Mechanical Stretch-Induced NLRP3 Inflammasome Expression on Human Annulus Fibrosus Cells Modulated by Endoplasmic Reticulum Stress
Abstract
1. Introduction
2. Results
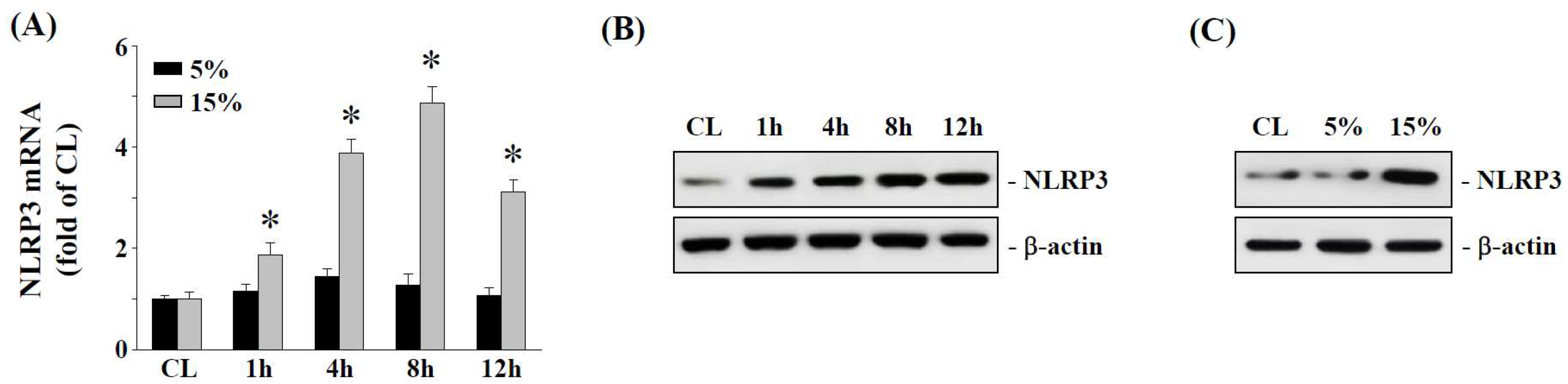
2.1. The 15% HCS Induced NLRP3 Expression in Human AF Cells
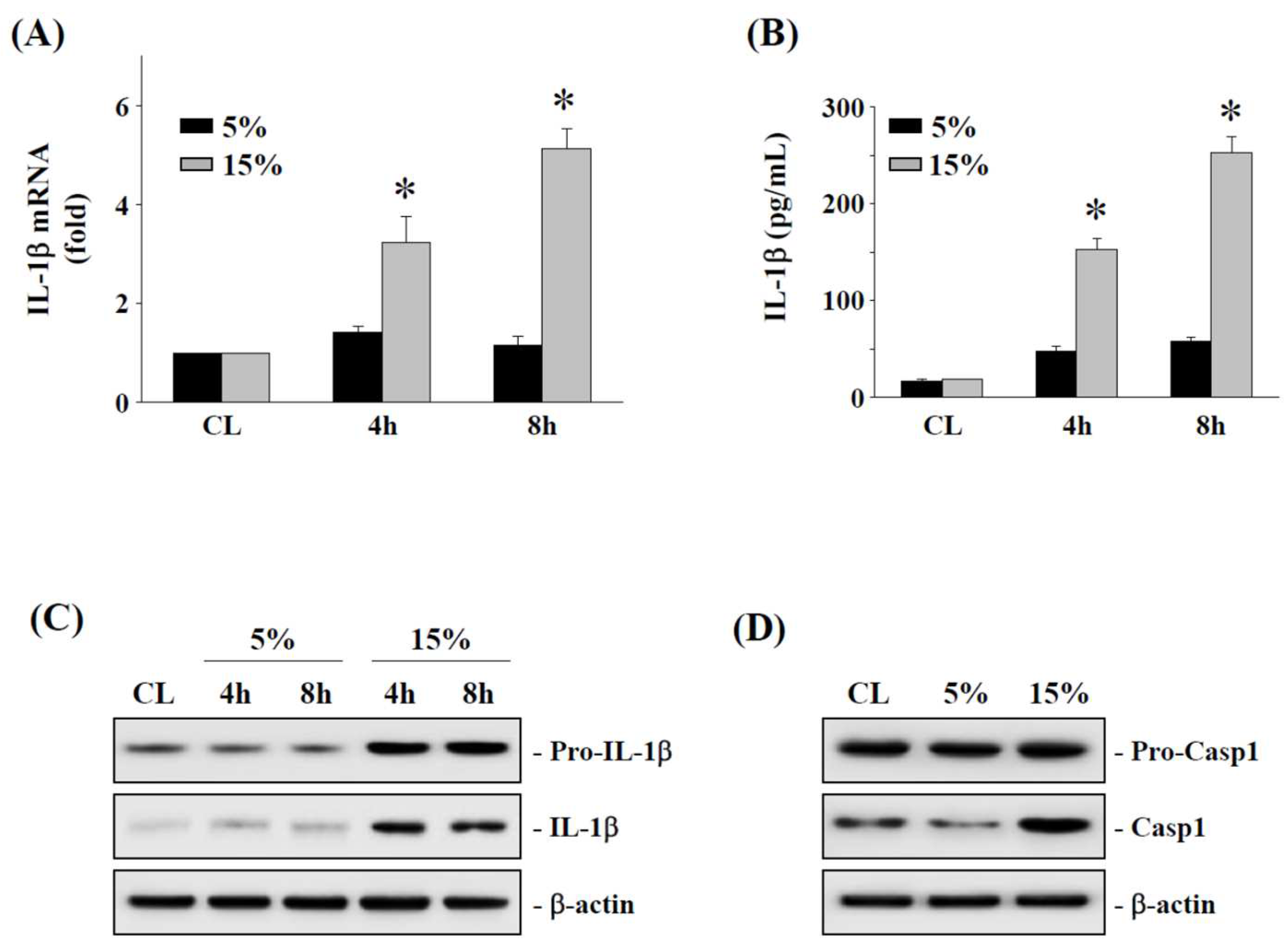
2.2. The 15% HCS Induced the Expression of IL-1β in Human AF Cells
2.3. Effects of 15% HCS on NOX2 Expression and ROS Production in Human AF Cells
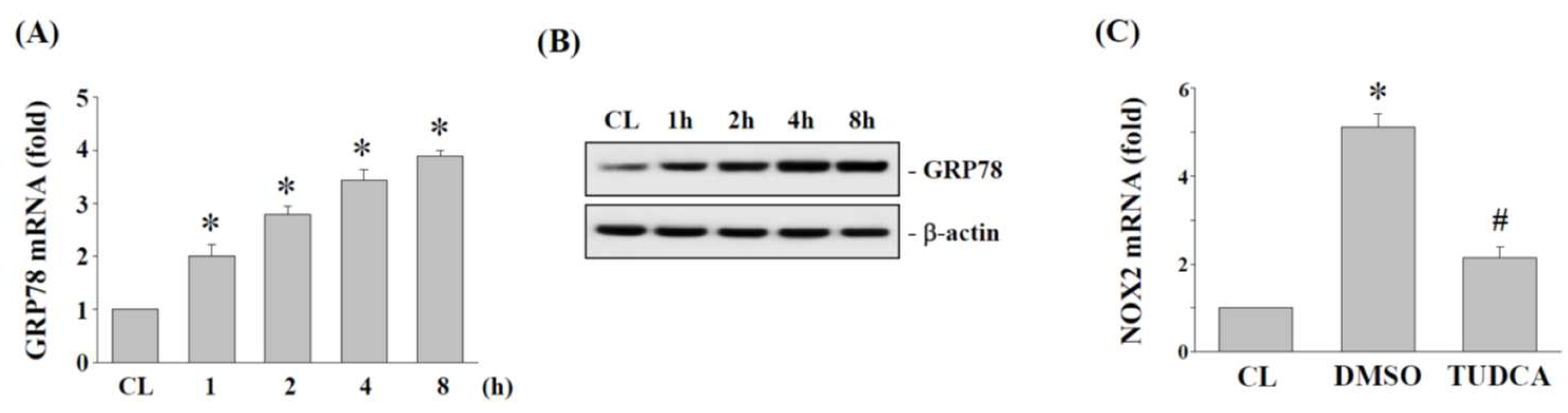
2.4. ER Stress Regulates NOX2 Expression in Human AF Cells under 15% HCS
2.5. TXNIP and NLRP3 Expression Is Regulated by NOX2 and ER Stress in Human AF Cells under 15% HCS
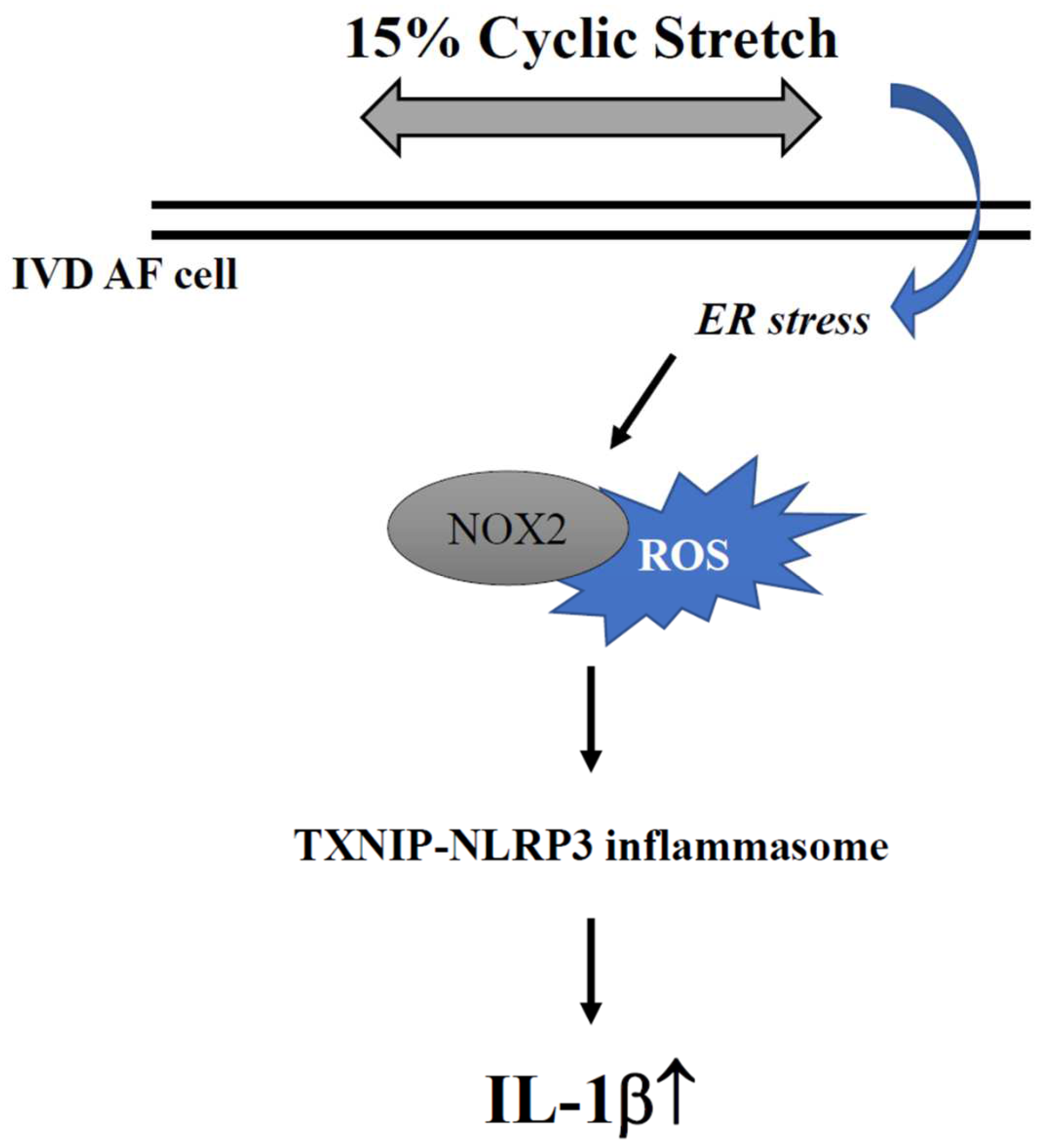
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Culture of AF Cell Lines
4.3. Cyclic Stretching
4.4. Real-Time Quantitative PCR
4.5. IL-1β Enzyme-Linked Immunosorbent Assay (ELISA)
4.6. Western Blot Analysis
4.7. siRNA Transfection
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luoma, K.; Riihimaki, H.; Luukkonen, R.; Raininko, R.; Viikari-Juntura, E.; Lamminen, A. Low back pain in relation to lumbar disc degeneration. Spine 2000, 25, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Katz, J.N. Lumbar disc disorders and low-back pain: Socioeconomic factors and consequences. J. Bone Joint Surg. Am. 2006, 88 (Suppl. S2), 21–24. [Google Scholar] [CrossRef] [PubMed]
- Gawri, R.; Rosenzweig, D.H.; Krock, E.; Ouellet, J.A.; Stone, L.S.; Quinn, T.M.; Haglund, L. High mechanical strain of primary intervertebral disc cells promotes secretion of inflammatory factors associated with disc degeneration and pain. Arthr. Res. Ther. 2014, 16, R21. [Google Scholar] [CrossRef]
- Urban, J.P.; Roberts, S. Degeneration of the intervertebral disc. Arthr. Res. Ther. 2003, 5, 120–130. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vo, N.V.; Hartman, R.A.; Patil, P.R.; Risbud, M.V.; Kletsas, D.; Iatridis, J.C.; Hoyland, J.A.; Le Maitre, C.L.; Sowa, G.A.; Kang, J.D. Molecular mechanisms of biological aging in intervertebral discs. J. Orthop. Res. 2016, 34, 1289–1306. [Google Scholar] [CrossRef]
