Adipose Tissue Inflammation and Pulmonary Dysfunction in Obesity
Abstract
1. Introduction
2. The Mechanical Effects and the Impact of Obesity on Lung Function
3. Adipose Tissue Dysfunction and Pulmonary Diseases
4. Obesity-Associated Pulmonary Diseases
4.1. Pulmonary Infections and Obesity
4.2. Obstructive Sleep Apnoea Syndrome and Obesity
4.3. Chronic Obstructive Pulmonary Disorder and Obesity
4.4. Asthma and Obesity
4.5. Obesity Hypoventilation Syndrome
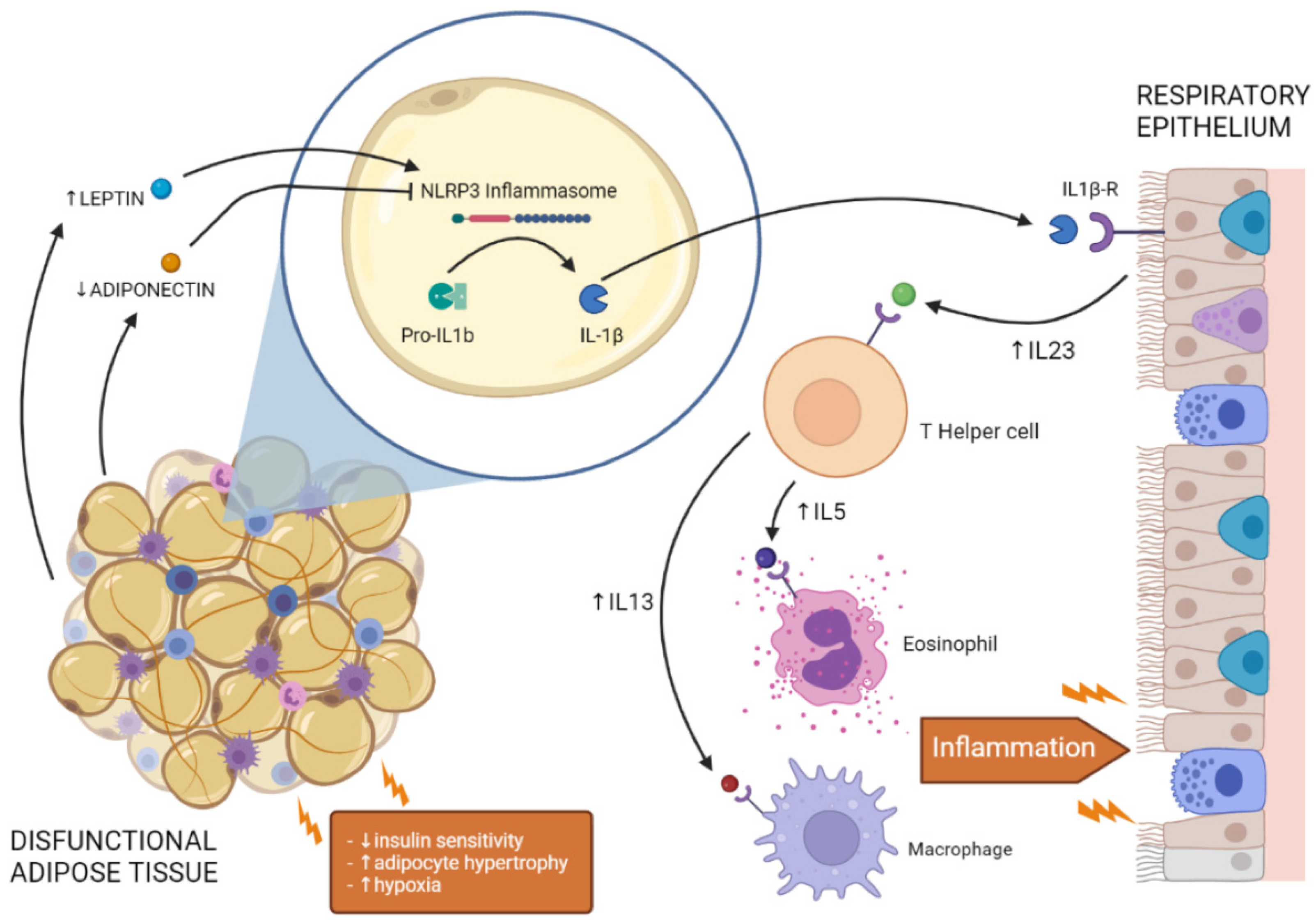
5. Mechanisms Underlying Obesity-Associated Pulmonary Dysfunction: Role of Adipose Tissue Mediators
5.1. Leptin, Adiponectin, and Pulmonary Dysfunction
5.2. Impairment of the NLRP3 Inflammasome in Obesity Leads to Pulmonary Dysfunction
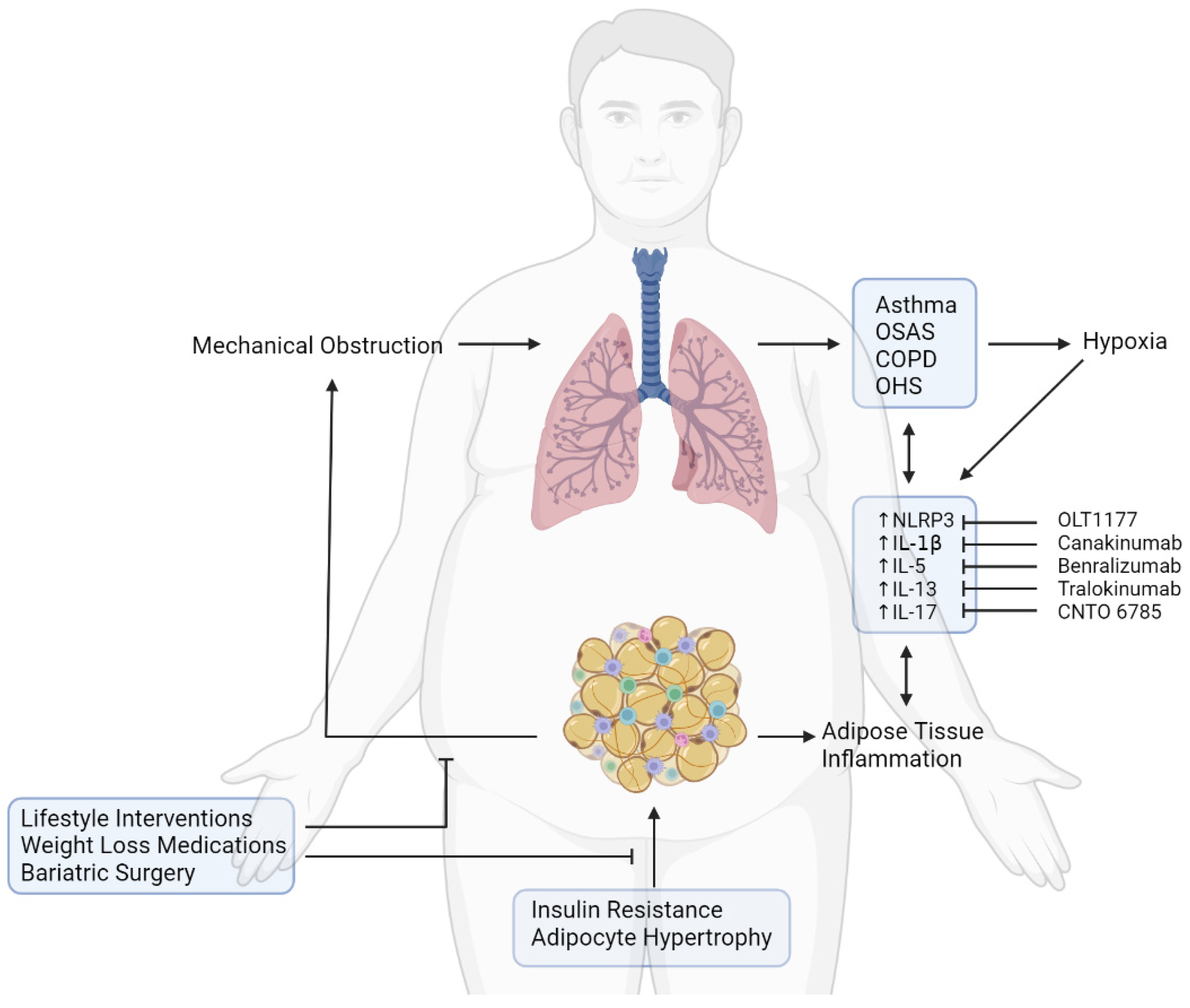
6. Therapeutic Targets to Prevent Pulmonary Dysfunction in Obesity
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dixon, A.E.; Peters, U. The effect of obesity on lung function. Expert Rev. Respir. Med. 2018, 12, 755–767. [Google Scholar] [CrossRef] [PubMed]
- Brock, J.M.; Billeter, A.; Müller-Stich, B.P.; Herth, F. Obesity and the Lung: What We Know Today. Respiration 2020, 99, 856–866. [Google Scholar] [CrossRef]
- Pépin, J.L.; Timsit, J.F.; Tamisier, R.; Borel, J.C.; Lévy, P.; Jaber, S. Prevention and care of respiratory failure in obese patients. Lancet Respir. Med. 2016, 4, 407–418. [Google Scholar] [CrossRef]
- Cignarelli, A.; Ciavarella, A.; Barbaro, M.; Kounaki, S.; Di Trani, A.; Falcone, V.A.; Quaranta, V.N.; Natalicchio, A.; Laviola, L.; Resta, O.; et al. Postprandial glucose and HbA1c are associated with severity of obstructive sleep apnoea in non-diabetic obese subjects. J. Endocrinol. Investig. 2021, 44, 2741–2748. [Google Scholar] [CrossRef] [PubMed]
- Perrini, S.; Cignarelli, A.; Quaranta, V.N.; Falcone, V.A.; Kounaki, S.; Porro, S.; Ciavarella, A.; Ficarella, R.; Barbaro, M.; Genchi, V.A.; et al. Correction of intermittent hypoxia reduces inflammation in obese subjects with obstructive sleep apnea. JCI Insight 2017, 2, e94379. [Google Scholar] [CrossRef]
- Grasemann, H.; Holguin, F. Oxidative stress and obesity-related asthma. Paediatr. Respir. Rev. 2020, 37, 18–21. [Google Scholar] [CrossRef]
- Perrotta, F.; Nigro, E.; Mollica, M.; Costigliola, A.; D’Agnano, V.; Daniele, A.; Bianco, A.; Guerra, G. Pulmonary Hypertension and Obesity: Focus on Adiponectin. Int. J. Mol. Sci. 2019, 20, 912. [Google Scholar] [CrossRef]
- Chan, S.M.; Selemidis, S.; Bozinovski, S.; Vlahos, R. Pathobiological mechanisms underlying metabolic syndrome (MetS) in chronic obstructive pulmonary disease (COPD): Clinical significance and therapeutic strategies. Pharmacol. Ther. 2019, 198, 160–188. [Google Scholar] [CrossRef]
