Impact of Metabolic Surgery on Gut Microbiota and Sera Metabolomic Patterns among Patients with Diabetes
Abstract
:1. Introduction
2. Results
2.1. Changes in Clinical and Circulating Biomarkers after Metabolic Surgery
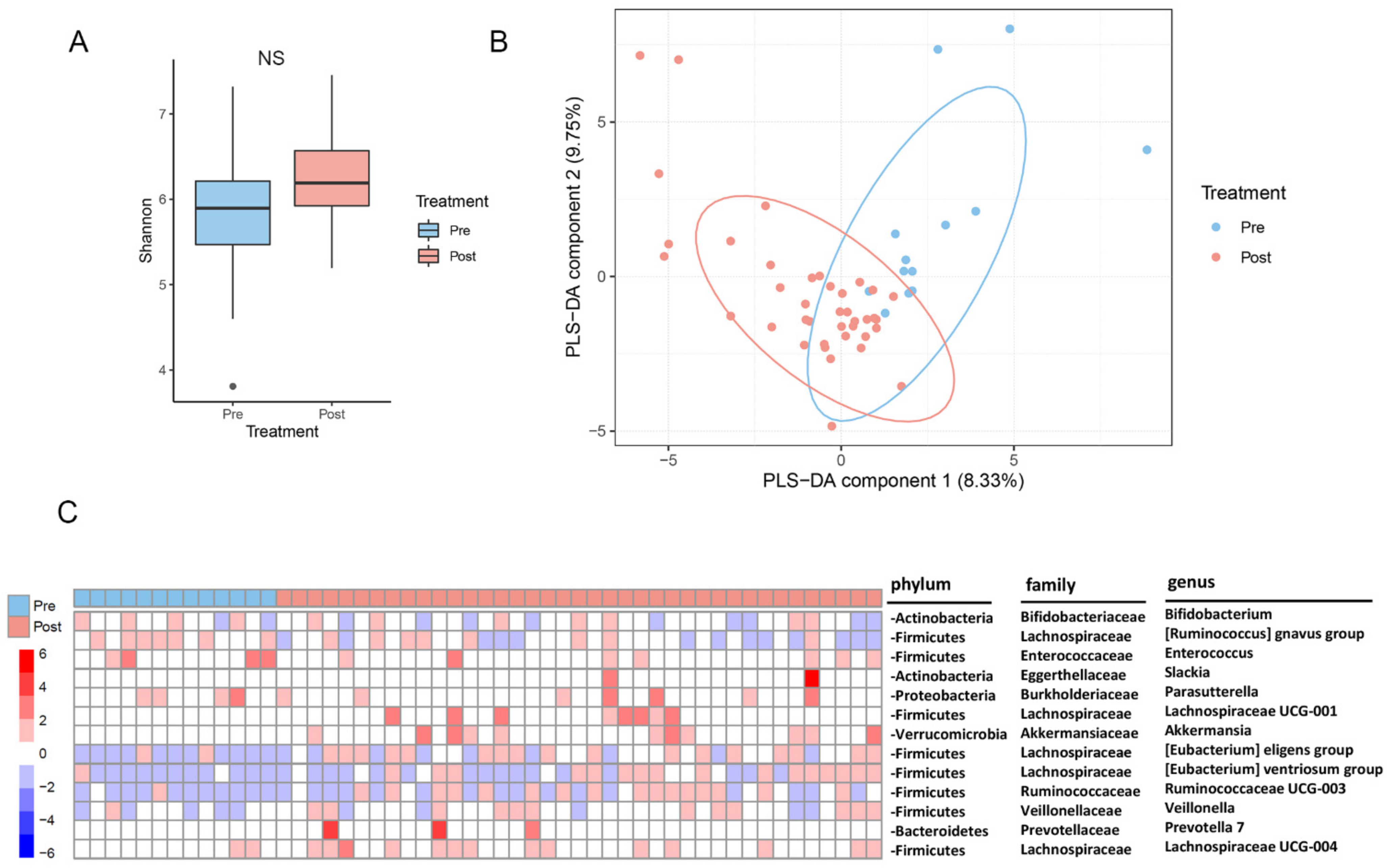
2.2. Changes in the Gut Microbiota after Metabolic Surgery
2.3. Changes in the Serum Metabolites after Metabolic Surgery
2.4. Changes of Branched Chain Amino Acids (BCAA) after Metabolic Surgery
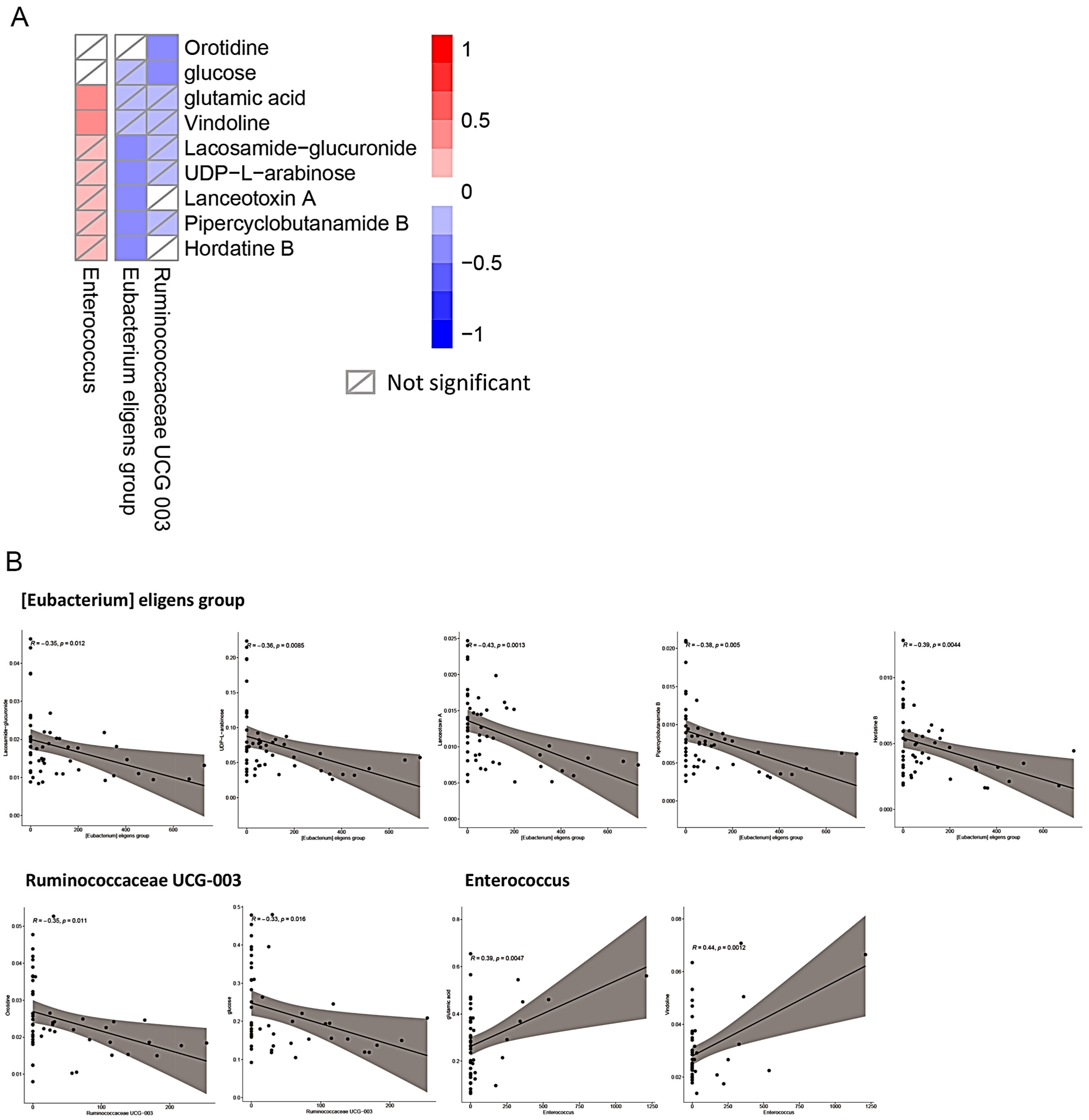
2.5. Association of Changes between the Gut Microbiota and Sera Metabolites after Metabolic Surgery
3. Discussion
4. Materials and Methods
4.1. Patients and Metabolic Surgery
4.2. Surgical Technique
4.2.1. Laparoscopic Gastric Bypass (GB)
4.2.2. Laparoscopic Sleeve Gastrectomy (SG)
4.2.3. Laparoscopic Duodenojejunal Bypass with Sleeve Gastrectomy (DJB–SG)
4.3. Study Protocol and Anthropometric Measurement
4.4. DNA Extraction from Human Stool Samples
4.5. 16S rDNA-Based Metagenomics Analysis Pipeline
4.6. Sera Metabolomics Analysis
4.6.1. Metabolites Extraction for Gas Chromatography-Mass Spectrometry (GC-MS)
4.6.2. Metabolites Extraction for Liquid Chromatography Mass Spectrometry (LC-MS)
4.6.3. Metabolomics Analysis
4.7. Correlation Analysis
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tan, S.Y.; Mei Wong, J.L.; Sim, Y.J.; Wong, S.S.; Mohamed Elhassan, S.A.; Tan, S.H.; Ling Lim, G.P.; Rong Tay, N.W.; Annan, N.C.; Bhattamisra, S.K.; et al. Type 1 and 2 diabetes mellitus: A review on current treatment approach and gene therapy as potential intervention. Diabetes Metab. Syndr. 2019, 13, 364–372. [Google Scholar] [CrossRef]
- Stenberg, E.; Thorell, A. Insulin resistance in bariatric surgery. Curr. Opin. Clin. Nutr. Metab. Care 2020, 23, 255–261. [Google Scholar] [CrossRef]
- Lee, J.; Cummings, B.P.; Martin, E.; Sharp, J.W.; Graham, J.L.; Stanhope, K.L.; Havel, P.J.; Raybould, H.E. Glucose sensing by gut endocrine cells and activation of the vagal afferent pathway is impaired in a rodent model of type 2 diabetes mellitus. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 302, R657–R666. [Google Scholar] [CrossRef]
- Grasset, E.; Puel, A.; Charpentier, J.; Collet, X.; Christensen, J.E.; Tercé, F.; Burcelin, R. A Specific Gut Microbiota Dysbiosis of Type 2 Diabetic Mice Induces GLP-1 Resistance through an Enteric NO-Dependent and Gut-Brain Axis Mechanism. Cell Metab. 2017, 26, 278. [Google Scholar] [CrossRef] [Green Version]