- Walter, B.A.; Korecki, C.L.; Purmessur, D.; Roughley, P.J.; Michalek, A.J.; Iatridis, J.C. Complex loading affects intervertebral disc mechanics and biology. Osteoarthr. Cartil. 2011, 19, 1011–1018. [Google Scholar] [CrossRef]
- Khan, A.N.; Jacobsen, H.E.; Khan, J.; Filippi, C.G.; Levine, M.; Lehman, R.A.; Jr Riew, K.D.; Lenke, L.G.; Chahine, N.O. Inflammatory biomarkers of low back pain and disc degeneration: A review. Ann. N. Y. Acad. Sci. 2017, 1410, 68–84. [Google Scholar] [CrossRef]
- Huang, Y.C.; Urban, J.P.; Luk, K.D. Intervertebral disc regeneration: Do nutrients lead the way? Nat. Rev. Rheumatol. 2014, 10, 561–566. [Google Scholar] [CrossRef]
- Chan, D.; Song, Y.; Sham, P.; Cheung, K.M. Genetics of disc degeneration. Eur. Spine J. 2006, 15 (Suppl. S3), S317–S325. [Google Scholar] [CrossRef]
- Adams, M.A.; Freeman, B.J.; Morrison, H.P.; Nelson, I.W.; Dolan, P. Mechanical initiation of intervertebral disc degeneration. Spine 2000, 25, 1625–1636. [Google Scholar] [CrossRef]
- Humzah, M.D.; Soames, R.W. Human intervertebral disc: Structure and function. Anat. Rec. 1988, 220, 337–356. [Google Scholar] [CrossRef] [PubMed]
- Stokes, I.A.F.; Iatridis, J.C. Mechanical conditions that accelerate intervertebral disc degeneration: Overload versus immobilization. Spine 2004, 29, 2724–2732. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Lin, Z.; Deng, K.; Shao, B.; Yang, D. Tension induces intervertebral disc degeneration via endoplasmic reticulum stress-mediated autophagy. Biosci. Rep. 2019, 39, BSR20190578. [Google Scholar] [CrossRef] [PubMed]
- Goswami, P.; Gupta, S.; Biswas, J.; Joshi, N.; Swarnkar, S.; Nath, C.; Singh, S. Endoplasmic Reticulum Stress Plays a Key Role in Rotenone-Induced Apoptotic Death of Neurons. Mol. Neurobiol. 2016, 53, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Nakka, V.P.; Prakash-Babu, P.; Vemuganti, R. Crosstalk Between Endoplasmic Reticulum Stress, Oxidative Stress, and Autophagy: Potential Therapeutic Targets for Acute CNS Injuries. Mol. Neurobiol. 2016, 53, 532–544. [Google Scholar] [CrossRef]
