Anti-Inflammatory and Analgesic Properties of the Cannabis Terpene Myrcene in Rat Adjuvant Monoarthritis
Abstract
:1. Introduction
2. Results
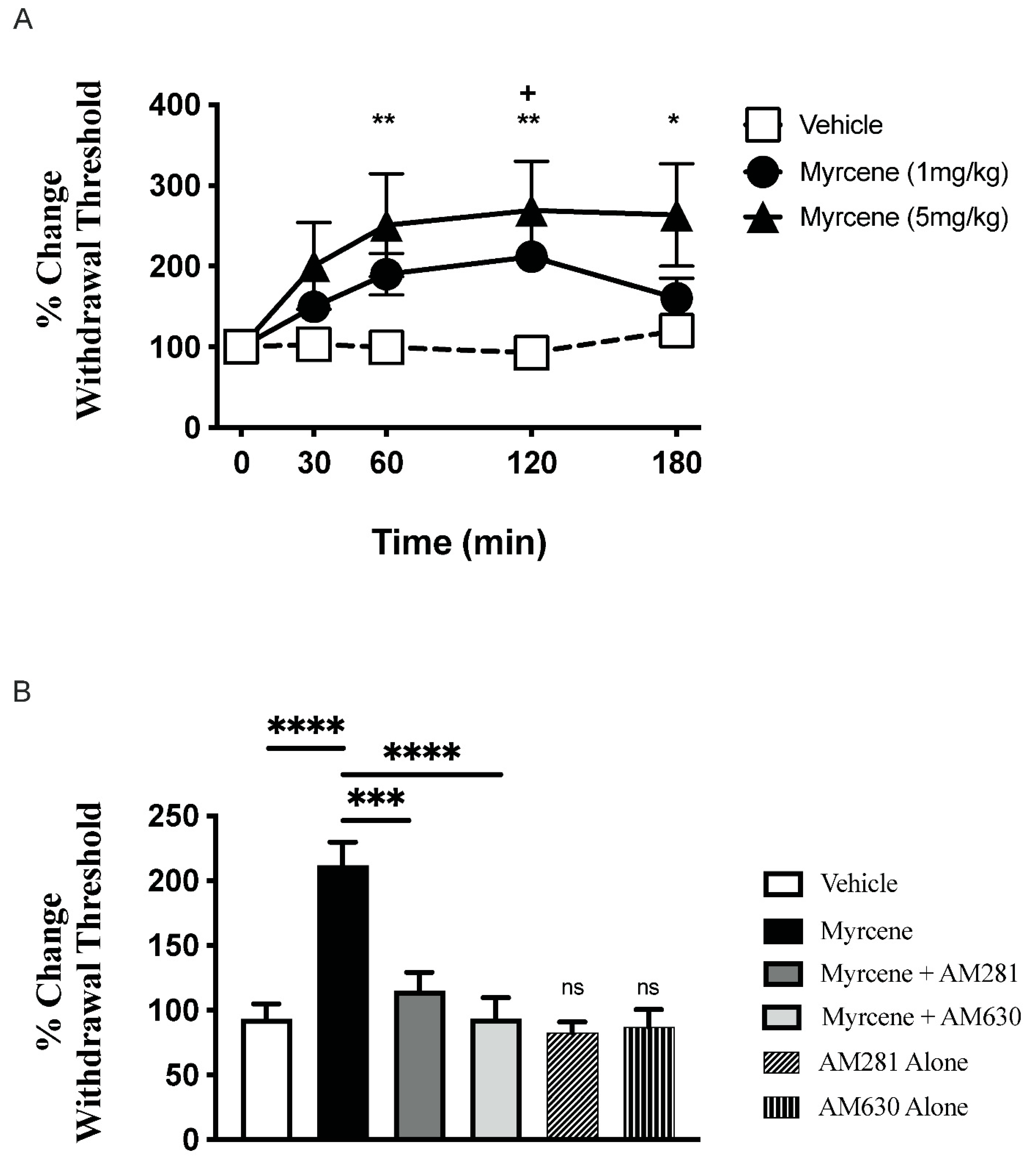
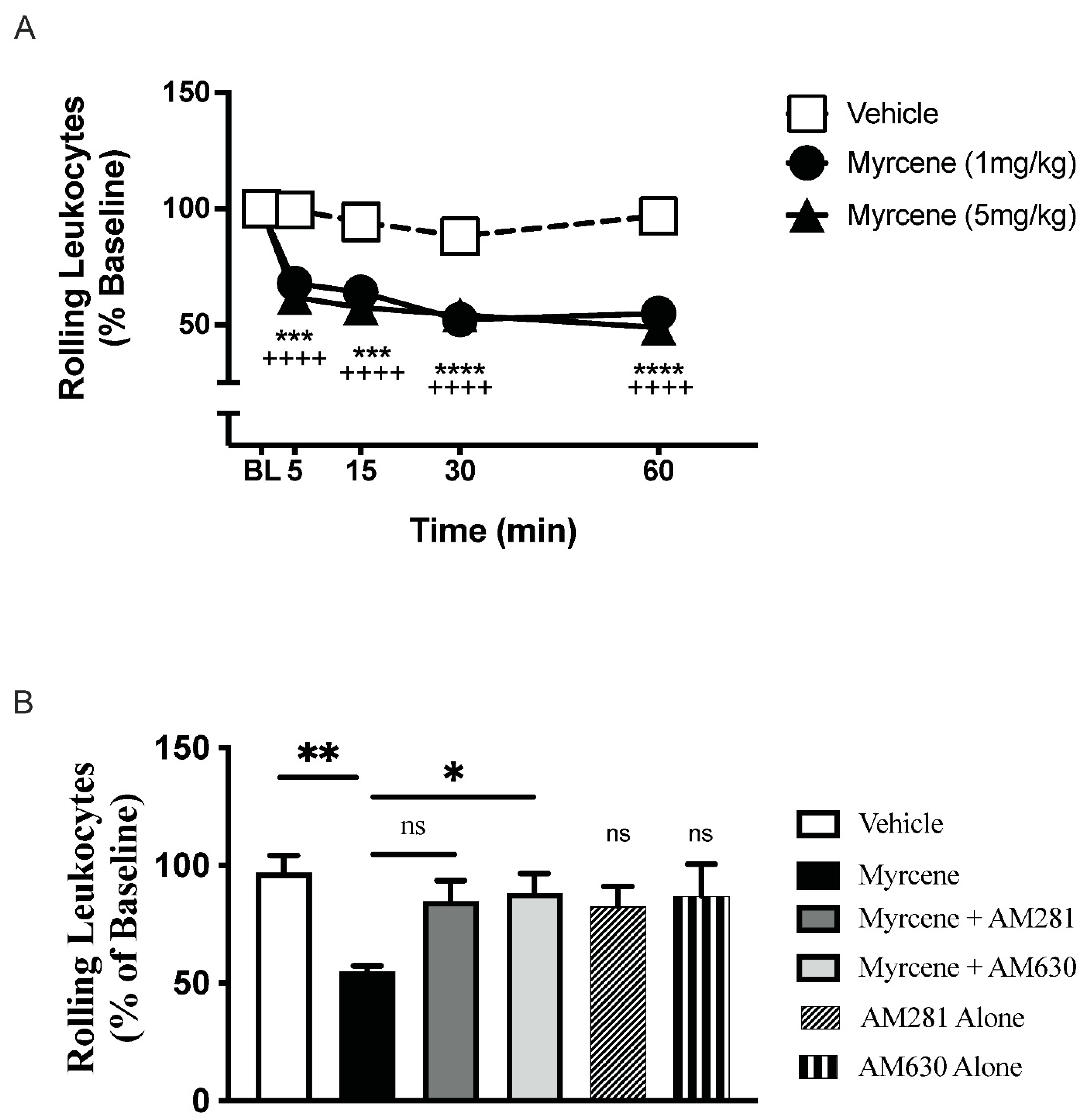
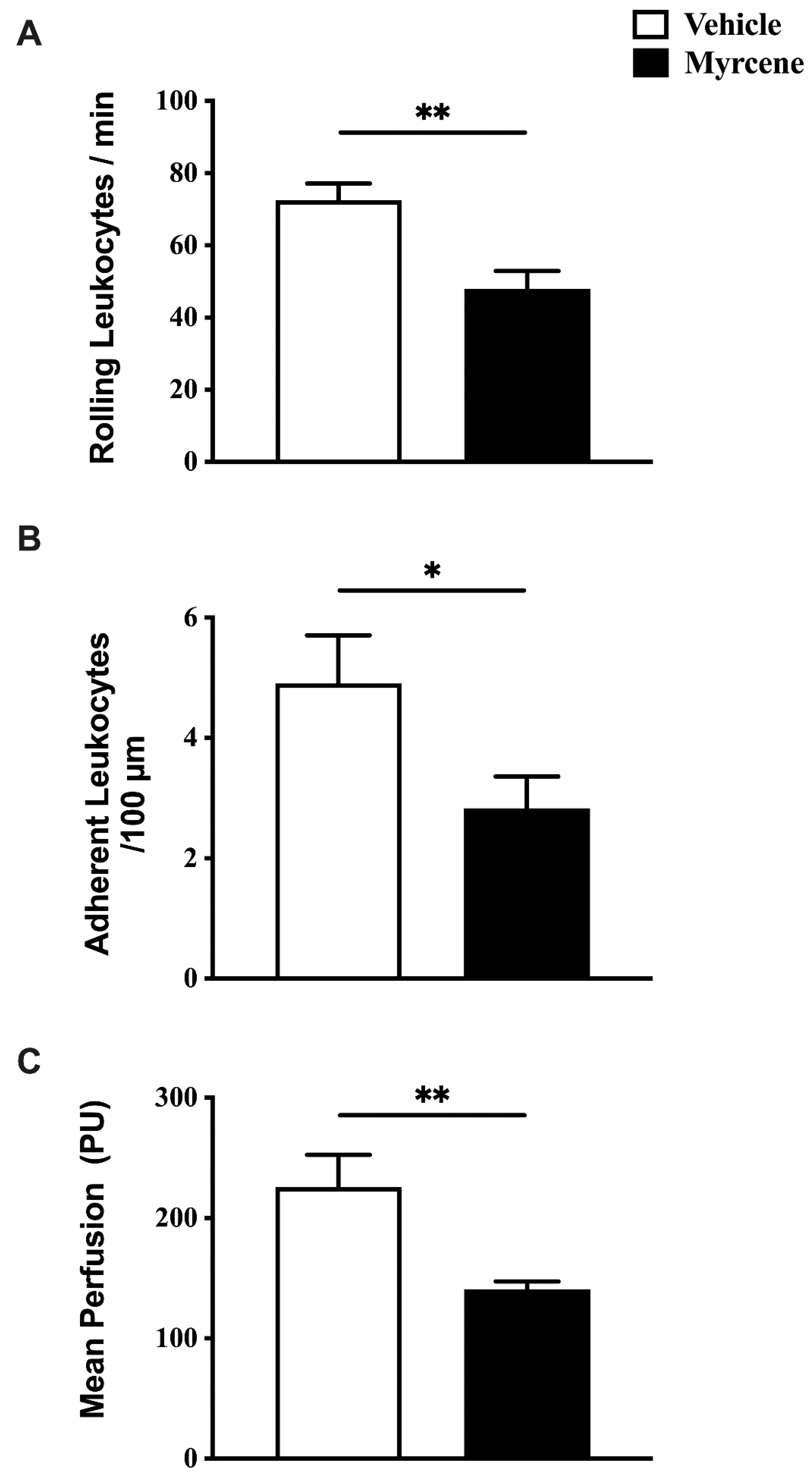
2.1. Acute Effects of Myrcene on Joint Pain and Inflammation
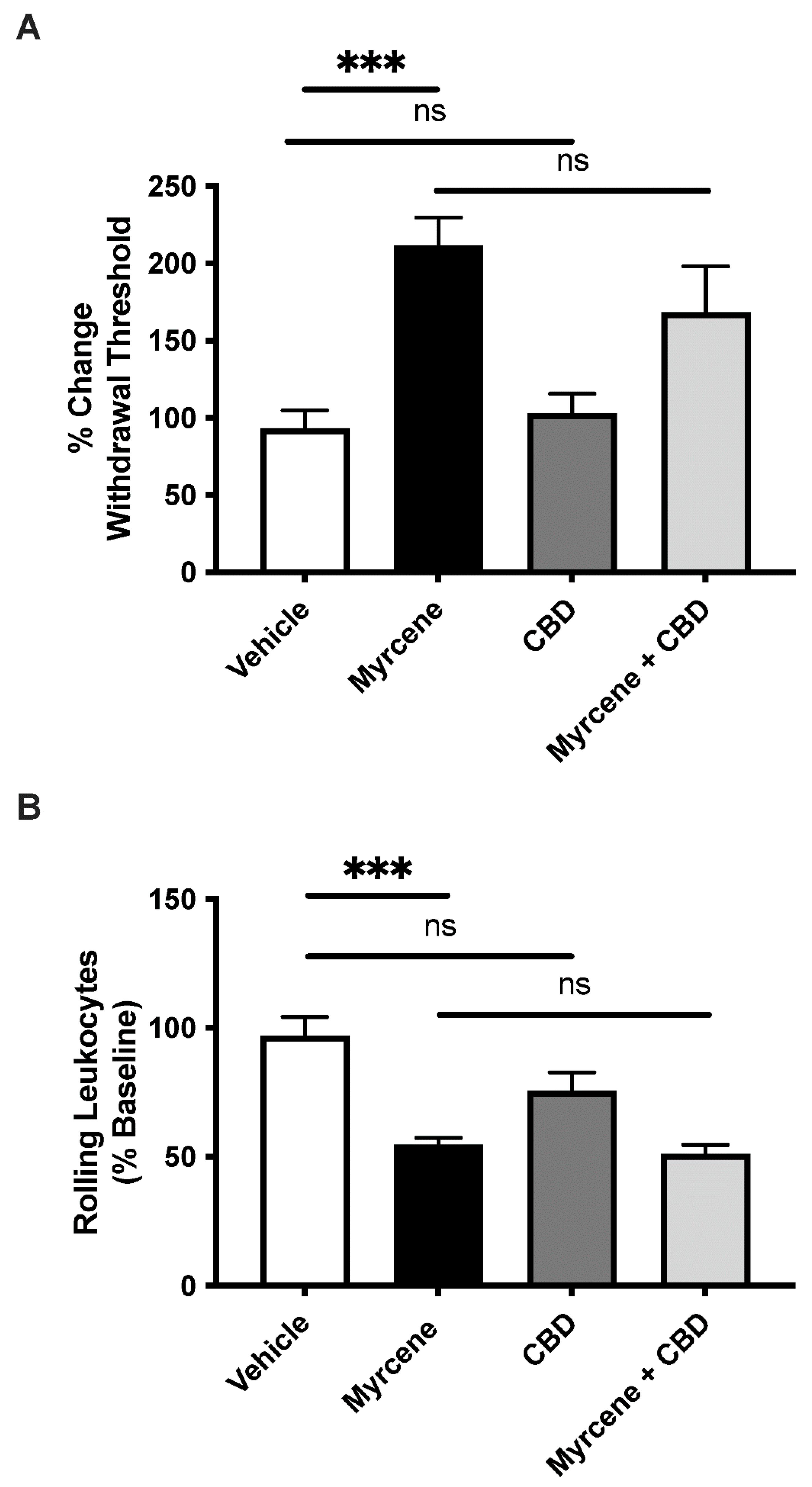
2.2. Combination Effects of Myrcene and CBD
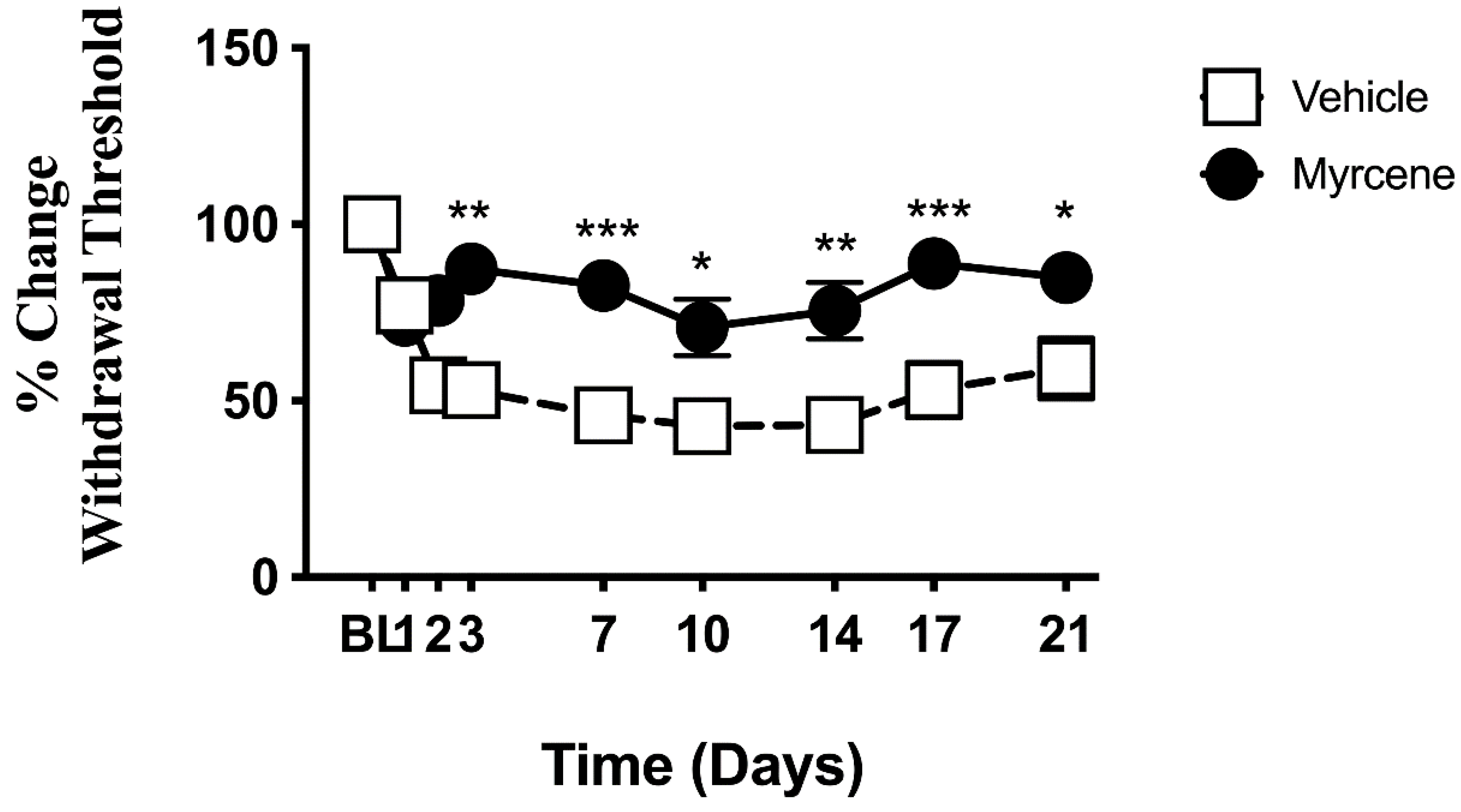
2.3. Effect of Chronic Myrcene on Hindlimb Pain and Inflammation
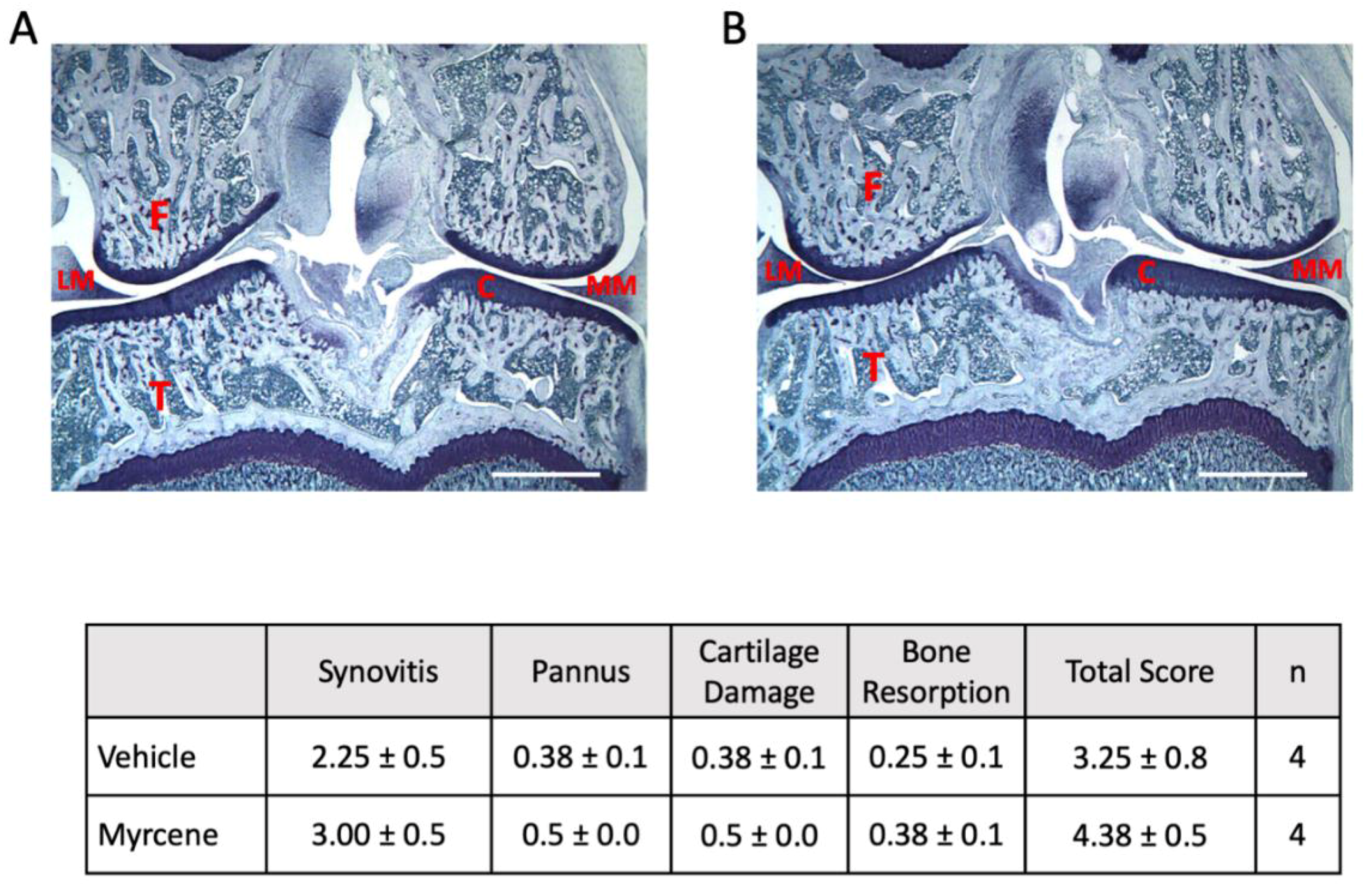
2.4. Repeated Myrcene Treatment and Joint Histopathology
3. Discussion
Study Limitations
4. Methods
4.1. Induction of Adjuvant Monoarthritis
4.2. Pain Behavior Assessment
4.3. Joint Inflammation Assessment
4.4. Cytokine Analysis
4.5. Joint Histopathology
4.6. Experimental Treatment Protocols
4.7. Reagents
4.8. Statistics
5. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Radwan, M.M.; Chandra, S.; Gul, S.; ElSohly, M.A. Cannabinoids, Phenolics, Terpenes and Alkaloids of Cannabis. Molecules 2021, 26, 2774. [Google Scholar] [CrossRef] [PubMed]
- Anil, S.M.; Peeri, H.; Koltai, H. Medical Cannabis Activity Against Inflammation: Active Compounds and Modes of Action. Front. Pharmacol. 2022, 13, 908198. [Google Scholar] [CrossRef] [PubMed]
- Liktor-Busa, E.; Keresztes, A.; LaVigne, J.; Streicher, J.M.; Largent-Milnes, T.M. Analgesic Potential of Terpenes Derived from Cannabis sativa. Pharmacol. Rev. 2021, 73, 98–126. [Google Scholar] [CrossRef]
