Genetic Background Matters: Population-Based Studies in Model Organisms for Translational Research
Abstract
1. Precision Medicine in Humans
2. Rodents as Model Organisms in Genetic Research: Advantages and Limitations
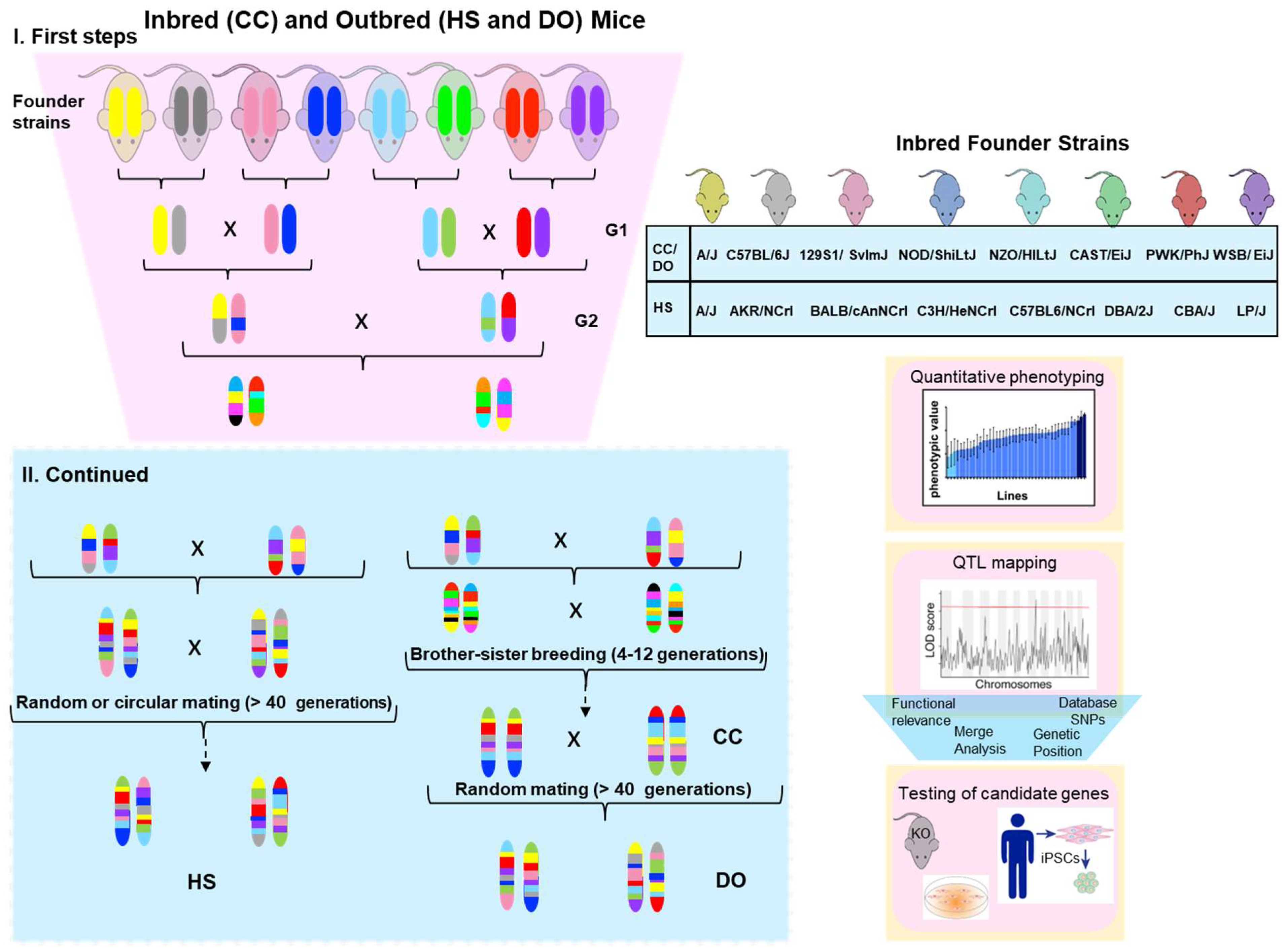
2.1. Hybrid Mouse Diversity Panel
2.2. The Collaborative Cross (CC) Panel
2.3. Heterogeneous Stock and Diversity Outbred Populations
3. Drosophila melanogaster as a Model Organism in Genetic Research: Advantages and Limitations
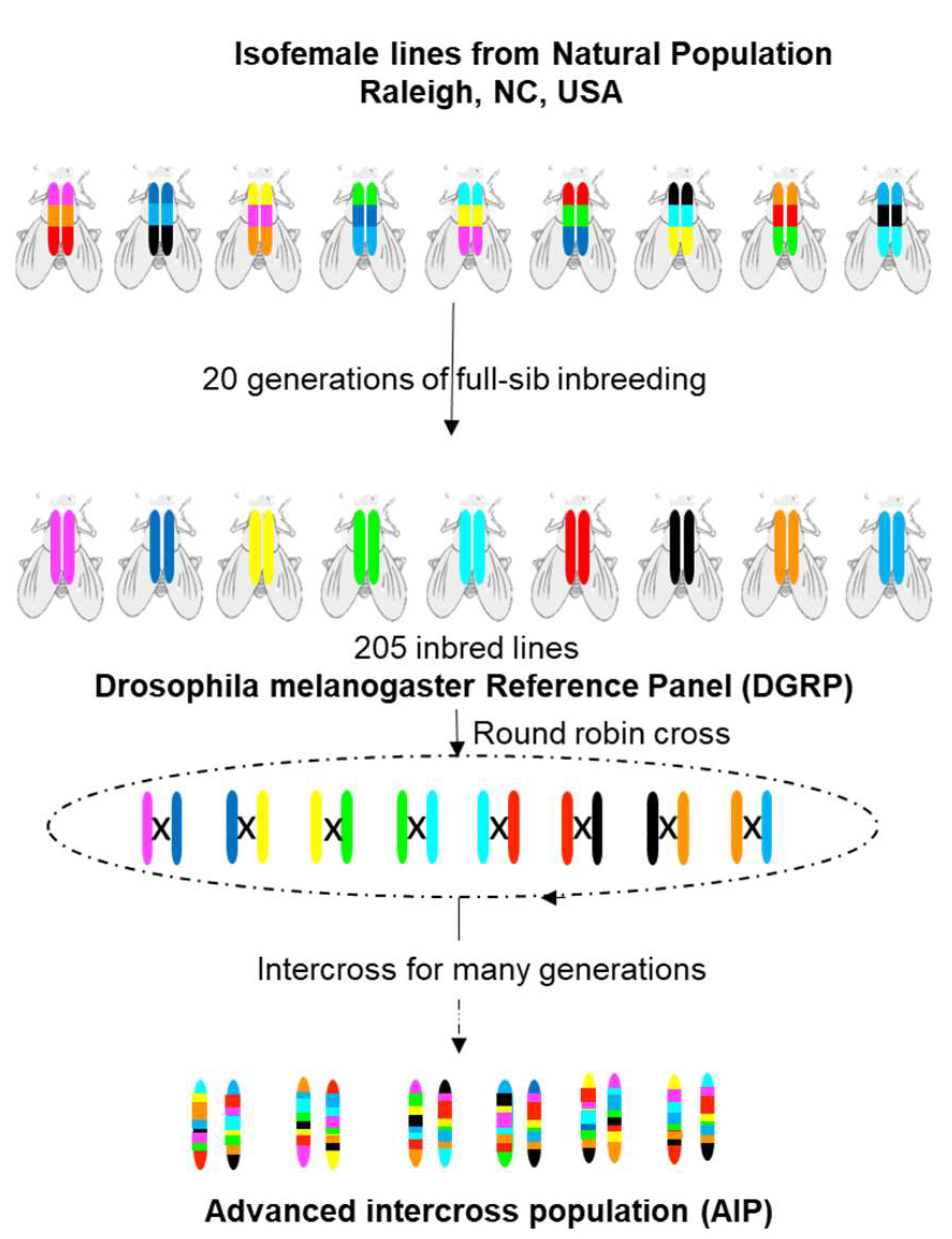
3.1. Drosophila melanogaster Genetic Reference Panel (DGRP)
3.2. DGRP for Mapping Physiological and Pathophysiological Traits
3.3. Lines Derived from DGRP and DSRP
4. Saccharomyces cerevisiae as a Model Organism in Genetic Research: Advantages and Limitations
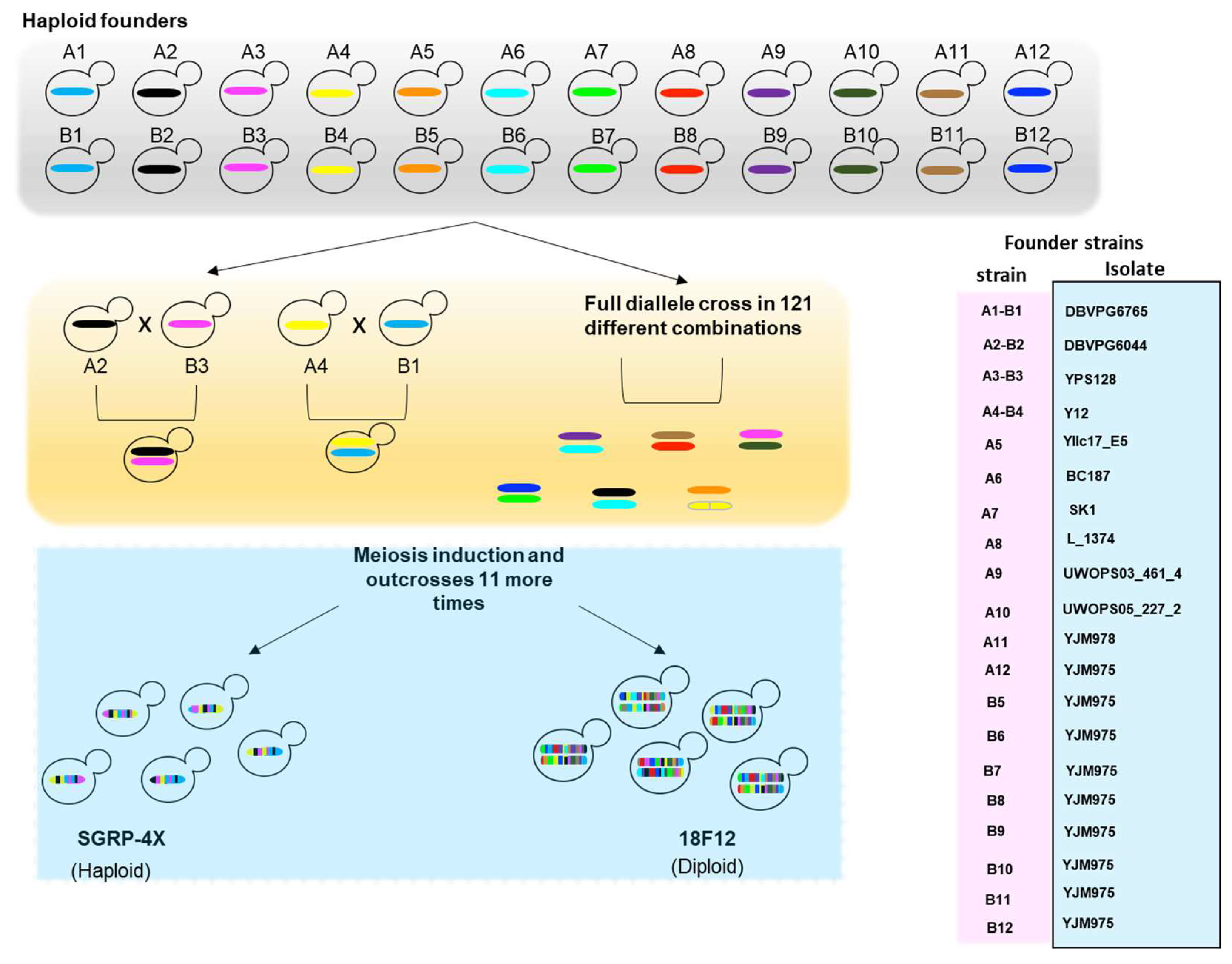
4.1. Analysis of Segregating Populations from Pairwise Crosses
4.2. Multi-Parent Populations (MPPs)
4.3. Genome-Wide Association Studies (GWAS) in S. cerevisiae
5. Practical Considerations and Concluding Remarks
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Ashley, E.A. Towards precision medicine. Nat. Rev. Genet. 2016, 17, 507–522. [Google Scholar] [CrossRef] [PubMed]
- Rahit, K.M.T.H.; Tarailo-Graovac, M. Genetic Modifiers and Rare Mendelian Disease. Genes 2020, 11, 239. [Google Scholar] [CrossRef] [PubMed]
- Canales, C.P.; Walz, K. The Mouse, a Model Organism for Biomedical Research. In Cellular and Animal Models in Human Genomics Research; Academic Press: Cambridge, MA, USA, 2019; pp. 119–140. [Google Scholar] [CrossRef]
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A Programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 2012, 337, 816–821. [Google Scholar] [CrossRef] [PubMed]
- Platt, R.J.; Chen, S.; Zhou, Y.; Yim, M.J.; Swiech, L.; Kempton, H.R.; Dahlman, J.E.; Parnas, O.; Eisenhaure, T.M.; Jovanovic, M.; et al. CRISPR-Cas9 Knockin Mice for Genome Editing and Cancer Modeling. Cell 2014, 159, 440–455. [Google Scholar] [CrossRef] [PubMed]
- Swiech, L.; Heidenreich, M.; Banerjee, A.; Habib, N.; Li, Y.; Trombetta, J.J.; Sur, M.; Zhang, F. In vivo interrogation of gene function in the mammalian brain using CRISPR-Cas9. Nat. Biotechnol. 2014, 33, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Calderón, J.F.; Klein, A.D. Controversies on the potential therapeutic use of rapamycin for treating a lysosomal cholesterol storage disease. Mol. Genet. Metab. Rep. 2018, 15, 135–136. [Google Scholar] [CrossRef]
- Durán, A.; Rebolledo-Jaramillo, B.; Olguin, V.; Rojas-Herrera, M.; Heras, M.L.; Calderón, J.F.; Zanlungo, S.; Priestman, D.A.; Platt, F.M.; Klein, A.D. Identification of genetic modifiers of murine hepatic β-glucocerebrosidase activity. Biochem. Biophys. Rep. 2021, 28, 101105. [Google Scholar] [CrossRef]
- Parra, J.; Klein, A.D.; Castro, J.; Morales, M.G.; Mosqueira, M.; Valencia, I.; Cortés, V.; Rigotti, A.; Zanlungo, S. Npc1 deficiency in the C57BL/6J genetic background enhances Niemann–Pick disease type C spleen pathology. Biochem. Biophys. Res. Commun. 2011, 413, 400–406. [Google Scholar] [CrossRef]
- Klein, A.D.; Ferreira, N.-S.; Ben-Dor, S.; Duan, J.; Hardy, J.; Cox, T.M.; Merril, A.H., Jr.; Futerman, A.H. Identification of Modifier Genes in a Mouse Model of Gaucher Disease. Cell Rep. 2016, 16, 2546–2553. [Google Scholar] [CrossRef]