- Umbrello, M.; Fumagalli, J.; Pesenti, A.; Chiumello, D. Pathophysiology and Management of Acute Respiratory Distress Syndrome in Obese Patients. Semin. Respir. Crit. Care Med. 2019, 40, 40–56. [Google Scholar] [CrossRef]
- Anderson, M.R.; Shashaty, M.G. Impact of Obesity in Critical Illness. Chest 2021, 160, 2135–2145. [Google Scholar] [CrossRef]
- Hornung, F.; Rogal, J.; Loskill, P.; Löffler, B.; Deinhardt-Emmer, S. The Inflammatory Profile of Obesity and the Role on Pulmonary Bacterial and Viral Infections. Int. J. Mol. Sci. 2021, 22, 3456. [Google Scholar] [CrossRef] [PubMed]
- Landecho, M.; Marin-Oto, M.; Recalde-Zamacona, B.; Bilbao, I.; Frühbeck, G. Obesity as an adipose tissue dysfunction disease and a risk factor for infections—Covid-19 as a case study. Eur. J. Intern. Med. 2021, 91, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Henning, R.J. Obesity and Obesity-Induced Inflammatory Disease Contribute to Atherosclerosis: A Review of the Pathophysiology and Treatment of Obesity. Am. J. Cardiovasc. Dis. 2021, 11, 504. [Google Scholar] [PubMed]
- Cordeiro, A.; Ribamar, A.; Ramalho, A. Adipose tissue dysfunction and MAFLD in obesity on the scene of COVID-19. Clin. Res. Hepatol. Gastroenterol. 2021, 46, 101807. [Google Scholar] [CrossRef]
- Kuvat, N.; Tanriverdi, H.; Armutcu, F. The relationship between obstructive sleep apnea syndrome and obesity: A new perspective on the pathogenesis in terms of organ crosstalk. Clin. Respir. J. 2020, 14, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Jutant, E.-M.; Tu, L.; Humbert, M.; Guignabert, C.; Huertas, A. The Thousand Faces of Leptin in the Lung. Chest 2020, 159, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Sideleva, O.; Suratt, B.T.; Black, K.E.; Tharp, W.G.; Pratley, R.E.; Forgione, P.; Dienz, O.; Irvin, C.G.; Dixon, A.E. Obesity and Asthma. Am. J. Respir. Crit. Care Med. 2012, 186, 598–605. [Google Scholar] [CrossRef]
- Skyba, P.; Ukropec, J.; Pobeha, P.; Ukropcova, B.; Joppa, P.; Kurdiova, T.; Stroffekova, K.; Brusik, M.; Klimes, I.; Tkac, I.; et al. Metabolic Phenotype and Adipose Tissue Inflammation in Patients with Chronic Obstructive Pulmonary Disease. Mediat. Inflamm. 2010, 2010, 173498. [Google Scholar] [CrossRef]
- De Oliveira, P.D.; Wehrmeister, F.C.; Horta, B.L.; Pérez-Padilla, R.; de França, G.V.A.; Gigante, D.P.; Barros, F.C.; Ong, K.K.; de Lucia Rolfe, E.; Menezes, A.M.B. Visceral and Subcutaneous Abdominal Adiposity and Pulmonary Function in 30-Year-Old Adults: A Cross-Sectional Analysis Nested in a Birth Cohort. BMC Pulm. Med. 2017, 17, 157. [Google Scholar] [CrossRef]
