PAX8 in the Junction between Development and Tumorigenesis
Abstract
1. Introduction
2. Paired Box Domain Family of Developmental Proteins
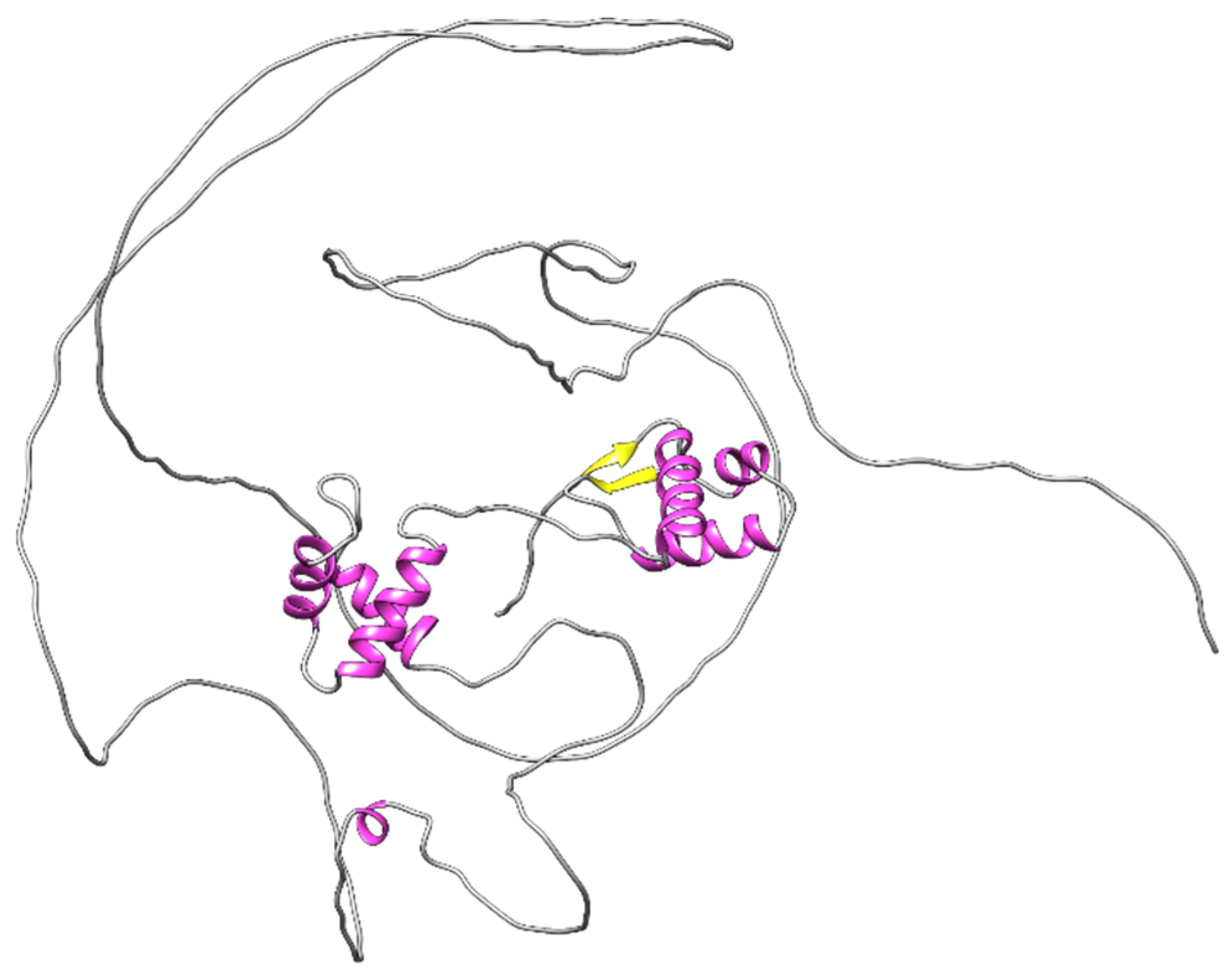
3. PAX8 Is a Member of the Paired Box Domain Gene Family
4. PAX8 in Thyroid Organogenesis and Cancer
4.1. The Role of PAX8 in Thyroid Organogenesis
4.2. The Role of PAX8 in Thyroid Carcinoma
5. PAX8 in Kidney Development and Cancer
5.1. The Role of PAX8 in Kidney Development
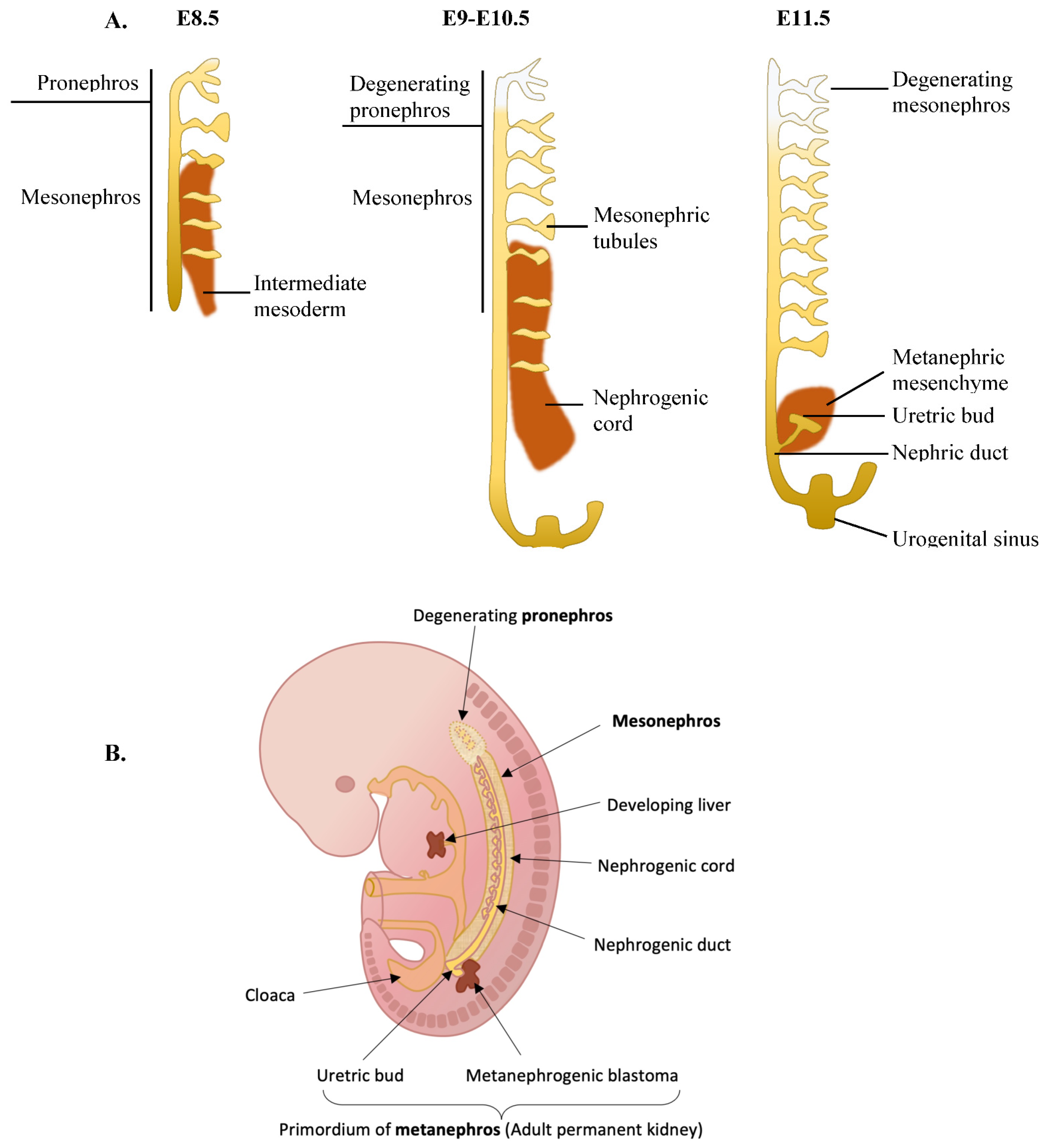
5.2. The Role of PAX8 in Renal Cancers
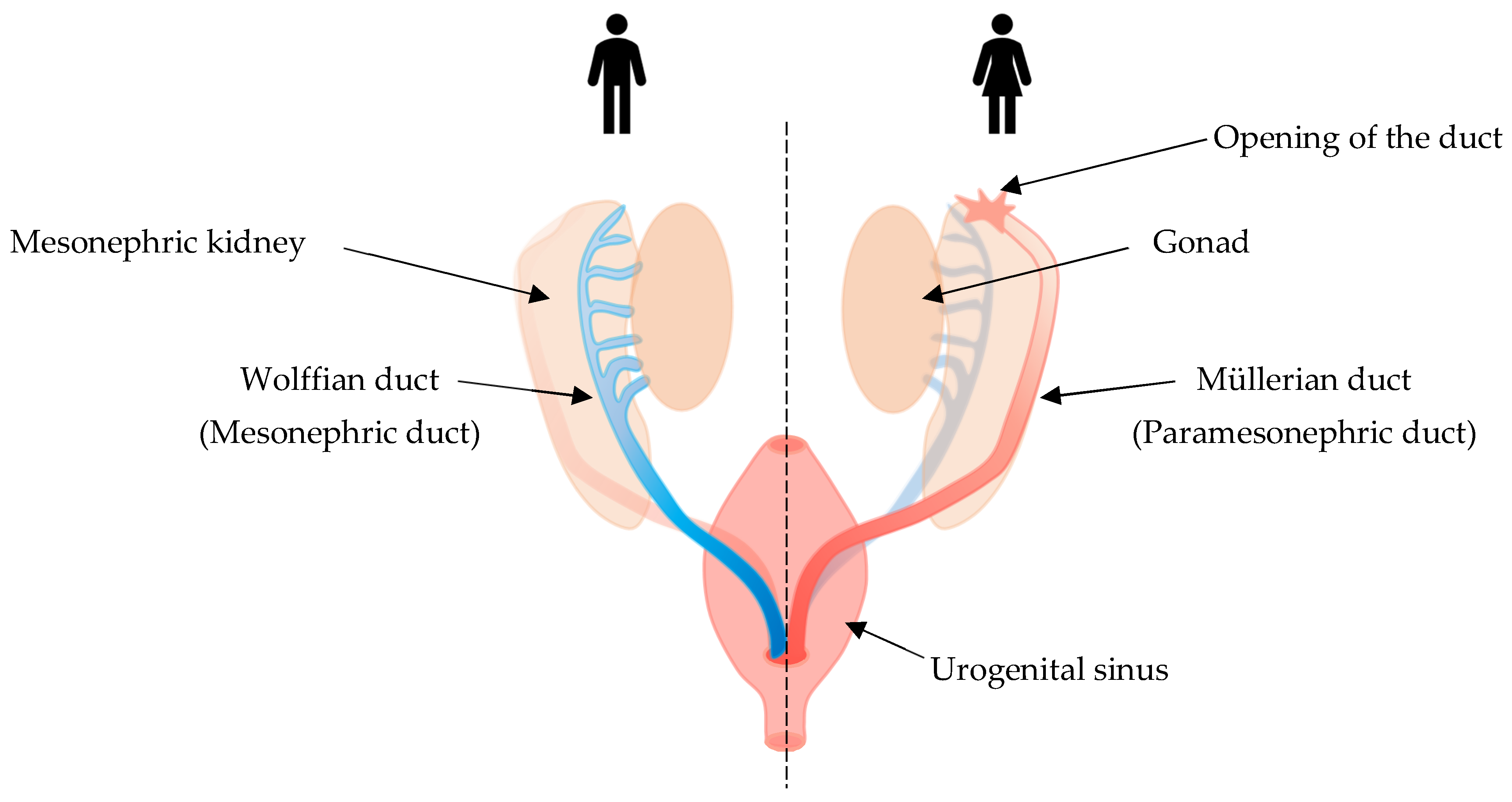
6. PAX8 Expression in Female Reproductive Tract Development and Cancer
6.1. The Role of PAX8 in Female Reproductive Tract Organogenesis
6.2. The Role of PAX8 in Tumors of the Female Genital System
7. PAX8 in the Male Reproductive System—Development and Cancer
7.1. The Role of PAX8 in Male Reproductive System Organogenesis
7.2. The Role of PAX8 in Male Genital Tract Neoplasms
8. PAX8 in Other Organs
9. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Perets, R.; Drapkin, R. It’s Totally Tubular…Riding The New Wave of Ovarian Cancer Research. Cancer Res. 2016, 76, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Cheung, H.W.; Cowley, G.S.; Weir, B.A.; Boehm, J.S.; Rusin, S.; Scott, J.A.; East, A.; Ali, L.D.; Lizotte, P.H.; Wong, T.C.; et al. Systematic investigation of genetic vulnerabilities across cancer cell lines reveals lineage-specific dependencies in ovarian cancer. Proc. Natl. Acad. Sci. USA 2011, 108, 12372–12377. [Google Scholar] [CrossRef] [PubMed]
- Ghannam-Shahbari, D.; Jacob, E.; Kakun, R.R.; Wasserman, T.; Korsensky, L.; Sternfeld, O.; Kagan, J.; Bublik, D.R.; Aviel-Ronen, S.; Levanon, K.; et al. PAX8 activates a p53-p21-dependent pro-proliferative effect in high grade serous ovarian carcinoma. Oncogene 2018, 37, 2213–2224. [Google Scholar] [CrossRef] [PubMed]
- Di Palma, T.; Lucci, V.; de Cristofaro, T.; Filippone, M.G.; Zannini, M. A role for PAX8 in the tumorigenic phenotype of ovarian cancer cells. BMC Cancer 2014, 14, 292. [Google Scholar] [CrossRef] [PubMed]
- Aiello, N.; Stanger, B.Z. Echoes of the embryo: Using the developmental biology toolkit to study cancer. Dis. Model. Mech. 2016, 9, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Stanger, B.Z. The tumor as organizer model. Science 2019, 363, 1038–1039. [Google Scholar] [CrossRef] [PubMed]
- Murray, M.J.; Lessey, B.A. Embryo Implantation and Tumor Metastasis: Common Pathways of Invasion and Angiogenesis. Semin. Reprod. Med. 1999, 17, 275–290. [Google Scholar] [CrossRef]
- Evans, M.J.; Kaufman, M.H. Establishment in culture of pluripotential cells from mouse embryos. Nature 1981, 292, 154–156. [Google Scholar] [CrossRef]
- Matsui, Y.; Zsebo, K.; Hogan, B.L. Derivation of pluripotential embryonic stem cells from murine primordial germ cells in culture. Cell 1992, 70, 841–847. [Google Scholar] [CrossRef]
- Cousin, H. Spemann–Mangold Grafts. Cold Spring Harb. Protoc. 2019, 2019, pdb-rot097345. [Google Scholar] [CrossRef]
- Oudin, M.; Weaver, V.M. Physical and Chemical Gradients in the Tumor Microenvironment Regulate Tumor Cell Invasion, Migration, and Metastasis. Cold Spring Harb. Symp. Quant. Biol. 2016, 81, 189–205. [Google Scholar] [CrossRef]
- Wellenstein, M.D.; de Visser, K.E. Cancer-Cell-Intrinsic Mechanisms Shaping the Tumor Immune Landscape. Immunity 2018, 48, 399–416. [Google Scholar] [CrossRef]
- Folkman, J. Tumor angiogenesis: Therapeutic implications. N. Engl. J. Med. 1971, 285, 1182–1186. [Google Scholar] [CrossRef]
- Buscail, L.; Bournet, B.; Cordelier, P. Role of oncogenic KRAS in the diagnosis, prognosis and treatment of pancreatic cancer. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 153–168. [Google Scholar] [CrossRef]
- Meškytė, E.M.; Keskas, S.; Ciribilli, Y. MYC as a Multifaceted Regulator of Tumor Microenvironment Leading to Metastasis. Int. J. Mol. Sci. 2020, 21, 7710. [Google Scholar] [CrossRef]
- Semenza, G.L.; Wang, G.L. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol. Cell Biol. 1992, 12, 5447–5454. [Google Scholar]
- Hardy, L.R.; Pergande, M.; Esparza, K.; Heath, K.N.; Önyüksel, H.; Cologna, S.M.; Burdette, J.E. Proteomic analysis reveals a role for PAX8 in peritoneal colonization of high grade serous ovarian cancer that can be targeted with micelle encapsulated thiostrepton. Oncogene 2019, 38, 6003–6016. [Google Scholar] [CrossRef]
- Chaves-Moreira, D.; Mitchell, M.A.; Arruza, C.; Rawat, P.; Sidoli, S.; Nameki, R.; Reddy, J.; Corona, R.I.; Afeyan, L.K.; Klein, I.A.; et al. The transcription factor PAX8 promotes angiogenesis in ovarian cancer through interaction with SOX17. Sci. Signal. 2022, 15. [Google Scholar] [CrossRef]
