Rationale and Clinical Research Progress on PD-1/PD-L1-Based Immunotherapy for Metastatic Triple-Negative Breast Cancer
Abstract
:1. Introduction
2. Immune Checkpoint and Immune Checkpoint Blockade Therapy
3. PD-1/PD-L1 Inhibitors Currently Used for Clinical Treatment
3.1. PD-1 Inhibitors
3.2. PD-L1 Inhibitors
4. Monotherapy with PD-1/PD-L1 Inhibitors
5. Combination Therapy with PD-1/PD-L1 Inhibitors
5.1. Combination with Chemotherapy Drugs
5.2. Combination with Small Molecule Inhibitors
6. New Targets for Immunotherapy
7. Adverse Events Associated with PD-1/PD-L1 Inhibitors
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Azamjah, N.; Soltan-Zadeh, Y.; Zayeri, F. Global Trend of Breast Cancer Mortality Rate: A 25-Year Study. Asian Pac. J. Cancer Prev. APJCP 2019, 20, 2015–2020. [Google Scholar] [CrossRef]
- Kumar, P.; Aggarwal, R. An overview of triple-negative breast cancer. Arch. Gynecol. Obstet. 2016, 293, 247–269. [Google Scholar] [CrossRef]
- Xiao, Y.; Gao, W. Therapeutic pattern and progress of neoadjuvant treatment for triple-negative breast cancer. Oncol. Lett. 2022, 24, 219. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Z.; Hu, X.; Wang, B.; Wang, L.; Yang, W.; Liu, Y.; Liu, G.; Di, G.; Hu, Z.; et al. Cisplatin and gemcitabine as the first line therapy in metastatic triple negative breast cancer. Int. J. Cancer 2015, 136, 204–211. [Google Scholar] [CrossRef]
- Ge, J.; Zuo, W.; Chen, Y.; Shao, Z.; Yu, K. The advance of adjuvant treatment for triple-negative breast cancer. Cancer Biol. Med. 2021, 19, 187–201. [Google Scholar] [CrossRef]
- Nouri Rouzbahani, F.; Shirkhoda, M.; Memari, F.; Dana, H.; Mahmoodi Chalbatani, G.; Mahmoodzadeh, H.; Samarghandi, N.; Gharagozlou, E.; Mohammadi Hadloo, M.H.; Maleki, A.R.; et al. Immunotherapy a New Hope for Cancer Treatment: A Review. Pak. J. Biol. Sci. 2018, 21, 135–150. [Google Scholar] [CrossRef] [Green Version]
- Kitano, A.; Ono, M.; Yoshida, M.; Noguchi, E.; Shimomura, A.; Shimoi, T.; Kodaira, M.; Yunokawa, M.; Yonemori, K.; Shimizu, C.; et al. Tumour-infiltrating lymphocytes are correlated with higher expression levels of PD-1 and PD-L1 in early breast cancer. ESMO Open 2017, 2, 000150. [Google Scholar] [CrossRef] [Green Version]
- Mori, H.; Kubo, M.; Yamaguchi, R.; Nishimura, R.; Osako, T.; Arima, N.; Okumura, Y.; Okido, M.; Yamada, M.; Kai, M.; et al. The combination of PD-L1 expression and decreased tumor-infiltrating lymphocytes is associated with a poor prognosis in triple-negative breast cancer. Oncotarget 2017, 8, 15584–15592. [Google Scholar] [CrossRef] [Green Version]
- Loi, S.; Michiels, S.; Salgado, R.; Sirtaine, N.; Jose, V.; Fumagalli, D.; Kellokumpu-Lehtinen, P.L.; Bono, P.; Kataja, V.; Desmedt, C.; et al. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: Results from the FinHER trial. Ann. Oncol. 2014, 25, 1544–1550. [Google Scholar] [CrossRef]
- Lee, S.E.; Park, H.Y.; Lim, S.D.; Han, H.S.; Yoo, Y.B.; Kim, W.S. Concordance of Programmed Death-Ligand 1 Expression between SP142 and 22C3/SP263 Assays in Triple-Negative Breast Cancer. J. Breast Cancer 2020, 23, 303–313. [Google Scholar] [CrossRef]
- Van Parijs, L.; Abbas, A.K. Homeostasis and self-tolerance in the immune system: Turning lymphocytes off. Science 1998, 280, 243–248. [Google Scholar] [CrossRef]
- O’Donnell, J.S.; Teng, M.W.L.; Smyth, M.J. Cancer immunoediting and resistance to T cell-based immunotherapy. Nat. Rev. Clin. Oncol. 2019, 16, 151–167. [Google Scholar] [CrossRef]
- Han, Y.; Liu, D.; Li, L. PD-1/PD-L1 pathway: Current researches in cancer. Am. J. Cancer Res. 2020, 10, 727–742. [Google Scholar]
- Klein, C.; Jamois, C.; Nielsen, T. Anti-CD20 treatment for B-cell malignancies: Current status and future directions. Expert Opin. Biol. Ther. 2021, 21, 161–181. [Google Scholar] [CrossRef]
- Zhu, F.-C.; Hu, S.-Y.; Hong, Y.; Hu, Y.-M.; Zhang, X.; Zhang, Y.-J.; Pan, Q.-J.; Zhang, W.-H.; Zhao, F.-H.; Zhang, C.-F.; et al. Efficacy, immunogenicity, and safety of the HPV-16/18 AS04-adjuvanted vaccine in Chinese women aged 18–25 years: Event-triggered analysis of a randomized controlled trial. Cancer Med. 2017, 6, 12–25. [Google Scholar] [CrossRef] [Green Version]
- June, C.H.; O’Connor, R.S.; Kawalekar, O.U.; Ghassemi, S.; Milone, M.C. CAR T cell immunotherapy for human cancer. Science 2018, 359, 1361–1365. [Google Scholar] [CrossRef] [Green Version]
