Microenvironment in Oral Potentially Malignant Disorders: Multi-Dimensional Characteristics and Mechanisms of Carcinogenesis
Abstract
:1. Introduction
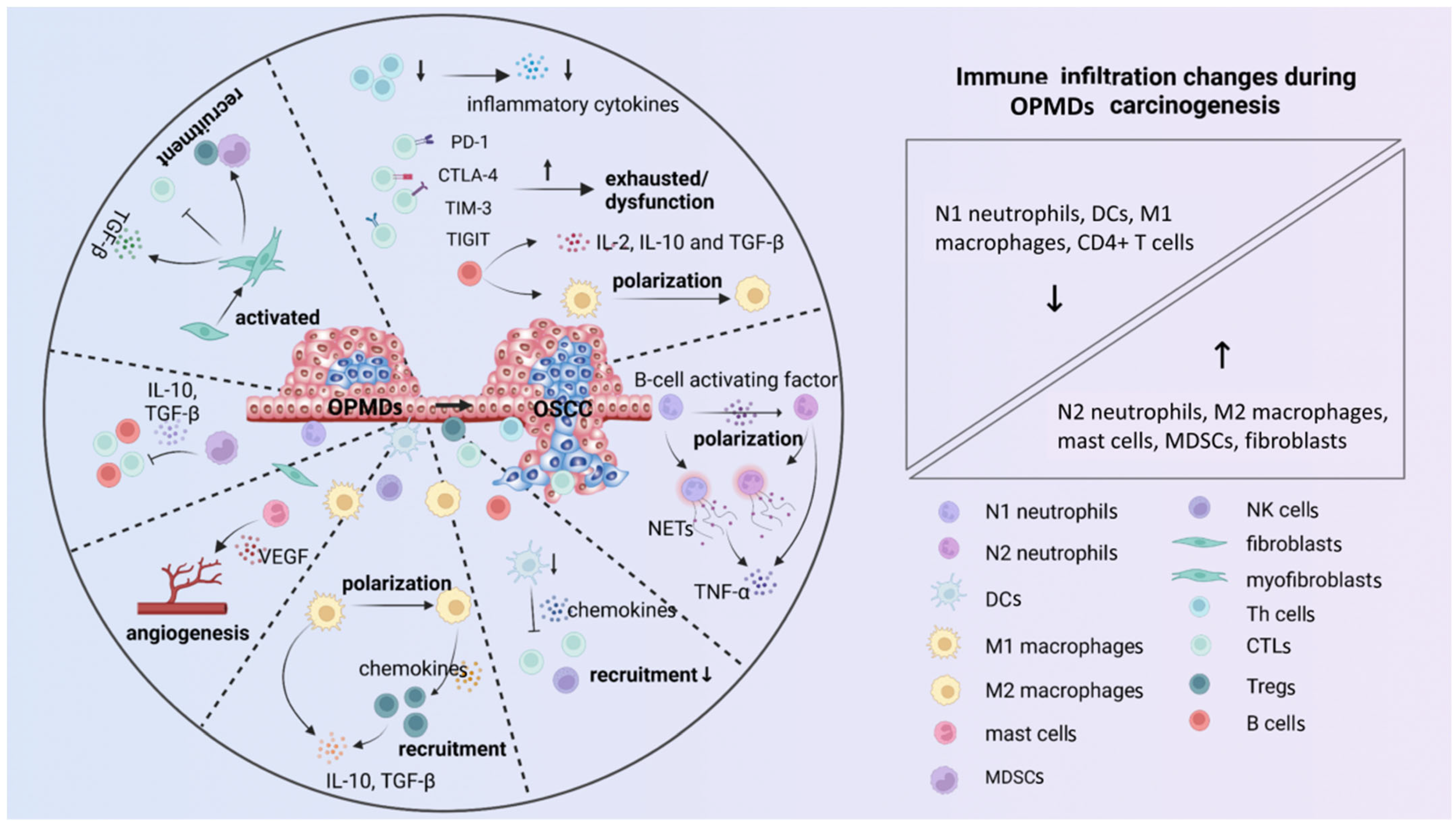
2. Immune Microenvironment
2.1. Cellular Components
2.2. Cytokines and Immune Checkpoints
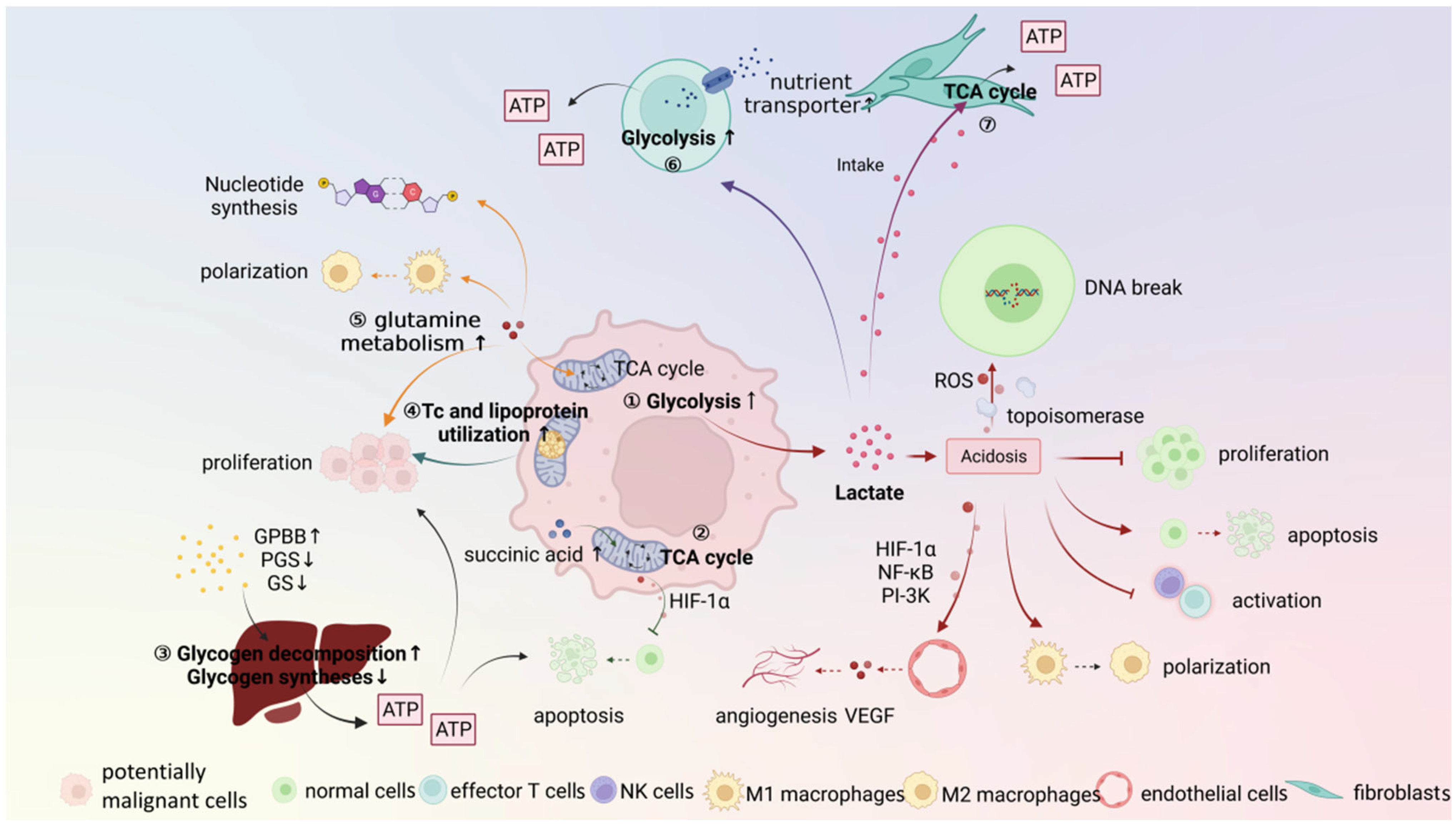
3. Metabolic Microenvironment
3.1. Potentially Malignant Cells
3.2. Stroma Cells
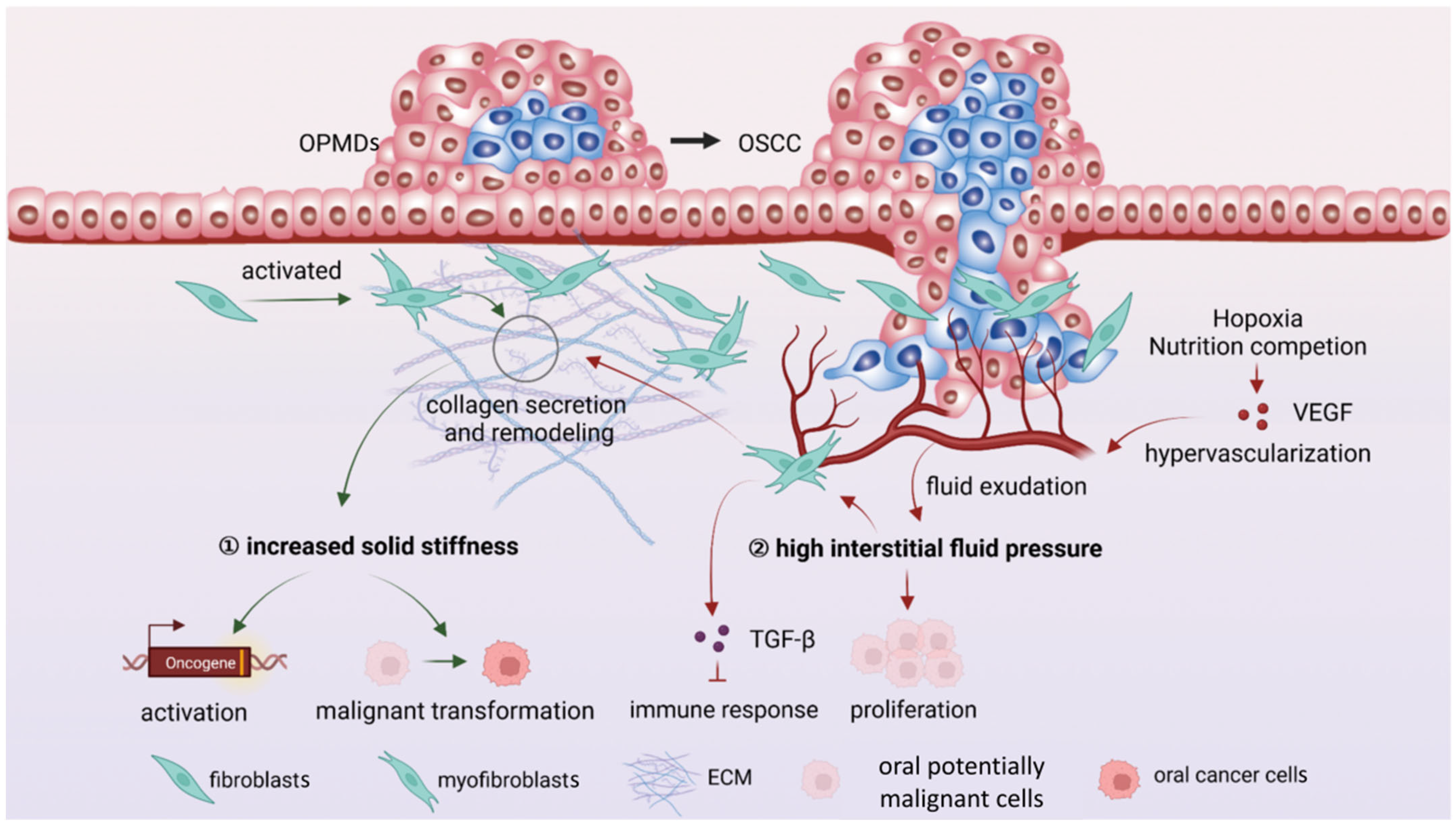
4. Mechanical Microenvironment
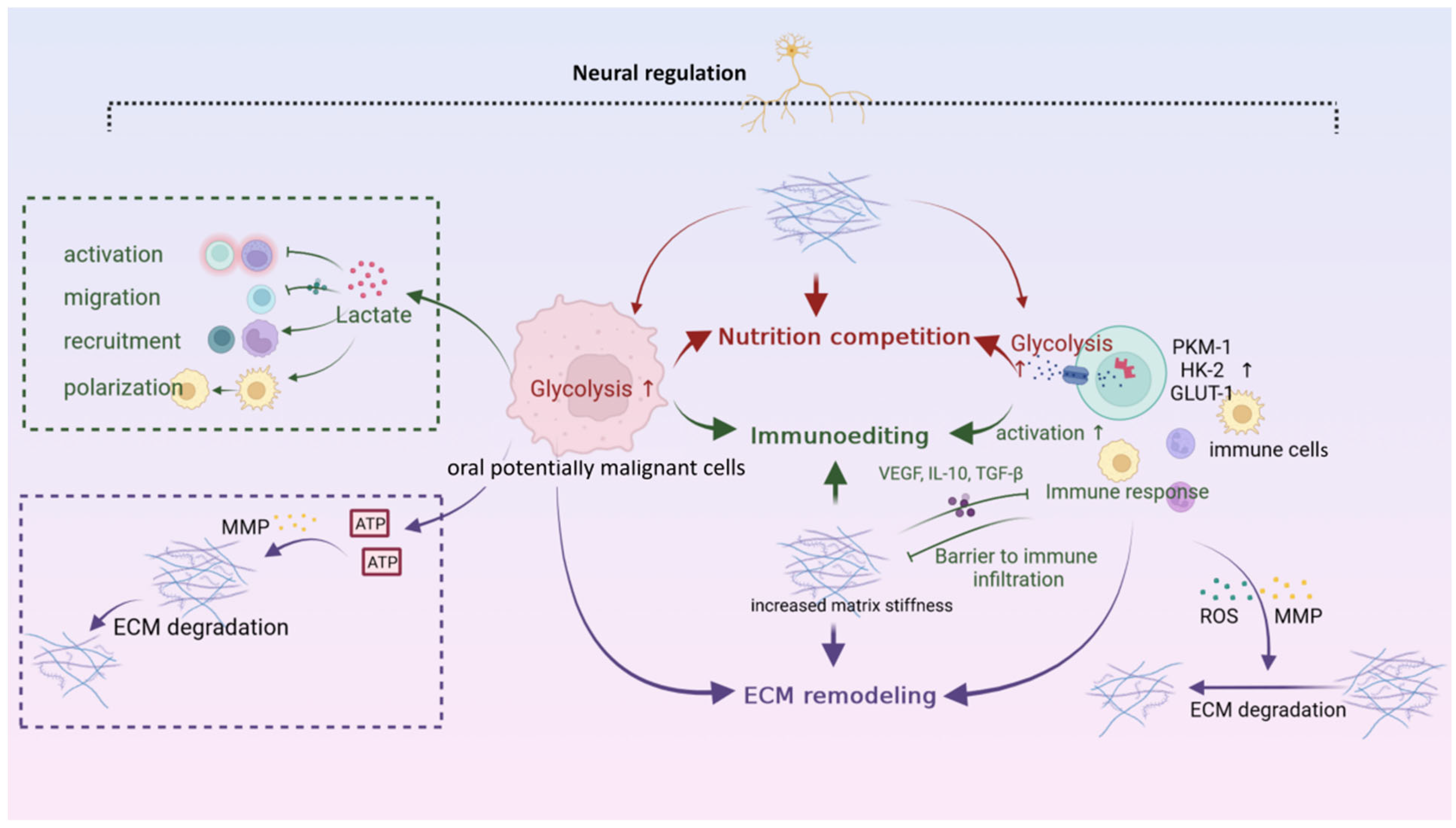
5. Neural Microenvironment
6. Interaction Networks among Microenvironments
7. Microenvironment-Based Targeting Strategies
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| OPMDs | Oral potentially malignant disorders |
| OSCC | Oral squamous cell carcinoma |
| OLK | Oral leukoplakia |
| OLP | Oral lichen planus |
| OSF | Oral submucous fibrosis |
| OLL | Oral lichenoid lesions |
| OEL | Oral erythroleukoplakia |
| ECM | Extracellular matrix |
| IL | Interleukin |
| TNF-α | Tumor necrosis factor-α |
| NETs | Neutrophil extracellular traps |
| DCs | Dendritic cells |
| 4NQO | 4-nitroquinoline-oxide |
| MDSCs | Myeloid-derived suppressor cells |
| Th cells | Helper T cells |
| CTLs | Cytotoxic T lymphocytes |
| IFN-γ | Interferon-γ |
| Tregs | Regulatory T cells |
| PD-1 | Programmed cell death protein-1 |
| CTLA-4 | Cytotoxic T-lymphocyte antigen-4 |
| TIGIT | Ig and ITIM domains |
| LAG-3 | Lymphocyte-activation gene-3 |
| TIM-3 | T cell immunoglobulin and mucin domain-containing protein-3 |
| VEGF | Vascular endothelial growth factor |
| TCA | Tricarboxylic acid |
| CAFs | Cancer-associated fibroblasts |
| MMP | Matrix metalloproteinase |
References
- Warnakulasuriya, S.; Kujan, O.; Aguirre-Urizar, J.M.; Bagan, J.V.; González-Moles, M.; Kerr, A.R.; Lodi, G.; Mello, F.W.; Monteiro, L.; Ogden, G.R.; et al. Oral potentially malignant disorders: A consensus report from an international seminar on nomenclature and classification, convened by the WHO Collaborating Centre for Oral Cancer. Oral Dis. 2021, 27, 1862–1880. [Google Scholar] [CrossRef] [PubMed]
- Iocca, O.; Sollecito, T.P.; Alawi, F.; Weinstein, G.S.; Newman, J.G.; De Virgilio, A.; Di Maio, P.; Spriano, G.; Pardiñas López, S.; Shanti, R.M. Potentially malignant disorders of the oral cavity and oral dysplasia: A systematic review and meta-analysis of malignant transformation rate by subtype. Head Neck 2020, 42, 539–555. [Google Scholar] [CrossRef] [PubMed]
- Sekikawa, S.; Kawachi, H.; Ogane, S.; Saito, H.; Takano, M.; Nomura, T.; Katakura, A.; Takano, N.; Shibahara, T. Which Factors Affect the Long-Term Survival of Patients with Oral Squamous Cell Carcinoma with Distant Metastasis? J. Oral Maxillofac. Surg. Off. J. Am. Assoc. Oral Maxillofac. Surg. 2020, 78, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Kujan, O.; Agag, M.; Smaga, M.; Vaishnaw, Y.; Idrees, M.; Shearston, K.; Farah, C.S. PD-1/PD-L1, Treg-related proteins, and tumour-infiltrating lymphocytes are associated with the development of oral squamous cell carcinoma. Pathology 2022, 54, 409–416. [Google Scholar] [CrossRef]
- Kujan, O.; Shearston, K.; Farah, C.S. The role of hypoxia in oral cancer and potentially malignant disorders: A review. J. Oral Pathol. Med. Off. Publ. Int. Assoc. Oral Pathol. Am. Acad. Oral Pathol. 2017, 46, 246–252. [Google Scholar] [CrossRef]
- Ai, R.; Tao, Y.; Hao, Y.; Jiang, L.; Dan, H.; Ji, N.; Zeng, X.; Zhou, Y.; Chen, Q. Microenvironmental regulation of the progression of oral potentially malignant disorders towards malignancy. Oncotarget 2017, 8, 81617–81635. [Google Scholar] [CrossRef] [Green Version]
- Sharma, P.; Sandhu, S.V.; Bhandari, R.; Verma, I.; Bhullar, R.K.; Khangura, R.K. Estimation of cortisol levels in patients with premalignant disorders and oral squamous cell carcinoma. J. Oral Maxillofac. Pathol. JOMFP 2018, 22, 27–34. [Google Scholar]
- Noël, G.; Langouo Fontsa, M.; Willard-Gallo, K. The impact of tumor cell metabolism on T cell-mediated immune responses and immuno-metabolic biomarkers in cancer. Semin. Cancer Biol. 2018, 52 Pt 2, 66–74. [Google Scholar] [CrossRef]
- Haas, R.; Smith, J.; Rocher-Ros, V.; Nadkarni, S.; Montero-Melendez, T.; D’Acquisto, F.; Bland, E.J.; Bombardieri, M.; Pitzalis, C.; Perretti, M.; et al. Lactate Regulates Metabolic and Pro-inflammatory Circuits in Control of T Cell Migration and Effector Functions. PLoS Biol. 2015, 13, e1002202. [Google Scholar] [CrossRef]
- Bertero, T.; Oldham, W.M.; Cottrill, K.A.; Pisano, S.; Vanderpool, R.R.; Yu, Q.; Zhao, J.; Tai, Y.; Tang, Y.; Zhang, Y.Y.; et al. Vascular stiffness mechanoactivates YAP/TAZ-dependent glutaminolysis to drive pulmonary hypertension. J. Clin. Investig. 2016, 126, 3313–3335. [Google Scholar] [CrossRef] [Green Version]
- Kalluri, R. The biology and function of fibroblasts in cancer. Nature reviews. Cancer 2016, 16, 582–598. [Google Scholar]
