]
- Candia, R.; Ruiz, A.; Torres-Robles, R.; Chávez-Tapia, N.; Méndez-Sánchez, N.; Arrese, M. Risk of non-alcoholic fatty liver disease in patients with psoriasis: A systematic review and meta-analysis. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 656–662. [Google Scholar] [CrossRef]
- Yamanaka, K.; Yamamoto, O.; Honda, T. Pathophysiology of psoriasis: A review. J. Dermatol. 2021, 48, 722–731. [Google Scholar] [CrossRef]
- Musunuru, K.; Kathiresan, S. Genetics of Common, Complex Coronary Artery Disease. Cell 2019, 177, 132–145. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Wang, Y.; Wang, Z.; Liu, L.; Yang, Z.; Wang, M.; Xu, Y.; Ye, D.; Zhang, J.; Lin, Y.; et al. Roles and Mechanisms of Interleukin-12 Family Members in Cardiovascular Diseases: Opportunities and Challenges. Front. Pharmacol. 2020, 11, 129. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Cai, Z.-R.; Zhang, B.; Cai, X.; Li, W.; Guo, Z.; Ma, L. Functional polymorphisms in interleukin-23 receptor and susceptibility to coronary artery disease. DNA Cell Biol. 2014, 33, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Jiang, H.; Ma, T.; Qiu, H.; Guo, M.; Zhang, X. The association between IL-17A and IL-23R polymorphisms and coronary artery disease risk in a Middle Eastern Chinese population. J. Clin. Lab. Anal. 2019, 33, e22893. [Google Scholar] [CrossRef] [PubMed]
- Kave, M.; Shadman, M.; Alizadeh, A.; Samadi, M. Analysis of the association between IL-23R rs11209026 polymorphism and incidence of atherosclerosis. Int. J. Immunogenet. 2015, 42, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.P.; Duffin, K.C.; Helms, C.; Ding, J.; Stuart, P.E.; Goldgar, D.; Gudjonsson, J.E.; Li, Y.; Tejasvi, T.; Feng, B.J.; et al. Genome-wide scan reveals association of psoriasis with IL-23 and NF-kappaB pathways. Nat. Genet 2009, 41, 199–204. [Google Scholar] [CrossRef]
- Chen, H.; Poon, A.; Yeung, C.; Helms, C.; Pons, J.; Bowcock, A.M.; Kwok, P.Y.; Liao, W. A genetic risk score combining ten psoriasis risk loci improves disease prediction. PLoS ONE 2011, 6, e19454. [Google Scholar] [CrossRef]
- Eiris, N.; Santos-Juanes, J.; Coto-Segura, P.; Gómez, J.; Alvarez, V.; Morales, B.; Queiro, R.; Díaz, M.; Corao, A.I.; Coto, E.; et al. Resequencing of the IL12B gene in psoriasis patients with the rs6887695/rs3212227 risk genotypes. Cytokine 2012, 60, 27–29. [Google Scholar] [CrossRef][Green Version]
- Vázquez-Vázquez, C.; Posadas-Sánchez, R.; Pérez-Hernández, N.; Rodríguez-Pérez, J.M.; Fragoso, J.M.; Cardoso-Saldaña, G.; Vargas-Alarcón, G. The rs2066808 Polymorphism Located Near the IL-23A Gene Is Associated with Premature Coronary Artery Disease in Mexican Population (GEA Study). DNA Cell Biol. 2019, 38, 880–886. [Google Scholar] [CrossRef]
- Cargill, M.; Schrodi, S.J.; Chang, M.; Garcia, V.E.; Brandon, R.; Callis, K.P.; Matsunami, N.; Ardlie, K.; Civello, D.; Catanese, J.; et al. A large-scale genetic association study confirms IL12B and leads to the identification of IL23R as psoriasis-risk genes. Am. J. Hum. Genet 2007, 80, 273–290. [Google Scholar] [CrossRef]
- Ogawa, K.; Okada, Y. The current landscape of psoriasis genetics in 2020. J. Dermatol. Sci. 2020, 99, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhou, H. A Meta-Analysis of the Relationship between Tumor Necrosis Factor-α Polymorphisms and Psoriasis. Dermatology 2021, 237, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Zhao, S.-R.; Li, Y.; Liu, F.; Gong, Y.; Xing, J.; Xu, Z.S. Association of tumor necrosis factor-α gene polymorphisms and coronary artery disease susceptibility: A systematic review and meta-analysis. BMC Med. Genet 2020, 21, 29. [Google Scholar] [CrossRef]
- Kaluza, W.; Reuss, E.; Grossmann, S.; Hug, R.; Schopf, R.E.; Galle, P.R.; Maerker-Hermann, E.; Hoehler, T. Different transcriptional activity and in vitro TNF-alpha production in psoriasis patients carrying the TNF-alpha 238A promoter polymorphism. J. Invest. Dermatol. 2000, 114, 1180–1183. [Google Scholar] [CrossRef]
- Rolski, F.; Błyszczuk, P. Complexity of TNF-α Signaling in Heart Disease. J. Clin. Med. 2020, 9, 3267. [Google Scholar] [CrossRef] [PubMed]
- Brånén, L.; Hovgaard, L.; Nitulescu, M.; Bengtsson, E.; Nilsson, J.; Jovinge, S. Inhibition of tumor necrosis factor-alpha reduces atherosclerosis in apolipoprotein E knockout mice. Arter. Thromb. Vasc. Biol. 2004, 24, 2137–2142. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, X.; Bian, F.; Wu, P.; Xing, S.; Xu, G.; Li, W.; Chi, J.; Ouyang, C.; Zheng, T.; et al. TNF-α promotes early atherosclerosis by increasing transcytosis of LDL across endothelial cells: Crosstalk between NF-κB and PPAR-γ. J. Mol. Cell Cardiol. 2014, 72, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.Y.; Twu, Y.C. Tumor necrosis factor-alpha -mediated protein kinases in regulation of scavenger receptor and foam cell formation on macrophage. J. Biol. Chem. 2000, 275, 41035–41048. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, S.; Zhang, H.; Hill, M.A.; Zhang, C.; Park, Y. Interaction of IL-6 and TNF-α contributes to endothelial dysfunction in type 2 diabetic mouse hearts. PLoS ONE 2017, 12, e0187189. [Google Scholar] [CrossRef]
- Kaptoge, S.; Seshasai, S.R.K.; Gao, P.; Freitag, D.F.; Butterworth, A.S.; Borglykke, A.; Di Angelantonio, E.; Gudnason, V.; Rumley, A.; Lowe, G.D.; et al. Inflammatory cytokines and risk of coronary heart disease: New prospective study and updated meta-analysis. Eur. Heart J. 2014, 35, 578–589. [Google Scholar] [CrossRef]
