Abstract
Extracellular vesicles are particles released from cells and delimited by a lipid bilayer. They have been widely studied, including extensive investigation in cardiovascular diseases. Many scientists have explored their role in atrial fibrillation. Patients suffering from atrial fibrillation have been evidenced to present altered levels of these particles as well as changed amounts of their contents such as micro-ribonucleic acids (miRs). Although many observations have been made so far, a large randomized clinical trial is needed to assess the previous findings. This review aims to thoroughly summarize current research regarding extracellular vesicles in atrial fibrillation.
1. Introduction
Atrial fibrillation (AF) is defined by the European Society of Cardiology (ESC) as a supraventricular tachyarrhythmia with uncoordinated atrial electrical activation and consequently ineffective atrial contraction [1]. Not only does it worsen the quality of the affected patients’ life [2] but also increases morbidity and mortality due to, e.g., increased risk of stroke [3]. It is currently the most common sustained cardiac arrhythmia in adults all over the world with an estimated prevalence between 2% and 4% [4].
Several risk factors for AF progression have been identified, such as age, heart failure, hypertension, chronic kidney disease, chronic pulmonary diseases, diabetes mellitus, previous stroke, and left atrial size [5]. However, it has not been explicitly established if biomarkers exhibit any added predictive value in terms of AF progression; moreover, in “2020 ESC Guidelines for the diagnosis and management of atrial fibrillation”, the role of biomarkers in AF management has been pointed out as having gaps in evidence [1]. Thus, research investigating the facility of biomarkers in AF patients is warranted.
According to the International Society for Extracellular Vesicles (ISEV), extracellular vesicles (EVs) are defined as particles naturally released from cells and delimited by a lipid bilayer; moreover, they cannot replicate, as they do not contain a functional nucleus [6]. Historically, several terms describing different types of EVs have been used, such as exosomes, ectosomes, or microparticles [7]. Nevertheless, the current scientific consensus advises against using these terms unless markers of subcellular origin can be established [6]. EVs play a crucial role in cell-to-cell communication [8]. Therefore, their importance in different diseases has been thoroughly studied by many research groups [9]. EVs have also been extensively investigated in various cardiovascular diseases, in terms of their involvement in pathophysiology or being potential biomarkers, as their concentrations can be determined with the use of the accessible techniques, e.g., flow cytometry (Figure 1) [10,11,12].
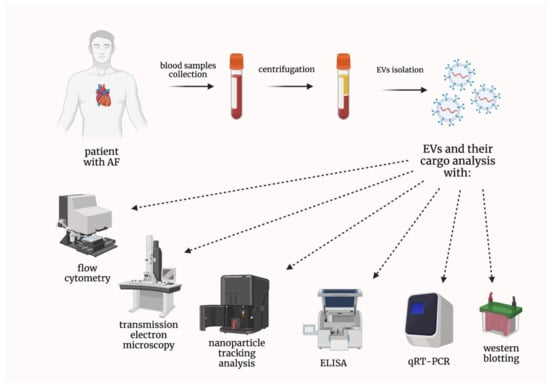
Figure 1.
Techniques used in the analysis of both extracellular vesicles and their cargo. AF—atrial fibrillation; ELISA—enzyme-linked immunosorbent assay; EVs—extracellular vesicles; qRT-PCR—quantitative real-time reverse transcription polymerase chain reaction.
Because EVs have been suggested to play a potential role in AF [13,14,15,16,17], the purpose of this review is to summarize the current data on EVs’ diagnostic and prognostic utility in AF patients.
2. Extracellular Vesicles in Patients with Atrial Fibrillation
We analyzed the available literature for original scientific papers concerning EVs in AF. After a meticulous analysis of the pertinent studies, we divided them into the following groups: preclinical studies, EVs in AF patients treated with anticoagulants and other drugs, EVs containing nucleic acids in AF patients, EVs in AF patients undergoing ablation or other invasive procedures, and other research studies concerning EVs in AF patients.
2.1. Preclinical Studies
It was proven that cardiomyocytes, treated with the EVs derived from myofibroblasts, showed the downregulation of L-type calcium channel Cav1.2. This downregulation is considered to be a characteristic feature of the ionic remodeling associated with AF [18]. Additionally, EVs derived from the angiotensin II-treated human cardiac myocytes encouraged macrophages to conducted M1 polarization and, consequently, proinflammatory state; moreover, it was evidenced that Plasmacytoma Variant Translocation 1 (PVT1) contained in EVs promoted extracellular matrix remodeling in atrial fibroblasts [19].
Another research group established that beagles undergoing rapid atrial pacing for 7 days showed a rise in the atrial and plasma EVs release; moreover, it was effectively hampered by GW4869, a commonly used agent inhibiting EVs generation. It was also suggested that miR-21-5p could play a role, as it was upregulated in both atrial and plasma EVs [20]. EVs originating from mouse adipose-tissue-derived mesenchymal stem cells and transfected with the X-inactive specific transcript (XIST) caused a decrease in inflammation and myocardial pyroptosis [21]. In a recent study, EVs from bone marrow mesenchymal stem cells were transduced with the nuclear factor-erythroid 2-related factor 2 (Nrf2). These EVs injected into rats with AF not only shortened AF duration and reduced cardiomyocyte apoptosis but also minimized atrial fibrosis [22]. It was also proven that EVs derived from bone marrow mesenchymal stem cells overexpressing miR-148a reduced cardiomyocyte apoptosis by inhibiting SPARC-associated modular calcium-binding protein 2 (SMOC2) [23]. The preclinical studies discussed in this subsection are graphically summarized in Figure 2.