- Monk, M.; Holding, C. Human embryonic genes re-expressed in cancer cells. Oncogene 2001, 20, 8085–8091. [Google Scholar] [CrossRef]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, W.; Li, Y. Stemness-related markers in cancer. Cancer Transl. Med. 2017, 3, 87–95. [Google Scholar] [CrossRef]
- Gwak, J.M.; Kim, M.; Kim, H.J.; Jang, M.H.; Park, S.Y. Expression of embryonal stem cell transcription factors in breast cancer: Oct4 as an indicator for poor clinical outcome and tamoxifen resistance. Oncotarget 2017, 8, 36305–36318. [Google Scholar] [CrossRef]
- Bhatlekar, S.; Fields, J.Z.; Boman, B.M. HOX genes and their role in the development of human cancers. Klin. Wochenschr. 2014, 92, 811–823. [Google Scholar] [CrossRef]
- Montavon, T.; Soshnikova, N. Hox gene regulation and timing in embryogenesis. Semin. Cell Dev. Biol. 2014, 34, 76–84. [Google Scholar] [CrossRef]
- Domsch, K.; Papagiannouli, F.; Lohmann, I. Chapter Five—he HOX–Apoptosis Regulatory Interplay in Development and Disease. In Current Topics in Developmental Biology; Steller, H., Ed.; Academic Press: Cambridge, MA, USA, 2015; Volume 114, pp. 121–158. [Google Scholar]
- Paço, A.; de Bessa Garcia, S.A.; Castro, J.L.; Costa-Pinto, A.; Freitas, R. Roles of the HOX Proteins in Cancer Invasion and Metastasis. Cancers 2020, 13, 10. [Google Scholar] [CrossRef]
- Smith, J.; Zyoud, A.; Allegrucci, C. A Case of Identity: HOX Genes in Normal and Cancer Stem Cells. Cancers 2019, 11, 512. [Google Scholar] [CrossRef]
- Mark, M.; Rijli, F.M.; Chambon, P. Homeobox Genes in Embryogenesis and Pathogenesis. Pediatr. Res. 1997, 42, 421–429. [Google Scholar] [CrossRef]
- Lou, Y.; Fallah, Y.; Yamane, K.; E Berg, P. BP1, a potential biomarker for breast cancer prognosis. Biomarkers Med. 2018, 12, 535–545. [Google Scholar] [CrossRef]
- Tan, Y.; Testa, J. DLX Genes: Roles in Development and Cancer. Cancers 2021, 13, 3005. [Google Scholar] [CrossRef]
- Holland, P.W.H. Evolution of homeobox genes. Wiley Interdiscip. Rev. Dev. Biol. 2012, 2, 31–45. [Google Scholar] [CrossRef]
- Holland, P.W.H.; Booth, H.A.F.; Bruford, E. Classification and nomenclature of all human homeobox genes. BMC Biol. 2007, 5, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Lang, D.; Powell, S.K.; Plummer, R.S.; Young, K.P.; Ruggeri, B.A. PAX genes: Roles in development, pathophysiology, and cancer. Biochem. Pharmacol. 2007, 73, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Chi, N.; Epstein, J.A. Getting your Pax straight: Pax proteins in development and disease. Trends Genet. 2002, 18, 41–47. [Google Scholar] [CrossRef]
- Robson, E.J.D.; He, S.-J.; Eccles, M.R. A PANorama of PAX genes in cancer and development. Nat. Cancer 2006, 6, 52–62. [Google Scholar] [CrossRef]
- Noll, M. Evolution and role of Pax genes. Curr. Opin. Genet. Dev. 1993, 3, 595–605. [Google Scholar] [CrossRef]
- Balczarek, K.A.; Lai, Z.C.; Kumar, S. Evolution of functional diversification of the paired box (Pax) DNA-binding domains. Mol. Biol. Evol. 1997, 14, 829–842. [Google Scholar] [CrossRef]
- Thompson, B.; Davidson, E.A.; Liu, W.; Nebert, D.W.; Bruford, E.A.; Zhao, H.; Dermitzakis, E.T.; Thompson, D.C.; Vasiliou, V. PAX Genes in Cancer; Friends specific variant expression and involvement in human disease. Hum. Genet. 2021, 140, 381–400. [Google Scholar] [CrossRef]
- Li, C.G.; Eccles, M.R. PAX Genes in Cancer; Friends or Foes? Front. Genet. 2012, 3, 6. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Varadi, M.; Anyango, S.; Deshpande, M.; Nair, S.; Natassia, C.; Yordanova, G.; Yuan, D.; Stroe, O.; Wood, G.; Laydon, A.; et al. AlphaFold Protein Structure Database: Massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res. 2022, 50, D439–D444. [Google Scholar] [CrossRef]
- Mansouri, A.; Chowdhury, K.; Gruss, P. Follicular cells of the thyroid gland require Pax8 gene function. Nat. Genet. 1998, 19, 87–90. [Google Scholar] [CrossRef]
- Campagnolo, M.; Pesaresi, A.; Zelezetsky, I.; Geremia, S.; Randaccio, L.; Bisca, A.; Tell, G. Structural Studies on Pax-8 Prd Domain/DNA Complex. J. Biomol. Struct. Dyn. 2007, 24, 429–441. [Google Scholar] [CrossRef]
- Codutti, L.; van Ingen, H.; Vascotto, C.; Fogolari, F.; Corazza, A.; Tell, G.; Quadrifoglio, F.; Viglino, P.; Boelens, R.; Esposito, G. The Solution Structure of DNA-free Pax-8 Paired Box Domain Accounts for Redox Regulation of Transcriptional Activity in the Pax Protein Family. J. Biol. Chem. 2008, 283, 33321–33328. [Google Scholar] [CrossRef]
- Kozmik, Z.; Kurzbauer, R.; Dörfler, P.; Busslinger, M. Alternative splicing of Pax-8 gene transcripts is developmentally regulated and generates isoforms with different transactivation properties. Mol. Cell. Biol. 1993, 13, 6024–6035. [Google Scholar]