- Sathasivam, H.P.; Kist, R.; Sloan, P.; Thomson, P.; Nugent, M.; Alexander, J.; Haider, S.; Robinson, M. Predicting the clinical outcome of oral potentially malignant disorders using transcriptomic-based molecular pathology. Br. J. Cancer 2021, 125, 413–421. [Google Scholar] [CrossRef]
- Wen, L.; Mu, W.; Lu, H.; Wang, X.; Fang, J.; Jia, Y.; Li, Q.; Wang, D.; Wen, S.; Guo, J.; et al. Porphyromonas gingivalis Promotes Oral Squamous Cell Carcinoma Progression in an Immune Microenvironment. J. Dent. Res. 2020, 99, 666–675. [Google Scholar] [CrossRef]
- Farlow, J.L.; Brenner, J.C.; Lei, Y.L.; Chinn, S.B. Immune deserts in head and neck squamous cell carcinoma: A review of challenges and opportunities for modulating the tumor immune microenvironment. Oral Oncol. 2021, 120, 105420. [Google Scholar] [CrossRef]
- Zhang, Q.F.; Yin, W.W.; Xia, Y.; Yi, Y.Y.; He, Q.F.; Wang, X.; Ren, H.; Zhang, D.Z. Liver-infiltrating CD11b(-)CD27(-) NK subsets account for NK-cell dysfunction in patients with hepatocellular carcinoma and are associated with tumor progression. Cell. Mol. Immunol. 2017, 14, 819–829. [Google Scholar] [CrossRef] [Green Version]
- Lei, X.; Lei, Y.; Li, J.K.; Du, W.X.; Li, R.G.; Yang, J.; Li, J.; Li, F.; Tan, H.B. Immune cells within the tumor microenvironment: Biological functions and roles in cancer immunotherapy. Cancer Lett. 2020, 470, 126–133. [Google Scholar] [CrossRef]
- Davidson, S.; Coles, M.; Thomas, T.; Kollias, G.; Ludewig, B.; Turley, S.; Brenner, M.; Buckley, C.D. Fibroblasts as immune regulators in infection, inflammation and cancer. Nat. Rev. Immunol. 2021, 21, 704–717. [Google Scholar] [CrossRef]
- Hinshaw, D.C.; Shevde, L.A. The Tumor Microenvironment Innately Modulates Cancer Progression. Cancer Res. 2019, 79, 4557–4566. [Google Scholar] [CrossRef] [Green Version]
- Goff, S.L.; Danforth, D.N. The Role of Immune Cells in Breast Tissue and Immunotherapy for the Treatment of Breast Cancer. Clin. Breast Cancer 2021, 21, e63–e73. [Google Scholar] [CrossRef]
- Rosales, C.; Lowell, C.A.; Schnoor, M.; Uribe-Querol, E. Neutrophils: Their Role in Innate and Adaptive Immunity 2017. J. Immunol. Res. 2017, 2017, 9748345. [Google Scholar] [CrossRef]
- Thornhill, M.H.; Sankar, V.; Xu, X.J.; Barrett, A.W.; High, A.S.; Odell, E.W.; Speight, P.M.; Farthing, P.M. The role of histopathological characteristics in distinguishing amalgam-associated oral lichenoid reactions and oral lichen planus. J. Oral Pathol. Med. Off. Publ. Int. Assoc. Oral Pathol. Am. Acad. Oral Pathol. 2006, 35, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Fine, N.; Hassanpour, S.; Borenstein, A.; Sima, C.; Oveisi, M.; Scholey, J.; Cherney, D.; Glogauer, M. Distinct Oral Neutrophil Subsets Define Health and Periodontal Disease States. J. Dent. Res. 2016, 95, 931–938. [Google Scholar] [CrossRef] [PubMed]
- Jab O Ska, E.; S Odczyk, B.E.; Wawrusiewicz-Kurylonek, N.; Dziemia Czyk-Pakie, A.D.; Garley, M.; Ratajczak-Wrona, W.; Borys, J.; Kr Towski, A. Comparison of B-Cell Activating Factor Expression in Neutrophils in Patients with Potentially Malignant Disorders and Patients with Cancer in the Same Site. Clin. Lab. 2016, 62, 1507–1514. [Google Scholar]
- Moonen, C.G.J.; Hirschfeld, J.; Cheng, L.; Chapple, I.L.C.; Loos, B.G.; Nicu, E.A. Oral Neutrophils Characterized: Chemotactic, Phagocytic, and Neutrophil Extracellular Trap (NET) Formation Properties. Front. Immunol. 2019, 10, 635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jablonska, E.; Garley, M.; Surazynski, A.; Grubczak, K.; Iwaniuk, A.; Borys, J.; Moniuszko, M.; Ratajczak-Wrona, W. Neutrophil extracellular traps (NETs) formation induced by TGF-β in oral lichen planus—Possible implications for the development of oral cancer. Immunobiology 2020, 225, 151901. [Google Scholar] [CrossRef] [PubMed]
- Chadwick, J.W.; Macdonald, R.; Ali, A.A.; Glogauer, M.; Magalhaes, M.A. TNFα Signaling Is Increased in Progressing Oral Potentially Malignant Disorders and Regulates Malignant Transformation in an Oral Carcinogenesis Model. Front. Oncol. 2021, 11, 741013. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.J.; Owens, D.M.; Stamp, G.; Arnott, C.; Burke, F.; East, N.; Holdsworth, H.; Turner, L.; Rollins, B.; Pasparakis, M.; et al. Mice deficient in tumor necrosis factor-alpha are resistant to skin carcinogenesis. Nat. Med. 1999, 5, 828–831. [Google Scholar] [CrossRef]
- Mignogna, M.D.; Fedele, S.; Lo Russo, L.; Lo Muzio, L.; Bucci, E. Immune activation and chronic inflammation as the cause of malignancy in oral lichen planus: Is there any evidence? Oral Oncol. 2004, 40, 120–130. [Google Scholar] [CrossRef]
- Al-Benna, S.; Shai, Y.; Jacobsen, F.; Steinstraesser, L. Oncolytic activities of host defense peptides. Int. J. Mol. Sci. 2011, 12, 8027–8051. [Google Scholar] [CrossRef] [Green Version]
- Wculek, S.K.; Cueto, F.J.; Mujal, A.M.; Melero, I.; Krummel, M.F.; Sancho, D. Dendritic cells in cancer immunology and immunotherapy. Nat. Rev. Immunol. 2020, 20, 7–24. [Google Scholar] [CrossRef]