- Ogunwande, I.A.; Avoseh, O.N.; Olasunkanmi, K.N.; Lawal, O.A.; Ascrizzi, R.; Flamini, G. Chemical composition, anti-nociceptive and anti-inflammatory activities of essential oil of Bougainvillea glabra. J. Ethnopharmacol. 2019, 232, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Sousa, O.V.; Silverio, M.S.; Del-Vechio-Vieira, G.; Matheus, F.C.; Yamamoto, C.H.; Alves, M.S. Antinociceptive and anti-inflammatory effects of the essential oil from Eremanthus erythropappus leaves. J. Pharm. Pharmacol. 2008, 60, 771–777. [Google Scholar] [CrossRef]
- Suh, H.R.; Chung, H.J.; Park, E.H.; Moon, S.W.; Park, S.J.; Park, C.W.; Kim, Y.I.; Han, H.C. The effects of Chamaecyparis obtusa essential oil on pain-related behavior and expression of pro-inflammatory cytokines in carrageenan-induced arthritis in rats. Biosci. Biotechnol. Biochem. 2016, 80, 203–209. [Google Scholar] [CrossRef]
- Rao, V.S.; Menezes, A.M.; Viana, G.S. Effect of myrcene on nociception in mice. J. Pharm. Pharmacol. 1990, 42, 877–878. [Google Scholar] [CrossRef]
- Lorenzetti, B.B.; Souza, G.E.; Sarti, S.J.; Santos Filho, D.; Ferreira, S.H. Myrcene mimics the peripheral analgesic activity of lemongrass tea. J. Ethnopharmacol. 1991, 34, 43–48. [Google Scholar] [CrossRef]
- Paula-Freire, L.I.; Andersen, M.L.; Molska, G.R.; Kohn, D.O.; Carlini, E.L. Evaluation of the antinociceptive activity of Ocimum gratissimum L. (Lamiaceae) essential oil and its isolated active principles in mice. Phytother. Res. 2013, 27, 1220–1224. [Google Scholar] [CrossRef]
