Regiospecific Grafting of Chitosan Oligomers Brushes onto Silicon Wafers
Abstract
:1. Introduction
2. Results and Discussion
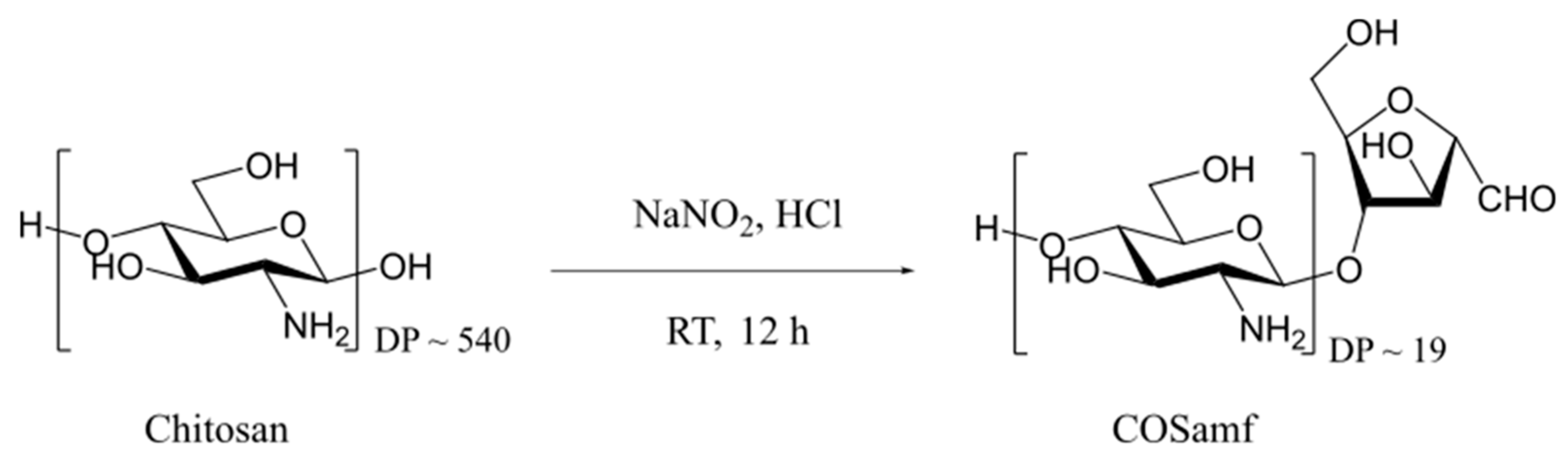
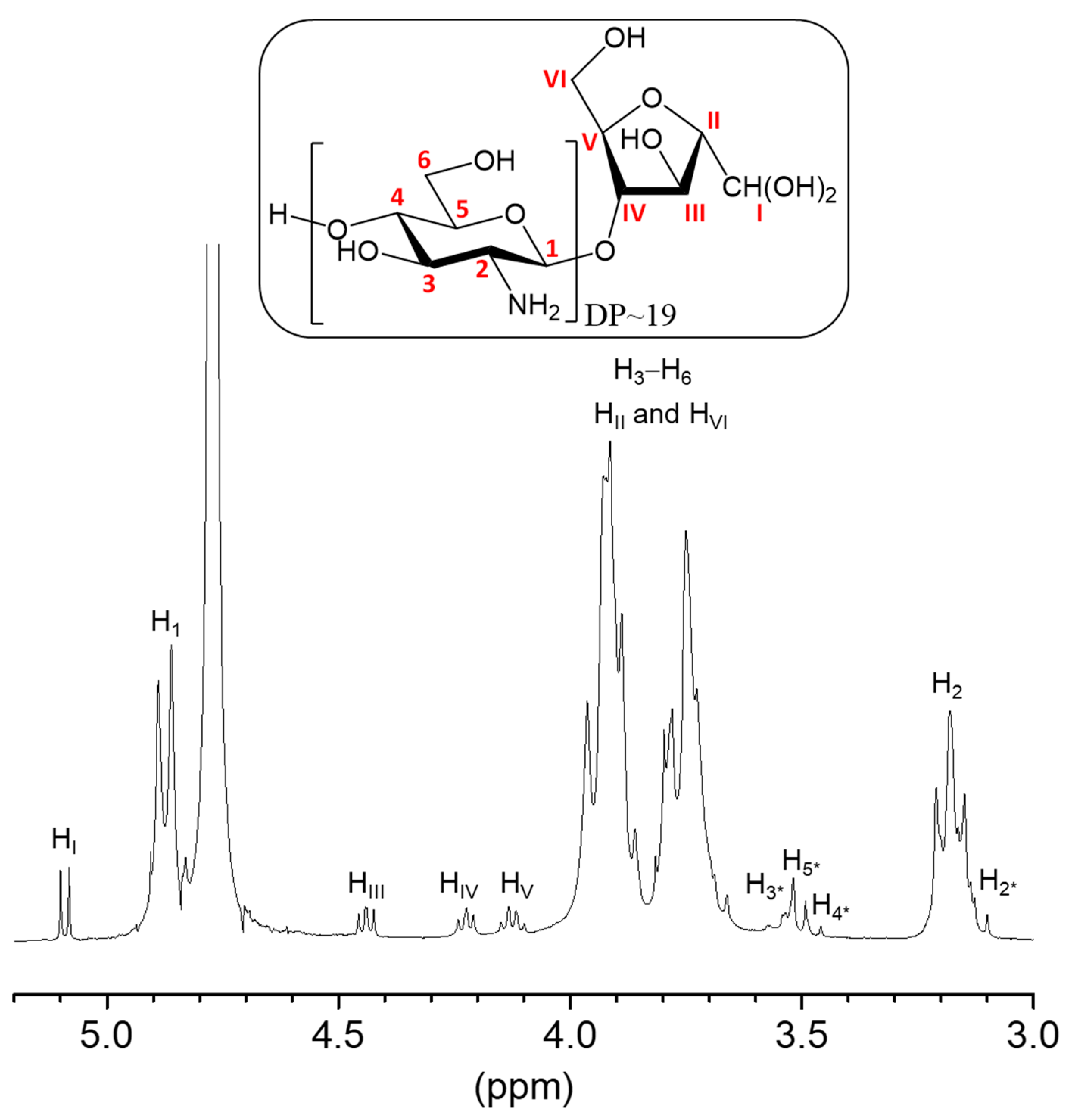
2.1. Synthesis and Characterization of Amf-Terminated Chitosan Oligomers (COSamf)
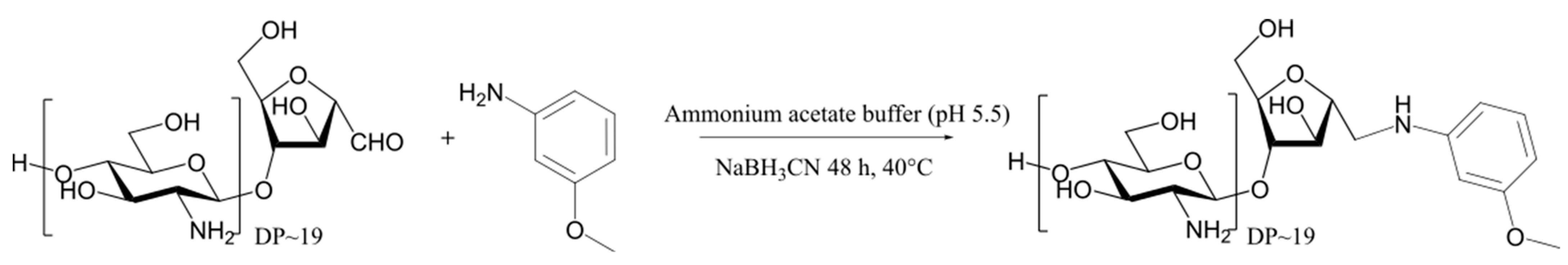
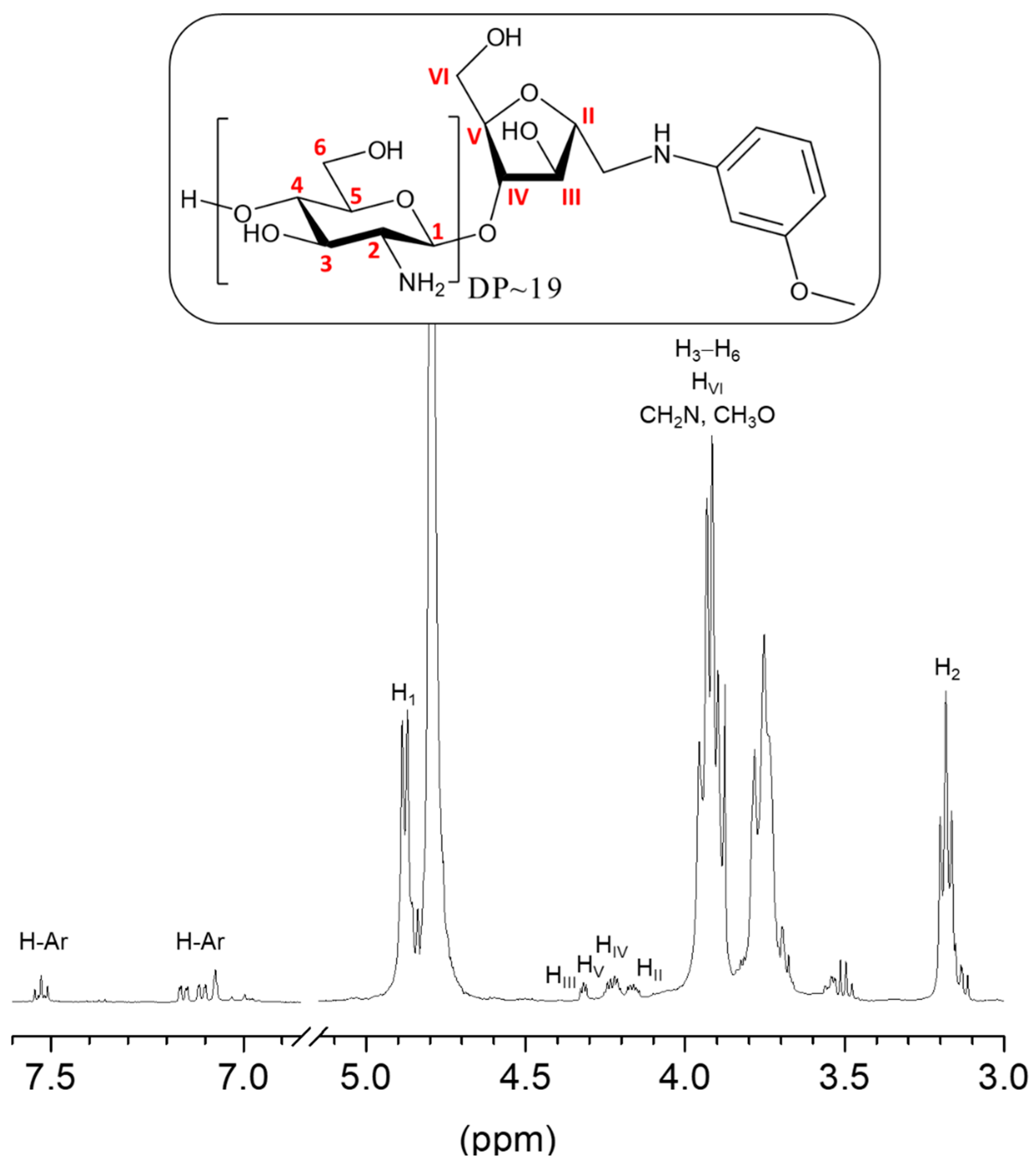
2.2. Reductive Amination of COSamf with 3-Methoxyaniline
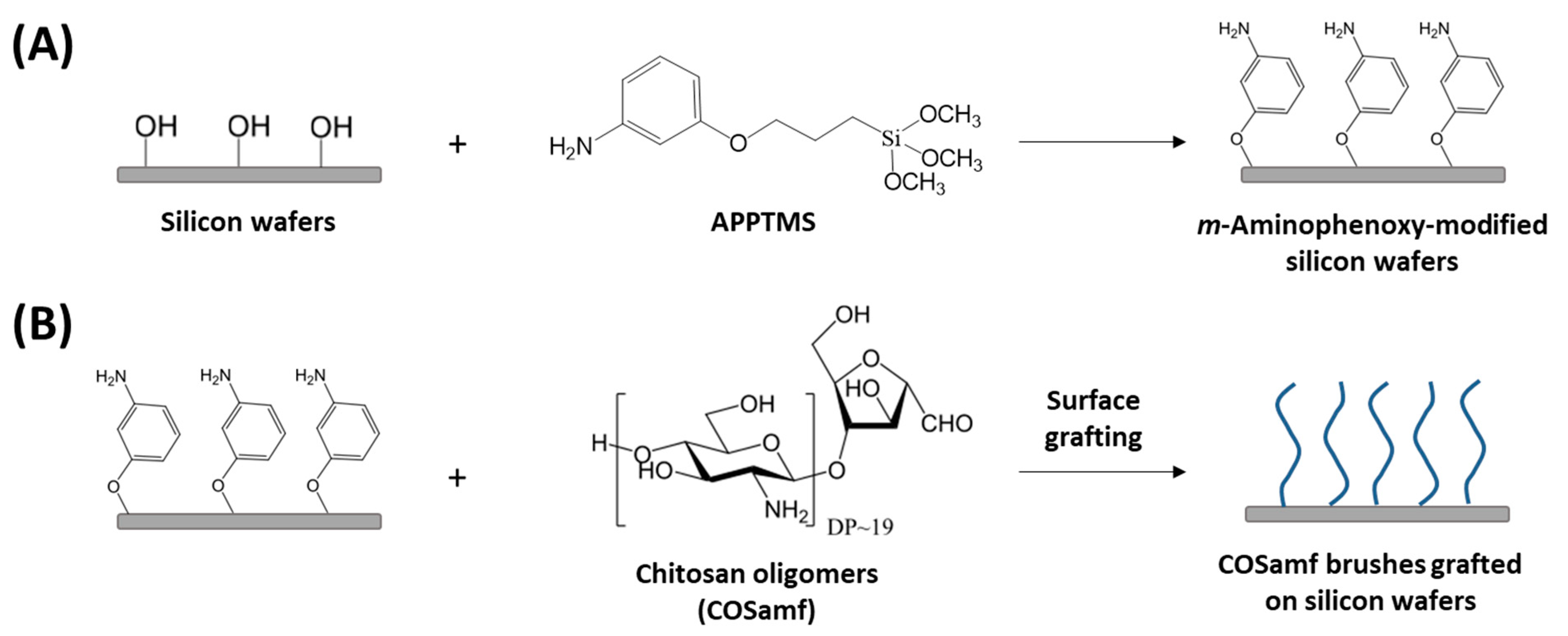
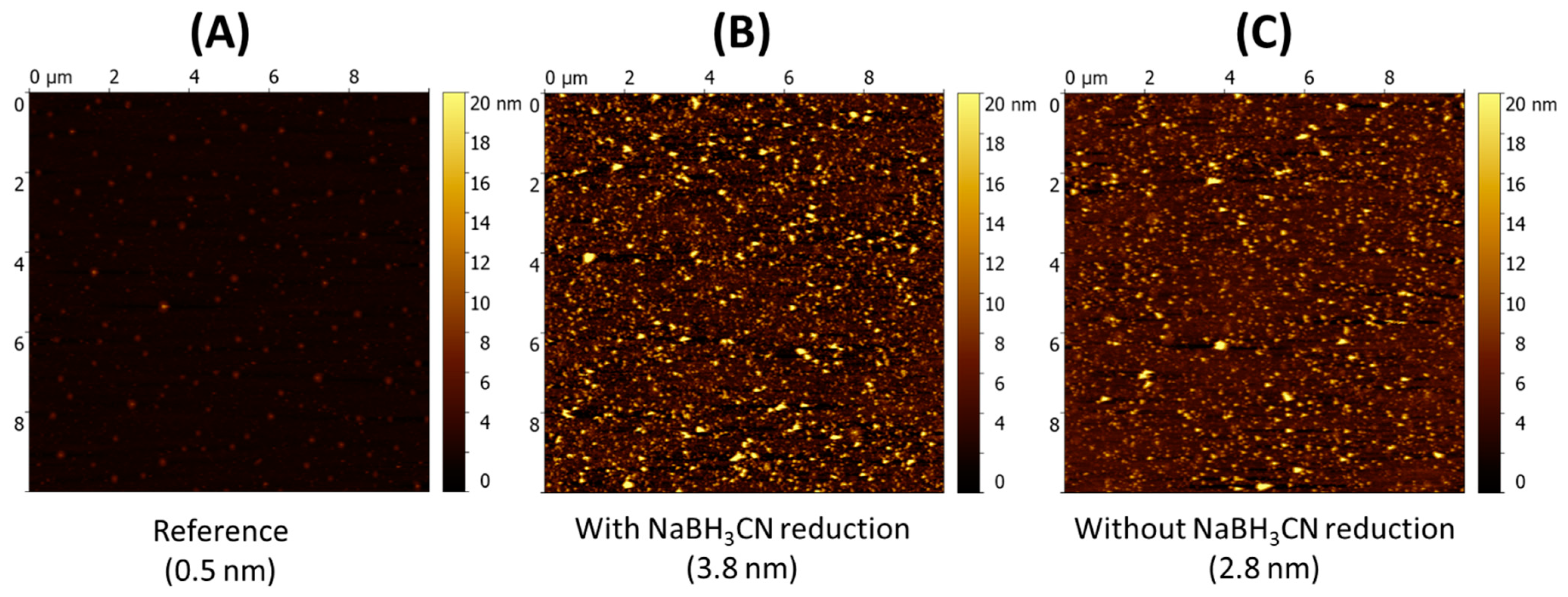
2.3. Grafting of COSamf onto M-Aminophenoxy-Modified Silicon Wafers
2.3.1. Silanization of Silicon Wafers with APPTMS
2.3.2. Grafting of COSamf Brushes Onto Silicon Wafers
- (a)
- Optimization of the grafting process using ATR-FTIR
- (b)
- Physicochemical characterization of the grafted COSamf brushes
3. Materials and Methods
3.1. Materials
3.2. Synthesis and Characterization of Amf-Terminated Chitosan Oligomers (COSamf)
3.2.1. Synthesis of COSamf (Average DP ~19 and DA ~0%)
3.2.2. Reductive Amination of COSamf with 3-Methoxyaniline
3.3. Functionalization and Characterization of Silicon Wafers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elieh-Ali-Komi, D.; Hamblin, M.R. Chitin and Chitosan: Production and Application of Versatile Biomedical Nanomaterials. Int. J. Adv. Res. 2016, 4, 411–427. [Google Scholar]