- Rodriguez-Gil, J.L.; Watkins-Chow, D.E.; Baxter, L.L.; Elliot, G.; Harper, U.L.; Wincovitch, S.M.; Wedel, J.C.; Incao, A.A.; Huebecker, M.; Boehm, F.J.; et al. Genetic background modifies phenotypic severity and longevity in a mouse model of Niemann-Pick disease type C1. Dis. Model. Mech. 2020, 13, dmm042614. [Google Scholar] [CrossRef]
- Klein, A.D. Modeling diseases in multiple mouse strains for precision medicine studies. Physiol. Genom. 2017, 49, 177–179. [Google Scholar] [CrossRef] [PubMed]
- Seok, J.; Warren, H.S.; Alex, G.C.; Michael, N.M.; Henry, V.B.; Xu, W.; Richards, D.R.; McDonald-Smith, G.P.; Gao, H.; Hennessy, L.; et al. Genomic Responses in Mouse Models Poorly Mimic Human Inflammatory Diseases. Proc. Natl. Acad. Sci. USA 2013, 110, 3507–3512. [Google Scholar] [CrossRef] [PubMed]
- Bennett, B.J.; Farber, C.R.; Orozco, L.; Kang, H.M.; Ghazalpour, A.; Siemers, N.; Neubauer, M.; Neuhaus, I.; Yordanova, R.; Guan, B.; et al. A high-resolution association mapping panel for the dissection of complex traits in mice. Genome Res. 2010, 20, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Lusis, A.J.; Seldin, M.M.; Allayee, H.; Bennett, B.J.; Civelek, M.; Davis, R.C.; Eskin, E.; Farber, C.; Hui, S.; Mehrabian, M.; et al. The Hybrid Mouse Diversity Panel: A resource for systems genetics analyses of metabolic and cardiovascular traits. J. Lipid Res. 2016, 57, 925–942. [Google Scholar] [CrossRef] [PubMed]
- Ghazalpour, A.; Rau, C.D.; Farber, C.R.; Bennett, B.J.; Orozco, L.D.; Van Nas, A.; Pan, C.; Allayee, H.; Beaven, S.W.; Civelek, M.; et al. Hybrid mouse diversity panel: A panel of inbred mouse strains suitable for analysis of complex genetic traits. Mamm. Genome 2012, 23, 680–692. [Google Scholar] [CrossRef] [PubMed]
- Petkov, P.; Graber, J.; Churchill, G.A.; DiPetrillo, K.; King, B.; Paigen, K. Evidence of a Large-Scale Functional Organization of Mammalian Chromosomes. PLoS Genet. 2005, 1, e33. [Google Scholar] [CrossRef]
- Flint, J.; Mott, R. Applying mouse complex-trait resources to behavioural genetics. Nature 2008, 456, 724–727. [Google Scholar] [CrossRef]
- Kang, E.Y.; Han, B.; Furlotte, N.; Joo, J.W.J.; Shih, D.; Davis, R.C.; Lusis, A.J.; Eskin, E. Meta-Analysis Identifies Gene-by-Environment Interactions as Demonstrated in a Study of 4,965 Mice. PLoS Genet. 2014, 10, e1004022. [Google Scholar] [CrossRef]
- Neuner, S.M.; Heuer, S.E.; Huentelman, M.J.; O’Connell, K.M.S.; Kaczorowski, C.C. Harnessing Genetic Complexity to Enhance Translatability of Alzheimer’s Disease Mouse Models: A Path toward Precision Medicine. Neuron 2019, 101, 399–411. [Google Scholar] [CrossRef]
- Srivastava, A.; Morgan, A.P.; Najarian, M.L.; Sarsani, V.K.; Sigmon, J.S.; Shorter, J.R.; Kashfeen, A.; McMullan, R.C.; Williams, L.H.; Giusti-Rodríguez, P.; et al. Genomes of the Mouse Collaborative Cross. Genetics 2017, 206, 537–556. [Google Scholar] [CrossRef]
- Yang, H.; Wang, J.R.; Didion, J.; Buus, R.J.; Bell, T.A.; Welsh, C.E.; Bonhomme, F.; Yu, A.H.-T.; Nachman, M.W.; Piálek, J.; et al. Subspecific origin and haplotype diversity in the laboratory mouse. Nat. Genet. 2011, 43, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Aylor, D.L.; Valdar, W.; Foulds-Mathes, W.; Buus, R.J.; Verdugo, R.A.; Baric, R.S.; Ferris, M.T.; Frelinger, J.A.; Heise, M.; Frieman, M.B.; et al. Genetic analysis of complex traits in the emerging Collaborative Cross. Genome Res. 2011, 21, 1213–1222. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.; de Villena, F.P.-M.; Wang, W.; McMillan, L.; Threadgill, D.W. The polymorphism architecture of mouse genetic resources elucidated using genome-wide resequencing data: Implications for QTL discovery and systems genetics. Mamm. Genome 2007, 18, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Saul, M.C.; Philip, V.M.; Reinholdt, L.G.; Chesler, E.J. High-Diversity Mouse Populations for Complex Traits. Trends Genet. 2019, 35, 501–514. [Google Scholar] [CrossRef] [PubMed]