- Szczepanek-Parulska, E.; Szaflarski, W.; Piątek, K.; Budny, B.; Jaszczyńska-Nowinka, K.; Biczysko, M.; Wierzbicki, T.; Skrobisz, J.; Zabel, M.; Ruchała, M. Alternative 3’ acceptor site in the exon 2 of human PAX8 gene resulting in the expression of unknown mRNA variant found in thyroid hemiagenesis and some types of cancers. Acta Biochim. Pol. 2013, 60, 573–578. [Google Scholar] [CrossRef]
- Johansson, E.; Andersson, L.; Örnros, J.; Carlsson, T.; Ingeson-Carlsson, C.; Liang, S.; Dahlberg, J.; Jansson, S.; Parrillo, L.; Zoppoli, P.; et al. Revising the embryonic origin of thyroid C cells in mice and humans. Development 2015, 142, 3519–3528. [Google Scholar]
- Mauchamp, J.; Mirrione, A.; Alquier, C.; André, F. Follicle-like structure and polarized monolayer: Role of the extracellular matrix on thyroid cell organization in primary culture. Biol. Cell 1998, 90, 369–380. [Google Scholar] [CrossRef]
- Fernández, L.P.; López-Márquez, A.; Santisteban, P. Thyroid transcription factors in development, differentiation and disease. Nat. Rev. Endocrinol. 2014, 11, 29–42. [Google Scholar] [CrossRef]
- Fagman, H.; Amendola, E.; Parrillo, L.; Zoppoli, P.; Marotta, P.; Scarfò, M.; De Luca, P.; de Carvalho, D.P.; Ceccarelli, M.; De Felice, M.; et al. Gene expression profiling at early organogenesis reveals both common and diverse mechanisms in foregut patterning. Dev. Biol. 2011, 359, 163–175. [Google Scholar] [CrossRef]
- Veis, D.J.; Sorenson, C.M.; Shutter, J.R.; Korsmeyer, S.J. Bcl-2-deficient mice demonstrate fulminant lymphoid apoptosis, polycystic kidneys, and hypopigmented hair. Cell 1993, 75, 229–240. [Google Scholar] [CrossRef]
- Parlato, R.; Rosica, A.; Rodriguez-Mallon, A.; Affuso, A.; Postiglione, M.P.; Arra, C.; Mansouri, A.; Kimura, S.; Di Lauro, R.; De Felice, M. An integrated regulatory network controlling survival and migration in thyroid organogenesis. Dev. Biol. 2004, 276, 464–475. [Google Scholar] [CrossRef]
- De Felice, M.; Ovitt, C.; Biffali, E.; Rodriguez-Mallon, A.; Arra, C.; Anastassiadis, K.; Macchia, P.E.; Mattei, M.-G.; Mariano, A.; Schöler, H.; et al. A mouse model for hereditary thyroid dysgenesis and cleft palate. Nat. Genet. 1998, 19, 395–398. [Google Scholar] [CrossRef]
- Fisher, D.A.; Dussault, J.H.; Sack, J.; Chopra, I.J. Ontogenesis of hypothalamic pituitary thyroid function and metabolism in man, sheep, and rat. Recent Prog. Horm. Res. 1977, 33, 59–116. [Google Scholar]
- Sgalitzer, K.E. Contribution to the study of the morphogenesis of the thyroid gland. J. Anat. 1941, 75, 389–405. [Google Scholar]
- Kaufman, M.H.; Bard, J.B.L. The Anatomical Basis of Mouse Development; Gulf Professional Publishing: Houston, TX, USA, 1999. [Google Scholar]
- Polak, M.; Sura-Trueba, S.; Chauty, A.; Szinnai, G.; Carré, A.; Castanet, M. Molecular Mechanisms of Thyroid Dysgenesis. Horm. Res. Paediatr. 2004, 62, 14–21. [Google Scholar] [CrossRef]
- De Felice, M.; Di Lauro, R. Thyroid Development and Its Disorders: Genetics and Molecular Mechanisms. Endocr. Rev. 2004, 25, 722–746. [Google Scholar] [CrossRef]
- di Magliano, M.P.; Di Lauro, R.; Zannini, M. Pax8 has a key role in thyroid cell differentiation. Proc. Natl. Acad. Sci. USA 2000, 97, 13144–13149. [Google Scholar] [CrossRef]
- D’Andrea, B.; Iacone, R.; Di Palma, T.; Nitsch, R.; Baratta, M.G.; Nitsch, L.; Di Lauro, R.; Zannini, M. Functional Inactivation of the Transcription Factor Pax8 through Oligomerization Chain Reaction. Mol. Endocrinol. 2006, 20, 1810–1824. [Google Scholar] [CrossRef] [PubMed]
- Macchia, P.E.; Lapi, P.; Krude, H.; Pirro, M.T.; Missero, C.; Chiovato, L.; Souabni, A.; Baserga, M.; Tassi, V.; Pinchera, A.; et al. PAX8 mutations associated with congenital hypothyroidism caused by thyroid dysgenesis. Nat. Genet. 1998, 19, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Hermanns, P.; Grasberger, H.; Cohen, R.; Freiberg, C.; Dörr, H.G.; Refetoff, S.; Pohlenz, J. Two cases of thyroid dysgenesis caused by different novel PAX8 mutations in the DNA-binding region: In vitro studies reveal different pathogenic mechanisms. Thyroid 2013, 23, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Nettore, I.C.; Cacace, V.; De Fusco, C.; Colao, A.; Macchia, P.E. The molecular causes of thyroid dysgenesis: A systematic review. J. Endocrinol. Investig. 2013, 36, 654–664. [Google Scholar]
- Hermanns, P.; Grasberger, H.; Refetoff, S.; Pohlenz, J. Mutations in the NKX2.5 gene and the PAX8 promoter in a girl with thyroid dysgenesis. J. Clin. Endocrinol. Metab. 2011, 96, E977–E981. [Google Scholar] [CrossRef]
- Hermanns, P.; Shepherd, S.; Mansor, M.; Schulga, J.; Jones, J.; Donaldson, M.; Pohlenz, J. A new mutation in the promoter region of the PAX8 gene causes true congenital hypothyroidism with thyroid hypoplasia in a girl with Down’s syndrome. Thyroid 2014, 24, 939–944. [Google Scholar] [CrossRef]
