Tomato Root Colonization by Exogenously Inoculated Arbuscular Mycorrhizal Fungi Induces Resistance against Root-Knot Nematodes in a Dose-Dependent Manner
Abstract
:1. Introduction
2. Results
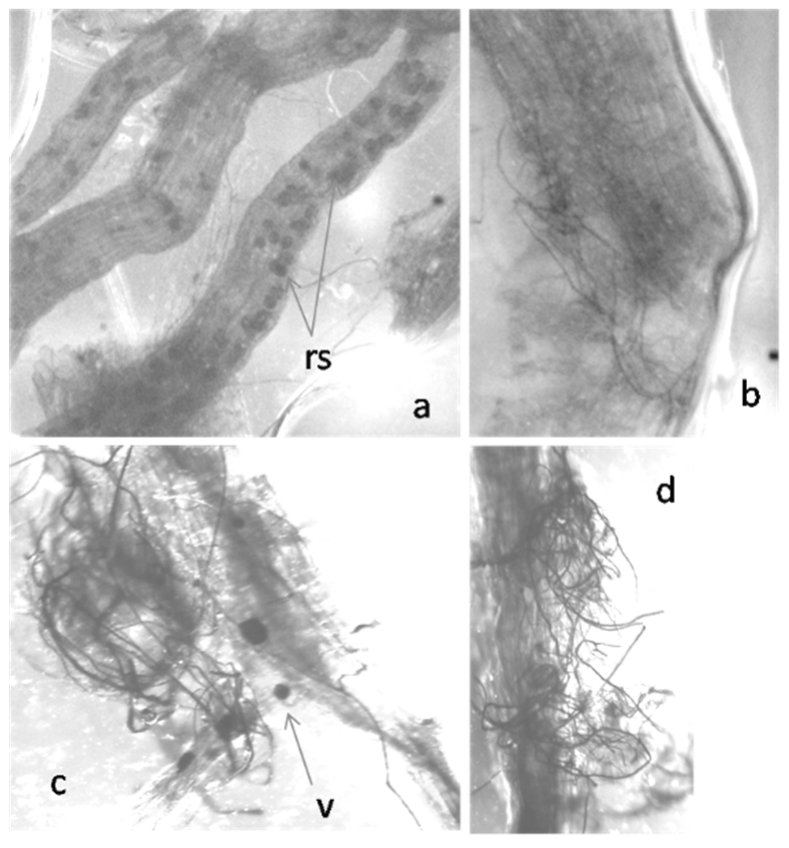
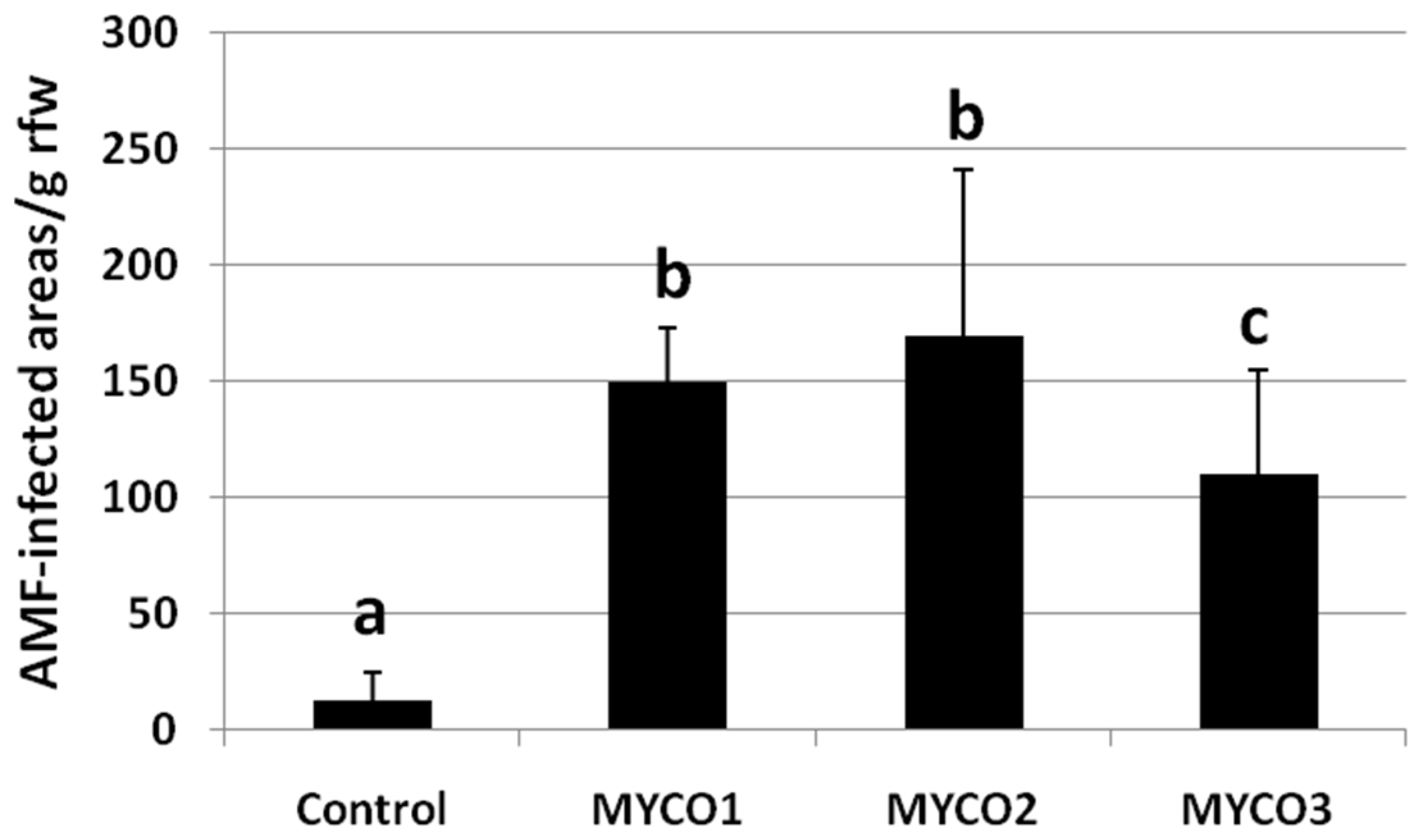
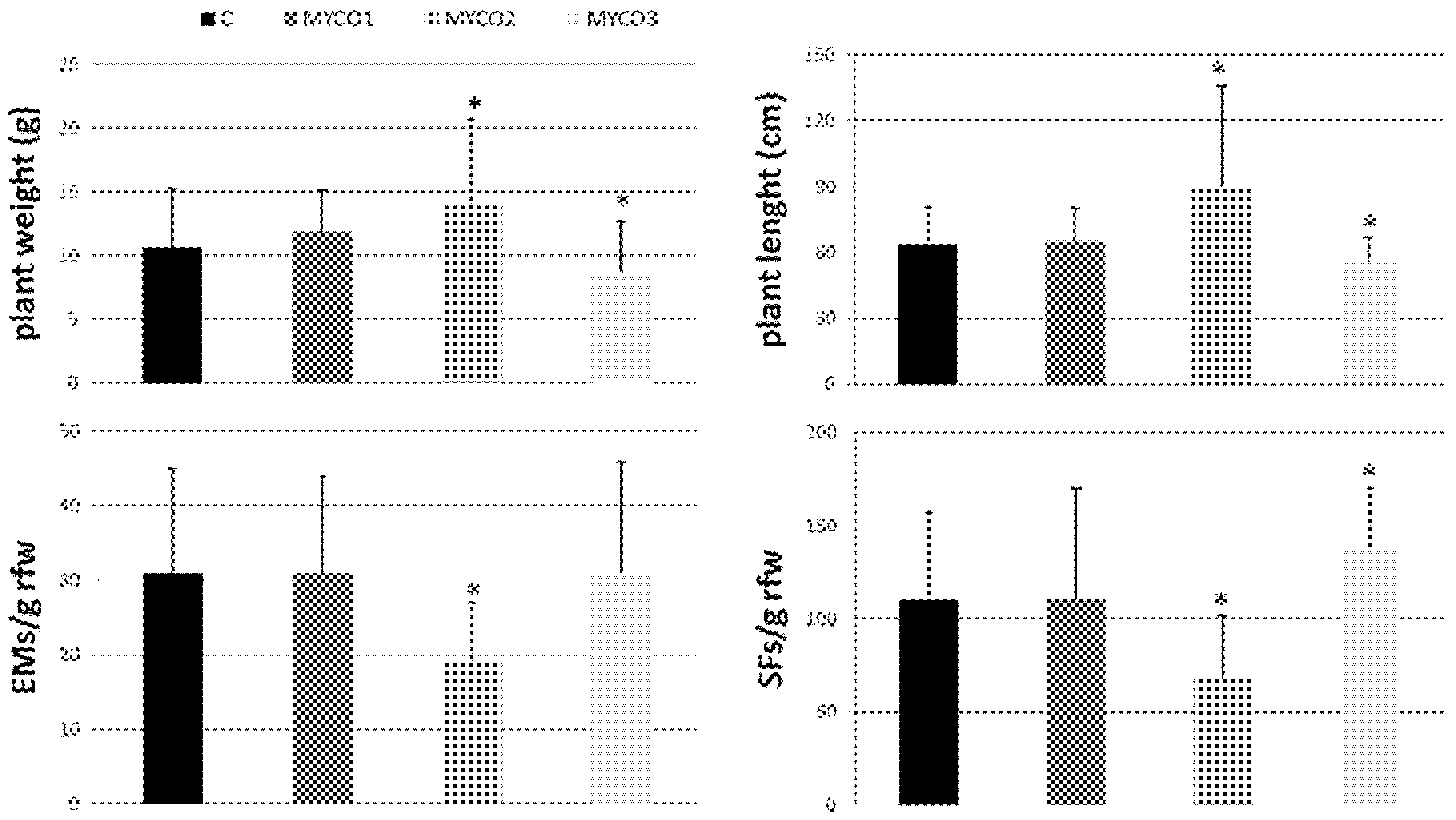
2.1. Colonization of Exogenously Added AMF to Tomato Roots
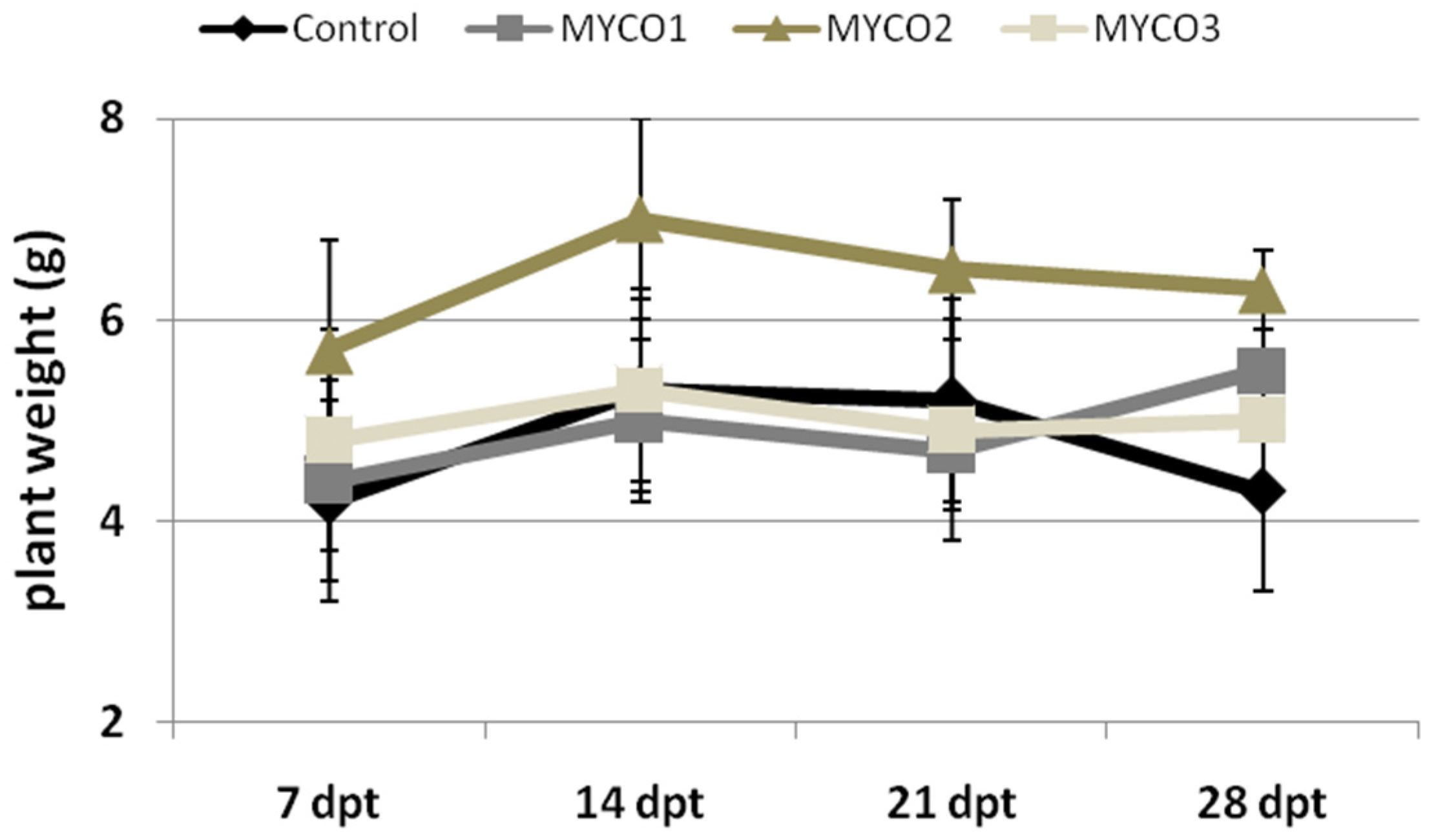
2.2. Myco Treatments to Tomato Roots Induces Resistance to RKNs in a Dose-Dependent Manner
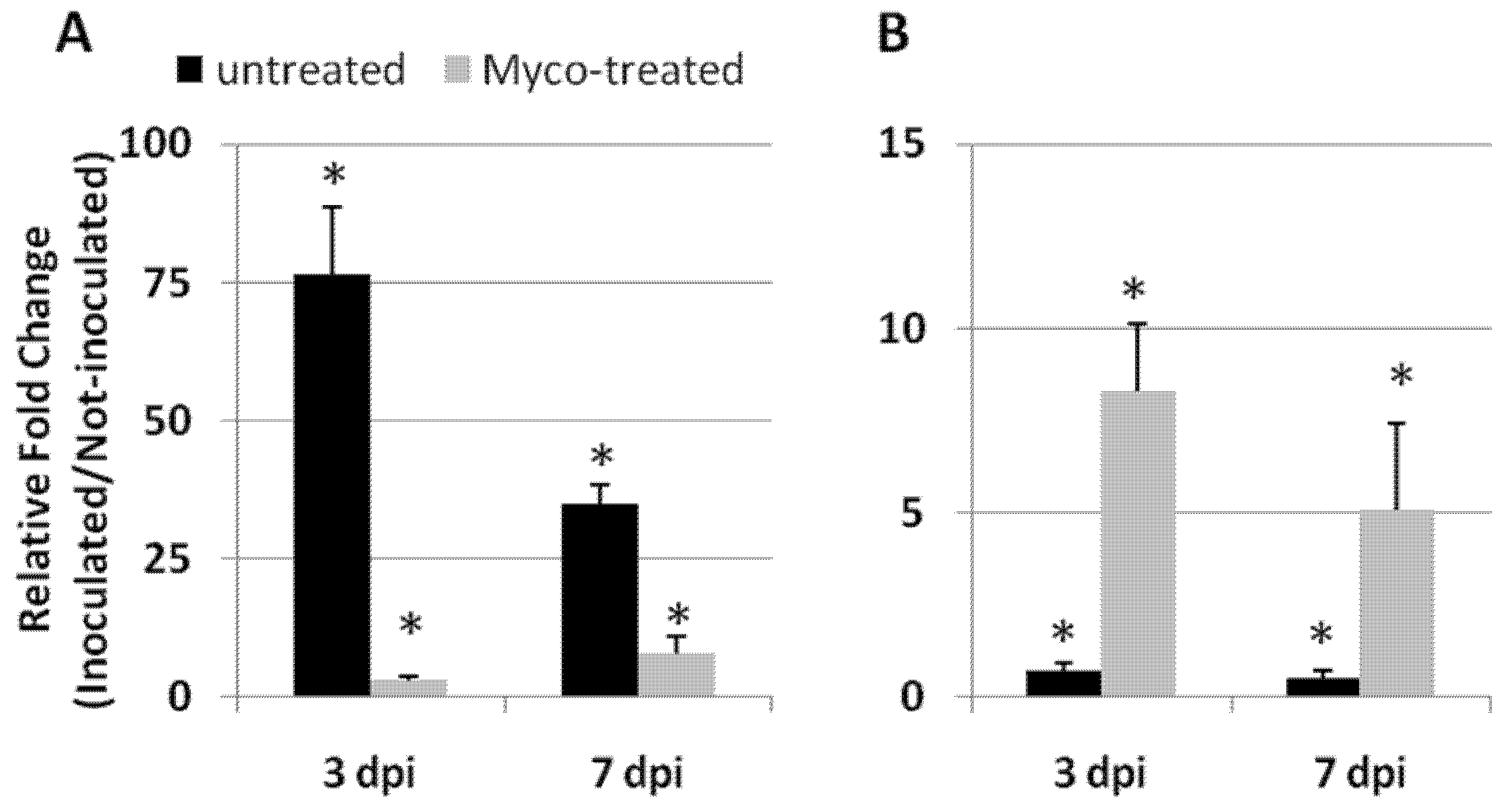
2.3. Resistance Induction against RKNs Is Mediated by AMF Colonization
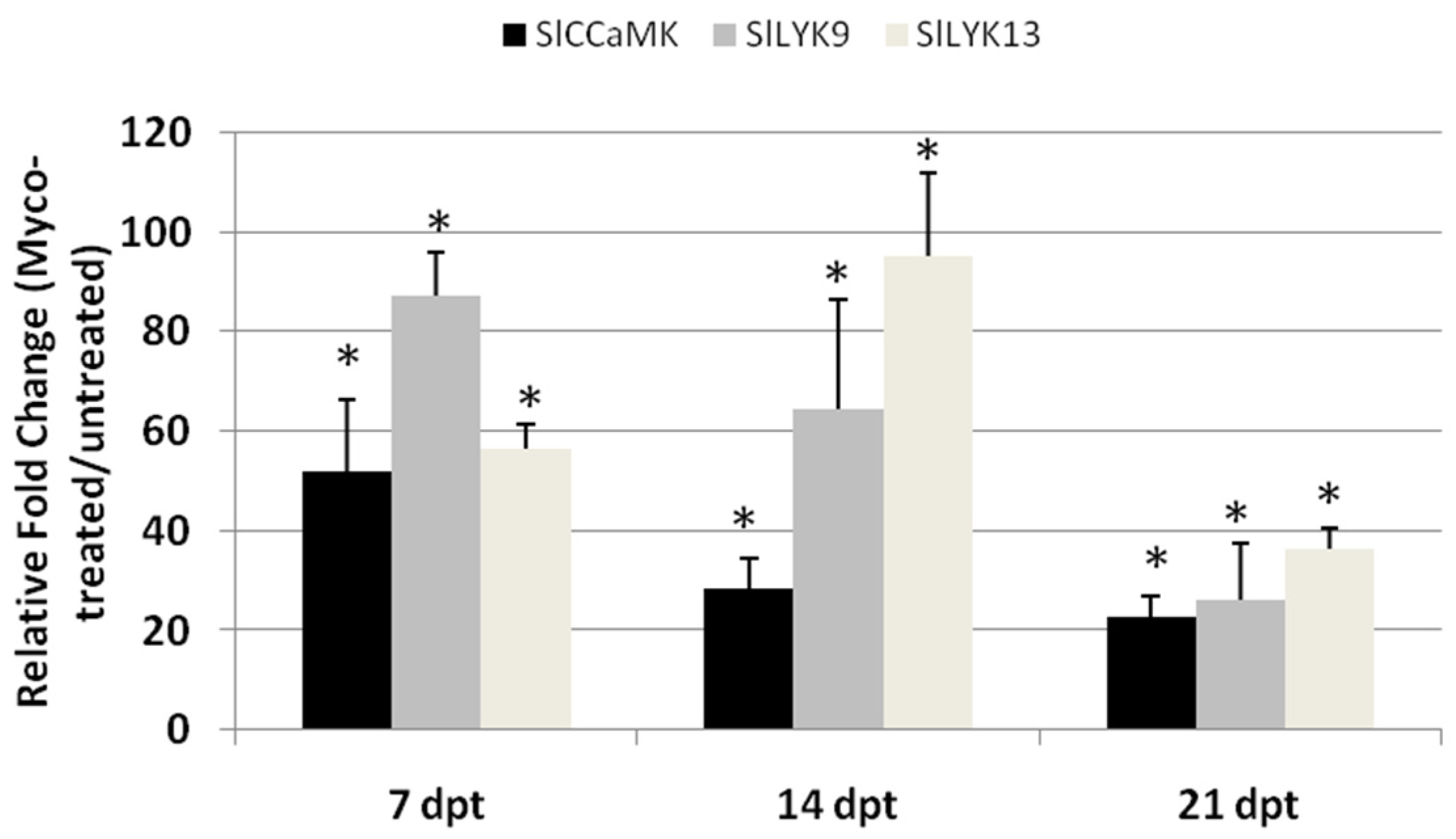
2.4. AMF-Mediated Plant Immunity against RKNs
3. Discussion
4. Materials and Methods
4.1. Treatment of Plants with Arbuscular Mycorrhizal Fungi
4.2. Root Colonization
4.3. Nematode Inoculations and Infection Level Determination
4.4. RNA Extraction, cDNA Synthesis, and Quantitative Real-Time Polymerase Chain Reaction