- Keele, G.; Zhang, T.; Pham, D.; Vincent, M.; Genomics, T.B.-C. Undefined Regulation of Protein Abundance in Genetically Diverse Mouse Populations; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Ram, R.; Morahan, G. Complex Trait Analyses of the Collaborative Cross: Tools and Databases. Syst. Genet. 2016, 1488, 121–129. [Google Scholar] [CrossRef]
- Molenhuis, R.T.; Bruining, H.; Brandt, M.J.V.; Van Soldt, P.E.; Atamni, H.J.A.-T.; Burbach, J.P.H.; Iraqi, F.A.; Mott, R.F.; Kas, M.J.H. Modeling the quantitative nature of neurodevelopmental disorders using Collaborative Cross mice. Mol. Autism 2018, 9, 63. [Google Scholar] [CrossRef]
- Noll, K.; Ferris, M.T.; Heise, M.T. The Collaborative Cross: A Systems Genetics Resource for Studying Host-Pathogen Interactions. Cell Host Microbe 2019, 25, 484–498. [Google Scholar] [CrossRef]
- Rogala, A.R.; Morgan, A.P.; Christensen, A.M.; Gooch, T.J.; Bell, T.A.; Miller, D.R.; Godfrey, V.L.; De Villena, F.P.-M. The Collaborative Cross as a Resource for Modeling Human Disease: CC011/Unc, a New Mouse Model for Spontaneous Colitis. Mamm. Genome 2014, 25, 95–108. [Google Scholar] [CrossRef]
- Mathes, W.F.; Aylor, D.L.; Miller, D.R.; Churchill, G.A.; Chesler, E.J.; de Villena, F.P.-M.; Threadgill, D.W.; Pomp, D. Architecture of energy balance traits in emerging lines of the Collaborative Cross. Am. J. Physiol. Metab. 2011, 300, E1124–E1134. [Google Scholar] [CrossRef]
- Mao, J.-H.; Langley, S.A.; Huang, Y.; Hang, M.; Bouchard, K.E.; Celniker, S.E.; Brown, J.B.; Jansson, J.; Karpen, G.H.; Snijders, A.M. Identification of genetic factors that modify motor performance and body weight using Collaborative Cross mice. Sci. Rep. 2015, 5, 16247. [Google Scholar] [CrossRef]
- Atamni, H.J.A.-T.; Ziner, Y.; Mott, R.; Wolf, L.; Iraqi, F.A. Glucose tolerance female-specific QTL mapped in collaborative cross mice. Mamm. Genome 2016, 28, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Lorè, N.I.; Sipione, B.; He, G.; Strug, L.J.; Atamni, H.J.; Dorman, A.; Mott, R.; Iraqi, F.A.; Bragonzi, A. Collaborative Cross Mice Yield Genetic Modifiers for Pseudomonas aeruginosa Infection in Human Lung Disease. mBio 2020, 11, e00097–e00117. [Google Scholar] [CrossRef]
- Woods, L.C.S. QTL mapping in outbred populations: Successes and challenges. Physiol. Genom. 2014, 46, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Talbot, C.J.; Nicod, A.; Cherny, S.S.; Fulker, D.W.; Collins, A.C.; Flint, J. High-resolution mapping of quantitative trait loci in outbred mice. Nat. Genet. 1999, 21, 305–308. [Google Scholar] [CrossRef]
- Woods, L.C.S.; Mott, R. Heterogeneous Stock Populations for Analysis of Complex Traits. Syst. Genet. 2016, 1488, 31–44. [Google Scholar] [CrossRef]
- Gatti, D.M.; Svenson, K.L.; Shabalin, A.; Wu, L.-Y.; Valdar, W.; Simecek, P.; Goodwin, N.; Cheng, R.; Pomp, D.; Palmer, A.; et al. Quantitative Trait Locus Mapping Methods for Diversity Outbred Mice. G3 Genes|Genomes|Genet. 2014, 4, 1623–1633. [Google Scholar] [CrossRef] [PubMed]
