Development of Injectable Calcium Sulfate and Self-Setting Calcium Phosphate Composite Bone Graft Materials for Minimally Invasive Surgery
Abstract
:1. Introduction
2. Results
2.1. The Synthesis of Calcium Sulfate Hemihydrate (CSH, CaSO4·0.5H2O)
2.2. Altering the Handling Property of CSH/CaP Composite Bone Materials by Citric Acid
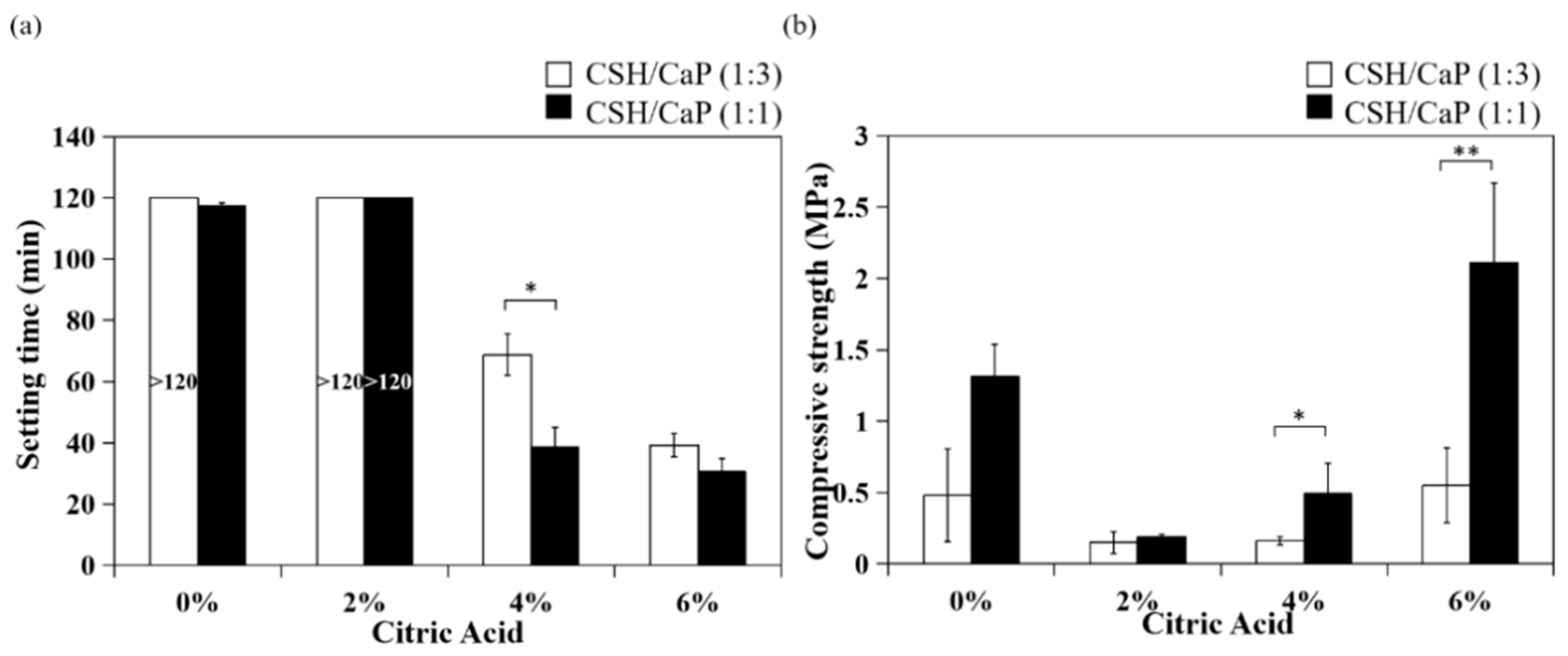
2.3. Effects of CSH/CaP Ratio on Mechanical Properties
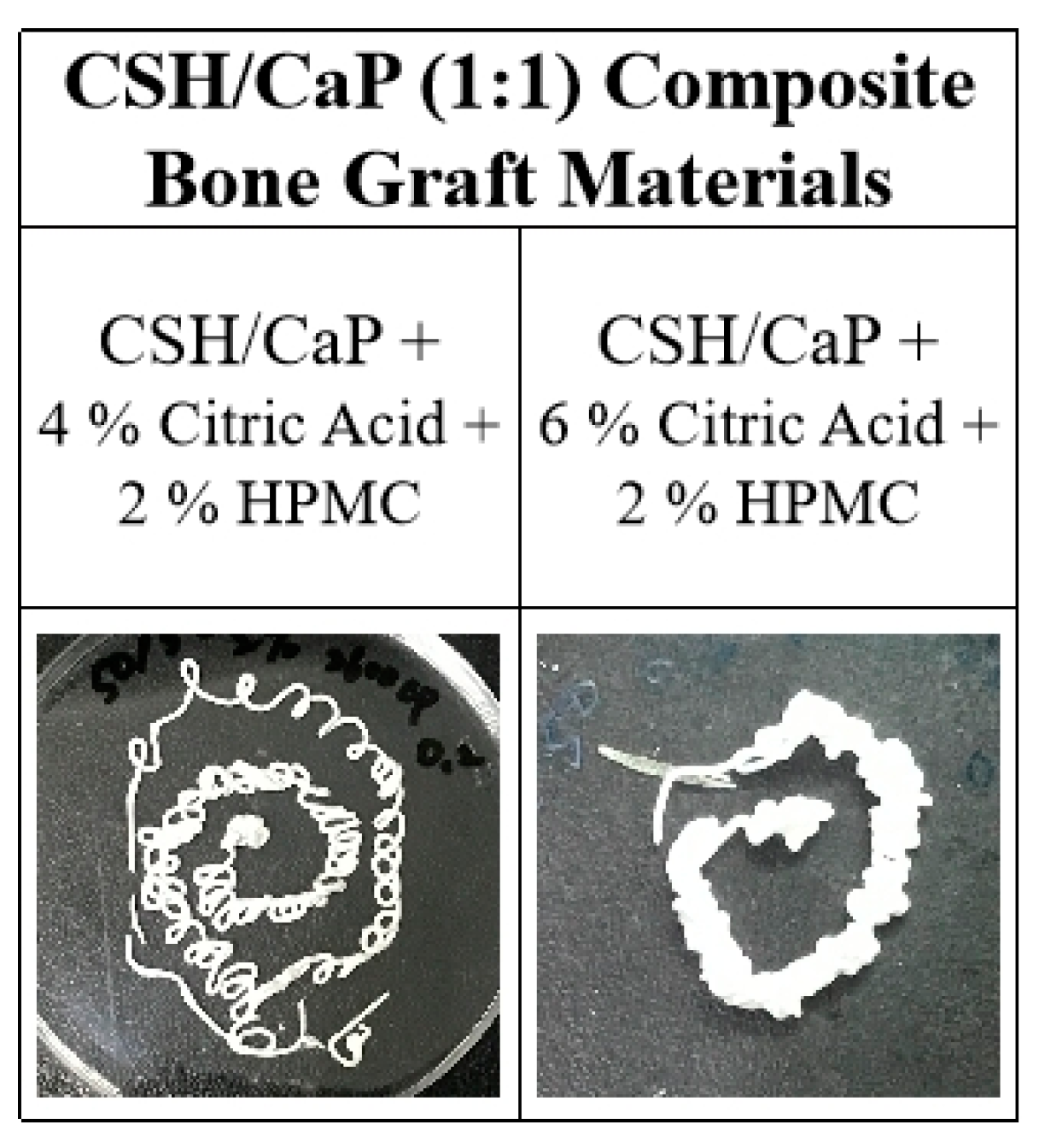
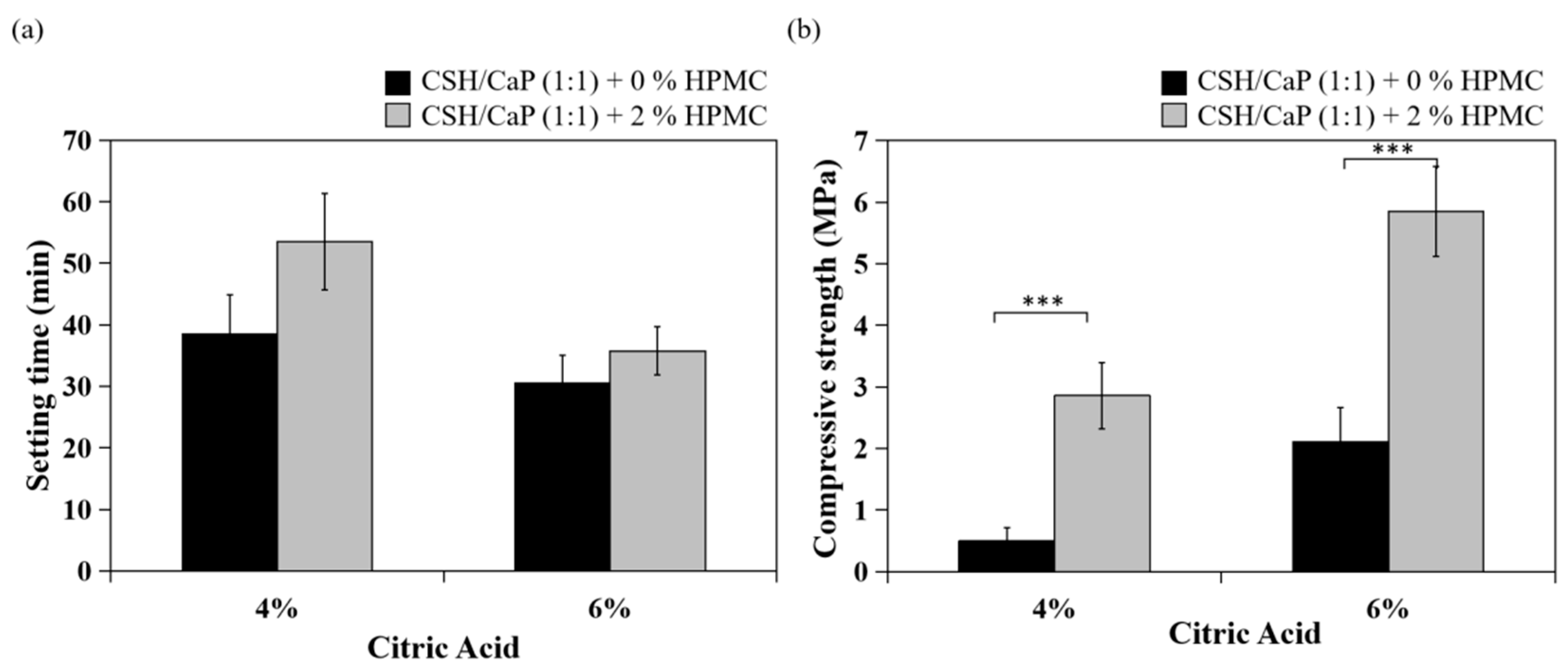
2.4. Effects of Hydroxyl Propyl methyl Cellulose (HPMC) on the Properties CSH/CaP Composite Bone Graft Materials
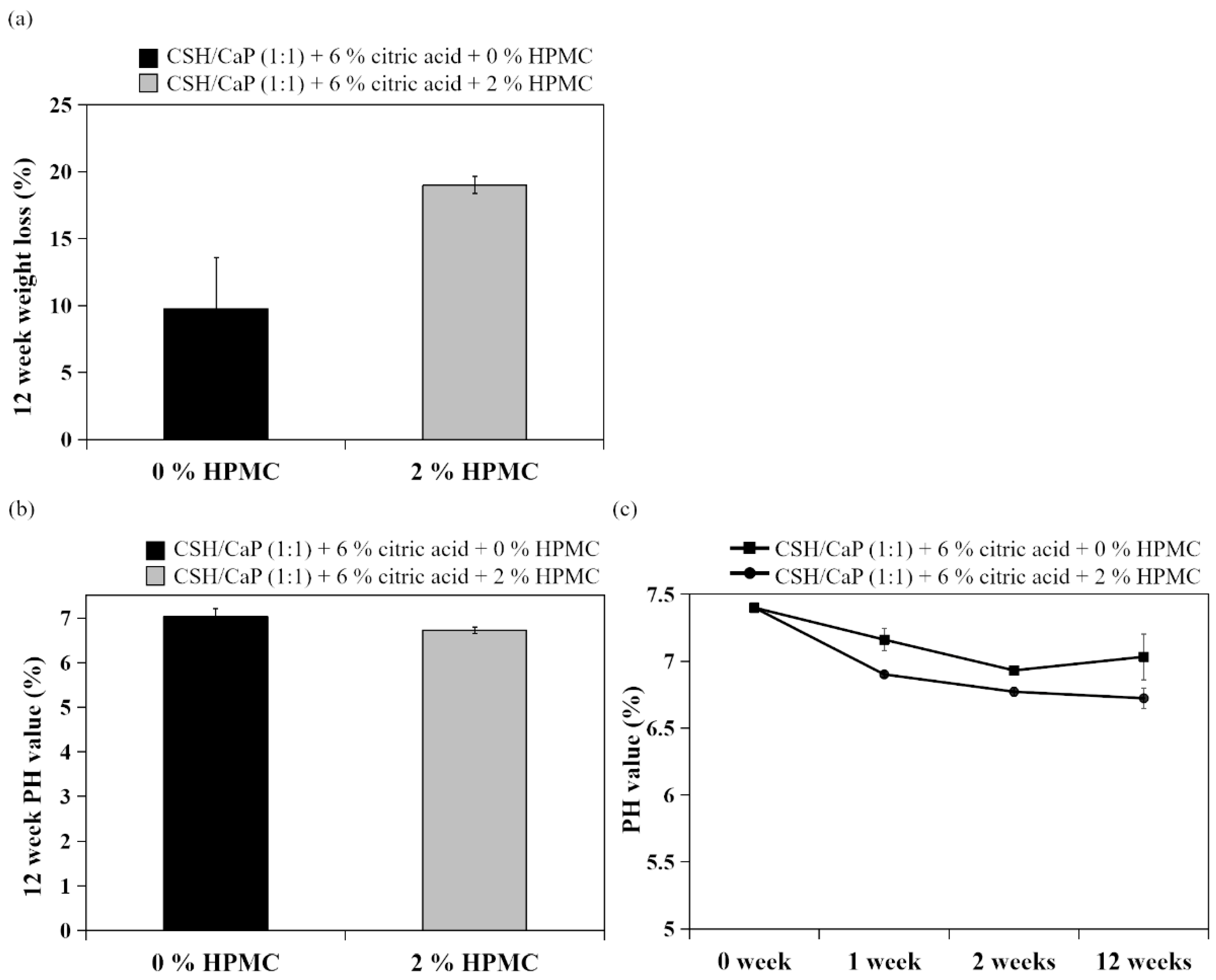
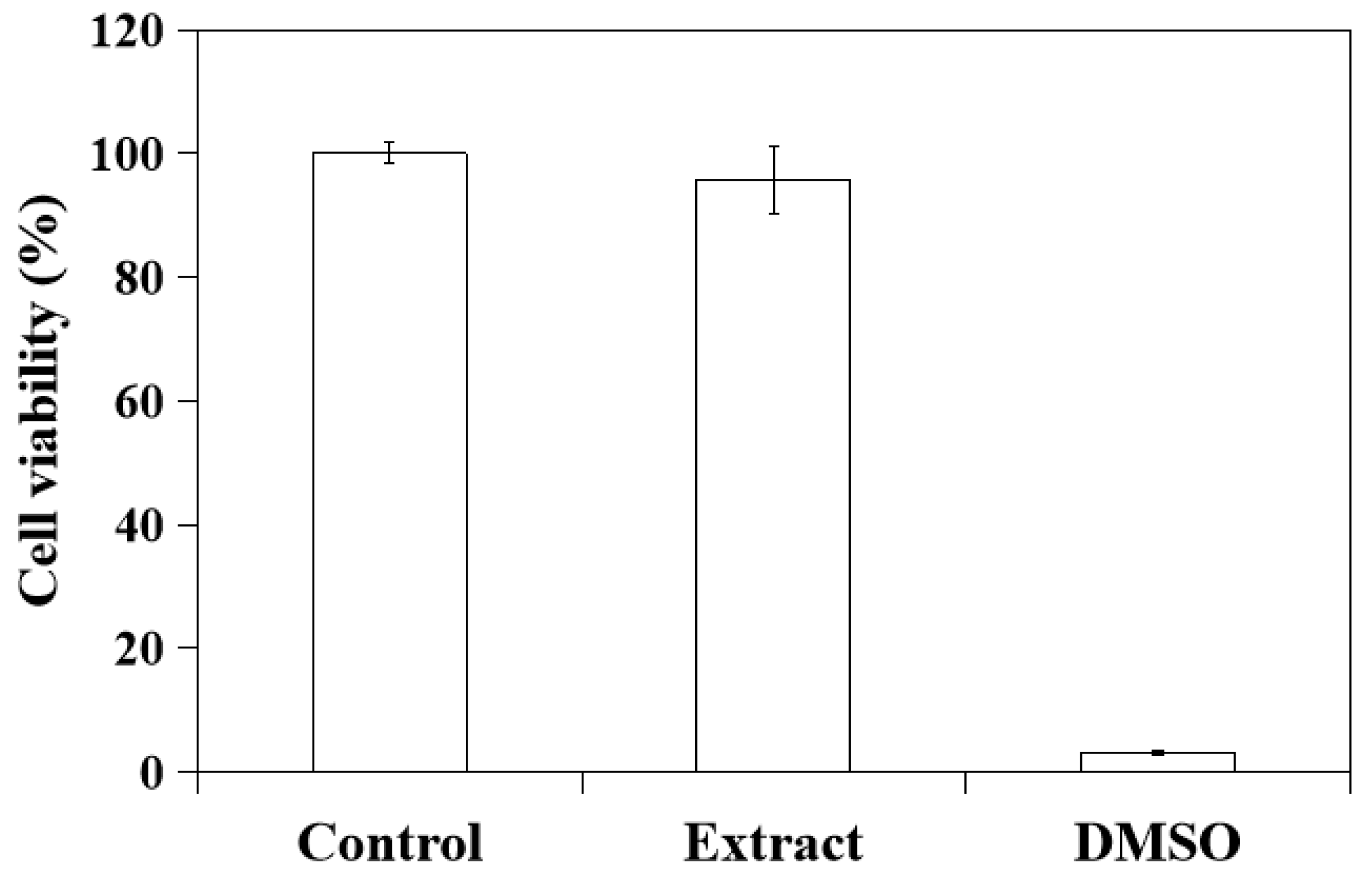
2.5. In Vitro Cytotoxicity of CSH/CaP Paste
3. Discussion
3.1. Effects of Citric Acid on Setting Time and Injectability
3.2. Effects of the Ratio of CSH and CaP
3.3. Effects of HPMC on Anti-Washout Ability and Mechanical Property
3.4. Possible Clinical Applications
4. Materials and Methods
4.1. Calcium Sulfate Hemihydrate (CSH) Preparation
4.2. Scanning Electron Microscope (SEM)
4.3. Fourier Transform Infrared Spectroscopy (FTIR)
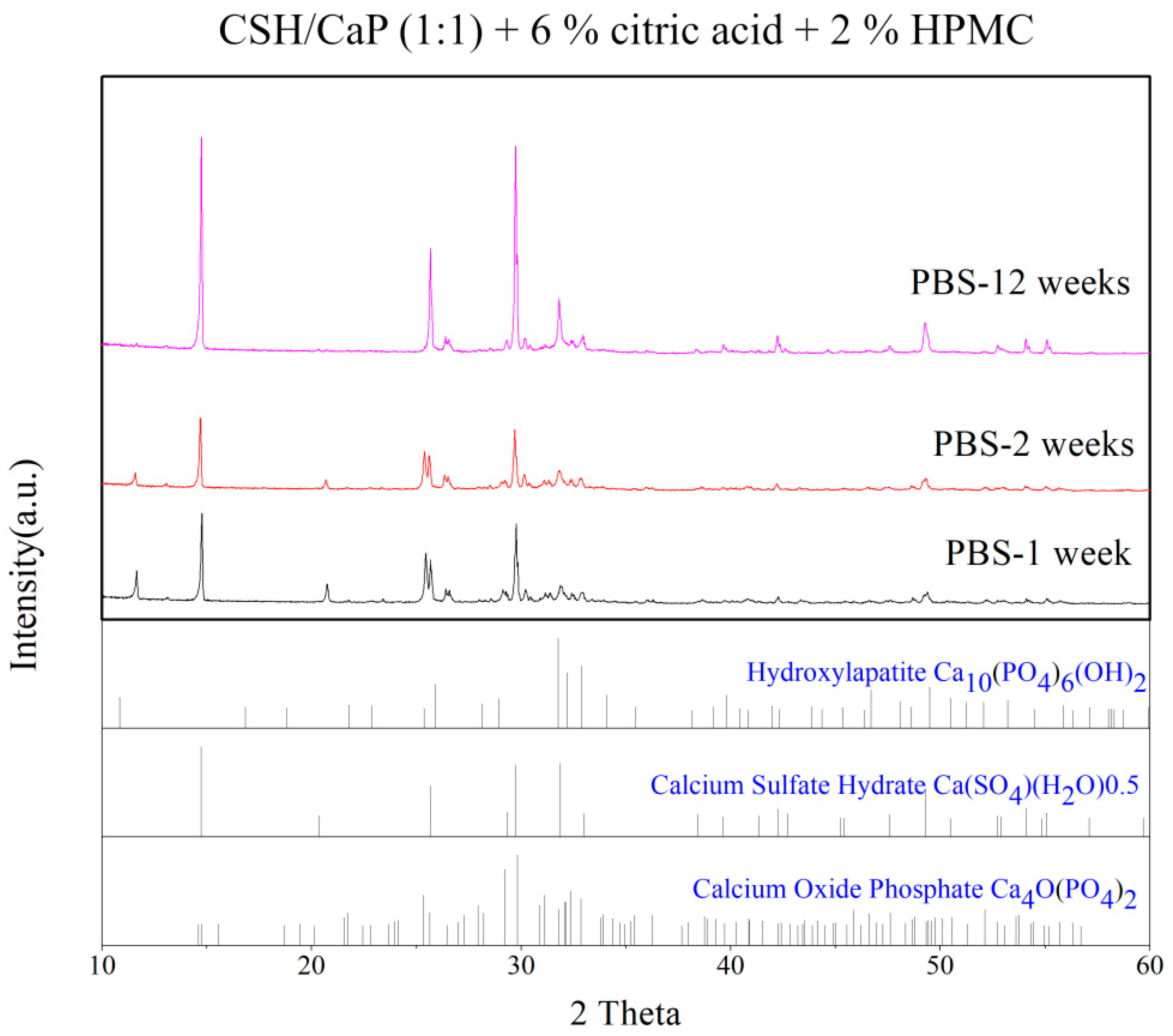
4.4. X-ray Diffraction (XRD)
4.5. CSH/CaP Composite Bone Graft Formulation and Paste Preparation
4.6. Setting Time Measurement
4.7. Injectability
4.8. Mechanical Compression Testing
4.9. Degradation Test and pH Value Measurement
4.10. Cell Viability
4.11. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Campana, V.; Milano, G.I.U.S.E.P.P.E.; Pagano, E.; Barba, M.; Cicione, C.; Salonna, G.; Logroscino, G. Bone substitutes in orthopaedic surgery: From basic science to clinical practice. J. Mater. Sci. Mater. Med. 2014, 25, 2445–2461. [Google Scholar] [CrossRef]
- Wang, W.; Yeung, K.W.K. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef] [PubMed]