- Silva, L.C.; Fonseca, F.P.; Almeida, O.P.; Mariz, B.A.; Lopes, M.A.; Radhakrishnan, R.; Sharma, M.; Kowalski, L.P.; Vargas, P.A. CD1a+ and CD207+ cells are reduced in oral submucous fibrosis and oral squamous cell carcinoma. Med. Oral Patol. Oral Y Cir. Bucal 2020, 25, e49–e55. [Google Scholar] [CrossRef]
- De Costa, A.M.; Justis, D.N.; Schuyler, C.A.; Young, M.R. Administration of a vaccine composed of dendritic cells pulsed with premalignant oral lesion lysate to mice bearing carcinogen-induced premalignant oral lesions stimulates a protective immune response. Int. Immunopharmacol. 2012, 13, 322–330. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Yrigoyen, M.; Cassetta, L.; Pollard, J.W. Macrophage targeting in cancer. Ann. New York Acad. Sci. 2021, 1499, 18–41. [Google Scholar] [CrossRef]
- Yunna, C.; Mengru, H.; Lei, W.; Weidong, C. Macrophage M1/M2 polarization. Eur. J. Pharmacol. 2020, 877, 173090. [Google Scholar] [CrossRef]
- Kouketsu, A.; Sato, I.; Oikawa, M.; Shimizu, Y.; Saito, H.; Tashiro, K.; Yamashita, Y.; Takahashi, T.; Kumamoto, H. Regulatory T cells and M2-polarized tumour-associated macrophages are associated with the oncogenesis and progression of oral squamous cell carcinoma. Int. J. Oral Maxillofac. Surg. 2019, 48, 1279–1288. [Google Scholar] [CrossRef]
- Shigeoka, M.; Koma, Y.I.; Nishio, M.; Komori, T.; Yokozaki, H. CD163(+) macrophages infiltration correlates with the immunosuppressive cytokine interleukin 10 expression in tongue leukoplakia. Clin. Exp. Dent. Res. 2019, 5, 627–637. [Google Scholar] [CrossRef] [Green Version]
- Bouaoud, J.; Foy, J.P.; Tortereau, A.; Michon, L.; Lavergne, V.; Gadot, N.; Boyault, S.; Valantin, J.; De Souza, G.; Zrounba, P.; et al. Early changes in the immune microenvironment of oral potentially malignant disorders reveal an unexpected association of M2 macrophages with oral cancer free survival. Oncoimmunology 2021, 10, 1944554. [Google Scholar] [CrossRef]
- Shigeoka, M.; Koma, Y.I.; Nishio, M.; Akashi, M.; Yokozaki, H. Alteration of Macrophage Infiltrating Compartment: A Novel View on Oral Carcinogenesis. Pathobiol. J. Immunopathol. Mol. Cell. Biol. 2021, 88, 327–337. [Google Scholar] [CrossRef]
- Elieh Ali Komi, D.; Wöhrl, S.; Bielory, L. Mast Cell Biology at Molecular Level: A Comprehensive Review. Clin. Rev. Allergy Immunol. 2020, 58, 342–365. [Google Scholar] [CrossRef]
- Jyothsna, M.; Rammanohar, M.; Kumar, K. Histomorphometric Analysis of Angiogenesis using CD31 Immunomarker and Mast Cell Density in Oral Premalignant and Malignant Lesions: A Pilot Study. J. Clin. Diagn. Res. JCDR 2017, 11, ZC37–ZC40. [Google Scholar] [CrossRef]
- Sundararajan, A.; Muthusamy, R.; Gopal Siva, K.; Harikrishnan, P.; Kumar, S.C.K.; Rathinasamy, S.K. Correlation of Mast Cell and Angiogenesis in Oral Lichen Planus, Dysplasia (Leukoplakia), and Oral Squamous Cell Carcinoma. Rambam Maimonides Med. J. 2021, 12, e0016. [Google Scholar] [CrossRef] [PubMed]
- Law, A.M.K.; Valdes-Mora, F.; Gallego-Ortega, D. Myeloid-Derived Suppressor Cells as a Therapeutic Target for Cancer. Cells 2020, 9, 561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pang, X.; Fan, H.Y.; Tang, Y.L.; Wang, S.S.; Cao, M.X.; Wang, H.F.; Dai, L.L.; Wang, K.; Yu, X.H.; Wu, J.B.; et al. Myeloid derived suppressor cells contribute to the malignant progression of oral squamous cell carcinoma. PLoS ONE 2020, 15, e0229089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Accolla, R.S.; Lombardo, L.; Abdallah, R.; Raval, G.; Forlani, G.; Tosi, G. Boosting the MHC Class II-Restricted Tumor Antigen Presentation to CD4+ T Helper Cells: A Critical Issue for Triggering Protective Immunity and Re-Orienting the Tumor Microenvironment Toward an Anti-Tumor State. Front. Oncol. 2014, 4, 32. [Google Scholar] [CrossRef] [Green Version]
- Stasikowska-Kanicka, O.; Wągrowska-Danilewicz, M.; Danilewicz, M. CD8+ and CD163+ infiltrating cells and PD-L1 immunoexpression in oral leukoplakia and oral carcinoma. APMIS Acta Pathol. Microbiol. Et Immunol. Scand. 2018, 126, 732–738. [Google Scholar] [CrossRef]
- Chaves, A.L.F.; Silva, A.G.; Maia, F.M.; Lopes, G.F.M.; de Paulo, L.F.B.; Muniz, L.V.; Dos Santos, H.B.; Soares, J.M.A.; Souza, A.A.; de Oliveira Barbosa, L.A.; et al. Reduced CD8(+) T cells infiltration can be associated to a malignant transformation in potentially malignant oral epithelial lesions. Clin. Oral Investig. 2019, 23, 1913–1919. [Google Scholar] [CrossRef]
- Sakata, J.; Yoshida, R.; Matsuoka, Y.; Kawahara, K.; Arita, H.; Nakashima, H.; Hirosue, A.; Naito, H.; Takeshita, H.; Kawaguchi, S.; et al. FOXP3 lymphocyte status may predict the risk of malignant transformation in oral leukoplakia. J. Oral Maxillofac. Surg. Med. Pathol. 2020, 32, 33–39. [Google Scholar] [CrossRef]
- Xie, W.; Shen, J.; Wang, D.; Guo, J.; Li, Q.; Wen, S.; Dai, W.; Wen, L.; Lu, H.; Fang, J.; et al. Dynamic changes of exhaustion features in T cells during oral carcinogenesis. Cell Prolif. 2022, 55, e13207. [Google Scholar] [CrossRef]