- Logan, R.W.; Robledo, R.F.; Recla, J.M.; Philip, V.M.; Bubier, J.A.; Jay, J.J.; Harwood, C.; Wilcox, T.; Gatti, D.M.; Bult, C.J.; et al. High-precision genetic mapping of behavioral traits in the diversity outbred mouse population. Genes Brain Behav. 2013, 12, 424–437. [Google Scholar] [CrossRef]
- Valdar, W.; Solberg, L.C.; Gauguier, D.; Burnett, S.; Klenerman, P.; Cookson, W.O.; Taylor, M.; Rawlins, J.N.P.; Mott, R.; Flint, J. Genome-wide genetic association of complex traits in heterogeneous stock mice. Nat. Genet. 2006, 38, 879–887. [Google Scholar] [CrossRef]
- Svenson, K.L.; Gatti, D.M.; Valdar, W.; Welsh, C.E.; Cheng, R.; Chesler, E.J.; Palmer, A.A.; McMillan, L.; Churchill, G.A. High-Resolution Genetic Mapping Using the Mouse Diversity Outbred Population. Genetics 2012, 190, 437–447. [Google Scholar] [CrossRef]
- Keane, T.M.; Goodstadt, L.; Danecek, P.; White, M.A.; Wong, K.; Yalcin, B.; Heger, A.; Agam, A.; Slater, G.; Goodson, M.; et al. Mouse genomic variation and its effect on phenotypes and gene regulation. Nature 2011, 477, 289–294. [Google Scholar] [CrossRef]
- Parker, C.C.; Palmer, A.A. Dark Matter: Are Mice the Solution to Missing Heritability? Front. Genet. 2011, 2, 32. [Google Scholar] [CrossRef] [PubMed]
- Chesler, E.J. Out of the bottleneck: The Diversity Outcross and Collaborative Cross mouse populations in behavioral genetics research. Mamm. Genome 2013, 25, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Koyuncu, D.; Niazi, M.K.K.; Tavolara, T.; Abeijon, C.; Ginese, M.L.; Liao, Y.; Mark, C.; Specht, A.; Gower, A.C.; Restrepo, B.I.; et al. CXCL1: A new diagnostic biomarker for human tuberculosis discovered using Diversity Outbred mice. PLoS Pathog. 2021, 17, e1009773. [Google Scholar] [CrossRef] [PubMed]
- Kazama, H. Systems neuroscience in Drosophila: Conceptual and technical advantages. Neuroscience 2015, 296, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Jaiswal, M.; Charng, W.-L.; Gambin, T.; Karaca, E.; Mirzaa, G.; Wiszniewski, W.; Sandoval, H.; Haelterman, N.A.; Xiong, B.; et al. A Drosophila Genetic Resource of Mutants to Study Mechanisms Underlying Human Genetic Diseases. Cell 2014, 159, 200–214. [Google Scholar] [CrossRef]
- Reiter, L.T.; Potocki, L.; Chien, S.; Gribskov, M.; Bier, E. A Systematic Analysis of Human Disease-Associated Gene Sequences in Drosophila melanogaster. Genome Res. 2001, 11, 1114–1125. [Google Scholar] [CrossRef] [PubMed]
- Myers, E.W.; Sutton, G.G.; Delcher, A.L.; Dew, I.M.; Fasulo, D.P.; Flanigan, M.J.; Kravitz, S.A.; Mobarry, C.M.; Reinert, K.H.J.; Remington, K.A.; et al. A Whole-Genome Assembly of Drosophila. Science 2000, 287, 2196–2204. [Google Scholar] [CrossRef]
- Mackay, T.F.C.; Richards, S.; Stone, E.A.; Barbadilla, A.; Ayroles, J.F.; Zhu, D.; Casillas, S.; Han, Y.; Magwire, M.M.; Cridland, J.M.; et al. The Drosophila melanogaster Genetic Reference Panel. Nature 2012, 482, 173–178. [Google Scholar] [CrossRef]
- Huang, W.; Massouras, A.; Inoue, Y.; Peiffer, J.; Ràmia, M.; Tarone, A.M.; Turlapati, L.; Zichner, T.; Zhu, D.; Lyman, R.F.; et al. Natural variation in genome architecture among 205 Drosophila melanogaster Genetic Reference Panel lines. Genome Res. 2014, 24, 1193–1208. [Google Scholar] [CrossRef]
- Anholt, R.R.H.; Mackay, T.F.C. The road less traveled: From genotype to phenotype in flies and humans. Mamm. Genome 2017, 29, 5–23. [Google Scholar] [CrossRef]
