Upregulation of Mineralocorticoid Receptor Contributes to Development of Salt-Sensitive Hypertension after Ischemia–Reperfusion Injury in Rats
Abstract
1. Introduction
2. Results
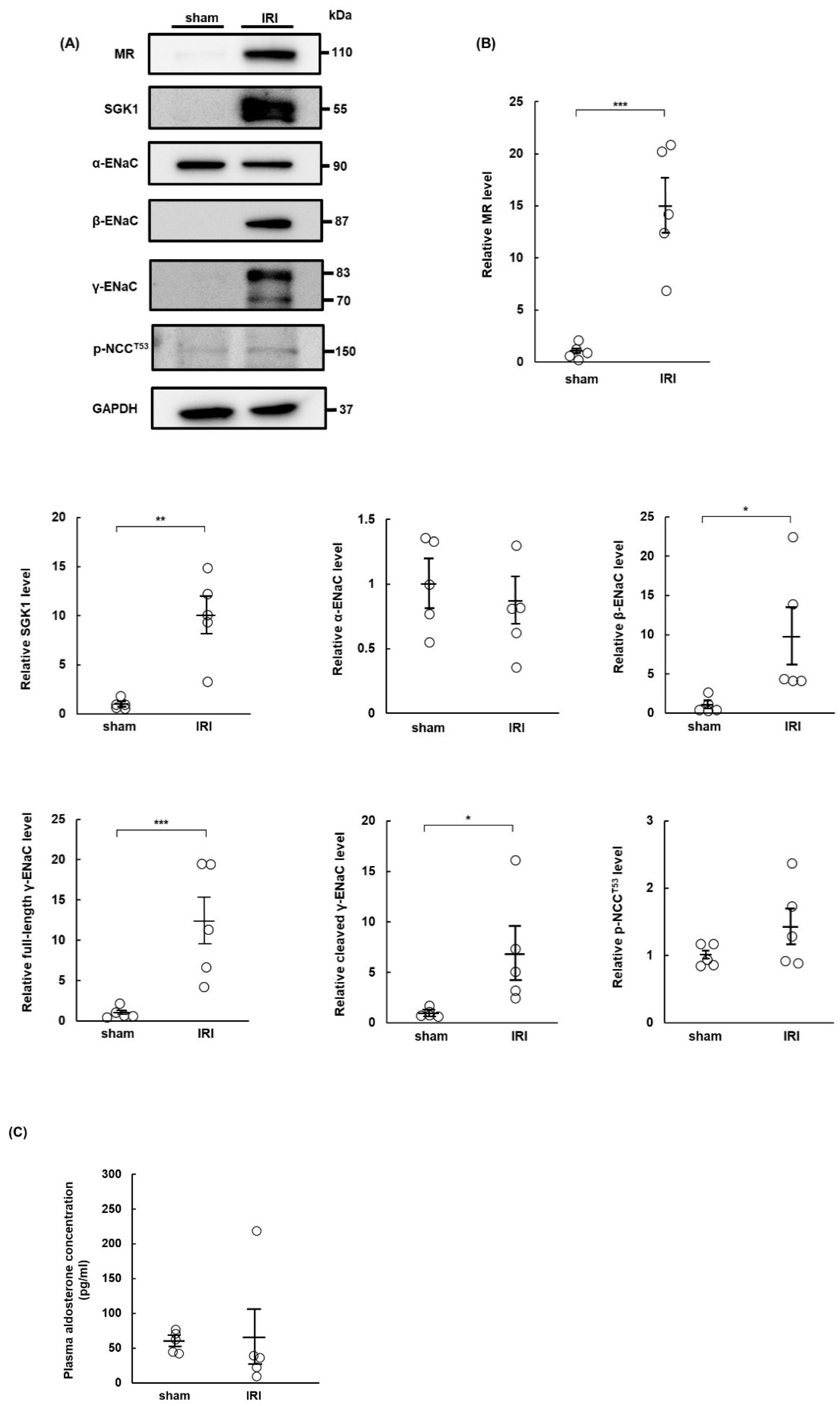
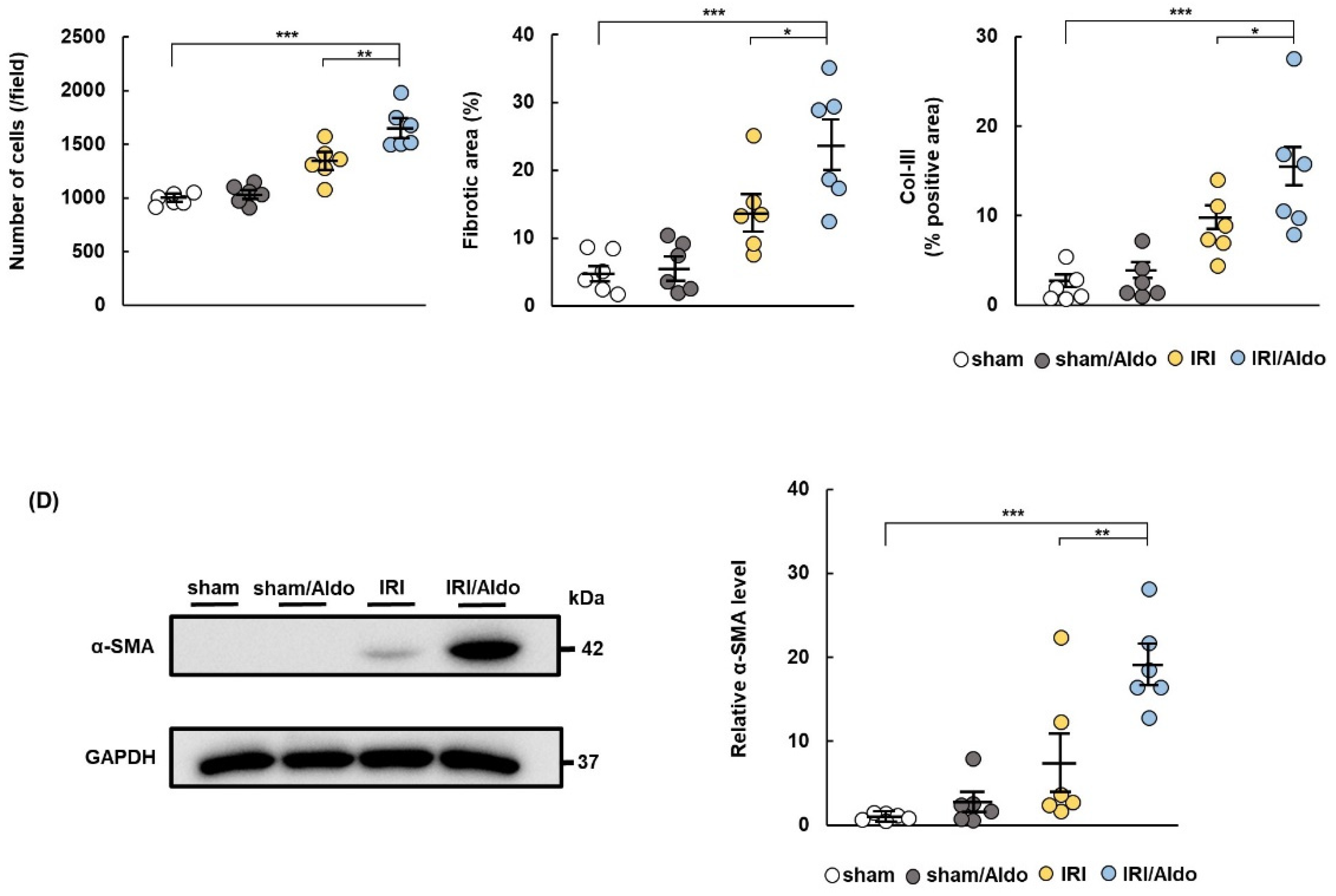
2.1. MR Expression Increases in IRI Rats at Day 7 Post-Surgery
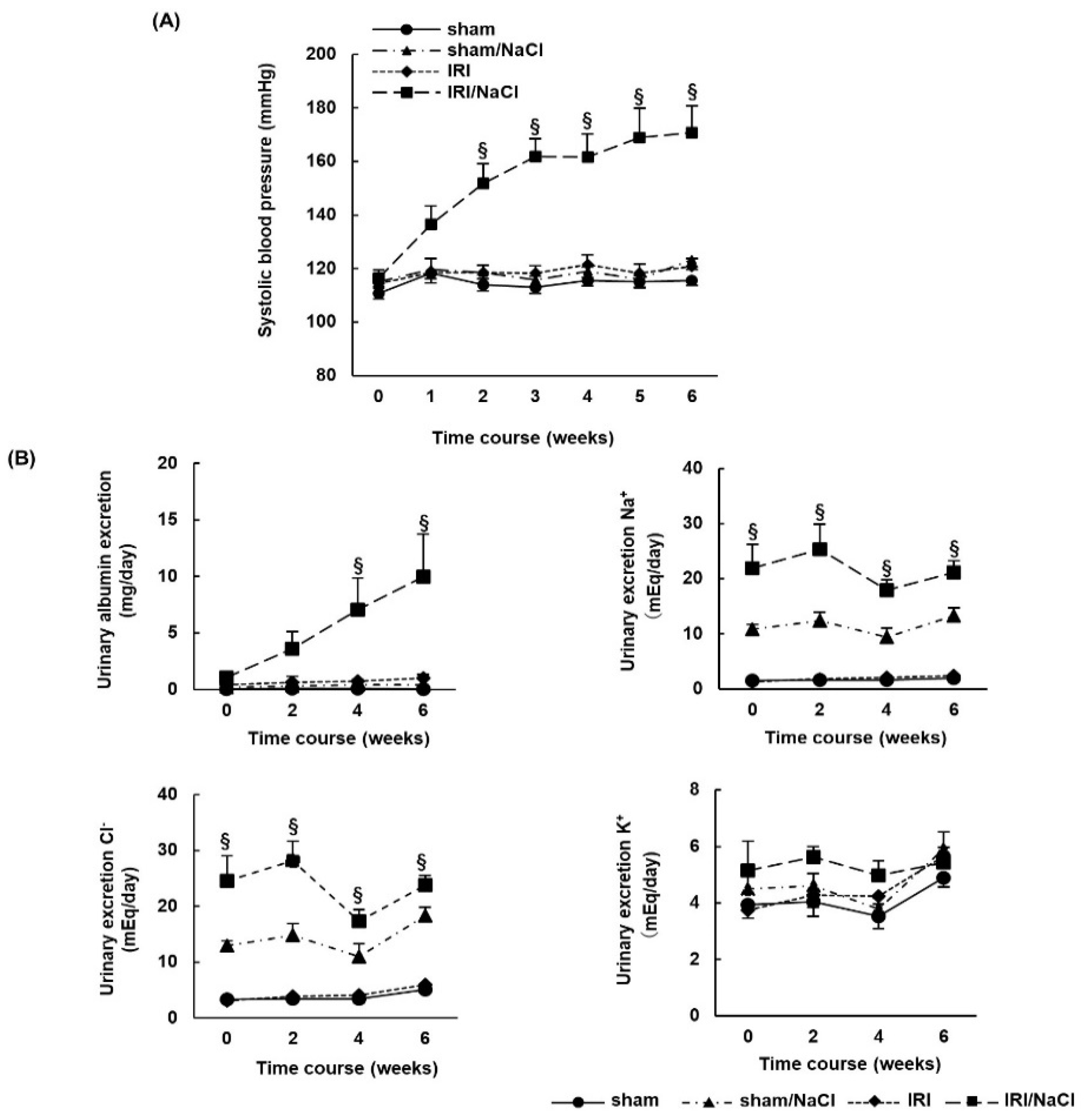
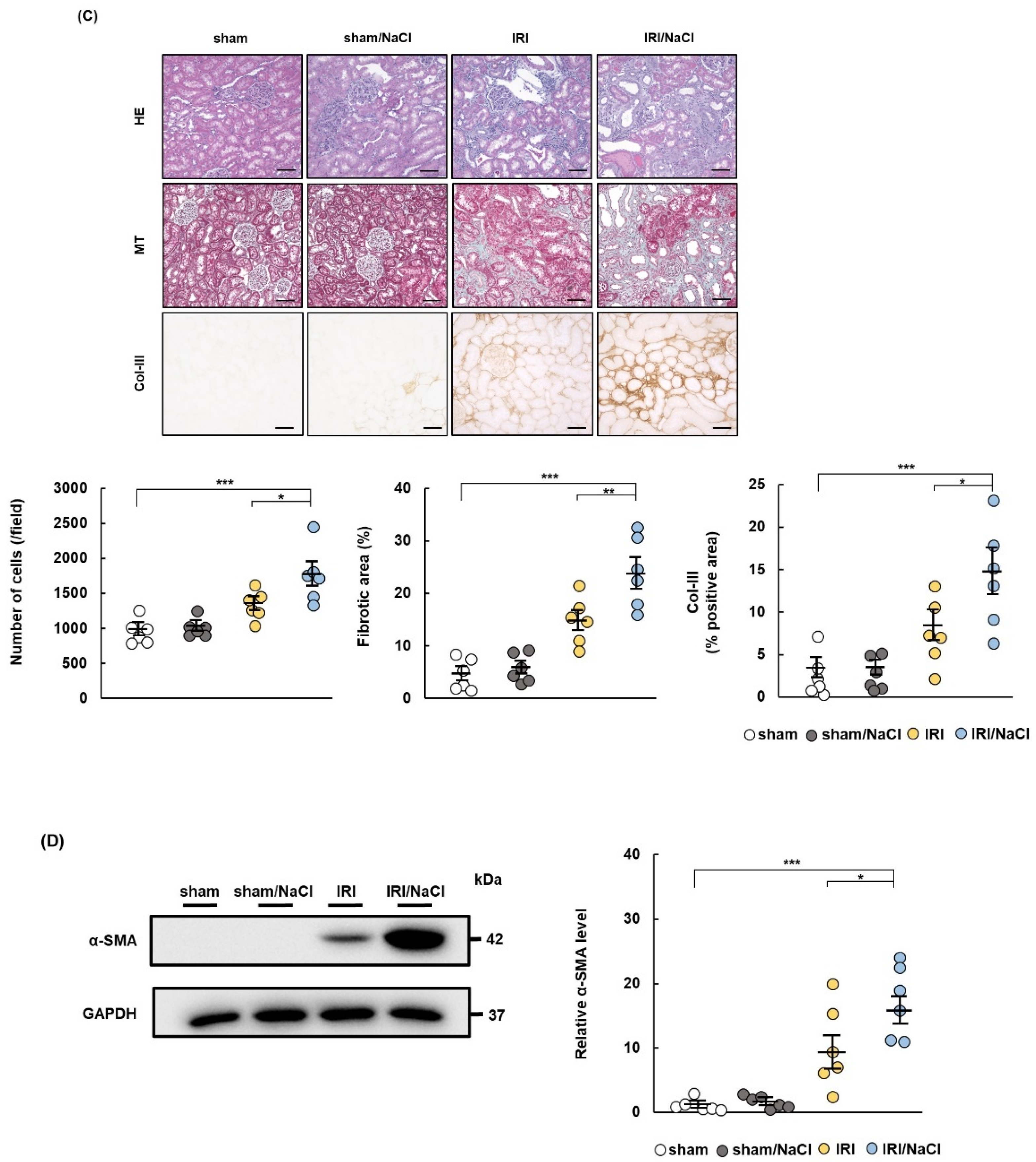
2.2. NaCl Overload Causes Hypertension and Renal Damage in IRI Rats
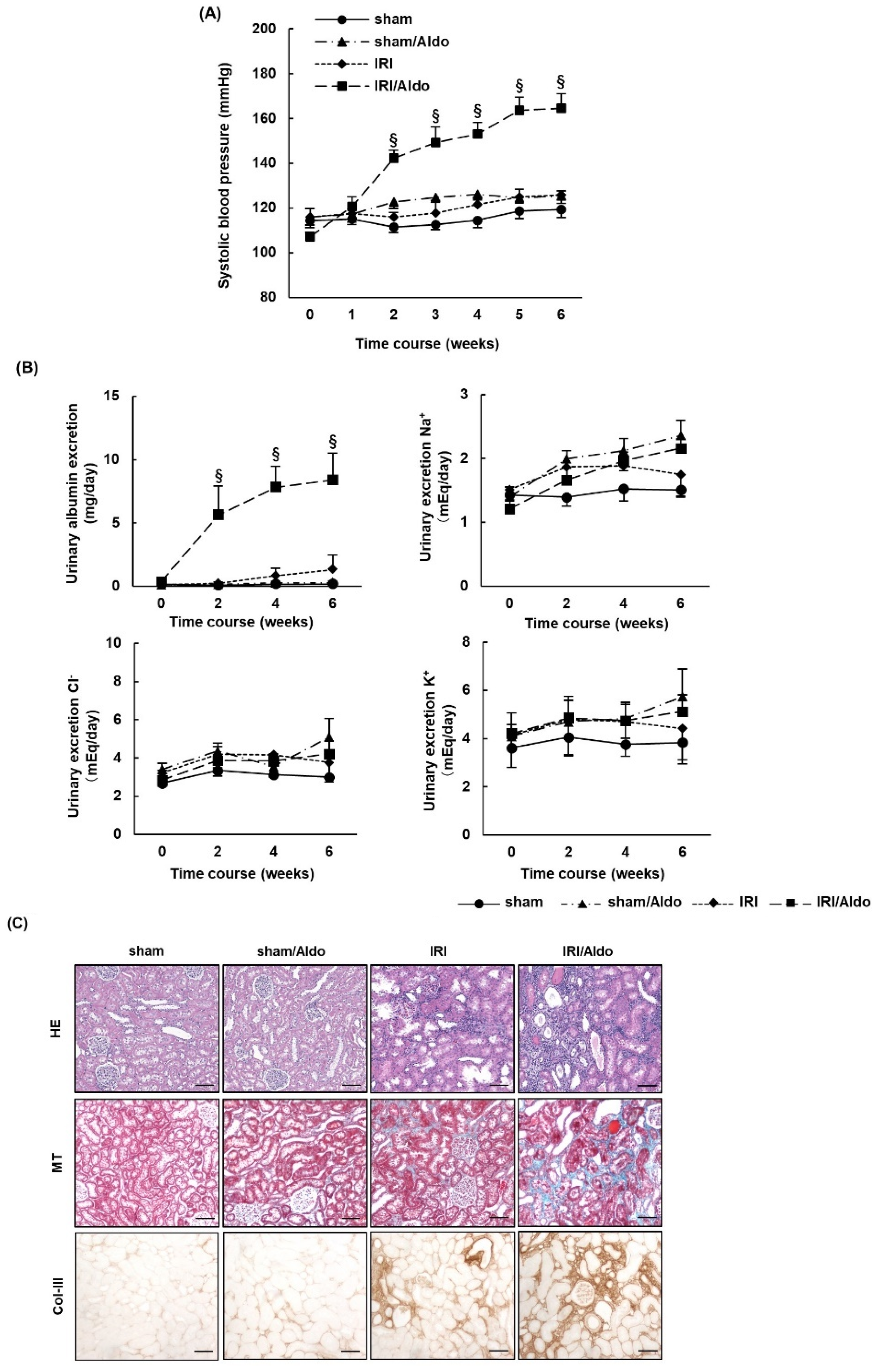
2.3. Aldosterone Infusion Induces Hypertension and Renal Damage in IRI Rats
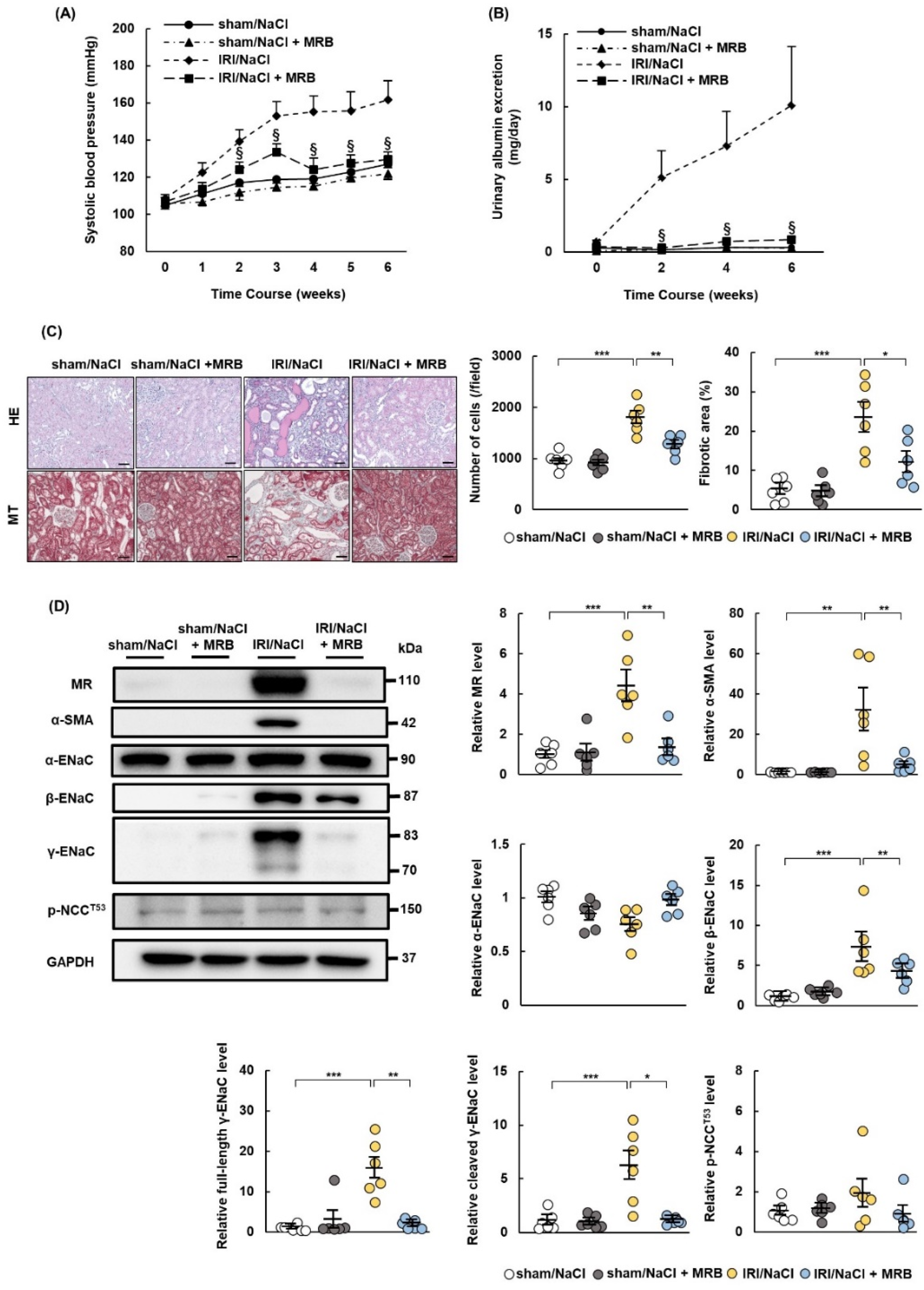
2.4. MRB Ameliorates Hypertension and Renal Damage in IRI/NaCl Rats
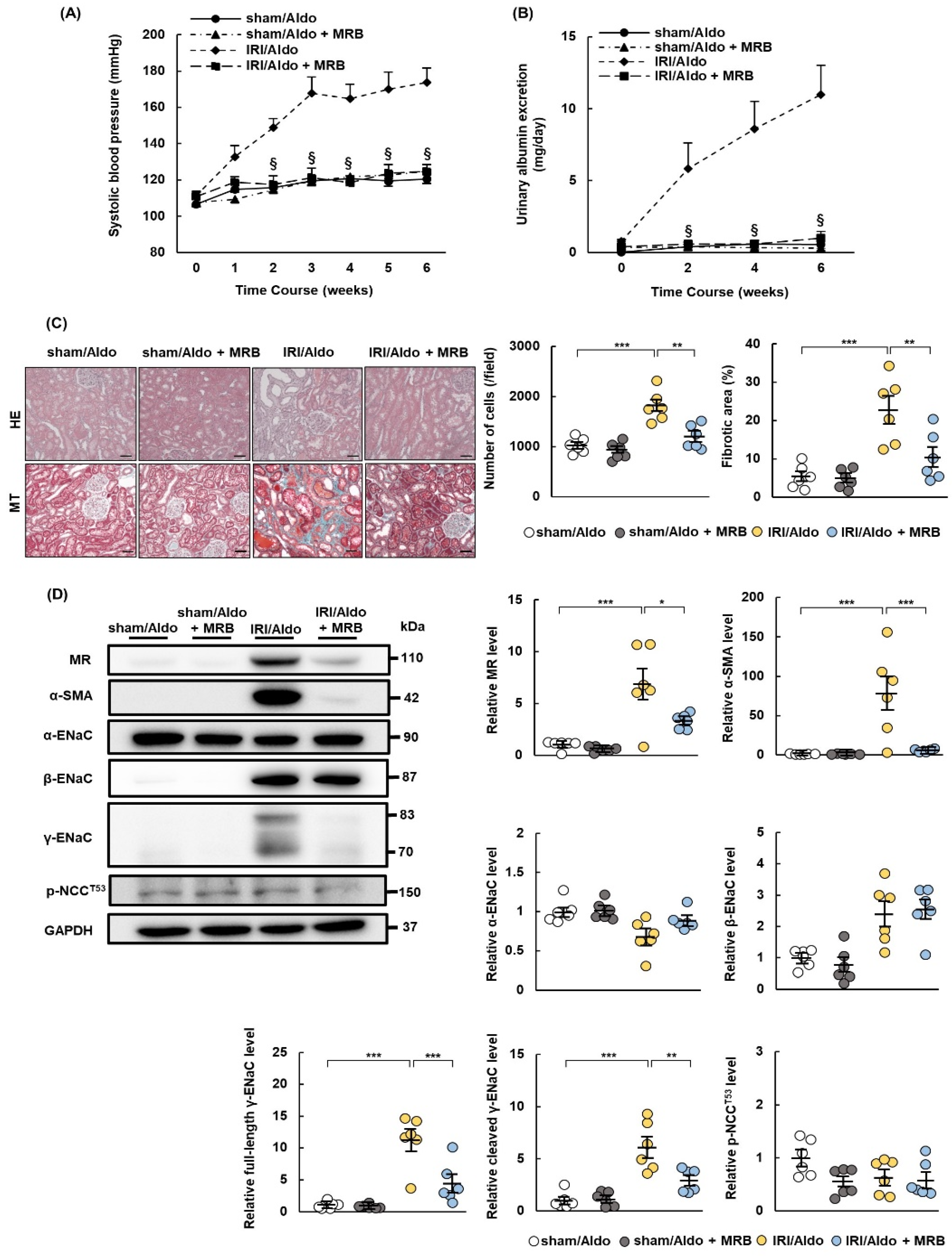
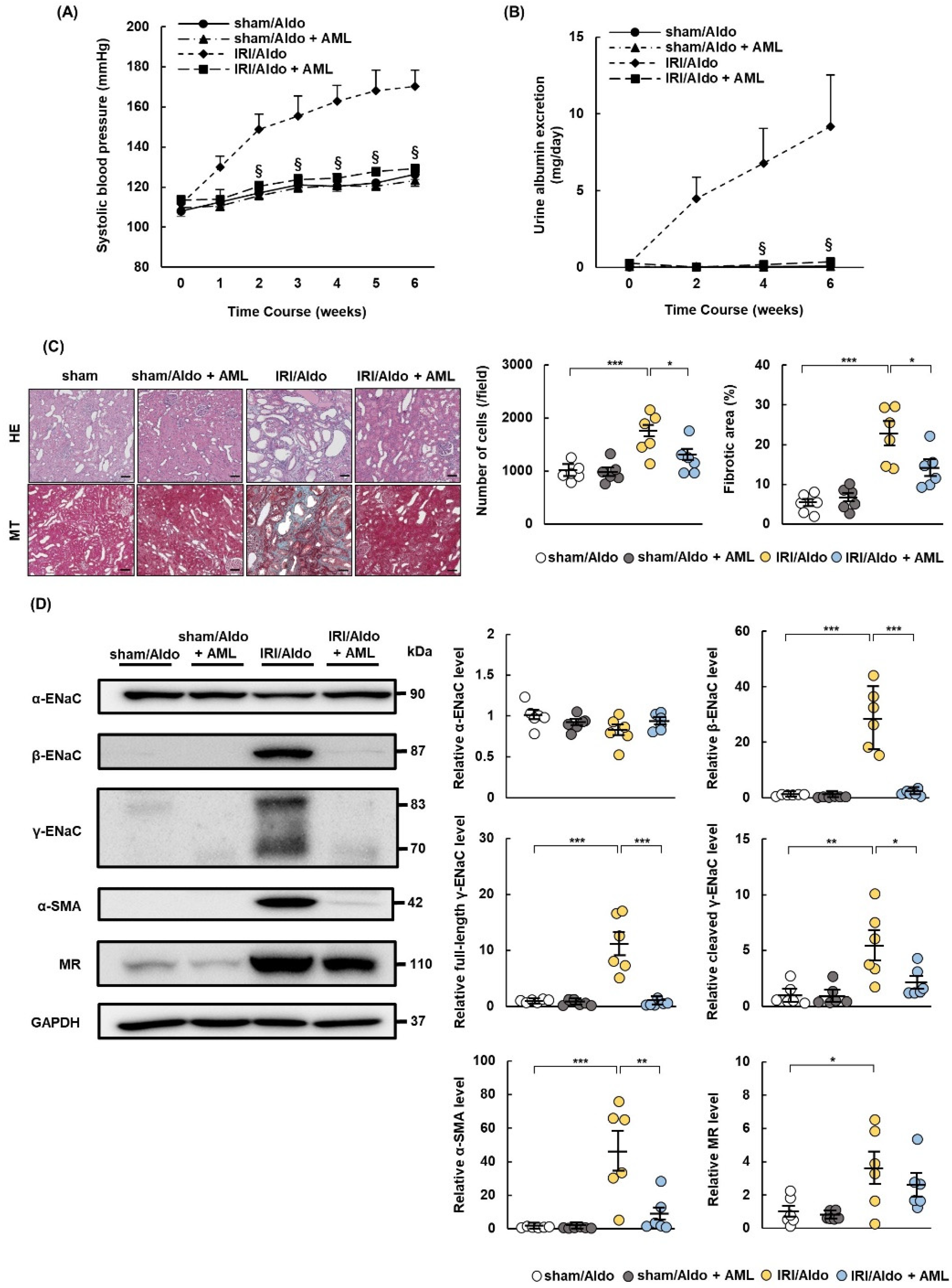
2.5. MRB Ameliorates Hypertension and Renal Damage in IRI/Aldo Rats
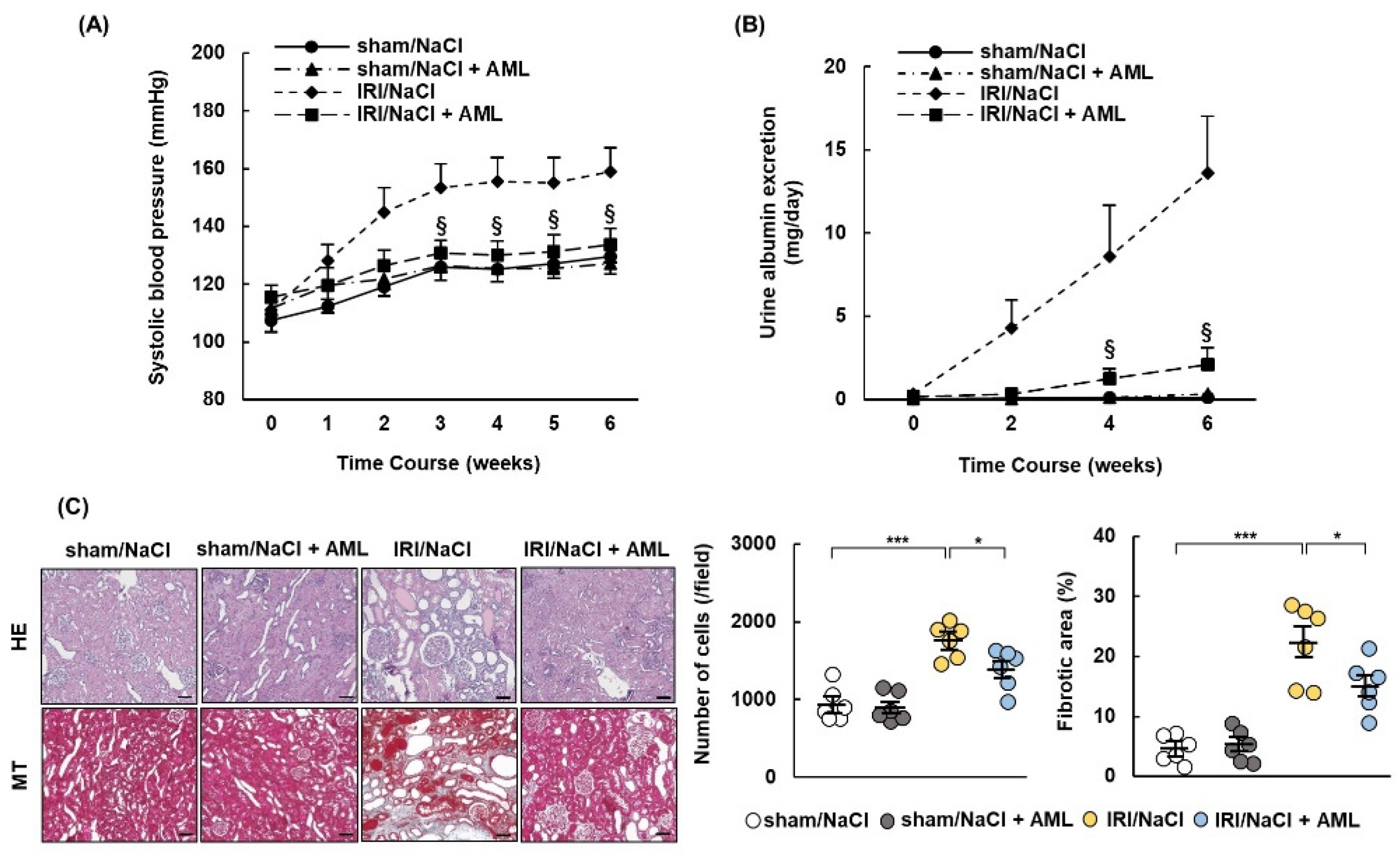
2.6. AML Ameliorates Hypertension and Renal Damage in IRI/NaCl Rats
2.7. AML Ameliorates Hypertension and Renal Damage in IRI/Aldo Rats
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Experimental Protocol
4.3. Measurement of Biological Parameters
4.4. Histology and Immunohistochemistry Analysis
4.5. Western Blot Analysis
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stevens, P.E.; Levin, A. Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members. Evaluation and Management of Chronic Kidney Disease: Synopsis of the Kidney Disease: Improving Global Outcomes 2012 Clinical Practice Guideline. Ann. Intern. Med. 2013, 158, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Coresh, J.; Selvin, E.; Stevens, L.A.; Manzi, J.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Levey, A.S. Prevalence of chronic kidney disease in the United States. JAMA 2007, 298, 2038–2047. [Google Scholar] [CrossRef] [PubMed]
- Bello, A.K.; Levin, A.; Lunney, M.; Osman, M.A.; Ye, F.; Ashuntantang, G.E.; Bellorin-Font, E.; Benghanem Gharbi, M.; Davison, S.N.; Ghnaimat, M.; et al. Status of care for end stage kidney disease in countries and regions worldwide: International cross sectional survey. BMJ 2019, 367, l5873. [Google Scholar] [CrossRef] [PubMed]
- GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Vittinghoff, E.; Lin, F.; Shlipak, M.G. The incidence of end-stage renal disease is increasing faster than the prevalence of chronic renal insufficiency. Ann. Intern. Med. 2004, 141, 95–101. [Google Scholar] [CrossRef]
- Coca, S.G.; Singanamala, S.; Parikh, C.R. Chronic kidney disease after acute kidney injury: A systematic review and meta-analysis. Kidney Int. 2012, 81, 442–448. [Google Scholar] [CrossRef]
- Heung, M.; Steffick, D.E.; Zivin, K.; Gillespie, B.W.; Banerjee, T.; Hsu, C.Y.; Powe, N.R.; Pavkov, M.E.; Williams, D.E.; Saran, R.; et al. Centers for Disease Control and Prevention CKD Surveillance Team. Acute Kidney Injury Recovery Pattern and Subsequent Risk of CKD: An Analysis of Veterans Health Administration Data. Am. J. Kidney Dis. 2016, 67, 742–752. [Google Scholar] [CrossRef]
- Fiorentino, M.; Grandaliano, G.; Gesualdo, L.; Castellano, G. Acute Kidney Injury to Chronic Kidney Disease Transition. Contrib. Nephrol. 2018, 193, 45–54. [Google Scholar] [CrossRef]
- Kurzhagen, J.T.; Dellepiane, S.; Cantaluppi, V.; Rabb, H. AKI: An increasingly recognized risk factor for CKD development and progression. J. Nephrol. 2020, 33, 1171–1187. [Google Scholar] [CrossRef]
- Venkatachalam, M.A.; Weinberg, J.M.; Kriz, W.; Bidani, A.K. Failed Tubule Recovery, AKI-CKD Transition, and Kidney Disease Progression. J. Am. Soc. Nephrol. 2015, 26, 1765–1776. [Google Scholar] [CrossRef]
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation 2020, 141, e139–e596. [Google Scholar] [CrossRef] [PubMed]
- Itoga, N.K.; Tawfik, D.S.; Lee, C.K.; Maruyama, S.; Leeper, N.J.; Chang, T.I. Association of Blood Pressure Measurements With Peripheral Artery Disease Events. Circulation 2018, 138, 1805–1814. [Google Scholar] [CrossRef] [PubMed]
- Erbel, R.; Lehmann, N.; Möhlenkamp, S.; Churzidse, S.; Bauer, M.; Kälsch, H.; Schmermund, A.; Moebus, S.; Stang, A.; Roggenbuck, U.; et al. Heinz Nixdorf Recall Study Investigators. Subclinical coronary atherosclerosis predicts cardiovascular risk in different stages of hypertension: Result of the Heinz Nixdorf Recall Study. Hypertension 2012, 59, 44–53. [Google Scholar] [CrossRef]