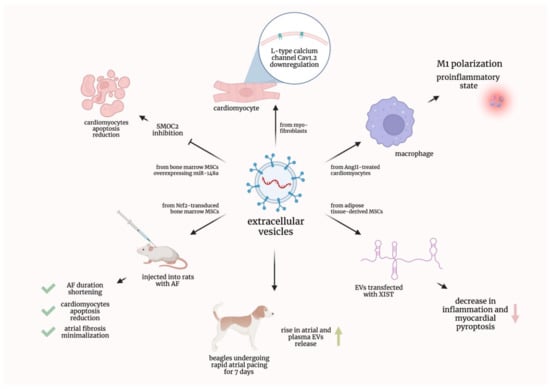
Figure 2.
Graphical summarization of the preclinical studies concerning extracellular vesicles in atrial fibrillation. AF—atrial fibrillation; AngII—Angiotensin II; EVs—extracellular vesicles; miR—micro-ribonucleic acid; MSCs—mesenchymal stem cells; Nrf2—nuclear factor-erythroid 2-related factor 2; SMOC2—SPARC-associated modular calcium-binding protein 2; XIST—X-inactive specific transcript.
2.2. EVs in Atrial Fibrillation Patients Treated with Anticoagulants and Other Drugs
Chirinos et al. investigated the association between digoxin use in patients suffering from non-valvular AF and the level of EVs originating from endothelium and platelets. They showed that patients taking digoxin exhibited increased levels of endothelial EVs [24]. Lau et al. studied the population of AF patients treated with warfarin. It was shown that endothelial and platelet EVs collectively negatively correlated with the estimated glomerular filtration rate (eGFR), making them potential nephrotoxicity biomarkers. However, platelet-derived EVs alone did not present any correlation with the eGFR [25]. Lenart-Migdalska et al. studied the impact of dabigatran intake on platelet and endothelial EV concentration in non-valvular AF patients. Platelet-derived EVs were increased in patients treated with dabigatran; moreover, dabigatran concentration correlated negatively with the concentration change among these EVs. Endothelial-derived EVs did not exhibit such relationships [26]. The same research group evidenced that non-valvular AF patients treated with rivaroxaban presented increased levels of both platelet- and endothelial-derived EVs after drug administration [27]. Moreover, Weiss et al. demonstrated that non-valvular AF patients treated with rivaroxaban had a significantly altered proteomic profile of EVs compared with those treated with warfarin; proinflammatory proteins and complement factors were decreased, whereas negative regulators of inflammatory pathways were elevated in patients treated with rivaroxaban [28]. Complementary to the aforementioned studies, Duarte et al. showed that AF patients treated with rivaroxaban or warfarin presented increased levels of platelet-derived EVs compared with the control subjects with no AF. However, no differences in endothelial-derived EVs were noted [29]. Further improvement in this field may lead to the treatment individualization with EVs being potential predictors of treatment response in AF patients. All studies discussed in this subsection with additional data are summarized in Table 1.

Table 1.
The summary of recent studies regarding extracellular vesicles in atrial fibrillation patients treated with anticoagulants and other drugs.
2.3. EVs Containing Nucleic Acids in Atrial Fibrillation Patients
Wang et al. investigated EVs containing micro-ribonucleic acids (miRs). They proved that patients suffering from non-valvular AF presented increased levels of miR-483-5p and decreased levels of miR-142-5p, miR-223-3p, and miR-223-5p compared with controls in sinus rhythm. Moreover, miR-483-5p, miR-142-5p, and miR-223-3p were shown to be related to AF by univariate logistic analysis, whereas multivariate logistic analyses proved miR-483-5p to be independently correlated with AF [30]. Mun et al. researched differences in the expression of miRs in circulating EVs between subjects with supraventricular tachycardia and patients suffering from persistent AF. The study revealed that the latter group presented increased levels of miR-107, miR-320d, miR-103a-3p, miR-486-5p, and let-7b-5p [31]. Soltész et al. proved that there were no differences in the EV-contained mitochondrial deoxyribonucleic acid (mtDNA) copy numbers between AF patients and healthy controls [32]. Additionally, Wei et al. demonstrated that AF patients had increased levels of miR-92b-3p, miR-1306-5p, and miR-let-7b-3p contained in the EVs compared with control patients in sinus rhythm [33].
Liu et al. found that patients suffering from congenital heart disease (CHD) and AF presented different concentrations of miRs associated with EVs when compared with CHD patients in sinus rhythm. Quantitative analysis showed reduced levels of miR-382-3p and miR-450a-2-3p as well as increased levels of miR-3126-5p in AF patients [34]. Wang et al. showed that AF patients had higher expression of EV-contained miR-107 compared with healthy controls [35].