- Ni, Y.-N.; Yu, H.; Xu, H.; Li, W.-J.; Liang, B.-M.; Yang, L.; Liang, Z.-A. High Visceral Adipose Tissue to Subcutaneous Adipose Tissue Ratio as a Predictor of Mortality in Acute Respiratory Distress Syndrome. Am. J. Med. Sci. 2019, 357, 213–222. [Google Scholar] [CrossRef]
- Grailer, J.J.; Canning, B.A.; Kalbitz, M.; Haggadone, M.D.; Dhond, R.M.; Andjelkovic, A.V.; Zetoune, F.S.; Ward, P.A. Critical Role for the NLRP3 Inflammasome during Acute Lung Injury. J. Immunol. 2014, 192, 5974–5983. [Google Scholar] [CrossRef]
- Lasithiotaki, I.; Giannarakis, I.; Tsitoura, E.; Samara, K.D.; Margaritopoulos, G.A.; Choulaki, C.; Vasarmidi, E.; Tzanakis, N.; Voloudaki, A.; Sidiropoulos, P.; et al. NLRP3 inflammasome expression in idiopathic pulmonary fibrosis and rheumatoid lung. Eur. Respir. J. 2016, 47, 910–918. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yan, Z.; Schwartz, D.E.; Yu, J.; Malik, A.B.; Hu, G. Activation of NLRP3 Inflammasome in Alveolar Macrophages Contributes to Mechanical Stretch-Induced Lung Inflammation and Injury. J. Immunol. 2013, 190, 3590–3599. [Google Scholar] [CrossRef] [PubMed]
- Unamuno, X.; Gómez-Ambrosi, J.; Ramírez, B.; Rodríguez, A.; Becerril, S.; Valentí, V.; Moncada, R.; Silva, C.; Salvador, J.; Frühbeck, G.; et al. NLRP3 inflammasome blockade reduces adipose tissue inflammation and extracellular matrix remodeling. Cell. Mol. Immunol. 2019, 18, 1045–1057. [Google Scholar] [CrossRef] [PubMed]
- Grassi, L.; Kacmarek, R.; Berra, L. Ventilatory Mechanics in the Patient with Obesity. Anesthesiology 2020, 132, 1246–1256. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.N.; Bajwa, E.K.; Thompson, B.T.; Christiani, D.C. Body mass index is associated with the development of acute respiratory distress syndrome. Thorax 2009, 65, 44–50. [Google Scholar] [CrossRef]
- Van Son, J.; Oussaada, S.M.; Şekercan, A.; Beudel, M.; Dongelmans, D.A.; van Assen, S.; Eland, I.A.; Moeniralam, H.S.; Dormans, T.P.J.; van Kalkeren, C.A.J.; et al. Overweight and Obesity Are Associated With Acute Kidney Injury and Acute Respiratory Distress Syndrome, but Not With Increased Mortality in Hospitalized COVID-19 Patients: A Retrospective Cohort Study. Front. Endocrinol. 2021, 12, 1661. [Google Scholar] [CrossRef]
- McGillis, J.P. White Adipose Tissue, Inert No More! Endocrinology 2005, 146, 2154–2156. [Google Scholar] [CrossRef]
- Fu, S.; Liu, L.; Han, L.; Yu, Y. Leptin promotes IL-18 secretion by activating the NLRP3 inflammasome in RAW 264.7 cells. Mol. Med. Rep. 2017, 16, 9770–9776. [Google Scholar] [CrossRef][Green Version]
- Moradi, F. The Relationship between Circulating Levels of IL-18 and Leptin, HsCRP, Blood Pressure and Cardiorespiratory Function in Obese and Lean Men. Hormozgan Med. J. 2016, 20, 233–240. [Google Scholar]
