Role of PsnWRKY70 in Regulatory Network Response to Infection with Alternaria alternata (Fr.) Keissl in Populus
Abstract
:1. Introduction
2. Results
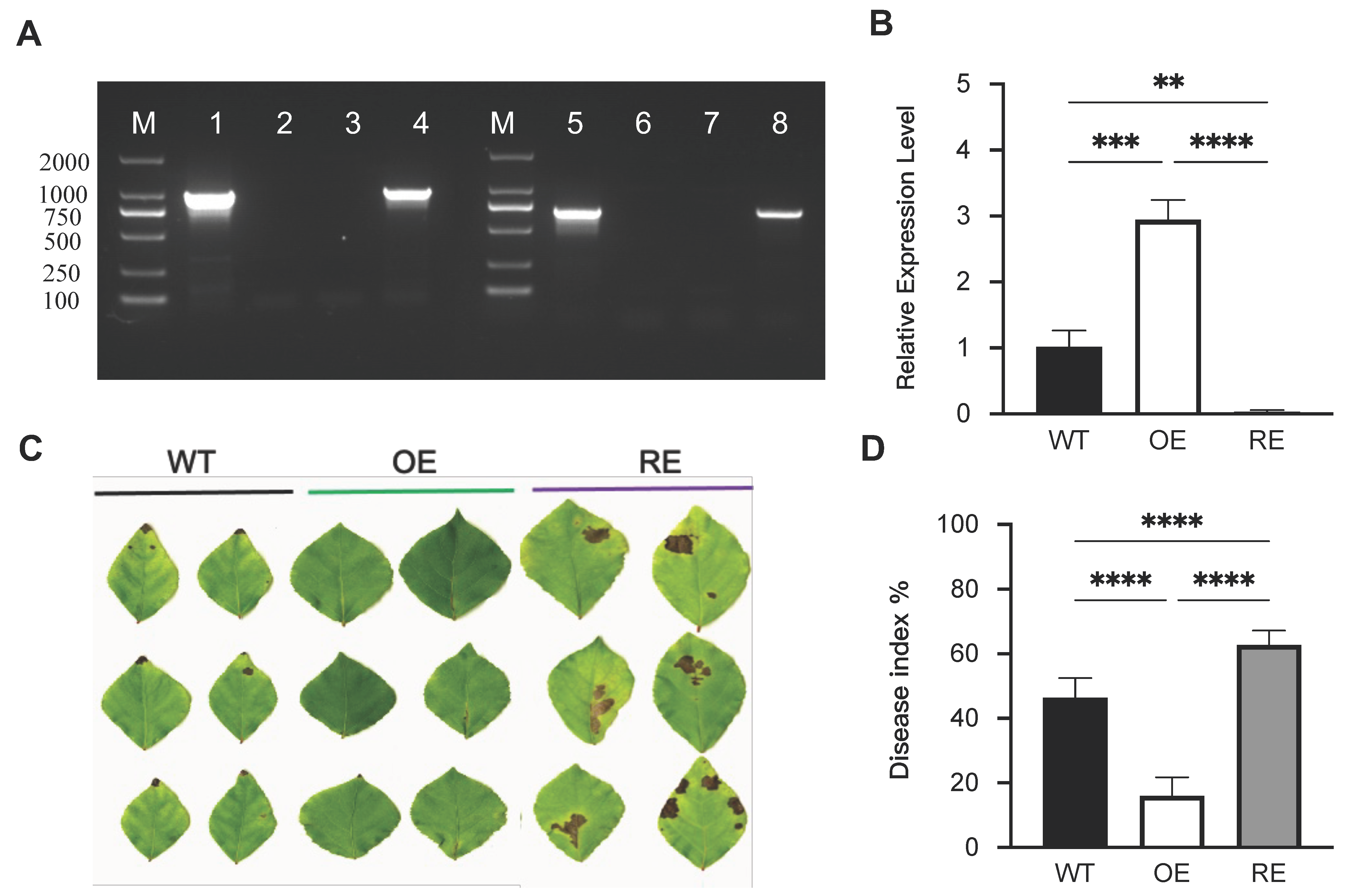
2.1. PsnWRKY70 Reduces Infection with A. alternata
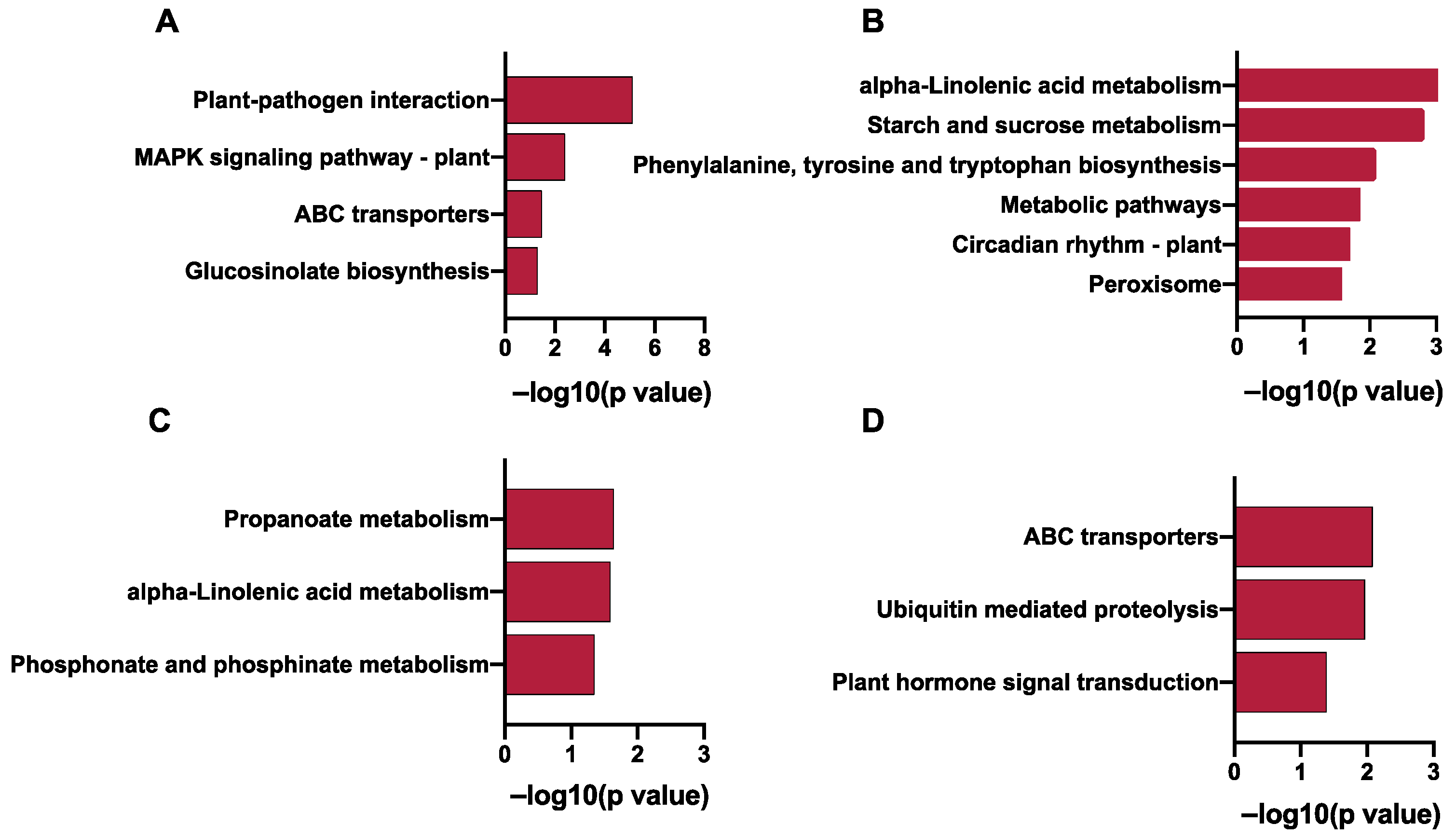
2.2. Transcriptime Analysis
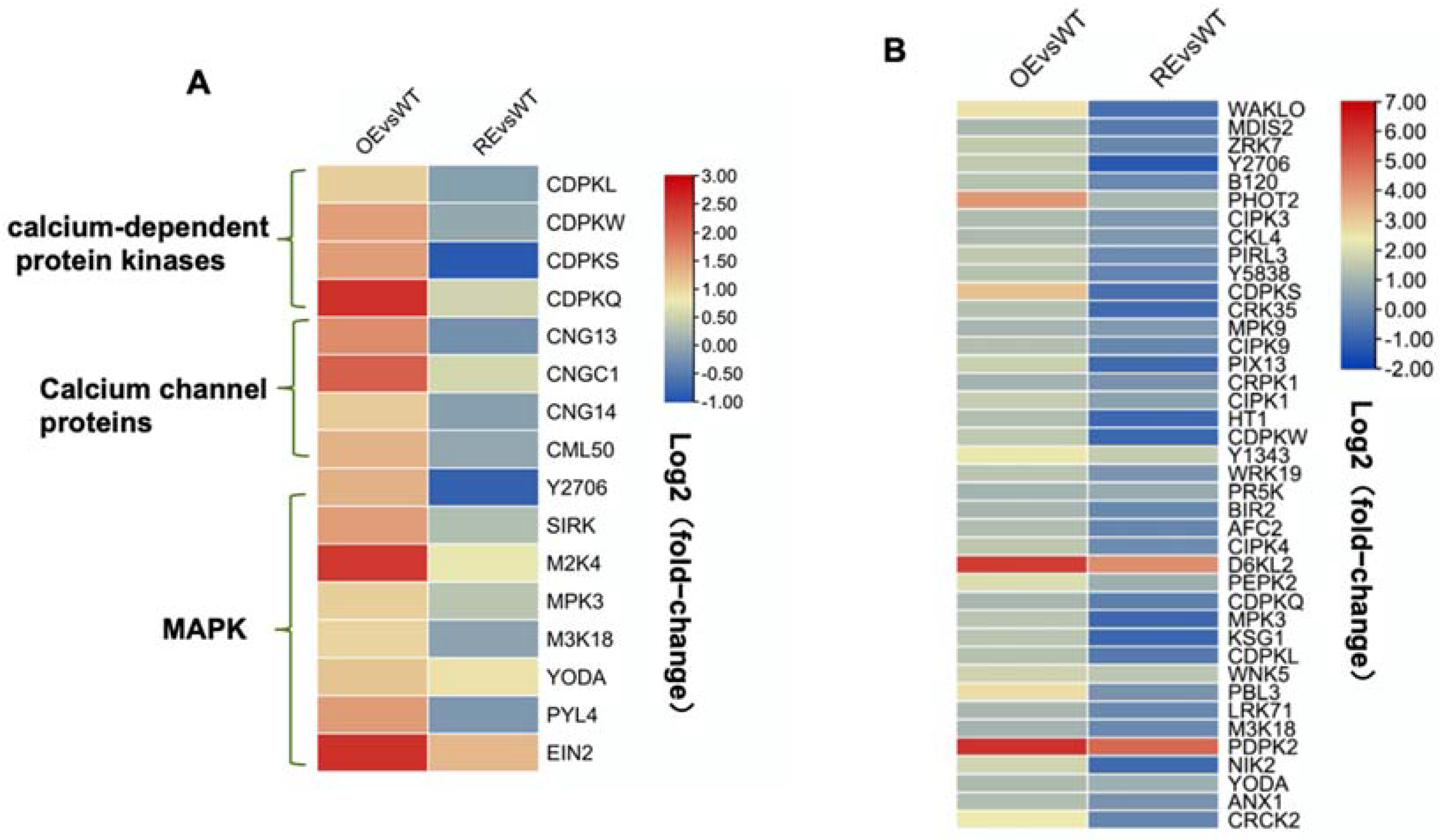
2.3. PsnWRKY70 Activates the Expression of MAPK and Ca2+ Signaling-Related Genes
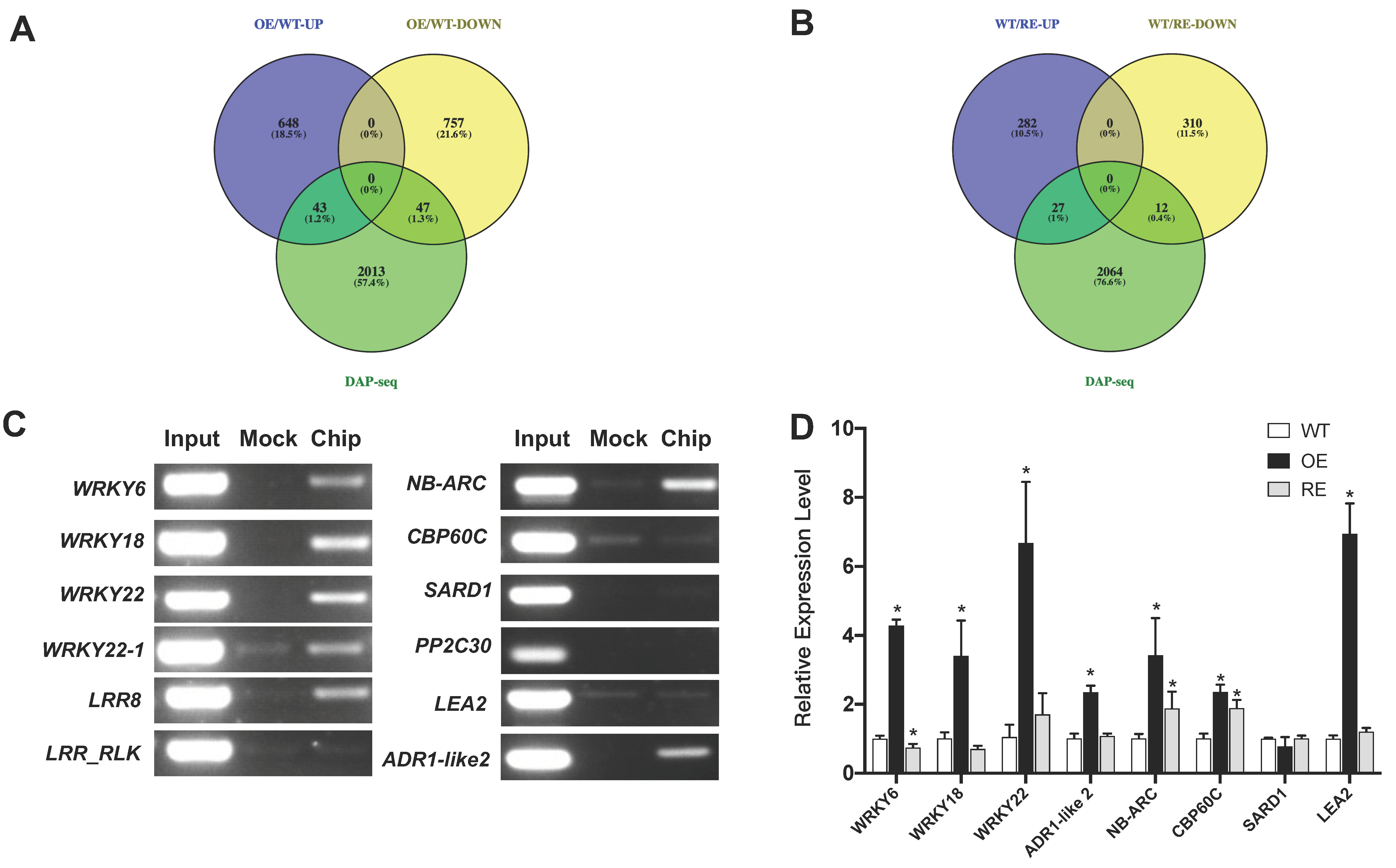
2.4. Genome-Wide Binding Targets of PsnWRKY70
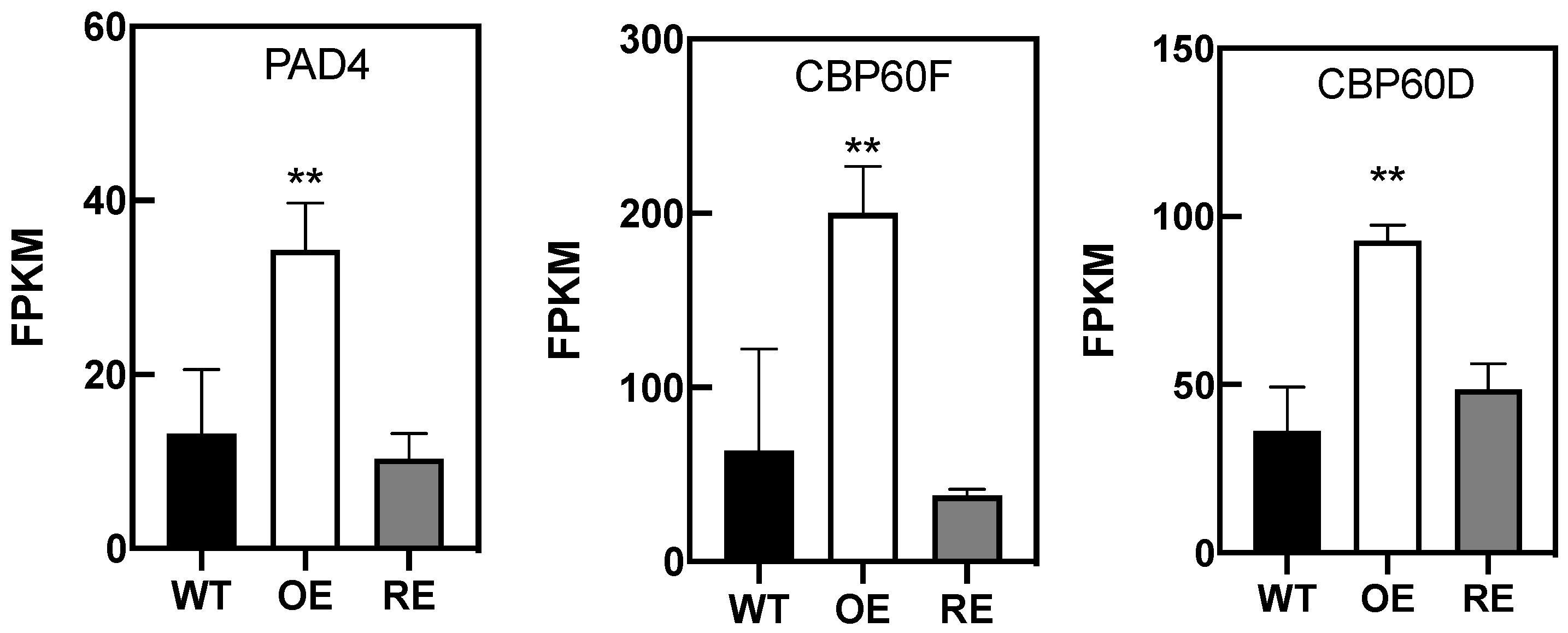
2.5. Target Genes Were Regulated by PsnWRKY70
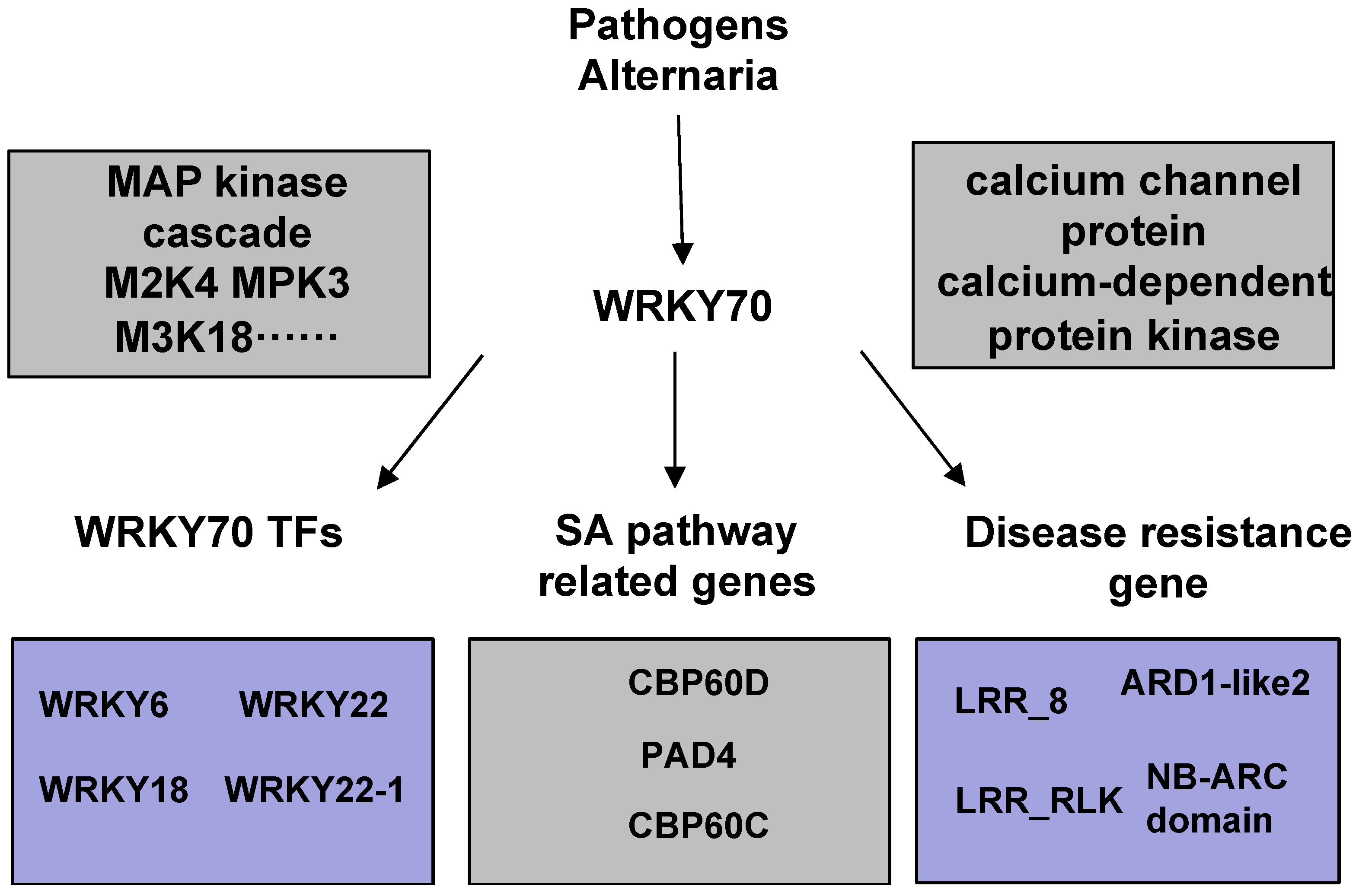
3. Discussion
3.1. PsnWRKY70 Induces Other WRKYs to Enhance Disease Resistance
3.2. PsnWRKY70 Plays a Key Role in PTI and ETI Pathways
4. Materials and Methods
4.1. Plant Material
4.2. Evaluation of Disease Severity
4.3. Transcriptome Analysis
4.4. Quantitative Real-Time Polymerase Chain Reaction (qPCR)
4.5. DAP-Seq Analysis
4.6. CHIP-PCR
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peng, X.; Wang, H.; Jang, J.-C.; Xiao, T.; He, H.; Jiang, D.; Tang, X. OsWRKY80-OsWRKY4 Module as a Positive Regulatory Circuit in Rice Resistance against Rhizoctonia solani. Rice 2016, 9, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, M.; Wu, R.; Song, Z.; Dong, B.; Chen, T.; Wang, M.; Cao, H.; Du, T.; Wang, S.; Li, N.; et al. Sorbitol Reduces Sensitivity to Alternaria by Promoting Ceramide Kinases (CERK) Expression through Transcription Factor Pswrky25 in Populus (Populus simonii Carr.). Genes 2022, 13, 405. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Liu, H.; Jia, Z.; Fang, Q.; Luo, K. Combined expression of antimicrobial genes (Bbchit1 and LJAMP2) in transgenic poplar enhances resistance to fungal pathogens. Tree Physiol. 2012, 32, 1313–1320. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.; Hu, X.; Ma, J.; Hettenhausen, C.; Wang, L.; Sun, G.; Wu, J. Requirement of ABA signalling-mediated stomatal closure for resistance of wild tobacco to Alternaria alternata. Plant Pathol. 2013, 63, 1070–1077. [Google Scholar] [CrossRef]
