Genome-Wide Analysis of the RAV Gene Family in Wheat and Functional Identification of TaRAV1 in Salt Stress
Abstract
:1. Introduction
2. Results
2.1. Identification and Phylogenetic Analyses of Wheat RAV Genes
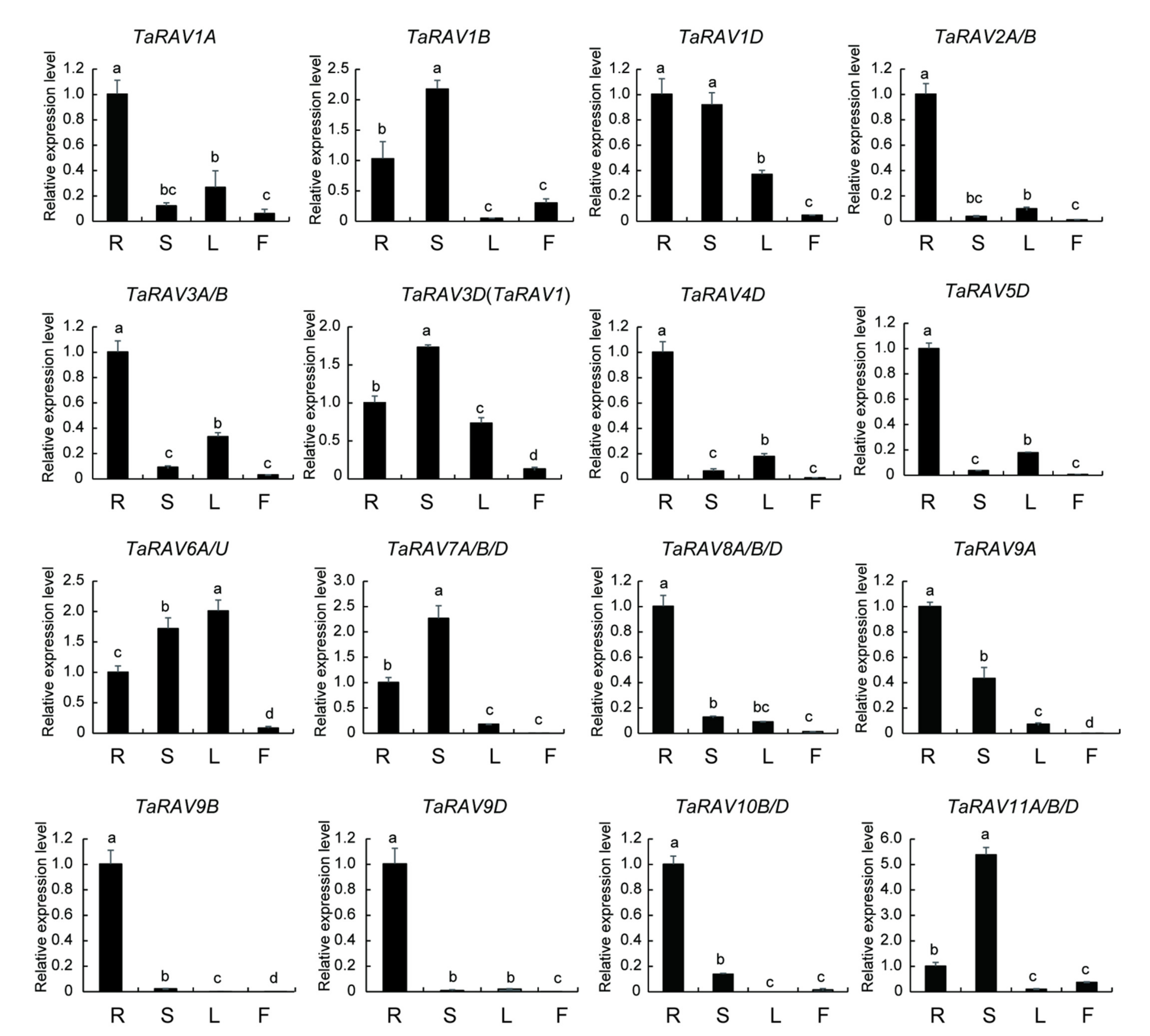
2.2. Expression Patterns of TaRAV Genes in Different Tissues
2.3. Expression Patterns of TaRAV1
2.4. TaRAV1 Is Localized in the Nucleus
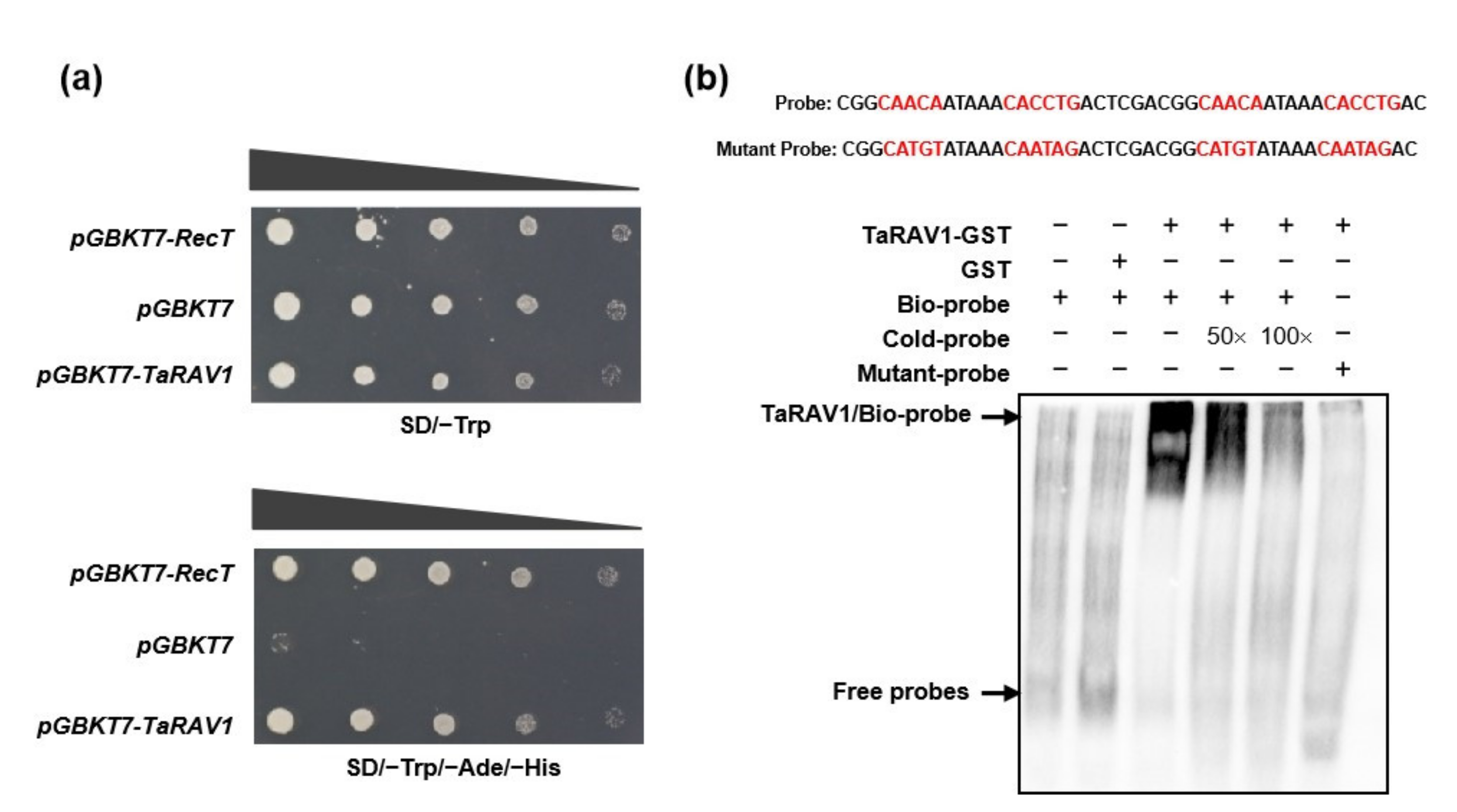
2.5. TaRAV1 Shows Both Transcription Activation and DNA Binding Activities
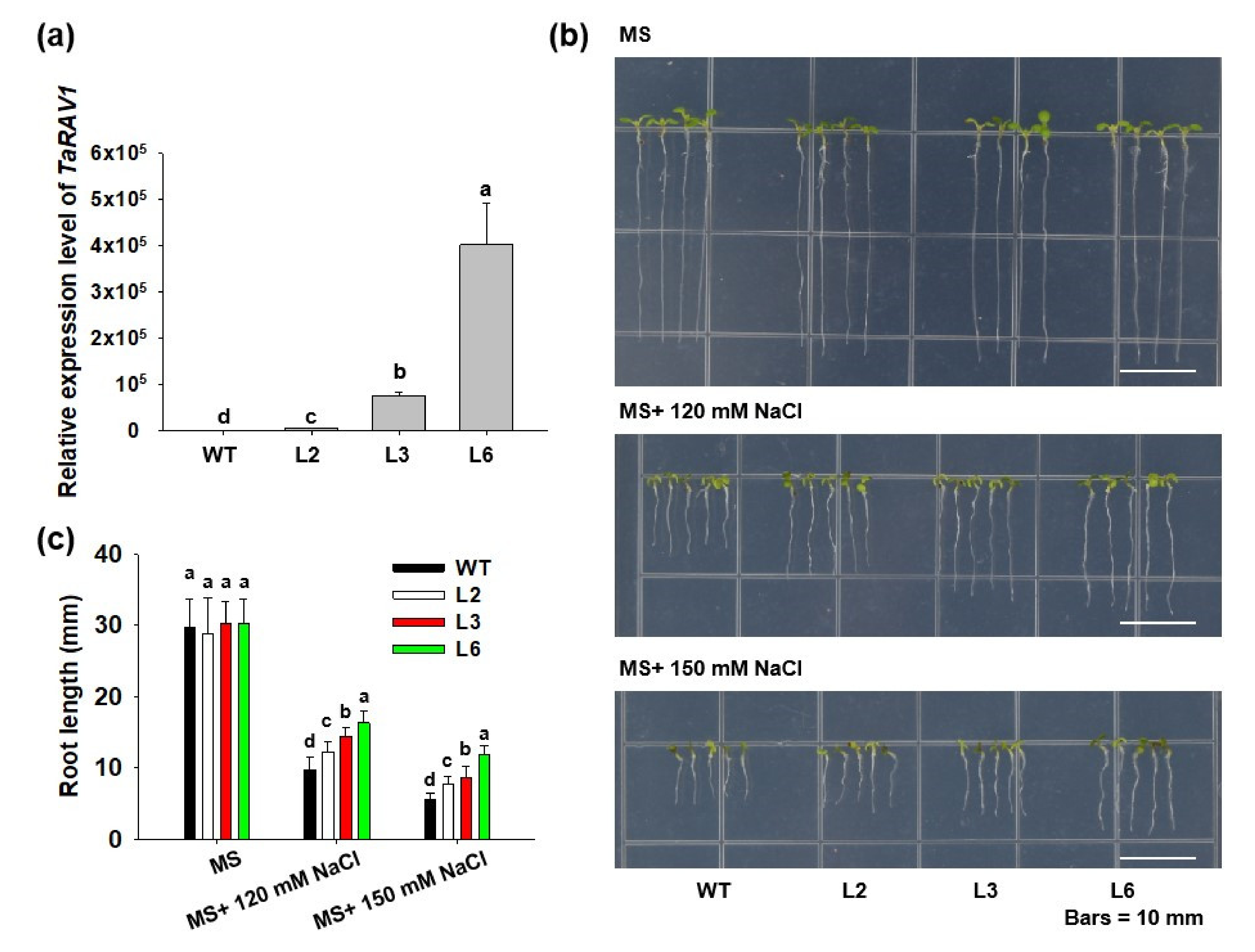
2.6. Overexpressing TaRAV1 in Arabidopsis Enhances Salt Tolerance
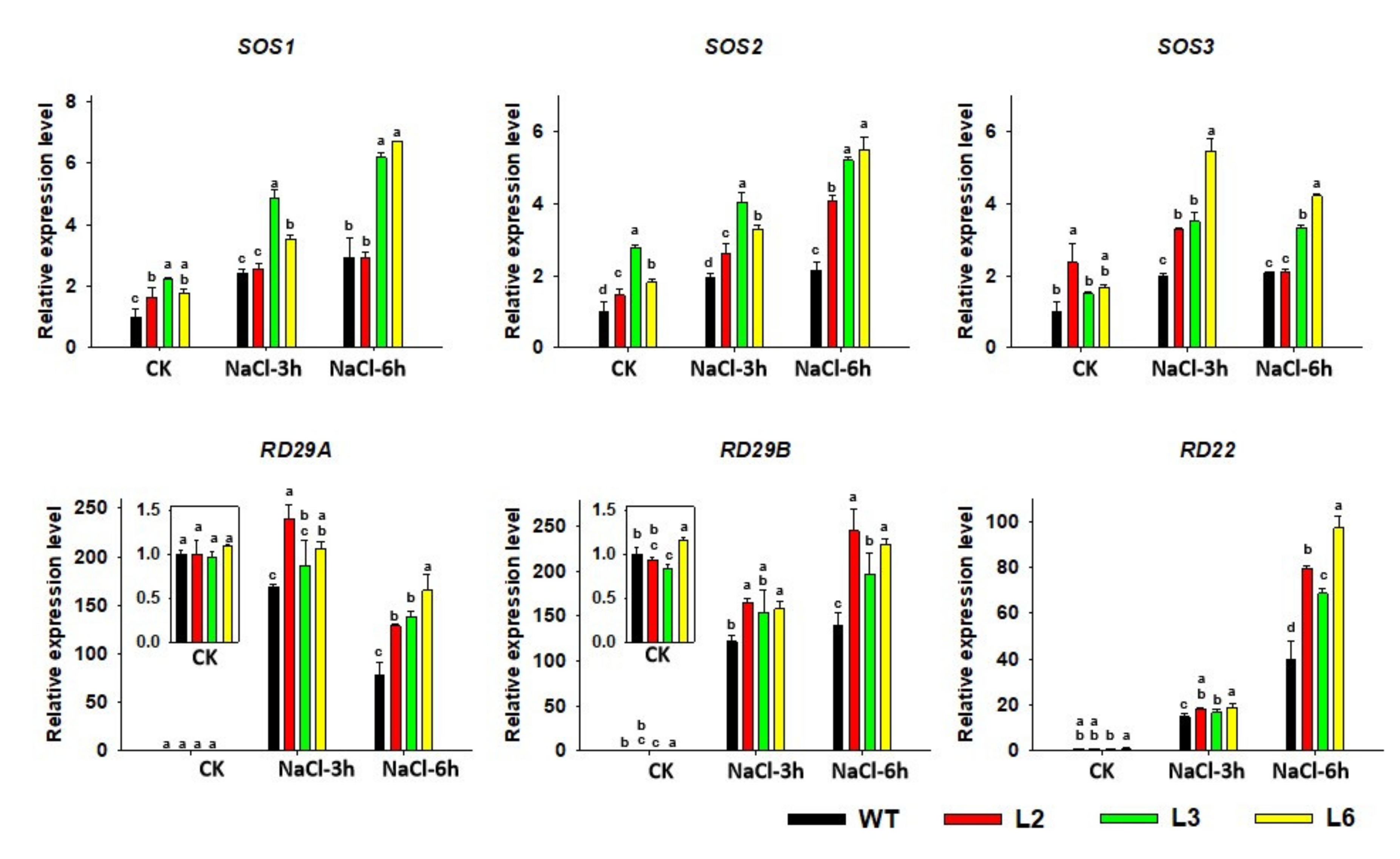
2.7. The Expression of Salt-Responsive Genes Is Altered in TaRAV1 Transgenic Arabidopsis
3. Discussion
3.1. Wheat Has More RAV Genes Than Diploid Plant Species
3.2. TaRAV1 May Work as a Transcription Factor in Wheat
3.3. TaRAV1 Plays an Important Role in Salt Response
4. Materials and Methods
4.1. Identification and Phylogenetic Analysis of RAVs
4.2. Plant Materials and Growth Conditions
4.3. Quantitative Real-Time PCR Analysis
4.4. Electrophoretic Mobility Shift Assay (EMSA)
4.5. Subcellular Localization Assay
4.6. Transcriptional Activity Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| RAV transcription factors | Related to ABI3/VP1 transcription factor |
| LAV families | Leafy Cotyledon2 (LEC2)-Abscisic Acid Insensitive3 (ABI3)-VAL |
| ARF families | Auxin Response Factor families |
| REM families | Reproductive Meristem families |
| TaRAV1 | Wheat Related to ABI3/VP1,1 |
| TF | transcription factor |
| ABA | Abscisic acid |
| GA | Gibberellin |
| GFP | Green fluorescent protein |
References
- Khan, M.S.; Rizvi, A.; Saif, S.; Zaidi, A. Phosphate-Solubilizing Microorganisms in Sustainable Production of Wheat: Current Perspective; Springer: Berlin/Heidelberg, Germany, 2017; pp. 51–81. [Google Scholar]
- Shewry, P.R.; Hey, S.J. The contribution of wheat to human diet and health. Food Energy Secur. 2015, 4, 178–202. [Google Scholar] [CrossRef]