- Terness, P.; Chuang, J.J.; Bauer, T.; Jiga, L.; Opelz, G. Regulation of human auto- and alloreactive T cells by indoleamine 2,3-dioxygenase (IDO)-producing dendritic cells: Too much ado about IDO? Blood 2005, 105, 2480–2486. [Google Scholar] [CrossRef]
- Naimi, A.; Mohammed, R.N.; Raji, A.; Chupradit, S.; Yumashev, A.V.; Suksatan, W.; Shalaby, M.N.; Thangavelu, L.; Kamrava, S.; Shomali, N.; et al. Tumor immunotherapies by immune checkpoint inhibitors (ICIs); the pros and cons. Cell Commun. Signal. CCS 2022, 20, 44. [Google Scholar] [CrossRef]
- Lu, R.M.; Hwang, Y.C.; Liu, I.J.; Lee, C.C.; Tsai, H.Z.; Li, H.J.; Wu, H.C. Development of therapeutic antibodies for the treatment of diseases. J. Biomed. Sci. 2020, 27, 1. [Google Scholar] [CrossRef]
- Liu, J.; Fu, M.; Wang, M.; Wan, D.; Wei, Y.; Wei, X. Cancer vaccines as promising immuno-therapeutics: Platforms and current progress. J. Hematol. Oncol. 2022, 15, 28. [Google Scholar] [CrossRef]
- Rohaan, M.W.; Wilgenhof, S.; Haanen, J. Adoptive cellular therapies: The current landscape. Virchows Arch. Int. J. Pathol. 2019, 474, 449–461. [Google Scholar] [CrossRef] [Green Version]
- Vallinayagam, L.; Adil, A.; Ahmed, N.; Rishi, A.; Jamal, S. Small molecule inhibitors as emerging cancer therapeutics. Intergrative Cancer Sci. Ther. 2014, 1, 39–46. [Google Scholar]
- Jiang, W.J.; Deng, X.-Y.; Li, X.-L.; Li, X.-Y.; Zeng, Z.-Y.; Xiong, W.; Li, G.-Y.; Xiong, F.; Guo, C. Immunotherapy targeted to immune checkpoint—A revolutionary breakthrough in cancer therapy written on the occasion of the 2018 Nobel Prize in Physiology or Medicine. Prog. Biochem. Biophys. 2018, 45, 1178–1186. [Google Scholar]
- Boussiotis, V.A. Molecular and Biochemical Aspects of the PD-1 Checkpoint Pathway. N. Engl. J. Med. 2016, 375, 1767–1778. [Google Scholar] [CrossRef] [Green Version]
- Qiu, X.Y.; Hu, D.X.; Chen, W.-Q.; Chen, R.Q.; Qian, S.R.; Li, C.Y.; Li, Y.J.; Xiong, X.X.; Liu, D.; Pan, F.; et al. PD-L1 confers glioblastoma multiforme malignancy via Ras binding and Ras/Erk/EMT activation. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 1754–1769. [Google Scholar] [CrossRef]
- Sharma, P.; Allison, J.P. Immune checkpoint targeting in cancer therapy: Toward combination strategies with curative potential. Cell 2015, 161, 205–214. [Google Scholar] [CrossRef] [Green Version]
- Topalian, S.L.; Taube, J.M.; Anders, R.A.; Pardoll, D.M. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat. Reviews. Cancer 2016, 16, 275. [Google Scholar] [CrossRef]
- Puzanov, I.; Diab, A.; Abdallah, K.; Bingham, C.; Brogdon, C.; Dadu, R.; Hamad, L.; Kim, S.; Lacouture, M.; LeBoeuf, N.; et al. Managing toxicities associated with immune checkpoint inhibitors: Consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J. Immun.Therapy. Cancer 2017, 5, 95. [Google Scholar] [CrossRef] [Green Version]
- Kwok, G.; Yau, T.C.C.; Chiu, J.W.; Tse, E.; Kwong, Y.L. Pembrolizumab (Keytruda). Hum. Vaccines 2016, 12, 2777–2789. [Google Scholar] [CrossRef] [Green Version]
- Vandeveer, A.J.; Fallon, J.K.; Tighe, R.; Sabzevari, H.; Schlom, J.; Greiner, J.W. Systemic Immunotherapy of Non-Muscle Invasive Mouse Bladder Cancer with Avelumab, an Anti-PD-L1 Immune Checkpoint Inhibitor. Cancer Immunol. Res. 2016, 4, 452–462. [Google Scholar] [CrossRef] [Green Version]
- Sundar, R.; Cho, B.-C.; Brahmer, J.R.; Soo, R.A. Nivolumab in NSCLC: Latest evidence and clinical potential. Ther. Adv. Med. Oncol. 2015, 7, 85–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rittmeyer, A.; Barlesi, F.; Waterkamp, D.; Park, K.; Ciardiello, F.; von Pawel, J.; Gadgeel, S.M.; Hida, T.; Kowalski, D.M.; Dols, M.C.; et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): A phase 3, open-label, multicentre randomised controlled trial. Lancet 2017, 389, 255–265. [Google Scholar] [CrossRef]
- Balar, A.V.; Galsky, M.D.; Rosenberg, J.E.; Powles, T.; Petrylak, D.P.; Bellmunt, J.; Loriot, Y.; Necchi, A.; Hoffman-Censits, J.; Perez-Gracia, J.L.; et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: A single-arm, multicentre, phase 2 trial. Lancet 2017, 389, 67–76. [Google Scholar] [CrossRef] [Green Version]