- Xin, J.; Liu, Y.; Li, H.; Chen, S.; Jiang, J.; Song, A.; Fang, W.; Chen, F. CmMLO17 and its partner CmKIC potentially support Alternaria alternata growth in Chrysanthemum morifolium. Hortic. Res. 2021, 8, 101. [Google Scholar] [CrossRef] [PubMed]
- Monaghan, J.; Zipfel, C. Plant pattern recognition receptor complexes at the plasma membrane. Curr. Opin. Plant Biol. 2012, 15, 349–357. [Google Scholar] [CrossRef]
- Nicaise, V.; Roux, M.; Zipfel, C. Recent Advances in PAMP-Triggered Immunity against Bacteria: Pattern Recognition Receptors Watch over and Raise the Alarm. Plant Physiol. 2009, 150, 1638–1647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ngou, B.P.M.; Ahn, H.-K.; Ding, P.; Jones, J.D.G. Mutual potentiation of plant immunity by cell-surface and intracellular receptors. Nature 2021, 592, 110–115. [Google Scholar] [CrossRef]
- Kadota, Y.; Sklenar, J.; Derbyshire, P.; Stransfeld, L.; Asai, S.; Ntoukakis, V.; Jones, J.D.; Shirasu, K.; Menke, F.; Jones, A.; et al. Direct Regulation of the NADPH Oxidase RBOHD by the PRR-Associated Kinase BIK1 during Plant Immunity. Mol. Cell 2014, 54, 43–55. [Google Scholar] [CrossRef] [Green Version]
- Peng, Y.; van Wersch, R.; Zhang, Y. Convergent and Divergent Signaling in PAMP-Triggered Immunity and Effector-Triggered Immunity. Mol. Plant-Microbe Interact. 2018, 31, 403–409. [Google Scholar] [CrossRef] [Green Version]
- Couto, D.; Zipfel, C. Regulation of pattern recognition receptor signalling in plants. Nat. Rev. Immunol. 2016, 16, 537–552. [Google Scholar] [CrossRef] [PubMed]
- Lapin, D.; Bhandari, D.D.; Parker, J.E. Origins and Immunity Networking Functions of EDS1 Family Proteins. Annu. Rev. Phytopathol. 2020, 58, 253–276. [Google Scholar] [CrossRef] [PubMed]
- Adachi, H.; Nakano, T.; Miyagawa, N.; Ishihama, N.; Yoshioka, M.; Katou, Y.; Yaeno, T.; Shirasu, K.; Yoshioka, H. WRKY Transcription Factors Phosphorylated by MAPK Regulate a Plant Immune NADPH Oxidase in Nicotiana benthamiana. Plant Cell 2015, 27, 2645–2663. [Google Scholar] [CrossRef] [Green Version]