- Rodella, L.F.; Ricci, F.; Borsani, E.; Stacchiotti, A.; Foglio, E.; Favero, G.; Rezzani, R.; Mariani, C.; Bianchi, R. Aluminium exposure induces Alzheimer’s disease-like histopathological alterations in mouse brain. Histol. Histopathol. 2008, 23, 433–439. [Google Scholar]
- Wang, H.Q.; Takahashi, R. Expanding insights on the involvement of endoplasmic reticulum stress in Parkinson’s disease. Antioxid. Redox Signal. 2007, 9, 553–561. [Google Scholar] [CrossRef]
- Rellmann, Y.; Eidhof, E.; Dreier, R. Review: ER stress-induced cell death in osteoarthritic cartilage. Cell Signal. 2021, 78, 109880. [Google Scholar] [CrossRef]
- Zhao, C.Q.; Zhang, Y.H.; Jiang, S.D.; Jiang, L.S.; Dai, L.Y. Both endoplasmic reticulum and mitochondria are involved in disc cell apoptosis and intervertebral disc degeneration in rats. Age 2010, 32, 161–177. [Google Scholar] [CrossRef]
- Wang, W.; Qing, X.; Wang, B.; Ma, K.; Wei, Y.; Shao, Z. Tauroursodeoxycholic Acid Protects Nucleus Pulposus Cells from Compression-Induced Apoptosis and Necroptosis via Inhibiting Endoplasmic Reticulum Stress. Evid. Based Complement. Alternat. Med. 2018, 2018, 6719460. [Google Scholar] [CrossRef]
- Sun, Y.; Leng, P.; Song, M.; Li, D.; Guo, P.; Xu, X.; Gao, H.; Li, Z.; Li, C.; Zhang, H. Piezo1 activates the NLRP3 inflammasome in nucleus pulposus cell-mediated by Ca2+/NF-κB pathway. Int. Immunopharmacol. 2020, 85, 106681. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.H.; Zhao, C.Q.; Jiang, L.S.; Dai, L.Y. Cyclic stretch-induced apoptosis in rat annulus fibrosus cells is mediated in part by endoplasmic reticulum stress through nitric oxide production. Eur. Spine J. 2011, 20, 1233–1243. [Google Scholar] [CrossRef] [PubMed]
- Rannou, F.; Lee, T.S.; Zhou, R.H.; Chin, J.; Lotz, J.C.; Mayoux-Benhamou, M.A.; Barbet, J.P.; Chevrot, A.; Shyy, J.Y. Intervertebral disc degeneration: The role of the mitochondrial pathway in annulus fibrosus cell apoptosis induced by overload. Am. J. Pathol. 2004, 164, 915–924. [Google Scholar] [CrossRef]