- Haugen, H.J.; Lyngstadaas, S.P.; Rossi, F.; Perale, G. Bone grafts: Which is the ideal biomaterial? J. Clin. Periodontol. 2019, 46 (Suppl. 21), 92–102. [Google Scholar] [CrossRef]
- Barone, A.W.; Andreana, S.; Dziak, R. Current Use of Calcium Sulfate Bone Grafts. Med. Res. Arch. 2020, 8. [Google Scholar] [CrossRef]
- Evaniew, N.; Tan, V.; Parasu, N.; Jurriaans, E.; Finlay, K.; Deheshi, B.; Ghert, M. Use of a calcium sulfate-calcium phosphate synthetic bone graft composite in the surgical management of primary bone tumors. Orthopedics 2013, 36, e216–e222. [Google Scholar] [CrossRef] [Green Version]
- Guan, Q.; Sui, Y.; Zhang, F.; Yu, W.; Bo, Y.; Wang, P.; Jin, J. Preparation of α-calcium sulfate hemihydrate from industrial by-product gypsum: A review. Physicochem. Probl. Miner. Processing 2021, 57, 168–181. [Google Scholar] [CrossRef]
- Laurencin, C.; Khan, Y.; El-Amin, S.F. Bone graft substitutes. Expert Rev. Med. Devices 2006, 3, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Roberts, T.T.; Rosenbaum, A.J. Bone grafts, bone substitutes and orthobiologics. Organogenesis 2012, 8, 114–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, M.V.; Puleo, D.A. Calcium sulfate: Properties and clinical applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 88B, 597–610. [Google Scholar] [CrossRef]
- Jeong, J.; Kim, J.H.; Shim, J.H.; Hwang, N.S.; Heo, C.Y. Bioactive calcium phosphate materials and applications in bone regeneration. Biomater. Res. 2019, 23, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burguera, E.F.; Xu, H.H.; Weir, M.D. Injectable and rapid-setting calcium phosphate bone cement with dicalcium phosphate dihydrate. J. Biomed. Mater. Res. Part B Appl. Biomater. 2006, 77, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.; Hannink, G. Injectable bone-graft substitutes: Current products, their characteristics and indications, and new developments. Injury 2011, 42, S30–S34. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, H.; Liu, X.; Lian, X.; Guo, Z.; Jiang, H.J.; Cui, F.Z. Improved workability of injectable calcium sulfate bone cement by regulation of self-setting properties. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 1048–1053. [Google Scholar] [CrossRef]
- Coetzee, A.S. Regeneration of bone in the presence of calcium sulfate. Arch. Otolaryngol. 1980, 106, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.-J.; Waris, R.A.; Ruslin, M.; Lin, Y.-H.; Chen, C.-S.; Ou, K.-L. An innovative α-calcium sulfate hemihydrate bioceramic as a potential bone graft substitute. J. Am. Ceram. Soc. 2018, 101, 419–427. [Google Scholar] [CrossRef]
- Lewis, K.N.; Thomas, M.V.; Puleo, D.A. Mechanical and degradation behavior of polymer-calcium sulfate composites. J. Mater. Sci. Mater. Med. 2006, 17, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.C.; Su, C.Y.; Lai, C.C.; Tsou, Y.S.; Zheng, Y.; Fang, H.W. Preparation and Characterization of Moldable Demineralized Bone Matrix/Calcium Sulfate Composite Bone Graft Materials. J. Funct. Biomater. 2021, 12, 56. [Google Scholar] [CrossRef] [PubMed]
- Chiang, H.-H.; Su, C.-Y.; Hsu, L.-H.; Yang, M.-H.; Fang, H.-W. Improved Anti-Washout Property of Calcium Sulfate/Tri-Calcium Phosphate Premixed Bone Substitute with Glycerin and Hydroxypropyl Methylcellulose. Appl. Sci. 2021, 11, 8136. [Google Scholar] [CrossRef]
- Wang, Y.-W.; Meldrum, F.C. Additives stabilize calcium sulfate hemihydrate (bassanite) in solution. J. Mater. Chem. 2012, 22, 22055–22062. [Google Scholar] [CrossRef]
- Moseke, C.; Gbureck, U. Tetracalcium phosphate: Synthesis, properties and biomedical applications. Acta Biomater. 2010, 6, 3815–3823. [Google Scholar] [CrossRef]