- Yuan, M.; Jiang, Z.; Bi, G.; Nomura, K.; Liu, M.; Wang, Y.; Cai, B.; Zhou, J.; He, S.; Xin, X. Pattern-recognition receptors are required for NLR-mediated plant immunity. Nature 2021, 592, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.; Hwang, S.H.; Fang, I.R.; Kwon, S.I.; Park, S.R.; Ahn, I.; Kim, J.B.; Hwang, D.J. Molecular characterization of Oryza sativa WRKY6, which binds to W-box-like element 1 of the Oryza sativa pathogenesis-related (PR) 10a promoter and confers reduced susceptibility to pathogens. New Phytol. 2015, 208, 846–859. [Google Scholar] [CrossRef] [PubMed]
- Thieffry, A.; López-Márquez, D.; Bornholdt, J.; Malekroudi, M.G.; Bressendorff, S.; Barghetti, A.; Sandelin, A.; Brodersen, P. PAMP-triggered genetic reprogramming involves widespread alternative transcription initiation and an immediate transcription factor wave. Plant Cell 2022, 34, 2615–2637. [Google Scholar] [CrossRef]
- Winkelmüller, T.M.; Entila, F.; Anver, S.; Piasecka, A.; Song, B.; Dahms, E.; Sakakibara, H.; Gan, X.; Kułak, K.; Sawikowska, A.; et al. Gene expression evolution in pattern-triggered immunity within Arabidopsis thaliana and across Brassicaceae species. Plant Cell 2021, 33, 1863–1887. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Tian, A.; Zou, H.; Xie, Z.; Lei, G.; Huang, J.; Wang, C.; Wang, H.; Zhang, J.; Chen, S. Soybean WRKY-type transcription factor genes, GmWRKY13, GmWRKY21, and GmWRKY54, confer differ-ential tolerance to abiotic stresses in transgenic Arabidopsis plants. Plant Biotechnol. J. 2008, 6, 486–503. [Google Scholar] [CrossRef] [PubMed]
- Rushton, P.J.; Macdonald, H.; Huttly, A.K.; Lazarus, C.M.; Hooley, R. Members of a new family of DNA-binding proteins bind to a conserved cis-element in the promoters of al-pha-Amy2 genes. Plant Mol. Biol. 1995, 29, 691–702. [Google Scholar] [CrossRef]