- Vincent, K.M.; Sharp, J.W.; Raybould, H.E. Intestinal glucose-induced calcium-calmodulin kinase signaling in the gut-brain axis in awake rats. Neurogastroenterol. Motil. 2011, 23, e282–e293. [Google Scholar] [CrossRef] [Green Version]
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.R.; Ismagilov, R.F.; Mazmanian, S.K.; Hsiao, E.Y. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 2015, 161, 264–276. [Google Scholar] [CrossRef] [Green Version]
- Fournel, A.; Drougard, A.; Duparc, T.; Marlin, A.; Brierley, S.M.; Castro, J.; Le-Gonidec, S.; Masri, B.; Colom, A.; Lucas, A.; et al. Apelin targets gut contraction to control glucose metabolism via the brain. Gut 2017, 66, 258–269. [Google Scholar] [CrossRef] [Green Version]
- Michel, K.; Zeller, F.; Langer, R.; Nekarda, H.; Kruger, D.; Dover, T.J.; Brady, C.A.; Barnes, N.M.; Schemann, M. Serotonin excites neurons in the human submucous plexus via 5-HT3 receptors. Gastroenterology 2005, 128, 1317–1326. [Google Scholar] [CrossRef]
- Abot, A.; Lucas, A.; Bautzova, T.; Bessac, A.; Fournel, A.; Le-Gonidec, S.; Valet, P.; Moro, C.; Cani, P.D.; Knauf, C. Galanin enhances systemic glucose metabolism through enteric Nitric Oxide Synthase-expressed neurons. Mol. Metab. 2018, 10, 100–108. [Google Scholar] [CrossRef]
- Cleary, J.L.; Condren, A.R.; Zink, K.E.; Sanchez, L.M. Calling all hosts: Bacterial communication in situ. Chem 2017, 2, 334–358. [Google Scholar] [CrossRef] [Green Version]
- Clemente, J.C.; Ursell, L.K.; Parfrey, L.W.; Knight, R. The impact of the gut microbiota on human health: An integrative view. Cell 2012, 148, 1258–1270. [Google Scholar] [CrossRef] [Green Version]
- Sekirov, I.; Russell, S.L.; Antunes, L.C.; Finlay, B.B. Gut microbiota in health and disease. Physiol. Rev. 2010, 90, 859–904. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, P.J.; Cryan, J.F.; Dinan, T.G.; Clarke, G. Irritable bowel syndrome: A microbiome-gut-brain axis disorder? World J. Gastroenterol. 2014, 20, 14105–14125. [Google Scholar] [CrossRef]
- Mayer, E.A. The neurobiology of stress and gastrointestinal disease. Gut 2000, 47, 861–869. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.Y.; Fujimiya, M.; Laviano, A.; Chang, F.Y.; Lin, H.C.; Lee, S.D. Modulation of ingestive behavior and gastrointestinal motility by ghrelin in diabetic animals and humans. J. Chin. Med. Assoc. 2010, 73, 225–229. [Google Scholar] [CrossRef] [Green Version]