- Liu, M.; Li, X.C.; Lu, L.; Cao, Y.; Sun, R.R.; Chen, S.; Zhang, P.Y. Cardiovascular disease and its relationship with chronic kidney disease. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 2918–2926. [Google Scholar] [PubMed]
- Tozawa, M.; Iseki, K.; Iseki, C.; Kinjo, K.; Ikemiya, Y.; Takishita, S. Blood pressure predicts risk of developing end-stage renal disease in men and women. Hypertension 2003, 41, 1341–1345. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, K.; Ishida, K.; Sairenchi, T.; Takahashi, H.; Ohba, S.; Shiigai, T.; Narita, M.; Koyama, A. Risk factors for chronic kidney disease in a community-based population: A 10-year follow-up study. Kidney Int. 2007, 71, 159–166. [Google Scholar] [CrossRef]
- Hamrahian, S.M.; Falkner, B. Hypertension in Chronic Kidney Disease. Adv. Exp. Med. Biol. 2017, 956, 307–325. [Google Scholar] [CrossRef]
- Klag, M.J.; Whelton, P.K.; Randall, B.L.; Neaton, J.D.; Brancati, F.L.; Ford, C.E.; Shulman, N.B.; Stamler, J. Blood pressure and end-stage renal disease in men. N. Engl. J. Med. 1996, 334, 13–18. [Google Scholar] [CrossRef]
- Askenazi, D.J.; Feig, D.I.; Graham, N.M.; Hui-Stickle, S.; Goldstein, S.L. 3–5 year longitudinal follow-up of pediatric patients after acute renal failure. Kidney Int. 2006, 69, 184–189. [Google Scholar] [CrossRef]
- Fu, Y.; Tang, C.; Cai, J.; Chen, G.; Zhang, D.; Dong, Z. Rodent models of AKI-CKD transition. Am. J. Physiol. Renal Physiol. 2018, 315, F1098–F1106. [Google Scholar] [CrossRef]
- Spurgeon-Pechman, K.R.; Donohoe, D.L.; Mattson, D.L.; Lund, H.; James, L.; Basile, D.P. Recovery from acute renal failure predisposes hypertension and secondary renal disease in response to elevated sodium. Am. J. Physiol. Renal Physiol. 2007, 293, F269–F278. [Google Scholar] [CrossRef] [PubMed]