- Wu, F.; Yang, J.; Liu, J.; Wang, Y.; Mu, J.; Zeng, Q.; Deng, S.; Zhou, H. Signaling pathways in cancer-associated fibroblasts and targeted therapy for cancer. Signal Transduct. Target. Ther. 2021, 6, 218. [Google Scholar] [CrossRef]
- Khalid, A.; Siddiqui, S.; Faizi, N.; Hassan, J.; Nehal, N.; Siddiqui, A. Role of Stromal Myofibroblasts in the Progression of Oral Lesions from Dysplasia to Invasive Carcinoma. Indian J. Med. Paediatr. Oncol. 2021, 40, 536–541. [Google Scholar] [CrossRef]
- Cheng, R.; Li, D.; Shi, X.; Gao, Q.; Wei, C.; Li, X.; Li, Y.; Zhou, H. Reduced CX3CL1 Secretion Contributes to the Susceptibility of Oral Leukoplakia-Associated Fibroblasts to Candida albicans. Front. Cell. Infect. Microbiol. 2016, 6, 150. [Google Scholar] [CrossRef]
- Di Cosola, M.; Cazzolla, A.P.; Charitos, I.A.; Ballini, A.; Inchingolo, F.; Santacroce, L. Candida albicans and Oral Carcinogenesis. A Brief Review. J. Fungi 2021, 7, 476. [Google Scholar] [CrossRef]
- Berraondo, P.; Sanmamed, M.F.; Ochoa, M.C.; Etxeberria, I.; Aznar, M.A.; Pérez-Gracia, J.L.; Rodríguez-Ruiz, M.E.; Ponz-Sarvise, M.; Castañón, E.; Melero, I. Cytokines in clinical cancer immunotherapy. Br. J. Cancer 2019, 120, 6–15. [Google Scholar] [CrossRef] [Green Version]
- Woodford, D.; Johnson, S.D.; De Costa, A.M.; Young, M.R. An Inflammatory Cytokine Milieu is Prominent in Premalignant Oral Lesions, but Subsides when Lesions Progress to Squamous Cell Carcinoma. J. Clin. Cell. Immunol. 2014, 5, 230. [Google Scholar] [CrossRef] [Green Version]
- Johnson, S.D.; Levingston, C.; Young, M.R. Premalignant Oral Lesion Cells Elicit Increased Cytokine Production and Activation of T-cells. Anticancer. Res. 2016, 36, 3261–3270. [Google Scholar]
- Darvin, P.; Toor, S.M.; Sasidharan Nair, V.; Elkord, E. Immune checkpoint inhibitors: Recent progress and potential biomarkers. Exp. Mol. Med. 2018, 50, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Yagyuu, T.; Hatakeyama, K.; Imada, M.; Kurihara, M.; Matsusue, Y.; Yamamoto, K.; Obayashi, C.; Kirita, T. Programmed death ligand 1 (PD-L1) expression and tumor microenvironment: Implications for patients with oral precancerous lesions. Oral Oncol. 2017, 68, 36–43. [Google Scholar] [CrossRef]
- Dave, K.; Ali, A.; Magalhaes, M. Increased expression of PD-1 and PD-L1 in oral lesions progressing to oral squamous cell carcinoma: A pilot study. Sci. Rep. 2020, 10, 9705. [Google Scholar] [CrossRef]
- Ries, J.; Agaimy, A.; Wehrhan, F.; Baran, C.; Bolze, S.; Danzer, E.; Frey, S.; Jantsch, J.; Möst, T.; Büttner-Herold, M.; et al. Importance of the PD-1/PD-L1 Axis for Malignant Transformation and Risk Assessment of Oral Leukoplakia. Biomedicines 2021, 9, 194. [Google Scholar] [CrossRef]
- Shi, Y.; Xie, T.X.; Leach, D.G.; Wang, B.; Young, S.; Osman, A.A.; Sikora, A.G.; Ren, X.; Hartgerink, J.D.; Myers, J.N.; et al. Local Anti-PD-1 Delivery Prevents Progression of Premalignant Lesions in a 4NQO-Oral Carcinogenesis Mouse Model. Cancer Prev. Res. 2021, 14, 767–778. [Google Scholar] [CrossRef]
- Monteiro de Oliveira Novaes, J.A.; Hirz, T.; Guijarro, I.; Nilsson, M.; Pisegna, M.A.; Poteete, A.; Barsoumian, H.B.; Fradette, J.J.; Chen, L.N.; Gibbons, D.L.; et al. Targeting of CD40 and PD-L1 Pathways Inhibits Progression of Oral Premalignant Lesions in a Carcinogen-induced Model of Oral Squamous Cell Carcinoma. Cancer Prev. Res. 2021, 14, 313–324. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, Z.; Mao, F.; Cai, L.; Dan, H.; Jiang, L.; Zeng, X.; Li, T.; Zhou, Y.; Chen, Q. PD-1 blockade prevents the progression of oral carcinogenesis. Carcinogenesis 2021, 42, 891–902. [Google Scholar] [CrossRef]
- Wang, J.; Xie, T.; Wang, B.; William, W.N., Jr.; Heymach, J.V.; El-Naggar, A.K.; Myers, J.N.; Caulin, C. PD-1 Blockade Prevents the Development and Progression of Carcinogen-Induced Oral Premalignant Lesions. Cancer Prev. Res. 2017, 10, 684–693. [Google Scholar] [CrossRef] [Green Version]
- Wei, F.; Wang, D.; Wei, J.; Tang, N.; Tang, L.; Xiong, F.; Guo, C.; Zhou, M.; Li, X.; Li, G.; et al. Metabolic crosstalk in the tumor microenvironment regulates antitumor immunosuppression and immunotherapy resisitance. Cell. Mol. Life Sci. CMLS 2021, 78, 173–193. [Google Scholar] [CrossRef]
- Boedtkjer, E.; Pedersen, S.F. The Acidic Tumor Microenvironment as a Driver of Cancer. Annu. Rev. Physiol. 2020, 82, 103–126. [Google Scholar] [CrossRef] [Green Version]
- Jayanth, V.R.; Bayne, M.T.; Varnes, M.E. Effects of extracellular and intracellular pH on repair of potentially lethal damage, chromosome aberrations and DNA double-strand breaks in irradiated plateau-phase A549 cells. Radiat. Res. 1994, 139, 152–162. [Google Scholar] [CrossRef]
- Flinck, M.; Kramer, S.H.; Pedersen, S.F. Roles of pH in control of cell proliferation. Acta Physiol. 2018, 223, e13068. [Google Scholar] [CrossRef] [PubMed]