Interestingly, Liu et al. demonstrated that AF patients presented decreased expression levels of EV-incorporated LINC00636 (Long Intergenic Non-Protein Coding RNA) and miR-450a-2-3p compared with non-AF patients. Moreover, the expression levels of these two RNAs positively correlated with each other [36]. Importantly, Chen et al. proved that AF patients showed an increased expression of myocardial infarction associated transcript (MIAT) in serum-derived EVs when compared with healthy individuals. Interestingly, the highest MIAT expression was observed in patients with permanent AF. It was also evidenced that MIAT, abundant in those EVs, promoted atrial fibrosis and thus compounded atrial remodeling and subsequent AF [37].
Siwaponanan et al. evidenced that AF patients presented increased levels of miR-106b-3p, miR-590-5p, miR-339-3p, miR-378-3p, miR-328-3p, and miR-532-3p derived from EVs. These miRs were suggested to be possibly involved in processes such as arrhythmogenesis or structural remodeling in AF [38]. Similarly, Zhu et al. investigated EV-contained miRs expression levels in AF patients. They presented an increase in miR-124-3p, miR-378d, miR-2110, and miR-3180-3p levels as well as a decrease in miR-223-5p, miR-574-3p, miR-125a-3p, and miR-1299 levels when compared with patients in sinus rhythm [39]. Finally, Mun et al. proved that patients with persistent AF showed a significant downregulation of miR-30a-5p in small EVs [40]. Just as EVs alone, miRs and other small molecules contained in EVs can become not only diagnostic but also predictive tools. All studies discussed in this subsection with additional data are summarized in Table 2.

Table 2.
Summary of recent studies concerning extracellular vesicles containing nucleic acids in atrial fibrillation patients.
2.4. EVs in Atrial Fibrillation Patients Undergoing Ablation or Other Invasive Procedures
Herrera-Siklódy et al. studied the differences between AF patients undergoing cryoablation and radiofrequency (RF) ablation in terms of EVs as cellular damage markers. In both groups of patients, they observed an increase in platelet- and leukocyte-derived (but not endothelial-derived) EVs after the ablation procedure. However, there were no significant differences between the groups [41]. Jesel et al. compared the concentrations of EVs between the right and the left atria in AF patients undergoing ablation. They investigated procoagulant EVs derived from platelets, leukocytes, and endothelial cells. Interestingly, only endothelial-derived EVs presented atrial-specific differences, being increased in the right atrium [42]. Liles et al. measured the levels of tissue-factor extracellular vesicles (TF-EVs) in non-valvular AF patients prior to ablation surgery. They compared patients and healthy controls and divided patients based on the type of their anticoagulant therapy. It was shown that TF-EVs were increased in AF patients compared with healthy controls; however, after dividing AF patients into two groups, not-treated with anticoagulants and treated with anticoagulants, only the latter showed increased levels of TF-EVs compared with healthy controls. Consistently, this group also presented increased levels of TF-EVs compared with the first group. Interestingly, there were no differences in TF-EV levels between AF patients treated with warfarin and AF patients treated with apixaban or rivaroxaban [43]. Pourtau et al. proved the diminished tissue-factor-dependent procoagulant activity of EVs in both paroxysmal and persistent AF patients undergoing ablation compared with healthy controls; however, only the paroxysmal AF patients showed decreased fibrinolytic activity of EVs compared with controls. Moreover, 10 AF patients in sinus rhythm (for 10 days before ablation) were subjected to induced AF. After 20 minutes of acute AF, these patients presented decreased procoagulant activity of EVs with unaltered fibrinolytic activity [44].
Meng et al. demonstrated that AF patients had elevated levels of TF-EVs and EVs derived from platelets, endothelial cells, and leukocytes (but not from erythrocytes) compared with healthy controls. It was also proven that both endothelial-derived EVs (>355/μL) as well as leukocyte-derived EVs (>639/μL) were risk factors for the early recurrence of atrial fibrillation (ERAF). Additionally, the former were shown to be an independent predictor of the ERAF [45].
Mørk et al. analyzed EVs (particularly TF-EVs) in patients with or without AF undergoing cardiac surgery procedures. Total EVs, as well as TF-EVs, were shown to be increased in patients suffering from AF in all measurements, from venous blood preoperatively/intraoperatively and directly from the left atrial appendage intraoperatively [46]. Amabile et al. showed that non-valvular AF patients who underwent left atrial appendage occlusion presented increased levels of annexin V-positive, and platelet-, erythrocyte-, and leukocyte-derived EVs after the intervention. In patients undergoing coronary angiography, who served as a control group, only annexin V-positive EVs were proven to be increased after the procedure [47]. Shaihov-Teper et al. investigated the population of patients with or without AF undergoing elective heart surgery. Organ cultures, grown from epicardial fat, secreted more EVs in samples from AF patients. Moreover, EV contents, proinflammatory and profibrotic cytokines as well as profibrotic miR, were also elevated in this group [48]. Perhaps ongoing and future research will provide evidence that will lead to the EVs’ inclusion into the qualification criteria for invasive procedures such as ablation. All studies discussed in this subsection with additional data are summarized in Table 3.

Table 3.
Summary of recent studies regarding extracellular vesicles in atrial fibrillation patients undergoing ablation and other procedures.