- Grasberger, H.; Ringkananont, U.; LeFrancois, P.; Abramowicz, M.; Vassart, G.; Refetoff, S. Thyroid transcription factor 1 rescues PAX8/p300 synergism impaired by a natural PAX8 paired domain mutation with dominant negative activity. Mol. Endocrinol. 2005, 19, 1779–1791. [Google Scholar] [CrossRef]
- Di Palma, T.; Zampella, E.; Filippone, M.G.; Macchia, P.E.; Ris-Stalpers, C.; De Vroede, M.; Zannini, M. Characterization of a novel loss-of-function mutation of PAX8 associated with congenital hypothyroidism. Clin. Endocrinol. 2010, 73, 808–814. [Google Scholar] [CrossRef]
- Fernández-Méndez, C.; Santisteban, P. A Critical Balance Between PAX8 and the Hippo Mediator TAZ Determines Sodium/Iodide Symporter Expression and Function. Thyroid 2022, 32, 315–325. [Google Scholar] [CrossRef]
- Di Palma, T.; D’Andrea, B.; Liguori, G.L.; Liguoro, A.; de Cristofaro, T.; Del Prete, D.; Pappalardo, A.; Mascia, A.; Zannini, M. TAZ is a coactivator for Pax8 and TTF-1, two transcription factors involved in thyroid differentiation. Exp. Cell Res. 2009, 315, 162–175. [Google Scholar] [CrossRef]
- Dralle, H.; Machens, A.; Basa, J.; Fatourechi, V.; Franceschi, S.; Hay, I.D.; Nikiforov, Y.E.; Pacini, F.; Pasieka, J.L.; Sherman, S.I. Follicular cell-derived thyroid cancer. Nat. Rev. Dis. Primers 2015, 1, 15077. [Google Scholar] [CrossRef]
- Parameswaran, R.; Brooks, S.; Sadler, G.P. Molecular pathogenesis of follicular cell derived thyroid cancers. Int. J. Surg. 2010, 8, 186–193. [Google Scholar] [CrossRef]
- LiVolsi, V.A. Papillary thyroid carcinoma: An update. Mod. Pathol. 2011, 24, S1–S9. [Google Scholar] [CrossRef]
- Enewold, L.; Zhu, K.; Ron, E.; Marrogi, A.J.; Stojadinovic, A.; Peoples, G.E.; Devesa, S.S. Rising Thyroid Cancer Incidence in the United States by Demographic and Tumor Characteristics, 1980–2005. Cancer Epidemiol. Biomark. Prev. 2009, 18, 784–791. [Google Scholar] [CrossRef]
- Pstrąg, N.; Ziemnicka, K.; Bluyssen, H.; Wesoły, J. Thyroid cancers of follicular origin in a genomic light: In-depth overview of common and unique molecular marker candidates. Mol. Cancer 2018, 17, 1–17. [Google Scholar] [CrossRef]
- Nonaka, D.; Tang, Y.; Chiriboga, L.; Rivera, M.; Ghossein, R.A. Diagnostic utility of thyroid transcription factors Pax8 and TTF-2 (FoxE1) in thyroid epithelial neoplasms. Mod. Pathol. 2007, 21, 192–200. [Google Scholar] [CrossRef]
- Bishop, J.A.; Sharma, R.; Westra, W.H. PAX8 immunostaining of anaplastic thyroid carcinoma: A reliable means of discerning thyroid origin for undifferentiated tumors of the head and neck. Hum. Pathol. 2011, 42, 1873–1877. [Google Scholar] [CrossRef] [PubMed]
- Laury, A.R.; Perets, R.; Piao, H.; Krane, J.F.; Barletta, J.A.; French, C.; Chirieac, L.R.; Lis, R.; Loda, M.; Hornick, J.L.; et al. A Comprehensive Analysis of PAX8 Expression in Human Epithelial Tumors. Am. J. Surg. Pathol. 2011, 35, 816–826. [Google Scholar] [CrossRef] [PubMed]
- French, C.A.; Alexander, E.K.; Cibas, E.S.; Nose, V.; Laguette, J.; Faquin, W.; Garber, J.; Moore, F.; Fletcher, J.A.; Larsen, P.R.; et al. Genetic and Biological Subgroups of Low-Stage Follicular Thyroid Cancer. Am. J. Pathol. 2003, 162, 1053–1060. [Google Scholar] [CrossRef]
- Tai, T.-A.C.; Jennermann, C.; Brown, K.K.; Oliver, B.B.; MacGinnitie, M.A.; Wilkison, W.O.; Brown, H.R.; Lehmann, J.M.; Kliewer, S.A.; Morris, D.C.; et al. Activation of the Nuclear Receptor Peroxisome Proliferator-activated Receptor γ Promotes Brown Adipocyte Differentiation. J. Biol. Chem. 1996, 271, 29909–29914. [Google Scholar] [CrossRef]
- Raman, P.; Koenig, R.J. PAX8-PPARγ fusion protein in thyroid carcinoma. Nat. Rev. Endocrinol. 2014, 10, 616–623. [Google Scholar] [CrossRef]
- Rosignolo, F.; Sponziello, M.; Durante, C.; Puppin, C.; Mio, C.; Baldan, F.; Di Loreto, C.; Russo, D.; Filetti, S.; Damante, G. Expression of PAX8 Target Genes in Papillary Thyroid Carcinoma. PLoS ONE 2016, 11, e0156658. [Google Scholar] [CrossRef]
- Credendino, S.C.; Bellone, M.L.; Lewin, N.; Amendola, E.; Sanges, R.; Basu, S.; Sepe, R.; Decaussin-Petrucci, M.; Tinto, N.; Fusco, A.; et al. A ceRNA Circuitry Involving the Long Noncoding RNA Klhl14-AS, Pax8, and Bcl2 Drives Thyroid Carcinogenesis. Cancer Res. 2019, 79, 5746–5757. [Google Scholar] [CrossRef]
- Dupain, C.; Ali, H.M.; Mouhoub, T.A.; Urbinati, G.; Massaad-Massade, L. Induction of TTF-1 or PAX-8 expression on proliferation and tumorigenicity in thyroid carcinomas. Int. J. Oncol. 2016, 49, 1248–1258. [Google Scholar] [CrossRef]
- Bouchard, M.; Pfeffer, P.; Busslinger, M. Functional equivalence of the transcription factors Pax2 and Pax5 in mouse development. Development 2000, 127, 3703–3713. [Google Scholar] [CrossRef]