4.5. Experimental Design and Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zipfel, C.; Oldoryd, G.E.D. Plant signalling in symbiosis and immunity. Nature 2017, 543, 328–336. [Google Scholar] [CrossRef] [PubMed]
- El-Sappah, A.H.; Islam, M.M.; El-Awady, H.H.; Yan, S.; Qi, S.; Liu, J.; Cheng, G.-T.; Liang, Y. Tomato natural resistance genes in controlling the root-knot nematode. Genes 2019, 10, 925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hewezi, T.; Baum, T.J. Gene silencing in nematode feeding sites. Adv. Bot. Res. 2015, 73, 221–239. [Google Scholar] [CrossRef]
- Molinari, S.; Leonetti, P. Bio-control agents activate plant immune response and prime susceptible tomato against root-knot nematodes. PLoS ONE 2019, 14, e0213230. [Google Scholar] [CrossRef] [PubMed]
- Molinari, S. Systemic acquired resistance activation in Solanaceous crops as a management strategy against root-knot nematodes. Pest Manag. Sci. 2016, 72, 888–896. [Google Scholar] [CrossRef]
- Molinari, S.; Baser, N. Induction of resistance to root-knot nematodes by SAR elicitors in tomato. Crop Prot. 2010, 29, 1354–1362. [Google Scholar] [CrossRef]