- Ashraf, M. Stress-induced changes in wheat grain composition and quality. Crit. Rev. Food Sci. Nutr. 2014, 54, 1576–1583. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.B.; Foley, R.C.; Onate-Sanchez, L. Transcription factors in plant defense and stress responses. Curr. Opin. Plant Biol. 2002, 5, 430–436. [Google Scholar] [CrossRef]
- Gong, Z.; Xiong, L.; Shi, H.; Yang, S.; Herrera-Estrella, L.R.; Xu, G.; Chao, D.; Li, J.; Wang, P.Y.; Qin, F.; et al. Plant abiotic stress response and nutrient use efficiency. Sci. China Life Sci. 2020, 63, 635–674. [Google Scholar] [CrossRef] [PubMed]
- Matías-Hernández, L.; Aguilar-Jaramillo, A.E.; Marín-González, E.; SuárezLópez, P.; Pelaz, S. RAV genes: Regulation of floral induction and beyond. Ann. Bot. 2014, 114, 1459–1470. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Deng, D.; Zhang, R.; Wang, S.; Bian, Y.; Yin, Z. Systematic analysis of plant-specific B3 domain-containing proteins based on the genome resources of 11 sequenced species. Mol. Biol. Rep. 2012, 39, 6267–6282. [Google Scholar] [CrossRef]
- McCarty, D.R.; Hattori, T.; Carson, C.B.; Vasil, V.; Lazar, M.; Vasil, K. The vivapaurous-1 developmental gene of maize encodes a novel transcriptional activator. Cell 1991, 66, 895–905. [Google Scholar] [CrossRef]
- Marella, H.H.; Sakata, Y.; Quatrano, R.S. Characterization and functional analysis of ABSCISIC ACID INSENSITIVE3-like genes from Physcomitrella patens. Plant J. 2006, 46, 1032–1044. [Google Scholar] [CrossRef]
- Swaminathan, K.; Peterson, K.; Jack, T. The plant B3 superfamily. Trends Plant Sci. 2008, 13, 647–655. [Google Scholar] [CrossRef]
- Mizoi, J.; Shinozaki, K.; Yamaguchi-Shinozaki, K. AP2/ERF family transcription factors in plant abiotic stress responses. Biochim. Biophys. Acta 2012, 1819, 86–96. [Google Scholar] [CrossRef]
- Okamuro, J.K.; Caster, B.; Villarroel, R.; Montagu, M.V.; Jofuku, K.D. The AP2 domain of APETALA2 defines a large new family of DNA binding proteins in Arabidopsis. Proc. Natl. Acad. Sci. USA 1997, 94, 7076–7081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saleh, A.; Pagés, M. Plant AP2/ERF transcription factors. Genetika 2003, 35, 37–50. [Google Scholar] [CrossRef]