- McDermott, D.F.; Sosman, J.A.; Sznol, M.; Massard, C.; Gordon, M.S.; Hamid, O.; Powderly, J.D.; Infante, J.R.; Fassò, M.; Wang, Y.V.; et al. Atezolizumab, an Anti-Programmed Death-Ligand 1 Antibody, in Metastatic Renal Cell Carcinoma: Long-Term Safety, Clinical Activity, and Immune Correlates From a Phase Ia Study. J. Clin. Oncol. 2016, 34, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Emens, L.A.; Cruz, C.; Eder, J.P.; Braiteh, F.; Chung, C.; Tolaney, S.M.; Kuter, I.; Nanda, R.; Cassier, P.A.; Delord, J.-P.; et al. Long-term Clinical Outcomes and Biomarker Analyses of Atezolizumab Therapy for Patients With Metastatic Triple-Negative Breast Cancer: A Phase 1 Study. JAMA Oncol. 2019, 5, 74–82. [Google Scholar] [CrossRef]
- Seiwert, T.Y.; Burtness, B.; Weiss, J. A phase Ib study of MK-3475 in patients with human papillomavirus (HPV)-associated and non-HPV-associated head and neck (H/N) cancer. Mccarthy 2014, 32, 6011. [Google Scholar] [CrossRef]
- Muro, K.; Bang, Y.; Shankaran, V.; Geva, R.; Catenacci, D.V.T.; Eder, S.G.P.; Berger, R.; Gonzalez, E.J.; Ray, A.; Dolled-Filhart, M. Relationship between PD-L1 expression and clinical outcomes in patients (Pts) with advanced gastric cancer treated with the anti-PD-1 monoclonal antibody pembrolizumab (Pembro; MK-3475) in KEYNOTE-012. J. Clin. Oncol. 2015, 33, 3. [Google Scholar] [CrossRef]
- Chow, L.Q.; Burtness, B.; Weiss, J.; Berger, R.; Eder, J.P.; Gonzalez, E.J.; Pulini, J.; Johnson, J.; Dolled-Filhart, M.; Emancipator, K. A phase Ib study of pembrolizumab (Pembro; MK-3475) in patients (Pts) with human papiilloma virus (HPV)-positive and negative head and neck cancer. Ann. Oncol. 2014, 25 (Suppl. S4), 1–41. [Google Scholar] [CrossRef]
- Adams, S.; Schmid, P.; Rugo, H.S.; Winer, E.P.; Loirat, D.; Awada, A.; Cescon, D.W.; Iwata, H.; Campone, M.; Nanda, R.; et al. Pembrolizumab monotherapy for previously treated metastatic triple-negative breast cancer: Cohort A of the phase II KEYNOTE-086 study. Ann. Oncol. 2019, 30, 397–404. [Google Scholar] [CrossRef] [Green Version]
- Winer, E.P.; Lipatov, O.; Im, S.-A.; Goncalves, A.; Muñoz-Couselo, E.; Lee, K.S.; Schmid, P.; Tamura, K.; Testa, L.; Witzel, I.; et al. Pembrolizumab versus investigator-choice chemotherapy for metastatic triple-negative breast cancer (KEYNOTE-119): A randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 499–511. [Google Scholar] [CrossRef]
- Dirix, L.Y.; Takacs, I.; Jerusalem, G.; Nikolinakos, P.; Arkenau, H.T.; Forero-Torres, A.; Boccia, R.; Lippman, M.E.; Somer, R.; Smakal, M.; et al. Avelumab, an anti-PD-L1 antibody, in patients with locally advanced or metastatic breast cancer: A phase 1b JAVELIN Solid Tumor study. Breast Cancer Res. Treat. 2018, 167, 671–686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmid, P.; Rugo, H.S.; Adams, S.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Diéras, V.; Henschel, V.; Molinero, L.; Chui, S.Y.; et al. Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): Updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2020, 21, 44–59. [Google Scholar] [CrossRef]
- Miles, D.; Gligorov, J.; André, F.; Cameron, D.; Schneeweiss, A.; Barrios, C.; Xu, B.; Wardley, A.; Kaen, D.; Andrade, L.; et al. Primary results from IMpassion131, a double-blind, placebo-controlled, randomised phase III trial of first-line paclitaxel with or without atezolizumab for unresectable locally advanced/metastatic triple-negative breast cancer. Ann. Oncol. 2021, 32, 994–1004. [Google Scholar] [CrossRef] [PubMed]
- Loibl, S.; Untch, M.; Burchardi, N.; Huober, J.; Sinn, B.V.; Blohmer, J.U.; Grischke, E.M.; Furlanetto, J.; Tesch, H.; Hanusch, C.; et al. A randomised phase II study investigating durvalumab in addition to an anthracycline taxane-based neoadjuvant therapy in early triple-negative breast cancer: Clinical results and biomarker analysis of GeparNuevo study. Ann. Oncol. 2019, 30, 1279–1288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghebeh, H.; Al-Sayed, A.; Eiada, R.; Cabangon, L.; Ajarim, D.; Suleman, K.; Tulbah, A.; Al-Tweigeri, T. Weekly Paclitaxel given concurrently with Durvalumab has a favorable safety profile in triple-negative metastatic breast cancer. Sci. Rep. 2021, 11, 19154. [Google Scholar] [CrossRef]