- Grebe, A.; Hoss, F.; Latz, E. NLRP3 Inflammasome and the IL-1 Pathway in Atherosclerosis. Circ. Res. 2018, 122, 1722–1740. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.M.; Kim, J.J.; Kim, H.J.; Shong, M.; Ku, B.J.; Jo, E.K. Upregulated NLRP3 inflammasome activation in patients with type 2 diabetes. Diabetes 2013, 62, 194–204. [Google Scholar] [CrossRef]
- Ising, C.; Venegas, C.; Zhang, S.; Scheiblich, H.; Schmidt, S.V.; Vieira-Saecker, A.; Schwartz, S.; Albasset, S.; McManus, R.M.; Tejera, D.; et al. NLRP3 inflammasome activation drives tau pathology. Nature 2019, 575, 669–673. [Google Scholar] [CrossRef]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef]
- Cordaro, M.; Fusco, R.; D’Amico, R.; Siracusa, R.; Peritore, A.F.; Gugliandolo, E.; Genovese, T.; Crupi, R.; Mandalari, G.; Cuzzocrea, S.; et al. Cashew (Anacardium occidentale L.) Nuts Modulate the Nrf2 and NLRP3 Pathways in Pancreas and Lung after Induction of Acute Pancreatitis by Cerulein. Antioxidants 2020, 9, 992. [Google Scholar] [CrossRef]
- D’Amico, R.; Fusco, R.; Siracusa, R.; Impellizzeri, D.; Peritore, A.F.; Gugliandolo, E.; Interdonato, L.; Sforza, A.M.; Crupi, R.; Cuzzocrea, S.; et al. Inhibition of P2X7 Purinergic Receptor Ameliorates Fibromyalgia Syndrome by Suppressing NLRP3 Pathway. Int. J. Mol. Sci. 2021, 22, 6471. [Google Scholar] [CrossRef]
- Liao, T.; Ding, L.; Wu, P.; Zhang, L.; Li, X.; Xu, B.; Zhang, H.; Ma, Z.; Xiao, Y.; Wang, P. Chrysin Attenuates the NLRP3 Inflammasome Cascade to Reduce Synovitis and Pain in KOA Rats. Drug Des. Devel. Ther. 2020, 14, 3015–3027. [Google Scholar] [CrossRef]
- Chao-Yang, G.; Peng, C.; Hai-Hong, Z. Roles of NLRP3 inflammasome in intervertebral disc degeneration. Osteoarthr. Cartil. 2021, 29, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Yang, M.; Lan, M.; Liu, C.; Zhang, Y.; Huang, B.; Liu, H.; Zhou, Y. ROS: Crucial Intermediators in the Pathogenesis of Intervertebral Disc Degeneration. Oxid. Med. Cell Longev. 2017, 2017, 5601593. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.Y.; Ahmad, N.; Haqqi, T.M. Oxidative stress and inflammation in osteoarthritis pathogenesis: Role of polyphenols. Biomed. Pharmacother. 2020, 129, 110452. [Google Scholar] [CrossRef]