- Rufino, A.T.; Ribeiro, M.; Sousa, C.; Judas, F.; Salgueiro, L.; Cavaleiro, C.; Mendes, A.F. Evaluation of the anti-inflammatory, anti-catabolic and pro-anabolic effects of E-caryophyllene, myrcene and limonene in a cell model of osteoarthritis. Eur. J. Pharmacol. 2015, 750, 141–150. [Google Scholar] [CrossRef]
- Schuelert, N.; McDougall, J.J. Cannabinoid-mediated antinociception is enhanced in rat osteoarthritic knees. Arthritis Rheum. 2008, 58, 145–153. [Google Scholar] [CrossRef] [PubMed]
- McDougall, J.J. Cannabinoids and pain control in the periphery. In Peripheral Receptor Targets for Analgesia: Novel Approaches to Pain Management; Cairns, B.E., Ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2009; pp. 325–346. [Google Scholar]
- Schuelert, N.; Zhang, C.; Mogg, A.J.; Broad, L.M.; Hepburn, D.L.; Nisenbaum, E.S.; Johnson, M.P.; McDougall, J.J. Paradoxical effects of the cannabinoid CB2 receptor agonist GW405833 on rat osteoarthritic knee joint pain. Osteoarthr. Cartil. 2010, 18, 1536–1543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howlett, A.C. The cannabinoid receptors. Prostaglandins Other Lipid Mediat. 2002, 68–69, 619–631. [Google Scholar] [CrossRef]
- Turcotte, C.; Blanchet, M.R.; Laviolette, M.; Flamand, N. The CB2 receptor and its role as a regulator of inflammation. Cell. Mol. Life Sci. CMLS 2016, 73, 4449–4470. [Google Scholar] [CrossRef] [Green Version]
- Bouaboula, M.; Rinaldi, M.; Carayon, P.; Carillon, C.; Delpech, B.; Shire, D.; Le Fur, G.; Casellas, P. Cannabinoid-receptor expression in human leukocytes. Eur. J. Biochem. 1993, 214, 173–180. [Google Scholar] [CrossRef]