- Tazawa, R.; Uchida, K.; Fujimaki, H.; Miyagi, M.; Inoue, G.; Sekiguchi, H.; Murata, K.; Takata, K.; Kawakubo, A.; Takaso, M. Elevated leptin levels induce inflammation through IL-6 in skeletal muscle of aged female rats. BMC Musculoskelet. Disord. 2019, 20, 199. [Google Scholar] [CrossRef] [PubMed]
- Salvator, H.; Grassin-Delyle, S.; Brollo, M.; Couderc, L.-J.; Abrial, C.; Victoni, T.; Naline, E.; Devillier, P. Adiponectin Inhibits the Production of TNF-α, IL-6 and Chemokines by Human Lung Macrophages. Front. Pharmacol. 2021, 12, 2178. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.-Q.; Dhlamini, Q.; Chen, C.; Li, X.; Bellusci, S.; Zhang, J.-S. FGF10 and Lipofibroblasts in Lung Homeostasis and Disease: Insights Gained from the Adipocytes. Front. Cell Dev. Biol. 2021, 9, 1299. [Google Scholar] [CrossRef] [PubMed]
- Porro, S.; Genchi, V.A.; Cignarelli, A.; Natalicchio, A.; Laviola, L.; Giorgino, F.; Perrini, S. Dysmetabolic adipose tissue in obesity: Morphological and functional characteristics of adipose stem cells and mature adipocytes in healthy and unhealthy obese subjects. J. Endocrinol. Investig. 2020, 44, 921–941. [Google Scholar] [CrossRef] [PubMed]
- Perrini, S.; Porro, S.; Nigro, P.; Cignarelli, A.; Caccioppoli, C.; Genchi, V.A.; Martines, G.; De Fazio, M.; Capuano, P.; Natalicchio, A.; et al. Reduced SIRT1 and SIRT2 expression promotes adipogenesis of human visceral adipose stem cells and associates with accumulation of visceral fat in human obesity. Int. J. Obes. 2019, 44, 307–319. [Google Scholar] [CrossRef]
- E Gyllenhammer, L.; Alderete, T.; Toledo-Corral, C.; Weigensberg, M.; I Goran, M. Saturation of subcutaneous adipose tissue expansion and accumulation of ectopic fat associated with metabolic dysfunction during late and post-pubertal growth. Int. J. Obes. 2015, 40, 601–606. [Google Scholar] [CrossRef]
- Britton, K.A.; Fox, C.S. Ectopic Fat Depots and Cardiovascular Disease. Circulation 2011, 124, e837–e841. [Google Scholar] [CrossRef]
- Cignarelli, A.; Genchi, V.A.; Perrini, S.; Natalicchio, A.; Laviola, L.; Giorgino, F. Insulin and Insulin Receptors in Adipose Tissue Development. Int. J. Mol. Sci. 2019, 20, 759. [Google Scholar] [CrossRef]
- Freedland, E.S. Role of a critical visceral adipose tissue threshold (CVATT) in metabolic syndrome: Implications for controlling dietary carbohydrates: A review. Nutr. Metab. 2004, 1, 12. [Google Scholar] [CrossRef]
- Thijs, W.; Dehnavi, R.A.; Hiemstra, P.; de Roos, A.; Melissant, C.F.; Janssen, K.; Tamsma, J.T.; Rabe, K.F. Association of lung function measurements and visceral fat in men with metabolic syndrome. Respir. Med. 2013, 108, 351–357. [Google Scholar] [CrossRef]