- Bai, F.; Zheng, W.; Dong, Y.; Wang, J.; Garstka, M.A.; Li, R.; An, J.; Ma, H. Serum levels of adipokines and cytokines in psoriasis patients: A systematic review and meta-analysis. Oncotarget 2018, 9, 1266–1278. [Google Scholar] [CrossRef] [PubMed]
- Arican, O.; Aral, M.; Sasmaz, S.; Ciragil, P. Serum levels of TNF-alpha, IFN-gamma, IL-6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediators. Inflamm. 2005, 2005, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Tsuji, H.; Hashimoto, Y.; Ishida-Yamamoto, A.; Iizuka, H. Serum cytokines and growth factor levels in Japanese patients with psoriasis. Clin. Exp. Dermatol. 2010, 35, 645–649. [Google Scholar] [CrossRef]
- Michalak-Stoma, A.; Bartosińska, J.; Kowal, M.; Juszkiewicz-Borowiec, M.; Gerkowicz, A.; Chodorowska, G. Serum levels of selected Th17 and Th22 cytokines in psoriatic patients. Dis. Markers 2013, 35, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Jääskeläinen, A.E.; Seppälä, S.; Kakko, T.; Jaakkola, U.; Kallio, J. Systemic treatment with neuropeptide Y receptor Y1-antagonist enhances atherosclerosis and stimulates IL-12 expression in ApoE deficient mice. Neuropeptides 2013, 47, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.S.; Yen, H.C.; Pan, C.C.; Chau, L.Y. The role of interleukin 12 in the development of atherosclerosis in ApoE-deficient mice. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 734–742. [Google Scholar] [CrossRef] [PubMed]
- Davenport, P.; Tipping, P.G. The role of interleukin-4 and interleukin-12 in the progression of atherosclerosis in apolipoprotein E-deficient mice. Am. J. Pathol. 2003, 163, 1117–1125. [Google Scholar] [CrossRef]
- Hauer, A.D.; Uyttenhove, C.; de Vos, P.; Stroobant, V.; Renauld, J.C.; van Berkel, T.J.C.; van Snick, J.; Kuiper, J. Blockade of interleukin-12 function by protein vaccination attenuates atherosclerosis. Circulation 2005, 112, 1054–1062. [Google Scholar] [CrossRef]
- Uyemura, K.; Demer, L.L.; Castle, S.C.; Jullien, D.; Berliner, J.A.; Gately, M.K.; Warrier, R.R.; Pham, N.; Fogelman, A.M.; Modlin, R.L. Cross-regulatory roles of interleukin (IL)-12 and IL-10 in atherosclerosis. J. Clin. Invest. 1996, 97, 2130–2138. [Google Scholar] [CrossRef]
- Allam, G.; Abdel-Moneim, A.; Gaber, A.M. The pleiotropic role of interleukin-17 in atherosclerosis. Biomed. Pharmacother. Biomed. Pharmacother. 2018, 106, 1412–1418. [Google Scholar] [CrossRef]
- Taleb, S.; Tedgui, A.; Mallat, Z. IL-17 and Th17 cells in atherosclerosis: Subtle and contextual roles. Arterioscler Thromb. Vasc. Biol. 2015, 35, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.; Gregersen, I.; Holm, S.; Daissormont, I.; Bjerkeli, V.; Krohg-Sørensen, K.; Dahl, T.B.; Russell, D.; Bundgaard, D.; Alteheld, L.H.; et al. Interleukin 23 levels are increased in carotid atherosclerosis: Possible role for the interleukin 23/interleukin 17 axis. Stroke 2015, 46, 793–799. [Google Scholar] [CrossRef]
- von Stebut, E.; Boehncke, W.-H.; Ghoreschi, K.; Gori, T.; Kaya, Z.; Thaci, D.; Schäffler, A. IL-17A in Psoriasis and Beyond: Cardiovascular and Metabolic Implications. Front. Immunol. 2019, 10, 3096. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.; Prasad, K.-M.R.; Butcher, M.; Dobrian, A.; Kolls, J.K.; Ley, K.; Galkina, E. Blockade of interleukin-17A results in reduced atherosclerosis in apolipoprotein E-deficient mice. Circulation 2010, 121, 1746–1755. [Google Scholar] [CrossRef] [PubMed]
- Erbel, C.; Dengler, T.J.; Wangler, S.; Lasitschka, F.; Bea, F.; Wambsganss, N.; Hakimi, M.; Böckler, D.; Katus, H.A.; Gleissner, C.A. Expression of IL-17A in human atherosclerotic lesions is associated with increased inflammation and plaque vulnerability. Basic Res. Cardiol. 2011, 106, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Erbel, C.; Chen, L.; Bea, F.; Wangler, S.; Celik, S.; Lasitschka, F.; Wang, Y.; Böckler, D.; Katus, H.A.; Dengler, T.J. Inhibition of IL-17A Attenuates Atherosclerotic Lesion Development in ApoE-Deficient Mice. J. Immunol. 2009, 183, 8167. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Jiang, Y.; Ma, T.; Zhu, F.; Gao, F.; Zhang, P.; Guo, C.; Wang, Q.; Wang, X.; Ma, C.; et al. A critical function of Th17 proinflammatory cells in the development of atherosclerotic plaque in mice. J. Immunol. 2010, 185, 5820–5827. [Google Scholar] [CrossRef]
- Madhur, M.S.; Funt, S.A.; Li, L.; Vinh, A.; Chen, W.; Lob, H.E.; Iwakura, Y.; Blinder, Y.; Rahman, A.; Quyyumi, A.A.; et al. Role of interleukin 17 in inflammation, atherosclerosis, and vascular function in apolipoprotein e-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1565–1572. [Google Scholar] [CrossRef]
- Danzaki, K.; Matsui, Y.; Ikesue, M.; Ohta, D.; Ito, K.; Kanayama, M.; Kurotaki, D.; Morimoto, J.; Iwakura, Y.; Yagita, H.; et al. Interleukin-17A Deficiency Accelerates Unstable Atherosclerotic Plaque Formation in Apolipoprotein E-Deficient Mice. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 273–280. [Google Scholar] [CrossRef]
- Karbach, S.; Croxford, A.L.; Oelze, M.; Schüler, R.; Minwegen, D.; Wegner, J.; Koukes, L.; Yogev, N.; Ullmann , A.; Knorr, M.; et al. Interleukin 17 drives vascular inflammation, endothelial dysfunction, and arterial hypertension in psoriasis-like skin disease. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2658–2668. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, H.; Loyd, C.M.; Fu, W.; Diaconu, D.; Liu, S.; Cooper, K.D.; McCormick, T.S.; Simon, D.I.; Ward, N.L. Chronic skin-specific inflammation promotes vascular inflammation and thrombosis. J. Invest. Dermatol. 2012, 132, 2067–2075. [Google Scholar] [CrossRef] [PubMed]
