Abstract
In recent years, many natural foods and herbs rich in phytochemicals have been proposed as health supplements for patients with metabolic syndrome (MetS). Theaflavins (TFs) are a polyphenol hydroxyl substance with the structure of diphenol ketone, and they have the potential to prevent and treat a wide range of MetS. However, the stability and bioavailability of TFs are poor. TFs have the marvelous ability to alleviate MetS through antiobesity and lipid-lowering (AMPK-FoxO3A-MnSOD, PPAR, AMPK, PI3K/Akt), hypoglycemic (IRS-1/Akt/GLUT4, Ca2+/CaMKK2-AMPK, SGLT1), and uric-acid-lowering (XO, GLUT9, OAT) effects, and the modulation of the gut microbiota (increasing beneficial gut microbiota such as Akkermansia and Prevotella). This paper summarizes and updates the bioavailability of TFs, and the available signaling pathways and molecular evidence on the functionalities of TFs against metabolic abnormalities in vitro and in vivo, representing a promising opportunity to prevent MetS in the future with the utilization of TFs.
1. Introduction
Metabolic syndrome (MetS) presents a multiplex risk of atherosclerotic cardiovascular disease and type 2 diabetes, which globally occur commonly, with prevalence from 10 to 40%, leading to the requirement of controlling its risk factors [1]. MetS is defined by a cluster of interconnected factors that are marked by the presence of clusters of hyperglycemia, obesity, dyslipidemia, and hypertension [2], and it is usually linked to an increased risk of developing central obesity, insulin resistance, atherogenic dyslipidemia (high triglycerides, low HDL cholesterol) and hyperuricemia [3]. Its pathophysiology is primarily attributed to insulin resistance associated with excess fatty acid flux [4].
Phytochemical dietary interventions are gaining considerable attention as a prophylactic and therapeutic strategy due to their safety, better tolerability, and economic benefits. Dietary interventions with flavan-3-ols have beneficial effects on metabolic syndrome (MetS). Tea is the major single contributor (75%) to flavan-3-ol monomer intake, followed by pome fruits with 6%, while theaflavins are the main source of primary flavan-3-ol intake [5]. In countries where tea is consumed sparingly, flavonoid intake relies mainly on proanthocyanidins from berries, contributing to preventing MetS [6]. The average concentration of flavanols in mg per 100 g fresh weight (mg per 100 mL) is 58% for blueberries, 39% for red wine, and 28% for black tea [7]. The dietary intake of flavan-3-ol monomer, proanthocyanidins, and theaflavin varies significantly among countries, and the average habitual intake of flavan-3-ol is much lower than that used in most dietary intervention studies [5]. Theaflavins (TFs) are one of the major functional phytochemicals from black and dark tea, forming the unique flavor of special teas and contributing to their health benefits [8,9]. They account for 2–20 g/kg of the dry weight of solids in brewed black tea [10]. The main structure of TFs is a seven-membered benzotropolone ring, followed by decarboxylation and simultaneous fusion with the epicatechin ring B or epicatechin gallate [11]. There are more than 20 identified types of TFs, and theaflavin (TF1), theaflavin-3-gallate (TF2A), theaflavin-3′-gallate (TF2B), and theaflavin-3,3′-digallate (TF3) are the major characterized structures [12] (Figure 1).
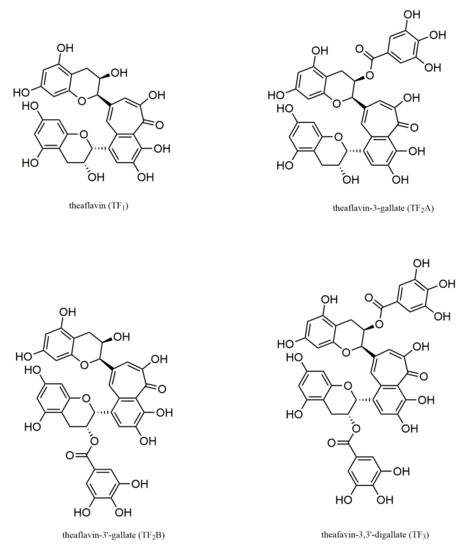
Figure 1.
The main monomer forms of theaflavins.
The antioxidant, lipid-regulatory, anti-inflammatory, antitumor, and antiviral bioactivity of TFs has recently been reviewed [13]. However, the effects and the underlying mechanism of the cell signaling pathway and gut microbiota modulation of TFs on MetS have not been systematically reviewed. The fact that theaflavin has such a multitude of effects gives it great research value and application advancement. Nevertheless, due to its low bioavailability and poor stability, the applications of phytochemicals such as theaflavins are limited [14]. Since the stability and bioavailability of TFs are crucial for their function, they are reviewed in our paper. The effects of pH, digestive enzymes, efflux transport, and metabolism are crucial, and these factors render encapsulation and structure modifications the major methods used for improving the stability and bioavailability of TFs. The purpose of this review is to investigate the functional and regulatory molecular evidence of the response to the treatment of TFs on MetS, with an emphasis on redox homeostasis and gut microbiota balance.
Disciplinary literature (Scopus, Web of Science) and publisher (ScienceDirect, SpringerLink) databases were searched to identify original contemporary full-text articles published on the subject of theaflavin stability, bioavailability, and functionality. Most cited publications were found by using the following search terms: “theaflavin degradation”, “theaflavin thermal stability”, “theaflavin gut bacteria”, “theaflavin stabilization”, “tea polyphenol intestinal microbiome”, “theaflavin obesity”, and “theaflavin diabetes”. The relevance of the given list of publications was further assessed by examining the title and the abstract. Of the total number of reviewed papers, 80% were published in the last 10 years, of which 66% were published in the last 5 years.
2. Stability and Bioavailability of Theaflavins
Theaflavins (TFs) in powdered form are quite stable. There is no significantly change in TFs in a simulated tropical environment for 6 months and dry conditions at 65 and 75 °C for 14 days [15]. Heating at a higher temperature (100 °C) for 3 h results in the complete degradation of TFs in aqueous solution, while 56% of which are degraded when heated at 70 °C for 3 h [16]. TF3 and TF2B are more stable compared with TF1 and TF2A in boiling water [15]. TFs, as a type of typical polyphenols, exhibit pH-dependent stability. TFs exhibited high stability during 24 h of incubation in sodium acetate buffer at pH 5.5 with simulated gastric juice (0.2% NaCl, 0.24% hydrochloric acid) [17]. On the other hand, TFs are more susceptible to be degraded and form naphthoquinone in alkaline solutions, resulting in the instability of TFs [17]. Under alkaline conditions, TF3 and TF2B are more stable than TF1 and TF2A, with pH ranging from 5.0 to 7.4 [15]. At a pH of 7.4, the degradation rate of TFs was 34.8% after incubation for 8 h; when the pH was increased to 8.5, the color of the solution rapidly turned into dark brown, and 78.4% of the TFs were degraded after 2 h of incubation [17]. The instability of TFs regards temperature and pH limiting their application in functional foods and medicinal use.
Bioavailability regards to the proportion of the particular nutrients that are digested, absorbed, and metabolized through normal pathways [18]. TFs are relatively stable during in vitro gastric digestion, which is majorly influenced by pH and digestive enzymes [19]. The effects of pH on the stability of TFs were documented above. In the intestinal phase, TFs exhibited poor bioavailability with apparent permeability coefficient (Papp) values ranging from 0.44 × 10−7 to 3.64 × 10−7 cm/s [20]. In addition, P-glycoprotein (P-gp), multidrug resistance-associated proteins (MRPs), and breast-cancer resistance proteins (BCRPs) are all involved in the efflux transport of the four TFs, of which P-gp and MRPs have the strongest secretory effect on TFs [20]. Intestinal microbial communities are capable of enzymatically transforming compounds into metabolites, thus potentially affecting their bioavailability [21]. However, the bioavailability of TFs has received limited attention in comparison with that of green tea polyphenols.
