Identification of Leukocyte Surface P2X7 as a Biomarker Associated with Alzheimer’s Disease
Abstract
:1. Introduction
2. Results
2.1. P2X7 Downregulation in AD
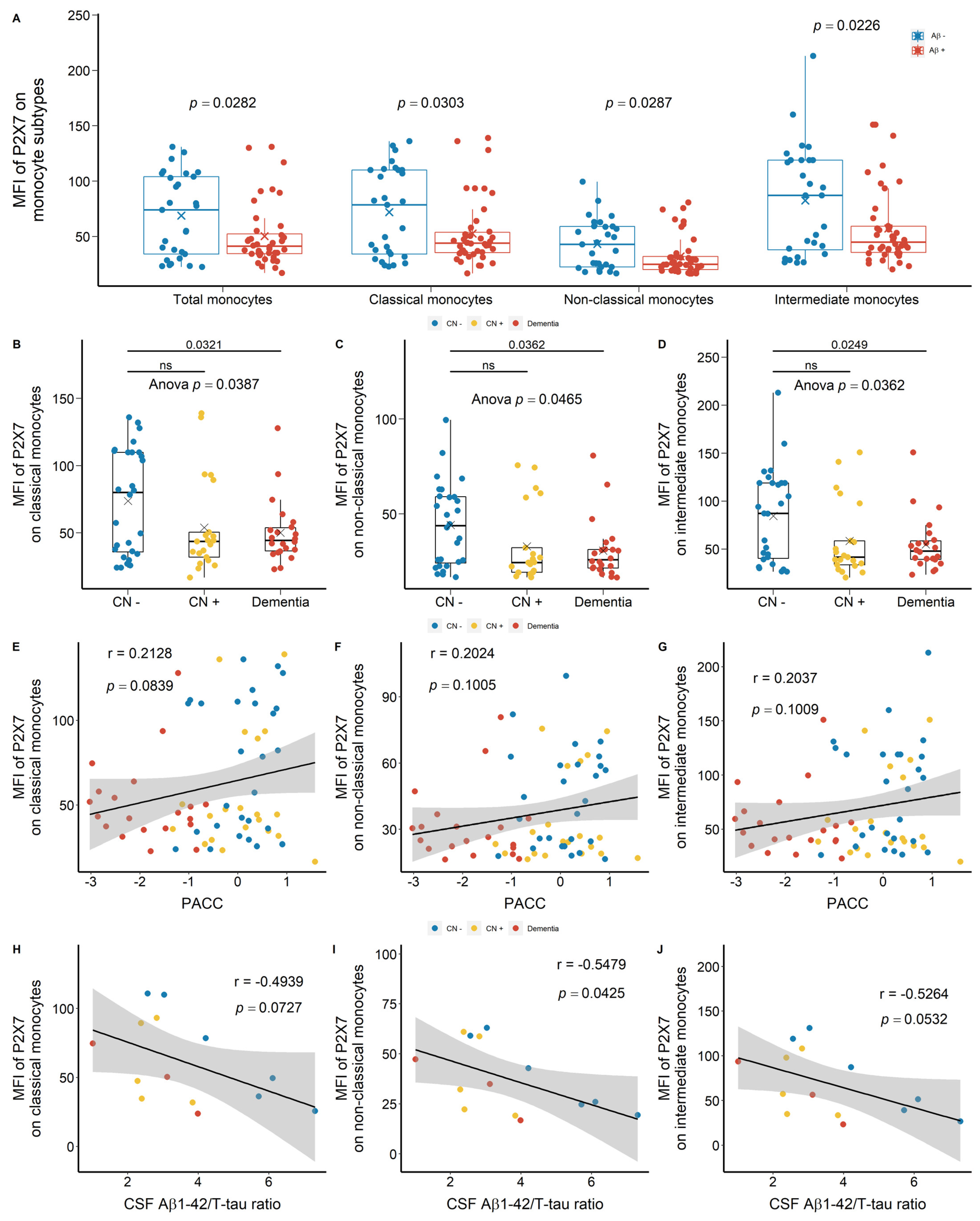
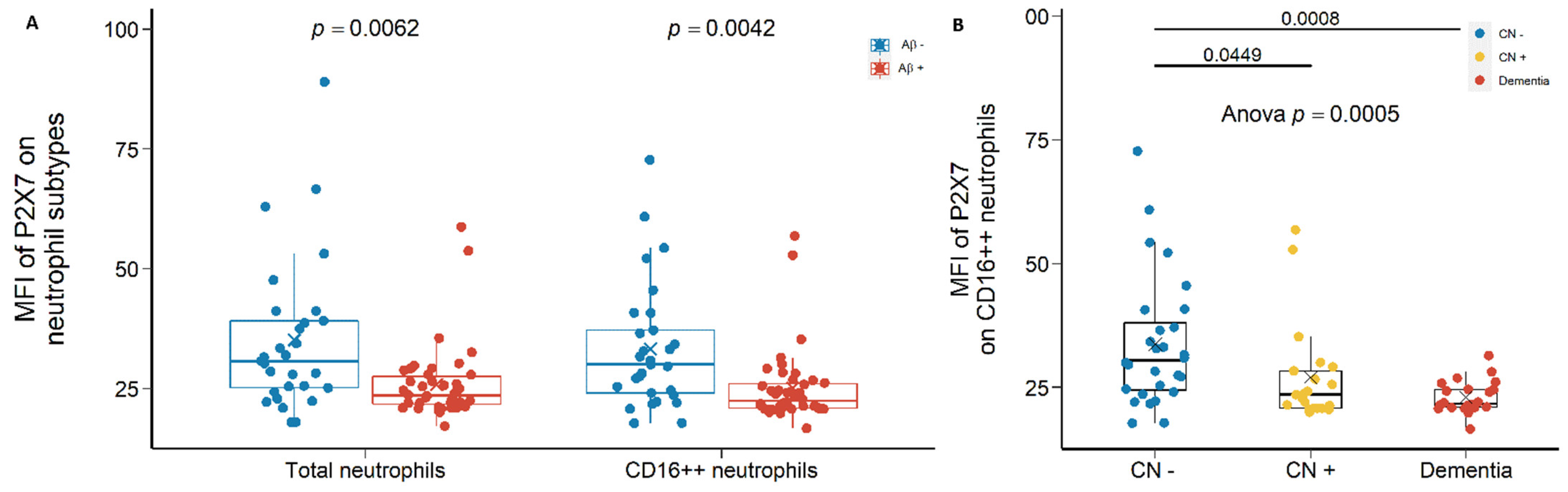
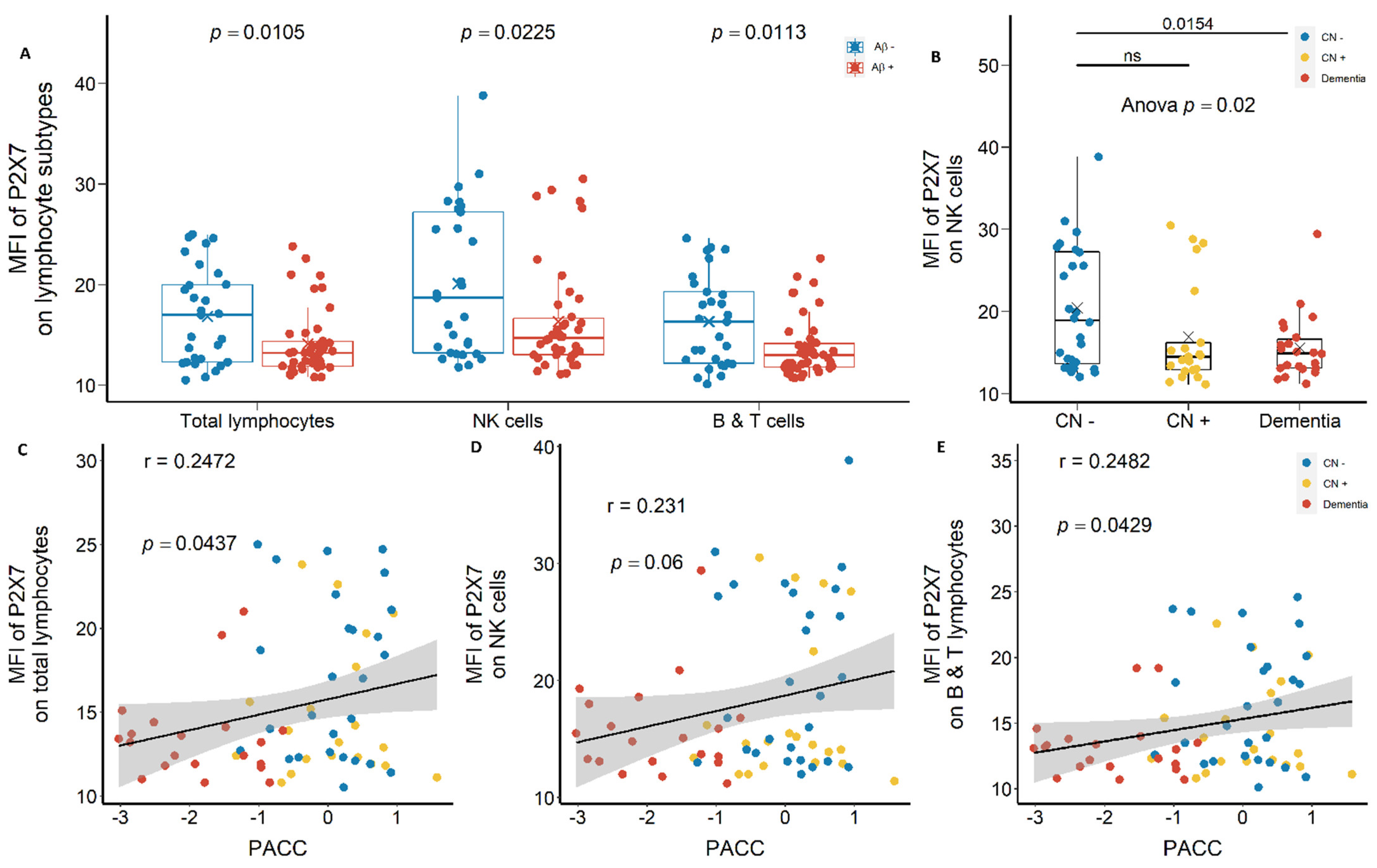
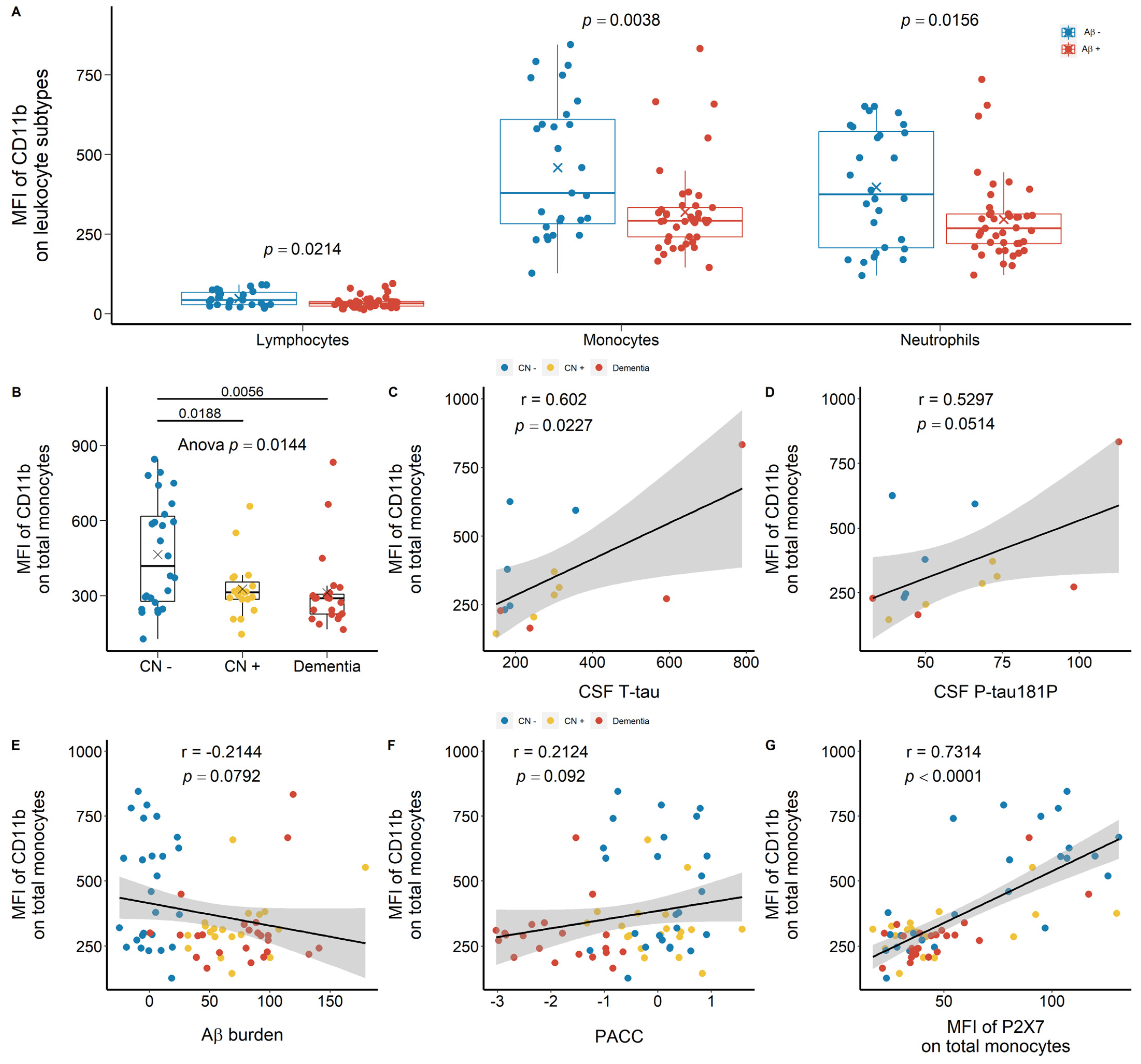
2.1.1. Reduced Leukocyte Surface P2X7 Expression in Aβ +Ve Cases in Discovery Cohort
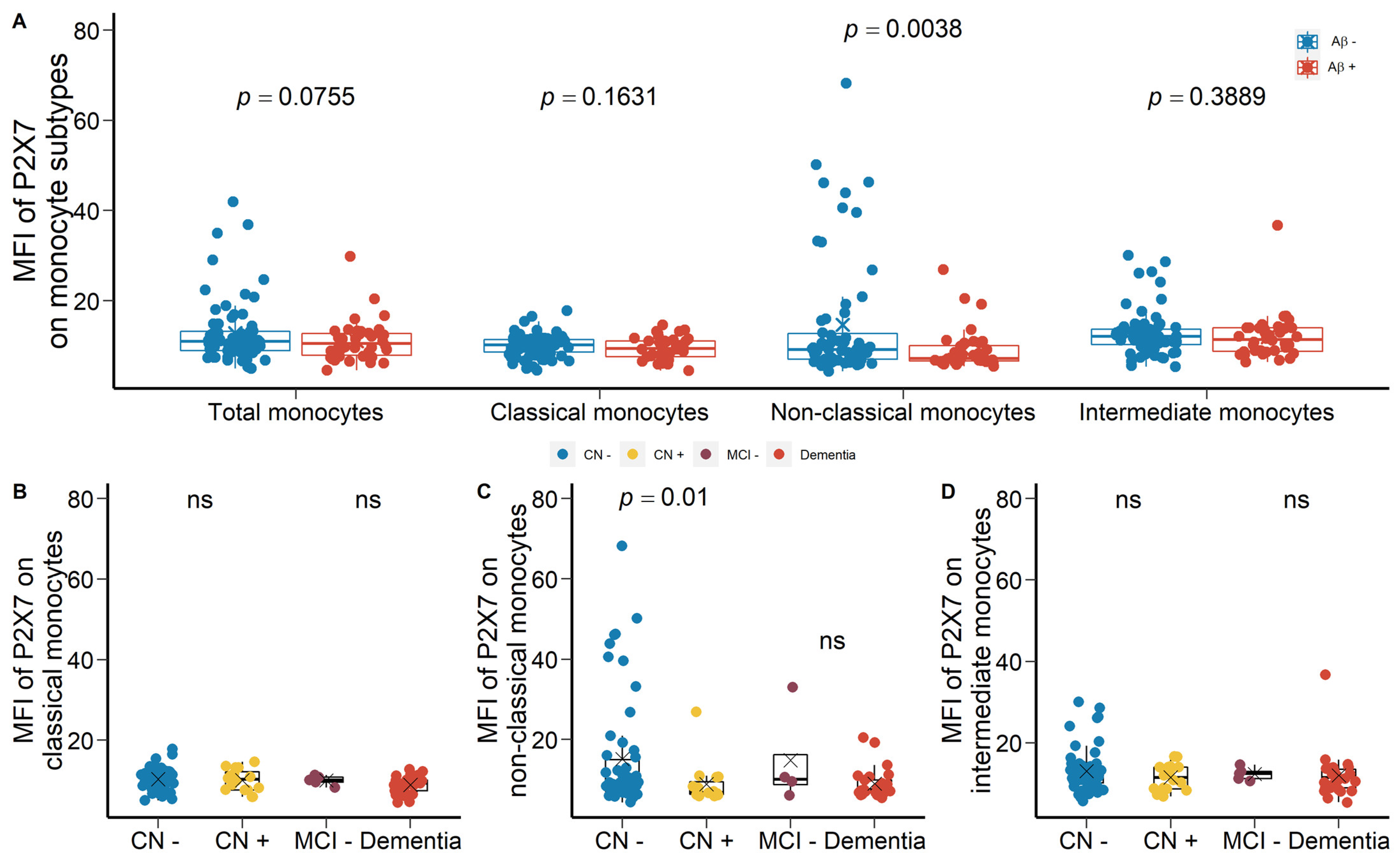
2.1.2. Reduced Monocyte Surface P2X7 Expressions in Aβ +Ve Cases in Validation Cohort
2.2. Strong Correlation between P2X7 Expressions and Integrin Expressions
2.3. Altered Percentage of Monocyte Subtypes in MCI and AD
2.4. Unchanged Polygenic Risk Scores (PRS) of P2X7 in AD
3. Discussion
3.1. Reduced Peripheral Leukocyte Surface P2X7 Expressions in Aβ +Ve Cases
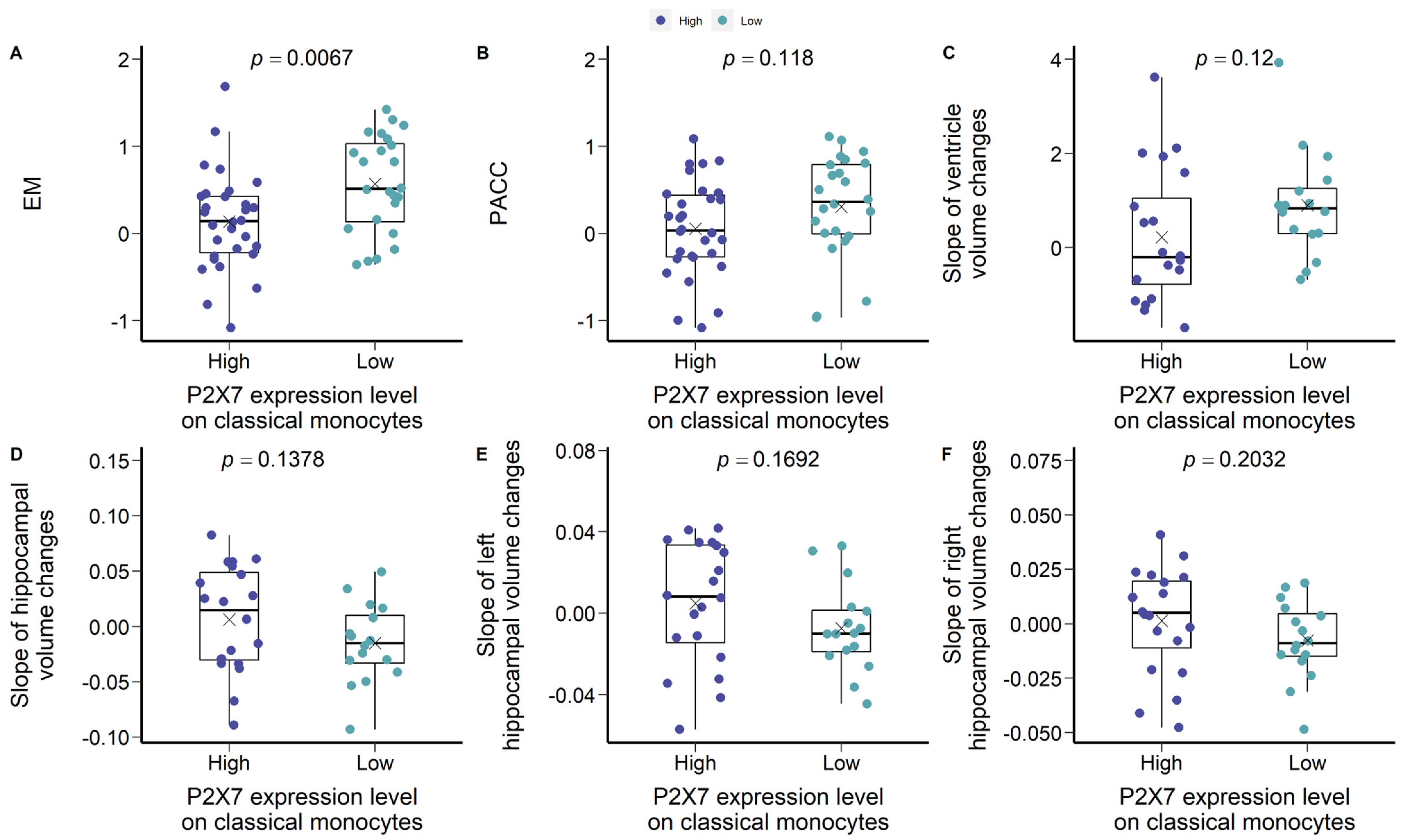
3.2. Are Low P2X7, CD11b, and CD11c Expressions Indicative of Pre-Clinical AD?
3.3. Altered Peripheral Monocyte Subpopulations in AD
3.4. P2X7 Alterations in AD Were Not Caused by Genetic Factors
3.5. Limitations of Current Study
4. Materials and Methods
4.1. Flow Study: Study Individuals, Ethical Approvals, and Blood Samples
4.2. PRS Study: Study Individuals and Ethical Approvals
4.3. Flow Study: Materials and Immune Staining
4.4. Flow Study: Magnetic Resonance Imaging (MRI)
4.5. Flow Study: PET-Aβ Imaging
4.6. Flow Study: EM Score
4.7. Flow Study: PACC Score
4.8. Flow Study: CSF Measurements of Biomarkers
4.9. Flow Study: Calculation of Progressive Changes
4.10. Flow Study: Statistical Rational
4.11. PRS Study: Genotype Data, PRS Calculation, and Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| ADNI | Alzheimer’s Disease Neuroimaging Initiative |
| AIBL | Australian Imaging, Biomarker & Lifestyle Flagship Study of Ageing |
| Aβ | Beta-amyloid |
| BBB | Blood-brain barrier |
| CL | Centiloid |
| CN | Cognitively normal |
| CNS | Central nervous system |
| CR3 | Complement receptor 3 |
| CR4 | Complement receptor 4 |
| CSF | Cerebrospinal fluid |
| EM | Episodic memory |
| GWAS | Genome-wide association studies |
| MCI | Mild cognitive impairment |
| MFI | Mean fluorescence intensity |
| MRI | Magnetic resonance imaging |
| NF-κB | Nuclear factor kappa light chain enhancer of activated B cells |
| NK cell | Natural killer cell |
| P2X7 | P2X purinoceptor 7 |
| PACC | Preclinical Alzheimer’s Cognitive Composite |
| PET | Positron emission tomography |
| P-tau181P | Tau phosphorylated at threonine 181 |
| SNP | Single nucleotide polymorphism |
| SP1 | Specificity protein 1 |
