Mitochondrial Dysfunction Affects the Synovium of Patients with Rheumatoid Arthritis and Osteoarthritis Differently
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics
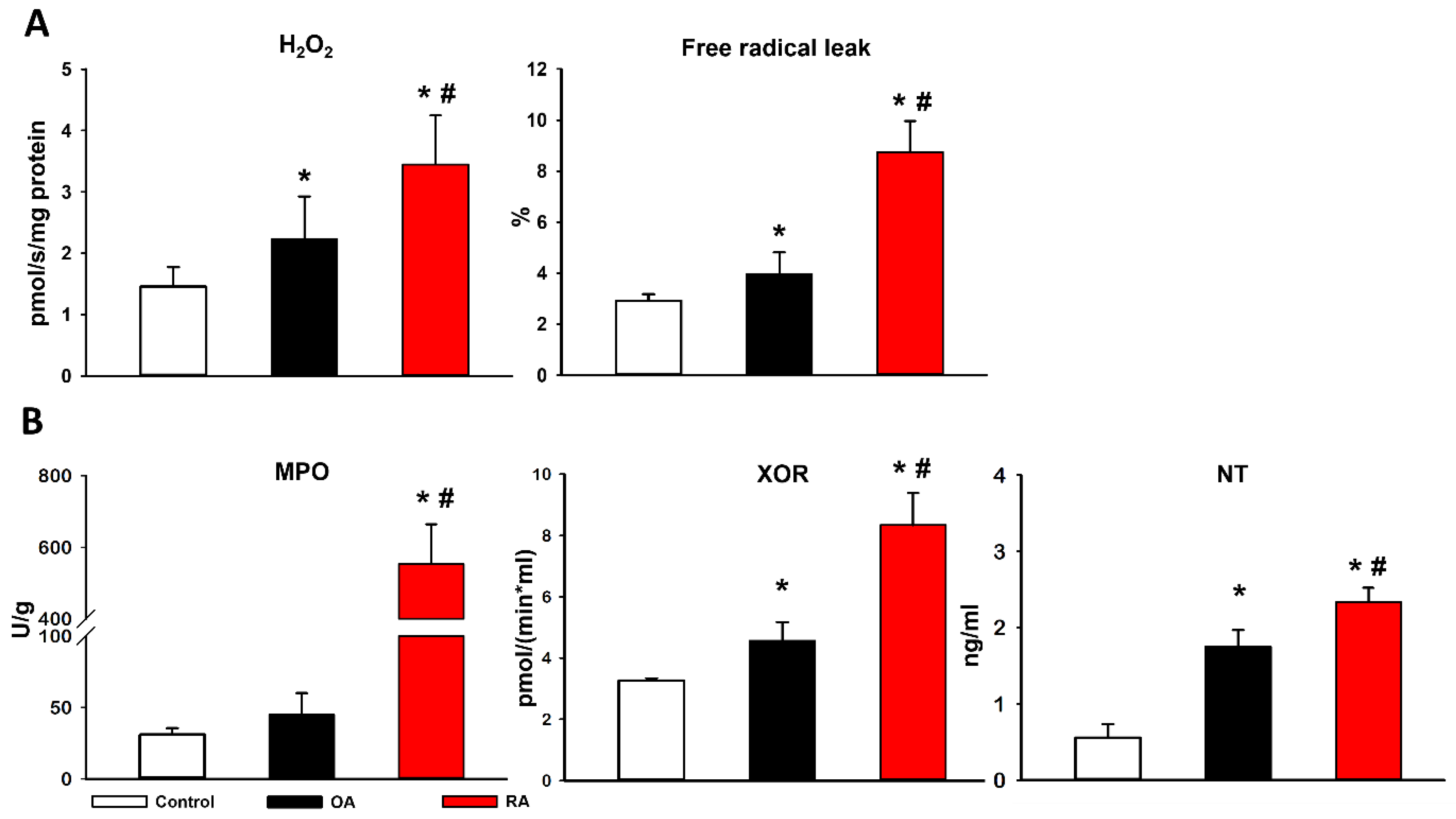
2.2. Mitochondrial Functional Measurements
2.3. Cytochrome C Release and ETC
2.4. Mitochondrial Hydrogen Peroxide (H2O2) Production and Biochemical Analyses
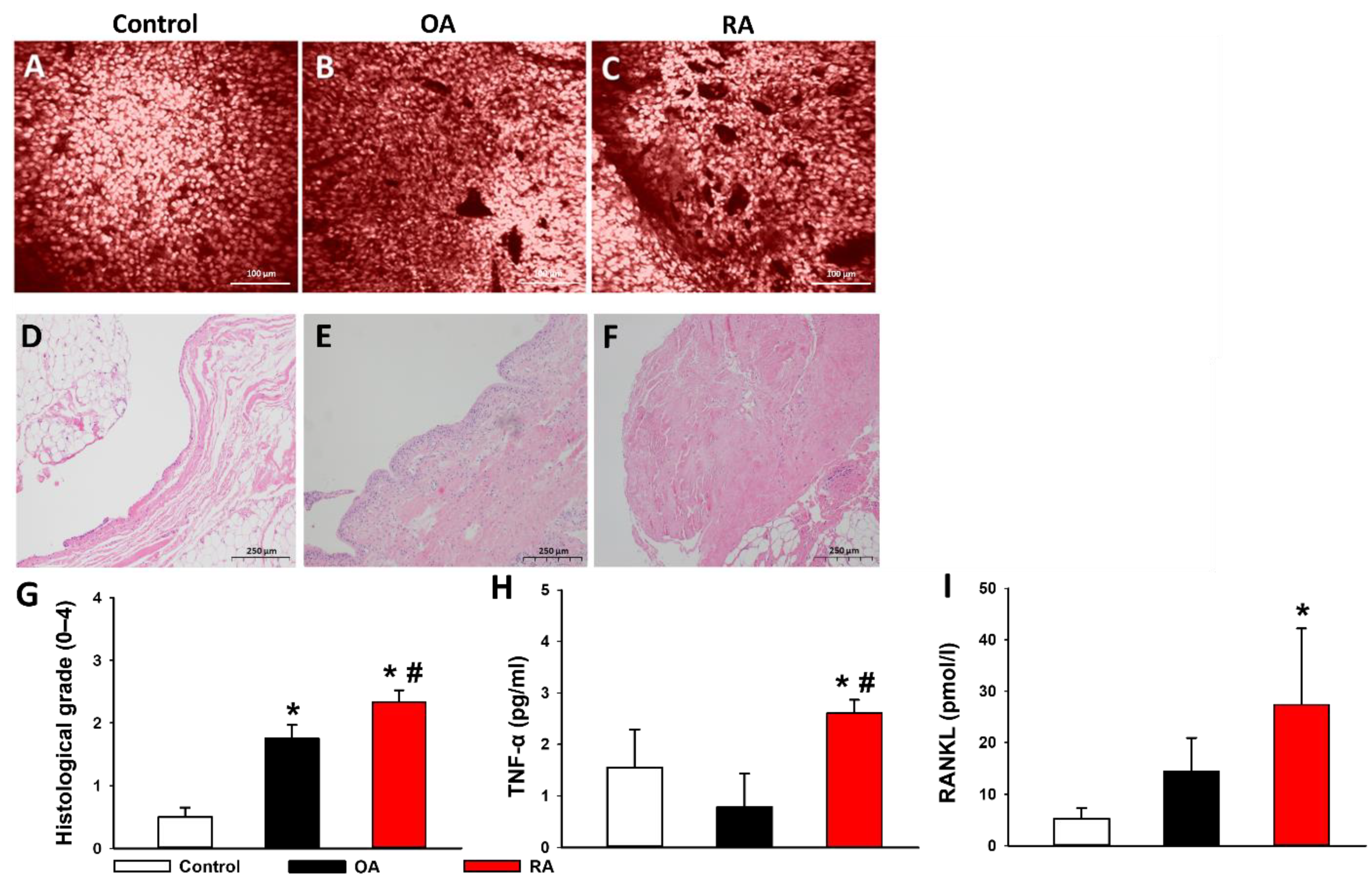
2.5. Histopathological Evaluation
2.6. Proinflammatory Cytokines in the Synovial Fluid
3. Discussion
4. Materials and Methods
4.1. Ethical Approval
4.2. Study Design
4.3. Patient Allocation
4.4. Sampling
4.5. Examination of Mitochondrial Functions
4.6. Biochemical Analyses
4.6.1. Tissue Xanthine Oxidoreductase (XOR) Activity
4.6.2. Tissue Myeloperoxidase (MPO) Activity
4.6.3. Nitrotyrosine (NT) Levels
4.6.4. Laboratory Testing of Synovial Fluid
4.7. Histopathological Analysis
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACR/EULAR | American College of Rheumatology/European League Against Rheumatism |
| ATP | adenosine triphosphate |
| BMI | body mass index |
| C | complexes |
| CLSEM | confocal laser scanning endomicroscope |
| CRP | C-reactive protein |
| DAMPs | damage-associated molecular patterns |
| ETC | electron transport chain |
| FCCP | p-trifluoromethoxy-phenyl-hydrazine |
| HClO | hypochlorous acid |
| H2O2 | hydrogen peroxide |
| ICD | International Statistical Classification of Diseases and Related Health Problems |
| KL | Kellgren-Lawrence |
| MPO | myeloperoxidase |
| NSAIDs | nonsteroidal anti-inflammatory drugs |
| NT | nitrotyrosine |
| OA | osteoarthritis |
| OxPhos | oxidative phosphorylation |
| RA | rheumatoid arthritis |
| RANKL | nuclear factor kappa-beta ligand |
| RCR | respiratory control ratio |
| ROS | reactive oxygen species |
| SCID | severe combined immunodeficiency |
| TP | total protein |
| TNF-α | tumor necrosis factor alpha |
| WBC | white blood cell count |
| XOR | xanthine oxidoreductase |
References
- Pap, T.; Korb-Pap, A. Cartilage damage in osteoarthritis and rheumatoid arthritis—Two unequal siblings. Nat. Rev. Rheumatol. 2015, 11, 606–615. [Google Scholar] [CrossRef] [PubMed]