- Osnato, M.; Matias-Hernandez, L.; Aguilar-Jaramillo, A.E.; Kater, M.M.; Pelaz, S. Genes of the RAV family control heading date and carpel development in rice. Plant Physiol. 2020, 183, 1663–1680. [Google Scholar] [CrossRef]
- Zhao, S.P.; Xu, Z.S.; Zheng, W.J.; Wang, Y.X.; Chen, T.F.; Zhou, Y.B.; Min, D.H.; Ma, Y.Z.; Chai, S.C.; Zhang, X.H. Genome-wide analysis of the RAV family in soybean and functional identifification of GmRAV-03 involvement in salt and drought stresses and exogenous ABA treatment. Front. Plant Sci. 2017, 8, 905. [Google Scholar] [CrossRef] [Green Version]
- Kabir, N.; Lin, H.; Kong, X.; Liu, L.; Qanmber, G.; Wang, Y.X.; Zhang, L.; Sun, Z.; Yang, Z.; Yu, Y.; et al. Identification, evolutionary analysis and functional diversification of RAV gene family in cotton (G. hirsutum L.). Planta 2022, 255, 14. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Deng, Z.; Liang, C.; Sun, H.; Wang, R. Genome-wide analysis of RAV transcription factors and functional characterization of anthocyanin-biosynthesis-related RAV genes in pear. Int. J. Mol. Sci. 2021, 22, 5567. [Google Scholar] [CrossRef]
- Peng, Z.; Wang, M.; Zhang, L.; Jiang, Y.; Zhao, C.; Shahid, M.Q.; Bai, Y.; Hao, J.; Peng, J.; Gao, Y.; et al. EjRAV1/2 delay flowering through transcriptional repression of EjFTs and EjSOC1s in Loquat. Front. Plant Sci. 2021, 12, 816086. [Google Scholar] [CrossRef]
- Kagaya, Y.; Ohmiya, K.; Hattori, T. RAV1, a novel DNA-binding protein, binds to bipartite recognition sequence through two distinct DNA-binding domains uniquely found in higher plants. Nucleic Acids Res. 1999, 27, 470–478. [Google Scholar] [CrossRef]