- Gatenby, R.A.; Gawlinski, E.T. The glycolytic phenotype in carcinogenesis and tumor invasion: Insights through mathematical models. Cancer Res. 2003, 63, 3847–3854. [Google Scholar] [PubMed]
- Angadi, V.C.; Angadi, P.V. GLUT-1 immunoexpression in oral epithelial dysplasia, oral squamous cell carcinoma, and verrucous carcinoma. J. Oral Sci. 2015, 57, 115–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakazato, K.; Mogushi, K.; Kayamori, K.; Tsuchiya, M.; Takahashi, K.I.; Sumino, J.; Michi, Y.; Yoda, T.; Uzawa, N. Glucose metabolism changes during the development and progression of oral tongue squamous cell carcinomas. Oncol. Lett. 2019, 18, 1372–1380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gholizadeh, N.; Alipanahi Ramandi, M.; Motiee-Langroudi, M.; Jafari, M.; Sharouny, H.; Sheykhbahaei, N. Serum and salivary levels of lactate dehydrogenase in oral squamous cell carcinoma, oral lichen planus and oral lichenoid reaction. BMC Oral Health 2020, 20, 314. [Google Scholar] [CrossRef]
- Goetze, K.; Walenta, S.; Ksiazkiewicz, M.; Kunz-Schughart, L.A.; Mueller-Klieser, W. Lactate enhances motility of tumor cells and inhibits monocyte migration and cytokine release. Int. J. Oncol. 2011, 39, 453–463. [Google Scholar] [CrossRef]
- Colegio, O.R.; Chu, N.Q.; Szabo, A.L.; Chu, T.; Rhebergen, A.M.; Jairam, V.; Cyrus, N.; Brokowski, C.E.; Eisenbarth, S.C.; Phillips, G.M.; et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature 2014, 513, 559–563. [Google Scholar] [CrossRef]
- Sonveaux, P.; Copetti, T.; De Saedeleer, C.J.; Végran, F.; Verrax, J.; Kennedy, K.M.; Moon, E.J.; Dhup, S.; Danhier, P.; Frérart, F.; et al. Targeting the lactate transporter MCT1 in endothelial cells inhibits lactate-induced HIF-1 activation and tumor angiogenesis. PLoS ONE 2012, 7, e33418. [Google Scholar] [CrossRef]
- Yang, Q.; Sun, H.; Wang, X.; Yu, X.; Zhang, J.; Guo, B.; Hexige, S. Metabolic changes during malignant transformation in primary cells of oral lichen planus: Succinate accumulation and tumour suppression. J. Cell. Mol. Med. 2020, 24, 1179–1188. [Google Scholar] [CrossRef]
- Aizawa, H.; Yamada, S.I.; Xiao, T.; Shimane, T.; Hayashi, K.; Qi, F.; Tanaka, H.; Kurita, H. Difference in glycogen metabolism (glycogen synthesis and glycolysis) between normal and dysplastic/malignant oral epithelium. Arch. Oral Biol. 2017, 83, 340–347. [Google Scholar] [CrossRef] [Green Version]
- Behl, I.; Calado, G.; Malkin, A.; Flint, S.; Galvin, S.; Healy, C.M.; Pimentel, M.L.; Byrne, H.J.; Lyng, F.M. A pilot study for early detection of oral premalignant diseases using oral cytology and Raman micro-spectroscopy: Assessment of confounding factors. J. Biophotonics 2020, 13, e202000079. [Google Scholar] [CrossRef]
- Mehta, R.; Gurudath, S.; Dayansoor, S.; Pai, A.; Ganapathy, K.S. Serum lipid profile in patients with oral cancer and oral precancerous conditions. Dent. Res. J. 2014, 11, 345–350. [Google Scholar]
- Georgakopoulos-Soares, I.; Chartoumpekis, D.V.; Kyriazopoulou, V.; Zaravinos, A. EMT Factors and Metabolic Pathways in Cancer. Front. Oncol. 2020, 10, 499. [Google Scholar] [CrossRef]
- Chen, X.; Yi, C.; Yang, M.J.; Sun, X.; Liu, X.; Ma, H.; Li, Y.; Li, H.; Wang, C.; He, Y.; et al. Metabolomics study reveals the potential evidence of metabolic reprogramming towards the Warburg effect in precancerous lesions. J. Cancer 2021, 12, 1563–1574. [Google Scholar] [CrossRef]
- Wei, J.; Xie, G.; Ge, S.; Qiu, Y.; Liu, W.; Lu, A.; Chen, T.; Li, H.; Zhou, Z.; Jia, W. Metabolic transformation of DMBA-induced carcinogenesis and inhibitory effect of salvianolic acid b and breviscapine treatment. J. Proteome Res. 2012, 11, 1302–1316. [Google Scholar] [CrossRef]
- Yang, J.; Shi, X.; Yang, M.; Luo, J.; Gao, Q.; Wang, X.; Wu, Y.; Tian, Y.; Wu, F.; Zhou, H. Glycolysis reprogramming in cancer-associated fibroblasts promotes the growth of oral cancer through the lncRNA H19/miR-675-5p/PFKFB3 signaling pathway. Int. J. Oral Sci. 2021, 13, 12. [Google Scholar] [CrossRef]
- Pérez-Escuredo, J.; Van Hée, V.F.; Sboarina, M.; Falces, J.; Payen, V.L.; Pellerin, L.; Sonveaux, P. Monocarboxylate transporters in the brain and in cancer. Biochim. Biophys. Acta 2016, 1863, 2481–2497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cribbs, A.P.; Terlecki-Zaniewicz, S.; Philpott, M.; Baardman, J.; Ahern, D.; Lindow, M.; Obad, S.; Oerum, H.; Sampey, B.; Mander, P.K.; et al. Histone H3K27me3 demethylases regulate human Th17 cell development and effector functions by impacting on metabolism. Proc. Natl. Acad. Sci. USA 2020, 117, 6056–6066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hadden, M.; Mittal, A.; Samra, J.; Zreiqat, H.; Sahni, S.; Ramaswamy, Y. Mechanically stressed cancer microenvironment: Role in pancreatic cancer progression. Biochim. Et Biophys. Acta Rev. Cancer 2020, 1874, 188418. [Google Scholar] [CrossRef] [PubMed]