Despite some publications having revealed the low bioavailability of TFs, it was surprising that 70% of TFs are more relatively available in the prostate than in EGCG, and a mouse model (mice given a decaffeinated black tea diet) showed that TF1 is found in the prostate in conjugated free form [22]. TF1 acts as a novel substrate for organic anion-transporting polypeptides (OATPs) 2B1, inhibiting transport and thus reducing pharmaceutical uptake [23]. Therefore, the low bioavailability of TFs can be improved by synergistic effects [24]. Encapsulation methods and structural modifications were used to improve the bioavailability of phenol components [25]. It would be interesting to fabricate emulsions and gel structures to optimize the bioavailability of TFs. Currently, the delivery systems used to encapsulate TFs include emulsifying systems (liposomes, Pickering emulsions) and nanoparticle systems (based on proteins and polysaccharides) [26]. Encapsulation or interaction with polymers including proteins, lipids, and carbohydrates may contribute to improving the stability and bioavailability of TFs. Bovine serum albumin, zein, and liposomes, as carriers for TFs, showed excellent potential to further optimize the bioavailability of nanoparticles [27,28,29]. Hydrophobic interaction and hydrogen bonding are the dominant interactions between TF3 and bovine serum albumin, and the microenvironment around bovine serum albumin enhances hydrophobicity with the increase in the α-helical structure, which could potentially be used to develop nanoparticles with excellent biochemical properties [30]. Ding et al. dissolved phospholipid S75, cholesterol, Tween-80, and TF3 in a 16:2.4:4:1 mass ratio with ethanol, and combined them with dynamic high-pressure microfluidization to produce nanoliposomes; these nanoliposomes significantly improved the in vitro digestibility of TF3 in adverse environments, including weakly alkaline pH and digesting with pancreatin (after 2 h of incubation in simulated intestinal juice, the residual amount of TF3 in nanoliposomes and free fluid was 48.42% and 18.24%, respectively) [31]. Srivastava et al. synthesized poly (lactic-coglycolide) nanoparticles (PLGA-NPs), and PLGA-NPs loaded with TF1 (encapsulation rate of 18%) showed potential for 7,12-dimethylbenzanthracene (DMBA)-induced DNA repair gene, and potential to inhibit DNA damage-response genes [32]. Nanocomplexes using chitosan (CS) complexed with caseinophosphopeptides (CPPs) via electrostatic interaction encapsulated TF3 with high encapsulation efficiency and low cytotoxicity. More importantly, the CS-CPP nanocomplexes significantly improved the intestinal permeability of TF3 in a caco-2 monolayer model [33]. The application of encapsulated TFs in the food industry, and their biological fate and mechanism in vivo need to be further explored. The preparation methods of TFs also include solvent extraction isolation, chemical reagent oxidation, and polyphenol oxidase [34], which are not efficient for industrial production [35], leading to the high price of pure TFs.
3. Metabolic Syndrome and Theaflavins
3.1. Antiobesity and Lipid-Lowering Effects
Obesity is defined as abnormal or excessive fat accumulation that presents a risk to health. It is a multifactorial disease that results from chronic positive energy balance and the excess energy stored in adipose tissue in the form of triglycerides, expanding adipose tissue size and increasing body fat deposits [36]. Abdominal obesity is the most frequently observed component of MetS [37]. The effects of TFs on obesity regard to the regulation of the lipid metabolism, the inhibition of lipogenesis, the promotion of favorable lipolysis, and the β-oxidation and induction of energy dissipation (Figure 2).
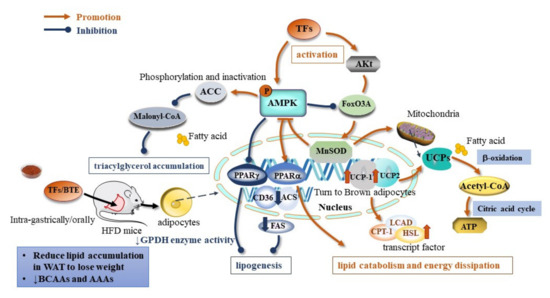
Figure 2.
Molecular mechanism of TFs on antiobesity effect (↓ indicates down-regulation).
TFs are associated with lipolysis through the AMPK–FoxO3A–MnSOD pathway. The transfection of 3T3-L1 adipocytes with a reporter gene constructed from GFP driven by the superoxide dismutase (MnSOD) promoter leads to the upregulation of peroxisome proliferator-activated receptor γ (PPARγ) and PPARα, while it downregulates the expression of the CD36, ACS, and FAS genes. TF3 increases CPT-1, LCAD, and HSL transcript levels, and promotes the expression profile of genes that favor lipolysis and β-oxidation, i.e., genes that induce energy-dissipation-related genes and brown-fat-related proteins: mitochondrial uncoupling protein-1 (UCP-1) and UCP-2 [38] (Table 1). The family of UCPs are mitochondrial inner membrane carriers that regulate electrochemical potential and dissipate energy in the form of heat [39]. Among them, UCP-2 is specific to white adipocytes, and functions similarly to brown adipocyte-specific UCP-1. The induction of UCP-1 and UCP-2 in adipose tissue increased systemic energy expenditure and alleviated metabolic disturbances [40].
TFs reduced food intake in mice and prevented obesity by inhibiting lipid uptake in vivo. A Yinghong no. 9 black tea infusion (Y9 BTI) (containing 0.56% TFs) significantly downregulated the expression of liver kinase B1 (LKB1), adenosine monophosphate-activated protein kinase (AMPK), and cell surface death receptor (FAS) with higher expression of AMPK phosphorylation (p-AMPK) and activated the acetyl coenzyme a carboxylase (ACC) [41]. The oral administration of black tea extract (BTE) of mice slightly reduced the levels of certain systemic inflammatory markers (IL-1β, iNOS, and Cox2) in adipose tissue [41]. Xu et al. [42] demonstrated that the oral supplementation of Huang Jinya black tea extract in C57BL/6 mice with HFD-induced obesity decreased the body weight with 14.40% and 18.44% in HJBT150 and HJBT300, respectively. Lipid accumulation comprising total fat mass and adipocyte size was reduced in the WAT of BTE-treated mice. It reduced the corresponding mRNA levels associated with lipogenesis peroxisome proliferator-activated receptor (PPAR) γ, CCAAT/enhancer-binding protein (CEBP) α, fatty acid-binding protein 4 (Fabp4), fatty acid synthase (FAS), acetyl-CoA carboxylase (ACC) 1, and stearoyl-CoA desaturase-1 (SCD1), [42]. Pancreatic lipase (PL) plays an important role in fat metabolism and is an effective target for weight control. Theaflavin-3,3′-digallate, theaflavin-3′-gallate, theaflavin-3 gallate, and theaflavin had inhibitory effects on pancreatic lipase, with IC50 of 1.9, 4.2, 3.0, and >10 μmol/L, respectively [43]. Thus, TFs exhibited antiobesity activity mainly through affecting the appetite, reducing lipogenesis, and promoting lipolysis to facilitate lipid accumulation and control WAT expansion.
Dyslipidemia is highly related with many diseases, such as hyperlipidemia, atherosclerosis, and nonalcoholic fatty liver, which lead to complications that endanger human health [44]. Black tea extract alleviated hyperlipidemia in HFD-induced mice, followed by a reduction in triglyceride (TG), free fatty acid (FFA), and total cholesterol levels [42]. TFs effectively reduced lipid levels in HFD-fed ApoE−/− and C57BL/6J mice treated with TFs with elevated levels of serum TG, TC, and LDL-C, and increased the level of HDL-C [45]. Oxidative stress is linked with the balance of lipid metabolism [46]. TF1 reduces ROS and MDA levels, and maintains antioxidant enzyme activity in both in vivo and in vitro experiments. The administration of TF1 (5, 10 μmol/L) promoted the activity of antioxidant enzymes (SOD, CAT, GSH-Px), and inhibited the process of atherosclerotic plaque formation and aortic histological alterations [45]. In addition, TF1 upregulated the Nrf2/HO-1 signaling pathway in vascular endothelial cells by increased microRNA-24 (miR-24) levels, invoking its activation on Nrf2 [45]. A dose-dependent reduction in lipid droplets and deposition in HepG2 cells was observed through the treatment of TF3, and the lipid droplets and deposition were reduced in vivo and in vitro in HepG2 cells [47]. TF3 downregulated the levels of SREBP-Ic and FAS, with increased activity of CPT1 and phosphorylation of ACC directly bound to and inhibiting the activation of plasma kinase (PK), further confirming its effect on the stimulation of adenosine monophosphate activated protein kinase (AMPK). Regarding AMPK stimulation and downstream targets, the proposed mechanism for a new target for nonalcoholic steatohepatitis treatment is the TF3–PK-AMPK regulatory axis to alleviate lipid deposition [47]. The mRNA levels of PPARα, carnitine palmitoyltransferase 1α (Cpt1α), L-bifunctional enzyme (Ehhadh), and acyl coenzyme a oxidase (Acox1) were significantly increased with black tea extract in vitro to mediate hepatic fatty acid oxidation, decreased the protein levels of FAS, ACC1, and sterol regulatory element binding protein 1 (Srebpl), and ultimately inhibited reduced fatty acid synthesis [42]. The phosphorylation of serine residues of insulin receptor substrate-1 (IRS1) and PI3K-p85 caused the phosphorylation of Akt, which increased synergistic insulin signaling in the insulin/Akt signaling pathway in the WAT of HJBT300 mice [42]. In addition, TFs also significantly reduced ROS production in steatosis hepatocytes and LPS-stimulated tumor necrosis factor-a (TNF-a) production in RAW264.7 cells [48].

Table 1.
Effects of theaflavins on diseases caused by metabolic abnormalities (↓ indicates down-regulation, ↑ indicates up-regulation).
Table 1.