- de la Paz Sánchez-Martínez, M.; Blanco-Favela, F.; Mora-Ruiz, M.D.; Chávez-Rueda, A.K.; Bernabe-García, M.; Chávez-Sánchez, L. IL-17-differentiated macrophages secrete pro-inflammatory cytokines in response to oxidized low-density lipoprotein. Lipids. Health Dis. 2017, 16, 196. [Google Scholar] [CrossRef] [PubMed]
- Taleb, S.; Romain, M.; Ramkhelawon, B.; Uyttenhove, C.; Pasterkamp, G.; Herbin, O.; Esposito, B.; Perez, N.; Yasukawa, H.; Van Snick, J.; et al. Loss of SOCS3 expression in T cells reveals a regulatory role for interleukin-17 in atherosclerosis. J. Exp. Med. 2009, 206, 2067–2077. [Google Scholar] [CrossRef] [PubMed]
- Gisterå, A.; Hansson, G.K. The immunology of atherosclerosis. Nat. Rev. Nephrol. 2017, 13, 368–380. [Google Scholar] [CrossRef]
- Eid, R.E.; Rao, D.A.; Zhou, J.; Lo, S.L.; Ranjbaran, H.; Gallo, A.; Sokol, S.I.; Pfau, S.; Pober, J.S.; Tellides, G. Interleukin-17 and interferon-gamma are produced concomitantly by human coronary artery-infiltrating T cells and act synergistically on vascular smooth muscle cells. Circulation 2009, 119, 1424–1432. [Google Scholar] [CrossRef]
- Sun, J.; Yu, H.; Liu, H.; Pu, D.; Gao, J.; Jin, X.; Liu, X.; Yan, A. Correlation of pre-operative circulating inflammatory cytokines with restenosis and rapid angiographic stenotic progression risk in coronary artery disease patients underwent percutaneous coronary intervention with drug-eluting stents. J. Clin. Lab. Anal. 2020, 34, e23108. [Google Scholar] [CrossRef]
- Cohen, A.D.; Weitzman, D.; Dreiher, J. Psoriasis and hypertension: A case-control study. Acta. Derm. Venereol. 2010, 90, 23–26. [Google Scholar] [CrossRef]
- Mills, K.T.; Stefanescu, A.; He, J. The global epidemiology of hypertension. Nat. Rev. Nephrol. 2020, 16, 223–237. [Google Scholar] [CrossRef]
- Qureshi, A.A.; Choi, H.K.; Setty, A.R.; Curhan, G.C. Psoriasis and the risk of diabetes and hypertension: A prospective study of US female nurses. Arch. Dermatol. 2009, 145, 379–382. [Google Scholar] [CrossRef]
- Takeshita, J.; Wang, S.; Shin, D.B.; Mehta, N.N.; Kimmel, S.E.; Margolis, D.J.; Troxel, A.B.; Gelfand, J.M. Effect of psoriasis severity on hypertension control: A population-based study in the United Kingdom. JAMA Dermatol. 2015, 151, 161–169. [Google Scholar] [CrossRef]
- Ogretmen, Z.; Hiz, M.M.; Silan, F.; Uludag, A.; Ozdemirc, O. Association of endothelial nitric oxide synthase Glu298Asp gene polymorphism in psoriasis cases with hypertension. Ann. Saudi. Med. 2014, 34, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Casas, J.P.; Cavalleri, G.L.; Bautista, L.E.; Smeeth, L.; Humphries, S.E.; Hingorani, A.D. Endothelial nitric oxide synthase gene polymorphisms and cardiovascular disease: A HuGE review. Am. J. Epidemiol. 2006, 164, 921–935. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Li, Y.; Zuo, X.-B.; Tang, H.-Y.; Tang, X.-F.; Gao, J.-P.; Sheng, Y.-J.; Yin, X.-Y.; Zhou, F.-S.; Zhang, C.; et al. Identification of a missense variant in LNPEP that confers psoriasis risk. J. Invest. Dermatol. 2014, 134, 359–365. [Google Scholar] [CrossRef]
- Albiston, A.L.; McDowall, S.G.; Matsacos, D.; Sim, P.; Clune, E.; Mustafa, T.; Lee, J.; Mendelsohn, F.A.; Simpson, R.J.; Connolly, L.M.; et al. Evidence that the angiotensin IV (AT(4)) receptor is the enzyme insulin-regulated aminopeptidase. J. Biol. Chem. 2001, 276, 48623–48626. [Google Scholar] [CrossRef]
- Enhörning, S.; Leosdottir, M.; Wallström, P.; Gullberg, B.; Berglund, G.; Wirfält, E.; Melander, O. Relation between human vasopressin 1a gene variance, fat intake, and diabetes. Am. J. Clin. Nutr. 2009, 89, 400–406. [Google Scholar] [CrossRef]
- Patel, B.M.; Mehta, A.A. Aldosterone and angiotensin: Role in diabetes and cardiovascular diseases. Eur. J. Pharmacol. 2012, 697, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Nakada, T.-A.; Russell, J.A.; Wellman, H.; Boyd, J.H.; Nakada, E.; Thain, K.R.; Thair, S.A.; Hirasawa, H.; Oda, S.; Walley, K.R. Leucyl/cystinyl aminopeptidase gene variants in septic shock. Chest 2011, 139, 1042–1049. [Google Scholar] [CrossRef]
- Shibata, K.; Kajiyama, H.; Ino, K.; Nawa, A.; Nomura, S.; Mizutani, S.; Kikkawa, F. P-LAP/IRAP-induced cell proliferation and glucose uptake in endometrial carcinoma cells via insulin receptor signaling. BMC Cancer 2007, 7, 15. [Google Scholar] [CrossRef]
- Ramezani, M.; Zavattaro, E.; Sadeghi, M. Angiotensin-converting enzyme gene insertion/deletion polymorphism and susceptibility to psoriasis: A systematic review and meta-analysis. BMC Med. Genet. 2020, 21, 8. [Google Scholar] [CrossRef]
- Weger, W.; Hofer, A.; Wolf, P.; El-Shabrawi, Y.; Renner, W.; Kerl, H.; Salmhofer, W. The angiotensin-converting enzyme insertion/deletion and the endothelin -134 3A/4A gene polymorphisms in patients with chronic plaque psoriasis. Exp. Dermatol. 2007, 16, 993–998. [Google Scholar] [CrossRef]
- Veletza, S.; Karpouzis, A.; Giassakis, G.; Caridha, R.; Papaioakim, M. Assessment of insertion/deletion polymorphism of the angiotensin converting enzyme gene in psoriasis. J. Dermatol. Sci. 2008, 49, 85–87. [Google Scholar] [CrossRef] [PubMed]
- Lamba, G.; Palaniswamy, C.; Singh, T.; Shah, D.; Lal, S.; Vinnakota, R.; Charrow, E.J.; Forman, L. Psoriasis induced by losartan therapy: A case report and review of the literature. Am. J. Ther. 2011, 18, e78–e80. [Google Scholar] [CrossRef] [PubMed]
- Silva, I.M.S.; Assersen, K.B.; Willadsen, N.N.; Jepsen, J.; Artuc, M.; Steckelings, U.M. The role of the renin-angiotensin system in skin physiology and pathophysiology. Exp. Dermatol. 2020, 29, 891–901. [Google Scholar] [CrossRef] [PubMed]