- Rushton, P.J.; Torres, J.T.; Parniske, M.; Wernert, P.; Hahlbrock, K.; Somssich, I. Interaction of elicitor-induced DNA-binding proteins with elicitor response elements in the promoters of parsley PR1 genes. EMBO J. 1996, 15, 5690–5700. [Google Scholar] [CrossRef]
- Jiang, Y.; Duan, Y.; Yin, J.; Ye, S.; Zhu, J.; Zhang, F.; Lu, W.; Fan, D.; Luo, K. Genome-wide identification and characterization of the Populus WRKY transcription factor family and analysis of their expression in response to biotic and abiotic stresses. J. Exp. Bot. 2014, 65, 6629–6644. [Google Scholar] [CrossRef]
- Liu, F.; Li, X.; Wang, M.; Wen, J.; Yi, B.; Shen, J.; Ma, C.; Fu, T.; Tu, J. Interactions of WRKY15 and WRKY33 transcription factors and their roles in the resistance of oilseed rape to Scle-rotinia infection. Plant Biotechnol. J. 2018, 16, 911–925. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.; Jiang, J.; Li, K.; Liu, G. Populus simonii x Populus nigra WRKY70 is involved in salt stress and leaf blight disease responses. Tree Physiol. 2017, 37, 827–844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Shi, L.; Yang, S.; Qiu, S.; Ma, X.; Cai, J.; Guan, D.; Wang, Z.; He, S. A conserved double-W box in the promoter of CaWRKY40 mediates autoregulation during response to pathogen attack and heat stress in pepper. Mol. Plant Pathol. 2020, 22, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Turck, F.; Zhou, A.; Somssich, I.E. Stimulus-dependent, promoter-specific binding of transcription factor WRKY1 to Its native promoter and the defense-related gene PcPR1-1 in Parsley. Plant Cell 2004, 16, 2573–2585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.; Zhu, Z.; Gao, J.; Zhou, X.; Zhu, S.; Wang, X.; Wang, X.; Ren, G.; Kuai, B. The NPR1-WRKY46-WRKY6 signaling cascade mediates probenazole/salicylic acid-elicited leaf senescence in Arabidopsis thaliana. J. Integr. Plant Biol. 2021, 63, 924–936. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Liu, H.; Deng, Y.; Xiao, J.; Li, X.; Wang, S. The WRKY45-2 WRKY13 WRKY42 Transcriptional Regulatory Cascade Is Required for Rice Resistance to Fungal Pathogen. Plant Physiol. 