Effects of theaflavins on diseases caused by metabolic abnormalities (↓ indicates down-regulation, ↑ indicates up-regulation).
| Type | Related Disease | Cell Line/Animal Model | Treatment | Effects | References |
|---|---|---|---|---|---|
| Hyperlipidemia | Obesity | Mouse 3T3-L1 fibroblast | 0, 25, 50 μM TF3, 48 h | ↓ FAS expression | [38] |
| ↓ upregulation of CD36 and ACS | |||||
| ↑ gene expression of lipid catabolism and β-oxidation | |||||
| ↑ CPT-1L, CAD and HSL transcript levels | |||||
| ↑ UCP-1, UCP-2 | |||||
| ↑ Akt (Ser473) | |||||
| ↑ PPARα gene expression | |||||
| ↓ PPARγ upregulation | |||||
| ↓ phosphorylated FoxO3A | |||||
| ↓ inactive FoxO3A protein level | |||||
| ↑ MnSOD | |||||
| ↑ GFP intensity | |||||
| ICR mice | 0.5, 1.0, or 2.0 g/kg Y9 BTI for two weeks, | ↓ diet consumption | [41] | ||
| ↓ abdominal adipose weight | |||||
| ↑ fecal triglyceride | |||||
| ↓ lipid absorption | |||||
| ↑ Protein intake | |||||
| ↑ LKB1 and AMPK | |||||
| ↑ FAS | |||||
| ↑ phosphorylation of ACC. | |||||
| ↓ l IL-1β, iNOS, and Cox-2 | |||||
| C57BL/6 mice with HFD-induced obesity | 150, 300 mg/kg/day black tea extract for 9 weeks, orally | ↓ Body weight | [42] | ||
| ↓ food intake and body weight | |||||
| ↓ Liver and kidney weight | |||||
| ↓ WAT lipid accumulation | |||||
| ↓ total WAT mass | |||||
| ↓ adipocyte hypertrophy | |||||
| ↓ BCAAs and AAAs content | |||||
| ↑ PPP metabolites | |||||
| ↓ PPARα, Cpt1a, Ehhadhm and Acox1 | |||||
| ↓ FAS, Acc1 and Srebp1 | |||||
| ↑ p-Acc1 levels | |||||
| ↓ p-Irs1 (Ser 318) and PI3K-p85 levels | |||||
| ↑ Akt phosphorylation | |||||
| ↑ p-AMPK levels | |||||
| ↑ insulin signalling synergistically | |||||
| ↑ EDRs | |||||
| ↓ phospho-elF2α (Ser52) | |||||
| ↓ chol | |||||
| ↓ hepatotoxicity | |||||
| ↑ mRNA level (WAT lipolysis) | |||||
| fatty liver | HepG2 | 5 μM TF3, 4 h | ↓ SREBP-1c | [47] | |
| ↓ FAS | |||||
| ↑ CPT1 activity | |||||
| ↑ ACC phosphorylation | |||||
| ↓ PK activity | |||||
| ↓ hepatic lipid accumulation | |||||
| ↓ liver steatosis | |||||
| Dyslipidemia | Atherosclerosis | HUVEC (CRL-1730) | 5, 10 μmol/L TF1, 2 h | ↓ ROS | [45] |
| ↓ MDA | |||||
| ↑ SOD, CAT, and GSH-Px | |||||
| ↑ Nrf2 | |||||
| ↑ down-stream protein HO-1 | |||||
| ↑ miR-24 | |||||
| ApoE-/-mice, C57BL/6J mice | 5, 10 mg/kg TF for 12 weeks, intragastrically | ↓ serum TG, TC, and LDL-C elevation | |||
| ↑ HDL-C | |||||
| ↓ vacuoles size and number | |||||
| ↓ atherosclerotic lesion area | |||||
| ↓ MMP-2 | |||||
| ↓ MMP-9 | |||||
| ↓ ROS | |||||
| ↓ MDA | |||||
| ↑ antioxidant enzymes activities | |||||
| Dysglycemia | type 2 diebete | C2C12(T2D) | 20 μM TF1, 48 h | ↑ Ca2+ abundance | [49] |
| ↑ mitochondrial abundance | |||||
| ↑ CaMKK2 | |||||
| ↑ AMPK | |||||
| ↑ PGC-1α | |||||
| ↑ SIRT1 | |||||
| ↑ mitochondrial metabolic activity | |||||
| ↑ 2-NBDG uptake | |||||
| ↑ total GLUT4 | |||||
| HepG2 | 2.5, 5, 10 µg/mL TFs, 24 h | ↑ membrane bound GLUT4 | [50] | ||
| ↓ IRS-1 (Ser307) | |||||
| ↑ Akt (Ser473) | |||||
| ↑ glucose uptake | |||||
| ↑ insulin sensitivity | |||||
| ↑ mtDNA copy number | |||||
| ↓ PGC-1β | |||||
| ↑ PRC | |||||
| ↓ TC uptake | |||||
| ↓ blood glucose level | |||||
| HFD-induced mice | TF1, TF2a, TF3 100 mg kg/d, and TFs 200 mg kg/d for 9 weeks | ↓ serum glucose | [51] | ||
| ↓ TC, TG, LDL and HLD | |||||
| ↑ SIRT6 expression | |||||
| ↓ SREBP-1 and FASN expression | |||||
| ↓ Serum glucose | |||||
| ↑ glucose tolerance | |||||
| SDT rats | 2 mL theaflavin extract in 0.5% CMC, 25 mg/kg/day for 10-, 16-, 22-, 24- and 28-wk, orally | ↑ plasma insulin levels | [52] | ||
| ↑ GLP and GLP1 | |||||
| ↑ incretin secretion | |||||
| the development of pre-diabetes in control, affect glucose transporter expression | |||||
| ↓ blood glucose levels | |||||
| ↑ plasma insulin | |||||
| streptozotocin-induced diabetic rats | theaflavin (25, 50 and 100 mg/kg b.wt.) in 0.5 mL water for 30 days, intra- gastrically | ↓ HOMA-IR index | [53] | ||
| ↑ total hemoglobin | |||||
| ↓ HbA1C | |||||
| ↓ hexose, hexosamine, fucose, | |||||
| and sialic acid in plasma | |||||
| ↓ TCA cycle key enzymes activities | |||||
| ↑ plasma insulin level | |||||
| ↓ TG | |||||
| ↓ FFA | |||||
| 1 µM alloxan and 4% glucose induced diabetic Zebrafish model. | TF3 (0.5, 2, 4, 6.7, 10, and 20 µg/mL) or metformin hydrochloride (10 µg/mL) for 24 h | ↓ glucose level | [54] | ||
| ↓ PEPCK level | |||||
| ↑ GCK expression | |||||
| ↑ β cell regeneration rates | |||||
| uric acid metabolism | Hyperuricemia | Kunming male mice of SPF einjected with PO-induced Hyperuricemia | 20, 50 and 100 mg/kg/day TF, TF-3-G and TFDG for 7 days, intragastrically | ↓ SUA values | [55] |
| ↓ serum Cr values | |||||
| ↓ ADA | |||||
| ↓ XOD | |||||
| ↓ URAT1 | |||||
| ↓ GLUT9 | |||||
| ↑ ABCG2 mRNA | |||||
| ↓ OAT1/2 | |||||
| ↑ OCTN1, OAT1 and OAT2 mRNA | |||||
| ↓ inflammatory cells | |||||
| ↑ Nrf2 and HO-1 |
3.2. Hypoglycemic Activity
Type 2 diabetes (T2D) is characterized by relative insulin deficiency caused by pancreatic β-cell dysfunction and insulin resistance in the target organs, contributed by genetic and environmental factors [56]. Sarcopenia may be a cause and consequence of T2D in the aging population, and daily protein intake involving certain amino acids and amino compounds could improve muscle strength, muscle function, and protein synthesis, playing key role in T2D status [57]. Amino acid deprivation was also related to stress response in a HepG2/C3A cell model [58]. TFs act as enzyme inhibitors to slow carbohydrate absorption, controlling starch digestion and regulating postprandial hyperglycemia [59]. TF2A had a strong inhibitory effect (92.3% inhibition ratio) on α-glucosidase (α-GC) via competitive inhibition mode, and stable complexes are spontaneously formed by hydrophobic interactions, resulting in a change in the α-GC secondary structure [60]. TFs can inhibit the activity of α-amylase, thereby delaying the digestion of starch. TF2B and TF2A have stronger inhibitory effects than those of TF1, and TF2B is a competitive inhibitor, while TF1 and TF2A are mixed inhibitors with both competitive and noncompetitive inhibitions [61].