- Da Sylva, T.R.; Connor, A.; Mburu, Y.; Keystone, E.; Wu, G. Somatic mutations in the mitochondria of rheumatoid arthritis synoviocytes. Arthritis Res. Ther. 2005, 7, R844–R851. [Google Scholar] [CrossRef] [PubMed]
- Fearon, U.; Canavan, M.; Biniecka, M.; Veale, D.J. Hypoxia, mitochondrial dysfunction and synovial invasiveness in rheumatoid arthritis. Nat. Rev. Rheumatol. 2016, 12, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Blanco, F.J.; Rego, I.; Ruiz-Romero, C. The role of mitochondria in osteoarthritis. Nat. Rev. Rheumatol. 2011, 7, 161–169. [Google Scholar] [CrossRef]
- Lepetsos, P.; Papavassiliou, A.G. ROS/oxidative stress signaling in osteoarthritis. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2016, 1862, 576–591. [Google Scholar] [CrossRef]
- Rego-Pérez, I.; Durán-Sotuela, A.; Ramos-Louro, P.; Blanco, F.J. Mitochondrial Genetics and Epigenetics in Osteoarthritis. Front. Genet. 2020, 10, 1335. [Google Scholar] [CrossRef]
- Roh, J.S.; Sohn, D.H. Damage-Associated Molecular Patterns in Inflammatory Diseases. Immune Netw. 2018, 18, e27. [Google Scholar] [CrossRef]
- Martin, J.A.; Martini, A.; Molinari, A.; Morgan, W.; Ramalingam, W.; Buckwalter, J.A.; McKinley, T.O. Mitochondrial electron transport and glycolysis are coupled in articular cartilage. Osteoarthr. Cartil. 2012, 20, 323–329. [Google Scholar] [CrossRef]
- Rego, I.; Fernandez-Moreno, M.; Fernandez-Lopez, C.; Gomez-Reino, J.J.; Gonzalez, A.; Arenas, J.; Blanco, F.J. Role of Eu-ropean mitochondrial DNA haplogroups in the prevalence of hip osteoarthritis in Galicia, Northern Spain. Ann. Rheum. Dis. 2010, 69, 210–213. [Google Scholar] [CrossRef]
- Woetzel, D.; Huber, R.; Kupfer, P.; Pohlers, D.; Pfaff, M.; Driesch, D.; Häupl, T.; Koczan, D.; Stiehl, P.; Guthke, R.; et al. Identification of rheumatoid arthritis and osteoarthritis patients by transcriptome-based rule set generation. Arthritis Res. Ther. 2014, 16, R84. [Google Scholar] [CrossRef]