| T-tau | Total tau |
References
- Scheltens, P.; De Strooper, B.; Kivipelto, M.; Holstege, H.; Chételat, G.; Teunissen, C.E.; Cummings, J.; van der Flier, W.M. Alzheimer’s disease. Lancet 2021, 397, 1577–1590. [Google Scholar] [CrossRef]
- Selkoe, D.J.; Hardy, J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 2016, 8, 595–608. [Google Scholar] [CrossRef] [PubMed]
- De Strooper, B.; Karran, E. The cellular phase of Alzheimer’s disease. Cell 2016, 164, 603–615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Laws, S.M.; Miles, L.A.; Wiley, J.S.; Huang, X.; Masters, C.L.; Gu, B.J. Genomics of Alzheimer’s disease implicates the innate and adaptive immune systems. Cell. Mol. Life Sci. 2021, 78, 7397–7426. [Google Scholar] [CrossRef] [PubMed]
- Villemagne, V.L.; Burnham, S.; Bourgeat, P.; Brown, B.; Ellis, K.A.; Salvado, O.; Szoeke, C.; Macaulay, S.L.; Martins, R.; Maruff, P.; et al. Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s disease: A prospective cohort study. Lancet Neurol. 2013, 12, 357–367. [Google Scholar] [CrossRef]
- Fowler, C.; Rainey-Smith, S.R.; Bird, S.; Bomke, J.; Bourgeat, P.; Brown, B.M.; Burnham, S.C.; Bush, A.I.; Chadunow, C.; Collins, S.; et al. Fifteen Years of the Australian Imaging, Biomarkers and Lifestyle (AIBL) Study: Progress and Observations from 2359 Older Adults Spanning the Spectrum from Cognitive Normality to Alzheimer’s Disease. J. Alzheimer’s Dis. Rep. 2021, 5, 443–468. [Google Scholar] [CrossRef]
- Podlesny-Drabiniok, A.; Marcora, E.; Goate, A.M. Microglial phagocytosis: A disease-associated process emerging from Alzheimer’s disease genetics. Trends Neurosci. 2020, 43, 965–979. [Google Scholar] [CrossRef]
- Hohsfield, L.A.; Humpel, C. Migration of blood cells to β-amyloid plaques in Alzheimer’s disease. Exp. Gerontol. 2015, 65, 8–15. [Google Scholar] [CrossRef] [Green Version]
- Dionisio-Santos, D.A.; Olschowka, J.A.; O’Banion, M.K. Exploiting microglial and peripheral immune cell crosstalk to treat Alzheimer’s disease. J. Neuroinflamm. 2019, 16, 74. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Gu, B.J.; Masters, C.L.; Wang, Y.J. A systemic view of Alzheimer disease—Insights from amyloid-beta metabolism beyond the brain. Nat. Rev. Neurol. 2017, 13, 612–623. [Google Scholar] [CrossRef]