- Krustev, E.; Reid, A.; McDougall, J.J. Tapping into the endocannabinoid system to ameliorate acute inflammatory flares and associated pain in mouse knee joints. Arthritis Res. Ther. 2014, 16, 437. [Google Scholar] [CrossRef] [Green Version]
- McDougall, J.J.; Yu, V.; Thomson, J. In vivo effects of CB2 receptor-selective cannabinoids on the vasculature of normal and arthritic rat knee joints. Br. J. Pharmacol. 2008, 153, 358–366. [Google Scholar] [CrossRef] [Green Version]
- McKenna, M.; McDougall, J.J. Cannabinoid control of neurogenic inflammation. Br. J. Pharmacol. 2020, 177, 4386–4399. [Google Scholar] [CrossRef]
- Philpott, H.T.; McDougall, J.J. Combatting joint pain and inflammation by dual inhibition of monoacylglycerol lipase and cyclooxygenase-2 in a rat model of osteoarthritis. Arthritis Res. Ther. 2020, 22, 9. [Google Scholar] [CrossRef] [Green Version]
- Stasilowicz, A.; Tomala, A.; Podolak, I.; Cielecka-Piontek, J. Cannabis sativa L. as a Natural Drug Meeting the Criteria of a Multitarget Approach to Treatment. Int. J. Mol. Sci. 2021, 22, 778. [Google Scholar] [CrossRef]
- Philpott, H.T.; O’Brien, M.; McDougall, J.J. Attenuation of early phase inflammation by cannabidiol prevents pain and nerve damage in rat osteoarthritis. Pain 2017, 158, 2442–2451. [Google Scholar] [CrossRef] [PubMed]
- Hammell, D.C.; Zhang, L.P.; Ma, F.; Abshire, S.M.; McIlwrath, S.L.; Stinchcomb, A.L.; Westlund, K.N. Transdermal cannabidiol reduces inflammation and pain-related behaviours in a rat model of arthritis. Eur. J. Pain 2016, 20, 936–948. [Google Scholar] [CrossRef] [PubMed]