- Song, G.G.; Bae, S.-C.; Kim, J.-H.; Lee, Y.H. The angiotensin-converting enzyme insertion/deletion polymorphism and susceptibility to rheumatoid arthritis, vitiligo and psoriasis: A meta-analysis. J. Renin. Angiotensin. Aldosterone. Syst. 2015, 16, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Purzycka-Bohdan, D.; Kisielnicka, A.; Bohdan, M.; Szczerkowska-Dobosz, A.; Sobalska-Kwapis, M.; Nedoszytko, B.; Nowicki, R.J. Analysis of the Potential Genetic Links between Psoriasis and Cardiovascular Risk Factors. Int. J. Mol. Sci. 2021, 22, 9063. [Google Scholar] [CrossRef]
- Mastrolonardo, M.; Picardi, A.; Alicino, D.; Bellomo, A.; Pasquini, P. Cardiovascular reactivity to experimental stress in psoriasis: A controlled investigation. Acta. Derm. Venereol. 2006, 86, 340–344. [Google Scholar] [CrossRef]
- Halıgür, B.D.; Cicek, D.; Bulut, S.; Berilgen, M.S. The investigation of autonomic functions in patients with psoriasis. Int. J. Dermatol. 2012, 51, 557–563. [Google Scholar] [CrossRef]
- Bonifati, C.; Mussi, A.; Carducci, M.; Pittarello, A.; D’Auria, L.; Venuti, A.; Bagnato, A.; Salani, D.; Fazio, M.; Ameglio, F. Endothelin-1 levels are increased in sera and lesional skin extracts of psoriatic patients and correlate with disease severity. Acta. Derm. Venereol. 1998, 78, 22–26. [Google Scholar] [CrossRef]
- Armstrong, A.W.; Lin, S.W.; Chambers, C.J.; Sockolov, M.E.; Chin, D.L. Psoriasis and hypertension severity: Results from a case-control study. PLoS ONE 2011, 6, e18227. [Google Scholar] [CrossRef]
- Ena, P.; Madeddu, P.; Glorioso, N.; Cerimele, D.; Rappelli, A. High prevalence of cardiovascular diseases and enhanced activity of the renin-angiotensin system in psoriatic patients. Acta. Cardiol. 1985, 40, 199–205. [Google Scholar]
- Suárez-Fariñas, M.; Li, K.; Fuentes-Duculan, J.; Hayden, K.; Brodmerkel, C.K.J. Expanding the Psoriasis Disease Profile: Interrogation of the Skin and Serum of Patients with Moderate-to-Severe Psoriasis. J. Invest. Dermatol. 2015, 135, 2901–2902. [Google Scholar] [CrossRef] [PubMed]
- Piaserico, S.; Osto, E.; Famoso, G.; Zanetti, I.; Gregori, D.; Poretto, A.; Iliceto, S.; Peserico, A.; Tona, F. Treatment with tumor necrosis factor inhibitors restores coronary microvascular function in young patients with severe psoriasis. Atherosclerosis 2016, 251, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Yoshizumi, M.; Perrella, M.A.; Burnett, J.C.J.; Lee, M.E. Tumor necrosis factor downregulates an endothelial nitric oxide synthase mRNA by shortening its half-life. Circ. Res. 1993, 73, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Ma, L.; Hall, J.E.; Liu, X.; Ying, Z. Dual regulation of tumor necrosis factor-α on myosin light chain phosphorylation in vascular smooth muscle. Am. J. Physiol. Heart Circ. Physiol. 2015, 308, H398–H406. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Park, Y.; Wu, J.; Chen, X.; Lee, S.; Yang, J.; Dellsperger, K.C.; Zhang, C. Role of TNF-alpha in vascular dysfunction. Clin. Sci. 2009, 116, 219–230. [Google Scholar] [CrossRef]
- Sriramula, S.; Haque, M.; Majid, D.S.A.; Francis, J. Involvement of tumor necrosis factor-alpha in angiotensin II-mediated effects on salt appetite, hypertension, and cardiac hypertrophy. Hypertension 2008, 51, 1345–1351. [Google Scholar] [CrossRef]
- Yao, W.; Sun, Y.; Wang, X.; Niu, K. Elevated Serum Level of Interleukin 17 in a Population With Prehypertension. J. Clin. Hypertens. 2015, 17, 770–774. [Google Scholar] [CrossRef] [PubMed]
- Madhur, M.S.; Lob, H.E.; McCann, L.A.; Iwakura, Y.; Blinder, Y.; Guzik, T.J.; Harrison, D.J. Interleukin 17 promotes angiotensin II-induced hypertension and vascular dysfunction. Hypertension 2010, 55, 500–507. [Google Scholar] [CrossRef]
- Wu, J.; Saleh, M.A.; Kirabo, A.; Itani, H.A.; Montaniel, K.R.C.; Xiao, L.; Chen, W.; Mernaugh, R.L.; Cai, H.; Bernstein, K.E.; et al. Immune activation caused by vascular oxidation promotes fibrosis and hypertension. J. Clin. Invest. 2016, 126, 50–67. [Google Scholar] [CrossRef]
- Huang, L.-H.; Zinselmeyer, B.H.; Chang, C.-H.; Saunders, B.T.; Elvington, A.; Baba, O.; Broekelmann, T.J.; Qi, L.; Rueve, J.S.; Swartz, M.A.; et al. Interleukin-17 Drives Interstitial Entrapment of Tissue Lipoproteins in Experimental Psoriasis. Cell Metab. 2019, 29, 475–487. [Google Scholar] [CrossRef]
- Amador, C.A.; Barrientos, V.; Peña, J.; Herrada, A.A.; González, M.; Valdés, S.; Carrasco, L.; Alzamora, R.; Figueroa, F.; Kalergis, A.M.; et al. Spironolactone decreases DOCA-salt-induced organ damage by blocking the activation of T helper 17 and the downregulation of regulatory T lymphocytes. Hypertension 2014, 63, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Saleh, M.A.; Norlander, A.E.; Madhur, M.S. Inhibition of Interleukin 17-A but not Interleukin-17F Signaling Lowers Blood Pressure and Reduces End-organ Inflammation in Angiotensin II-induced Hypertension. JACC Basic Transl. Sci. 2016, 1, 606–616. [Google Scholar] [CrossRef] [PubMed]
- Azfar, R.S.; Gelfand, J.M. Psoriasis and metabolic disease: Epidemiology and pathophysiology. Curr. Opin. Rheumatol. 2008, 20, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Coto-Segura, P.; Eiris-Salvado, N.; González-Lara, L.; Queiro-Silva, R.; Martinez-Camblor, P.; Maldonado-Seral, C.; García-García, B.; Palacios-García, L.; Gomez-Bernal, S.; Santos-Juanes, J.; et al. Psoriasis, psoriatic arthritis and type 2 diabetes mellitus: A systematic review and meta-analysis. Br. J. Dermatol. 2013, 169, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Wan, M.T.; Shin, D.B.; Hubbard, R.A.; Noe, M.H.; Mehta, N.N.; Gelfand, J.M. Psoriasis and the risk of diabetes: A prospective population-based cohort study. J. Am. Acad. Dermatol. 2018, 78, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Vebman, J.I.; Choy, A.; Yao, C.J. Review of the Prevalence of Cardiovascular and Metabolic Comorbidities of Psoriasis. SKIN J. Cutan. Med. 2020, 23, 179. [Google Scholar] [CrossRef]