- Blanco, F.J.; Valdes, A.; Rego-Pérez, I. Mitochondrial DNA variation and the pathogenesis of osteoarthritis phenotypes. Nat. Rev. Rheumatol. 2018, 14, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Fu, P.; Wang, L.; Xiang, C. Mitochondria: Potential Targets for Osteoarthritis. Front. Med. 2020, 7, 581402. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, X.; Lotz, M.; Terkeltaub, R.; Liu-Bryan, R. Mitochondrial Biogenesis Is Impaired in Osteoarthritis Chondrocytes but Reversible via Peroxisome Proliferator-Activated Receptor γ Coactivator 1α. Arthritis Rheumatol. 2015, 67, 2141–2153. [Google Scholar] [CrossRef] [PubMed]
- Panga, V.; Kallor, A.A.; Nair, A.; Harshan, S.; Raghunathan, S. Mitochondrial dysfunction in rheumatoid arthritis: A com-prehensive analysis by integrating gene expression, protein-protein interactions and gene ontology data. PLoS ONE 2019, 14, e0224632. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, Z.; Cao, Y.; Cui, Y.; Yang, X.; Meng, Z.; Wang, R. Effect of chondrocyte mitochondrial dysfunction on cartilage degeneration: A possible pathway for osteoarthritis pathology at the subcellular level. Mol. Med. Rep. 2019, 20, 3308–3316. [Google Scholar] [CrossRef] [PubMed]
- Maneiro, E.; Martín, M.A.; De Andres, M.C.; López-Armada, M.J.; Fernández-Sueiro, J.L.; Del Hoyo, P.; Galdo, F.; Arenas, J.; Blanco, F.J. Mitochondrial respiratory activity is altered in osteoarthritic human articular chondrocytes. Arthritis Care Res. 2003, 48, 700–708. [Google Scholar] [CrossRef] [PubMed]
- Mathiessen, A.; Conaghan, P.G. Synovitis in osteoarthritis: Current understanding with therapeutic implications. Arthritis Res. Ther. 2017, 19, 1–9. [Google Scholar] [CrossRef]
- Lehmann, J.; Jungel, A.; Lehmann, I.; Busse, F.; Biskop, M.; Saalbach, A.; Emmrich, F.; Sack, U. Grafting of fibroblasts isolated from the synovial membrane of rheumatoid arthritis (RA) patients induces chronic arthritis in SCID mice-A novel model for studying the arthritogenic role of RA fibroblasts in vivo. J. Autoimmun. 2000, 15, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Gavriilidis, C.; Miwa, S.; von Zglinicki, T.; Taylor, R.W.; Young, D.A. Mitochondrial dysfunction in osteoarthritis is associated with down-regulation of superoxide dismutase 2. Arthritis Care Res. 2012, 65, 378–387. [Google Scholar] [CrossRef]
- June, R.K.; Liu-Bryan, R.; Long, F.; Griffin, T.M. Emerging role of metabolic signaling in synovial joint remodeling and oste-oarthritis. J. Orthop. Res. 2016, 34, 2048–2058. [Google Scholar] [CrossRef]