2.5. Other Research Studies Concerning EVs in Atrial Fibrillation Patients
Choudhury et al. proved that both patients suffering from AF and patients in sinus rhythm suffering from other cardiovascular diseases had increased levels of platelet EVs compared with healthy controls in sinus rhythm. Moreover, they evidenced that neither AF type (paroxysmal or permanent) nor applied therapy (aspirin or warfarin) influenced the level of platelet EVs [49]. Ederhy et al. showed increased levels of annexin V-positive EVs in AF patients compared with control subjects with or without cardiovascular risk factors. The level of platelet- and endothelial-derived EVs showed no differences between AF patients and control subjects with cardiovascular risk factors; however, they were increased in those two groups as compared with control subjects without cardiovascular risk factors [50]. Azzam et al. proved that patients suffering from valvular AF had increased levels of platelet-derived EVs compared with age-matched healthy volunteers in sinus rhythm. Moreover, it appeared that the severity of mitral stenosis correlated with EV levels [51].
Wang et al. investigated non-valvular AF patients, both with paroxysmal AF and persistent AF. It emerged that the latter group had increased levels of EVs compared with normal controls and paroxysmal AF patients. What is more interesting is it was shown that patients with persistent AF had increased levels of EV-bound interleukin-1β and P-selectin [52]. Hayashi et al. compared the levels of P-selectin-positive EVs in platelet-rich plasma between non-valvular AF patients and control subjects; there were no differences between these groups. However, they proved that the induction of AF in paroxysmal AF patients resulted in increased levels of EVs expressing P-selectin [53]. Idriss et al. proved that both mitral valve disease (MVD) patients with AF and MVD patients in sinus rhythm had increased levels of EVs showing positive binding to anti-CD41a as compared with healthy controls; however, there were no differences between these two groups of MVD patients [54].
Wang et al. studied non-valvular AF patients with or without left atrial thrombi. Both groups showed increased phosphatidyl-serine (PS)-exposing EV levels compared with control subjects; moreover, the patients with thrombi had elevated EV levels compared with the patients without thrombi. Both groups presented an increased procoagulant activity to be effectively inhibited by the addition of lactadherin. Interestingly, the amount of EVs positively correlated with the thrombus diameter [55]. Siwaponanan et al. demonstrated that non-valvular AF patients had increased levels of total EVs and increased levels of platelet- and endothelial-derived EVs compared with healthy controls [56].
Thulin et al. investigated AF patients (some of whom were stroke cases) selected from a large randomized clinical trial and a cohort of the randomly selected control individuals of age 70. It was shown that AF patients presented increased levels of PS-positive EVs and EVs derived from platelets, leukocytes, and erythrocytes (but not from endothelial cells) compared with controls. Moreover, there were no differences in the EV levels among the AF patients when comparing the stroke cases to the others [57]. Wang et al. classified non-valvular AF patients as “low to moderate risk” or “high risk” of stroke using the CHADS2 score. The latter group was evidenced to have increased levels of annexin V-positive and platelet-derived EVs as compared with the lower-risk group. Moreover, it was shown that EVs derived from AF patients bound to platelet receptor CD36 and activated platelets [58].
Voukalis et al. evidenced that AF patients presented increased levels of apoptotic EVs (annexin V-positive) when compared with ischemic disease patients in sinus rhythm; however, both groups had similar levels of platelet-derived EVs [59]. Ni et al. conducted a proteomic analysis of serum EVs comparing paroxysmal AF patients with healthy subjects. These two groups were proven to have different expression levels of many proteins mainly involved in anticoagulation, complement system, and protein folding, as indicated by the bioinformatic analysis [60]. Zietzer et al. proved that patients suffering from AF had higher levels of large EVs derived from platelets in the left atrial appendage than patients with no AF. Moreover, patients with permanent AF presented higher levels of these EVs when compared with non-permanent AF patients [61]. All studies discussed in this subsection with additional data are summarized in Table 4.

Table 4.
Summary of other research studies concerning extracellular vesicles in atrial fibrillation patients.
3. Conclusions and Future Perspectives
Multiple studies concerning EVs in patients with AF have been conducted so far. EV plasma concentration changes of different origins have been found in patients suffering from AF; moreover, several correlations between EV concentrations and the type of AF or the type of anticoagulant treatment have been identified. Not only EV concentration per se have been found to be affected but also their cargos, including miRs and long non-coding RNAs. However, most studies have enrolled a rather small sample of patients; therefore, large randomized clinical trials evaluating the most important findings and possibly assessing other undiscovered features are needed. Although the detailed role of EVs in AF remains not fully discovered, it is surely crucial; thus, subsequent research in this field is highly required.
Further improvement of our knowledge about EVs in various diseases, particularly in AF, would undoubtedly contribute to the deeper understanding of the molecular basis and thus to better diagnostic and therapeutic strategies to deal with these conditions. Establishing the cut-off values of certain EV concentrations for AF diagnosis would be of great importance. Furthermore, the discovered correlations between EV concentration and various diseases could probably lead to the development of, e.g., directed therapy. Nevertheless, future research is needed to fill the gaps in the body of evidence indicated by the ESC concerning the role of biomarkers in AF management.