2015, 167, 1087–1099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Chen, Z. Potentiation of Developmentally Regulated Plant Defense Response by AtWRKY18, a Pathogen-Induced Arabidopsis Transcription Factor. Plant Physiol. 2002, 129, 706–716. [Google Scholar] [CrossRef] [Green Version]
- Jones, J.D.G.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef] [Green Version]
- Dodds, P.N.; Rathjen, J.P. Plant immunity: Towards an integrated view of plant–pathogen interactions. Nat. Rev. Genet. 2010, 11, 539–548. [Google Scholar] [CrossRef]
- Pruitt, R.N.; Locci, F.; Wanke, F.; Zhang, L.; Saile, S.C.; Joe, A.; Karelina, D.; Hua, C.; Fröhlich, K.; Wan, W.L.; et al. The EDS1-PAD4-ADR1 node mediates Arabidopsis pattern-triggered immunity. Nature 2021, 598, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Cox, K.L., Jr.; He, P. Functions of Calcium-Dependent Protein Kinases in Plant Innate Immunity. Plants 2014, 3, 160–176. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Liu, X.; Zhang, A.; Ren, Y.; Wu, F.; Wang, G.; Xu, Y.; Lei, C.; Zhu, S.; Pan, T.; et al. A cyclic nucleotide-gated channel mediates cytoplasmic calcium elevation and disease resistance in rice. Cell Res. 2019, 29, 820–831. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Hou, C.; Ren, Z.; Wang, C.; Zhao, F.; Dahlbeck, D.; Hu, S.; Zhang, L.; Niu, Q.; Li, L.; et al. A calmodulin-gated calcium channel links pathogen patterns to plant immunity. Nature 2019, 572, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Han, X.; Feng, D.; Yuan, D.; Huang, L. Signaling Crosstalk between Salicylic Acid and Ethylene/Jasmonate in Plant Defense: Do We Understand What They Are Whispering? Int. J. Mol. Sci. 2019, 20, 671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Xu, S.; Ding, P.; Wang, D.; Cheng, Y.; He, J.; Gao, M.; Xu, F.; Li, Y.; Zhu, Z.; et al. Control of salicylic acid synthesis and systemic acquired resistance by two members of a plant-specific family of transcription factors. Proc. Natl. Acad. Sci. USA 2010, 107, 18220–18225. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Tsuda, K.; Truman, W.; Sato, M.; Nguyen, L.V.; Katagiri, F.; Glazebrook, J. CBP60g and SARD1 play partially redundant critical roles in salicylic acid signaling. Plant J. 2011, 67, 1029–1041. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Lu, Y.; Bethke, G.; Harrison, B.T.; Hatsugai, N.; Katagiri, F.; Glazebrook, J. WRKY70 prevents axenic activation of plant immunity by direct repression of SARD1. New Phytol. 2017, 217, 700–712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pertea, M.; Kim, D.; Pertea, G.M.; Leek, J.T.; Salzberg, S.L. Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nat. Protoc. 2016, 11, 1650–1667. [Google Scholar] [CrossRef]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.-C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef] [Green Version]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene ontology: Tool for the unification of biology. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gene Ontology, C. The Gene Ontology resource: Enriching a GOld mine. Nucleic Acids Res. 2021, 49, D325–D334. [Google Scholar] [CrossRef] [PubMed]
- Mi, H.; Muruganujan, A.; Ebert, D.; Huang, X.; Thomas, P.D. PANTHER version 14: More genomes, a new PANTHER GO-slim and improvements in enrichment analysis tools. Nucleic Acids Res. 2018, 47, D419–D426. [Google Scholar] [CrossRef]
- Bu, D.; Luo, H.; Huo, P.; Wang, Z.; Zhang, S.; He, Z.; Wu, Y.; Zhao, L.; Liu, J.; Guo, J.; et al. KOBAS-i: Intelligent prioritization and exploratory visualization of biological functions for gene enrichment analysis. Nucleic Acids Res. 2021, 49, W317–W325. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Y.; Wang, F.; Wang, G.; Wang, C.; Wang, W.; Chen, K.; Gu, C.; Yu, Q.; Jiang, J. Growth adaptability and foreign gene stability of TaLEA transgenic Populus simonii × nigra. Ann. For. Sci. 2021, 78, 42. [Google Scholar] [CrossRef]