- Vetter, M.R.; Gibley, C.W. Morphogenesis and histochemistry of the developing mouse kidney. J. Morphol. 1966, 120, 135–155. [Google Scholar] [CrossRef]
- Davidson, A.J. Mouse kidney development. In StemBook; Harvard Stem Cell Institut: Cambridge, MA, USA, 2008. [Google Scholar]
- Tan, W.-H.; Gilmore, E.C.; Baris, H.N. Human Developmental Genetics. In Emery and Rimoin’s Principles and Practice of Medical Genetics, 6th ed.; Academic Press: Cambridge, MA, USA, 2013; pp. 1–63. [Google Scholar] [CrossRef]
- Birchmeier, W. Epithelial Morphogenesis in Development and Disease; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Sainio, K.; Hellstedt, P.; Kreidberg, J.; Saxen, L.; Sariola, H. Differential regulation of two sets of mesonephric tubules by WT-1. Development 1997, 124, 1293–1299. [Google Scholar] [CrossRef]
- Smith, C.; Mackay, S. Morphological development and fate of the mouse mesonephros. J. Anat. 1991, 174, 171–184. [Google Scholar]
- Bouchard, M.; Souabni, A.; Mandler, M.; Neubüser, A.; Busslinger, M. Nephric lineage specification by Pax2 and Pax8. Genes Dev. 2002, 16, 2958–2970. [Google Scholar] [CrossRef]
- Dressler, G.R.; Wilkinson, J.E.; Rothenpieler, U.W.; Patterson, L.T.; Williams-Simons, L.; Westphal, H. Deregulation of Pax-2 expression in transgenic mice generates severe kidney abnormalities. Nature 1993, 362, 65–67. [Google Scholar] [CrossRef]
- Dressler, G.; Deutsch, U.; Chowdhury, K.; Nornes, H.; Gruss, P. Pax2, a new murine paired-box-containing gene and its expression in the developing excretory system. Development 1990, 109, 787–795. [Google Scholar] [CrossRef]
- Sanyanusin, P.; Schimmenti, L.; McNoe, L.A.; Ward, T.A.; Pierpont, M.E.M.; Sullivan, M.J.; Dobyns, W.; Eccles, M.R. Mutation of the PAX2 gene in a family with optic nerve colobomas, renal anomalies and vesicoureteral reflux. Nat. Genet. 1995, 9, 358–364. [Google Scholar] [CrossRef]
- Tong, G.-X.; Yu, W.M.; Beaubier, N.T.; Weeden, E.M.; Hamele-Bena, D.; Mansukhani, M.M.; O’Toole, K.M. Expression of PAX8 in normal and neoplastic renal tissues: An immunohistochemical study. Mod. Pathol. 2009, 22, 1218–1227. [Google Scholar] [CrossRef]
- Laszczyk, A.M.; Higashi, A.Y.; Patel, S.R.; Johnson, C.; Soofi, A.; Abraham, S.; Dressler, G.R. Pax2 and Pax8 Proteins Regulate Urea Transporters and Aquaporins to Control Urine Concentration in the Adult Kidney. J. Am. Soc. Nephrol. 2020, 31, 1212–1225. [Google Scholar] [CrossRef] [PubMed]
- Kousta, E.; Papathanasiou, A.; Skordis, N. Sex determination and disorders of sex development according to the revised nomenclature and classification in 46, XX individuals. Hormones 2010, 9, 218–231. [Google Scholar] [CrossRef]
- Tagge, E.P.; Hanson, P.; Re, G.G.; Othersen, H.; Smith, C.D.; Garvin, A. Paired box gene expression in Wilms’ tumor. J. Pediatr. Surg. 1994, 29, 134–141. [Google Scholar] [CrossRef]
- Sefidbakht, S.; Khorsand-Rahimzadeh, A.; Omidi, S.; Mohsenpourian, S.; Mirzaian, E. Expression of PAX2 and PAX8 in Wilms Tumor: A Tissue Microarray-based Immunohistochemical Study. Iran. J. Pathol. 2021, 16, 310–315. [Google Scholar] [CrossRef]
- Poleev, A.; Fickenscher, H.; Mundlos, S.; Winterpacht, A.; Zabel, B.; Fidler, A.; Gruss, P.; Plachov, D. PAX8, a human paired box gene: Isolation and expression in developing thyroid, kidney and Wilms’ tumors. Development 1992, 116, 611–623. [Google Scholar] [CrossRef]
- Ozcan, A.; de la Roza, G.; Ro, J.Y.; Shen, S.S.; Truong, L.D. PAX2 and PAX8 Expression in Primary and Metastatic Renal Tumors: A Comprehensive Comparison. Arch. Pathol. Lab. Med. 2012, 136, 1541–1551. [Google Scholar] [CrossRef]
- Bleu, M.; Gaulis, S.; Lopes, R.; Sprouffske, K.; Apfel, V.; Holwerda, S.; Pregnolato, M.; Yildiz, U.; Cordo’, V.; Dost, A.F.M.; et al. PAX8 activates metabolic genes via enhancer elements in Renal Cell Carcinoma. Nat. Commun. 2019, 10, 1–10. [Google Scholar] [CrossRef]
- Li, C.G.; Nyman, J.E.; Braithwaite, A.W.; Eccles, M.R. PAX8 promotes tumor cell growth by transcriptionally regulating E2F1 and stabilizing RB protein. Oncogene 2011, 30, 4824–4834. [Google Scholar] [CrossRef]
- Coalson, R.E.; Tomasek, J.J. Urogenital System. In Embryology; Springer: Berlin/Heidelberg, Germany, 1992; pp. 95–102. [Google Scholar] [CrossRef]
- Kobayashi, A.; Behringer, R.R. Developmental genetics of the female reproductive tract in mammals. Nat. Rev. Genet. 2003, 4, 969–980. [Google Scholar] [CrossRef]
- Wilson, D.; Bordoni, B. Embryology, Mullerian Ducts (Paramesonephric Ducts). In StatPearls; StatPearls Publishing: Tampa, FL, USA, 2022. [Google Scholar]
- Aimen, F.M.; Atef, Y.; Majed, G.; Manel, M.; Monia, M.; Radhouane, A.; Hedi, R.; Khaled, N. Spontaneous pregnancy after vaginoplasty in a patient presenting a congenital vaginal aplasia. Asian Pac. J. Reprod. 2016, 5, 351–353. [Google Scholar] [CrossRef]