- Ober, U.; Huang, W.; Magwire, M.; Schlather, M.; Simianer, H.; Mackay, T.F.C. Correction: Accounting for Genetic Architecture Improves Sequence Based Genomic Prediction for a Drosophila Fitness Trait. PLoS ONE 2015, 10, e0132980. [Google Scholar] [CrossRef]
- Edwards, S.M.; Sørensen, I.F.; Sarup, P.; Mackay, T.F.; Sørensen, P. Genomic Prediction for Quantitative Traits Is Improved by Mapping Variants to Gene Ontology Categories in Drosophila melanogaster. Genetics 2016, 203, 1871–1883. [Google Scholar] [CrossRef]
- Garlapow, M.E.; Huang, W.; Yarboro, M.T.; Peterson, K.R.; Mackay, T.F.C. Quantitative Genetics of Food Intake in Drosophila melanogaster. PLoS ONE 2015, 10, e0138129. [Google Scholar] [CrossRef]
- Negron, Y.L.S.; Hansen, N.F.; Harbison, S.T. The Sleep Inbred Panel, a Collection of Inbred Drosophila melanogaster with Extreme Long and Short Sleep Duration. G3 Genes|Genomes|Genet. 2018, 8, 2865–2873. [Google Scholar] [CrossRef] [PubMed]
- Bevers, R.P.J.; Litovchenko, M.; Kapopoulou, A.; Braman, V.S.; Robinson, M.R.; Auwerx, J.; Hollis, B.; Deplancke, B. Mitochondrial haplotypes affect metabolic phenotypes in the Drosophila Genetic Reference Panel. Nat. Metab. 2019, 1, 1226–1242. [Google Scholar] [CrossRef]
- Harbison, S.T.; Negron, Y.L.S.; Hansen, N.F.; Lobell, A.S. Selection for long and short sleep duration in Drosophila melanogaster reveals the complex genetic network underlying natural variation in sleep. PLoS Genet. 2017, 13, e1007098. [Google Scholar] [CrossRef] [PubMed]
- Unckless, R.L.; Rottschaefer, S.M.; Lazzaro, B.P. A Genome-Wide Association Study for Nutritional Indices in Drosophila. G3 Genes|Genomes|Genet. 2015, 5, 417–425. [Google Scholar] [CrossRef]
- Lafuente, E.; Duneau, D.; Beldade, P. Genetic basis of thermal plasticity variation in Drosophila melanogaster body size. PLoS Genet. 2018, 14, e1007686. [Google Scholar] [CrossRef] [PubMed]
- Harbison, S.T.; Kumar, S.; Huang, W.; McCoy, L.J.; Smith, K.R.; Mackay, T.F.C. Genome-Wide Association Study of Circadian Behavior in Drosophila melanogaster. Behav. Genet. 2018, 49, 60–82. [Google Scholar] [CrossRef]
- Chow, C.Y.; Kelsey, K.J.; Wolfner, M.F.; Clark, A.G. Candidate genetic modifiers of retinitis pigmentosa identified by exploiting natural variation in Drosophila. Hum. Mol. Genet. 2015, 25, 651–659. [Google Scholar] [CrossRef]
- Lavoy, S.; Chittoor-Vinod, V.G.; Chow, C.Y.; Martin, I. Genetic Modifiers of Neurodegeneration in a Drosophila Model of Parkinson’s Disease. Genetics 2018, 209, 1345–1356. [Google Scholar] [CrossRef]
- Klein, A.D.; Mazzulli, J.R. Is Parkinson’s disease a lysosomal disorder? Brain 2018, 141, 2255–2262. [Google Scholar] [CrossRef]
- Marder, K.; Wang, Y.; Alcalay, R.N.; Mejia-Santana, H.; Tang, M.-X.; Lee, A.; Raymond, D.; Mirelman, A.; Saunders-Pullman, R.; Clark, L.; et al. Age-specific penetrance of LRRK2 G2019S in the Michael J. Fox Ashkenazi Jewish LRRK2 Consortium. Neurology 2015, 85, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Olivares, G.H.; Olguín, P.; Klein, A.D. Modeling Parkinson’s Disease Heterogeneity to Accelerate Precision Medicine. Trends Mol. Med. 2019, 25, 1052–1055. [Google Scholar] [CrossRef] [PubMed]
- Mackay, T.F.; Huang, W. Charting the genotype–phenotype map: Lessons from the Drosophila melanogaster Genetic Reference Panel. Wiley Interdiscip. Rev. Dev. Biol. 2017, 7, e289. [Google Scholar] [CrossRef] [PubMed]