- O’Malley, R.C.; Huang, S.-S.C.; Song, L.; Lewsey, M.G.; Bartlett, A.; Nery, J.R.; Galli, M.; Gallavotti, A.; Ecker, J.R. Cistrome and Epicistrome Features Shape the Regulatory DNA Landscape. Cell 2016, 165, 1280–1292. [Google Scholar] [CrossRef] [Green Version]
- Yao, J.; Shen, Z.; Zhang, Y.; Wu, X.; Wang, J.; Sa, G.; Zhang, Y.; Zhang, H.; Deng, C.; Liu, J.; et al. Populus euphratica WRKY1 binds the promoter of H+-ATPase gene to enhance gene expression and salt tolerance. J. Exp. Bot. 2019, 71, 1527–1539. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Liu, T.; Meyer, C.A.; Eeckhoute, J.; Johnson, D.S.; Bernstein, B.E.; Nusbaum, C.; Myers, R.M.; Brown, M.; Li, W.; et al. Model-based analysis of ChIP-Seq (MACS). Genome Biol. 2008, 9, R137. [Google Scholar] [CrossRef] [Green Version]
- Machanick, P.; Bailey, T.L. MEME-ChIP: Motif analysis of large DNA, datasets. Bioinformatics 2011, 27, 1696–1697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heinz, S.; Benner, C.; Spann, N.; Bertolino, E.; Lin, Y.C.; Laslo, P.; Cheng, J.X.; Murre, C.; Singh, H. Glass CKSimple combinations of lineage-determining transcription factors prime cis-regulatory elements required for mac-rophage and B cell identities. Mol. Cell 2010, 38, 576–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Lin, Y.; Li, Q.; Shi, R.; Lin, C.-Y.; Chen, H.; Chuang, L.; Qu, G.-Z.; Sederoff, R.R.; Chiang, V.L. A robust chromatin immunoprecipitation protocol for studying transcription factor–DNA interactions and histone modifications in wood-forming tissue. Nat. Protoc. 2014, 9, 2180–2193. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Huang, H.; Han, R.; Liu, C.; Qiu, Z.; Liu, G.; Chen, S.; Jiang, J. Negative feedback loop between BpAP1 and BpPI/BpDEF heterodimer in Betula platyphylla x B. pendula. Plant Sci. 2019, 289, 110280. [Google Scholar] [CrossRef]


| Gene | Description | Fold Change | p-Value | Peak |
|---|---|---|---|---|
| Potri.004G007500.1 | WRKY 6 | 1.1051 | 0.0192 | Chr04-461048-1 |