- Gu, B.J.; Huang, X.; Ou, A.; Rembach, A.; Fowler, C.; Avula, P.K.; Horton, A.; Doecke, J.D.; Villemagne, V.L.; Macaulay, S.L.; et al. Innate phagocytosis by peripheral blood monocytes is altered in Alzheimer’s disease. Acta Neuropathol. 2016, 132, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Munawara, U.; Catanzaro, M.; Xu, W.; Tan, C.; Hirokawa, K.; Bosco, N.; Dumoulin, D.; Khalil, A.; Larbi, A.; Lévesque, S.; et al. Hyperactivation of monocytes and macrophages in MCI patients contributes to the progression of Alzheimer’s disease. Immun. Ageing 2021, 18, 29. [Google Scholar] [CrossRef] [PubMed]
- Gu, B.J.; Wiley, J.S. P2X7 as a scavenger receptor for innate phagocytosis in the brain. Br. J. Pharmacol. 2018, 175, 4195–4208. [Google Scholar] [CrossRef]
- Jansen, W.J.; Janssen, O.; Tijms, B.M.; Vos, S.J.B.; Ossenkoppele, R.; Visser, P.J.; Amyloid Biomarker Study, G. Prevalence Estimates of Amyloid Abnormality Across the Alzheimer Disease Clinical Spectrum. JAMA Neurol. 2022, 79, 228–243. [Google Scholar] [CrossRef] [PubMed]
- Suppiah, S.; Didier, M.A.; Vinjamuri, S. The Who, When, Why, and How of PET Amyloid Imaging in Management of Alzheimer’s Disease-Review of Literature and Interesting Images. Diagnostics 2019, 9, 65. [Google Scholar] [CrossRef] [Green Version]
- McLarnon, J.G.; Ryu, J.K.; Walker, D.G.; Choi, H.B. Upregulated expression of purinergic P2X(7) receptor in Alzheimer disease and amyloid-beta peptide-treated microglia and in peptide-injected rat hippocampus. J. Neuropathol. Exp. Neurol. 2006, 65, 1090–1097. [Google Scholar] [CrossRef] [Green Version]
- Qin, J.; Zhang, X.; Wang, Z.; Li, J.; Zhang, Z.; Gao, L.; Ren, H.; Qian, M.; Du, B. Presenilin 2 deficiency facilitates Aβ-induced neuroinflammation and injury by upregulating P2X7 expression. Sci. China Life Sci. 2017, 60, 189–201. [Google Scholar] [CrossRef]
- Novikova, G.; Kapoor, M.; Tcw, J.; Abud, E.M.; Efthymiou, A.G.; Chen, S.X.; Cheng, H.; Fullard, J.F.; Bendl, J.; Liu, Y.; et al. Integration of Alzheimer’s disease genetics and myeloid genomics identifies disease risk regulatory elements and genes. Nat. Commun. 2021, 12, 1610. [Google Scholar] [CrossRef]
- Li, Q.-X.; Villemagne, V.L.; Doecke, J.D.; Rembach, A.; Sarros, S.; Varghese, S.; McGlade, A.; Laughton, K.M.; Pertile, K.K.; Fowler, C.J.; et al. Alzheimer’s Disease Normative Cerebrospinal Fluid Biomarkers Validated in PET Amyloid-β Characterized Subjects from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study. J. Alzheimer’s Dis. 2015, 48, 175–187. [Google Scholar] [CrossRef]
- Francistiová, L.; Bianchi, C.; Di Lauro, C.; Sebastián-Serrano, Á.; de Diego-García, L.; Kobolák, J.; Dinnyés, A.; Díaz-Hernández, M. The Role of P2X7 Receptor in Alzheimer’s Disease. Front. Mol. Neurosci. 2020, 13, 94. [Google Scholar] [CrossRef]