- Davies, N.K.; O’Sullivan, J.M.; Plank, L.D.; Murphy, R. Altered gut microbiome after bariatric surgery and its association with metabolic benefits: A systematic review. Surg. Obes. Relat. Dis. 2019, 15, 656–665. [Google Scholar] [CrossRef]
- Gutiérrez-Repiso, C.; Moreno-Indias, I.; de Hollanda, A.; Martín-Núñez, G.M.; Vidal, J.; Tinahones, F.J. Gut microbiota specific signatures are related to the successful rate of bariatric surgery. Am. J. Transl. Res. 2019, 11, 942–952. [Google Scholar]
- Wu, J.; Zhang, P.B.; Ren, Z.Q.; Zhou, F.; Hu, H.H.; Zhang, H.; Xue, K.K.; Xu, P.; Shao, X.Q. Changes of serum lipopolysaccharide, inflammatory factors, and cecal microbiota in obese rats with type 2 diabetes induced by Roux-en-Y gastric bypass. Nutrition 2019, 67–68, 110565. [Google Scholar] [CrossRef]
- Samczuk, P.; Luba, M.; Godzien, J.; Mastrangelo, A.; Hady, H.R.; Dadan, J.; Barbas, C.; Gorska, M.; Kretowski, A.; Ciborowski, M. “Gear mechanism” of bariatric interventions revealed by untargeted metabolomics. J. Pharm. Biomed. Anal. 2018, 151, 219–226. [Google Scholar] [CrossRef]
- Samczuk, P.; Ciborowski, M.; Kretowski, A. Application of Metabolomics to Study Effects of Bariatric Surgery. J. Diabetes Res. 2018, 2018, 6270875. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Cheng, Z.; Wang, Y.; Dai, Y.; Zhang, X.; Hu, S. Role of Bile Acids in Bariatric Surgery. Front. Physiol. 2019, 10, 374. [Google Scholar] [CrossRef]
- Huffman, K.M.; Shah, S.H.; Stevens, R.D.; Bain, J.R.; Muehlbauer, M.; Slentz, C.A.; Tanner, C.J.; Kuchibhatla, M.; Houmard, J.A.; Newgard, C.B.; et al. Relationships between circulating metabolic intermediates and insulin action in overweight to obese, inactive men and women. Diabetes Care 2009, 32, 1678–1683. [Google Scholar] [CrossRef] [Green Version]
- Newgard, C.B.; An, J.; Bain, J.R.; Muehlbauer, M.J.; Stevens, R.D.; Lien, L.F.; Haqq, A.M.; Shah, S.H.; Arlotto, M.; Slentz, C.A.; et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009, 9, 311–326. [Google Scholar] [CrossRef] [Green Version]
- Osataphan, S.; Patti, M.E. Trim the gut, lose the weight—and the bone. J. Clin. Investig. 2019, 129, 2184–2186. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.J.; Chen, C.Y.; Chong, K.; Lee, Y.C.; Chen, S.C.; Lee, S.D. Changes in postprandial gut hormones after metabolic surgery: A comparison of gastric bypass and sleeve gastrectomy. Surg. Obes. Relat. Dis. 2011, 7, 683–690. [Google Scholar] [CrossRef]