Besides the inhibitory effects of enzymes, the suppression of glucose absorption is of great importance in modulating diabetes. TFs might be involved in the regulation of specific components of the PGC family to alleviate diabetes [62]. In a palmitate-induced insulin resistance HepG2 cell model, TFs significantly increased glucose uptake and recovered the mitochondrial function, followed by upregulation of total membrane-bound glucose transporter protein 4 (GLUT4) and phosphorylated Akt (Ser473) levels, and the downregulation of PGC-1β mRNA levels and IRS-1 Ser307 phosphorylation levels [50]. Sodium-dependent glucose transporter 1 (SGLT1) is a transmembrane protein located in the apical membrane od enterocytes, and TF1 (40 μM) inhibited the expression of SGLT1 instead of glucose transporter protein 2 (GLUT2), which is a high-capacity transporter [63]. The mechanism of SGLT1 for transporting glucose involves the cotransport of Na+, and depolarizes the plasma membrane as a way to open the Ca2+ channels. TFs activated the CaMKK2-AMPK signaling pathway via Ca2+ influx and upregulated the expression of PGC-1α and SIRT1 in the C2C12 cell line, thereby promoting myotubular mitochondrial abundance and glucose uptake [49]. A TF mixture (25 mg/kg/day) improved impaired glucose tolerance and significantly lower blood glucose levels in prediabetic SDT rats, and increased insulin expression via the inhibition of gastric inhibitory polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) degradation [52]. Treatment with TFs reduced blood glucose levels and improved insulin resistance in mice, significantly reduced serum total cholesterol (TC), total cholesterol (TG), and low-density lipoprotein (LDL) levels, and inhibited alanine amino transferase (ALT), aspartate amino transferase (AST) activity [51]. The expression levels of sterol regulatory element-binding transcription factor 1 (SREBP-1), fatty acid synthase (FASN), which increases the expression of sirtuin 6 (SIRT6), were increased [51] High-fat diets and streptozotocin-induced diabetic rats were treated with different doses of TFs (25, 50, and 100 mg/kg b.wt/day) for 30 days, which resulted in glycated hemoglobin, hemoglobin, and glycoproteins (hexose, hexosamine, polystyrene and sialic acid), TCA cycle enzymes (isocitrate dehydrogenase, a-ketoglutarate dehydrogenase, succinate dehydrogenase and malate dehydrogenase) returned to near-normal levels, and the decreased homeostatic model assessment of insulin resistance (HOMA-IR) index in a dose-dependent manner [53]. Other pathophysiological mechanisms may explain hyperglycemia. Pancreatic β cells are responsible for producing, storing, and releasing insulin to maintain glucose homeostasis [64]. TF3 could restore the size of damaged islets, promote islet β cell proliferation in an alloxan-induced zebrafish model, increase insulin secretion, and regulate blood glucose through a reduction in the levels of phosphoenolpyruvate carboxykinase (PEPCK) and hexokinase isoenzyme (GCK) [54]. Thus, TFs could promote glucose homeostasis and prevent the development of insulin resistance by regulating the IRS-1/Akt/GLUT4 signaling pathway. In addition, TFs act as an enzyme inhibitor, reduces glucose transport activity, and increases mitochondrial abundance, thus delaying glucose transport and absorption in the intestine.
3.3. Uric Acid Lowering Effect
Hyperuricemia is an abnormal purine metabolic disease that occurs when uric acid is excessive in the blood, and is significantly correlated with cardiovascular diseases such as coronary heart disease, stroke, and hypertension [65]. TFs can be used as an inhibitor of xanthine oxidase (XO) to improve the antihyperuricemia effect of hyperuricemic mice. TF1 is a competitive inhibitor with a significant reversible inhibition of XO (IC50 values, 63.17 + 0.13 μmol/L), and the main driving factors are hydrophobicity and hydrogen bonding [66]. TF1 interacts with some residues around an active XO cavity, including Glu-879, Pro-1012, Val-1011, THR-1010, LYS-771, Glu-802, Pro-1076, LEU-873, LEU-1014, ASN-768, LEU-648 and Phi-649 [66]. TFs exerted significant UA-lowering effects on hyperuricemic mice, reducing serum BUN and Cr values while inhibiting ADA and XOD activity to improve renal damage in hyperuricemic mice [55]. TFs downregulated the gene and protein expression of glucose transporter 9 (GLUT9) and urate transporter 1 (URAT1), and dose-dependently upregulated the genes for organic anion transporter-1(OAT1), organic cation transporter n1 (OCTN1), OCT1/2, and OAT2 [55]. Accordingly, the mechanism of TFs in the prevention and treatment of hyperuricemia may be related to inhibiting xanthine oxidase activity, regulating the mRNA and protein expression levels of related anionic transporters.
4. The Interactions of Theaflavins and Gut Microbiota
The gut microbiota have been implicated in the pathogenesis of MetS as a constellation risk factor to progress greater metabolic defects [67]. Hypercaloric diets are regarded as the major contributor to the development of the obesity epidemic in the United States and the rest of the world [68]. For example, a high-fat diet usually leads to poor microbiotic health, which leads to the eventual onset of chronic disease [68]. While the gut microbiome is responsive to large swings in food and nutrients [69], sufficient evidence shows that polyphenols can alter the composition of gut microbiota by increasing or decreasing both beneficial and harmful microbes [70]. TFs impact the gut microbiota and contribute to its health-promoting effects [69]. After humans ingest black tea, only trace quantities of TFs are absorbed in the upper gastrointestinal tract [71], indicating that TFs would potentially be subjected to the bacteria-mediated catabolism [72]. Studies support that TFs that reach the large intestine undigested are modified by ring-cleavage, reduction, hydrolysis, decarboxylation, and dihydroxylation reactions [73,74]. TFs could be hydrolyzed by gut microflora such as Bifidobacteria and Lactobacilli, which convert them into their corresponding metabolites (TF1, TF2A, TF2B, gallic acid, and pyrogallol) [72,75]. Instead, the gallic acid released from TFs is further converted into 3-0 and 4-0 methyl gallate acid, o-benzyl gallate-1-sulfate and o-benzyl gallate-2-sulfate [76]. Furthermore, TFs could be transferred to some smaller phenolic compounds such as 5-(3′,4′-dihydroxyphenyl)-γ-valerolactone and 3-(3′,4′-dihydroxyphenyl) propionic acid [77]. With 200 mg/kg TFs to germ-free (GF) and conventional mice, TFs were transformed into small phenolic metabolites dihydro-and tetrahydro (DF-TF and TH-TF) through the cleavage of the C-ring by intestinal microorganisms in vivo, instead of reducing benzotropolone [78]. In addition, two key colonic metabolites of theaflavin, 3-4′-hydroxyphenylpropionic acid and gallic acid, protect neuronal cells from oxidative-stress-sensitive strains, and TFs might be involved in the neuroprotective effects of MPTP-induced dopaminergic neurodegeneration by increasing tyrosine hydroxylase (TH) and dopamine transporter (DAT) immune responses, and reducing the appearance of caspases in SN [76,79]. TFs also have a regulatory effect on the intestinal microflora. TFs and EGCG have similar flavan-3-ol building blocks, to the extent that they may exhibit similar intestinal microbiota-modulating effects [80]. Microbiomic analysis through 16S rRNA gene sequencing shows that polyphenon E and TFs treatments significantly alter the bacterial community structure in the cecum and colon, but not in the ileum [81]. Particularly, several typical species of probiotic and harmful intestinal microorganisms interact with TFs (Figure 3). A recent study on TF3 on the modulation of microbial metabolism during in vitro fecal fermentation showed the growth promotion of Bacteroides, Lachnoclostridium, Faecalibacterium, Parabacteroides, and Bifidobacterium, while Prevotella and Fusobacterium were significantly inhibited (p < 0.05) [77]. TF3 has a weaker inhibitory effect on E. coli than that of EGCG, and TF3 also shows a specificity rise in Dialiser levels [77].
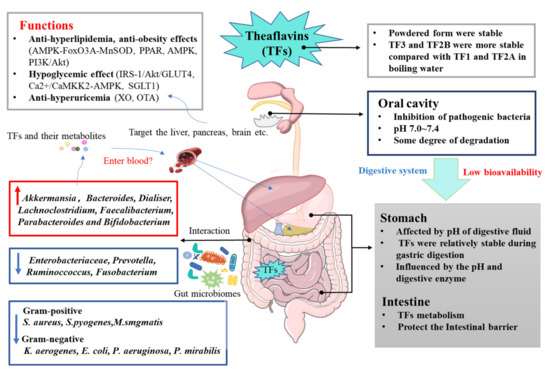
Figure 3.
Bioavailability of TFs and mechanism of TFs on metabolic syndrome.