- Eivazzadeh-Keihan, R.; Noruzi, E.B.; Mehrban, S.F.; Aliabadi, H.A.M.; Karimi, M.; Mohammadi, A.; Maleki, A.; Mahdavi, M.; Larijani, B.; Shalan, A.E. Review: The latest advances in biomedical applications of chitosan hydrogel as a powerful natural structure with eye-catching biological properties. J. Mater. Sci. 2022, 57, 3855–3891. [Google Scholar] [CrossRef]
- Wang, H.X.; Qan, J.; Ding, F.Y. Emerging Chitosan-Based Films for Food Packaging Applications. J. Agric. Food Chem. 2018, 66, 395–413. [Google Scholar] [CrossRef] [PubMed]
- Kou, S.J.; Peters, L.; Mucalo, M. Chitosan: A review of molecular structure, bioactivities and interactions with the human body and micro-organisms. Carbohydr. Polym. 2022, 282, 119132. [Google Scholar] [CrossRef] [PubMed]
- Hamed, I.; Özogul, F.; Regenstein, J.M. Industrial applications of crustacean by-products (chitin, chitosan, and chitooligosaccharides): A review. Trends Food Sci. Technol. 2016, 48, 40–50. [Google Scholar] [CrossRef]
- Morin-Crini, N.; Lichtfouse, E.; Torri, G.; Crini, G. Applications of chitosan in food, pharmaceuticals, medicine, cosmetics, agriculture, textiles, pulp and paper, biotechnology, and environmental chemistry. Environ. Chem. Lett. 2019, 17, 1667–1692. [Google Scholar]
- Wang, W.; Xue, C.; Mao, X. Chitosan: Structural modification, biological activity and application. Int. J. Biol. Macromol. 2020, 164, 4532–4546. [Google Scholar] [CrossRef]
- Muxika, A.; Etxabide, A.; Uranga, J.; Guerrero, P.; de la Caba, K. Chitosan as a bioactive polymer: Processing, properties and applications. Int. J. Biol. Macromol. 2017, 105, 1358–1368. [Google Scholar] [CrossRef]