- Sarda, S.; Fernández, E.; Nilsson, M.; Balcells, M.; Planell, J.A. Kinetic study of citric acid influence on calcium phosphate bone cements as water-reducing agent. J. Biomed. Mater. Res. 2002, 61, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, W.; Schnitzler, V.; Tancret, F.; Bouler, J.M. Calcium phosphate cements for bone substitution: Chemistry, handling and mechanical properties. Acta Biomater. 2014, 10, 1035–1049. [Google Scholar] [CrossRef] [PubMed]
- Blouin, S.; Moreau, M.F.; Weiss, P.; Daculsi, G.; Baslé, M.F.; Chappard, D. Evaluation of an injectable bone substitute (betaTCP/hydroxyapatite/hydroxy-propyl-methyl-cellulose) in severely osteopenic and aged rats. J. Biomed. Mater. Res. Part A 2006, 78, 570–580. [Google Scholar] [CrossRef] [PubMed]
- Barralet, J.E.; Hofmann, M.; Grover, L.M.; Gbureck, U.J.A.M. High-strength apatitic cement by modification with α-hydroxy acid salts. Adv. Mater. 2003, 15, 2091–2094. [Google Scholar] [CrossRef]
- Thai, V.V.; Lee, B.T. Fabrication of calcium phosphate-calcium sulfate injectable bone substitute using hydroxy-propyl-methyl-cellulose and citric acid. J. Mater. Sci. Mater. Med. 2010, 21, 1867–1874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.; Zhang, J.; Rethore, G.; Khairoun, K.; Pilet, P.; Tancret, F.; Weiss, P. A novel injectable, cohesive and toughened Si-HPMC (silanized-hydroxypropyl methylcellulose) composite calcium phosphate cement for bone substitution. Acta Biomater. 2014, 10, 3335–3345. [Google Scholar] [CrossRef] [PubMed]
- Schlickewei, C.W.; Laaff, G.; Andresen, A.; Klatte, T.O.; Rueger, J.M.; Ruesing, J.; Lehmann, W. Bone augmentation using a new injectable bone graft substitute by combining calcium phosphate and bisphosphonate as composite—An animal model. J. Orthop. Surg. Res. 2015, 10, 116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boyle, K.K.; Sosa, B.; Osagie, L.; Turajane, K.; Bostrom, M.P.G.; Yang, X. Vancomycin-laden calcium phosphate-calcium sulfate composite allows bone formation in a rat infection model. PLoS ONE 2019, 14, e0222034. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Wu, Z.; Wan, Y.; Yu, T.; Zhou, C. Manipulation of the degradation behavior of calcium phosphate and calcium sulfate bone cement system by the addition of micro-nano calcium phosphate. Ceram. Int. 2021, 47, 29213–29224. [Google Scholar] [CrossRef]
- Smirnov, V.V.; Antonova, O.S.; Goldberg, M.A.; Smirnov, S.V.; Shvorneva, L.I.; Egorov, A.A.; Barinov, S.M. Bone cements in the calcium phosphate–calcium sulfate system. Dokl. Chem. 2016, 467, 136–139. [Google Scholar] [CrossRef]
- Ishikawa, K.; Miyamoto, Y.; Kon, M.; Nagayama, M.; Asaoka, K. Non-decay type fast-setting calcium phosphate cement: Composite with sodium alginate. Biomaterials 1995, 16, 527–532. [Google Scholar] [CrossRef]
- Alkhraisat, M.H.; Rueda, C.; Marino, F.T.; Torres, J.; Jerez, L.B.; Gbureck, U.; Cabarcos, E.L. The effect of hyaluronic acid on brushite cement cohesion. Acta Biomater. 2009, 5, 3150–3156. [Google Scholar] [CrossRef] [PubMed]
- Cherng, A.; Takagi, S.; Chow, L.C. Effects of hydroxypropyl methylcellulose and other gelling agents on the handling properties of calcium phosphate cement. J. Biomed. Mater. Res. 1997, 35, 273–277. [Google Scholar] [CrossRef]
- Liu, H.; Li, H.; Cheng, W.; Yang, Y.; Zhu, M.; Zhou, C. Novel injectable calcium phosphate/chitosan composites for bone substitute materials. Acta Biomater. 2006, 2, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhang, J.; Weiss, P.; Tancret, F.; Bouler, J.-M. The influence of different cellulose ethers on both the handling and mechanical properties of calcium phosphate cements for bone substitution. Acta Biomater. 2013, 9, 5740–5750. [Google Scholar] [CrossRef]