- Schittenhelm, L.; Hilkens, C.M.; Morrison, V.L. β2 Integrins As Regulators of Dendritic Cell, Monocyte, and Macrophage Function. Front. Immunol. 2017, 8, 1866. [Google Scholar] [CrossRef] [PubMed]
- Hopperton, K.E.; Mohammad, D.; Trépanier, M.O.; Giuliano, V.; Bazinet, R.P. Markers of microglia in post-mortem brain samples from patients with Alzheimer’s disease: A systematic review. Mol. Psychiatry 2018, 23, 177–198. [Google Scholar] [CrossRef] [PubMed]
- Kapellos, T.S.; Bonaguro, L.; Gemünd, I.; Reusch, N.; Saglam, A.; Hinkley, E.R.; Schultze, J.L. Human Monocyte Subsets and Phenotypes in Major Chronic Inflammatory Diseases. Front. Immunol. 2019, 10, 2035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monoranu, C.M.; Hartmann, T.; Strobel, S.; Heinsen, H.; Riederer, P.; Distel, L.; Bohnert, S. Is There Any Evidence of Monocytes Involvement in Alzheimer’s Disease? A Pilot Study on Human Postmortem Brain. J. Alzheimers Dis. Rep. 2021, 5, 887–897. [Google Scholar] [CrossRef] [PubMed]
- Ellis, K.A.; Bush, A.I.; Darby, D.; De Fazio, D.; Foster, J.; Hudson, P.; Lautenschlager, N.T.; Lenzo, N.; Martins, R.N.; Maruff, P.; et al. The Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging: Methodology and baseline characteristics of 1112 individuals recruited for a longitudinal study of Alzheimer’s disease. Int. Psychogeriatr. 2009, 21, 672–687. [Google Scholar] [CrossRef] [Green Version]
- McKhann, G.M.; Knopman, D.S.; Chertkow, H.; Hyman, B.T.; Jack, C.R., Jr.; Kawas, C.H.; Klunk, W.E.; Koroshetz, W.J.; Manly, J.J.; Mayeux, R.; et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011, 7, 263–269. [Google Scholar] [CrossRef] [Green Version]
- Albert, M.S.; DeKosky, S.T.; Dickson, D.; Dubois, B.; Feldman, H.H.; Fox, N.C.; Gamst, A.; Holtzman, D.M.; Jagust, W.J.; Petersen, R.C.; et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011, 7, 270–279. [Google Scholar] [CrossRef] [Green Version]
- Sperling, R.A.; Aisen, P.S.; Beckett, L.A.; Bennett, D.A.; Craft, S.; Fagan, A.M.; Iwatsubo, T.; Jack, C.R., Jr.; Kaye, J.; Montine, T.J.; et al. Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011, 7, 280–292. [Google Scholar] [CrossRef] [Green Version]
- Buell, G.; Chessell, I.P.; Michel, A.D.; Collo, G.; Salazzo, M.; Herren, S.; Gretener, D.; Grahames, C.; Kaur, R.; Kosco-Vilbois, M.H.; et al. Blockade of human P2X7 receptor function with a monoclonal antibody. Blood 1998, 92, 3521–3528. [Google Scholar] [CrossRef]