- Wang, J.W.; Chen, P.Y.; Huang, H.H.; Yeh, C.; Chen, S.C.; Lee, W.J.; Chen, C.Y. Change of plasma amylin after bariatric surgery challenged by oral glucose is associated with remission of type 2 diabetes mellitus. J. Chin. Med. Assoc. 2021, 84, 1001–1006. [Google Scholar] [CrossRef]
- Chen, C.Y.; Asakawa, A.; Fujimiya, M.; Lee, S.D.; Inui, A. Ghrelin gene products and the regulation of food intake and gut motility. Pharmacol. Rev. 2009, 61, 430–481. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.Y.; Lee, W.J.; Asakawa, A.; Fujitsuka, N.; Chong, K.; Chen, S.C.; Lee, S.D.; Inui, A. Insulin secretion and interleukin-1β dependent mechanisms in human diabetes remission after metabolic surgery. Curr. Med. Chem. 2013, 20, 2374–2388. [Google Scholar] [CrossRef] [Green Version]
- Russel, S.M.; Valle, V.; Spagni, G.; Hamilton, S.; Patel, T.; Abdukadyrov, N.; Dong, Y.; Gangemi, A. Physiologic Mechanisms of Type II Diabetes Mellitus Remission Following Bariatric Surgery: A Meta-analysis and Clinical Implications. J. Gastrointest. Surg. 2020, 24, 728–741. [Google Scholar] [CrossRef]
- Ben-Porat, T.; Weiss-Sadan, A.; Rottenstreich, A.; Sherf-Dagan, S.; Schweiger, C.; Yosef-Levi, I.M.; Weiner, D.; Azulay, O.; Sakran, N.; Harari, R.; et al. Nutritional Management for Chronic Kidney Disease Patients who Undergo Bariatric Surgery: A Narrative Review. Adv. Nutr. 2019, 10, 122–132. [Google Scholar] [CrossRef]
- Liu, J.; Prudom, C.E.; Nass, R.; Pezzoli, S.S.; Oliveri, M.C.; Johnson, M.L.; Veldhuis, P.; Gordon, D.A.; Howard, A.D.; Witcher, D.R.; et al. Novel ghrelin assays provide evidence for independent regulation of ghrelin acylation and secretion in healthy young men. J. Clin. Endocrinol. Metab. 2008, 93, 1980–1987. [Google Scholar] [CrossRef]
- Chen, C.Y.; Inui, A.; Asakawa, A.; Fujino, K.; Kato, I.; Chen, C.C.; Ueno, N.; Fujimiya, M. Des-acyl ghrelin acts by CRF type 2 receptors to disrupt fasted stomach motility in conscious rats. Gastroenterology 2005, 129, 8–25. [Google Scholar] [CrossRef]
- Haghighi, S.; Amini, M.; Pournaghshband, Z.; Amini, P.; Hovsepian, S. Relationship between gamma-glutamyl transferase and glucose intolerance in first degree relatives of type 2 diabetics patients. J. Res. Med. Sci. 2011, 16, 123–129. [Google Scholar]