- Mattson, D.L.; Dasinger, J.H.; Abais-Battad, J.M. Amplification of Salt-Sensitive Hypertension and Kidney Damage by Immune Mechanisms. Am. J. Hypertens. 2021, 34, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Sanchez, C.E.; Warden, M.; Gomez-Sanchez, M.T.; Hou, X.; Gomez-Sanchez, E.P. Diverse immunostaining patterns of mineralocorticoid receptor monoclonal antibodies. Steroids 2011, 76, 1541–1545. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gorini, S.; Marzolla, V.; Mammi, C.; Armani, A.; Caprio, M. Mineralocorticoid Receptor and Aldosterone-Related Biomarkers of End-Organ Damage in Cardiometabolic Disease. Biomolecules 2018, 8, 96. [Google Scholar] [CrossRef] [PubMed]
- Funder, J.W. Aldosterone and Mineralocorticoid Receptors-Physiology and Pathophysiology. Int. J. Mol. Sci. 2017, 18, 1032. [Google Scholar] [CrossRef]
- Mistry, A.C.; Wynne, B.M.; Yu, L.; Tomilin, V.; Yue, Q.; Zhou, Y.; Al-Khalili, O.; Mallick, R.; Cai, H.; Alli, A.A.; et al. The sodium chloride cotransporter (NCC) and epithelial sodium channel (ENaC) associate. Biochem. J. 2016, 473, 3237–3252. [Google Scholar] [CrossRef]
- Terker, A.S.; Yarbrough, B.; Ferdaus, M.Z.; Lazelle, R.A.; Erspamer, K.J.; Meermeier, N.P.; Park, H.J.; McCormick, J.A.; Yang, C.L.; Ellison, D.H. Direct and Indirect Mineralocorticoid Effects Determine Distal Salt Transport. J. Am. Soc. Nephrol. 2016, 27, 2436–2445. [Google Scholar] [CrossRef]
- Hwang, I.K.; Yoo, K.Y.; Nam, Y.S.; Choi, J.H.; Lee, I.S.; Kwon, Y.G.; Kang, T.C.; Kim, Y.S.; Won, M.H. Mineralocorticoid and glucocorticoid receptor expressions in astrocytes and microglia in the gerbil hippocampal CA1 region after ischemic insult. Neurosci. Res. 2006, 54, 319–327. [Google Scholar] [CrossRef]
- Yamauchi, T.; Doi, S.; Nakashima, A.; Doi, T.; Sohara, E.; Uchida, S.; Masaki, T. Na +-Cl− cotransporter-mediated chloride uptake contributes to hypertension and renal damage in aldosterone-infused rats. Am. J. Physiol. Renal Physiol. 2018, 315, F300–F312. [Google Scholar] [CrossRef]
- Kakizoe, Y.; Kitamura, K.; Ko, T.; Wakida, N.; Maekawa, A.; Miyoshi, T.; Shiraishi, N.; Adachi, M.; Zhang, Z.; Masilamani, S.; et al. Aberrant ENaC activation in Dahl salt-sensitive rats. J. Hypertens. 2006, 27, 1679–1689. [Google Scholar] [CrossRef]