- Cortes, J.; Cescon, D.W.; Rugo, H.S.; Nowecki, Z.; Im, S.-A.; Yusof, M.M.; Gallardo, C.; Lipatov, O.; Barrios, C.H.; Holgado, E.; et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): A randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet 2020, 396, 1817–1828. [Google Scholar] [CrossRef]
- Castellano, D.; Albers, P.; Gschwend, J.E.; Culine, S.; Bullmunt, J.; Hussain, M.; Shen, X.; Nelson, B.; Powles, T. 1143 A phase III study of the efficacy and safety of adjuvant atezolizumab (anti-PDL1) vs observation in patients with muscle-invasive urothelial carcinoma of the bladder (IMvigor 010). Eur. Urol. Suppl. 2016, 15, 1143. [Google Scholar] [CrossRef]
- Voorwerk, L.; Slagter, M.; Horlings, H.M.; Sikorska, K.; van de Vijver, K.K.; de Maaker, M.; Nederlof, I.; Kluin, R.J.C.; Warren, S.; Ong, S.; et al. Immune induction strategies in metastatic triple-negative breast cancer to enhance the sensitivity to PD-1 blockade: The TONIC trial. Nat. Med. 2019, 25, 920–928. [Google Scholar] [CrossRef]
- Lord, C.J.; Ashworth, A. PARP inhibitors: Synthetic lethality in the clinic. Science 2017, 355, 1152–1158. [Google Scholar] [CrossRef]
- Stover, E.H.; Fuh, K.; Konstantinopoulos, P.A.; Matulonis, U.A.; Liu, J.F. Clinical assays for assessment of homologous recombination DNA repair deficiency. Gynecol Oncol. 2020, 159, 887–898. [Google Scholar] [CrossRef]
- Matulonis, U.A.; Shapira-Frommer, R.; Santin, A.D.; Lisyanskaya, A.S.; Pignata, S.; Vergote, I.; Raspagliesi, F.; Sonke, G.S.; Birrer, M.; Provencher, D.M.; et al. Antitumor activity and safety of pembrolizumab in patients with advanced recurrent ovarian cancer: Results from the phase II KEYNOTE-100 study. Ann. Oncol. 2019, 30, 1080–1087. [Google Scholar] [CrossRef] [PubMed]
- Kuai, M.; Le, X.; Li, Q.; Hu, G. Research Progress MEK Inhibitors. Anti-Tumor Pharm. 2017, 7, 10. [Google Scholar]
- Brufsky, A.; Kim, S.B.; Zvirbule, Ž; Eniu, A.; Mebis, J.; Sohn, J.H.; Wongchenko, M.; Chohan, S.; Amin, R.; Yan, Y. A phase II randomized trial of cobimetinib plus chemotherapy, with or without atezolizumab, as first-line treatment for patients with locally advanced or metastatic triple-negative breast cancer (COLET): Primary analysis. Ann. Oncol. 2021, 32, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Minor, M.; Alcedo, K.P.; Battaglia, R.A.; Snider, N.T. Cell type- and tissue-specific functions of ecto-5’-nucleotidase (CD73). Am. J. Physiol. Cell Physiol 2019, 317, 1079–1092. [Google Scholar] [CrossRef]
- Ochoa de Olza, M.; Navarro Rodrigo, B.; Zimmermann, S.; Coukos, G. Turning up the heat on non-immunoreactive tumours: Opportunities for clinical development. Lancet Oncol. 2020, 21, 419–430. [Google Scholar] [CrossRef]
- Ghalamfarsa, G.; Kazemi, M.H.; Raoofi Mohseni, S.; Masjedi, A.; Hojjat-Farsangi, M.; Azizi, G.; Yousefi, M.; Jadidi-Niaragh, F. CD73 as a potential opportunity for cancer immunotherapy. Expert Opin. Targets 2019, 23, 127–142. [Google Scholar] [CrossRef]
- Chen, S.; Wainwright, D.A.; Wu, J.D.; Wan, Y.; Matei, D.E.; Zhang, Y.; Zhang, B. CD73: An emerging checkpoint for cancer immunotherapy. Immunotherapy 2019, 11, 983–997. [Google Scholar] [CrossRef]
- Santa-Maria, C.A.; Kato, T.; Park, J.H.; Flaum, L.E.; Giles, F.J. Durvalumab and tremelimumab in metastatic breast cancer (MBC): Immunotherapy and immunopharmacogenomic dynamics. J. Clin. Oncol. 2017, 35, 3052. [Google Scholar] [CrossRef]
- Banerjee, S.; Dowsett, M.; Ashworth, A.; Martin, L.A. Mechanisms of disease: Angiogenesis and the management of breast cancer. Nat. Clin. Practice. Oncol. 2007, 4, 536–550. [Google Scholar] [CrossRef]
- Seymour, L.; Dajee, D.; Bezwoda, W.R. Tissue platelet derived-growth factor (PDGF) predicts for shortened survival and treatment failure in advanced breast cancer. Breast Cancer Res. Treat. 1993, 26, 247–252. [Google Scholar] [CrossRef]
- Hines, S.J.; Organ, C.; Kornstein, M.J.; Krystal, G.W. Coexpression of the c-kit and stem cell factor genes in breast carcinomas. Cell Growth Differ. Mol. Biol. J. Am. Assoc. Cancer Res. 1995, 6, 769–779. [Google Scholar]