- Elliot, J.G.; Donovan, G.M.; Wang, K.C.; Green, F.H.; James, A.L.; Noble, P.B. Fatty airways: Implications for obstructive disease. Eur. Respir. J. 2019, 54, 1900857. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Caruso, D.; Tuccinardi, D.; Risi, R.; Zerunian, M.; Polici, M.; Pucciarelli, F.; Tarallo, M.; Strigari, L.; Manfrini, S.; et al. Visceral fat shows the strongest association with the need of intensive care in patients with COVID-19. Metabolism 2020, 111, 154319. [Google Scholar] [CrossRef] [PubMed]
- Mourelatou, R.; Kostopoulou, E.; Rojas-Gil, A.P.; Kehagias, I.; Linos, D.; Kalfarentzos, F.E.; Spiliotis, B.E. Decreased adipocyte glucose transporter 4 (GLUT4) and aquaglyceroporin-7 (AQP7) in adults with morbid obesity: Possible early markers of metabolic dysfunction. Hormones 2019, 18, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Suganami, T.; Nishida, J.; Ogawa, Y. A Paracrine Loop Between Adipocytes and Macrophages Aggravates Inflammatory Changes: Role of free fatty acids and tumor necrosis factor α. Arter. Thromb. Vasc. Biol. 2005, 25, 2062–2068. [Google Scholar] [CrossRef]
- Ito, A.; Suganami, T.; Miyamoto, Y.; Yoshimasa, Y.; Takeya, M.; Kamei, Y.; Ogawa, Y. Role of MAPK Phosphatase-1 in the Induction of Monocyte Chemoattractant Protein-1 during the Course of Adipocyte Hypertrophy. J. Biol. Chem. 2007, 282, 25445–25452. [Google Scholar] [CrossRef]
- Perrini, S.; Ficarella, R.; Picardi, E.; Cignarelli, A.; Barbaro, M.; Nigro, P.; Peschechera, A.; Palumbo, O.; Carella, M.; De Fazio, M.; et al. Differences in Gene Expression and Cytokine Release Profiles Highlight the Heterogeneity of Distinct Subsets of Adipose Tissue-Derived Stem Cells in the Subcutaneous and Visceral Adipose Tissue in Humans. PLoS ONE 2013, 8, e57892. [Google Scholar] [CrossRef]
- Bjørndal, B.; Burri, L.; Staalesen, V.; Skorve, J.; Berge, R.K. Different Adipose Depots: Their Role in the Development of Metabolic Syndrome and Mitochondrial Response to Hypolipidemic Agents. J. Obes. 2011, 2011, 490650. [Google Scholar] [CrossRef]
- Sarr, O.; Strohm, R.J.; Macdonald, T.L.; Gaudio, N.; Reed, J.K.; Foute-Nelong, J.; Dyck, D.J.; Mutch, D. Subcutaneous and Visceral Adipose Tissue Secretions from Extremely Obese Men and Women both Acutely Suppress Muscle Insulin Signaling. Int. J. Mol. Sci. 2017, 18, 959. [Google Scholar] [CrossRef]
- Yu, J.-Y.; Choi, W.-J.; Lee, H.-S.; Lee, J.-W. Relationship between inflammatory markers and visceral obesity in obese and overweight Korean adults. Medicine 2019, 98, e14740. [Google Scholar] [CrossRef]