- Huang, H.H.; Lee, W.J.; Chen, S.C.; Chen, T.F.; Lee, S.D.; Chen, C.Y. Bile Acid and Fibroblast Growth Factor 19 Regulation in Obese Diabetics, and Non-Alcoholic Fatty Liver Disease after Sleeve Gastrectomy. J. Clin. Med. 2019, 8, 815. [Google Scholar] [CrossRef] [Green Version]
- Zachariah, P.J.; Chen, C.Y.; Lee, W.J.; Chen, S.C.; Ser, K.H.; Chen, J.C.; Lee, Y.C. Compared to Sleeve Gastrectomy, Duodenal-Jejunal Bypass with Sleeve Gastrectomy Gives Better Glycemic Control in T2DM Patients, with a Lower β-Cell Response and Similar Appetite Sensations: Mixed-Meal Study. Obes. Surg. 2016, 26, 2862–2872. [Google Scholar] [CrossRef]
- Guyton, K.; Alverdy, J.C. The gut microbiota and gastrointestinal surgery. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 43–54. [Google Scholar] [CrossRef]
- Ulker, İ.; Yildiran, H. The effects of bariatric surgery on gut microbiota in patients with obesity: A review of the literature. Biosci. Microbiota Food Health 2019, 38, 3–9. [Google Scholar] [CrossRef] [Green Version]
- Depommier, C.; Everard, A.; Druart, C.; Plovier, H.; Van Hul, M.; Vieira-Silva, S.; Falony, G.; Raes, J.; Maiter, D.; Delzenne, N.M.; et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: A proof-of-concept exploratory study. Nat. Med. 2019, 25, 1096–1103. [Google Scholar] [CrossRef]
- Hu, R.; Zeng, F.; Wu, L.; Wan, X.; Chen, Y.; Zhang, J.; Liu, B. Fermented carrot juice attenuates type 2 diabetes by mediating gut microbiota in rats. Food Funct. 2019, 10, 2935–2946. [Google Scholar] [CrossRef]
- Wei, X.; Tao, J.; Xiao, S.; Jiang, S.; Shang, E.; Zhu, Z.; Qian, D.; Duan, J. Xiexin Tang improves the symptom of type 2 diabetic rats by modulation of the gut microbiota. Sci. Rep. 2018, 8, 3685. [Google Scholar] [CrossRef]
- Hu, T.G.; Wen, P.; Shen, W.Z.; Liu, F.; Li, Q.; Li, E.N.; Liao, S.T.; Wu, H.; Zou, Y.X. Effect of 1-Deoxynojirimycin Isolated from Mulberry Leaves on Glucose Metabolism and Gut Microbiota in a Streptozotocin-Induced Diabetic Mouse Model. J. Nat. Prod. 2019, 82, 2189–2200. [Google Scholar] [CrossRef]
- Kang, X.; Zhan, L.; Lu, X.; Song, J.; Zhong, Y.; Wang, Y.; Yang, Y.; Fan, Z.; Jiang, X.; Sun, R. Characteristics of Gastric Microbiota in GK Rats with Spontaneous Diabetes: A Comparative Study. Diabetes Metab. Syndr. Obes. 2020, 13, 1435–1447. [Google Scholar] [CrossRef]