- Bourgeat, P.; Chételat, G.; Villemagne, V.L.; Fripp, J.; Raniga, P.; Pike, K.; Acosta, O.; Szoeke, C.; Ourselin, S.; Ames, D.; et al. Beta-amyloid burden in the temporal neocortex is related to hippocampal atrophy in elderly subjects without dementia. Neurology 2010, 74, 121–127. [Google Scholar] [CrossRef]
- Bourgeat, P.; Villemagne, V.L.; Dore, V.; Brown, B.; Macaulay, S.L.; Martins, R.; Masters, C.L.; Ames, D.; Ellis, K.; Rowe, C.C.; et al. Comparison of MR-less PiB SUVR quantification methods. Neurobiol. Aging 2015, 36 (Suppl. S1), S159–S166. [Google Scholar] [CrossRef] [PubMed]
- Tustison, N.J.; Avants, B.B.; Cook, P.A.; Zheng, Y.; Egan, A.; Yushkevich, P.A.; Gee, J.C. N4ITK: Improved N3 bias correction. IEEE Trans. Med. Imaging. 2010, 29, 1310–1320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rohlfing, T.; Rademacher, M.H.; Pfefferbaum, A. Volume reconstruction by inverse interpolation: Application to interleaved MR motion correction. Med. Image Comput. Comput. Assist. Interv. 2008, 11, 798–806. [Google Scholar] [CrossRef] [Green Version]
- Rowe, C.C.; Ellis, K.A.; Rimajova, M.; Bourgeat, P.; Pike, K.E.; Jones, G.; Fripp, J.; Tochon-Danguy, H.; Morandeau, L.; O’Keefe, G.; et al. Amyloid imaging results from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging. Neurobiol. Aging 2010, 31, 1275–1283. [Google Scholar] [CrossRef] [PubMed]
- Klunk, W.E.; Koeppe, R.A.; Price, J.C.; Benzinger, T.L.; Devous, M.D., Sr.; Jagust, W.J.; Johnson, K.A.; Mathis, C.A.; Minhas, D.; Pontecorvo, M.J.; et al. The Centiloid Project: Standardizing quantitative amyloid plaque estimation by PET. Alzheimers Dement. 2015, 11, 1–15.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bourgeat, P.; Doré, V.; Fripp, J.; Ames, D.; Masters, C.L.; Salvado, O.; Villemagne, V.L.; Rowe, C.C. Implementing the centiloid transformation for (11)C-PiB and β-amyloid (18)F-PET tracers using CapAIBL. Neuroimage 2018, 183, 387–393. [Google Scholar] [CrossRef]
- Lim, Y.Y.; Maruff, P.; Pietrzak, R.H.; Ames, D.; Ellis, K.A.; Harrington, K.; Lautenschlager, N.T.; Szoeke, C.; Martins, R.N.; Masters, C.L.; et al. Effect of amyloid on memory and non-memory decline from preclinical to clinical Alzheimer’s disease. Brain 2014, 137, 221–231. [Google Scholar] [CrossRef]
- Harrington, K.D.; Lim, Y.Y.; Ellis, K.A.; Copolov, C.; Darby, D.; Weinborn, M.; Ames, D.; Martins, R.N.; Savage, G.; Szoeke, C.; et al. The association of Aβ amyloid and composite cognitive measures in healthy older adults and MCI. Int. Psychogeriatr. 2013, 25, 1667–1677. [Google Scholar] [CrossRef]