- Hu, Y.X.; Wang, Y.X.; Liu, X.F.; Li, J.Y. Arabidopsis RAV1 is downregulated by brassinosteroid and may act as a negative regulator during plant development. Cell Res. 2004, 14, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Woo, H.R.; Kim, J.H.; Kim, J.; Kim, J.; Lee, U.; Song, I.J.; Kim, J.H.; Lee, H.Y.; Nam, H.G.; Lim, P.O. The RAV1 transcription factor positively regulates leaf senescence in Arabidopsis. J. Exp. Bot. 2010, 61, 3947–3957. [Google Scholar] [CrossRef] [Green Version]
- Feng, C.Z.; Chen, Y.; Wang, C.; Kong, Y.H.; Wu, W.H.; Chen, Y.F. Arabidopsis RAV1 transcription factor, phosphorylatedby SnRK2 kinases, regulates the expression of ABI3, ABI4, and ABI5 during seed germination and early seedling development. Plant J. 2014, 80, 654–668. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Kang, H.K.; Son, S.H.; Kim, S.K.; Nam, K.H. A subset of Arabidopsis RAV transcription factors modulates drought and salt stress responses independent of ABA. Plant Cell Physiol. 2014, 55, 1892–1904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mittal, A.; Gampala, S.; Ritchie, G.L.; Payton, P.; Burke, J.J.; Rock, C.D. Related to ABA-Insensitive3 (ABI3)/Viviparous1 and AtABI5 transcription factor coexpression in cotton enhances drought stress adaptation. Plant Biotechnol. J. 2014, 12, 578–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castillejo, C.; Pelaz, S. The balance between CONSTANS and TEMPRANILLO activities determines FT expression to trigger flowering. Curr. Bio. 2008, 18, 1338–1343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osnato, M.; Castillejo, C.; Matías-Hernández, L.; Pelaz, S. TEMPRANILLO genes link photoperiod and gibberellin pathways to control flowering in Arabidopsis. Nat. Commun. 2012, 3, 808. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Luo, Q.; Yang, C.; Han, Y.; Li, W. A RAV-like transcription factor controls photosynthesis and senescence in soybean. Planta 2008, 227, 1389–1399. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Guo, T.; Shen, Y.; Wang, Z.; Kang, J.; Zhang, J.; Yi, F.; Yang, Q.; Long, R. Overexpression of MtRAV3 enhances osmotic and salt tolerance and inhibits growth of Medicago truncatula. Plant Physiol. Bioch. 2021, 163, 154–165. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Zhang, K.; Yang, C. Functional roles of the birch BpRAV1 transcription factor in salt and osmotic stress response. Plant Sci. 2022, 315, 111131. [Google Scholar] [CrossRef]