- Molinari, S.; Fanelli, E.; Leonetti, P. Expression of tomato salicylic acid (SA)-responsive pathogenesis-related genes in Mi-1-mediated and SA-induced resistance to root-knot nematodes. Mol. Plant Pathol. 2014, 15, 255–264. [Google Scholar] [CrossRef]
- Pozo, M.J.; Azcón-Aguilar, C. Unravelling mycorrhiza-induced resistance. Curr. Opin. Plant Biol. 2007, 10, 393–398. [Google Scholar] [CrossRef]
- Berruti, A.; Lumini, E.; Balestrini, R.; Bianciotto, V. Arbuscular mycorrhizal fungi as natural biofertilizer: Let’s benefit from past successes. Front. Microbiol. 2016, 6, 1559. [Google Scholar] [CrossRef] [Green Version]
- Poveda, J.; Abril-Urias, P.; Escobar, C. Biological control of plant-parasitic nematodes by filamentous fungi inducers of resistance: Trichoderma, Mycorrhizal and Endophytic fungi. Front. Microbiol. 2020, 11, 992. [Google Scholar] [CrossRef]
- Vos, C.; Schouteden, N.; van Tuinenb, D.; Chatagnier, O.; Elsenc, A.; De Waele, D.; Panisa, B.; Gianinazzi-Pearson, V. Mycorrhiza-induced resistance against the root–knot nematode Meloidogyne incognita involves priming of defense gene responses in tomato. Soil Biol. Biochem. 2013, 60, 45–54. [Google Scholar] [CrossRef]
- Schouteden, N.; De Waele, D.; Panis, B.; Vos, C.M. Arbuscular mycorrhizal fungi for the biocontrol of plant-parasitic nematodes: A review of the mechanisms involved. Front. Microbiol. 2015, 6, 1280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hart, M.M.; Trevors, J.T. Microbe management: Application of mycorrhizal fungi in sustainable agriculture. Front Ecol. Environ. 2005, 3, 533–539. [Google Scholar] [CrossRef]
- Molinari, S. Factors determining the variability of performance of bio-control agents against root-knot nematodes in vegetable plants. Agronomy 2021, 11, 1602. [Google Scholar] [CrossRef]
- Buendia, L.; Wang, T.; Girardin, A.; Lefebvre, B. The LysM receptor-like kinase SlLYK10 regulates the arbuscular mycorrhizal symbiosis in tomato. New Phytol. 