- Angadi, P.V.; Kale, A.D.; Hallikerimath, S. Evaluation of myofibroblasts in oral submucous fibrosis: Correlation with disease severity. J. Oral Pathol. Med. Off. Publ. Int. Assoc. Oral Pathol. Am. Acad. Oral Pathol. 2011, 40, 208–213. [Google Scholar] [CrossRef]
- Pogoda, K.; Cieśluk, M.; Deptuła, P.; Tokajuk, G.; Piktel, E.; Król, G.; Reszeć, J.; Bucki, R. Inhomogeneity of stiffness and density of the extracellular matrix within the leukoplakia of human oral mucosa as potential physicochemical factors leading to carcinogenesis. Transl. Oncol. 2021, 14, 101105. [Google Scholar] [CrossRef]
- McCarthy, J.B.; El-Ashry, D.; Turley, E.A. Hyaluronan, Cancer-Associated Fibroblasts and the Tumor Microenvironment in Malignant Progression. Front. Cell Dev. Biol. 2018, 6, 48. [Google Scholar] [CrossRef]
- Sharma, M.; Hunter, K.D.; Fonseca, F.P.; Shetty, S.S.; Radhakrishnan, R. Role of Yes-associated protein and transcriptional coactivator with PDZ-binding motif in the malignant transformation of oral submucous fibrosis. Arch. Oral Biol. 2021, 128, 105164. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, G.; Luo, X.; Qiu, J.; Tang, C. Substrate stiffness regulates the proliferation, migration, and differentiation of epidermal cells. Burn. J. Int. Soc. Burn. Inj. 2012, 38, 414–420. [Google Scholar] [CrossRef]
- Chaudhuri, P.K.; Low, B.C.; Lim, C.T. Mechanobiology of Tumor Growth. Chem. Rev. 2018, 118, 6499–6515. [Google Scholar] [CrossRef]
- Jones, J.O.; Moody, W.M.; Shields, J.D. Microenvironmental modulation of the developing tumour: An immune-stromal dialogue. Mol. Oncol. 2021, 15, 2600–2633. [Google Scholar] [CrossRef]
- Shieh, A.C.; Rozansky, H.A.; Hinz, B.; Swartz, M.A. Tumor cell invasion is promoted by interstitial flow-induced matrix priming by stromal fibroblasts. Cancer Res 2011, 71, 790–800. [Google Scholar] [CrossRef] [Green Version]
- Jin, M.Z.; Jin, W.L. The updated landscape of tumor microenvironment and drug repurposing. Signal Transduct. Target. Ther. 2020, 5, 166. [Google Scholar] [CrossRef]
- Zahalka, A.H.; Frenette, P.S. Nerves in cancer. Nat. Rev. Cancer 2020, 20, 143–157. [Google Scholar] [CrossRef]
- Cirillo, N. Role of tissue-specific steroid metabolism in oral disease: Is there any clinical implication? Oral Dis. 2018, 24, 224–227. [Google Scholar] [CrossRef]
- Dioufa, N.; Farmaki, E.; Schally, A.V.; Kiaris, H.; Vlahodimitropoulos, D.; Papavassiliou, A.G.; Kittas, C.; Block, N.L.; Chatzistamou, I. Growth hormone-releasing hormone receptor splice variant 1 is frequently expressed in oral squamous cell carcinomas. Horm. Cancer 2012, 3, 172–180. [Google Scholar] [CrossRef]
- Santacroce, L.; Di Cosola, M.; Bottalico, L.; Topi, S.; Charitos, I.A.; Ballini, A.; Inchingolo, F.; Cazzolla, A.P.; Dipalma, G. Focus on HPV Infection and the Molecular Mechanisms of Oral Carcinogenesis. Viruses 2021, 13, 559. [Google Scholar] [CrossRef]
- Rathod, S.; Rathi, P.; Harkare, V. Trace elements as a diagnostic biomarker for premalignant lesions and malignant conditions. SRM J. Res. Dent. Sci. 2019, 10, 40. [Google Scholar] [CrossRef]
- Gottfried, E.; Kunz-Schughart, L.A.; Ebner, S.; Mueller-Klieser, W.; Hoves, S.; Andreesen, R.; Mackensen, A.; Kreutz, M. Tumor-derived lactic acid modulates dendritic cell activation and antigen expression. Blood 2006, 107, 2013–2021. [Google Scholar] [CrossRef]
- Mondanelli, G.; Ugel, S.; Grohmann, U.; Bronte, V. The immune regulation in cancer by the amino acid metabolizing enzymes ARG and IDO. Curr. Opin. Pharmacol. 2017, 35, 30–39. [Google Scholar] [CrossRef]
- Finlay, D.K.; Rosenzweig, E.; Sinclair, L.V.; Feijoo-Carnero, C.; Hukelmann, J.L.; Rolf, J.; Panteleyev, A.A.; Okkenhaug, K.; Cantrell, D.A. PDK1 regulation of mTOR and hypoxia-inducible factor 1 integrate metabolism and migration of CD8+ T cells. J. Exp. Med. 2012, 209, 2441–2453. [Google Scholar] [CrossRef] [Green Version]
- Vats, D.; Mukundan, L.; Odegaard, J.I.; Zhang, L.; Smith, K.L.; Morel, C.R.; Wagner, R.A.; Greaves, D.R.; Murray, P.J.; Chawla, A. Oxidative metabolism and PGC-1beta attenuate macrophage-mediated inflammation. Cell Metab. 2006, 4, 13–24. [Google Scholar] [CrossRef] [Green Version]
- Zanotelli, M.R.; Zhang, J.; Reinhart-King, C.A. Mechanoresponsive metabolism in cancer cell migration and metastasis. Cell Metab. 2021, 33, 1307–1321. [Google Scholar] [CrossRef] [PubMed]
- LeBleu, V.S.; O’Connell, J.T.; Gonzalez Herrera, K.N.; Wikman, H.; Pantel, K.; Haigis, M.C.; de Carvalho, F.M.; Damascena, A.; Domingos Chinen, L.T.; Rocha, R.M.; et al. PGC-1α mediates mitochondrial biogenesis and oxidative phosphorylation in cancer cells to promote metastasis. Nat. Cell Biol. 2014, 16, 1–15, 992–1003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brassart-Pasco, S.; Brézillon, S.; Brassart, B.; Ramont, L.; Oudart, J.B.; Monboisse, J.C. Tumor Microenvironment: Extracellular Matrix Alterations Influence Tumor Progression. Front. Oncol. 2020, 10, 397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santinon, G.; Brian, I.; Pocaterra, A.; Romani, P.; Franzolin, E.; Rampazzo, C.; Bicciato, S.; Dupont, S. dNTP metabolism links mechanical cues and YAP/TAZ to cell growth and oncogene-induced senescence. EMBO J. 2018, 37, e97780. [Google Scholar] [CrossRef] [PubMed]