- Felig, P. The glucose-alanine cycle. Metabolism 1973, 22, 179–207. [Google Scholar] [CrossRef]
- Petersen, K.F.; Dufour, S.; Cline, G.W.; Shulman, G.I. Regulation of hepatic mitochondrial oxidation by glucose-alanine cycling during starvation in humans. J. Clin. Investig. 2019, 129, 4671–4675. [Google Scholar] [CrossRef]
- Adeva, M.; González-Lucán, M.; Seco, M.; Donapetry, C. Enzymes involved in l-lactate metabolism in humans. Mitochondrion 2013, 13, 615–629. [Google Scholar] [CrossRef]
- Lee, W.J.; Hur, K.Y.; Lakadawala, M.; Kasama, K.; Wong, S.K.; Chen, S.C.; Lee, Y.C.; Ser, K.H. Predicting success of metabolic surgery: Age, body mass index, C-peptide, and duration score. Surg. Obes. Relat. Dis. 2013, 9, 379–384. [Google Scholar] [CrossRef]
- Huang, H.H.; Yeh, C.; Chen, J.C.; Lee, T.H.; Chen, S.C.; Lee, W.J.; Chen, C.Y. Does bariatric surgery influence plasma levels of fetuin-A and leukocyte cell-derived chemotaxin-2 in patients with type 2 diabetes mellitus? PeerJ 2018, 6, e4884. [Google Scholar] [CrossRef]
- Lee, W.J.; Lee, K.T.; Kasama, K.; Seiki, Y.; Ser, K.H.; Chun, S.C.; Chen, J.C.; Lee, Y.C. Laparoscopic single-anastomosis duodenal-jejunal bypass with sleeve gastrectomy (SADJB-SG): Short-term result and comparison with gastric bypass. Obes. Surg. 2014, 24, 109–113. [Google Scholar] [CrossRef]
- Lee, W.J.; Chong, K.; Ser, K.H.; Lee, Y.C.; Chen, S.C.; Chen, J.C.; Tsai, M.H.; Chuang, L.M. Gastric bypass vs sleeve gastrectomy for type 2 diabetes mellitus: A randomized controlled trial. Arch. Surg. 2011, 146, 143–148. [Google Scholar] [CrossRef] [Green Version]
- Nakayama, J.; Watanabe, K.; Jiang, J.; Matsuda, K.; Chao, S.H.; Haryono, P.; La-Ongkham, O.; Sarwoko, M.A.; Sujaya, I.N.; Zhao, L.; et al. Diversity in gut bacterial community of school-age children in Asia. Sci. Rep. 2015, 5, 8397. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.J.; Lin, C.S.; Lu, C.C.; Martel, J.; Ko, Y.F.; Ojcius, D.M.; Tseng, S.F.; Wu, T.R.; Chen, Y.Y.; Young, J.D.; et al. Ganoderma lucidum reduces obesity in mice by modulating the composition of the gut microbiota. Nat. Commun. 2015, 6, 7489. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.J.; Lu, C.C.; Lin, C.S.; Martel, J.; Ko, Y.F.; Ojcius, D.M.; Wu, T.R.; Tsai, Y.H.; Yeh, T.S.; Lu, J.J.; et al. Antrodia cinnamomea reduces obesity and modulates the gut microbiota in high-fat diet-fed mice. Int. J. Obes. 2018, 42, 231–243. [Google Scholar] [CrossRef] [Green Version]