- He, S.; Yang, J.; Li, X.; Gu, H.; Su, Q.; Qin, L. Visceral adiposity index is associated with lung function impairment: A population-based study. Respir. Res. 2021, 22, 2. [Google Scholar] [CrossRef]
- Gealekman, O.; Guseva, N.; Hartigan, C.; Apotheker, S.; Gorgoglione, M.; Gurav, K.; Tran, K.-V.; Straubhaar, J.; Nicoloro, S.; Czech, M.P.; et al. Depot-Specific Differences and Insufficient Subcutaneous Adipose Tissue Angiogenesis in Human Obesity. Circulation 2011, 123, 186–194. [Google Scholar] [CrossRef]
- Ryan, S.; Arnaud, C.; Fitzpatrick, S.F.; Gaucher, J.; Tamisier, R.; Pépin, J.-L. Adipose tissue as a key player in obstructive sleep apnoea. Eur. Respir. Rev. 2019, 28, 190006. [Google Scholar] [CrossRef] [PubMed]
- McMurray, F.; Patten, D.A.; Harper, M.-E. Reactive Oxygen Species and Oxidative Stress in Obesity-Recent Findings and Empirical Approaches. Obesity 2016, 24, 2301–2310. [Google Scholar] [CrossRef] [PubMed]
- D’Oria, R.; Schipani, R.; Leonardini, A.; Natalicchio, A.; Perrini, S.; Cignarelli, A.; Laviola, L.; Giorgino, F. The Role of Oxidative Stress in Cardiac Disease: From Physiological Response to Injury Factor. Oxidative Med. Cell. Longev. 2020, 2020, 5732956. [Google Scholar] [CrossRef] [PubMed]
- Incalza, M.A.; D’Oria, R.; Natalicchio, A.; Perrini, S.; Laviola, L.; Giorgino, F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vasc. Pharmacol. 2018, 100, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Kaess, B.M.; Pedley, A.; Massaro, J.M.; Murabito, J.; Hoffmann, U.; Fox, C.S. The ratio of visceral to subcutaneous fat, a metric of body fat distribution, is a unique correlate of cardiometabolic risk. Diabetologia 2012, 55, 2622–2630. [Google Scholar] [CrossRef]
- Camhi, S.M.; Katzmarzyk, P.T. Differences in body composition between metabolically healthy obese and metabolically abnormal obese adults. Int. J. Obes. 2013, 38, 1142–1145. [Google Scholar] [CrossRef]
- Lee, H.Y.; Yang, H.K.; Song, H.J.; Chang, H.J.; Kang, J.Y.; Lee, S.H.; Han, S.; Kim, Y.K. Metabolic health is more closely associated with decrease in lung function than obesity. PLoS ONE 2019, 14, e0209575. [Google Scholar] [CrossRef]
- Nouri-Keshtkar, M.; Taghizadeh, S.; Farhadi, A.; Ezaddoustdar, A.; Vesali, S.; Hosseini, R.; Totonchi, M.; Kouhkan, A.; Chen, C.; Zhang, J.-S.; et al. Potential Impact of Diabetes and Obesity on Alveolar Type 2 (AT2)-Lipofibroblast (LIF) Interactions After COVID-19 Infection. Front. Cell Dev. Biol. 2021, 9, 676150. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, G.; Wang, Y.; Ren, W.; Zhao, X.; Ji, F.; Zhu, Y.; Feng, F.; Gong, M.; Ju, X.; et al. Functional and Genetic Analysis of Viral Receptor ACE2 Orthologs Reveals a Broad Potential Host Range of SARS-CoV-2. Proc. Natl. Acad. Sci. USA 2021, 118, e2025373118. [Google Scholar] [CrossRef]