- Sohn, K.H.; Lee, S.C.; Jung, H.W.; Hong, J.K.; Hwang, B.K. Expression and functional roles of the pepper pathogen-induced transcription factor RAV1 in bacterial disease resistance, and drought and salt stress tolerance. Plant Mol. Biol. 2006, 61, 897–915. [Google Scholar] [CrossRef]
- Lee, S.C.; Choi, D.S.; Hwang, I.S.; Hwang, B.K. The pepper oxidoreductase CaOXR1 interacts with the transcription factor CaRAV1 and is required for salt and osmotic stress tolerance. Plant Mol. Biol. 2010, 73, 409–424. [Google Scholar] [CrossRef]
- Li, X.J.; Li, M.; Zhou, Y.; Hu, S.; Hu, R.; Chen, Y.; Li, X.B. Overexpression of cotton RAV1 gene in Arabidopsis confers transgenic plants high salinity and drought sensitivity. PLoS ONE 2015, 10, e0118056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.W.; Su, R.C.; Cheng, C.P.; You, S.J.; Hsieh, T.H.; Chao, T.C.; Chan, M.T. Tomato RAV transcription factor is a pivotal modulator involved in the AP2/EREBP-mediated defense pathway. Plant Physiol. 2011, 156, 213–227. [Google Scholar] [CrossRef] [Green Version]
- Wei, Y.; Chang, Y.; Zeng, H.; Liu, G.; He, C.; Shi, H. RAV transcription factors are essential for disease resistance against cassava bacterial blight via activation of melatonin biosynthesis genes. J. Pineal Res. 2018, 64, e12454. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Yan, Y.; Bai, Y.; Dong, Y.; Wei, Y.; Zeng, H.; Shi, H. Phosphorylation of RAV1/2 by KIN10 is essential for transcriptional activation of CAT6/7, which underlies oxidative stress response in cassava. Cell Rep. 2021, 37, 110119. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Yan, Y.; Lu, Y.; Liu, G.; Liu, J.; Shi, H. The co-modulation of RAV transcription factors in ROS burst and extensive transcriptional reprogramming underlies disease resistance in cassava. Plant Cell Rep. 2022, 41, 1261–1272. [Google Scholar] [CrossRef]