- Hayata, M.; Kakizoe, Y.; Uchimura, K.; Morinaga, J.; Yamazoe, R.; Mizumoto, T.; Onoue, T.; Ueda, M.; Shiraishi, N.; Adachi, M.; et al. Effect of a serine protease inhibitor on the progression of chronic renal failure. Am. J. Physiol. Renal Physiol. 2012, 303, F1126–F1135. [Google Scholar] [CrossRef] [PubMed]
- Passero, C.J.; Mueller, G.M.; Rondon-Berrios, H.; Tofovic, S.P.; Hughey, R.P.; Kleyman, T.R. Plasmin activates epithelial Na+ channels by cleaving the gamma subunit. J. Biol. Chem. 2008, 283, 36586–36591. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.; Bae, S.E.; Bell, J.E.; Seckl, J.R.; Macleod, M.R. Mineralocorticoid receptor mRNA expression is increased in human hippocampus following brief cerebral ischaemia. Neuropathol. Appl. Neurobiol. 2009, 35, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Mitsuishi, Y.; Shibata, H.; Kurihara, I.; Kobayashi, S.; Yokota, K.; Murai-Takeda, A.; Hayashi, T.; Jo, R.; Nakamura, T.; Morisaki, M.; et al. Epidermal growth factor receptor/extracellular signal-regulated kinase pathway enhances mineralocorticoid receptor transcriptional activity through protein stabilization. Mol. Cell. Endocrinol. 2018, 473, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Nieuwenhuijs-Moeke, G.J.; Pischke, S.E.; Berger, S.P.; Sanders, J.S.F.; Pol, R.A.; Struys, M.M.R.F.; Ploeg, R.J.; Leuvenink, H.G.D. Ischemia and Reperfusion Injury in Kidney Transplantation: Relevant Mechanisms in Injury and Repair. J. Clin. Med. 2020, 9, 253. [Google Scholar] [CrossRef]
- Morales-Buenrostro, L.E.; Ortega-Trejo, J.A.; Pérez-Villalva, R.; Marino, L.A.; González-Bobadilla, Y.; Juárez, H.; Zamora-Mejía, F.M.; González, N.; Espinoza, R.; Barrera-Chimal, J.; et al. Spironolactone reduces oxidative stress in living donor kidney transplantation: A randomized controlled trial. Am. J. Physiol. Renal Physiol. 2019, 317, F519–F528. [Google Scholar] [CrossRef]
- Medeiros, M.; Velásquez-Jones, L.; Hernández, A.M.; Ramón-García, G.; Valverde, S.; Fuentes, Y.; Vargas, A.; Patiño, M.; Pérez-Villalva, R.; Ortega-Trejo, J.A.; et al. Randomized Controlled Trial of Mineralocorticoid Receptor Blockade in Children with Chronic Kidney Allograft Nephropathy. Clin. J. Am. Soc. Nephrol. 2017, 12, 1291–1300. [Google Scholar] [CrossRef]