2016, 210, 184–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeom, S.; Baek, H.; Oh, S.; Kang, W.; Lee, S.J.; Lee, J.M.; Seo, E.; Rose, J.K.C.; Kim, B.; Choi, D. Use of a secretion trap screen in pepper following Phytophthora capsici infection reveals novel functions of secreted plant proteins in modulating cell death. Mol. Plant-Microbe Interact. 2011, 24, 671–684. [Google Scholar] [CrossRef] [Green Version]
- Miao, Y.; Lv, D.; Wang, P.; Wang, X.C.; Chen, J.; Miao, C.; Song, C.P. An Arabidopsis glutathione peroxidase functions as both a redox transducer and a scavenger in abscisic acid and drought stress responses. Plant Cell 2006, 18, 2749–2766. [Google Scholar] [CrossRef] [Green Version]
- Hwang, I.S.; Choi, D.S.; Kim, N.H.; Kim, D.S.; Hwang, B.K. Pathogenesis-related protein 4b interacts with leucine-rich repeat protein 1 to suppress PR4b-triggered cell death and defense response in pepper. Plant J. 2014, 77, 521–533. [Google Scholar] [CrossRef]
- Tommerup, I.S. Methods for the study of the population biology of vesicular-arbuscular mycorrhizal fungi. In Methods in Microbiology; Techniques for the study of mycorrhiza; Norris, J.R., Read, D.J., Varma, A.K., Eds.; Academic Press: London, UK, 1992; Volume 24, pp. 1–21. [Google Scholar]
- Dodd, J.C.; Thomson, B.D. The screening and selection of inoculant arbuscular-mychorrizal and ectomycorrhizal fungi. Plant Soil 1994, 159, 149–158. [Google Scholar] [CrossRef]
- Calvet, C.; Pinochet, J.; Hernández-Dorrego, A.; Estaún, V.; Camprubí, A. Field microplot performance of the peach-almond hybrid GF-677 after inoculation with arbuscular mycorrhizal fungi in a replant soil infested with root-knot nematodes. Mycorrhiza 2001, 10, 295–300. [Google Scholar] [CrossRef]
- Jarecki, M.K.; Chong, C.; Voroney, R.P. Evaluation of compost leachates for plant growth in hydroponic culture. J. Plant Nutr. 2005, 28, 651–667. [Google Scholar] [CrossRef]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−∆∆Ct method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]

| Plant Weight (g) | EMs/g rfw | SFs/g rfw | ||||