- Cignarelli, A.; Castellana, M.; Castellana, G.; Perrini, S.; Brescia, F.; Natalicchio, A.; Garruti, G.; Laviola, L.; Resta, O.; Giorgino, F. Effects of CPAP on Testosterone Levels in Patients with Obstructive Sleep Apnea: A Meta-Analysis Study. Front. Endocrinol. 2019, 10, 551. [Google Scholar] [CrossRef] [PubMed]
- Divo, M.; Cote, C.; de Torres, J.P.; Casanova, C.; Marin, J.M.; Pinto-Plata, V.; Zulueta, J.; Cabrera, C.; Zagaceta, J.; Hunninghake, G.; et al. Comorbidities and Risk of Mortality in Patients with Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2012, 186, 155–161. [Google Scholar] [CrossRef]
- Vogelmeier, C.F.; Criner, G.J.; Martinez, F.J.; Anzueto, A.; Barnes, P.J.; Bourbeau, J.; Celli, B.R.; Chen, R.; Decramer, M.; Fabbri, L.M.; et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report. GOLD Executive Summary. Am. J. Respir. Crit. Care Med. 2017, 195, 557–582. [Google Scholar] [CrossRef] [PubMed]
- Chapman, D.G.; Irvin, C.G. Mechanisms of airway hyper-responsiveness in asthma: The past, present and yet to come. Clin. Exp. Allergy 2015, 45, 706–719. [Google Scholar] [CrossRef] [PubMed]
- Peters, U.; Dixon, A.E.; Forno, E. Obesity and asthma. J. Allergy Clin. Immunol. 2018, 141, 1169–1179. [Google Scholar] [CrossRef]
- Shah, N.M.; Shrimanker, S.; Kaltsakas, G. Defining obesity hypoventilation syndrome. Breathe 2021, 17, 210089. [Google Scholar] [CrossRef]
- Masa, J.F.; Pépin, J.L.; Borel, J.C.; Mokhlesi, B.; Murphy, P.B.; Sánchez-Quiroga, M. Obesity hypoventilation syndrome. Eur. Respir. Rev. 2019, 28, 180097. [Google Scholar] [CrossRef]
- Balistreri, C.R.; Caruso, C.; Candore, G. The Role of Adipose Tissue and Adipokines in Obesity-Related Inflammatory Diseases. Mediat. Inflamm. 2010, 2010, 802078. [Google Scholar] [CrossRef]
- Ramos-Ramírez, P.; Malmhäll, C.; Johansson, K.; Adner, M.; Lötvall, J.; Bossios, A. Lung Regulatory T Cells Express Adiponectin Receptor 1: Modulation by Obesity and Airway Allergic Inflammation. Int. J. Mol. Sci. 2020, 21, 8990. [Google Scholar] [CrossRef]
- Jaswal, S.; Saini, V.; Kaur, J.; Gupta, S.; Kaur, H.; Garg, K. Association of Adiponectin with Lung Function Impairment and Disease Severity in Chronic Obstructive Pulmonary Disease. Int. J. Appl. Basic Med. Res. 2018, 8, 14–18. [Google Scholar] [CrossRef]
- Thyagarajan, B.; Jacobs, D.R.; Smith, L.J.; Kalhan, R.; Gross, M.D.; Sood, A. Serum adiponectin is positively associated with lung function in young adults, independent of obesity: The CARDIA study. Respir. Res. 2010, 11, 176. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Makita, H.; Östling, J.; Thomsen, L.H.; Konno, S.; Nagai, K.; Shimizu, K.; Pedersen, J.H.; Ashraf, H.; Bruijnzeel, P.L.B.; et al. Lower Leptin/Adiponectin Ratio and Risk of Rapid Lung Function Decline in Chronic Obstructive Pulmonary Disease. Ann. Am. Thorac. Soc. 2014, 11, 1511–1519. [Google Scholar] [CrossRef] [PubMed]