- Armstrong, J.S. Mitochondrial Medicine: Pharmacological targeting of mitochondria in disease. J. Cereb. Blood Flow Metab. 2007, 151, 1154–1165. [Google Scholar] [CrossRef] [PubMed]
- Baeten, D.; Demetter, P.; Cuvelier, C.; Bosch, F.V.D.; Kruithof, E.; Van Damme, N.; Verbruggen, G.; Mielants, H.; Veys, E.M.; De Keyser, F. Comparative study of the synovial histology in rheumatoid arthritis, spondyloarthropathy, and osteoarthritis: Influence of disease duration and activity. Ann. Rheum. Dis. 2000, 59, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Sharma, L.K.; Lu, J.; Bai, Y. Mitochondrial Respiratory Complex I: Structure, Function and Implication in Human Diseases. Curr. Med. Chem. 2009, 16, 1266–1277. [Google Scholar] [CrossRef] [PubMed]
- Lambert, A.J.; Brand, M. Inhibitors of the Quinone-binding Site Allow Rapid Superoxide Production from Mitochondrial NADH:Ubiquinone Oxidoreductase (Complex I). J. Biol. Chem. 2004, 279, 39414–39420. [Google Scholar] [CrossRef] [PubMed]
- Detaille, D.; Pasdois, P.; Sémont, A.; Dos Santos, P.; Diolez, P. An old medicine as a new drug to prevent mitochondrial complex I from producing oxygen radicals. PLoS ONE 2019, 14, e0216385. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, U.; Anwar, A.; Savage, R.S.; Costa, M.L.; Mackay, N.; Filer, A.; Raza, K.; Watts, R.A.; Winyard, P.G.; Tarr, J.; et al. Biomarkers of early stage osteoarthritis, rheumatoid arthritis and musculoskeletal health. Sci. Rep. 2015, 5, 9259. [Google Scholar] [CrossRef] [PubMed]
- Garrido, C.; Galluzzi, L.; Brunet, M.; Puig, P.E.; Didelot, C.; Kroemer, G. Mechanisms of cytochrome c release from mito-chondria. Cell Death Differ. 2006, 13, 1423–1433. [Google Scholar] [CrossRef]
- Martinou, I.; Desagher, S.; Eskes, R.; Antonsson, B.; André, E.; Fakan, S.; Martinou, J.-C. The Release of Cytochrome c from Mitochondria during Apoptosis of NGF-deprived Sympathetic Neurons Is a Reversible Event. J. Cell Biol. 1999, 144, 883–889. [Google Scholar] [CrossRef]
- Dickerson, T.J.; Suzuki, E.; Stanecki, C.; Shin, H.-S.; Qui, H.; Adamopoulos, I.E. Rheumatoid and pyrophosphate arthritis synovial fibroblasts induce osteoclastogenesis independently of RANKL, TNF and IL-6. J. Autoimmun. 2012, 39, 369–376. [Google Scholar] [CrossRef]