- Liaqat, F.; Eltem, R. Chitooligosaccharides and their biological activities: A comprehensive review. Carbohydr. Polym. 2018, 184, 243–259. [Google Scholar] [CrossRef]
- Liang, S.; Sun, Y.; Dai, X. A Review of the Preparation, Analysis and Biological Functions of Chitooligosaccharide. Int. J. Mol. Sci. 2018, 19, 2197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naveed, M.; Phil, L.; Sohail, M.; Hasnat, M.; Baig, M.M.F.A.; Ihsan, A.U.; Shumzaid, M.; Kakar, M.U.; Khan, T.M.; Akabar, M.D.; et al. Chitosan oligosaccharide (COS): An overview. Int. J. Biol. Macromol. 2019, 129, 827–843. [Google Scholar] [CrossRef] [PubMed]
- Lodhi, G.; Kim, Y.S.; Hwang, J.W.; Kim, S.K.; Jeon, Y.J.; Je, J.Y.; Ahn, C.B.; Moon, S.H.; Jeon, B.T.; Park, P.J. Chitooligosaccharide and its derivatives: Preparation and biological applications. Biomed Res. Int. 2014, 2014, 654913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mourya, V.K.; Inamdar, N.N.; Choudhari, Y.M. Chitooligosaccharides: Synthesis, characterization and applications. Polym. Sci.-A 2011, 53, 583–612. [Google Scholar] [CrossRef]
- Li, K.; Xing, R.; Liu, S.; Li, P. Advances in preparation, analysis and biological activities of single chitooligosaccharides. Carbohydr. Polym. 2016, 139, 178–190. [Google Scholar] [CrossRef]
- Cord-Landwehr, S.; Richter, C.; Wattjes, J.; Sreekumar, S.; Singh, R.; Basa, S.; El Gueddari, N.E.; Moerschbacher, B.M. Patterns matter part 2: Chitosan oligomers with defined patterns of acetylation. React. Funct. Polym. 2020, 151, 104577. [Google Scholar] [CrossRef]