- Zhang, Z.; Shi, Y.; Ma, Y.; Yang, X.; Yin, X.; Zhang, Y.; Xiao, Y.; Liu, W.; Li, Y.; Li, S.; et al. The strawberry transcription factor FaRAV1 positively regulates anthocyanin accumulation by activation of FaMYB10 and anthocyanin pathway genes. Plant Biotechnol. J. 2020, 18, 2267–2279. [Google Scholar] [CrossRef] [Green Version]
- Tian, J.; Wang, C.; Xia, J.; Wu, L.; Xu, G.; Wu, W.; Li, D.; Qin, W.; Han, X.; Chen, Q.; et al. Teosinte ligule allele narrows plant architecture and enhances high-density maize yields. Science 2019, 365, 658–664. [Google Scholar] [CrossRef]
- Tavares, E.Q.P.; Souza1, A.P.D.; Romim, G.H.; Grandis, A.; Plasencia, A.; Buckeridge, M.S. The control of endopolygalacturonase expression by the sugarcane RAV transcription factor during aerenchyma formation. J. Exp. Bot. 2019, 70, 497–506. [Google Scholar] [CrossRef]
- Rosa, S.; Ntoukakis, V.; Ohmido, N.; Pendle, A.; Abranches, R.; Shaw, P. Cell difffferentiation and development in Arabidopsis are associated with changes in histone dynamics at the single-cell level. Plant Cell 2014, 26, 4821–4833. [Google Scholar] [CrossRef] [Green Version]
- Halfter, U.; Ishitani, M.; Zhu, J.K. The Arabidopsis SOS2 protein kinase physically interacts with and is activated by the calcium-binding protein SOS3. Proc. Natl. Acad. Sci. USA 2000, 97, 3735–3740. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ishitani, M.; Halfter, U.; Kim, C.S.; Zhu, J.K. The Arabidopsis thaliana SOS2 gene encodes a protein kinase that is required for salt tolerance. Proc. Natl. Acad. Sci. USA 2000, 97, 3730–3734. [Google Scholar] [CrossRef]
- Shi, H.; Ishitani, M.; Kim, C.; Zhu, J.K. The Arabidopsis thaliana salt tolerance gene SOS1 encodes a putative Na+/H+ antiporter. Proc. Natl. Acad. Sci. USA 2000, 97, 6896–6901. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi-Shinozaki, K.; Shinozaki, K. The plant hormone abscisic acid mediates the drought-induced expression but not the seed-specific expression of RD22, a gene responsive to dehydration stress in Arabidopsis thaliana. Mol. Gen. Genet. 