- Samavati, L.; Lee, I.; Mathes, I.; Lottspeich, F.; Hüttemann, M. Tumor Necrosis Factor α Inhibits Oxidative Phosphorylation through Tyrosine Phosphorylation at Subunit I of Cytochrome c Oxidase. J. Biol. Chem. 2008, 283, 21134–21144. [Google Scholar] [CrossRef]
- Halliwell, B. Oxidative stress and neurodegeneration: Where are we now? J. Neurochem. 2006, 97, 1634–1658. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Halliwell, B. Evidence for nitric oxide-mediated oxidative damage in chronic inflammation Nitrotyrosine in serum and synovial fluid from rheumatoid patients. FEBS Lett. 1994, 350, 9–12. [Google Scholar] [CrossRef]
- Misko, T.P.; Radabaugh, M.R.; Highkin, M.; Abrams, M.; Friese, O.; Gallavan, R.; Bramson, C.; Le Graverand, M.P.H.; Lohmander, L.S.; Roman, D. Characterization of nitrotyrosine as a biomarker for arthritis and joint injury. Osteoarthr. Cartil. 2013, 21, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Strzepa, A.; Lobo, F.M.; Majewska-Szczepanik, M.; Szczepanik, M. Antibiotics and autoimmune and allergy diseases: Caus-ative factor or treatment? Int. Immunopharmacol. 2018, 65, 328–341. [Google Scholar] [CrossRef] [PubMed]
- Kohn, M.D.; Sassoon, A.A.; Fernando, N.D. Classifications in Brief: Kellgren-Lawrence Classification of Osteoarthritis. Clin. Orthop. Relat. Res. 2016, 474, 1886–1893. [Google Scholar] [CrossRef]
- Braun, H.J.; Gold, G.E. Diagnosis of osteoarthritis: Imaging. Bone 2011, 51, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.M.; Cake, M.A.; Ghosh, P.; Schiavinato, A.; Read, R.A.; Little, C.B. Significant synovial pathology in a menis-cectomy model of osteoarthritis: Modification by intra-articular hyaluronan therapy. Rheumatology 2008, 47, 1172–1178. [Google Scholar] [CrossRef][Green Version]
- Komary, Z.; Tretter, L.; Adam-Vizi, V. Membrane potential-related effect of calcium on reactive oxygen species generation in isolated brain mitochondria. Biochim. Biophys. Acta 2010, 1797, 922–928. [Google Scholar] [CrossRef][Green Version]