- Tsugawa, H.; Cajka, T.; Kind, T.; Ma, Y.; Higgins, B.; Ikeda, K.; Kanazawa, M.; VanderGheynst, J.; Fiehn, O.; Arita, M. MS-DIAL: Data-independent MS/MS deconvolution for comprehensive metabolome analysis. Nat. Methods 2015, 12, 523–526. [Google Scholar] [CrossRef]
- Kind, T.; Wohlgemuth, G.; Lee, D.Y.; Lu, Y.; Palazoglu, M.; Shahbaz, S.; Fiehn, O. FiehnLib: Mass spectral and retention index libraries for metabolomics based on quadrupole and time-of-flight gas chromatography/mass spectrometry. Anal. Chem. 2009, 81, 10038–10048. [Google Scholar] [CrossRef] [Green Version]
- Dunn, W.B.; Broadhurst, D.; Begley, P.; Zelena, E.; Francis-McIntyre, S.; Anderson, N.; Brown, M.; Knowles, J.D.; Halsall, A.; Haselden, J.N.; et al. Procedures for large-scale metabolic profiling of serum and plasma using gas chromatography and liquid chromatography coupled to mass spectrometry. Nat. Protoc. 2011, 6, 1060–1083. [Google Scholar] [CrossRef]


| Pre-Surgery | 3 Months | 12 Months | 24 Months | |
|---|---|---|---|---|
| Age (year-old) | 42.4 (8.6) | |||
| Gender | Male (n = 8); Female (n = 5) | |||
| Surgery | Gastric bypass (n = 6); Sleeve gastrectomy (n = 4); DJB–SG (n = 3) | |||
| Weight (kg) | 98.0 (15.6) | 80.3 (12.3) ** | 71.1 (13.0) **** | 71.2 (12.1) **** |
| BMI (kg/m2) | 35.6 (3.2) | 29.1 (2.3) **** | 25.8 (3.0) **** | 26.0 (3.1) **** |
| Systolic BP (mmHg) | 145.8 (13.8) | 134.8 (16.5) | 132.8 (18.1) | 130.8 (20.9) |
| Diastolic BP (mmHg) | 90.0 (12.5) | 77.9 (10.1) | 79.2 (12.3) | 78.9 (17.0) |
| Total cholesterol (mg/dL) | 198.9 (35.3) | 171.0 (29.0) * | 166.5 (21.7) * | 159.6 (20.7) ** |
| HDL (mg/dL) | 39.2 (7.3) | 37.1 (6.4) | 48.9 (9.1) * | 50.9 (10.2) ** |
| LDL (mg/dL) | 123.7 (27.8) | 104.5 (43.0) | 100.6 (23.0) | 97.1 (20.6) |
| Triglycerides (mg/dL) | 222.9 (173.6) | 118.7 (65.4) * | 79.25 (31.7) ** | 85 (35.4) ** |
| Fasting glucose (mg/dL) | 136.5 (43.5) | 98.9 (20.1) ** | 96.6 (16.3) ** | 101.6 (19.2) ** |
| Fasting insulin (mU/L) | 41.0 (68.8) | 7.7 (3.3) | 10.4 (13.8) | 11.4 (13.2) |
| C-peptide (ng/mL) | 3.3 (1.5) | 2.0 (0.8) ** | 1.6 (0.5) *** | 1.5 (0.6) *** |
| HbA1c (%) | 7.9 (1.4) | 5.8 (0.4) *** | 6.0 (1.8) ** | 5.9 (1.0) *** |
| HOMA-IR | 14.3 (24.3) | 1.9 (0.8) * | 2.8 (4.6) | 3.2 (4.1) |
| Uric acid (mg/dL) | 6.7 (1.5) | 6.1 (1.0) | 5.64 (1.4) | 5.9 (1.2) |
| Creatinine (mg/dL) | 0.83 (0.12) | 0.80 (0.13) | 0.77 (0.12) | 0.76 (0.23) |