- Risbud, M.V. Shapiro IM: Role of cytokines in intervertebral disc degeneration: Pain and disc content. Nat. Rev. Rheumatol. 2014, 10, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Johnson, Z.I.; Schoepflin, Z.R.; Choi, H.; Shapiro, I.M.; Risbud, M.V. Disc in flames: Roles of TNF-α and IL-1β in intervertebral disc degeneration. Eur. Cell Mater. 2015, 30, 104–117. [Google Scholar] [CrossRef] [PubMed]
- Broberg, K.B. On the mechanical behaviour of intervertebral discs. Spine 1983, 8, 151–165. [Google Scholar] [CrossRef]
- Setton, L.A.; Chen, J. Mechanobiology of the intervertebral disc and relevance to disc degeneration. J. Bone Joint Surg. Am. 2006, 88 (Suppl. S2), 52–57. [Google Scholar]
- Wang, D.; He, X.; Zheng, C.; Wang, C.; Peng, P.; Gao, C.; Xu, X.; Ma, Y.; Liu, M.; Yang, L.; et al. Endoplasmic Reticulum Stress: An Emerging Therapeutic Target for Intervertebral Disc Degeneration. Front. Cell Dev. Biol. 2022, 9, 819139. [Google Scholar] [CrossRef]
- Casas, C. GRP78 at the Centre of the Stage in Cancer and Neuroprotection. Front. Neurosci. 2017, 11, 177. [Google Scholar] [CrossRef]
- Bertolotti, A.; Zhang, Y.; Hendershot, L.M.; Harding, H.P.; Ron, D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat. Cell Biol. 2000, 2, 326–332. [Google Scholar] [CrossRef]
- Chen, K.; Keaney, J.F., Jr. Evolving concepts of oxidative stress and reactive oxygen species in cardiovascular disease. Curr. Atheroscler. Rep. 2012, 14, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; Kim, S.R.; Lee, Y.C. Impact of oxidative stress on lung diseases. Respirology 2009, 14, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Salim, S. Oxidative Stress and the Central Nervous System. J. Pharmacol. Exp. Ther. 2017, 360, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Pratsinis, H.; Papadopoulou, A.; Neidlinger-Wilke, C.; Brayda-Bruno, M.; Wilke, H.J.; Kletsas, D. Cyclic tensile stress of human annulus fibrosus cells induces MAPK activation: Involvement in proinflammatory gene expression. Osteoarthr. Cartil. 