- Strifler, G.; Tuboly, E.; Szél, E.; Kaszonyi, E.; Cao, C.; Kaszaki, J.; Meszaros, A.; Boros, M.; Hartmann, P. Inhaled Methane Limits the Mitochondrial Electron Transport Chain Dysfunction during Experimental Liver Ischemia-Reperfusion Injury. PLoS ONE 2016, 11, e0146363. [Google Scholar] [CrossRef]
- Beckman, J.S.; Parks, D.A.; Pearson, J.D.; Marshall, P.A.; Freeman, B.A. A sensitive fluorometric assay for measuring xanthine dehydrogenase and oxidase in tissues. Free Radic. Biol. Med. 1989, 6, 607–615. [Google Scholar] [CrossRef]
- Kuebler, W.; Abels, C.; Schuerer, L.; Goetz, A. Measurement of Neutrophil Content in Brain and Lung Tissue by a Modified Myeloperoxidase Assay. Int. J. Microcirc. 1996, 16, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-P.; Walton, M.; Wang, A.; Anderson, P.; Wang, T.; Kirk, T.; Zheng, M. The development of confocal arthroscopy as optical histology for rotator cuff tendinopathy. J. Microsc. 2015, 259, 269–275. [Google Scholar] [CrossRef] [PubMed]


| Demographics | All Patients (n = 109) | RA Group (n = 24) | OA Group (n = 47) | Control Group (n = 38) |
|---|---|---|---|---|
| Age (y) (mean ± SD) | 47 ± 21 | 49 ± 15 | 52 ± 11 | 45 ± 8 |
| Female n (%) | 56 (51) | 19 (79) | 21 (45) | 16 (42) |
| Male n (%) | 53 (49) | 5 (21) | 26 (55) | 2215 (58) |
| OA Risk Factors | ||||
| Age >50 years n (%) | 48 (44) | 11 (46) | 30 (64) | 7 (18) |
| BMI (mean ± SD) | 29 ± 5 | 28 ± 4 | 33 ± 6 | 26 ± 3 |
| BMI ≥30 n (%) | 32 (29) | 2 (1) | 25 (53) | 5 (13) |
| Joint trauma in the anamnesis n (%) | 64 (59) | 3 (1) | 23 (49) | 38 (100) |
| Disease Severity | ||||
| ACR/EULAR score (mean ± SD) | 7 ± 1 | |||
| ACR/EULAR score (median [IQR]) | 7 [6,7] | |||
| Kellgren–Lawrence Score (mean ± SD) | 4 ± 1 | 4 ± 1 | ||
| Kellgren–Lawrence Score (median [IQR]) | 4 [3,4] | 4 [3,4] | ||
| VAS (mean ± SD) | 8 ± 2 | 5 ± 3 | ||
| Takes NSAIDs daily n (%) | 11 (46) | 18 (38) | ||
| Needs walking aid n (%) | 12 (50) | 19 (40) | ||
| Labor Results | ||||
| WBC (G/L) (mean ± SD) | 9.8 ± 3.6 | 10.8 ± 3.3 | 10.3 ± 3.8 | 8.3 ± 3.0 |
| WBC (G/L) (median [IQR]) | 9.2 [7.5–12.0] | 10.1 [8.8–13.2] | 10.0 [7.9–12.1] | 7.6 [6.1–10.0] |
| CRP (mg/L) (mean ± SD) | 7.0 ± 7.0 | 11.2 ± 8.9 | 7.3 ± 6.1 | 3.5 ± 4.3 |
| CRP (mg/L) (median [IQR]) | 5.5 [2.9–8.8] | 6.9 [5.4–15.6] | 5.6 [3.5–10.4] | 2.8 [0.0–5.5] |