- Wang, X.; Chen, L.; Xiang, H.; Ye, J. Influence of anti-washout agents on the rheological properties and injectability of a calcium phosphate cement. J. Biomed. Mater. Res. Part B Appl. Biomater. 2007, 81B, 410–418. [Google Scholar] [CrossRef]
- Hong, H.R.; Jang, Y.J. Correlation between remnant inferior turbinate volume and symptom severity of empty nose syndrome. Laryngoscope 2016, 126, 1290–1295. [Google Scholar] [CrossRef]
- Bergmark, R.W.; Gray, S.T. Surgical Management of Turbinate Hypertrophy. Otolaryngol. Clin. N. Am. 2018, 51, 919–928. [Google Scholar] [CrossRef]
- Passali, D.; La Rosa, R.; Passali, G.C.; Ciprandi, G. The Empty Nose Syndrome: A pragmatic classification in clinical practice. Acta Bio-Med. Atenei Parm. 2021, 92, e2021288. [Google Scholar]
- Leong, S.C. The clinical efficacy of surgical interventions for empty nose syndrome: A systematic review. Laryngoscope 2015, 125, 1557–1562. [Google Scholar] [CrossRef]
- Lamb, M.; Bacon, D.R.; Zeatoun, A.; Onourah, P.; Thorp, B.D.; Abramowitz, J.; Senior, B.A. Mental health burden of empty nose syndrome compared to chronic rhinosinusitis and chronic rhinitis. Int. Forum Allergy Rhinol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.J.; Fu, C.H.; Wu, C.L.; Tam, Y.Y.; Huang, C.C.; Chang, P.H.; Wu, M.H. Evaluation of depression and anxiety in empty nose syndrome after surgical treatment. Laryngoscope 2016, 126, 1284–1289. [Google Scholar] [CrossRef] [PubMed]
- Kuan, E.C.; Suh, J.D.; Wang, M.B. Empty nose syndrome. Curr. Allergy Asthma Rep. 2015, 15, 493. [Google Scholar] [CrossRef] [PubMed]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009.
- ISO 10993-12:2021; Biological Evaluation of Medical Devices—Part 12: Sample Preparation and Reference Materials. International Organization for Standardization: Geneva, Switzerland, 2021.