- Schmitz, C.; González Auza, L.; Koberidze, D.; Rasche, S.; Fischer, R.; Bortesi, L. Conversion of Chitin to Defined Chitosan Oligomers: Current Status and Future Prospects. Mar. Drugs 2019, 17, 452. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.S.; Eckmann, D.M.; Lee, D.; Hickok, N.J.; Composto, R.J. Symmetric pH-dependent swelling and antibacterial properties of chitosan brushes. Langmuir 2011, 27, 12458–12465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.S.; Yee, M.Q.; Eckmann, Y.Y.; Hickok, N.J.; Eckmann, D.M.; Composto, R.J. Reversible swelling of chitosan and quaternary ammonium modified chitosan brush layers: Effects of pH and counter anion size and functionality. J. Mater. Chem. 2012, 22, 19605–19616. [Google Scholar] [CrossRef]
- Huang, H.; Jin, Y.; Xue, M.; Yu, L.; Fu, Q.; Ke, Y.; Chu, C.; Liang, X. A novel click chitooligosaccharide for hydrophilic interaction liquid chromatography. Chem. Commun. 2009, 45, 6973–6975. [Google Scholar] [CrossRef]
- Illy, N.; Robitzer, M.; Auvergne, R.; Caillol, S.; David, G.; Boutevin, B. Synthesis of water-soluble allyl-functionalized oligochitosan and its modification by thiol-ene addition in water. J. Polym. Sci. Part A-1 Polym. Chem. 2014, 52, 39–48. [Google Scholar] [CrossRef]
- Jain, A.; Gulbake, A.; Shilpi, S.; Jain, A.; Hurkat, P.; Jain, S.K. A New Horizon in Modifications of Chitosan: Syntheses and Applications. Crit. Rev. Ther. Drug Carrier Syst. 2013, 30, 91–181. [Google Scholar] [CrossRef]