| TP (g/L) (mean ± SD) | 70.1 ± 2.9 | 69.2 ± 2.5 | 70.5 ± 3.0 | 70.2 ± 2.7 |
| TP (g/L) (median [IQR]) | 69.4 [68.5–71.0] | 72.2 [67.5–69.8] | 69.6 [69.2–71.0] | 69.5 [68.5–71.6] |
| RF positive n (%) | 15 (63) | |||
| Comorbidities | ||||
| Presence of comorbidities n (%) | 63 (58) | 21 (88) | 38 (81) | 4 (118) |
| Primary hypertension | 26 (24) | 6 (25) | 18 (38) | 2 (5) |
| Diabetes | 12 (11) | 2 (8) | 8 (17) | 2(5) |
| NIDDM | 9 (8) | 2 (8) | 5 (10) | 2 (5) |
| IDDM | 3 (3) | 0 (0) | 3 (6) | 0 (0) |
| Gout | 3 (3) | 1 (4) | 2 (4) | 0 (0) |
| Other | 22 (31) | 12 (71) | 10 (32) | 0 (0) |
| Operation Type | ||||
| Arthroscopy n (%) | 47 (43) | 2 (8) | 12 (26) | 33 (87) |
| Open surgery n (%) | 62 (57) | 22 (92) | 35 (74) | 5 (13) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jávor, P.; Mácsai, A.; Butt, E.; Baráth, B.; Jász, D.K.; Horváth, T.; Baráth, B.; Csonka, Á.; Török, L.; Varga, E.; et al. Mitochondrial Dysfunction Affects the Synovium of Patients with Rheumatoid Arthritis and Osteoarthritis Differently. Int. J. Mol. Sci. 2022, 23, 7553. https://doi.org/10.3390/ijms23147553
Jávor P, Mácsai A, Butt E, Baráth B, Jász DK, Horváth T, Baráth B, Csonka Á, Török L, Varga E, et al. Mitochondrial Dysfunction Affects the Synovium of Patients with Rheumatoid Arthritis and Osteoarthritis Differently. International Journal of Molecular Sciences. 2022; 23(14):7553. https://doi.org/10.3390/ijms23147553
Chicago/Turabian StyleJávor, Péter, Attila Mácsai, Edina Butt, Bálint Baráth, Dávid Kurszán Jász, Tamara Horváth, Bence Baráth, Ákos Csonka, László Török, Endre Varga, and et al. 2022. "Mitochondrial Dysfunction Affects the Synovium of Patients with Rheumatoid Arthritis and Osteoarthritis Differently" International Journal of Molecular Sciences 23, no. 14: 7553. https://doi.org/10.3390/ijms23147553
APA StyleJávor, P., Mácsai, A., Butt, E., Baráth, B., Jász, D. K., Horváth, T., Baráth, B., Csonka, Á., Török, L., Varga, E., & Hartmann, P. (2022). Mitochondrial Dysfunction Affects the Synovium of Patients with Rheumatoid Arthritis and Osteoarthritis Differently. International Journal of Molecular Sciences, 23(14), 7553. https://doi.org/10.3390/ijms23147553