- Robboy, S.J.; Kurita, T.; Baskin, L.; Cunha, G.R. New insights into human female reproductive tract development. Differentiation 2017, 97, 9–22. [Google Scholar] [CrossRef]
- Joki-Erkkilä, M.M.; Heinonen, P.K. Presenting and long-term clinical implications and fecundity in females with obstructing vaginal malformations. J. Pediatr. Adolesc. Gynecol. 2003, 16, 307–312. [Google Scholar] [CrossRef]
- Sanfilippo, J.S.; Lara-Torre, E.; Gomez-Lobo, V. Sanfilippo’s Textbook of Pediatric and Adolescent Gynecology, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Kobayashi, A.; Shawlot, W.; Kania, A.; Behringer, R.R. Requirement of Lim1 for female reproductive tract development. Development 2004, 131, 539–549. [Google Scholar] [CrossRef]
- Orvis, G.D.; Behringer, R.R. Cellular mechanisms of Müllerian duct formation in the mouse. Dev. Biol. 2007, 306, 493–504. [Google Scholar] [CrossRef]
- Vainio, S.; Heikkilä, M.; Kispert, A.; Chin, N.; McMahon, A.P. Female development in mammals is regulated by Wnt-4 signalling. Nature 1999, 397, 405–409. [Google Scholar] [CrossRef]
- Carroll, T.J.; Park, J.-S.; Hayashi, S.; Majumdar, A.; McMahon, A.P. Wnt9b Plays a Central Role in the Regulation of Mesenchymal to Epithelial Transitions Underlying Organogenesis of the Mammalian Urogenital System. Dev. Cell 2005, 9, 283–292. [Google Scholar] [CrossRef]
- Mittag, J.; Winterhager, E.; Bauer, K.; Grümmer, R. Congenital Hypothyroid Female Pax8-Deficient Mice Are Infertile Despite Thyroid Hormone Replacement Therapy. Endocrinology 2007, 148, 719–725. [Google Scholar] [CrossRef]
- Bowen, N.J.; Logani, S.; Dickerson, E.B.; Kapa, L.B.; Akhtar, M.; Benigno, B.B.; McDonald, J.F. Emerging roles for PAX8 in ovarian cancer and endosalpingeal development. Gynecol. Oncol. 2007, 104, 331–337. [Google Scholar] [CrossRef]
- Dubeau, L. Pathogenesis of serous, extra-uterine Müllerian epithelial cancer and therapeutic implications. Transl. Cancer Res. 2015, 4, 3–13. [Google Scholar] [PubMed]
- Nonaka, D.; Chiriboga, L.; Soslow, R.A. Expression of Pax8 as a Useful Marker in Distinguishing Ovarian Carcinomas From Mammary Carcinomas. Am. J. Surg. Pathol. 2008, 32, 1566–1571. [Google Scholar] [CrossRef] [PubMed]
- Chai, H.; Ren, Q.; Fan, Q.; Ye, L.; Du, G.; Du, H.; Xu, W.; Li, Y.; Zhang, L.; Cheng, Z. PAX8 is a potential marker for the diagnosis of primary epithelial ovarian cancer. Oncol. Lett. 2017, 14, 5871–5875. [Google Scholar] [CrossRef]
- Laury, A.R.; Hornick, J.L.; Perets, R.; Krane, J.F.; Corson, J.; Drapkin, R.; Hirsch, M.S. PAX8 Reliably Distinguishes Ovarian Serous Tumors From Malignant Mesothelioma. Am. J. Surg. Pathol. 2010, 34, 627–635. [Google Scholar] [CrossRef]
- Tong, G.-X.; Devaraj, K.; Hamele-Bena, D.; Yu, W.M.; Turk, A.; Chen, X.; Wright, J.D.; Greenebaum, E. Pax8: A marker for carcinoma of Müllerian origin in serous effusions. Diagn. Cytopathol. 2010, 39, 562–566. [Google Scholar] [CrossRef]
- Holmes, B.J.; Gown, A.M.; Vang, R.; Ronnett, B.M.; Yemelyanova, A. PAX8 Expression in Uterine Malignant Mesodermal Mixed Tumor (Carcinosarcoma). Int. J. Gynecol. Pathol. 2014, 33, 425–431. [Google Scholar] [CrossRef]
- Tacha, D.E.; Zhou, D.; Cheng, L. Expression of PAX8 in normal and neoplastic tissues: A comprehensive immunohistochemical study. Appl. Immunohistochem. Mol. Morphol. 2011, 19, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Tavanafar, Z.; Heidarpour, M. Diagnostic utility of PAX8 in differentiation of mullerian from non-mullerian tumors. Adv. Biomed. Res. 2014, 3, 96. [Google Scholar] [CrossRef]
- Rodgers, L.H.; Hainmhire, E.; Young, A.N.; Burdette, J.E. Loss of PAX8 in high-grade serous ovarian cancer reduces cell survival despite unique modes of action in the fallopian tube and ovarian surface epithelium. Oncotarget 2016, 7, 32785–32795. [Google Scholar] [CrossRef]
- Lin, L.; Shi, K.; Zhou, S.; Cai, M.-C.; Zhang, C.; Sun, Y.; Zang, J.; Cheng, L.; Ye, K.; Ma, P.; et al. SOX17 and PAX8 constitute an actionable lineage-survival transcriptional complex in ovarian cancer. Oncogene 2022, 41, 1767–1779. [Google Scholar] [CrossRef]
- Bleu, M.; Mermet-Meillon, F.; Apfel, V.; Barys, L.; Holzer, L.; Salvy, M.B.; Lopes, R.; Barbosa, I.A.M.; Delmas, C.; Hinniger, A.; et al. PAX8 and MECOM are interaction partners driving ovarian cancer. Nat. Commun. 2021, 12, 1–12. [Google Scholar] [CrossRef]
- Elias, K.M.; Emori, M.M.; Westerling, T.; Long, H.; Budina-Kolomets, A.; Li, F.; Macduffie, E.; Davis, M.; Holman, A.; Lawney, B.; et al. Epigenetic remodeling regulates transcriptional changes between ovarian cancer and benign precursors. JCI Insight 2016, 1. [Google Scholar] [CrossRef] [PubMed]