- Lim, Y.Y.; Snyder, P.J.; Pietrzak, R.H.; Ukiqi, A.; Villemagne, V.L.; Ames, D.; Salvado, O.; Bourgeat, P.; Martins, R.N.; Masters, C.L.; et al. Sensitivity of composite scores to amyloid burden in preclinical Alzheimer’s disease: Introducing the Z-scores of Attention, Verbal fluency, and Episodic memory for Nondemented older adults composite score. Alzheimers Dement. 2016, 2, 19–26. [Google Scholar] [CrossRef] [Green Version]
- Donohue, M.C.; Sperling, R.A.; Salmon, D.P.; Rentz, D.M.; Raman, R.; Thomas, R.G.; Weiner, M.; Aisen, P.S. The preclinical Alzheimer cognitive composite: Measuring amyloid-related decline. JAMA Neurol. 2014, 71, 961–970. [Google Scholar] [CrossRef] [Green Version]
- Vanderstichele, H.; Bibl, M.; Engelborghs, S.; Le Bastard, N.; Lewczuk, P.; Molinuevo, J.L.; Parnetti, L.; Perret-Liaudet, A.; Shaw, L.M.; Teunissen, C.; et al. Standardization of preanalytical aspects of cerebrospinal fluid biomarker testing for Alzheimer’s disease diagnosis: A consensus paper from the Alzheimer’s Biomarkers Standardization Initiative. Alzheimers Dement. 2012, 8, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.W.; Mak, T.S.; O’Reilly, P.F. Tutorial: A guide to performing polygenic risk score analyses. Nat. Protoc. 2020, 15, 2759–2772. [Google Scholar] [CrossRef] [PubMed]
- Shemon, A.N.; Sluyter, R.; Fernando, S.L.; Clarke, A.L.; Da-Ung, L.P.; Skarratt, K.K.; Saunders, B.M.; Tan, K.S.; Gu, B.J.; Fuller, S.J.; et al. A Thr357 to Ser polymorphism in homozygous and compound heterozygous subjects causes absent or reduced P2X7 function and impairs ATP-induced mycobacterial killing by macrophages. J. Biol. Chem. 2006, 281, 2079–2086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, B.J.; Baird, P.N.; Vessey, K.A.; Skarratt, K.K.; Fletcher, E.L.; Fuller, S.J.; Richardson, A.J.; Guymer, R.H.; Wiley, J.S. A rare functional haplotype of the P2RX4 and P2RX7 genes leads to loss of innate phagocytosis and confers increased risk of age-related macular degeneration. FASEB J. 2013, 27, 1479–1487. [Google Scholar] [CrossRef]
- Gu, B.J.; Field, J.; Dutertre, S.; Ou, A.; Kilpatrick, T.J.; Lechner-Scott, J.; Scott, R.; Lea, R.; Taylor, B.V.; Stankovich, J.; et al. A rare P2X7 variant Arg307Gln with absent pore formation function protects against neuroinflammation in multiple sclerosis. Hum. Mol. Genet. 2015, 24, 5644–5654. [Google Scholar] [CrossRef]
- Stokes, L.; Scurrah, K.; Ellis, J.A.; Cromer, B.A.; Skarratt, K.K.; Gu, B.J.; Harrap, S.B.; Wiley, J.S. A loss-of-function polymorphism in the human P2X4 receptor is associated with increased pulse pressure. Hypertension 2011, 58, 1086–1092. [Google Scholar] [CrossRef] [Green Version]