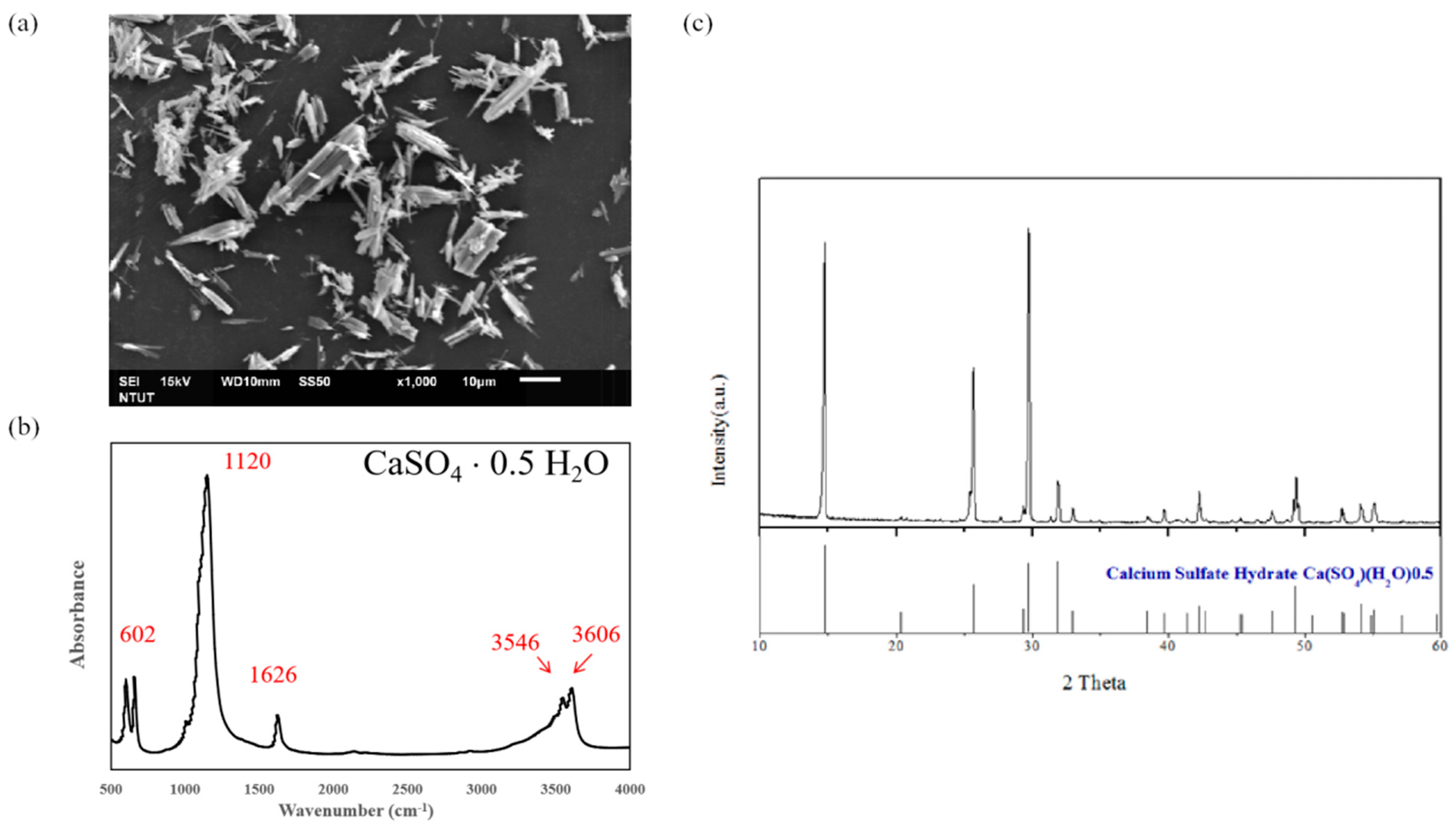
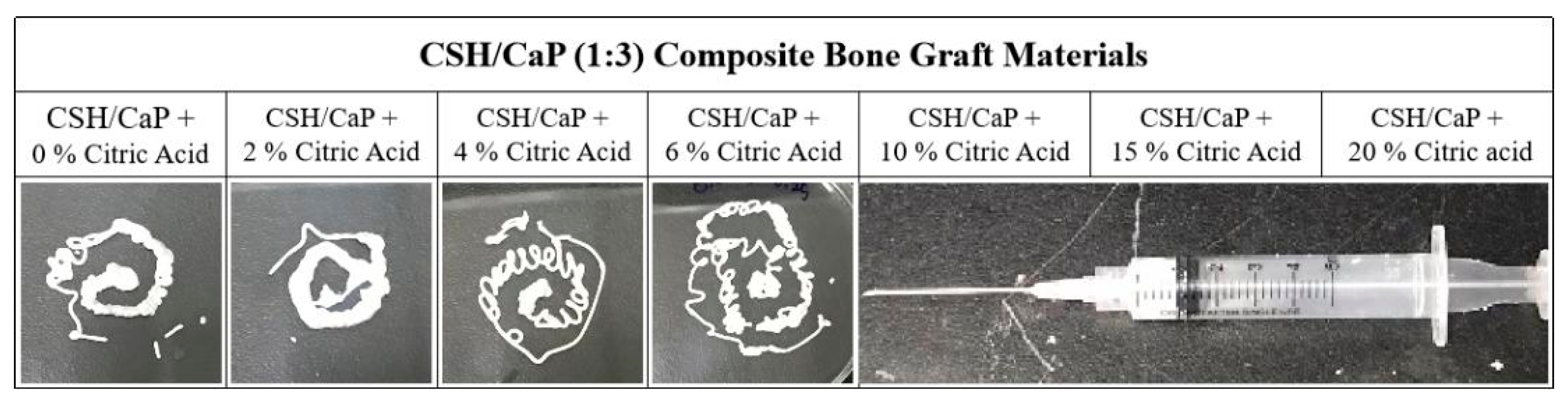
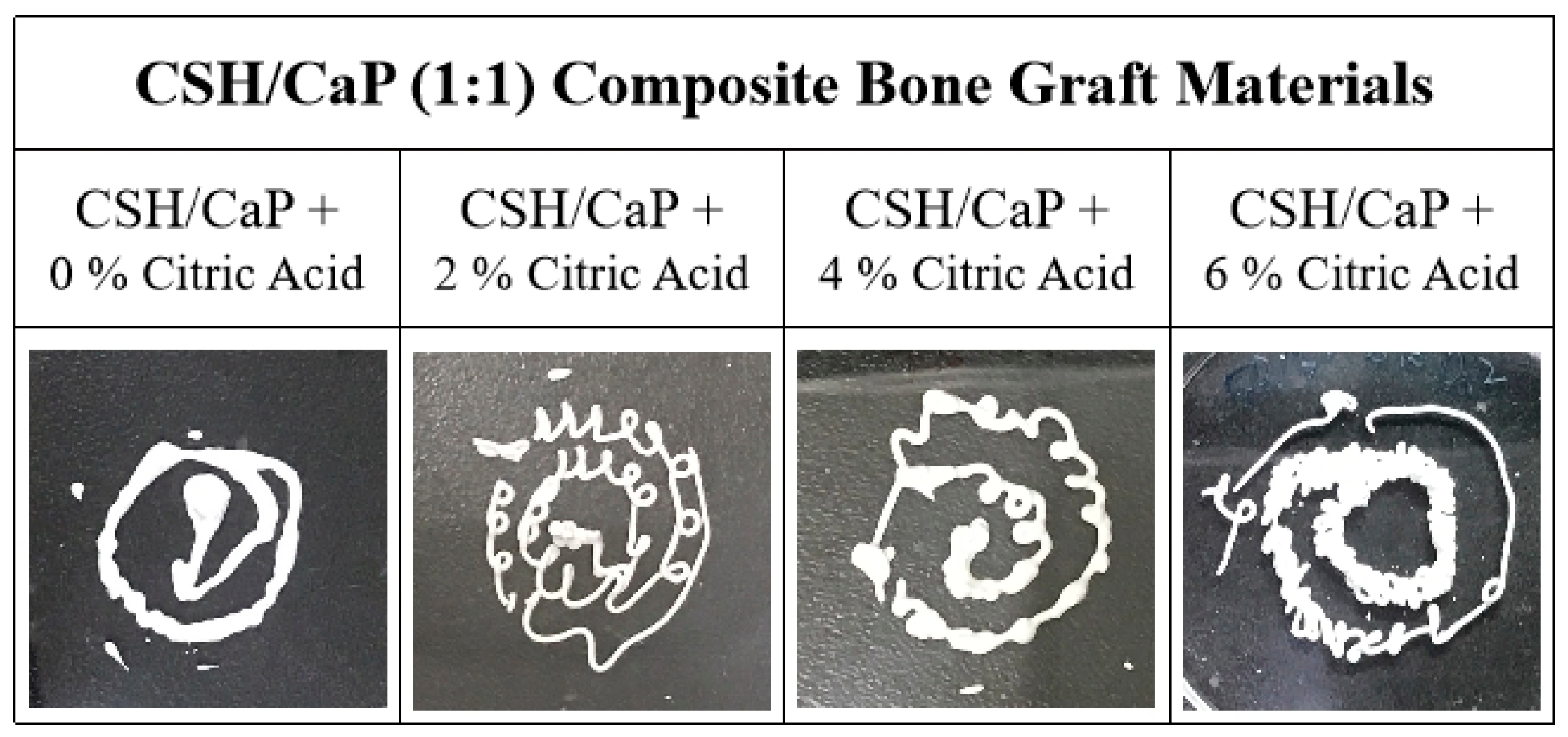
| CSH/CaP (1:3) Composite Bone Graft Materials | |||||||
|---|---|---|---|---|---|---|---|
| Powder component | CSH/CaP + 0% Citric Acid | CSH/CaP + 2% Citric Acid | CSH/CaP + 4% Citric Acid | CSH/CaP + 6% Citric Acid | CSH/CaP + 10% Citric Acid | CSH/CaP + 15% Citric Acid | CSH/CaP + 20% Citric acid |
| L/P | 0.2 | 0.2 | 0.2 | 0.25 | 0.25 | 0.3 | 0.4 |
| Working time | 97 min | 64 min | 16 min | 15 min | 15 min | 10 min | 8 min |
| Setting time | >120 min | >120 min | 68 min | 39 min | >60 min | 31 min | 40 min |
| Injectability | Yes | Yes | Yes | Yes | No | No | No |
| CSH/CaP (1:1) Composite Bone Graft Materials | ||||
|---|---|---|---|---|
| Powder component | CSH/CaP + 0% Citric Acid | CSH/CaP + 2% Citric Acid | CSH/CaP + 4% Citric Acid | CSH/CaP + 6% Citric Acid |
| L/P | 0.2 | 0.2 | 0.2 | 0.2 |
| Working time | 61 min | 50 min | 23 min | 15 min |
| Setting time | 117 min | >120 min | 39 min | 31 min |
| Injectability | Yes | Yes | Yes | Yes |
| CSH/CaP (1:1) Composite Bone Graft Materials | ||
|---|---|---|
| Powder component | CSH/CaP (1:1) + 4% Citric Acid + 2% HPMC | CSH/CaP (1:1) + 6% Citric Acid + 2% HPMC |
| L/P | 0.2 | 0.25 |
| Working time | 11 min | 10 min |
| Setting time | 54 min | 36 min |