- Swarup, S.; Huang, W.; Mackay, T.F.C.; Anholt, R.R.H. Analysis of natural variation reveals neurogenetic networks for Drosophila olfactory behavior. Proc. Natl. Acad. Sci. USA 2012, 110, 1017–1022. [Google Scholar] [CrossRef] [PubMed]
- Shorter, J.; Couch, C.; Huang, W.; Carbone, M.A.; Peiffer, J.; Anholt, R.R.H.; Mackay, T.F.C. Genetic architecture of natural variation in Drosophila melanogaster aggressive behavior. Proc. Natl. Acad. Sci. USA 2015, 112, E3555–E3563. [Google Scholar] [CrossRef] [PubMed]
- Long, A.D.; Macdonald, S.J.; King, E.G. Dissecting complex traits using the Drosophila Synthetic Population Resource. Trends Genet. 2014, 30, 488–495. [Google Scholar] [CrossRef]
- Duina, A.A.; Miller, M.E.; Keeney, J.B. Budding Yeast for Budding Geneticists: A Primer on the Saccharomyces cerevisiae Model System. Genetics 2014, 197, 33–48. [Google Scholar] [CrossRef]
- Walberg, M.W. Applicability of Yeast Genetics to Neurologic Disease. Arch. Neurol. 2000, 57, 1129–1134. [Google Scholar] [CrossRef][Green Version]
- Mancera, E.; Bourgon, R.; Brozzi, A.; Huber, W.; Steinmetz, L.M. High-resolution mapping of meiotic crossovers and non-crossovers in yeast. Nature 2008, 454, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, A.; Borde, V. Methods to Map Meiotic Recombination Proteins in Saccharomyces cerevisiae. In Homologous Recombination; Humana: New York, NY, USA, 2020; pp. 295–306. [Google Scholar] [CrossRef]
- Botstein, D.; Fink, G.R. Yeast: An Experimental Organism for Modern Biology. Science 1988, 240, 1439–1443. [Google Scholar] [CrossRef] [PubMed]
- Giaever, G.; Nislow, C. The Yeast Deletion Collection: A Decade of Functional Genomics. Genetics 2014, 197, 451–465. [Google Scholar] [CrossRef] [PubMed]
- Galardini, M.; Busby, B.P.; Vieitez, C.; Dunham, A.S.; Typas, A.; Beltrao, P. The impact of the genetic background on gene deletion phenotypes in Saccharomyces cerevisiae. Mol. Syst. Biol. 2019, 15, e8831. [Google Scholar] [CrossRef] [PubMed]
- Parts, L.; Batté, A.; Lopes, M.; Yuen, M.W.; Laver, M.; Luis, B.S.; Yue, J.; Pons, C.; Eray, E.; Aloy, P.; et al. Natural variants suppress mutations in hundreds of essential genes. Mol. Syst. Biol. 2021, 17, e10138. [Google Scholar] [CrossRef] [PubMed]
- Khurana, V.; Lindquist, S. Modelling neurodegeneration in Saccharomyces cerevisiae: Why cook with baker’s yeast? Nat. Rev. Neurosci. 2010, 11, 436–449. [Google Scholar] [CrossRef]
- Ehrenreich, I.M.; Magwene, P.M. Genetic Dissection of Heritable Traits in Yeast Using Bulk Segregant Analysis. Cold Spring Harb. Protoc. 2017, 2017, pdb.prot088989. [Google Scholar] [CrossRef] [PubMed]
- Bloom, J.S.; Boocock, J.; Treusch, S.; Sadhu, M.J.; Day, L.; Oates-Barker, H.; Kruglyak, L. Rare variants contribute disproportionately to quantitative trait variation in yeast. eLife 2019, 8, e49212. [Google Scholar] [CrossRef]
- Lander, E.S.; Botstein, D. Mapping mendelian factors underlying quantitative traits using RFLP linkage maps. Genetics 1989, 121, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Michelmore, R.W.; Paran, I.; Kesseli, R.V. Identification of markers linked to disease-resistance genes by bulked segregant analysis: A rapid method to detect markers in specific genomic regions by using segregating populations. Proc. Natl. Acad. Sci. USA 1991, 88, 9828–9832. [Google Scholar] [CrossRef]
- Liti, G.; Carter, D.M.; Moses, A.M.; Warringer, J.; Parts, L.; James, S.A.; Davey, R.P.; Roberts, I.N.; Burt, A.; Koufopanou, V.; et al. Population genomics of domestic and wild yeasts. Nature 2009, 458, 337–341. [Google Scholar] [CrossRef]