| Potri.014G195100.7 | LRR_8 | 1.3831 | 0.0453 | Chr14-17455217-2 |
| Potri.019G122500.2 | LRR_RLK | 1.5968 | 0.0015 | Chr19-14761541-1 |
| Potri.014G035700.1 | ADR1-like 2 | 1.3115 | 0.0008 | Chr14-2249744-1 |
| Potri.011G043300.1 | CBP60-C | 1.7518 | 0.0050 | Chr11-3380308-1 |
| Potri.006G263600.1 | WRKY18 | 1.4573 | 0.0089 | Chr06-25984884-1 |
| Potri.002G164400.4 | WRKY22 | 1.1219 | 0.0045 | Chr02-12589328-2 |
| Potri.T001933.3 | NB-ARC | 1.4444 | 0.0281 | scaffold_45-136830-2 |
| Potri.014G090300.1. | WRKY22-1 | 1.0772 | 0.0047 | Chr14-5869875-1 |
| Potri.003G133400.1 | LEA_2 | 1.2516 | 0.0002 | Chr03-15146326-1 |
| Potri.019G097300.1 | LRR | 3.1536 | 0.0385 | Chr19-13468226-1 |
| Potri.013G010700.2 | SARD1 | 1.4677 | 7.7484 × 10−6 | Chr13-680718-1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Bai, X.-D.; Chen, K.; Gu, C.-R.; Yu, Q.-B.; Jiang, J.; Liu, G.-F. Role of PsnWRKY70 in Regulatory Network Response to Infection with Alternaria alternata (Fr.) Keissl in Populus. Int. J. Mol. Sci. 2022, 23, 7537. https://doi.org/10.3390/ijms23147537
Wang W, Bai X-D, Chen K, Gu C-R, Yu Q-B, Jiang J, Liu G-F. Role of PsnWRKY70 in Regulatory Network Response to Infection with Alternaria alternata (Fr.) Keissl in Populus. International Journal of Molecular Sciences. 2022; 23(14):7537. https://doi.org/10.3390/ijms23147537
Chicago/Turabian StyleWang, Wei, Xiang-Dong Bai, Kun Chen, Chen-Rui Gu, Qi-Bin Yu, Jing Jiang, and Gui-Feng Liu. 2022. "Role of PsnWRKY70 in Regulatory Network Response to Infection with Alternaria alternata (Fr.) Keissl in Populus" International Journal of Molecular Sciences 23, no. 14: 7537. https://doi.org/10.3390/ijms23147537
APA StyleWang, W., Bai, X.-D., Chen, K., Gu, C.-R., Yu, Q.-B., Jiang, J., & Liu, G.-F. (2022). Role of PsnWRKY70 in Regulatory Network Response to Infection with Alternaria alternata (Fr.) Keissl in Populus. International Journal of Molecular Sciences, 23(14), 7537. https://doi.org/10.3390/ijms23147537