Author Contributions
Conceptualization and methodology, G.P.; writing—original draft preparation, G.P.; writing—review and editing, A.G., D.B., P.B., P.L. and M.G.; visualization, G.P.; supervision, A.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research study received no external funding.
Data Availability Statement
Not applicable.
Acknowledgments
The figures were created with BioRender.com.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef] [PubMed]
- Thrall, G.; Lane, D.; Carroll, D.; Lip, G.Y. Quality of life in patients with atrial fibrillation: A systematic review. Am. J. Med. 2006, 119, 448.e1–448.e19. [Google Scholar] [CrossRef] [PubMed]
- Odutayo, A.; Wong, C.X.; Hsiao, A.J.; Hopewell, S.; Altman, D.G.; Emdin, C.A. Atrial fibrillation and risks of cardiovascular disease, renal disease, and death: Systematic review and meta-analysis. BMJ 2016, 354, i4482. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R.; et al. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation 2019, 139, e56–e528. [Google Scholar] [CrossRef]
- Deng, H.; Bai, Y.; Shantsila, A.; Fauchier, L.; Potpara, T.S.; Lip, G.Y.H. Clinical scores for outcomes of rhythm control or arrhythmia progression in patients with atrial fibrillation: A systematic review. Clin. Res. Cardiol. 2017, 106, 813–823. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Yáñez-Mó, M.; Siljander, P.R.; Andreu, Z.; Zavec, A.B.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef]
- Mathieu, M.; Martin-Jaular, L.; Lavieu, G.; Théry, C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 2019, 21, 9–17. [Google Scholar] [CrossRef]
- Shah, R.; Patel, T.; Freedman, J.E. Circulating Extracellular Vesicles in Human Disease. N. Engl. J. Med. 2018, 379, 958–966. [Google Scholar] [CrossRef]
- Thulin, Å.; Christersson, C.; Alfredsson, J.; Siegbahn, A. Circulating cell-derived microparticles as biomarkers in cardiovascular disease. Biomark Med. 2016, 10, 1009–1022. [Google Scholar] [CrossRef]
- Voukalis, C.; Shantsila, E.; Lip, G.Y.H. Microparticles and cardiovascular diseases. Ann. Med. 2019, 51, 193–223. [Google Scholar] [CrossRef]
- Martins-Marques, T.; Girão, H. The good, the bad and the ugly: The impact of extracellular vesicles on the cardiovascular system. J. Physiol. 2022. [Google Scholar] [CrossRef]
- Jesel, L.; Abbas, M.; Toti, F.; Cohen, A.; Arentz, T.; Morel, O. Microparticles in atrial fibrillation: A link between cell activation or apoptosis, tissue remodelling and thrombogenicity. Int. J. Cardiol. 2013, 168, 660–669. [Google Scholar] [CrossRef]
- Chen, C.; Chen, Q.; Cheng, K.; Zou, T.; Pang, Y.; Ling, Y.; Xu, Y.; Zhu, W. Exosomes and Exosomal Non-coding RNAs Are Novel Promises for the Mechanism-Based Diagnosis and Treatments of Atrial Fibrillation. Front. Cardiovasc. Med. 2021, 8, 782451. [Google Scholar] [CrossRef]
- Huang, S.; Deng, Y.; Xu, J.; Liu, J.; Liu, L.; Fan, C. The Role of Exosomes and Their Cargos in the Mechanism, Diagnosis, and Treatment of Atrial Fibrillation. Front. Cardiovasc. Med. 2021, 8, 712828. [Google Scholar] [CrossRef]
- Xiang, K.; Akram, M.; Elbossaty, W.F.; Yang, J.; Fan, C. Exosomes in atrial fibrillation: Therapeutic potential and role as clinical biomarkers. Heart Fail. Rev. 2021, 27, 1211–1221. [Google Scholar] [CrossRef]
- Berezin, A.E.; Berezin, A.A. Extracellular Vesicles and Thrombogenicity in Atrial Fibrillation. Int. J. Mol. Sci. 2022, 23, 1774. [Google Scholar] [CrossRef]
- Li, S.; Gao, Y.; Liu, Y.; Li, J.; Yang, X.; Hu, R.; Liu, J.; Zhang, Y.; Zuo, K.; Li, K.; et al. Myofibroblast-Derived Exosomes Contribute to Development of a Susceptible Substrate for Atrial Fibrillation. Cardiology 2020, 145, 324–332. [Google Scholar] [CrossRef]
- Cao, F.; Li, Z.; Ding, W.; Yan, L.; Zhao, Q. Angiotensin II-Treated Cardiac Myocytes Regulate M1 Macrophage Polarization via Transferring Exosomal PVT1. J. Immunol. Res. 2021, 2021, 1994328. [Google Scholar] [CrossRef]