- Cao, J.; Zhang, J.; Wang, Z.; Wang, B.; Lv, F.; Wang, L.; Hu, X. Hypothyroidism as a potential biomarker of efficacy of famitinib, a novel VEGFR-2 inhibitor in metastatic breast cancer. Cancer Chemother. Pharmacol. 2014, 74, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Jiang, Y.Z.; Wu, S.Y.; Wu, J.; Di, G.H.; Liu, G.Y.; Yu, K.D.; Fan, L.; Li, J.J.; Hou, Y.F.; et al. Famitinib with Camrelizumab and Nab-Paclitaxel for Advanced Immunomodulatory Triple-Negative Breast Cancer (FUTURE-C-Plus): An Open-Label, Single-Arm, Phase II Trial. Clin. Cancer Res. 2022, 28, 2807–2817. [Google Scholar] [CrossRef] [PubMed]
- Mediratta, K.; El-Sahli, S.; D’Costa, V.; Wang, L. Current Progresses and Challenges of Immunotherapy in Triple-Negative Breast Cancer. Cancers 2020, 12, 3529. [Google Scholar] [CrossRef] [PubMed]
- Esteva, F.J.; Hubbard-Lucey, V.M.; Tang, J.; Pusztai, L. Immunotherapy and targeted therapy combinations in metastatic breast cancer. Lancet Oncol. 2019, 20, 175–186. [Google Scholar] [CrossRef]
- Gupta, G.K.; Collier, A.L.; Lee, D.; Hoefer, R.A.; Zheleva, V.; Siewertsz van Reesema, L.L.; Tang-Tan, A.M.; Guye, M.L.; Chang, D.Z.; Winston, J.S.; et al. Perspectives on Triple-Negative Breast Cancer: Current Treatment Strategies, Unmet Needs, and Potential Targets for Future Therapies. Cancers 2020, 12, 2392. [Google Scholar] [CrossRef]
- Flosbach, M.; Oberle, S.G.; Scherer, S.; Zecha, J.; von Hoesslin, M.; Wiede, F.; Chennupati, V.; Cullen, J.G.; List, M.; Pauling, J.K.; et al. PTPN2 Deficiency Enhances Programmed T Cell Expansion and Survival Capacity of Activated T Cells. Cell Rep. 2020, 32, 107957. [Google Scholar] [CrossRef]
- LaFleur, M.W.; Nguyen, T.H.; Coxe, M.A.; Miller, B.C.; Yates, K.B.; Gillis, J.E.; Sen, D.R.; Gaudiano, E.F.; Al Abosy, R.; Freeman, G.J.; et al. PTPN2 regulates the generation of exhausted CD8 T cell subpopulations and restrains tumor immunity. Nat. Immunol. 2019, 20, 1335–1347. [Google Scholar] [CrossRef]
- Manguso, R.T.; Pope, H.W.; Zimmer, M.D.; Brown, F.D.; Yates, K.B.; Miller, B.C.; Collins, N.B.; Bi, K.; LaFleur, M.W.; Juneja, V.R.; et al. In vivo CRISPR screening identifies Ptpn2 as a cancer immunotherapy target. Nature 2017, 547, 413–418. [Google Scholar] [CrossRef] [Green Version]
- Jevtovic, A.; Pantic, J.; Jovanovic, I.; Milovanovic, M.; Stanojevic, I.; Vojvodic, D.; Arsenijevic, N.; Lukic, M.L.; Radosavljevic, G.D. Interleukin-33 pretreatment promotes metastatic growth of murine melanoma by reducing the cytotoxic capacity of CD8 T cells and enhancing regulatory T cells. Cancer Immunol. Immunother. 2020, 69, 1461–1475. [Google Scholar] [CrossRef]
- Larsen, K.M.; Minaya, M.K.; Vaish, V.; Peña, M.M.O. The Role of IL-33/ST2 Pathway in Tumorigenesis. Int. J. Mol. Sci. 2018, 19, 2676. [Google Scholar] [CrossRef] [Green Version]
- Jovanovic, I.P.; Pejnovic, N.N.; Radosavljevic, G.D.; Pantic, J.M.; Milovanovic, M.Z.; Arsenijevic, N.N.; Lukic, M.L. Interleukin-33/ST2 axis promotes breast cancer growth and metastases by facilitating intratumoral accumulation of immunosuppressive and innate lymphoid cells. Int. J. Cancer 2014, 134, 1669–1682. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Shi, L.; Meng, D.; Zhou, H.; Ma, J.; Wu, Y.; Wu, Y.; Gu, Y.; Xie, W.; Zhang, J.; et al. PD-1 blockade combined with IL-33 enhances the antitumor immune response in a type-1 lymphocyte-mediated manner. Cancer Treat. Res. Commun. 2021, 28, 100379. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, M.Z.; Geller, D.A.; Gajovic, N.M.; Jurisevic, M.M.; Arsenijevic, N.N.; Jovanovic, M.M.; Supic, G.M.; Vojvodic, D.V.; Jovanovic, I.P. Dual blockage of PD-L/PD-1 and IL33/ST2 axes slows tumor growth and improves antitumor immunity by boosting NK cells. Life Sci. 2022, 289, 120214. [Google Scholar] [CrossRef] [PubMed]
- Lodyga, M.; Hinz, B. TGF-β1—A truly transforming growth factor in fibrosis and immunity. Semin Cell Dev. Biol 2020, 101, 123–139. [Google Scholar] [CrossRef] [PubMed]
- Jiang, A.; Qin, Y.; Springer, T.A. Loss of LRRC33-dependent TGFβ1 activation enhances anti-tumor immunity and checkpoint blockade therapy. Cancer Immunol. Res. 2022, 10, 453–467. [Google Scholar] [CrossRef]
- Peña-Asensio, J.; Calvo, H.; Torralba, M.; Miquel, J.; Sanz-de-Villalobos, E.; Larrubia, J.-R. Anti-PD-1/PD-L1 Based Combination Immunotherapy to Boost Antigen-Specific CD8 T Cell Response in Hepatocellular Carcinoma. Cancers 2021, 13, 1922. [Google Scholar] [CrossRef]