TFs could positively reshape the composition of gut flora [82]. TFs play a beneficial role in regulating leaky and dysregulated intestinal homeostasis by controlling the LMD signaling pathway by blocking the incorporation of LMD, and could delay intestinal epithelial dysfunction, thereby preventing DSS-induced colitis in mice [83]. TF3 has the potential to be a broad-spectrum pharmaceutical as an antibacterial agent capable of inhibiting the growth of Gram-positive and -negative, and acid-resistant groups of bacteria with an antisporulating agent (250 ug/mL of TF3 inhibited bacterial growth by 99.97%, and 625 ug/mL TF2B inhibited spore germination by 99.92%). For example, it inhibited the growth Gram-negative bacteria Klebsiella aerogenes (K. aerogenes), Escherichia coli (E. coli), Pseudomonas aeruginosa (P. aeruginosa), and Proteus mirabilis (P. mirabilis), and Gram-positive bacteria Staphylococcus aureus (S. aureus), Staphylococcus aureus (S. aureus), Streptococcus pyogenes (S. pyogenes), and Mycobacterium smegmatis (M. smgmatis) [84,85]. TFs on the pathogenic bacterial population in the oral cavity were also investigated [86]. In addition, Lagha reported that TFs possessed similar activity to that of EGCG, which could reduce the relative abundance of lipopolysaccharide-producing bacteria [87]. Furthermore, by exposing TF1 to germ-free (GF) (mice colonized with specific-pathogen-free microbiota) and conventionalized mice, the gut microbiota enhanced the amination and MGO conjugation of TF1, which then removed these endogenous metabolic toxins [88]. Interestingly, gallic acyl ester substitution at the 3-position hydroxyl group of the C-ring contributed to bacterial Escherichia coli β-glucuronidase (EcGUS) inhibition and effectively alleviated drug-induced gastrointestinal toxicity, as described by Sun et al. [89]. TF-3-G, TF-3′-G, and TFDG inhibited EcGUS more strongly than GCG, ECG, and EGCG did. Thus, TFs are capable of exerting a prebiotic effect on gut microbiota by increasing the abundance of potentially beneficial bacteria (e.g., Lachnoclostridium and Bifidobacterium) and decreasing the abundance of potentially harmful bacteria (e.g., Prevotella and Faecalibaculum), and provided enteroprotective benefits by combining toxic metabolites to reduce their toxic effects.
The modulation effects of TFs on MetS through gut microbiota were mainly targeted on the gut barrier, gut–brain axis, and gut–liver/adipose axis, especially by increasing the beneficial gut microbiota and metabolites such as short-chain fatty acids (SCFAs), bile acids (BAs), and amino acids (AAs) [42,90,91,92]. As one significant structural component of the intestinal barrier, gut microbiota play an imperative role in sustaining the intestinal microecosystem, while TFs and their metabolies could reshape microbial profiles and confer protective actions onto the gut barrier [82,93].
The impaired intestinal epithelial barrier function and immune tolerance to intestinal flora in susceptible hosts may be the reasons for a series of intestinal inflammatory diseases such as inflammatory bowel disease (IBD) [94,95]. TFs inhibited neutrophil adhesion, ICAM-1 and VCAM-1 mRNA, and protein expression in LPS-induced RIE cells [96], suggesting that the TFs could be beneficial for the treatment of IBD. The circadian rhythm (CR) of the host and the gut microbes that interfere with each other were suggested to cause various chronic disease problems, such as fatty liver, type 2 diabetes, and chronic gastroenteritis, which affect the metabolism via multi-organ crosstalk (enteric–liver–brain axis etc.) [96,97,98]. A recent study found that TFs significantly modulated the circadian clock oscillations of the intestinal flora and the transcription of circadian clock genes induced by continuous dark (CD) treatment in mice [99]. However, many studies fall short because of the lack of animal models with comparable genetic susceptibilities with the human biology, especially when the role and specific mechanism of TFs in the regulation of human intestinal flora on metabolism remain unrevealed. Thus, clinical trials and the establishment of animal models more closely related to the human intestinal flora are necessary. Moreover, there are very few studies on the effects of pure TFs on MetS by targeting gut microbiota, and further evidence on the gut–brain and gut–liver/adipose axes is needed.
5. Conclusions and Future Perspective
As the pathogenesis of metabolic diseases involves multiple pathways, treatment targeting multiple factors is expected to address the driving forces of these diseases’ progression. TFs, which are relatively abundant in fermented teas such as black tea, are advantageous in preventing and treating MetS through modulation on the lipid and glucose metabolism, and the gut microbiota (Figure 3). However, the bioavailability of TFs is quite low, which has led to the further advanced strategies for enhancing TF stability, such as encapsulation and structural modification. Interestingly, the use of TFs targets chronic diseases by influencing multisignaling networks. Thus, more investigations are required to develop an understanding of their beneficial role by multiomic integration, particularly in clinical studies and applications. According to the available evidence, the administration of theaflavins as a nutritional supplement in a well-balanced, nutrient-dense diet or the oral use of theaflavin-enriched tea beverages and loaded tablets may be beneficial for people with chronic diseases. Furthermore, few articles have reported on pure TFs in relation to such aspects, with the majority of studies focusing on black or postfermented teas. Therefore, more indepth studies are required to explore the complex relationships between different TFs and the intestinal flora. The dosage and timing of administration also need to be considered, which might be beneficial for the development of more personalized nutritional and functional foods, and even clinical treatments of TFs.
Author Contributions
M.S., original draft and editing; Y.L., original draft; J.W., C.L., and Z.Z., software and visualization; J.Y., supervision and editing; S.Q. and C.Z., review and editing. All listed authors have made a substantial, direct, and intellectual contribution to the work, and approved it for publication. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Hunan Province Innovative Postdoctoral Project (2021RC2080) and the Natural Science Foundation of Hunan Province (2021JJ40246).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available in the paper.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Li, X.; Li, S.; Chen, M.; Wang, J.; Xie, B.; Sun, Z. (−)-Epigallocatechin-3-gallate (EGCG) inhibits starch digestion and improves glucose homeostasis through direct or indirect activation of PXR/CAR-mediated phase II metabolism in diabetic mice. Food Funct. 2018, 9, 4651–4663. [Google Scholar] [CrossRef]
- Gregory, K.; Panagiota, P.; Eva, K.; George, C. Metabolic syndrome: Definitions and controversies. BMC Med. 2011, 9, 48. [Google Scholar]
- Cardiology Research and Practice. Retracted: A Comprehensive Review on Metabolic Syndrome. Cardiol. Res. Pract. 2019, 2019, 4301528. [Google Scholar]
- Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z. The metabolic syndrome. Lancet 2005, 365, 1415–1428. [Google Scholar] [CrossRef]