- Yao, Y.; He, S.; Wang, Y.; Cao, Z.; Liu, D.; Fu, Y.; Chen, H.; Wang, X.; Zhao, Q. Blockade of Exosome Release Suppresses Atrial Fibrillation by Alleviating Atrial Fibrosis in Canines With Prolonged Atrial Pacing. Front. Cardiovasc. Med. 2021, 8, 699175. [Google Scholar] [CrossRef]
- Yan, B.; Liu, T.; Yao, C.; Liu, X.; Du, Q.; Pan, L. LncRNA XIST shuttled by adipose tissue-derived mesenchymal stem cell-derived extracellular vesicles suppresses myocardial pyroptosis in atrial fibrillation by disrupting miR-214-3p-mediated Arl2 inhibition. Lab. Investig. 2021, 101, 1427–1438. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Fan, Y.; Wu, L.; Zhang, C.; Chu, M.; Wang, Y.; Zhuang, W. Exosomes from Bone Marrow Mesenchymal Stem Cells with Overexpressed Nrf2 Inhibit Cardiac Fibrosis in Rats with Atrial Fibrillation. Cardiovasc. Ther. 2022, 2022, 2687807. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Man, Y.; Chen, Z. microRNA-148a in Exosomes Derived from Bone Marrow Mesenchymal Stem Cells Alleviates Cardiomyocyte Apoptosis in Atrial Fibrillation by Inhibiting SMOC2. Mol. Biotechnol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Chirinos, J.A.; Castrellon, A.; Zambrano, J.P.; Jimenez, J.J.; Jy, W.; Horstman, L.L.; Willens, H.J.; Castellanos, A.; Myerburg, R.J.; Ahn, Y.S. Digoxin use is associated with increased platelet and endothelial cell activation in patients with nonvalvular atrial fibrillation. Heart Rhythm. 2005, 2, 525–529. [Google Scholar] [CrossRef]
- Lau, Y.C.; Xiong, Q.; Blann, A.D.; Lip, G.Y. Relationship between renal function and circulating microparticles, soluble P-selectin and E-selectin levels in atrial fibrillation. J. Thromb. Thrombolysis 2017, 43, 18–23. [Google Scholar] [CrossRef]
- Lenart-Migdalska, A.; Drabik, L.; Kaźnica-Wiatr, M.; Tomkiewicz-Pająk, L.; Podolec, P.; Olszowska, M. Flow Cytometric Assessment of Endothelial and Platelet Microparticles in Patients With Atrial Fibrillation Treated With Dabigatran. Clin. Appl. Thromb. Hemost. 2020, 26, 1076029620972467. [Google Scholar] [CrossRef]
- Lenart-Migdalska, A.; Drabik, L.; Kaźnica-Wiatr, M.; Tomkiewicz-Pająk, L.; Podolec, P.; Olszowska, M. Increased Levels of Platelets and Endothelial-Derived Microparticles in Patients With Non-Valvular Atrial Fibrillation During Rivaroxaban Therapy. Clin. Appl. Thromb. Hemost. 2021, 27, 10760296211019465. [Google Scholar] [CrossRef]
- Weiss, L.; Keaney, J.; Szklanna, P.B.; Prendiville, T.; Uhrig, W.; Wynne, K.; Kelliher, S.; Ewins, K.; Comer, S.P.; Egan, K.; et al. Nonvalvular atrial fibrillation patients anticoagulated with rivaroxaban compared with warfarin exhibit reduced circulating extracellular vesicles with attenuated pro-inflammatory protein signatures. J. Thromb. Haemost. 2021, 19, 2583–2595. [Google Scholar] [CrossRef]
- Duarte, R.C.F.; Rios, D.R.A.; Figueiredo, E.L.; Caiaffa, J.R.S.; Silveira, F.R.; Lanna, R.; Alves, L.C.V.; Martins, G.L.; Reis, H.J.; Reis, E.A.; et al. Thrombin Generation and other hemostatic parameters in patients with atrial fibrillation in use of warfarin or rivaroxaban. J. Thromb. Thrombolysis 2021, 51, 47–57. [Google Scholar] [CrossRef]
- Wang, S.; Min, J.; Yu, Y.; Yin, L.; Wang, Q.; Shen, H.; Yang, J.; Zhang, P.; Xiao, J.; Wang, Z. Differentially expressed miRNAs in circulating exosomes between atrial fibrillation and sinus rhythm. J. Thorac. Dis. 2019, 11, 4337–4348. [Google Scholar] [CrossRef]
- Mun, D.; Kim, H.; Kang, J.Y.; Park, H.; Park, H.; Lee, S.H.; Yun, N.; Joung, B. Expression of miRNAs in circulating exosomes derived from patients with persistent atrial fibrillation. FASEB J. 2019, 33, 5979–5989. [Google Scholar] [CrossRef]
- Soltész, B.; Urbancsek, R.; Pös, O.; Hajas, O.; Forgács, I.N.; Szilágyi, E.; Nagy-Baló, E.; Szemes, T.; Csanádi, Z.; Nagy, B. Quantification of peripheral whole blood, cell-free plasma and exosome encapsulated mitochondrial DNA copy numbers in patients with atrial fibrillation. J. Biotechnol. 2019, 299, 66–71. [Google Scholar] [CrossRef]
- Wei, Z.; Bing, Z.; Shaohuan, Q.; Yanran, W.; Shuo, S.; Bi, T.; Feiyu, Z.; Heng, Z.; Qin, G.; Pinfang, K. Expression of miRNAs in plasma exosomes derived from patients with atrial fibrillation. Clin. Cardiol. 2020, 43, 1450–1459. [Google Scholar] [CrossRef]