- Verrico, C.D.; Wesson, S.; Konduri, V.; Hofferek, C.J.; Vazquez-Perez, J.; Blair, E.; Dunner, K., Jr.; Salimpour, P.; Decker, W.K.; Halpert, M.M. A randomized, double-blind, placebo-controlled study of daily cannabidiol for the treatment of canine osteoarthritis pain. Pain 2020, 161, 2191–2202. [Google Scholar] [CrossRef]
- Fitzcharles, M.A.; Clauw, D.J.; Hauser, W. A cautious hope for cannabidiol (CBD) in rheumatology care. Arthritis Care Res. 2020. [Google Scholar] [CrossRef]
- Vela, J.; Dreyer, L.; Petersen, K.K.; Lars, A.N.; Duch, K.S.; Kristensen, S. Cannabidiol treatment in hand osteoarthritis and psoriatic arthritis: A randomized, double-blind placebo-controlled trial. Pain 2021, 163, 1206–1214. [Google Scholar] [CrossRef] [PubMed]
- Evans, A.T.; Formukong, E.A.; Evans, F.J. Actions of cannabis constituents on enzymes of arachidonate metabolism: Anti-inflammatory potential. Biochem. Pharmacol. 1987, 36, 2035–2037. [Google Scholar] [CrossRef]
- Russo, E.B. The Case for the Entourage Effect and Conventional Breeding of Clinical Cannabis: No “Strain,” No Gain. Front. Plant. Sci. 2018, 9, 1969. [Google Scholar] [CrossRef]
- Karniol, I.G.; Carlini, E.A. Pharmacological interaction between cannabidiol and delta 9-tetrahydrocannabinol. Psychopharmacologia 1973, 33, 53–70. [Google Scholar] [CrossRef]
- LaVigne, J.E.; Hecksel, R.; Keresztes, A.; Streicher, J.M. Cannabis sativa terpenes are cannabimimetic and selectively enhance cannabinoid activity. Sci. Rep. 2021, 11, 8232. [Google Scholar] [CrossRef]