- Khan, M.A.B.; Hashim, M.J.; King, J.K.; Govender, R.D.; Mustafa, H.; Al Kaabi, J. Epidemiology of Type 2 Diabetes—Global Burden of Disease and Forecasted Trends. J. Epidemiol. Glob. Health 2020, 10, 107–111. [Google Scholar] [CrossRef]
- Gyldenløve, M.; Storgaard, H.; Holst, J.J.; Vilsbøll, T.; Knop, F.K.; Skov, L. Patients with psoriasis are insulin resistant. J. Am. Acad. Dermatol. 2015, 72, 599–605. [Google Scholar] [CrossRef]
- Wolf, N.; Quaranta, M.; Prescott, N.J.; Allen, M.; Smith, R.; Burden, A.D.; Worthington, J.; Griffiths, C.E.; Mathew, C.G.; Barker, J.N.; et al. Psoriasis is associated with pleiotropic susceptibility loci identified in type II diabetes and Crohn disease. J. Med. Genet 2008, 45, 114–116. [Google Scholar] [CrossRef]
- Li, Y.; Liao, W.; Chang, M.; Schrodi, S.J.; Bui, N.; Catanese, J.J.; Poon, A.; Matsunami, N.; Callis-Duffin, K.P.; Leppert, M.F.; et al. Further genetic evidence for three psoriasis-risk genes: ADAM33, CDKAL1, and PTPN22. J. Invest. Dermatol. 2009, 129, 629–634. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Z.; Rani, P.L.; Fu, X.; Yu, W.; Bao, F.; Yu, G.; Li, J.; Li, L.; Sun, L.; et al. Identification of PTPN22, ST6GAL1 and JAZF1 as psoriasis risk genes demonstrates shared pathogenesis between psoriasis and diabetes. Exp. Dermatol. 2017, 26, 1112–1117. [Google Scholar] [CrossRef] [PubMed]
- Eirís, N.; González-Lara, L.; Santos-Juanes, J.; Queiro, R.; Coto, E.; Coto-Segura, P. Genetic variation at IL12B, IL23R and IL23A is associated with psoriasis severity, psoriatic arthritis and type 2 diabetes mellitus. J. Dermatol. Sci. 2014, 75, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Friis, N.U.; Hoffmann, N.; Gyldenløve, M.; Skov, L.; Vilsbøll, T.; Knop, F.K.; Storgaard, H. Glucose metabolism in patients with psoriasis. Br. J. Dermatol. 2019, 180, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Boehncke, S.; Thaci, D.; Beschmann, H.; Ludwig, R.J.; Ackermann, H.; Badenhoop, K.; Boehncke, W.H. Psoriasis patients show signs of insulin resistance. Br. J. Dermatol. 2007, 157, 1249–1251. [Google Scholar] [CrossRef]
- Davidovici, B.B.; Sattar, N.; Prinz, J.C.; Puig, L.; Emery, P.; Barker, J.N.; van de Kerkhof, P.; Nestle, F.O.; Girolomoni, G.; Krueger, J.G.; et al. Psoriasis and systemic inflammatory diseases: Potential mechanistic links between skin disease and co-morbid conditions. J. Invest. Dermatol. 2010, 130, 1785–1796. [Google Scholar] [CrossRef] [PubMed]
- Donath, M.Y. Targeting inflammation in the treatment of type 2 diabetes: Time to start. Nat. Rev. Drug Discov. 2014, 13, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef]
- Uysal, K.T.; Wiesbrock, S.M.; Hotamisligil, G.S. Functional analysis of tumor necrosis factor (TNF) receptors in TNF-alpha-mediated insulin resistance in genetic obesity. Endocrinology 1998, 139, 4832–4838. [Google Scholar] [CrossRef]
- Uysal, K.T.; Wiesbrock, S.M.; Marino, M.W.; Hotamisligil, G.S. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature 1997, 389, 610–614. [Google Scholar] [CrossRef]
- Ventre, J.; Doebber, T.; Wu, M.; MacNaul, K.; Stevens, K.; Pasparakis, M.; Kollias, G.; Moller, D.E. Targeted disruption of the tumor necrosis factor-alpha gene: Metabolic consequences in obese and nonobese mice. Diabetes 1997, 46, 1526–1531. [Google Scholar] [CrossRef]
- Pina, T.; Armesto, S.; Lopez-Mejias, R.; Genre, F.; Ubilla, B.; Gonzalez-Lopez, M.A.; Gonzalez-Vela, M.C.; Corrales, A.; Blanco, R.; Garcia-Unzueta, M.T.; et al. Anti-TNF-α therapy improves insulin sensitivity in non-diabetic patients with psoriasis: A 6-month prospective study. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 1325–1330. [Google Scholar] [CrossRef] [PubMed]
- Owczarczyk-Saczonek, A.; Placek, W. Interleukin-17 as a factor linking the pathogenesis of psoriasis with metabolic disorders. Int. J. Dermatol. 2017, 56, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Zareian, P.; Mirzaii Dizgah, I. Serum Interleukin 17 in Type 2 Diabetes Mellitus. J. Arch. Mil. Med. 2014, 2, e24689. [Google Scholar] [CrossRef]
- Sumarac-Dumanovic, M.; Jeremic, D.; Pantovic, A.; Janjetovic, K.; Stamenkovic-Pejkovic, D.; Cvijovic, G.; Stevanovic, D.; Micic, D.; Trajkovic, V. Therapeutic improvement of glucoregulation in newly diagnosed type 2 diabetes patients is associated with a reduction of IL-17 levels. Immunobiology 2013, 218, 1113–1118. [Google Scholar] [CrossRef]
- Zúñiga, L.A.; Shen, W.-J.; Joyce-Shaikh, B.; Pyatnova, E.A.; Richards, A.G.; Thom, C.; Andrade, S.M.; Cua, D.J.; Kraemer, F.B.; Butcher, E.C. IL-17 regulates adipogenesis, glucose homeostasis, and obesity. J. Immunol. 2010, 185, 6947–6959. [Google Scholar] [CrossRef]
- Ohshima, K.; Mogi, M.; Jing, F.; Iwanami, J.; Tsukuda, K.; Min, L.-J.; Higaki, J.; Horiuchi, M. Roles of interleukin 17 in angiotensin II type 1 receptor-mediated insulin resistance. Hypertension 2012, 59, 493–499. [Google Scholar] [CrossRef]
- Gaffen, S.L.; Jain, R.; Garg, A.V.; Cua, D.J. The IL-23-IL-17 immune axis: From mechanisms to therapeutic testing. Nat. Rev. Immunol. 2014, 14, 585–600. [Google Scholar] [CrossRef]
- Shinjo, T.; Iwashita, M.; Yamashita, A.; Sano, T.; Tsuruta, M.; Matsunaga, H.; Sanui, T.; Asano, T.; Nishimura, F. IL-17A synergistically enhances TNFα-induced IL-6 and CCL20 production in 3T3-L1 adipocytes. Biochem. Biophys. Res. Commun. 2016, 477, 241–246. [Google Scholar] [CrossRef]