- Swinnen, S.; Thevelein, J.M.; Nevoigt, E. Genetic mapping of quantitative phenotypic traits in Saccharomyces cerevisiae. FEMS Yeast Res. 2012, 12, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Kessi-Pérez, E.I.; Molinet, J.; Martínez, C. Disentangling the genetic bases of Saccharomyces cerevisiae nitrogen consumption and adaptation to low nitrogen environments in wine fermentation. Biol. Res. 2020, 53, 2. [Google Scholar] [CrossRef] [PubMed]
- Haas, R.; Horev, G.; Lipkin, E.; Kesten, I.; Portnoy, M.; Buhnik-Rosenblau, K.; Soller, M.; Kashi, Y. Mapping Ethanol Tolerance in Budding Yeast Reveals High Genetic Variation in a Wild Isolate. Front. Genet. 2019, 10, 998. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Qi, Q.; Lin, Y.; Guo, Y.; Liu, Y.; Wang, Q. QTL Analysis Reveals Genomic Variants Linked to High-Temperature Fermentation Performance in the Industrial Yeast. Biotechnol. Biofuels 2019, 12, 59. [Google Scholar] [CrossRef]
- Liti, G.; Louis, E.J. Yeast Evolution and Comparative Genomics. Annu. Rev. Microbiol. 2005, 59, 135–153. [Google Scholar] [CrossRef][Green Version]
- Warringer, J.; Zörgö, E.; Cubillos, F.A.; Zia, A.; Gjuvsland, A.; Simpson, J.T.; Forsmark, A.; Durbin, R.; Omholt, S.W.; Louis, E.J.; et al. Trait Variation in Yeast Is Defined by Population History. PLoS Genet. 2011, 7, e1002111. [Google Scholar] [CrossRef]
- Perlstein, E.; Ruderfer, D.; Roberts, D.C.; Schreiber, S.L.; Kruglyak, L. Genetic basis of individual differences in the response to small-molecule drugs in yeast. Nat. Genet. 2007, 39, 496–502. [Google Scholar] [CrossRef]
- Cubillos, F.A.; Louis, E.J.; Liti, G. Generation of a large set of genetically tractable haploid and diploid Saccharomyces strains. FEMS Yeast Res. 2009, 9, 1217–1225. [Google Scholar] [CrossRef]
- Linder, R.A.; Majumder, A.; Chakraborty, M.; Long, A. Two Synthetic 18-Way Outcrossed Populations of Diploid Budding Yeast with Utility for Complex Trait Dissection. Genetics 2020, 215, 323–342. [Google Scholar] [CrossRef]
- Cubillos, F.; Parts, L.; Salinas, F.; Bergström, A.; Scovacricchi, E.; Zia, A.; Illingworth, C.J.R.; Mustonen, V.; Ibstedt, S.; Warringer, J.; et al. High-Resolution Mapping of Complex Traits with a Four-Parent Advanced Intercross Yeast Population. Genetics 2013, 195, 1141–1155. [Google Scholar] [CrossRef] [PubMed]
- Diouf, I.; Pascual, L. Multiparental Population in Crops: Methods of Development and Dissection of Genetic Traits. In Crop Breeding; Tripodi, P., Ed.; Humana: New York, NY, USA, 2020; Volume 2264, pp. 13–32. [Google Scholar] [CrossRef]
- Peter, J.; Schacherer, J. Population genomics of yeasts: Towards a comprehensive view across a broad evolutionary scale. Yeast 2015, 33, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Bendixsen, D.P.; Frazão, J.G.; Stelkens, R. Saccharomyces yeast hybrids on the rise. Yeast 2021, 39, 40–54. [Google Scholar] [CrossRef] [PubMed]
- Schacherer, J.; Shapiro, J.A.; Ruderfer, D.M.; Kruglyak, L. Comprehensive polymorphism survey elucidates population structure of Saccharomyces cerevisiae. Nature 2009, 458, 342–345. [Google Scholar] [CrossRef]
- Peter, J.; De Chiara, M.; Friedrich, A.; Yue, J.-X.; Pflieger, D.; Bergström, A.; Sigwalt, A.; Barre, B.; Freel, K.; Llored, A.; et al. Genome evolution across 1,011 Saccharomyces cerevisiae isolates. Nature 2018, 556, 339–344. [Google Scholar] [CrossRef]