- Santiago, M.; Sachdev, S.; Arnold, J.C.; McGregor, I.S.; Connor, M. Absence of Entourage: Terpenoids Commonly Found in Cannabis sativa Do Not Modulate the Functional Activity of Delta(9)-THC at Human CB1 and CB2 Receptors. Cannabis Cannabinoid Res. 2019, 4, 165–176. [Google Scholar] [CrossRef] [Green Version]
- Russo, E.B. Taming THC: Potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br. J. Pharmacol. 2011, 163, 1344–1364. [Google Scholar] [CrossRef] [PubMed]
- Jansen, C.; Shimoda, L.M.N.; Kawakami, J.K.; Ang, L.; Bacani, A.J.; Baker, J.D.; Badowski, C.; Speck, M.; Stokes, A.J.; Small-Howard, A.L.; et al. Myrcene and terpene regulation of TRPV1. Channels 2019, 13, 344–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katsuyama, S.; Mizoguchi, H.; Kuwahata, H.; Komatsu, T.; Nagaoka, K.; Nakamura, H.; Bagetta, G.; Sakurada, T.; Sakurada, S. Involvement of peripheral cannabinoid and opioid receptors in beta-caryophyllene-induced antinociception. Eur. J. Pain 2013, 17, 664–675. [Google Scholar] [CrossRef] [PubMed]
- Ley, K. Molecular mechanisms of leukocyte recruitment in the inflammatory process. Cardiovasc. Res. 1996, 32, 733–742. [Google Scholar] [CrossRef] [Green Version]
- Muller, W.A. Mechanisms of leukocyte transendothelial migration. Annu. Rev. Pathol. 2011, 6, 323–344. [Google Scholar] [CrossRef] [Green Version]
- d’Alessio, P.A.; Mirshahi, M.; Bisson, J.F.; Bene, M.C. Skin repair properties of d-Limonene and perillyl alcohol in murine models. Antiinflamm Antiallergy Agents Med. Chem. 2014, 13, 29–35. [Google Scholar] [CrossRef] [Green Version]