- Fantuzzi, G. Adiponectin in inflammatory and immune-mediated diseases. Cytokine 2013, 64, 1–10. [Google Scholar] [CrossRef]
- Scherer, P.E.; Williams, S.; Fogliano, M.; Baldini, G.; Lodish, H.F. A novel serum protein similar to C1q, produced exclusively in adipocytes. J. Biol. Chem. 1995, 270, 26746–26749. [Google Scholar] [CrossRef]
- Yamauchi, T.; Kamon, J.; Minokoshi, Y.; Ito, Y.; Waki, H.; Uchida, S.; Yamashita, S.; Noda, M.; Kita, S.; Ueki, K.; et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat. Med. 2002, 8, 1288–1295. [Google Scholar] [CrossRef] [PubMed]
- Howard, J.K.; Flier, J.S. Attenuation of leptin and insulin signaling by SOCS proteins. Trends. Endocrinol. Metab. 2006, 17, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Boehncke, W.-H. Systemic Inflammation and Cardiovascular Comorbidity in Psoriasis Patients: Causes and Consequences. Front. Immunol. 2018, 9, 579. [Google Scholar] [CrossRef]
- Gerdes, S.; Osadtschy, S.; Rostami-Yazdi, M.; Buhles, N.; Weichenthal, M.; Mrowietz, U. Leptin, adiponectin, visfatin and retinol-binding protein-4–mediators of comorbidities in patients with psoriasis? Exp. Dermatol. 2012, 21, 43–47. [Google Scholar] [CrossRef]
- Shibata, S.; Saeki, H.; Tada, Y.; Karakawa, M.; Komine, M.; Tamaki, K. Serum high molecular weight adiponectin levels are decreased in psoriasis patients. J. Dermatol. Sci. 2009, 55, 62–63. [Google Scholar] [CrossRef]
- Cerman, A.A.; Bozkurt, S.; Sav, A.; Tulunay, A.; Elbaşi, M.O.; Ergun, T. Serum leptin levels, skin leptin and leptin receptor expression in psoriasis. Br. J. Dermatol. 2008, 159, 820–826. [Google Scholar] [CrossRef]
- Gustafson, B.; Hammarstedt, A.; Andersson, C.X.; Smith, U. Inflamed adipose tissue: A culprit underlying the metabolic syndrome and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2276–2283. [Google Scholar] [CrossRef] [PubMed]
- Romanowska, M.; al Yacoub, N.; Seidel, H.; Donandt, S.; Gerken, H.; Phillip, S.; Haritonova, N.; Artuc, M.; Schweiger, S.; Sterry, W.; et al. PPARdelta enhances keratinocyte proliferation in psoriasis and induces heparin-binding EGF-like growth factor. J. Invest. Dermatol. 2008, 128, 110–124. [Google Scholar] [CrossRef]
- Ma, C.; Harskamp, C.T.; Armstrong, E.J.; Armstrong, A.W. The association between psoriasis and dyslipidaemia: A systematic review. Br. J. Dermatol. 2013, 168, 486–495. [Google Scholar] [CrossRef]
- Wu, S.; Li, W.-Q.; Han, J.; Sun, Q.; Qureshi, A.A. Hypercholesterolemia and risk of incident psoriasis and psoriatic arthritis in US women. Arthritis. Rheumatol. 2014, 66, 304–310. [Google Scholar] [CrossRef]
- Lu, Y.; Chen, H.; Nikamo, P.; Qi Low, H.; Helms, C.; Seielstad, M.; Liu, J.; Bowcock, A.M.; Stahle, M.; Liao, W. Association of cardiovascular and metabolic disease genes with psoriasis. J. Invest. Dermatol. 2013, 133, 836–839. [Google Scholar] [CrossRef] [PubMed]
- Mallbris, L.; Granath, F.; Hamsten, A.; Ståhle, M. Psoriasis is associated with lipid abnormalities at the onset of skin disease. J. Am. Acad. Dermatol. 2006, 54, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Liu, T.; Lu, L. Apolipoprotein E gene polymorphism in psoriasis: A meta-analysis. Arch. Med. Res. 2013, 44, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Asefi, M.; Vaisi-Raygani, A.; Bahrehmand, F.; Kiani, A.; Rahimi, Z.; Nomani, H.; Ebrahimi, A.; Tavilani, H.; Pourmotabbed, T. Paraoxonase 1 (PON1) 55 polymorphism, lipid profiles and psoriasis. Br. J. Dermatol. 2012, 167, 1279–1286. [Google Scholar] [CrossRef] [PubMed]
- Gudjonsson, J.E.; Ding, J.; Johnston, A.; Tejasvi, T.; Guzman, A.M.; Nair, R.P.; Voorhees, J.J.; Abecasis, G.R.; Elder, J.T. Assessment of the psoriatic transcriptome in a large sample: Additional regulated genes and comparisons with in vitro models. J. Invest. Dermatol. 2010, 130, 1829–1840. [Google Scholar] [CrossRef]
- Mehta, N.N.; Li, K.; Szapary, P.; Krueger, J.; Brodmerkel, C. Modulation of cardiometabolic pathways in skin and serum from patients with psoriasis. J. Transl. Med. 2013, 11, 194. [Google Scholar] [CrossRef]
- Shih, C.-M.; Chen, C.-C.; Chu, C.-K.; Wang, K.-H.; Huang, C.-Y.; Lee, A.-W. The Roles of Lipoprotein in Psoriasis. Int. J. Mol. Sci. 2020, 21, 859. [Google Scholar] [CrossRef]
- Holzer, M.; Wolf, P.; Curcic, S.; Birner-Gruenberger, R.; Weger, W.; Inzinger, M.; El-Gamal, D.; Wadsack, C.; Heinemann, A.; Marsche, C. Psoriasis alters HDL composition and cholesterol efflux capacity. J. Lipid Res. 2012, 53, 1618–1624. [Google Scholar] [CrossRef]
- Mehta, N.N.; Li, R.; Krishnamoorthy, P.; Yu, Y.; Farver, W.; Rodrigues, A.; Raper, A.; Wilcox, M.; Baer, A.; DerOhannesian, S.; et al. Abnormal lipoprotein particles and cholesterol efflux capacity in patients with psoriasis. Atherosclerosis 2012, 224, 218–221. [Google Scholar] [CrossRef]
- Paiva-Lopes, M.J.; Batuca, J.R.; Gouveia, S.; Alves, M.; Papoila, A.L.; Alves, J.D. Antibodies towards high-density lipoprotein components in patients with psoriasis. Arch. Dermatol. Res. 2020, 312, 93–102. [Google Scholar] [CrossRef]
- Paiva-Lopes, M.J.; Delgado Alves, J. Psoriasis-associated vascular disease: The role of HDL. J. Biomed. Sci. 2017, 24, 73. [Google Scholar] [CrossRef] [PubMed]
- Norata, G.D.; Catapano, A.L. HDL and adaptive immunity: A tale of lipid rafts. Atherosclerosis 2012, 225, 34–35. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Savanelli, M.C.; Di Somma, C.; Napolitano, M.; Megna, M.; Colao, A.; Savastano, S. Vitamin D and its role in psoriasis: An overview of the dermatologist and nutritionist. Rev. Endocr. Metab. Disord. 2017, 18, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Orgaz-Molina, J.; Buendía-Eisman, A.; Arrabal-Polo, M.A.; Ruiz, J.C.; Arias-Santiago, S. Deficiency of serum concentration of 25-hydroxyvitamin D in psoriatic patients: A case-control study. J. Am. Acad. Dermatol. 2012, 67, 931–938. [Google Scholar] [CrossRef]