2016, 24, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Tian, B.; Xu, Q.; Zhang, C.; Zhang, L.; Fang, H. Extensive mechanical tension promotes annulus fibrosus cell senescence through suppressing cellular autophagy. Biosci. Rep. 2019, 39, BSR20190163. [Google Scholar] [CrossRef]
- Yang, R.Z.; Xu, W.N.; Zheng, H.L.; Zheng, X.F.; Li, B.; Jiang, L.S.; Jiang, S.D. Involvement of oxidative stress-induced annulus fibrosus cell and nucleus pulposus cell ferroptosis in intervertebral disc degeneration pathogenesis. J. Cell Physiol. 2021, 236, 2725–2739. [Google Scholar] [CrossRef]
- Tang, P.; Gu, J.M.; Xie, Z.A.; Gu, Y.; Jie, Z.W.; Huang, K.M.; Wang, J.Y.; Fan, S.W.; Jiang, X.S.; Hu, Z.J. Honokiol alleviates the degeneration of intervertebral disc via suppressing the activation of TXNIP-NLRP3 inflammasome signal pathway. Free Radic. Biol. Med. 2018, 120, 368–379. [Google Scholar] [CrossRef]
- Yu, L.; Hao, Y.; Xu, C.; Zhu, G.; Cai, Y. LINC00969 promotes the degeneration of intervertebral disk by sponging miR-335-3p and regulating NLRP3 inflammasome activation. IUBMB Life 2019, 71, 611–618. [Google Scholar] [CrossRef]
- Tian, Y.; Bao, Z.; Ji, Y.; Mei, X.; Yang, H. Epigallocatechin-3-Gallate Protects H2O2-Induced Nucleus Pulposus Cell Apoptosis and Inflammation by Inhibiting cGAS/Sting/NLRP3 Activation. Drug Des. Devel. Ther. 2020, 14, 2113–2122. [Google Scholar] [CrossRef]
- Le Maitre, C.L.; Freemont, A.J.; Hoyland, J.A. The role of interleukin-1 in the pathogenesis of human intervertebral disc degeneration. Arthr. Res. Ther. 2005, 7, R732–R745. [Google Scholar] [CrossRef]