- Maria Marzaioli, A.; Bedini, E.; Lanzetta, R.; Perino, V.; Parrilli, M.; De Castro, C. Preparation and NMR characterization of glucosamine oligomers bearing an azide function using chitosan. Carbohydr. Polym. 2012, 90, 847–852. [Google Scholar] [CrossRef] [PubMed]
- Coudurier, M.; Faivre, J.; Crépet, A.; Ladavière, C.; Delair, T.; Schatz, C.; Trombotto, S. Reducing-end functionalization of 2,5-anhydro-d-mannofuranose-linked chitooligosaccharides by dioxyamine: Synthesis and characterization. Molecules 2020, 25, 1143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guerry, A.; Bernard, J.; Samain, E.; Fleury, E.; Cottaz, S.; Halila, S. Aniline-catalyzed reductive amination as a powerful method for the preparation of reducing end-”clickable” chitooligosaccharides. Bioconjug. Chem. 2013, 24, 544–549. [Google Scholar] [CrossRef]
- Moussa, A.; Crépet, A.; Ladavière, C.; Trombotto, S. Reducing-end “clickable” functionalizations of chitosan oligomers for the synthesis of chitosan-based diblock copolymers. Carbohydr. Polym. 2019, 219, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Pickenhahn, V.D.; Darras, V.; Dziopa, F.; Biniecki, K.; De Crescenzo, G.; Lavertu, M.; Buschmann, M.D. Regioselective thioacetylation of chitosan end-groups for nanoparticle gene delivery systems. Chem. Sci. 2015, 6, 4650–4664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salim, E.; Galais, A.; Trombotto, S. 4-(Hexyloxy)aniline-linked chitooligosaccharide-2,5-anhydro-d-mannofuranose. Molbank 2014, 2014, M815. [Google Scholar] [CrossRef]