- Park, B.V.; Freeman, Z.T.; Ghasemzadeh, A.; Chattergoon, M.A.; Rutebemberwa, A.; Steigner, J.; Winter, M.E.; Huynh, T.V.; Sebald, S.M.; Lee, S.-J.; et al. TGFβ1-Mediated SMAD3 Enhances PD-1 Expression on Antigen-Specific T Cells in Cancer. Cancer Discov. 2016, 6, 1366–1381. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.-M.; Luo, Y.-F.; Zeng, F.-F.; Su, C.; Liu, X.; Li, X.-P.; Lu, J. TGF-β1-Mediated PD-L1 Glycosylation Contributes to Immune Escape c-Jun/STT3A Pathway in Nasopharyngeal Carcinoma. Front. Oncol. 2022, 12, 815437. [Google Scholar] [CrossRef]
- Martin, C.J.; Datta, A.; Littlefield, C.; Kalra, A.; Chapron, C.; Wawersik, S.; Dagbay, K.B.; Brueckner, C.T.; Nikiforov, A.; Danehy, F.T.; et al. Selective inhibition of TGFβ1 activation overcomes primary resistance to checkpoint blockade therapy by altering tumor immune landscape. Sci. Transl. Med. 2020, 12, 8456. [Google Scholar] [CrossRef]
- Wu, Q.; Tian, A.L.; Li, B.; Leduc, M.; Forveille, S.; Hamley, P.; Galloway, W.; Xie, W.; Liu, P.; Zhao, L.; et al. IGF1 receptor inhibition amplifies the effects of cancer drugs by autophagy and immune-dependent mechanisms. J. Immunother. Cancer 2021, 9, 002722. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.; Yuan, C. Kita-Kyushu Lung Cancer Antigen-1 (KK-LC-1): A Promising Cancer Testis Antigen. Aging Dis. 2022, 13, 1267–1277. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Zhang, L.; Wang, Z.; Kong, X.; Zhai, J.; Fang, Y.; Wang, J. Efficacy and Safety of Anti-PD-1/ PD-L1 Monotherapy for Metastatic Breast Cancer: Clinical Evidence. Front. Pharmacol. 2021, 12, 653521. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.; Syed, Y.Y. Atezolizumab (in Combination with Nab-Paclitaxel): A Review in Advanced Triple-Negative Breast Cancer. Drugs 2020, 80, 601–607. [Google Scholar] [CrossRef]
- Villacampa, G.; Tolosa, P.; Salvador, F.; Sánchez-Bayona, R.; Villanueva, L.; Dienstmann, R.; Ciruelos, E.; Pascual, T. Addition of immune checkpoint inhibitors to chemotherapy versus chemotherapy alone in first-line metastatic triple-negative breast cancer: A systematic review and meta-analysis. Cancer Treat. Rev. 2022, 104, 102352. [Google Scholar] [CrossRef]
- Gumusay, O.; Callan, J.; Rugo, H.S. Immunotherapy toxicity: Identification and management. Breast Cancer Res. Treat. 2022, 192, 1–17. [Google Scholar] [CrossRef]
- Huo, X.; Shen, G.; Liu, Z.; Liang, Y.; Li, J.; Zhao, F.; Ren, D.; Zhao, J. Addition of immunotherapy to chemotherapy for metastatic triple-negative breast cancer: A systematic review and meta-analysis of randomized clinical trials. Crit. Rev. Oncol./Hematol. 2021, 168, 103530. [Google Scholar] [CrossRef]
- Sternschuss, M.; Yerushalmi, R.; Saleh, R.R.; Amir, E.; Goldvaser, H. Efficacy and safety of neoadjuvant immune checkpoint inhibitors in early-stage triple-negative breast cancer: A systematic review and meta-analysis. J. Cancer Res. Clin. Oncol. 2021, 147, 3369–3379. [Google Scholar] [CrossRef]
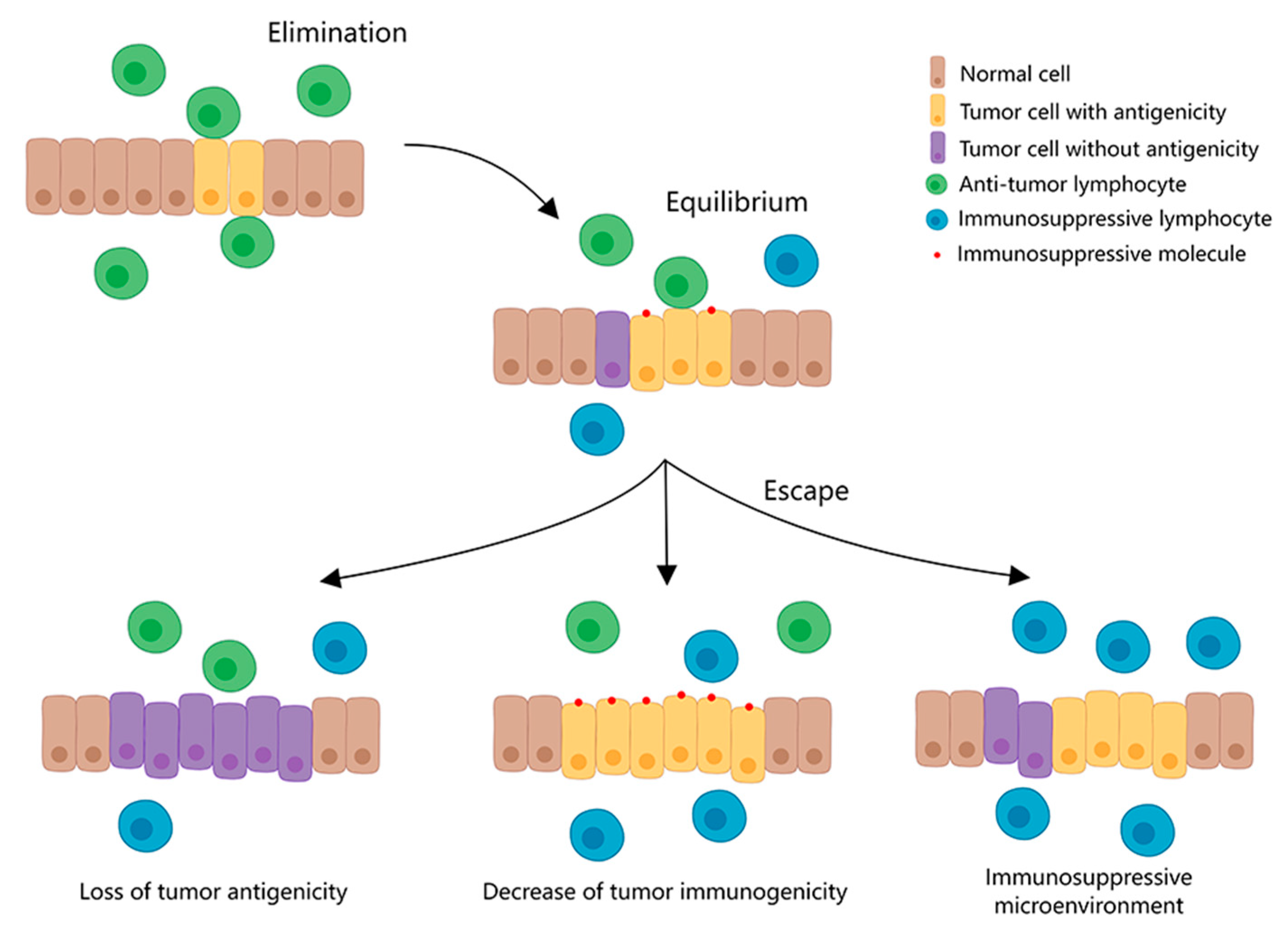
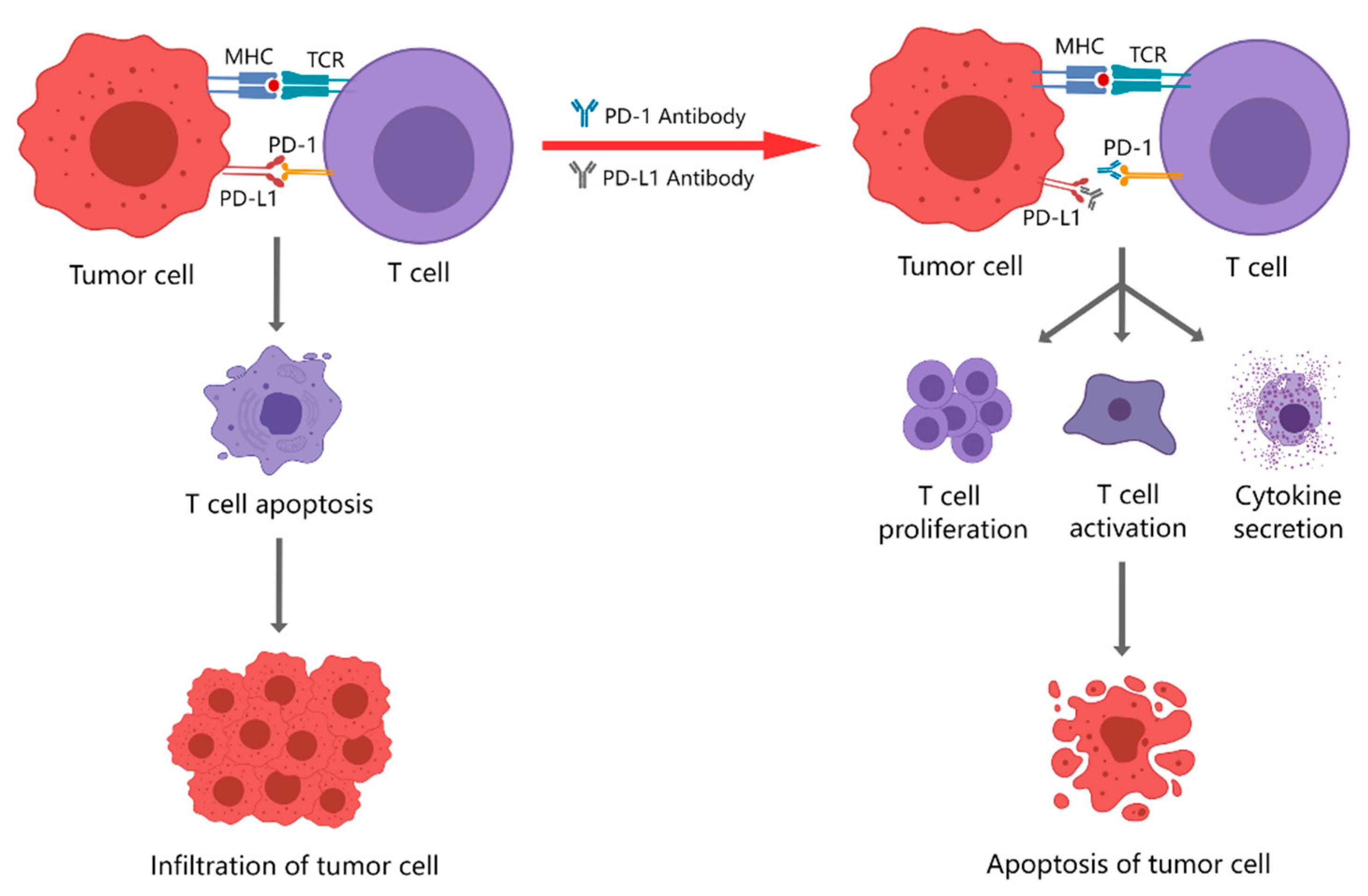
| Common Immunotherapy Approaches | Principle | Current Clinical Applications |
|---|---|---|
| Immune checkpoint blockade therapy | This is a type of therapy that blocks the action of immune checkpoints by artificially administering inhibitors of immune checkpoints or their ligands, thereby upregulating T cells activity and improving the body’s anti-tumor immune response. The most commonly used inhibitors are monoclonal antibodies to the corresponding molecules, such as PD-1/PD-L1 monoclonal antibodies and CTLA-4 monoclonal antibodies. | breast cancer, lung cancer, hepatocellular carcinoma, prostate cancer, melanoma, MSI-H/dMMR colorectal cancer RCC, lymphoma, MCC, urothelial cancer [18]. |
| Therapeutic antibodies | Laboratory-designed antibodies destroy tumor cells by inducing direct apoptosis, antibody-dependent cytotoxicity, and complement-dependent cytotoxicity. Common therapeutic antibodies include rituximab and panitumumab. | breast cancer, colorectal cancer, lymphoma, melanoma, head and neck cancer, NSCLC, RCC, cervical cancer [19]. |