- Hua, G.; Lv, X.; He, C.; Remmenga, S.W.; Rodabough, K.J.; Dong, J.; Yang, L.; Lele, S.M.; Yang, P.; Zhou, J.; et al. YAP induces high-grade serous carcinoma in fallopian tube secretory epithelial cells. Oncogene 2015, 35, 2247–2265. [Google Scholar] [CrossRef] [PubMed]
- Adler, E.K.; Corona, R.I.; Lee, J.M.; Rodriguez-Malave, N.; Mhawech-Fauceglia, P.; Sowter, H.; Hazelett, D.J.; Lawrenson, K.; Gayther, S.A. The PAX8 cistrome in epithelial ovarian cancer. Oncotarget 2017, 8, 108316–108332. [Google Scholar] [CrossRef]
- Crozier, M.A.; Copeland, L.J.; Silva, E.G.; Gershenson, D.M.; Stringer, C. Clear cell carcinoma of the ovary: A study of 59 cases. Gynecol. Oncol. 1989, 35, 199–203. [Google Scholar] [CrossRef]
- Kurman, R.J.; Shih, I.-M. The Origin and Pathogenesis of Epithelial Ovarian Cancer: A Proposed Unifying Theory. Am. J. Surg. Pathol. 2010, 34, 433–443. [Google Scholar] [CrossRef]
- Adler, E.; Mhawech-Fauceglia, P.; Gayther, S.A.; Lawrenson, K. PAX8 expression in ovarian surface epithelial cells. Hum. Pathol. 2015, 46, 948–956. [Google Scholar] [CrossRef]
- Ozcan, A.; Liles, N.; Coffey, D.; Shen, S.S.; Truong, L.D. PAX2 and PAX8 expression in primary and metastatic müllerian epithelial tumors: A comprehensive comparison. Am. J. Surg. Pathol. 2011, 35, 1837–1847. [Google Scholar] [CrossRef]
- Fares, B.; Berger, L.; Bangiev-Girsh, E.; Kakun, R.R.; Ghannam-Shahbari, D.; Tabach, Y.; Zohar, Y.; Gottlieb, E.; Perets, R. PAX8 plays an essential antiapoptotic role in uterine serous papillary cancer. Oncogene 2021, 40, 5275–5285. [Google Scholar] [CrossRef]
- Gurung, P.; Yetiskul, E.; Jialal, I. Physiology, Male Reproductive System. In StatPearls; StatPearls Publishing: Tampa, FL, USA, 2022. [Google Scholar]
- Drews, U. Local mechanisms in sex specific morphogenesis. Cytogenet. Genome Res. 2000, 91, 72–80. [Google Scholar] [CrossRef]
- Brune, R.; Bard, J.; Dubreuil, C.; Guest, E.; Hill, W.; Kaufman, M.; Stark, M.; Davidson, D.; Baldock, R. A Three-Dimensional Model of the Mouse at Embryonic Day 9. Dev. Biol. 1999, 216, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, M.; Souabni, A.; Busslinger, M. Tissue-specific expression of cre recombinase from thePax8 locus. Genesis 2004, 38, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Major, A.T.; Estermann, M.A.; Smith, C.A. Anatomy, Endocrine Regulation, and Embryonic Development of the Rete Testis. Endocrinology 2021, 162, bqab046. [Google Scholar] [CrossRef]
- Wistuba, J.; Mittag, J.; Luetjens, C.M.; Cooper, T.G.; Yeung, C.-H.; Nieschlag, E.; Bauer, K. Male congenital hypothyroid Pax8−/− mice are infertile despite adequate treatment with thyroid hormone. J. Endocrinol. 2007, 192, 99–109. [Google Scholar] [CrossRef][Green Version]
- Ozcan, A.; Shen, S.S.; Hamilton, C.; Anjana, K.; Coffey, D.; Krishnan, B.; Truong, L.D. PAX 8 expression in non-neoplastic tissues, primary tumors, and metastatic tumors: A comprehensive immunohistochemical study. Mod. Pathol. 2011, 24, 751–764. [Google Scholar] [CrossRef]
- Grote, D.; Souabni, A.; Busslinger, M.; Bouchard, M. Pax 2/8-regulated Gata 3 expression is necessary for morphogenesis and guidance of the nephric duct in the developing kidney. Development 2006, 133, 53–61. [Google Scholar] [CrossRef]
- Narlis, M.; Grote, D.; Gaitan, Y.; Boualia, S.K.; Bouchard, M. Pax2 and Pax8 Regulate Branching Morphogenesis and Nephron Differentiation in the Developing Kidney. J. Am. Soc. Nephrol. 2007, 18, 1121–1129. [Google Scholar] [CrossRef]
- Tong, G.-X.; Memeo, L.; Colarossi, C.; Hamele-Bena, D.; Magi-Galluzzi, C.; Zhou, M.; Lagana, S.M.; Harik, L.; Oliver-Krasinski, J.M.; Mansukhani, M.; et al. PAX8 and PAX2 Immunostaining Facilitates the Diagnosis of Primary Epithelial Neoplasms of the Male Genital Tract. Am. J. Surg. Pathol. 2011, 35, 1473–1483. [Google Scholar] [CrossRef]
- Zong, Y.; Xiong, Y.; Dresser, K.; Yang, M.; Bledsoe, J.R. Polyclonal PAX8 expression in carcinomas of the biliary tract — Frequent non-specific staining represents a potential diagnostic pitfall. Ann. Diagn. Pathol. 2021, 53, 151762. [Google Scholar] [CrossRef]
- Moretti, L.; Medeiros, L.J.; Kunkalla, K.; Williams, M.D.; Singh, R.R.; Vega, F. N-terminal PAX8 polyclonal antibody shows cross-reactivity with N-terminal region of PAX5 and is responsible for reports of PAX8 positivity in malignant lymphomas. Mod. Pathol. 2011, 25, 231–236. [Google Scholar] [CrossRef]
- Ordóñez, N.G. Value of PAX 8 Immunostaining in Tumor Diagnosis: A Review and Update. Adv. Anat. Pathol. 2012, 19, 140–151. [Google Scholar] [CrossRef]