- Bianchi, S.; Bigazzi, R.; Campese, V.M. Long-term effects of spironolactone on proteinuria and kidney function in patients with chronic kidney disease. Kidney Int. 2006, 70, 2116–2123. [Google Scholar] [CrossRef]
- Selye, H.; Stone, H. Influence of sodium chloride upon the actions of desoxycorticosteron acetate. Am. Heart J. 1949, 37, 1009–1016. [Google Scholar] [CrossRef]
- Bayorh, M.A.; Mann, G.; Walton, M.; Eatman, D. Effects of enalapril, tempol, and eplerenone on salt-induced hypertension in dahl salt-sensitive rats. Clin. Exp. Hypertens. 2006, 28, 121–132. [Google Scholar] [CrossRef]
- Bovée, D.M.; Uijl, E.; Severs, D.; Rubio-Beltrán, E.; van Veghel, R.; Maassen van den Brink, A.; Joles, J.A.; Zietse, R.; Cuevas, C.A.; Danser, A.H.J.; et al. Dietary salt modifies the blood pressure response to renin-angiotensin inhibition in experimental chronic kidney disease. Am. J. Physiol. Renal Physiol. 2021, 320, F654–F668. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, X.; Ke, Y.; Lu, Y.; Wang, F.; Fan, N.; Sun, H.; Zhang, H.; Liu, R.; Yang, J.; et al. GPR48 increases mineralocorticoid receptor gene expression. J. Am. Soc. Nephrol. 2012, 23, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Bock, J.S.; Gottlieb, S.S. Cardiorenal syndrome: New perspectives. Circulation 2010, 121, 2592–2600. [Google Scholar] [CrossRef] [PubMed]
- Kawarazaki, W.; Fujita, T. The Role of Aldosterone in Obesity-Related Hypertension. Am. J. Hypertens. 2016, 29, 415–423. [Google Scholar] [CrossRef]
- Jia, G.; Whaley-Connell, A.; Sowers, J.R. Diabetic cardiomyopathy: A hyperglycaemia- and insulin-resistance-induced heart disease. Diabetologia 2018, 61, 21–28. [Google Scholar] [CrossRef]
- Greene, A.S.; Yu, Z.Y.; Roman, R.J.; Cowley, A.W. Role of blood volume expansion in Dahl rat model of hypertension. Am. J. physiol. 1990, 258, H508–H514. [Google Scholar] [CrossRef]
- Hall, J.E. Renal dysfunction, rather than nonrenal vascular dysfunction, mediates salt-induced hypertension. Circulation 2016, 133, 894–906. [Google Scholar] [CrossRef]