| Cancer vaccine | Tumor antigens are introduced into patients in the form of tumor cells, tumor-related proteins or peptides, and genes that express tumor antigens, so as to activate patients′ own immune responses and reduce immune suppression caused by tumors, thus achieving control or clearance of the tumor. They can be divided into prophylactic and therapeutic vaccines, such as the cervical cancer vaccine and the Sipuleucel-T vaccine. | pancreatic cancer, lymphoma, breast cancer, NSCLC, gastric cancer, glioblastoma, cervical cancer, prostate cancer [20]. |
| Adoptive cellular immunotherapy | Immune cells are collected from the patient’s blood, and the collected immune cells are then genetically edited to change ordinary immune cells into immune cells that can recognize tumor cells, expanded and cultured, and then infused back into the patient with such immune cells that can trigger the killing effect of tumor cells, thus playing the role of anti-tumor immunity. The available immune cells are autologous lymphokine-activated killer cells, natural killer cells, cytokine-induced killer cells, cytotoxic T cells, and genetically modified T cells, etc. | melanoma, renal cell carcinoma, breast cancer, cervical cancer, gastrointestinal cancers, cholangiocarcinoma, pancreatic cancer, head and neck cancer, ovarian cancer, NSCLC [21]. |
| Small-molecule inhibitors | There are many small-molecule proteins in tumor cells and in the tumor microenvironment, which can promote the occurrence and development of tumors by inhibiting the anti-tumor immunity, and promoting the accumulation of abnormal mutations and the abnormal proliferation of tumor cells. By artificially providing inhibitors of these small-molecule proteins, the above abnormal responses can be cut off and tumor progression can be inhibited. Common small-molecule inhibitors include IDO inhibitors, PARP inhibitors, MEK inhibitors, VEGFR inhibitors, etc. | breast cancer, ovarian cancer, thyroid cancer, soft tissue sarcoma, colorectal cancer, melanoma, pancreatic cancer, renal cell carcinoma, NSCLC, leukemia [22]. |
| Generic Name | Approved for | R&D Company | Degree of Antibody Humanization | Antibody Type |
|---|---|---|---|---|
| Nivolumab | NSCLC, head and neck squamous cell carcinoma, pleural mesothelioma, gastroesophageal junction carcinoma, gastric cancer, melanoma. | Bristol-Myers Squibb Pharm EEIG (New York, NY, the US) | Fully human | IgG4 |
| Pembrolizumab | Melanoma, Hodgkin’s lymphoma, NSCLC, head and neck squamous cell carcinoma, esophageal cancer, advanced MSI-H/dMMR colorectal carcinoma. | Merck Sharp & Dohme Corp (Beijing, China) | Humanized | IgG4k |
| Camrelizumab | Non-squamous NSCLC, classical Hodgkin’s lymphoma, nasopharyngeal carcinoma, HCC, esophageal squamous carcinoma. | Suzhou Shengdiya Biopharmaceutical Co. (Suzhou, China) | Humanized | IgG4k |
| Toripalimab | Melanoma, nasopharyngeal carcinoma, uroepithelial carcinoma, esophageal squamous carcinoma. | Shanghai Junshi Biomedical Technology Co. (Shanghai, China) | Humanized | IgG4k |
| Tislelizumab | (Non-)squamous NSCLC, hepatocellular carcinoma, Hodgkin’s lymphoma, uroepithelial carcinoma. | Baekje Shenzhou (Shanghai) Biotechnology Co. (Shanghai, China) | Humanized | IgG4 |
| Penpulimab | Hodgkin’s lymphoma. | Zhongshan Kangfang Bio-pharmaceutical Co. (Zhongshan, China) | Humanized | IgG1 |
| Sinitilimab | Squamous lung cancer, non-squamous NSCLC, HCC, Hodgkin’s lymphoma. | Cinda Biopharma (Suzhou) Co. (Suzhou, China) | Fully human | IgG4 |
| Zimberelimab | Hodgkin’s lymphoma. | Guangzhou Yu Heng Biotechnology Co. (Guangzhou, China) | Fully human | IgG4 |
| Generic Name | Approved for | R&D Company | Degree of Antibody Humanization | Antibody Type |
|---|---|---|---|---|
| Atezolizumab | Breast cancer, uroepithelial cancer, (non-) small cell lung cancer, HCC. | Genentech (Roche) (San Francisco, the US) | Humanized | IgG1k |
| Durvalumab | (Non-) small cell lung cancer. | AstraZeneca (London, the UK) | Fully human | IgG1k |
| Avelumab | Metastatic MCC, uroepithelial carcinoma. | EMD Serono (Merck/Pfizer) (Darmstadt, Germany) | Fully human | IgG1 |
| Test Name | Identifiers | Test Arm | Control Arm |
|---|---|---|---|
| PCD4989g (Phase I) | NCT01375842 | ORR: 24% mOS: 17.6 months (95% CI: 10.2–N/A) trAEs: 62% | ORR: 6% mOS: 7.3 months (95% CI: 6.1–10.8) trAEs: 43% |
| KEYNOTE-01 (Phase Ib) | NCT01848834 | ORR: 18.5% (95% CI: 6.3–38.1%) mPFS: 1.9 months (95% CI: 1.3–4.3) mOS: 10.2 months (95% CI: 5.3–N/A) Level 3–5 trAEs: 15.6% | - |