- Allan, G.G.; Peyron, M. Molecular weight manipulation of chitosan I: Kinetics of depolymerization by nitrous acid. Carbohydr. Res. 1995, 277, 257–272. [Google Scholar] [CrossRef]
- Hussain, I.; Singh, T.; Chittenden, C. Preparation of chitosan oligomers and characterization: Their antifungal activities and decay resistance. Holzforschung 2012, 66, 119–125. [Google Scholar] [CrossRef]
- Tommeraas, K.; Varum, K.M.; Christensen, B.E.; Smidsrød, O. Preparation and characterization of oligosaccharides produced by nitrous acid depolymerization of chitosan. Carbohydr. Res. 2001, 333, 137–144. [Google Scholar] [CrossRef]
- Salim, E.; Ailincai, D.; Trombotto, S. Chitooligosaccharide-2,5-anhydro-d-mannonic acid. MolBank 2014, 2014, 4–7. [Google Scholar] [CrossRef] [Green Version]
- Schatz, C.; Viton, C.; Delair, T.; Pichot, C.; Domard, A. Typical physicochemical behaviors of chitosan in aqueous solution. Biomacromolecules 2003, 4, 641–648. [Google Scholar] [CrossRef]
- Haensch, C.; Hoeppener, S.; Schubert, U.S. Chemical modification of self-assembled silane based monolayers by surface reactions. Chem. Soc. Rev. 2010, 39, 2323–2334. [Google Scholar] [CrossRef] [PubMed]
- Diallo, M. Preparation of Model Surfaces Modified by Chitosan: Physicochemical Characterizations and Biological Response. Ph.D. Thesis, University of Lyon, Lyon, France, 30 May 2018. [Google Scholar]
- Vachoud, L.; Zydowicz, N.; Domard, A. Formation and characterisation of a physical chitin gel. Carbohydr. Res. 1997, 302, 169–177. [Google Scholar] [CrossRef]