- Hanukoglu, I.; Hanukoglu, A. Epithelial sodium channel (ENaC) family: Phylogeny, structure-function, tissue distribution, and associated inherited diseases. Gene 2016, 579, 95–132. [Google Scholar] [CrossRef]
- Zhao, Q.; Gu, D.; Hixson, J.E.; Liu, D.P.; Rao, D.C.; Jaquish, C.E.; Kelly, T.N.; Lu, F.; Ma, J.; Mu, J.; et al. Genetic Epidemiology Network of Salt Sensitivity Collaborative Research Group. Common variants in epithelial sodium channel genes contribute to salt sensitivity of blood pressure: The GenSalt study. Circ. Cardiovasc. Genet. 2011, 4, 375–380. [Google Scholar] [CrossRef]
- Saxena, A.; Hanukoglu, I.; Saxena, D.; Thompson, R.J.; Gardiner, R.M.; Hanukoglu, A. Novel mutations responsible for autosomal recessive multisystem pseudohypoaldosteronism and sequence variants in epithelial sodium channel alpha-, beta-, and gamma-subunit genes. J. Clin. Endocrinol. Metab. 2002, 87, 3344–3350. [Google Scholar] [CrossRef]
- Furuhashi, M.; Kitamura, K.; Adachi, M.; Miyoshi, T.; Wakida, N.; Ura, N.; Shikano, Y.; Shinshi, Y.; Sakamoto, K.; Hayashi, M.; et al. Liddle’s syndrome caused by a novel mutation in the proline-rich PY motif of the epithelial sodium channel beta-subunit. J. Clin. Endocrinol. Metab. 2005, 90, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Mejía-Vilet, J.M.; Ramírez, V.; Cruz, C.; Uribe, N.; Gamba, G.; Bobadilla, N.A. Renal ischemia-reperfusion injury is prevented by the mineralocorticoid receptor blocker spironolactone. Am. J. Physiol. Renal Physiol. 2007, 293, F78–F86. [Google Scholar] [CrossRef] [PubMed]
- Barrera-Chimal, J.; Rocha, L.; Amador-Martínez, I.; Pérez-Villalva, R.; González, R.; Cortés-González, C.; Uribe, N.; Ramírez, V.; Berman, N.; Gamba, G.; et al. Delayed spironolactone administration prevents the transition from acute kidney injury to chronic kidney disease through improving renal inflammation. Nephrol. Dial. Transplant. 2019, 34, 794–801. [Google Scholar] [CrossRef] [PubMed]
- Barrera-Chimal, J.; Pérez-Villalva, R.; Ortega, J.A.; Sánchez, A.; Rodríguez-Romo, R.; Durand, M.; Jaisser, F.; Bobadilla, N.A. Mild ischemic injury leads to long-term alterations in the kidney: Amelioration by spironolactone administration. Int. J. Biol. Sci. 2015, 11, 892–900. [Google Scholar] [CrossRef]