- Aghlara-Fotovat, S.; Nash, A.; Kim, B.; Krencik, R.; Veiseh, O. Targeting the extracellular matrix for immunomodulation: Applications in drug delivery and cell therapies. Drug Deliv. Transl. Res. 2021, 11, 2394–2413. [Google Scholar] [CrossRef]
- Winkler, J.; Abisoye-Ogunniyan, A.; Metcalf, K.J.; Werb, Z. Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nat. Commun. 2020, 11, 5120. [Google Scholar] [CrossRef]
- Madsen, D.H.; Jürgensen, H.J.; Siersbæk, M.S.; Kuczek, D.E.; Grey Cloud, L.; Liu, S.; Behrendt, N.; Grøntved, L.; Weigert, R.; Bugge, T.H. Tumor-Associated Macrophages Derived from Circulating Inflammatory Monocytes Degrade Collagen through Cellular Uptake. Cell Rep. 2017, 21, 3662–3671. [Google Scholar] [CrossRef] [Green Version]
- Barbazán, J.; Matic Vignjevic, D. Cancer associated fibroblasts: Is the force the path to the dark side? Curr. Opin. Cell Biol. 2019, 56, 71–79. [Google Scholar] [CrossRef]
- Qiao, G.; Bucsek, M.J.; Winder, N.M.; Chen, M.; Giridharan, T.; Olejniczak, S.H.; Hylander, B.L.; Repasky, E.A. β-Adrenergic signaling blocks murine CD8(+) T-cell metabolic reprogramming during activation: A mechanism for immunosuppression by adrenergic stress. Cancer Immunol. Immunother. CII 2019, 68, 11–22. [Google Scholar] [CrossRef] [Green Version]
- Zahalka, A.H.; Arnal-Estapé, A.; Maryanovich, M.; Nakahara, F.; Cruz, C.D.; Finley, L.W.S.; Frenette, P.S. Adrenergic nerves activate an angio-metabolic switch in prostate cancer. Science 2017, 358, 321–326. [Google Scholar] [CrossRef] [Green Version]
- Kim-Fuchs, C.; Le, C.P.; Pimentel, M.A.; Shackleford, D.; Ferrari, D.; Angst, E.; Hollande, F.; Sloan, E.K. Chronic stress accelerates pancreatic cancer growth and invasion: A critical role for beta-adrenergic signaling in the pancreatic microenvironment. Brain Behav. Immun. 2014, 40, 40–47. [Google Scholar] [CrossRef] [Green Version]
- Keshavarz-Fathi, M.; Rezaei, N. Cancer Immunoprevention: Current Status and Future Directions. Arch. Immunol. Ther. Exp. 2021, 69, 3. [Google Scholar] [CrossRef]
- Young, M.R.I. Redirecting the focus of cancer immunotherapy to premalignant conditions. Cancer Lett. 2017, 391, 83–88. [Google Scholar] [CrossRef]
- Young, M.R. Use of carcinogen-induced premalignant oral lesions in a dendritic cell-based vaccine to stimulate immune reactivity against both premalignant oral lesions and oral cancer. J. Immunother. 2008, 31, 148–156. [Google Scholar] [CrossRef] [Green Version]
- Dhodapkar, M.V.; Sexton, R.; Das, R.; Dhodapkar, K.M.; Zhang, L.; Sundaram, R.; Soni, S.; Crowley, J.J.; Orlowski, R.Z.; Barlogie, B. Prospective analysis of antigen-specific immunity, stem-cell antigens, and immune checkpoints in monoclonal gammopathy. Blood 2015, 126, 2475–2478. [Google Scholar] [CrossRef] [Green Version]
- Gurizzan, C.; Lorini, L.; Paderno, A.; Tomasoni, M.; Zigliani, G.; Bozzola, A.; Ardighieri, L.; Battocchio, S.; Bignotti, E.; Ravaggi, A.; et al. Immunotherapy for the prevention of high-risk oral disorders malignant transformation: The IMPEDE trial. BMC Cancer 2021, 21, 561. [Google Scholar] [CrossRef]
- Bhatia, S.; Tykodi, S.S.; Thompson, J.A. Treatment of metastatic melanoma: An overview. Oncology 2009, 23, 488–496. [Google Scholar]
- Johnson, S.D.; Young, M.R. Indomethacin Treatment of Mice with Premalignant Oral Lesions Sustains Cytokine Production and Slows Progression to Cancer. Front. Immunol. 2016, 7, 379. [Google Scholar] [CrossRef] [Green Version]
- Wei, J.; Wu, J.; Xu, W.; Nie, H.; Zhou, R.; Wang, R.; Liu, Y.; Tang, G.; Wu, J. Salvianolic acid B inhibits glycolysis in oral squamous cell carcinoma via targeting PI3K/AKT/HIF-1α signaling pathway. Cell Death Dis. 2018, 9, 599. [Google Scholar] [CrossRef] [Green Version]
- Ulrich, C.; Johannsen, A.; Röwert-Huber, J.; Ulrich, M.; Sterry, W.; Stockfleth, E. Results of a randomized, placebo-controlled safety and efficacy study of topical diclofenac 3% gel in organ transplant patients with multiple actinic keratoses. Eur. J. Dermatol. EJD 2010, 20, 482–488. [Google Scholar] [CrossRef]
- Ghanghas, P.; Jain, S.; Rana, C.; Sanyal, S.N. Chemopreventive action of non-steroidal anti-inflammatory drugs on the inflammatory pathways in colon cancer. Biomed. Pharmacother. 2016, 78, 239–247. [Google Scholar] [CrossRef]
- Malekpour-Dehkordi, Z.; Teimourian, S.; Nourbakhsh, M.; Naghiaee, Y.; Sharifi, R.; Mohiti-Ardakani, J. Metformin reduces fibrosis factors in insulin resistant and hypertrophied adipocyte via integrin/ERK, collagen VI, apoptosis, and necrosis reduction. Life Sci. 2019, 233, 116682. [Google Scholar] [CrossRef]