| KEYNOTE-086 (Phase II) | NCT02447003 | ORR: 21.4% mPFS: 2.1 months (95% CI: 1.9–2.0) mOS: 18 months (95% CI: 12.9–23.0) trAEs: 63.1% | ORR: 5.3% mPFS: 2.0 months (95% CI: 1.9–2.0) mOS: 9 months (95% CI: 7.6 –11.2) trAEs: 60.6% |
| KEYNOTE-119 (Phase III) | NCT02555657 | mOS: 9.9 months (95% CI: 8.3–11.4) cps≥20: ORR: 26% cps≥10: ORR: 18% mOS: 12.7 months (95% CI: 9.9–16.3) cps≥1: ORR: 12% mOS: 10.7 months (95% CI: 9.3–12.5) | mOS: 10.8 months (95% CI: 9.1–12.6) ORR: 12% ORR: 9% mOS: 11.6 months (95% CI: 8.3–13.7) ORR: 9% mOS: 10.2 months (95% CI: 7.9–12.6) |
| Test Name | Identifiers | Test Arm | Control Arm |
|---|---|---|---|
| IMpassion130 (phase III) | NCT02425891 | ITT: mPFS: 7.2 months mOS: 21.0 months (95% CI: 19.0–22.6) PD–L1–positive: ORR:53% mPFS: 7.5 months mOS: 25.0 months (95% CI: 19.6–30.7) | mPFS: 5.5 months mOS: 18.7 months (95%CI: 16.9–20.3) ORR:33% mPFS: 5.0 months mOS: 18.0 months (95% CI: 13.6–20.1) |
| IMpassion131 (phase III) | NCT03125902 | mPFS: 6.0 months mOS: 22.1 months | mPFS: 5.7 months mOS: 28.3 months |
| GeparNuevo (phase II) | - | normal cohort: pCR: 53.4% window cohort: pCR: 61.0% | pCR: 44.2% pCR: 41.4% |
| - | NCT02628132 | mPFS: 4.0–5.0 months | - |
| KEYNOTE-355 (phase III) | NCT02819518 | cps≥10: mPFS: 9.7 months cps≥1: mPFS: 7.6 months | mPFS: 5.6 months mPFS:5.6 months |
| KEYNOTE-522 (phase III) | NCT03036488 | pCR: 64.8% (95% CI: 59.9–69.5) | pCR: 51.2% (95% CI: 44.1–58.3) |
| TONIC trial (phase II) | - | doxorubicin cohort: ORR: 35% cisplatin cohort: ORR: 23% | - |
| Test Name | Identifiers | Result | |
|---|---|---|---|
| KEYNOTE-162 (phase I/II) | NCT02657889 | ORR: 21% PD-L1-positive: 33% tBRCA mutation: 47% mPFS: tBRCA mutation: 8.3months DCR: 49% tBRCA mutation: 80% | PD-L1-negative: 15% tBRCA wild-type: 11% tBRCA wild-type: 2.1months tBRCA wild-type: 33% |
| COLET (phase II) | NCT02322814 | ORR: C+P: 38.3% (95% CI: 24.40–52.20%) placebo+P: 20.9% (95% CI: 8.77–33.09%) C+A+P: 34.4% (95% CI: 18.57–53.19%) C+A+nab-P: 29.0% (95% CI: 14.22–48.04%) mPFS: C+A+P: 3.8 months C+A+nab-P: 7.0 months | |
| - | NCT04129996 | ORR: 81.3% (95% CI: 70.2–92.3) mPFS: 13.6 months (95% CI: 8.4–18.8) median DOR: 14.9 months | |
| First-Line | Second-Line | Third-Line | |
|---|---|---|---|
| Patients sensitive to paclitaxel treatment | 1. In early-stage TNBC, the current regimen remains anthracycline- or paclitaxel-based single-agent or combination chemotherapy. 2. For locally advanced or metastatic PD-L1-positive TNBC, the recommended regimen is PD-L1 inhibitors in combination with chemotherapy. | 1. single-agent chemotherapy 2. nab-paclitaxel in combination with PD-L1 inhibitors | 1 chemotherapeutic drug liposomes 2. PD-L1 inhibitors in combination with chemotherapy. |
| Patients who have failed paclitaxel therapy | 1. In early-stage TNBC, the current regimen remains anthracycline- or paclitaxel-based single-agent or combination chemotherapy. 2. For locally advanced or metastatic PD-L1-positive TNBC, the recommended regimen is PD-L1 inhibitors in combination with chemotherapy. | 1. single-agent chemotherapy 2. multi-drug combination chemotherapy | 1. chemotherapeutic drug liposomes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, Y.; Song, J.; Li, X.; Luo, N. Rationale and Clinical Research Progress on PD-1/PD-L1-Based Immunotherapy for Metastatic Triple-Negative Breast Cancer. Int. J. Mol. Sci. 2022, 23, 8878. https://doi.org/10.3390/ijms23168878
Ren Y, Song J, Li X, Luo N. Rationale and Clinical Research Progress on PD-1/PD-L1-Based Immunotherapy for Metastatic Triple-Negative Breast Cancer. International Journal of Molecular Sciences. 2022; 23(16):8878. https://doi.org/10.3390/ijms23168878
Chicago/Turabian StyleRen, Yifan, Jialong Song, Xinyi Li, and Na Luo. 2022. "Rationale and Clinical Research Progress on PD-1/PD-L1-Based Immunotherapy for Metastatic Triple-Negative Breast Cancer" International Journal of Molecular Sciences 23, no. 16: 8878. https://doi.org/10.3390/ijms23168878
APA StyleRen, Y., Song, J., Li, X., & Luo, N. (2022). Rationale and Clinical Research Progress on PD-1/PD-L1-Based Immunotherapy for Metastatic Triple-Negative Breast Cancer. International Journal of Molecular Sciences, 23(16), 8878. https://doi.org/10.3390/ijms23168878