- Johard, U.; Eklund, A.; Hed, J.; Lundahl, J. Terpenes enhance metabolic activity and alter expression of adhesion molecules (Mac-1 and L-selectin) on human granulocytes. Inflammation 1993, 17, 499–509. [Google Scholar] [CrossRef]
- Rogerio, A.P.; Andrade, E.L.; Leite, D.F.; Figueiredo, C.P.; Calixto, J.B. Preventive and therapeutic anti-inflammatory properties of the sesquiterpene alpha-humulene in experimental airways allergic inflammation. Br. J. Pharmacol. 2009, 158, 1074–1087. [Google Scholar] [CrossRef] [Green Version]
- Du, Y.; Luan, J.; Jiang, R.P.; Liu, J.; Ma, Y. Myrcene exerts anti-asthmatic activity in neonatal rats via modulating the matrix remodeling. Int. J. Immunopathol. Pharmacol. 2020, 34, 2058738420954948. [Google Scholar] [CrossRef]
- Islam, A.U.S.; Hellman, B.; Nyberg, F.; Amir, N.; Jayaraj, R.L.; Petroianu, G.; Adem, A. Myrcene Attenuates Renal Inflammation and Oxidative Stress in the Adrenalectomized Rat Model. Molecules 2020, 25, 4492. [Google Scholar] [CrossRef]
- Malfait, A.M.; Gallily, R.; Sumariwalla, P.F.; Malik, A.S.; Andreakos, E.; Mechoulam, R.; Feldmann, M. The nonpsychoactive cannabis constituent cannabidiol is an oral anti-arthritic therapeutic in murine collagen-induced arthritis. Proc. Natl. Acad. Sci. USA 2000, 97, 9561–9566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaplan, S.R.; Bach, F.W.; Pogrel, J.W.; Chung, J.M.; Yaksh, T.L. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods 1994, 53, 55–63. [Google Scholar] [CrossRef]