|---|---|---|---|---|---|---|
| Cntr | Myco2 | Cntr | Myco2 | Cntr | Myco2 | |
| MY | 15.4 ± 3.9 ab | 18.2 ± 2.5 a | 52 ± 15 a | 32 ± 10 b | 277 ± 81 a | 173 ± 28 c |
| MYAut | 16.4 ± 3.1 ab | 17.3 ± 4.4 ab | 58 ± 11 a | 66 ± 22 a | 173 ± 73 c | 214 ± 88 bc |
| MYAMPHB | 14.4 ± 3.2 b | 14.6 ± 3.1 b | 51 ± 20 a | 55 ± 22 a | 189 ± 48 bc | 243 ± 78 ab |
| Gene Acronym | Primer Sequence (5′-3′) |
|---|---|
| SlCCaMK | F: CATGGGTGAGGGGAGAGTTA R: CATAGCTGCTGCACGAAACTT |
| SlLYK9 | F: TGAGCAATTCAATCCATGTCA R: ATGGGGATAAAAGGGGTTG |
| SlLYK13 | F: ACTTCTTCTCAAATTGCACAACA R: AAGGGAGATTCGATTCGGGC |
| SlGPX | F: GTTTGCTTGCACACGGTTTA R: CGTCGTTGGTGGATACCTCT |
| SlPR-4b/P2 | F: TGACCAACACAGGAACAGGA R: GCCCAATCCATTAGTGTCCA |
| ACT-7 | F: CAGCAGATGTGGATCTCAAA R: CTGTGGACAATGGAAGGAC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molinari, S.; Akbarimotlagh, M.; Leonetti, P. Tomato Root Colonization by Exogenously Inoculated Arbuscular Mycorrhizal Fungi Induces Resistance against Root-Knot Nematodes in a Dose-Dependent Manner. Int. J. Mol. Sci. 2022, 23, 8920. https://doi.org/10.3390/ijms23168920
Molinari S, Akbarimotlagh M, Leonetti P. Tomato Root Colonization by Exogenously Inoculated Arbuscular Mycorrhizal Fungi Induces Resistance against Root-Knot Nematodes in a Dose-Dependent Manner. International Journal of Molecular Sciences. 2022; 23(16):8920. https://doi.org/10.3390/ijms23168920
Chicago/Turabian StyleMolinari, Sergio, Masoud Akbarimotlagh, and Paola Leonetti. 2022. "Tomato Root Colonization by Exogenously Inoculated Arbuscular Mycorrhizal Fungi Induces Resistance against Root-Knot Nematodes in a Dose-Dependent Manner" International Journal of Molecular Sciences 23, no. 16: 8920. https://doi.org/10.3390/ijms23168920
APA StyleMolinari, S., Akbarimotlagh, M., & Leonetti, P. (2022). Tomato Root Colonization by Exogenously Inoculated Arbuscular Mycorrhizal Fungi Induces Resistance against Root-Knot Nematodes in a Dose-Dependent Manner. International Journal of Molecular Sciences, 23(16), 8920. https://doi.org/10.3390/ijms23168920