- Hirai, A.; Odani, H.; Nakajima, A. Determination of degree of deacetylation of chitosan by 1H NMR spectroscopy. Polym. Bull. 1991, 26, 87–94. [Google Scholar] [CrossRef]
- Sugiyama, H.; Hisamichi, K.; Sakai, K.; Usui, T.; Ishiyama, J.I.; Kudo, H.; Ito, H.; Senda, Y. The conformational study of chitin and chitosan oligomers in solution. Bioorg. Med. Chem. 2001, 9, 211–216. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garreau, C.; Gablin, C.; Léonard, D.; Delair, T.; Sudre, G.; Trombotto, S. Regiospecific Grafting of Chitosan Oligomers Brushes onto Silicon Wafers. Int. J. Mol. Sci. 2022, 23, 8013. https://doi.org/10.3390/ijms23148013
Garreau C, Gablin C, Léonard D, Delair T, Sudre G, Trombotto S. Regiospecific Grafting of Chitosan Oligomers Brushes onto Silicon Wafers. International Journal of Molecular Sciences. 2022; 23(14):8013. https://doi.org/10.3390/ijms23148013
Chicago/Turabian StyleGarreau, Cyrielle, Corinne Gablin, Didier Léonard, Thierry Delair, Guillaume Sudre, and Stéphane Trombotto. 2022. "Regiospecific Grafting of Chitosan Oligomers Brushes onto Silicon Wafers" International Journal of Molecular Sciences 23, no. 14: 8013. https://doi.org/10.3390/ijms23148013
APA StyleGarreau, C., Gablin, C., Léonard, D., Delair, T., Sudre, G., & Trombotto, S. (2022). Regiospecific Grafting of Chitosan Oligomers Brushes onto Silicon Wafers. International Journal of Molecular Sciences, 23(14), 8013. https://doi.org/10.3390/ijms23148013