- Liu, L.; Chen, Y.; Shu, J.; Tang, C.E.; Jiang, Y.; Luo, F. Identification of microRNAs enriched in exosomes in human pericardial fluid of patients with atrial fibrillation based on bioinformatic analysis. J. Thorac. Dis. 2020, 12, 5617–5627. [Google Scholar] [CrossRef]
- Wang, S.; Li, L.; Hu, X.; Liu, T.; Jiang, W.; Wu, R.; Ren, Y.; Wang, M. Effects of Atrial Fibrillation-Derived Exosome Delivery of miR-107 to Human Umbilical Vein Endothelial Cells. DNA Cell Biol. 2021, 40, 568–579. [Google Scholar] [CrossRef]
- Liu, L.; Luo, F.; Lei, K. Exosomes Containing LINC00636 Inhibit MAPK1 through the miR-450a-2-3p Overexpression in Human Pericardial Fluid and Improve Cardiac Fibrosis in Patients with Atrial Fibrillation. Mediat. Inflamm. 2021, 2021, 9960241. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, X.; Li, H.; Li, Y.; Cheng, D.; Tang, Y.; Sang, H. Serum extracellular vesicles containing MIAT induces atrial fibrosis, inflammation and oxidative stress to promote atrial remodeling and atrial fibrillation via blockade of miR-485-5p-mediated CXCL10 inhibition. Clin. Transl. Med. 2021, 11, e482. [Google Scholar] [CrossRef]
- Siwaponanan, P.; Kaewkumdee, P.; Phromawan, W.; Udompunturak, S.; Chomanee, N.; Udol, K.; Pattanapanyasat, K.; Krittayaphong, R. Increased expression of six-large extracellular vesicle-derived miRNAs signature for nonvalvular atrial fibrillation. J. Transl. Med. 2022, 20, 4. [Google Scholar] [CrossRef]
- Zhu, P.; Li, H.; Zhang, A.; Li, Z.; Zhang, Y.; Ren, M.; Zhang, Y.; Hou, Y. MicroRNAs sequencing of plasma exosomes derived from patients with atrial fibrillation: miR-124-3p promotes cardiac fibroblast activation and proliferation by regulating AXIN1. J. Physiol. Biochem. 2022, 78, 85–98. [Google Scholar] [CrossRef]
- Mun, D.; Kim, H.; Kang, J.Y.; Yun, N.; Youn, Y.N.; Joung, B. Small extracellular vesicles derived from patients with persistent atrial fibrillation exacerbate arrhythmogenesis via miR-30a-5p. Clin. Sci. 2022, 136, 621–637. [Google Scholar] [CrossRef]
- Herrera Siklódy, C.; Arentz, T.; Minners, J.; Jesel, L.; Stratz, C.; Valina, C.M.; Weber, R.; Kalusche, D.; Toti, F.; Morel, O.; et al. Cellular damage, platelet activation, and inflammatory response after pulmonary vein isolation: A randomized study comparing radiofrequency ablation with cryoablation. Heart Rhythm. 2012, 9, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Jesel, L.; Arentz, T.; Herrera-Siklody, C.; Trenk, D.; Zobairi, F.; Abbas, M.; Weber, R.; Minners, J.; Toti, F.; Morel, O. Do atrial differences in endothelial damage, leukocyte and platelet activation, or tissue factor activity contribute to chamber-specific thrombogenic status in patients with atrial fibrillation? J. Cardiovasc. Electrophysiol. 2014, 25, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Liles, J.; Liles, J.; Wanderling, C.; Syed, M.; Hoppensteadt, D.; Fareed, J. Increased Level of Thrombotic Biomarkers in Patients with Atrial Fibrillation Despite Traditional and New Anticoagulant Therapy. Clin. Appl. Thromb. Hemost. 2016, 22, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Pourtau, L.; Sellal, J.M.; Lacroix, R.; Poncelet, P.; Bernus, O.; Clofent-Sanchez, G.; Hocini, M.; Haïssaguerre, M.; Dignat-George, F.; Sacher, F.; et al. Platelet function and microparticle levels in atrial fibrillation: Changes during the acute episode. Int. J. Cardiol. 2017, 243, 216–222. [Google Scholar] [CrossRef]
- Meng, H.; Kou, J.; Ma, R.; Ding, W.; Kou, Y.; Cao, M.; Dong, Z.; Bi, Y.; Thatte, H.S.; Shi, J. Prognostic implications and procoagulant activity of phosphatidylserine exposure of blood cells and microparticles in patients with atrial fibrillation treated with pulmonary vein isolation. Mol. Med. Rep. 2017, 16, 8579–8588. [Google Scholar] [CrossRef]
- Mørk, M.; Andreasen, J.J.; Rasmussen, L.H.; Lip, G.Y.H.; Pedersen, S.; Bæk, R.; Jørgensen, M.M.; Kristensen, S.R. Elevated blood plasma levels of tissue factor-bearing extracellular vesicles in patients with atrial fibrillation. Thromb. Res. 2019, 173, 141–150. [Google Scholar] [CrossRef]