- Vogiatzoglou, A.; Mulligan, A.A.; Luben, R.N.; Lentjes, M.A.H.; Heiss, C.; Kelm, M.; Merx, M.W.; Spencer, J.P.E.; Schroeter, H.; Kuhnle, G.G.C. Assessment of the dietary intake of total flavan-3-ols, monomeric flavan-3-ols, proanthocyanidins and theaflavins in the European Union. Br. J. Nutr. 2014, 111, 1463–1473. [Google Scholar] [CrossRef] [Green Version]
- Derrick, S.A.; Kristo, A.S.; Reaves, S.K.; Sikalidis, A.K. Effects of Dietary Red Raspberry Consumption on Pre-Diabetes and Type 2 Diabetes Mellitus Parameters. Int. J. Environ. Res. Public Health 2021, 18, 9364. [Google Scholar] [CrossRef]
- Pascual-Teresa, S.; Santos-Buelga, C.; Rivas-Gonzalo, J.C. Quantitative analysis of flavan-3-ols in Spanish foodstuffs and beverages. J. Agric. Food Chem. 2000, 48, 5331–5337. [Google Scholar] [CrossRef]
- Sharma, V.; Rao, L.J.M. A Thought on the Biological Activities of Black Tea. Crit. Rev. Food Sci. Nutr. 2009, 49, 379–404. [Google Scholar] [CrossRef]
- Bhuyan, L.P.; Borah, P.; Sabhapondit, S.; Gogoi, R.; Bhattacharyya, P. Spatial variability of theaflavins and thearubigins fractions and their impact on black tea quality. J. Food Sci. Technol. 2015, 52, 7984–7993. [Google Scholar] [CrossRef] [Green Version]
- Sang, S.; Lambert, J.D.; Tian, S.; Hong, J.; Hou, Z.; Ryu, J.-H.; Stark, R.E.; Rosen, R.T.; Huang, M.-T.; Yang, C.S. Enzymatic synthesis of tea theaflavin derivatives and their anti-inflammatory and cytotoxic activities. Bioorg. Med. Chem. 2004, 12, 459–467. [Google Scholar] [CrossRef]
- Beecher, G.R. Overview of dietary flavonoids: Nomenclature, occurrence and intake. J. Nutr. 2003, 133, 3248S–3254S. [Google Scholar] [CrossRef]
- Teng, J.; Gong, Z.; Deng, Y.; Chen, L.; Li, Q.; Shao, Y.; Lin, L.; Xiao, W. Purification, characterization and enzymatic synthesis of theaflavins of polyphenol oxidase isozymes from tea leaf (Camellia sinensis). LWT 2017, 84, 263–270. [Google Scholar] [CrossRef]
- Bag, S.; Mondal, A.; Majumder, A.; Banik, A. Tea and its phytochemicals: Hidden health benefits & modulation of signaling cascade by phytochemicals. Food Chem. 2022, 371, 131098. [Google Scholar] [PubMed]
- Rahim, R.A.; Jayusman, P.A.; Muhammad, N.; Ahmad, F.; Mokhtar, N.; Mohamed, I.N.; Mohamed, N.; Shuid, A.N. Recent Advances in Nanoencapsulation Systems Using PLGA of Bioactive Phenolics for Protection against Chronic Diseases. Int. J. Environ. Res. Public Health 2019, 16, 4962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lun, S.Y.; Kwok, L.L.; Yu, H.; Zhen-Yu, C. Stability of tea theaflavins and catechins. Food Chem. 2003, 83, 189–195. [Google Scholar] [CrossRef]
- Li, S.M.; Lo, C.Y.; Pan, M.H.; Lai, C.S.; Ho, C.T. Black tea: Chemical analysis and stability. Food Funct. 2013, 4, 10–18. [Google Scholar] [CrossRef]
- Jhoo, J.-W.; Lo, C.-Y.; Li, S.; Sang, S.; Ang, C.Y.W.; Heinze, T.M.; Ho, C.-T. Stability of black tea polyphenol, theaflavin, and identification of theanaphthoquinone as its major radical reaction product. J. Agric. Food Chem. 2005, 53, 6146–6150. [Google Scholar] [CrossRef]
- Garcia-Arieta, A. Interactions between active pharmaceutical ingredients and excipients affecting bioavailability: Impact on bioequivalence. Eur. J. Pharm. Sci. 2014, 65, 89–97. [Google Scholar] [CrossRef]
- Gomez-Mejia, E.; Rosales-Conrado, N.; Leon-Gonzalez, M.E.; Valverde, A.; Madrid, Y. A combined analytical-chemometric approach for the in vitro determination of polyphenol bioaccessibility by simulated gastrointestinal digestion. Anal. Bioanal. Chem. 2022, 414, 2739–2755. [Google Scholar] [CrossRef]
- Qu, F.; Ai, Z.; Liu, S.; Zhang, H.; Chen, Y.; Wang, Y.; Ni, D. Study on mechanism of low bioavailability of black tea theaflavins by using Caco-2 cell monolayer. Drug Deliv. 2021, 28, 1737–1747. [Google Scholar] [CrossRef]
- Flowers, S.A.; Bhat, S.; Lee, J.C. Potential Implications of Gut Microbiota in Drug Pharmacokinetics and Bioavailability. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2020, 40, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Henning, S.M.; Aronson, W.; Niu, Y.; Conde, F.; Lee, N.H.; Seeram, N.P.; Lee, R.P.; Lu, J.; Harris, D.M.; Moro, A. Tea polyphenols and theaflavins are present in prostate tissue of humans and mice after green and black tea consumption. J. Nutr. 2006, 136, 1839. [Google Scholar] [CrossRef]
- Kondo, A.; Narumi, K.; Okuhara, K.; Takahashi, Y.; Iseki, K. Black tea extract and theaflavin derivatives affect the pharmacokinetics of rosuvastatin by modulating organic anion transporting polypeptide (OATP) 2B1 activity. Biopharm. Drug Dispos. 2019, 40, 302–306. [Google Scholar] [CrossRef] [PubMed]
- Kondo, A.; Narumi, K.; Ogura, J.; Sasaki, A.; Yabe, K.; Kobayashi, T.; Furugen, A.; Kobayashi, M.; Iseki, K. Organic anion-transporting polypeptide (OATP) 2B1 contributes to the cellular uptake of theaflavin. Drug Metab. Pharmacokinet. 2017, 32, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Barrueta, T.; Martínez-Bustos, F.; Castaño-Tostado, E.; Lee, Y.; Miller, M.J.; Amaya-Llano, S.L. Encapsulation of probiotics in whey protein isolate and modified huauzontle’s starch: An approach to avoid fermentation and stabilize polyphenol compounds in a ready-to-drink probiotic green tea. LWT 2020, 124, 109131. [Google Scholar] [CrossRef]
- Yin, Z.Y.; Zheng, T.; Ho, C.T.; Huang, Q.R.; Wu, Q.L.; Zhang, M. Improving the stability and bioavailability of tea polyphenols by encapsulations: A review. Food Sci. Hum. Wellness 2022, 11, 537–556. [Google Scholar] [CrossRef]
- Xia, Y.A.; Xc, B.; Shuang, L.C.; Lla, D.; Jie, W.E.; Min, W.B.; Liang, Z. Studies on the interactions of theaflavin-3,3′-digallate with bovine serum albumin: Multi-spectroscopic analysis and molecular docking. Food Chem. 2021, 366, 130422. [Google Scholar]
- Ye, Q.; Li, T.; Li, J.; Liu, L.; Zhang, X. Development and evaluation of tea polyphenols loaded water in oil emulsion with zein as stabilizer. J. Drug Deliv. Sci. Technol. 2020, 56, 101528. [Google Scholar] [CrossRef]
- Hu, J.; Yang, J.; Guo, H.; Yao, X.; Qiu, H.; Gao, P.; Wang, Q. Effect of Theaflavin on Inflammatory and Remolding of Airway in the Asthma Mice. J. Biomater. Tissue Eng. 2021, 11, 1091–1098. [Google Scholar] [CrossRef]
- Yu, X.; Cai, X.H.; Li, S.; Luo, L.Y.; Wang, J.; Wang, M.; Zeng, L. Studies on the interactions of theaflavin-3,3′-digallate with bovine serum albumin: Multi-spectroscopic analysis and molecular docking. Food Chem. 2022, 366, 130422. [Google Scholar] [CrossRef]
- Ding, Y.; Zou, L.; Lu, C.; Tong, H.; Chen, B. In situ enzymatic synthesis and purification of theaflavin-3,3′-digallate monomer and incorporation into nanoliposome. Int. J. Food Sci. Technol. 2018, 53, 2552–2559. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Bhatnagar, P.; Singh, M. Synthesis of PLGA nanoparticles of tea polyphenols and their strong in vivo protective effect against chemically induced DNA damage. Int. J. Nanomed. 2013, 8, 1451, Erratum in Int. J. Nanomed. 2019, 14, 7001–7002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Y.K.; Zheng, T.; Jin, W.P.; Shi, Y.X.; Huang, Q.R. Enhancing Intestinal Permeability of Theaflavin-3,3’-digallate by Chitosan-Caseinophosphopeptides Nanocomplexes. J. Agric. Food Chem. 2022, 70, 2029–2041. [Google Scholar] [CrossRef] [PubMed]
- He, H.F. Research progress on theaflavins: Efficacy, formation, and preparation. Food Nutr. Res. 2017, 61, 1344521. [Google Scholar] [CrossRef] [Green Version]
- Kun, Z.; Jie, O.; Jianan, H.; Zhonghua, L. Research progress of black tea thearubigins: A review. Crit. Rev. Food Sci. Nutr. 2021, 61, 1556–1566. [Google Scholar]
- Chooi, Y.C.; Ding, C.; Magkos, F. The epidemiology of obesity. Metabolism 2019, 92, 6–10. [Google Scholar] [CrossRef] [Green Version]