- Kincse, G.; Bhattoa, P.H.; Herédi, E.; Varga, J.; Szegedi, A.; Kéri, J.; Gaàl, J. Vitamin D3 levels and bone mineral density in patients with psoriasis and/or psoriatic arthritis. J. Dermatol. 2015, 42, 679–684. [Google Scholar] [CrossRef]
- Grazio, S.; Naglić, Đ.B.; Anić, B.; Grubišić, F.; Bobek, D.; Bakula, M.; Kavanagh, H.S.; Kuna, A.T.; Cvijetić, S. Vitamin D serum level, disease activity and functional ability in different rheumatic patients. Am. J. Med. Sci. 2015, 349, 46–49. [Google Scholar] [CrossRef]
- Martins, D.; Wolf, M.; Pan, D.; Zadshir, A.; Tareen, N.; Thadhani, R.; Felsenfeld, A.; Levine, B.; Mehrotra, R.; Norris, K. Prevalence of cardiovascular risk factors and the serum levels of 25-hydroxyvitamin D in the United States: Data from the Third National Health and Nutrition Examination Survey. Arch. Intern. Med. 2007, 167, 1159–1165. [Google Scholar] [CrossRef]
- Zittermann, A.; Gummert, J.F.; Börgermann, J. The role of vitamin D in dyslipidemia and cardiovascular disease. Curr. Pharm. Des. 2011, 17, 933–942. [Google Scholar] [CrossRef]
- Dibaba, D.T. Effect of vitamin D supplementation on serum lipid profiles: A systematic review and meta-analysis. Nutr. Rev. 2019, 77, 890–902. [Google Scholar] [CrossRef]
- Zittermann, A.; Frisch, S.; Berthold, H.K.; Götting, C.; Kuhn, J.; Kleesiek, K.; Stehle, P.; Koertke, H.; Koerfer, R. Vitamin D supplementation enhances the beneficial effects of weight loss on cardiovascular disease risk markers. Am. J. Clin. Nutr. 2009, 89, 1321–1327. [Google Scholar] [CrossRef]
- Lin, S.H.; Lin, Y.F.; Lu, K.C.; Diang, L.K.; Chyr, S.H.; Liao, W.K.; Shieh, S.D. Effects of intravenous calcitriol on lipid profiles and glucose tolerance in uraemic patients with secondary hyperparathyroidism. Clin. Sci. 1994, 87, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Yeksan, M.; Türk, S.; Polat, M.; Ciğli, A.; Erdoğan, Y. Effects of 1,25 (OH)2D3 treatment on lipid levels in uremic hemodialysis patients. Int. J. Artif. Organs. 1992, 15, 704–707. [Google Scholar] [CrossRef] [PubMed]
- Gisondi, P.; Tessari, G.; Conti, A.; Piaserico, S.; Schianchi, S.; Peserico, A.; Giannetti, A.; Girolomoni, G. Prevalence of metabolic syndrome in patients with psoriasis: A hospital-based case-control study. Br. J. Dermatol. 2007, 157, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Guidolin, L.; Borin, M.; Fontana, E.; Caroppo, F.; Piaserico, S.; Fortina, A.B. Central Obesity in Children with Psoriasis. Acta. Derm. Venereol. 2018, 98, 282–283. [Google Scholar] [CrossRef]
- Sommer, D.M.; Jenisch, S.; Suchan, M.; Christophers, E.; Weichenthal, M. Increased prevalence of the metabolic syndrome in patients with moderate to severe psoriasis. Arch. Dermatol. Res. 2006, 298, 321. [Google Scholar] [CrossRef] [PubMed]
- Jensen, P.; Skov, L. Psoriasis and Obesity. Dermatology 2016, 232, 633–639. [Google Scholar] [CrossRef]
- Lønnberg, A.S.; Skov, L.; Skytthe, A.; Kyvik, K.O.; Pedersen, O.B.; Thomsen, S.F. Association of Psoriasis With the Risk for Type 2 Diabetes Mellitus and Obesity. JAMA Dermatol. 2016, 152, 761–767. [Google Scholar] [CrossRef]
- Tupikowska-Marzec, M.; Kolačkov, K.; Zdrojowy-Wełna, A.; Słoka, N.K.; Szepietowski, J.C.; Maj, J. The Influence of FTO Polymorphism rs9939609 on Obesity, Some Clinical Features, and Disturbance of Carbohydrate Metabolism in Patients with Psoriasis. Biomed. Res. Int. 2019, 2019, 7304345. [Google Scholar] [CrossRef]
- Voiculescu, V.M.; Solomon, I.; Popa, A.; Draghici, C.C.; Dobre, M.; Giurcaneanu, C.; Papagheorghe, L.M.L.; Lupu, M. Gene polymorphisms of TNF-238G/A, TNF-308G/A, IL10-1082G/A, TNFAIP3, and MC4R and comorbidity occurrence in a Romanian population with psoriasis. J. Med. Life 2018, 11, 69–74. [Google Scholar]
- Kara, N.; Aydin, F.; Senturk, N.; Gunes, S.; Canturk, M.T.; Bagci, H.; Bek, Y.; Turanli, A.Y. Lack of association between the G-2548A polymorphism of the leptin gene and psoriasis in a Turkish population. Int. J. Dermatol. 2007, 46, 1271–1274. [Google Scholar] [CrossRef]
- Torres, T.; Bettencourt, N.; Ferreira, J.; Carvalho, C.; Mendonça, D.; Vasconcelos, C.; Selores, M.; Silva, B. Lack of association between leptin, leptin receptor, adiponectin gene polymorphisms and epicardial adipose tissue, abdominal visceral fat volume and atherosclerotic burden in psoriasis patients. Arch. Physiol. Biochem. 2015, 121, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Turan, H.; Yaykasli, K.O.; Soguktas, H.; Yaykasli, E.; Aliagaoglu, C.; Erdem, T.; Karkucak, M.; Kaya, E.; Ucgun, T.; Bahadir, A. Omentin serum levels and omentin gene Val109Asp polymorphism in patients with psoriasis. Int. J. Dermatol. 2014, 53, 601–605. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhu, K.J.; Liu, J.L.; Xu, G.X.; Liu, W.; Jiang, F.X.; Zheng, H.F.; Quan, C. Omentin-1 plasma levels and omentin-1 expression are decreased in psoriatic lesions of psoriasis patients. Arch. Dermatol. Res. 2015, 307, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Yang, Y.; Liao, Y.; Shi, Y.; Zhang, L. Emerging Roles of Adipose Tissue in the Pathogenesis of Psoriasis and Atopic Dermatitis in Obesity. JID Innov. 2022, 2, 100064. [Google Scholar] [CrossRef]
- Chiricozzi, A.; Raimondo, A.; Lembo, S.; Fausti, F.; Dini, V.; Costanzo, A.; Monfrecola, G.; Balato, N.; Ayala, F.; Romanelli, M.; et al. Crosstalk between skin inflammation and adipose tissue-derived products: Pathogenic evidence linking psoriasis to increased adiposity. Expert Rev. Clin. Immunol. 2016, 12, 1299–1308. [Google Scholar] [CrossRef] [PubMed]