1993, 238, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi-Shinozaki, K. Characterization of the expression of a desiccation-responsive RD29 gene of Arabidopsis thaliana and analysis of its promoter in transgenic plants. Mol. Gen. Genet. 1993, 236, 331–340. [Google Scholar] [CrossRef]
- Shewry, P.R. Wheat. J. Exp. Bot. 2009, 60, 1537–1553. [Google Scholar] [CrossRef]
- Kagaya, Y.; Hattori, T. Arabidopsis transcription factors, RAV1 and RAV2, are regulated by touch-related stimuli in a dose-dependent and biphasic manner. Genes Genet. Syst. 2009, 84, 95–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhuang, J.; Sun, C.C.; Zhou, X.R.; Xiong, A.S.; Zhang, J. Isolation and characterization of an AP2/ERF-RAV transcription factor Bnarav-1-HY15 in Brassica napus L. HuYou15. Mol. Biol. Rep. 2010, 38, 3921–3928. [Google Scholar] [CrossRef] [PubMed]
- Bolser, D.M.; Staines, D.M.; Perry, E.; Kersey, P.J. Ensembl plants: Integrating tools for visualizing, mining, and analyzing plant genomic data. Methods Mol. Biol. 2017, 1533, 1–31. [Google Scholar]
- Mistry, J.; Chuguransky, S.; Williams, L.; Qureshi, M.; Salazar, G.A.; Sonnhammer, E.L.L.; Tosatto, S.C.E.; Paladin, L.; Raj, S.; Richardson, L.J.; et al. Pfam: The protein families database in 2021. Nucleic Acids Res. 2021, 49, D412–D419. [Google Scholar] [CrossRef]
- Marchler-Bauer, A.; Derbyshire, M.K.; Gonzales, N.R.; Lu, S.; Chitsaz, F.; Geer, L.Y.; Geer, R.C.; He, J.; Gwadz, M.; Hurwitz, D.I.; et al. CDD: NCBI’s conserved domain database. Nucleic Acids Res. 2015, 43, D222–D226. [Google Scholar] [CrossRef] [Green Version]
- Letunic, I.; Khedkar, S.; Bork, P. SMART: Recent updates, new developments and status in 2020. Nucleic Acids Res. 2021, 49, D458–D460. [Google Scholar] [CrossRef] [PubMed]