| Injectability | Yes | Yes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiu, Y.-H.; Chen, I.-C.; Su, C.-Y.; Tsai, H.-H.; Young, T.-H.; Fang, H.-W. Development of Injectable Calcium Sulfate and Self-Setting Calcium Phosphate Composite Bone Graft Materials for Minimally Invasive Surgery. Int. J. Mol. Sci. 2022, 23, 7590. https://doi.org/10.3390/ijms23147590
Chiu Y-H, Chen I-C, Su C-Y, Tsai H-H, Young T-H, Fang H-W. Development of Injectable Calcium Sulfate and Self-Setting Calcium Phosphate Composite Bone Graft Materials for Minimally Invasive Surgery. International Journal of Molecular Sciences. 2022; 23(14):7590. https://doi.org/10.3390/ijms23147590
Chicago/Turabian StyleChiu, Yu-Hsun, I-Cheng Chen, Chen-Ying Su, Hsin-Hua Tsai, Tai-Horng Young, and Hsu-Wei Fang. 2022. "Development of Injectable Calcium Sulfate and Self-Setting Calcium Phosphate Composite Bone Graft Materials for Minimally Invasive Surgery" International Journal of Molecular Sciences 23, no. 14: 7590. https://doi.org/10.3390/ijms23147590
APA StyleChiu, Y.-H., Chen, I.-C., Su, C.-Y., Tsai, H.-H., Young, T.-H., & Fang, H.-W. (2022). Development of Injectable Calcium Sulfate and Self-Setting Calcium Phosphate Composite Bone Graft Materials for Minimally Invasive Surgery. International Journal of Molecular Sciences, 23(14), 7590. https://doi.org/10.3390/ijms23147590