- Dopytalska, K.; Baranowska-Bik, A.; Roszkiewicz, M.; Bik, W.; Walecka, I. The role of leptin in selected skin diseases. Lipids Health Dis. 2020, 19, 215. [Google Scholar] [CrossRef]
- Stjernholm, T.; Ommen, P.; Langkilde, A.; Johansen, C.; Iversen, L.; Rosada, C.; Stenderup, K. Leptin deficiency in mice counteracts imiquimod (IMQ)-induced psoriasis-like skin inflammation while leptin stimulation induces inflammation in human keratinocytes. Exp. Dermatol. 2017, 26, 338–345. [Google Scholar] [CrossRef]
- Kyriakou, A.; Patsatsi, A.; Sotiriadis, D.; Goulis, D.G. Serum Leptin, Resistin, and Adiponectin Concentrations in Psoriasis: A Meta-Analysis of Observational Studies. Dermatology 2017, 233, 378–389. [Google Scholar] [CrossRef]
- Shibata, S.; Tada, Y.; Hau, C.S.; Mitsui, A.; Kamata, M.; Asano, Y.; Sugaya, M.; Kadono, T.; Masamoto, Y.; Kurokawa, M.; et al. Adiponectin regulates psoriasiform skin inflammation by suppressing IL-17 production from γδ-T cells. Nat. Commun. 2015, 6, 7687. [Google Scholar] [CrossRef]
- Johnston, A.; Arnadottir, S.; Gudjonsson, J.E.; Aphale, A.; Sigmarsdottir, A.A.; Gunnarsson, S.I.; Steinsson, J.T.; Elder, J.T.; Valdimarsson, H. Obesity in psoriasis: Leptin and resistin as mediators of cutaneous inflammation. Br. J. Dermatol. 2008, 159, 342–350. [Google Scholar] [CrossRef]
- Coban, M.; Tasli, L.; Turgut, S.; Özkan, S.; Tunç Ata, M.; Akın, F. Association of Adipokines, Insulin Resistance, Hypertension and Dyslipidemia in Patients with Psoriasis Vulgaris. Ann. Dermatol. 2016, 28, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Zou, Q.; Si, J.; Guo, Y.; Yu, J.; Shi, H. Association between serum visfatin levels and psoriasis and their correlation with disease severity: A meta-analysis. J. Int. Med. Res. 2021, 49, 3000605211002381. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Fuster, J.J.; Walsh, K. Adipokines: A link between obesity and cardiovascular disease. J. Cardiol. 2014, 63, 250–259. [Google Scholar] [CrossRef]
- Ziccardi, P.; Nappo, F.; Giugliano, G.; Esposito, K.; Marfella, R.; Cioffi, M.; D’Andrea, F.; Molinari, A.M.; Giugliano, D. Reduction of inflammatory cytokine concentrations and improvement of endothelial functions in obese women after weight loss over one year. Circulation 2002, 105, 804–809. [Google Scholar] [CrossRef]
- Kim, J.; Bajaj, M. Normal Adipose Tissue Biology: Adipocytokines and Inflammation. In Mitchell RNBT-P of, H.D.; McManus, L.M., Ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 488–497. [Google Scholar] [CrossRef]
- Kern, P.A.; Saghizadeh, M.; Ong, J.M.; Bosch, R.J.; Deem, R.; Simsolo, R.B. The expression of tumor necrosis factor in human adipose tissue. Regulation by obesity, weight loss, and relationship to lipoprotein lipase. J. Clin. Invest. 1995, 95, 2111–2119. [Google Scholar] [CrossRef]
- Wang, T.; He, C. Pro-inflammatory cytokines: The link between obesity and osteoarthritis. Cytokine Growth Factor Rev. 2018, 44, 38–50. [Google Scholar] [CrossRef]
- Winer, S.; Paltser, G.; Chan, Y.; Tsui, H.; Engleman, E.; Winer, D.; Dosch, H.M. Obesity predisposes to Th17 bias. Eur. J. Immunol. 2009, 39, 2629–2635. [Google Scholar] [CrossRef]
- Polak, K.; Bergler-Czop, B.; Szczepanek, M.; Wojciechowska, K.; Frątczak, A.; Kiss, N. Psoriasis and Gut Microbiome-Current State of Art. Int. J. Mol. Sci. 2021, 22, 4529. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-J.; Ho, H.J.; Tseng, C.-H.; Lai, Z.-L.; Shieh, J.-J.; Wu, C.-Y. Intestinal microbiota profiling and predicted metabolic dysregulation in psoriasis patients. Exp. Dermatol. 2018, 27, 1336–1343. [Google Scholar] [CrossRef]
- Targher, G.; Byrne, C.D.; Tilg, H. NAFLD and increased risk of cardiovascular disease: Clinical associations, pathophysiological mechanisms and pharmacological implications. Gut 2020, 69, 1691–1705. [Google Scholar] [CrossRef]
- Balak, D.M.W.; Piaserico, S.; Kasujee, I. Non-Alcoholic Fatty Liver Disease (NAFLD) in Patients with Psoriasis: A Review of the Hepatic Effects of Systemic Therapies. Psoriasis 2021, 11, 151–168. [Google Scholar] [CrossRef] [PubMed]
- Young, S.; Tariq, R.; Provenza, J.; Satapathy, S.K.; Faisal, K.; Choudhry, A.; Friedman, S.L.; Singal, A.K. Prevalence and Profile of Nonalcoholic Fatty Liver Disease in Lean Adults: Systematic Review and Meta-Analysis. Hepatol. Commun. 2020, 4, 953–972. [Google Scholar] [CrossRef] [PubMed]
- Ruiz de Morales, J.M.G.; Puig, L.; Daudén, E.; Cañete, J.D.; Pablos, J.L.; Martín, A.O.; Juanatey, C.G.; Adàn, A.; Montalbàn, X.; Borruel, N.; et al. Critical role of interleukin (IL)-17 in inflammatory and immune disorders: An updated review of the evidence focusing in controversies. Autoimmun. Rev. 2020, 19, 102429. [Google Scholar] [CrossRef] [PubMed]
- Rau, M.; Schilling, A.-K.; Meertens, J.; Hering, I.; Weiss, J.; Jurowich, C.; Kudlich, T.; Hermanns, H.M.; Bantel, H.; Beyersdorf, N.; et al. Progression from Nonalcoholic Fatty Liver to Nonalcoholic Steatohepatitis Is Marked by a Higher Frequency of Th17 Cells in the Liver and an Increased Th17/Resting Regulatory T Cell Ratio in Peripheral Blood and in the Liver. J. Immunol. 2016, 196, 97–105. [Google Scholar] [CrossRef]
- Gomes, A.L.; Teijeiro, A.; Burén, S.; Tummala, K.S.; Yilmaz, M.; Waisman, A.; Theurillat, J.P.; Perna, C.; Djouder, N. Metabolic Inflammation-Associated IL-17A Causes Non-alcoholic Steatohepatitis and Hepatocellular Carcinoma. Cancer Cell 2016, 30, 161–175. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).