- Gasteiger, E.; Gattiker, A.; Hoogland, C.; Ivanyi, I.; Appel, R.D.; Bairoch, A. ExPASy: The proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 2003, 31, 3784–3788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, C.S.; Chen, Y.C.; Lu, C.H.; Hwang, J.K. Prediction of protein subcellular localization. Proteins 2006, 64, 643–651. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, H.; Debarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.; Jin, H.; Marler, B.; Guo, H. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krzywinski, M.; Schein, J.; Birol, I.; Connors, J.; Gascoyne, R.; Horsman, D.; Jones, S.J.; Marra, M.A. Circos: An information aesthetic for comparative genomics. Genome Res. 2009, 19, 1639–1645. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Gong, Z.; Koiwa, H.; Niu, X.; Hasegawa, P.M. Bar-expressing peppermint (Mentha × Piperita L. var. Black Mitcham) plants are highly resistant to the glufosinate herbicide liberty. Mol. Breed. 2001, 8, 109–118. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Y.-X.; Chen, S.-K.; Wang, P.-D.; Peng, D.; Zhang, X.; Li, H.-F.; Feng, C.-Z. Genome-Wide Analysis of the RAV Gene Family in Wheat and Functional Identification of TaRAV1 in Salt Stress. Int. J. Mol. Sci. 2022, 23, 8834. https://doi.org/10.3390/ijms23168834
Luo Y-X, Chen S-K, Wang P-D, Peng D, Zhang X, Li H-F, Feng C-Z. Genome-Wide Analysis of the RAV Gene Family in Wheat and Functional Identification of TaRAV1 in Salt Stress. International Journal of Molecular Sciences. 2022; 23(16):8834. https://doi.org/10.3390/ijms23168834
Chicago/Turabian StyleLuo, Yun-Xin, Shou-Kun Chen, Peng-Dan Wang, De Peng, Xu Zhang, Hai-Feng Li, and Cui-Zhu Feng. 2022. "Genome-Wide Analysis of the RAV Gene Family in Wheat and Functional Identification of TaRAV1 in Salt Stress" International Journal of Molecular Sciences 23, no. 16: 8834. https://doi.org/10.3390/ijms23168834
APA StyleLuo, Y.-X., Chen, S.-K., Wang, P.-D., Peng, D., Zhang, X., Li, H.-F., & Feng, C.-Z. (2022). Genome-Wide Analysis of the RAV Gene Family in Wheat and Functional Identification of TaRAV1 in Salt Stress. International Journal of Molecular Sciences, 23(16), 8834. https://doi.org/10.3390/ijms23168834