- Kawai, S.; Takagi, Y.; Kaneko, S.; Kurosawa, T. Effect of three types of mixed anesthetic agents alternate to ketamine in mice. Exp. Anim. 2011, 60, 481–487. [Google Scholar] [CrossRef]
- Doi, T.; Doi, S.; Nakashima, A.; Ueno, T.; Yokoyama, Y.; Kohno, N.; Masaki, T. Mizoribine ameliorates renal injury and hypertension along with the attenuation of renal caspase-1 expression in aldosterone-salt-treated rats. PLoS ONE 2014, 9, e93513. [Google Scholar] [CrossRef]
- Ueno, T.; Nakashima, A.; Doi, S.; Kawamoto, T.; Honda, K.; Yokoyama, Y.; Doi, T.; Higashi, Y.; Yorioka, N.; Kato, Y.; et al. Mesenchymal stem cells ameliorate experimental peritoneal fibrosis by suppressing inflammation and inhibiting TGF-β1 signaling. Kidney Int. 2013, 84, 297–307. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsumoto, T.; Doi, S.; Nakashima, A.; Ike, T.; Sasaki, K.; Masaki, T. Upregulation of Mineralocorticoid Receptor Contributes to Development of Salt-Sensitive Hypertension after Ischemia–Reperfusion Injury in Rats. Int. J. Mol. Sci. 2022, 23, 7831. https://doi.org/10.3390/ijms23147831
Matsumoto T, Doi S, Nakashima A, Ike T, Sasaki K, Masaki T. Upregulation of Mineralocorticoid Receptor Contributes to Development of Salt-Sensitive Hypertension after Ischemia–Reperfusion Injury in Rats. International Journal of Molecular Sciences. 2022; 23(14):7831. https://doi.org/10.3390/ijms23147831
Chicago/Turabian StyleMatsumoto, Takumi, Shigehiro Doi, Ayumu Nakashima, Takeshi Ike, Kensuke Sasaki, and Takao Masaki. 2022. "Upregulation of Mineralocorticoid Receptor Contributes to Development of Salt-Sensitive Hypertension after Ischemia–Reperfusion Injury in Rats" International Journal of Molecular Sciences 23, no. 14: 7831. https://doi.org/10.3390/ijms23147831
APA StyleMatsumoto, T., Doi, S., Nakashima, A., Ike, T., Sasaki, K., & Masaki, T. (2022). Upregulation of Mineralocorticoid Receptor Contributes to Development of Salt-Sensitive Hypertension after Ischemia–Reperfusion Injury in Rats. International Journal of Molecular Sciences, 23(14), 7831. https://doi.org/10.3390/ijms23147831