- Engin, A. The definition and prevalence of obesity and metabolic syndrome. In Obesity and Lipotoxicity; Springer: Cham, Switzerland, 2017; pp. 1–17. [Google Scholar]
- Ko, H.-J.; Lo, C.-Y.; Wang, B.-J.; Chiou, R.Y.-Y.; Lin, S.-M. Theaflavin-3,3′-digallate, a black tea polyphenol, stimulates lipolysis associated with the induction of mitochondrial uncoupling proteins and AMPK-FoxO3A-MnSOD pathway in 3T3-L1 adipocytes. J. Funct. Foods 2015, 17, 271–282. [Google Scholar] [CrossRef]
- Zhang, L.; Wen, S.P.; Da-Hai, G.U.; Zhi-Gang, L.I.; Cao, Z.H.; Rong, H.; Chang-Rong, G.E.; Jia, J.J. The Polymorphisms of UCPs Genes Associated with Fat Metabolism. China Anim. Husb. Vet. Med. 2010, 37, 1513–1522. [Google Scholar]
- Berardi, M.; Chou, J. Fatty Acid Flippase Activity of UCP2 Is Essential for Its Proton Transport in Mitochondria. Cell Metab. 2014, 20, 541–552. [Google Scholar] [CrossRef] [Green Version]
- Pan, S.; Deng, X.; Sun, S.; Lai, X.; Sun, L.; Li, Q.; Xiang, L.; Zhang, L.; Huang, Y. Black tea affects obesity by reducing nutrient intake and activating AMP-activated protein kinase in mice. Mol. Biol. Rep. 2018, 45, 689–697. [Google Scholar] [CrossRef]
- Xu, J.; Li, M.; Zhang, Y.; Chu, S.; Huo, Y.; Zhao, J.; Wan, C. Huangjinya Black Tea Alleviates Obesity and Insulin Resistance via Modulating Fecal Metabolome in High-Fat Diet-Fed Mice. Molecular Nutr. Food Res. 2020, 64, e2000353. [Google Scholar] [CrossRef] [PubMed]
- Glisan, S.L.; Grove, K.A.; Yennawar, N.H.; Lambert, J.D. Inhibition of pancreatic lipase by black tea theaflavins: Comparative enzymology and in silico modeling studies. Food Chem. 2017, 216, 296–300. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Yu, X.H.; Ou, X.; Ouyang, X.P.; Tang, C.K. Hepatic cholesterol transport and its role in non-alcoholic fatty liver disease and atherosclerosis. Prog. Lipid Res. 2021, 83, 101109. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Deng, Z.; Zou, Y.; Liu, C.; Fu, H.; Gu, Y.; Chang, H. Theaflavin alleviates oxidative injury and atherosclerosis progress via activating microRNA-24-mediated Nrf2/HO-1 signal. Phytother. Res. 2021, 35, 3418–3427. [Google Scholar] [CrossRef]
- Arroyave-Ospina, J.C.; Wu, Z.; Geng, Y.; Han, M. Role of Oxidative Stress in the Pathogenesis of Non-Alcoholic Fatty Liver Disease: Implications for Prevention and Therapy. Antioxidants 2021, 10, 174. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; An, R.; Li, Q.; Sun, L.; Lai, X.; Chen, R.; Li, D.; Sun, S. Theaflavin TF3 Relieves Hepatocyte Lipid Deposition through Activating an AMPK Signaling Pathway by targeting Plasma Kallikrein. J. Agric. Food Chem. 2020, 68, 2673–2683. [Google Scholar] [CrossRef]
- Luo, X.Y.; Takahara, T.; Hou, J.; Kawai, K.; Sugiyama, T.; Tsukada, K.; Takemoto, M.; Takeuchi, M.; Zhong, L.; Li, X.K. Theaflavin attenuates ischemia-reperfusion injury in a mouse fatty liver model. Biochem. Biophys. Res. Commun. 2012, 417, 287–293. [Google Scholar] [CrossRef]
- Qu, Z.; Liu, A.; Liu, C.; Tang, Q.; Zhan, L.; Xiao, W.; Huang, J.; Liu, Z.; Zhang, S. Theaflavin Promotes Mitochondrial Abundance and Glucose Absorption in Myotubes by Activating the CaMKK2-AMPK Signal Axis via Calcium-Ion Influx. J. Agric. Food Chem. 2021, 69, 8144–8159. [Google Scholar] [CrossRef]
- Tong, T.; Ren, N.; Soomi, P.; Wu, J.; Guo, N.; Kang, H.; Kim, E.; Wu, Y.; He, P.; Tu, Y.; et al. Theaflavins Improve Insulin Sensitivity through Regulating Mitochondrial Biosynthesis in Palmitic Acid-Induced HepG2 Cells. Molecules 2018, 23, 3382. [Google Scholar] [CrossRef] [Green Version]
- Cai, X.; Liu, Z.; Dong, X.; Wang, Y.; Zhu, L.; Li, M.; Xu, Y. Hypoglycemic and lipid lowering effects of theaflavins in high-fat diet-induced obese mice. Food Funct. 2021, 12, 9922–9931. [Google Scholar] [CrossRef]
- Li, B.; Fu, L.; Kojima, R.; Yamamoto, A.; Matsui, T. Theaflavins prevent the onset of diabetes through ameliorating glucose tolerance mediated by promoted incretin secretion in spontaneous diabetic Torii rats. J. Funct. Foods 2021, 86, 104702. [Google Scholar] [CrossRef]
- Gothandam, K.; Ganesan, V.S.; Ayyasamy, T.; Ramalingam, S. Protective effect of theaflavin on glycoprotein components and TCA cycle enzymes in high-fat diet and streptozotocin-induced diabetic rats. J. Basic Appl. Zool. 2019, 80, 43. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y. Simultaneous Tests of Theaflavin-3,3′-digallate as an Anti-Diabetic Drug in Human Hepatoma G2 Cells and Zebrafish (Danio rerio). Nutrients 2021, 13, 4379. [Google Scholar]
- Tai, L.; Liu, Z.; Sun, M.; Xie, Q.; Cai, X.; Wang, Y.; Dong, X.; Xu, Y. Anti-hyperuricemic effects of three theaflavins isolated from black tea in hyperuricemic mice. J. Funct. Foods 2020, 66, 103803. [Google Scholar] [CrossRef]
- Chatterjee, S.; Khunti, K.; Davies, M.J. Type 2 diabetes. Lancet 2017, 389, 2239–2251. [Google Scholar] [CrossRef]
- Maykish, A.; Sikalidis, A.K. Utilization of Hydroxyl-Methyl Butyrate, Leucine, Glutamine and Arginine Supplementation in Nutritional Management of Sarcopenia-Implications and Clinical Considerations for Type 2 Diabetes Mellitus Risk Modulation. J. Pers. Med. 2020, 10, 19. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.I.; Dominy, J.E.; Sikalidis, A.K.; Hirschberger, L.L.; Wang, W.; Stipanuk, M.H. HepG2/C3A cells respond to cysteine deprivation by induction of the amino acid deprivation/integrated stress response pathway. Physiol. Genom. 2008, 33, 218–229. [Google Scholar] [CrossRef] [Green Version]
- He, J.H.; Chen, L.X.; Li, H. Progress in the discovery of naturally occurring anti-diabetic drugs and in the identification of their molecular targets. Fitoterapia 2019, 134, 270–289. [Google Scholar] [CrossRef]
- Li, S.; Yin, L.; Yi, J.; Zhang, L.; Yang, L. Insight into interaction mechanism between theaflavin-3-gallate and α-glucosidase using spectroscopy and molecular docking analysis. J. Food Biochem. 2021, 45, e13550. [Google Scholar] [CrossRef]
- Sun, L.; Warren, F.J.; Netzel, G.; Gidley, M.J. 3 or 3′-Galloyl substitution plays an important role in association of catechins and theaflavins with porcine pancreatic α-amylase: The kinetics of inhibition of α-amylase by tea polyphenols. J. Funct. Foods 2016, 26, 144–156. [Google Scholar] [CrossRef]
- Hana, A.; Wang, Y.; Liu, D. Dietary Flavonoids in the Prevention of T2D: An Overview. Nutrients 2018, 10, 438. [Google Scholar]
- Bl, A.; Lei, F.A.; Ca, A.; Amn, A.; Ay, B.; Tm, A. Theaflavins inhibit glucose transport across Caco-2 cells through the downregulation of the Ca2+/AMP-activated protein kinase-mediated glucose transporter SGLT1. J. Funct. Foods 2020, 75, 104273. [Google Scholar]
- Hudish, L.I.; Reusch, J.; Sussel, L. β Cell dysfunction during progression of metabolic syndrome to type 2 diabetes. J. Clin. Investig. 2019, 129, 4001–4008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Luo, L.; Hu, S.; Gan, R.; Zeng, L. The chemistry, processing, and preclinical anti-hyperuricemia potential of tea: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2022, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, Q.; Ye, Y.; Ran, M.; Jin, N. Inhibition of xanthine oxidase by theaflavin: Possible mechanism for anti-hyperuricaemia effect in mice. Process Biochem. 2020, 97, 11–18. [Google Scholar] [CrossRef]
- Dabke, K.; Hendrick, G.; Devkota, S. The gut microbiome and metabolic syndrome. J. Clin. Investig. 2019, 129, 4050–4057. [Google Scholar] [CrossRef]
- Herrema, H.; Niess, J.H. Intestinal microbial metabolites in human metabolism and type 2 diabetes. Diabetologia 2020, 63, 2533–2547. [Google Scholar] [CrossRef]