- Tacha, D.; Qi, W.; Zhou, D.; Bremer, R.; Cheng, L. PAX8 Mouse Monoclonal Antibody [BC12] Recognizes a Restricted Epitope and Is Highly Sensitive in Renal Cell and Ovarian Cancers But Does Not Cross-react With B Cells and Tumors of Pancreatic Origin. Appl. Immunohistochem. Mol. Morphol. 2013, 21, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, P.I.; Moreno, C.M.J.; Delgado, I.; Cobo-Vuilleumier, N.; Meier, R.; Gomez-Izquierdo, L.; Berney, T.; Garcia-Carbonero, R.; Rojas, A.; Gauthier, B.R. Immunohistochemical assessment of Pax8 expression during pancreatic islet development and in human neuroendocrine tumors. Histochem. Cell Biol. 2011, 136, 595–607. [Google Scholar] [CrossRef] [PubMed]
- Rieck, S.; White, P.; Schug, J.; Fox, A.J.; Smirnova, O.; Gao, N.; Gupta, R.K.; Wang, Z.V.; Scherer, P.E.; Keller, M.P.; et al. The Transcriptional Response of the Islet to Pregnancy in Mice. Mol. Endocrinol. 2009, 23, 1702–1712. [Google Scholar] [CrossRef] [PubMed]
- Martin-Montalvo, A.; López-Noriega, L.; Jimenez-Moreno, C.; Herranz, A.; Lorenzo, P.I.; Cobo-Vuilleumier, N.; Tamayo, A.; Gonzalez-Guerrero, C.; Hofsteede, J.S.; Lebreton, F.; et al. Transient PAX8 Expression in Islets During Pregnancy Correlates With β-Cell Survival, Revealing a Novel Candidate Gene in Gestational Diabetes Mellitus. Diabetes 2019, 68, 109–118. [Google Scholar] [CrossRef]
- Elbein, S.C.; Das, S.K.; Hallman, D.M.; Hanis, C.L.; Hasstedt, S.J. Genome-Wide Linkage and Admixture Mapping of Type 2 Diabetes in African American Families From the American Diabetes Association GENNID (Genetics of NIDDM) Study Cohort. Diabetes 2009, 58, 268–274. [Google Scholar] [CrossRef]
- Sangoi, A.R.; Ohgami, R.S.; Pai, R.K.; Beck, A.H.; McKenney, J.K.; Pai, R.K. PAX8 expression reliably distinguishes pancreatic well-differentiated neuroendocrine tumors from ileal and pulmonary well-differentiated neuroendocrine tumors and pancreatic acinar cell carcinoma. Mod. Pathol. 2010, 24, 412–424. [Google Scholar] [CrossRef]
- Long, K.B.; Srivastava, A.; Hirsch, M.S.; Hornick, J.L. PAX8 Expression in Well-differentiated Pancreatic Endocrine Tumors: Correlation With Clinicopathologic Features and Comparison With Gastrointestinal and Pulmonary Carcinoid Tumors. Am. J. Surg. Pathol. 2010, 34, 723–729. [Google Scholar] [CrossRef]
- Moreno, C.M.J.; Lorenzo, P.I.; Delgado, I.; Cobo-Vuilleumier, N.; Gomez-Izquierdo, L.; Garcia-Carbonero, R.; Rojas, A.; Gauthier, B.R. Pax8 Detection in Well-Differentiated Pancreatic Endocrine Tumors: How Reliable is it? Am. J. Surg. Pathol. 2011, 35, 1906–1908. [Google Scholar] [CrossRef]
- Plachov, D.; Chowdhury, K.; Walther, C.; Simon, D.; Guenet, J.; Gruss, P. Pax8, a murine paired box gene expressed in the developing excretory system and thyroid gland. Development 1990, 110, 643–651. [Google Scholar] [CrossRef]
- Khanlou, N.; Shintaku, P.; Yi, J.; Moatamed, N. Evaluation of PAX8 Expression in Brain Tissue and Related Neoplasms. Appl. Immunohistochem. Mol. Morphol. 2016, 24, 207–209. [Google Scholar] [CrossRef] [PubMed]
- Harter, P.N.; Baumgarten, P.; Zinke, J.; Schilling, K.; Baader, S.; Hartmetz, A.K.; Schittenhelm, J.; Beschorner, R.; Liebner, S.; Schulte, D.; et al. Paired box gene 8 (PAX8) expression is associated with sonic hedgehog (SHH)/wingless int (WNT) subtypes, desmoplastic histology and patient survival in human medulloblastomas. Neuropathol. Appl. Neurobiol. 2015, 41, 165–179. [Google Scholar] [CrossRef] [PubMed]
- Asano, M.; Gruss, P. Pax-5 is expressed at the midbrain-hindbrain boundary during mouse development. Mech. Dev. 1992, 39, 29–39. [Google Scholar] [CrossRef]
- Bouchard, M.; de Caprona, D.; Busslinger, M.; Xu, P.; Fritzsch, B. Pax2 and Pax8 cooperate in mouse inner ear morphogenesis and innervation. BMC Dev. Biol. 2010, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Hung, N.; Chen, Y.-J.; Taha, A.; Olivecrona, M.; Boet, R.; Wiles, A.; Warr, T.; Shaw, A.; Eiholzer, R.; Baguley, B.C.; et al. Increased paired box transcription factor 8 has a survival function in Glioma. BMC Cancer 2014, 14, 159. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kakun, R.R.; Melamed, Z.; Perets, R. PAX8 in the Junction between Development and Tumorigenesis. Int. J. Mol. Sci. 2022, 23, 7410. https://doi.org/10.3390/ijms23137410
Kakun RR, Melamed Z, Perets R. PAX8 in the Junction between Development and Tumorigenesis. International Journal of Molecular Sciences. 2022; 23(13):7410. https://doi.org/10.3390/ijms23137410
Chicago/Turabian StyleKakun, Reli Rachel, Zohar Melamed, and Ruth Perets. 2022. "PAX8 in the Junction between Development and Tumorigenesis" International Journal of Molecular Sciences 23, no. 13: 7410. https://doi.org/10.3390/ijms23137410
APA StyleKakun, R. R., Melamed, Z., & Perets, R. (2022). PAX8 in the Junction between Development and Tumorigenesis. International Journal of Molecular Sciences, 23(13), 7410. https://doi.org/10.3390/ijms23137410