- Gu, B.J.; Sluyter, R.; Skarratt, K.K.; Shemon, A.N.; Dao-Ung, L.P.; Fuller, S.J.; Barden, J.A.; Clarke, A.L.; Petrou, S.; Wiley, J.S. An Arg307 to Gln polymorphism within the ATP-binding site causes loss of function of the human P2X7 receptor. J. Biol. Chem. 2004, 279, 31287–31295. [Google Scholar] [CrossRef] [Green Version]
- Gu, B.J.; Zhang, W.; Worthington, R.A.; Sluyter, R.; Dao-Ung, P.; Petrou, S.; Barden, J.A.; Wiley, J.S. A Glu-496 to Ala polymorphism leads to loss of function of the human P2X7 receptor. J. Biol. Chem. 2001, 276, 11135–11142. [Google Scholar] [CrossRef] [Green Version]
- Cabrini, G.; Falzoni, S.; Forchap, S.L.; Pellegatti, P.; Balboni, A.; Agostini, P.; Cuneo, A.; Castoldi, G.; Baricordi, O.R.; Di Virgilio, F. A His-155 to Tyr polymorphism confers gain-of-function to the human P2X7 receptor of human leukemic lymphocytes. J. Immunol. 2005, 175, 82–89. [Google Scholar] [CrossRef] [Green Version]
- Wiley, J.S.; Dao-Ung, L.P.; Li, C.; Shemon, A.N.; Gu, B.J.; Smart, M.L.; Fuller, S.J.; Barden, J.A.; Petrou, S.; Sluyter, R. An Ile-568 to Asn polymorphism prevents normal trafficking and function of the human P2X7 receptor. J. Biol. Chem. 2003, 278, 17108–17113. [Google Scholar] [CrossRef] [Green Version]


| Demographics | Discovery | p 1 | Validation A 2 | p | Validation B 3 | p | |||
|---|---|---|---|---|---|---|---|---|---|
| Control (≤25 CL) | Case (>25 CL) | Control (≤25 CL) | Case (>25 CL) | Control (≤25 CL) | Case (>25 CL) | ||||
| Sample size, n (%) | 29 (39.7) | 44 (60.3) | - | 69 (64.5) | 38 (35.5) | - | 42 (47.7) | 46 (52.3) | - |
| Age in years, mean (SD) | 73.9 (4.3) | 76.9 (7.4) | 0.0343 | 73.7 (6.6) | 77.4 (8.8) | 0.0253 | 73.6 (13.2) | 73.5 (13.1) | ns |
| Sex (female), n (%) | 11 (37.9) | 22 (50.0) | ns | 39 (56.5) | 19 (50.0) | ns | 18 (42.9) | 17 (37.0) | ns |
| APOE ε4, n (%) | 7 (24.1) | 20 (45.5) | 0.0975 | 13 (18.8) | 18 (47.3) | 0.0025 | 6 (14.3) | 29 (63.0) | 0.0005 |
| Years of education, mean (SD) | 12.4 (2.9) | 12.3 (2.8) | ns | 14.7 (3.4) | 13 (3.1) | 0.0174 | 13.7 (3.4) | 12.5 (3.1) | ns |
| Aβ burden, CL mean (SD) | 1.7 (13.5) | 78.8 (33.1) | <0.0001 | 2.2 (7.8) | 102.1 (41.2) | <0.0001 | 3.7 (8.3) | 96.4 (44.5) | <0.0001 |
| CN, n (%) | 28 (96.6) | 22 (50.0) | - | 60 (87.0) | 16 (42.1) | - | 36 (85.7) | 8 (17.4) | - |
| MCI, n (%) | 0 (0.0) | 15 (34.1) | - | 5 (7.2) | 9 (26.7) | - | 5 (11.9) | 16 (34.8) | - |
| AD, n (%) | 1 (3.4) | 7 (15.9) | - | 4 (5.8) | 13 (34.2) | - | 1 (2.4) | 22 (47.8) | - |
| EM, mean (SD) | 0.0 (0.7) | −0.9 (1.3) | 0.0006 | 0.1 (0.9) | −1.3 (1.6) | <0.0001 | −0.5 (1.0) | −0.9 (1.6) | ns |