- Yin, R.; Kuo, H.-C.; Hudlikar, R.; Sargsyan, D.; Li, S.; Wang, L.; Wu, R.; Kong, A.-N. Gut microbiota, dietary phytochemicals and benefits to human health. Curr. Pharmacol. Rep. 2019, 5, 332–344. [Google Scholar] [CrossRef]
- Aravind, S.M.; Wichienchot, S.; Tsao, R.; Ramakrishnan, S.; Chakkaravarthi, S. Role of dietary polyphenols on gut microbiota, their metabolites and health benefits. Food Res. Int. 2021, 142, 110189. [Google Scholar] [CrossRef]
- Mulder, T.P.; van Platerink, C.J.; Schuyl, P.J.W.; van Amelsvoort, J.M. Analysis of theaflavins in biological fluids using liquid chromatography-electrospray mass spectrometry. J. Chromatogr. B Biomed. Sci. Appl. 2001, 760, 271–279. [Google Scholar] [CrossRef]
- Chen, H.D.; Parks, T.A.; Chen, X.X.; Gillitt, N.D.; Jobin, C.; Sang, S.M. Structural identification of mouse fecal metabolites of theaflavin 3,3′-digallate using liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2011, 1218, 7297–7306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, H.; Hu, Y.Z.; Zhang, B.W.; Shao, Z.P.; Roura, E.G.; Wang, S. Tea polyphenol-gut microbiota interactions: Hints on improving the metabolic syndrome in a multi-element and multi-target manner. Food Sci. Hum. Wellness 2022, 11, 11–21. [Google Scholar] [CrossRef]
- Chen, H.D.; Sang, S.M. Biotransformation of tea polyphenols by gut microbiota. J. Funct. Foods 2014, 7, 26–42. [Google Scholar] [CrossRef]
- Chen, H.D.; Hayek, S.; Guzman, J.R.; Gillitt, N.D.; Ibrahim, S.A.; Jobin, C.; Sang, S.M. The Microbiota Is Essential for the Generation of Black Tea Theaflavins-Derived Metabolites. PLoS ONE 2012, 7, 10. [Google Scholar] [CrossRef] [Green Version]
- Pereira-Caro, G.; Moreno-Rojas, J.M.; Brindani, N.; Del Rio, D.; Lean, M.E.J.; Hara, Y.; Crozier, A. Bioavailability of Black Tea Theaflavins: Absorption, Metabolism, and Colonic Catabolism. J. Agric. Food Chem. 2017, 65, 5365–5374. [Google Scholar] [CrossRef]
- Liu, Z.; Bruijn, W.; Bruins, M.E.; Vincken, J.P. Microbial Metabolism of Theaflavin-3,3′-digallate and Its Gut Microbiota Composition Modulatory Effects. J. Agric. Food Chem. 2020, 69, 232–245. [Google Scholar] [CrossRef]
- Shuwei, Z.; Ohland, C.; Jobin, C.; Sang, S. Degradation of black tea theaflavin through C-ring cleavage by gut microbiota. Food Sci. Hum. Wellness 2022, 11, 598–605. [Google Scholar]
- Anandhan, A.; Tamilselvam, K.; Radhiga, T.; Rao, S.; Essa, M.M.; Manivasagam, T. Theaflavin, a black tea polyphenol, protects nigral dopaminergic neurons against chronic MPTP/probenecid induced Parkinson’s disease. Brain Res. 2012, 1433, 104–113. [Google Scholar] [CrossRef]
- Owuor, P.O.; Obanda, M. The use of green tea (Camellia sinensis) leaf flavan-3-ol composition in predicting plain black tea quality potential. Food Chem. 2007, 100, 873–884. [Google Scholar] [CrossRef]
- Chen, T.; Liu, A.B.; Sun, S.; Ajami, N.J.; Ross, M.C.; Wang, H.; Zhang, L.; Reuhl, K.; Kobayashi, K.; Onishi, J.C. Green tea polyphenols modify the gut microbiome in db/db mice as Co-abundance groups correlating with the blood glucose lowering effect. Mol. Nutr. Food Res. 2019, 63, 1801064. [Google Scholar] [CrossRef]
- Meiyan, W.; Jianying, L.; Ting, H.; Hui, Z. Metabolic fate of tea polyphenols and their crosstalk with gut microbiota. Food Sci. Hum. Wellness 2022, 11, 455–466. [Google Scholar]
- Cai, Q.S.; Ji, S.M.; Li, M.W.; Zheng, S.; Zhou, X.H.; Guo, H.M.; Deng, S.Y.; Zhu, J.Y.; Li, D.X.; Xie, Z.W. Theaflavin-regulated Imd condensates control Drosophila intestinal homeostasis and aging. iScience 2021, 24, 35. [Google Scholar] [CrossRef]
- Yussof, A.; Cammalleri, B.; Fayemiwo, O.; Lopez, S.; Chu, T.C. Antibacterial and Sporicidal Activity Evaluation of Theaflavin-3,3′-digallate. Int. J Mol. Sci. 2022, 23, 21. [Google Scholar] [CrossRef] [PubMed]
- Appice, G.M. Comparative Evaluation of the Antibacterial, Anti-biofilm and Anti-spore Effects of Theaflavins and Palmitoyl-EGCG. Master’s Thesis, Seton Hall University, South Orange, NJ, USA, 2017. [Google Scholar]
- Kong, J.H.; Zhang, G.Q.; Xia, K.; Diao, C.H.; Yang, X.F.; Zuo, X.B.; Li, Y.D.; Liang, X.L. Tooth brushing using toothpaste containing theaflavins reduces the oral pathogenic bacteria in healthy adults. 3 Biotech 2021, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Lagha, A.B.; Grenier, D. Tea polyphenols inhibit the activation of NF-κB and the secretion of cytokines and matrix metalloproteinases by macrophages stimulated with Fusobacterium nucleatum. Sci. Rep. 2016, 6, 34520. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Ohland, C.; Jobin, C.; Sang, S. Black Tea Theaflavin Detoxifies Metabolic Toxins in the Intestinal Tract of Mice. Mol. Nutr. Food Res. 2021, 65, 2000887. [Google Scholar] [CrossRef]
- Sun, C.P.; Tian, X.G.; Feng, L.; Wang, C.; Li, J.X.; Huo, X.K.; Zhao, W.Y.; Ning, J.; Yu, Z.L.; Deng, S.; et al. Inhibition of gut bacterial beta-glucuronidase by chemical components from black tea: Inhibition interactions and molecular mechanism. Arab. J. Chem. 2021, 14, 11. [Google Scholar] [CrossRef]
- Sun, L.; Xu, H.; Ye, J.; Gaikwad, N.W. Comparative effect of black, green, oolong, and white tea intake on weight gain and bile acid metabolism. Nutrition 2019, 65, 208–215. [Google Scholar] [CrossRef]
- Sung-Bum, C.; Kang-Jin, P.; Ho-Seok, K.; Dae-Ho, C.; Gi-Hoon, L.; Cho-Yun, C.; Kyu-Yeol, K.; Young-Lan, P.; Song, Y.A.; Wan-Sik, L. Black tea extract prevents lipopolysaccharide-induced NF-κB signaling and attenuates dextran sulfate sodium-induced experimental colitis. BMC Complementary Altern. Med. 2011, 11, 91. [Google Scholar]
- Angiletta, C.J.; Griffin, L.E.; Steele, C.N.; Baer, D.J.; Novotny, J.A.; Davy, K.P.; Neilson, A.P. Impact of short-term flavanol supplementation on fasting plasma trimethylamine N-oxide concentrations in obese adults. Food Funct. 2018, 9, 5350–5361. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Liu, G.; Tang, M.; Fang, J.; Jiang, H. Epigallocatechin Gallate Can Protect Mice from Acute Stress Induced by LPS While Stabilizing Gut Microbes and Serum Metabolites Levels. Front. Immunol. 2021, 12, 640305. [Google Scholar] [CrossRef] [PubMed]
- Gadaleta, R.M.; Erpecum, K.V.; Oldenburg, B.; Willemsen, E.; Renooij, W.; Murzilli, S.; Klomp, L.; Siersema, P.D.; Schipper, M.; Danese, S. Farnesoid X receptor activation inhibits inflammation and preserves the intestinal barrier in inflammatory bowel disease. Gut 2011, 60, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Shanahan, F. Therapeutic Manipulation of Gut Flora. (research on inflammatory bowel disease). Science 2000, 289, 1311–1312. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.A.; Park, Y.L.; Yoon, S.H.; Kim, K.Y.; Cho, S.B.; Lee, W.S.; Chung, I.J.; Joo, Y.E. Black tea polyphenol theaflavin suppresses LPS-induced ICAM-1 and VCAM-1 expression via blockage of NF-κB and JNK activation in intestinal epithelial cells. Inflamm. Res. 2011, 60, 493–500. [Google Scholar] [CrossRef]
- Yan, R.; Ho, C.; Zhang, X. Interaction Between Tea Polyphenols and Intestinal Microbiota in Host Metabolic Diseases from the Perspective of the Gut-Brain Axis. Mol. Nutr. Food Res. 2020, 64, 2000187. [Google Scholar] [CrossRef]
- Hu, S.S.; Luo, L.Y.; Zeng, L. Tea combats circadian rhythm disorder syndrome via the gut-liver-brain axis: Potential mechanisms speculated. Crit. Rev. Food Sci. Nutr. 2022, 1–22. [Google Scholar] [CrossRef]
- Guo, T.; Song, D.; Ho, C.T.; Zhang, X.; Wu, Z. Omics Analyses of Gut Microbiota in a Circadian Rhythm Disorder Mouse Model Fed with Oolong Tea Polyphenols. J. Agric. Food Chem. 2019, 67, 8847–8854. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).