- Vitale-Cross, L.; Molinolo, A.A.; Martin, D.; Younis, R.H.; Maruyama, T.; Patel, V.; Chen, W.; Schneider, A.; Gutkind, J.S. Metformin prevents the development of oral squamous cell carcinomas from carcinogen-induced premalignant lesions. Cancer Prev. Res. 2012, 5, 562–573. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Yang, J.; Wei, C.; Zhou, G.; Wu, L.; Gao, Q.; He, X.; Shi, J.; Mei, Y.; Liu, Y.; et al. A personalized computational model predicts cancer risk level of oral potentially malignant disorders and its web application for promotion of non-invasive screening. J. Oral Patho. 2020, 49, 417–426. [Google Scholar] [CrossRef]
- Sarode, G.S.; Sarode, S.C.; Maniyar, N.; Sharma, N.; Yerwadekar, S.; Patil, S. Recent trends in predictive biomarkers for determining malignant potential of oral potentially malignant disorders. Oncol. Rev. 2019, 13, 424. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.; Gao, X.; Liu, W. Bibliometric analysis of the top-100 cited articles on oral potentially malignant disorders to guide research topic and direction. J. Dent. Sci. 2020, 15, 479–485. [Google Scholar] [CrossRef]
- Gurizzan, C.; Lorini, L.; Bossi, P. Oral potentially malignant disorders: New insights for future treatment. Curr. Opin. Otolaryngol. Head Neck Surg. 2021, 29, 138–142. [Google Scholar] [CrossRef]

| Drug Type | Drug | OPMDs Type | Result | Status | NCT Number | Last Update Date |
|---|---|---|---|---|---|---|
| mAb | Avelumab | OPMDs with dysplasia | No results posted. | Phase II; recruiting | NCT04504552 | 2020 |
| Sintilimab | High-risk OPMDs | No results posted. | Phase II; not yet recruiting | NCT04065737 | 2019 | |
| Nivolumab | OVH | No results posted. | Phase II; active, not recruiting | NCT03692325 | 2021 | |
| Pembrolizumab | OLK; OEL and OVH | No results posted. | Phase II; recruiting | NCT03603223 | 2021 | |
| Celecoxib | OLK | Could not prevent the disease progression. | Phase II; completed | NCT00101335 | 2013 | |
| Celecoxib | OPMDs with dysplasia | Prevented oral cancer from forming. | Phase II; completed | NCT00014404 | 2013 | |
| Celecoxib | OLK with dysplasia | No results posted. | Phase II; completed | NCT00052611 | 2018 | |
| Celecoxib | OPMDs | No results posted. | Phase II; completed | NCT00036283 | 2008 | |
| Vaccine | Hespecta vaccination | HPV16-positive OPMDs | No results posted. | Phase I; completed | NCT02821494 | 2021 |
| Plant extract and mAb | Green tea polyphenon E and erlotinib | OPMDs with dysplasia | No results posted. | Phase I; completed | NCT01116336 | 2018 |
| Plant extract | Bowman–Birk inhibitor Concentrate | OLK | Prevented oral cancer from forming. | Phase II; completed | NCT00330382 | 2014 |
| Plant | Green tea | OLK | No results posted. | Phase II; terminated | NCT00176566 | 2009 |
| Hypoglycemic drug | Metformin hydrochloride | OLK and OEL with dysplasia | Prevented oral cancer from forming. | Phase II; completed | NCT02581137 | 2021 |
| Pioglitazone hydrochloride | OLK | No significant effect. | Phase II; terminated | NCT00951379 | 2016 | |
| Rosiglitazone | OLK and OEL | Prevented oral cancer from forming. | Phase II; completed | NCT00369174 | 2013 | |
| Pioglitazone hydrochloride | OLK with dysplasia | Prevented oral cancer from forming. | Phase II; completed | NCT00099021 | 2019 | |
| TKI | Vandetanib | OPMDs with dysplasia | Prevented oral cancer from forming. | Phase II; completed | NCT01414426 | 2021 |
| EGFR and COX-2 inhibitor | OPMDs | No significant effect. | Phase I/II; completed | NCT00314262 | 2014 | |
| Analgesic–antipyretic | Aspirin mouthwash | OLK | Prevented oral cancer from forming. | Phase I; completed | NCT01238185 | 2013 |
| Sulindac | OLK and OEL | No results posted. | Not applicable; has results | NCT00299195 | 2020 | |
| Vitamin A derivative | SBS-101/Isotretinoin oral-adhesive film | OLK and OEL | No results posted. | Phase I; withdrawn | NCT03939364 | 2021 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, S.; Wang, S.; Shi, X.; Zhou, H. Microenvironment in Oral Potentially Malignant Disorders: Multi-Dimensional Characteristics and Mechanisms of Carcinogenesis. Int. J. Mol. Sci. 2022, 23, 8940. https://doi.org/10.3390/ijms23168940
Deng S, Wang S, Shi X, Zhou H. Microenvironment in Oral Potentially Malignant Disorders: Multi-Dimensional Characteristics and Mechanisms of Carcinogenesis. International Journal of Molecular Sciences. 2022; 23(16):8940. https://doi.org/10.3390/ijms23168940
Chicago/Turabian StyleDeng, Shuzhi, Shimeng Wang, Xueke Shi, and Hongmei Zhou. 2022. "Microenvironment in Oral Potentially Malignant Disorders: Multi-Dimensional Characteristics and Mechanisms of Carcinogenesis" International Journal of Molecular Sciences 23, no. 16: 8940. https://doi.org/10.3390/ijms23168940
APA StyleDeng, S., Wang, S., Shi, X., & Zhou, H. (2022). Microenvironment in Oral Potentially Malignant Disorders: Multi-Dimensional Characteristics and Mechanisms of Carcinogenesis. International Journal of Molecular Sciences, 23(16), 8940. https://doi.org/10.3390/ijms23168940