| Slope of EM, mean (SD) | 0.0 (0.1) | −0.1 (0.2) | 0.0005 | 0.1 (0.2) | 0.0 (0.2) | 0.0176 | 0.0 (0.1) | −0.1 (0.3) | ns |
| PACC, mean (SD) | 0.0 (0.7) | −0.8 (1.2) | 0.0006 | 0.0 (0.9) | −1.6 (1.6) | <0.0001 | −0.1 (0.8) | −1.0 (1.4) | ns |
| Slope of PACC, mean (SD) | 0.0 (0.1) | −0.2 (0.2) | 0.0004 | 0.1 (0.2) | −0.1 (0.2) | 0.0003 | 0.0 (0.1) | −0.1 (0.3) | ns |
| Demographics | AIBL | ADNI | Combined | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CN | MCI | AD | CN | MCI | AD | CN | MCI | AD | ||
| Sample size | n | 647 | 77 | 176 | 255 | 379 | 152 | 902 | 456 | 328 |
| Sex (women) | n | 377 | 40 | 102 | 134 | 154 | 61 | 511 | 194 | 163 |
| % | 0.583 | 0.520 | 0.580 | 0.526 | 0.406 | 0.401 | 0.567 | 0.425 | 0.497 | |
| PRS-pore | mean | 0.293 | 0.292 | 0.321 | 0.243 | 0.282 | 0.191 | 0.279 | 0.284 | 0.261 |
| SD | 0.958 | 1.063 | 0.984 | 0.958 | 1.063 | 0.984 | 0.947 | 0.940 | 0.962 | |
| PRS-phago | mean | −0.057 | −0.077 | −0.054 | −0.050 | −0.056 | −0.069 | −0.055 | −0.059 | −0.061 |
| SD | 0.192 | 0.240 | 0.187 | 0.170 | 0.207 | 0.197 | 0.186 | 0.213 | 0.192 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Huang, X.; Fowler, C.; Lim, Y.Y.; Laws, S.M.; Faux, N.; Doecke, J.D.; Trounson, B.; Pertile, K.; Rumble, R.; et al. Identification of Leukocyte Surface P2X7 as a Biomarker Associated with Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 7867. https://doi.org/10.3390/ijms23147867
Li Y, Huang X, Fowler C, Lim YY, Laws SM, Faux N, Doecke JD, Trounson B, Pertile K, Rumble R, et al. Identification of Leukocyte Surface P2X7 as a Biomarker Associated with Alzheimer’s Disease. International Journal of Molecular Sciences. 2022; 23(14):7867. https://doi.org/10.3390/ijms23147867
Chicago/Turabian StyleLi, Yihan, Xin Huang, Christopher Fowler, Yen Y. Lim, Simon M. Laws, Noel Faux, James D. Doecke, Brett Trounson, Kelly Pertile, Rebecca Rumble, and et al. 2022. "Identification of Leukocyte Surface P2X7 as a Biomarker Associated with Alzheimer’s Disease" International Journal of Molecular Sciences 23, no. 14: 7867. https://doi.org/10.3390/ijms23147867
APA StyleLi, Y., Huang, X., Fowler, C., Lim, Y. Y., Laws, S. M., Faux, N., Doecke, J. D., Trounson, B., Pertile, K., Rumble, R., Doré, V., Villemagne, V. L., Rowe, C. C., Wiley, J. S., Maruff, P., Masters, C. L., & Gu, B. J. (2022). Identification of Leukocyte Surface P2X7 as a Biomarker Associated with Alzheimer’s Disease. International Journal of Molecular Sciences, 23(14), 7867. https://doi.org/10.3390/ijms23147867