| AST (U/L) | 25.2 (13.4) | 21.9 (9.5) | 19.3 (10.6) | 20.8 (5.7) |
| ALT (U/L) | 40.0 (25.4) | 27.1 (12.4) | 28.6 (28.0) | 23.2 (11.4) |
| Albumin (g/dL) | 4.5 (0.2) | 4.3 (0.2) | 5.1 (2.8) | 4.4 (0.2) |
| ALP (U/L) | 68.3 (19.6) | 73.40 (18.6) | 72.91 (18.7) | 70.67 (18.0) |
| γ-GT (U/L) | 41.3 (24.6) | 22.8 (9.1) ** | 18.5 (9.2) *** | 18.2 (5.9) *** |
| Acyl-Ghrelin (pg/mL) | 40.3 (8.6) | 38.1 (7.1) | 42.0 (12.9) | 37.2 (18.2) |
| Des-Acyl-Ghrelin (pg/mL) | 110.2 (34.4) | 47.4 (29.4) ** | 50.7 (23.6) ** | 61.4 (34.7) ** |
| GLP-1 (ng/mL) | 0.059 (0.036) | 0.065 (0.058) | 0.086 (0.045) | 0.095 (0.044) |
| PYY (ng/mL) | 0.304 (0.121) | 0.324 (0.176) | 0.392 (0.174) | 0.391 (0.118) |
| GIP (ng/mL) | 0.469 (0.126) | 0.300 (0.204) | 0.386 (0.172) | 0.374 (0.216) |
| FGF-19 (pg/mL) | 61.9 (43.3) | 115.1 (108.6) | 145.7 (130.5) | 190.2 (184.0) |
| FGF-21 (pg/mL) | 341.4 (245.3) | 413.9 (367.8) | 161.7 (104.5) | 93.7 (43.3) |
| FGF-23 (pg/mL) | 41.8 (13.7) | 78.1 (77.8) | 36.2 (8.1) | 48.5 (12.4) |
| Total bile acids (μM) | 12.7 (13.4) | 7.3 (3.4) | 8.4 (9.5) | 14.0 (19.7) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, H.-H.; Lin, T.-L.; Lee, W.-J.; Chen, S.-C.; Lai, W.-F.; Lu, C.-C.; Lai, H.-C.; Chen, C.-Y. Impact of Metabolic Surgery on Gut Microbiota and Sera Metabolomic Patterns among Patients with Diabetes. Int. J. Mol. Sci. 2022, 23, 7797. https://doi.org/10.3390/ijms23147797
Huang H-H, Lin T-L, Lee W-J, Chen S-C, Lai W-F, Lu C-C, Lai H-C, Chen C-Y. Impact of Metabolic Surgery on Gut Microbiota and Sera Metabolomic Patterns among Patients with Diabetes. International Journal of Molecular Sciences. 2022; 23(14):7797. https://doi.org/10.3390/ijms23147797
Chicago/Turabian StyleHuang, Hsien-Hao, Tzu-Lung Lin, Wei-Jei Lee, Shu-Chun Chen, Wei-Fan Lai, Chia-Chen Lu, Hsin-Chih Lai, and Chih-Yen Chen. 2022. "Impact of Metabolic Surgery on Gut Microbiota and Sera Metabolomic Patterns among Patients with Diabetes" International Journal of Molecular Sciences 23, no. 14: 7797. https://doi.org/10.3390/ijms23147797
APA StyleHuang, H.-H., Lin, T.-L., Lee, W.-J., Chen, S.-C., Lai, W.-F., Lu, C.-C., Lai, H.-C., & Chen, C.-Y. (2022). Impact of Metabolic Surgery on Gut Microbiota and Sera Metabolomic Patterns among Patients with Diabetes. International Journal of Molecular Sciences, 23(14), 7797. https://doi.org/10.3390/ijms23147797