- Amabile, N.; Bagdadi, I.; Armero, S.; Elhadad, S.; Sebag, F.; Landolff, Q.; Saby, L.; Mechulan, A.; Boulanger, C.M.; Caussin, C. Impact of left atrial appendage closure on circulating microvesicles levels: The MICROPLUG study. Int. J. Cardiol. 2020, 307, 24–30. [Google Scholar] [CrossRef]
- Shaihov-Teper, O.; Ram, E.; Ballan, N.; Brzezinski, R.Y.; Naftali-Shani, N.; Masoud, R.; Ziv, T.; Lewis, N.; Schary, Y.; Levin-Kotler, L.P.; et al. Extracellular Vesicles From Epicardial Fat Facilitate Atrial Fibrillation. Circulation 2021, 143, 2475–2493. [Google Scholar] [CrossRef]
- Choudhury, A.; Chung, I.; Blann, A.D.; Lip, G.Y.H. Elevated platelet microparticle levels in nonvalvular atrial fibrillation: Relationship to p-selectin and antithrombotic therapy. Chest 2007, 131, 809–815. [Google Scholar] [CrossRef]
- Ederhy, S.; Di Angelantonio, E.; Mallat, Z.; Hugel, B.; Janower, S.; Meuleman, C.; Boccara, F.; Freyssinet, J.M.; Tedgui, A.; Cohen, A. Levels of circulating procoagulant microparticles in nonvalvular atrial fibrillation. Am. J. Cardiol. 2007, 100, 989–994. [Google Scholar] [CrossRef]
- Azzam, H.; Zagloul, M. Elevated platelet microparticle levels in valvular atrial fibrillation. Hematology 2009, 14, 357–360. [Google Scholar] [CrossRef]
- Wang, H.; Yan, H.M.; Tang, M.X.; Wang, Z.H.; Zhong, M.; Zhang, Y.; Deng, J.T.; Zhang, W. Increased serum levels of microvesicles in nonvalvular atrial fibrillation determinated by ELISA using a specific monoclonal antibody AD-1. Clin. Chim. Acta 2010, 411, 1700–1704. [Google Scholar] [CrossRef]
- Hayashi, M.; Takeshita, K.; Inden, Y.; Ishii, H.; Cheng, X.W.; Yamamoto, K.; Murohara, T. Platelet activation and induction of tissue factor in acute and chronic atrial fibrillation: Involvement of mononuclear cell-platelet interaction. Thromb. Res. 2011, 128, e113–e118. [Google Scholar] [CrossRef]
- Idriss, N.K.; Blann, A.D.; Sayed, D.M.; Gaber, M.A.; Hassen, H.A.; Kishk, Y.T. Circulating Endothelial Cells and Platelet Microparticles in Mitral Valve Disease With and Without Atrial Fibrillation. Angiology 2015, 66, 631–637. [Google Scholar] [CrossRef]
- Wang, L.; Bi, Y.; Yu, M.; Li, T.; Tong, D.; Yang, X.; Zhang, C.; Guo, L.; Wang, C.; Kou, Y.; et al. Phosphatidylserine-exposing blood cells and microparticles induce procoagulant activity in non-valvular atrial fibrillation. Int. J. Cardiol. 2018, 258, 138–143. [Google Scholar] [CrossRef]
- Siwaponanan, P.; Keawvichit, R.; Udompunturak, S.; Hunnangkul, S.; Reesukumal, K.; Sukapirom, K.; Pattanapanyasat, K.; Krittayaphong, R. Altered profile of circulating microparticles in nonvalvular atrial fibrillation. Clin. Cardiol. 2019, 42, 425–431. [Google Scholar] [CrossRef]
- Thulin, Å.; Lindbäck, J.; Granger, C.B.; Wallentin, L.; Lind, L.; Siegbahn, A. Extracellular vesicles in atrial fibrillation and stroke. Thromb. Res. 2020, 193, 180–189. [Google Scholar] [CrossRef]
- Wang, H.; Song, N.P.; Li, J.P.; Wang, Z.H.; Ti, Y.; Li, Y.H.; Zhang, W.; Zhong, M. The microvesicle/CD36 complex triggers a prothrombotic phenotype in patients with non-valvular atrial fibrillation. J. Cell. Mol. Med. 2020, 24, 7331–7340. [Google Scholar] [CrossRef]
- Voukalis, C.; Lip, G.Y.H.; Shantsila, E. Effects of antithrombotic drugs on the prothrombotic state in patients with atrial fibrillation: The west Birmingham atrial fibrillation project. Thromb. Res. 2021, 200, 149–155. [Google Scholar] [CrossRef]
- Ni, H.; Pan, W.; Jin, Q.; Xie, Y.; Zhang, N.; Chen, K.; Lin, T.; Lin, C.; Xie, Y.; Wu, J.; et al. Label-free proteomic analysis of serum exosomes from paroxysmal atrial fibrillation patients. Clin. Proteom. 2021, 18, 1. [Google Scholar] [CrossRef]
- Zietzer, A.; Al-Kassou, B.; Jamme, P.; Rolfes, V.; Steffen, E.; Bulic, M.; Hosen, M.R.; Goody, P.R.; Tiyerili, V.; Zimmer, S.; et al. Large extracellular vesicles in the left atrial appendage in patients with atrial fibrillation-the missing link? Clin. Res. Cardiol. 2022, 111, 34–49. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).