- Khurana, V.; Peng, J.; Chung, C.Y.; Auluck, P.K.; Fanning, S.; Tardiff, D.F.; Bartels, T.; Koeva, M.; Eichhorn, S.W.; Benyamini, H.; et al. Genome-Scale Networks Link Neurodegenerative Disease Genes to alpha-Synuclein through Specific Molecular Pathways. Cell Syst. 2017, 4, 157–170. [Google Scholar] [CrossRef]
- Jung, P.P.; Zhang, Z.; Paczia, N.; Jaeger, C.; Ignac, T.; May, P.; Linster, C.L. Natural variation of chronological aging in the Saccharomyces cerevisiae species reveals diet-dependent mechanisms of life span control. npj Aging Mech. Dis. 2018, 4, 3. [Google Scholar] [CrossRef]
- Breschi, A.; Gingeras, T.R.; Guigó, A.B.R. Comparative transcriptomics in human and mouse. Nat. Rev. Genet. 2017, 18, 425–440. [Google Scholar] [CrossRef]
- Adams, M.D.; Celniker, S.E.; Holt, R.A.; Evans, C.A.; Gocayne, J.D.; Amanatides, P.G.; Scherer, S.E.; Li, P.W.; Hoskins, R.A.; Galle, R.F.; et al. The Genome Sequence of Drosophila melanogaster. Science 2000, 287, 2185–2195. [Google Scholar] [CrossRef]
- Belda, I.; Ruiz, J.; Santos, A.; Van Wyk, N.; Pretorius, I.S. Saccharomyces cerevisiae. Trends Genet. 2019, 35, 956–957. [Google Scholar] [CrossRef]
- Zhu, F.; Nair, R.R.; Fisher, E.M.C.; Cunningham, T.J. Humanising the mouse genome piece by piece. Nat. Commun. 2019, 10, 1845. [Google Scholar] [CrossRef] [PubMed]
- Ugur, B.; Chen, K.; Bellen, H.J. Drosophila tools and assays for the study of human diseases. Dis. Models Mech. 2016, 9, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.G.; Snyder, M. Yeast as a Model for Human Disease. Curr. Protoc. Hum. Genet. 2006, 48, 15–21. [Google Scholar] [CrossRef] [PubMed]
| Mus musculus | Drosophila melanogaster | Saccharomyces cerevisiae | |
|---|---|---|---|
| Genome size (kb) | 2,725,521 [102] | 180,000 [103] | 12,070 [104] |
| Percentage of homolog genes to human disease-causing genes | 99 [105] | 70 [47,106] | 60 [107] |
| Costs to keep the panels | High | Medium | Very low |
| Complex behaviors | Yes | Yes | No |
| Discovery of cell-autonomous processes | Yes | Yes | Yes |
| Speed for throughput screenings and automatization of measurements | Slow | Fast | Very fast |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olguín, V.; Durán, A.; Las Heras, M.; Rubilar, J.C.; Cubillos, F.A.; Olguín, P.; Klein, A.D. Genetic Background Matters: Population-Based Studies in Model Organisms for Translational Research. Int. J. Mol. Sci. 2022, 23, 7570. https://doi.org/10.3390/ijms23147570
Olguín V, Durán A, Las Heras M, Rubilar JC, Cubillos FA, Olguín P, Klein AD. Genetic Background Matters: Population-Based Studies in Model Organisms for Translational Research. International Journal of Molecular Sciences. 2022; 23(14):7570. https://doi.org/10.3390/ijms23147570
Chicago/Turabian StyleOlguín, Valeria, Anyelo Durán, Macarena Las Heras, Juan Carlos Rubilar, Francisco A. Cubillos, Patricio Olguín, and Andrés D. Klein. 2022. "Genetic Background Matters: Population-Based Studies in Model Organisms for Translational Research" International Journal of Molecular Sciences 23, no. 14: 7570. https://doi.org/10.3390/ijms23147570
APA StyleOlguín, V., Durán, A., Las Heras, M., Rubilar, J. C., Cubillos, F. A., Olguín, P., & Klein, A. D. (2022). Genetic Background Matters: Population-Based Studies in Model Organisms for Translational Research. International Journal of Molecular Sciences, 23(14), 7570. https://doi.org/10.3390/ijms23147570