- Gerwin, N.; Bendele, A.M.; Glasson, S.; Carlson, C.S. The OARSI histopathology initiative-recommendations for histological assessments of osteoarthritis in the rat. Osteoarthr. Cartil. 2010, 18, S24–S34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| IL-1β | IL-10 | IL-6 | IL-17A | TNF-α | n | |
|---|---|---|---|---|---|---|
| Vehicle | 631 ± 258 | 277 ± 83 | 80 ±32 | 41 ± 16 | 84 ± 46 | 7 |
| Myrcene | 631 ± 204 | 263 ± 67 | 80 ± 24 | 40 ± 12 | 96 ± 36 | 7–8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McDougall, J.J.; McKenna, M.K. Anti-Inflammatory and Analgesic Properties of the Cannabis Terpene Myrcene in Rat Adjuvant Monoarthritis. Int. J. Mol. Sci. 2022, 23, 7891. https://doi.org/10.3390/ijms23147891
McDougall JJ, McKenna MK. Anti-Inflammatory and Analgesic Properties of the Cannabis Terpene Myrcene in Rat Adjuvant Monoarthritis. International Journal of Molecular Sciences. 2022; 23(14):7891. https://doi.org/10.3390/ijms23147891
Chicago/Turabian StyleMcDougall, Jason J., and Meagan K. McKenna. 2022. "Anti-Inflammatory and Analgesic Properties of the Cannabis Terpene Myrcene in Rat Adjuvant Monoarthritis" International Journal of Molecular Sciences 23, no. 14: 7891. https://doi.org/10.3390/ijms23147891
APA StyleMcDougall, J. J., & McKenna, M. K. (2022). Anti-Inflammatory and Analgesic Properties of the Cannabis Terpene Myrcene in Rat Adjuvant Monoarthritis. International Journal of Molecular Sciences, 23(14), 7891. https://doi.org/10.3390/ijms23147891