- Le Maitre, C.L.; Hoyland, J.A.; Freemont, A.J. Catabolic cytokine expression in degenerate and herniated human intervertebral discs: IL-1beta and TNF-alpha expression profile. Arthr. Res. Ther. 2007, 9, R77. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Che, M.; Xin, J.; Zheng, Z.; Li, J.; Zhang, S. The role of IL-1β and TNF-α in intervertebral disc degeneration. Biomed. Pharmacother. 2020, 131, 110660. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.H.; Jin, S.H.; Wang, M.Y.; Jin, X.L.; Lv, C.; Deng, Y.F.; Wang, J.L. Enhanced NLRP3, caspase-1, and IL-1β levels in degenerate human intervertebral disc and their association with the grades of disc degeneration. Anat. Rec. 2015, 298, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.F.; Huang, K.C.; Chang, H.I.; Lee, K.C.; Su, Y.P.; Chen, C.N. 2 dyn/cm(2) shear force upregulates kruppel-like factor 4 expression in human chondrocytes to inhibit the interleukin-1beta-activated nuclear factor-kappaB. J. Cell Physiol. 2018, 234, 958–968. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, H.-I.; Chen, C.-N.; Huang, K.-Y. Mechanical Stretch-Induced NLRP3 Inflammasome Expression on Human Annulus Fibrosus Cells Modulated by Endoplasmic Reticulum Stress. Int. J. Mol. Sci. 2022, 23, 7951. https://doi.org/10.3390/ijms23147951
Chang H-I, Chen C-N, Huang K-Y. Mechanical Stretch-Induced NLRP3 Inflammasome Expression on Human Annulus Fibrosus Cells Modulated by Endoplasmic Reticulum Stress. International Journal of Molecular Sciences. 2022; 23(14):7951. https://doi.org/10.3390/ijms23147951
Chicago/Turabian StyleChang, Hsin-I, Cheng-Nan Chen, and Kuo-Yuan Huang. 2022. "Mechanical Stretch-Induced NLRP3 Inflammasome Expression on Human Annulus Fibrosus Cells Modulated by Endoplasmic Reticulum Stress" International Journal of Molecular Sciences 23, no. 14: 7951. https://doi.org/10.3390/ijms23147951
APA StyleChang, H.-I., Chen, C.-N., & Huang, K.-Y. (2022). Mechanical Stretch-Induced NLRP3 Inflammasome Expression on Human Annulus Fibrosus Cells Modulated by Endoplasmic Reticulum Stress. International Journal of Molecular Sciences, 23(14), 7951. https://doi.org/10.3390/ijms23147951