- Hansel, N.N.; Gao, L.; Rafaels, N.M.; Mathias, R.A.; Neptune, E.R.; Tankersley, C.; Grant, A.V.; Connett, J.; Beaty, T.H.; Wise, R.A.; et al. Leptin receptor polymorphisms and lung function decline in COPD. Eur. Respir. J. 2009, 34, 103–110. [Google Scholar] [CrossRef]
- Sin, D.D. Impaired lung function and serum leptin in men and women with normal body weight: A population based study. Thorax 2003, 58, 695–698. [Google Scholar] [CrossRef]
- Siegrist-Kaiser, C.A.; Pauli, V.; Juge-Aubry, C.E.; Boss, O.; Pernin, A.; Chin, W.W.; Cusin, I.; Rohner-Jeanrenaud, F.; Burger, A.G.; Zapf, J.; et al. Direct effects of leptin on brown and white adipose tissue. J. Clin. Investig. 1997, 100, 2858–2864. [Google Scholar] [CrossRef] [PubMed]
- Genchi, V.; D’Oria, R.; Palma, G.; Caccioppoli, C.; Cignarelli, A.; Natalicchio, A.; Laviola, L.; Giorgino, F.; Perrini, S. Impaired Leptin Signalling in Obesity: Is Leptin a New Thermolipokine? Int. J. Mol. Sci. 2021, 22, 6445. [Google Scholar] [CrossRef]
- Torday, J.S.; E Sunday, M.; Wang, L.; Torres, E. Leptin mediates the parathyroid hormone-related protein paracrine stimulation of fetal lung maturation. Am. J. Physiol. Cell. Mol. Physiol. 2002, 282, L405–L410. [Google Scholar] [CrossRef]
- Maffei, M.; Halaas, J.; Ravussin, E.; Pratley, R.E.; Lee, G.H.; Zhang, Y.; Fei, H.; Kim, S.; Lallone, R.; Ranganathan, S.; et al. Leptin levels in human and rodent: Measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat. Med. 1995, 1, 1155–1161. [Google Scholar] [CrossRef]
- Poetsch, M.S.; Strano, A.; Guan, K. Role of Leptin in Cardiovascular Diseases. Front. Endocrinol. 2020, 11, 354. [Google Scholar] [CrossRef]
- Perrini, S. Leptin: A marker of renal injury. Intern. Emerg. Med. 2019, 14, 493–494. [Google Scholar] [CrossRef]
- Phipps, P.R.; Starritt, E.; Caterson, I.; Grunstein, R.R. Association of serum leptin with hypoventilation in human obesity. Thorax 2002, 57, 75–76. [Google Scholar] [CrossRef]
- Gavrila, A.; Chan, J.L.; Yiannakouris, N.; Kontogianni, M.; Miller, L.C.; Orlova, C.; Mantzoros, C.S. Serum Adiponectin Levels Are Inversely Associated with Overall and Central Fat Distribution but Are Not Directly Regulated by Acute Fasting or Leptin Administration in Humans: Cross-Sectional and Interventional Studies. J. Clin. Endocrinol. Metab. 2003, 88, 4823–4831. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.; Cho, J.Y.; Pham, A.; Ramsdell, J.; Broide, D.H. Adiponectin and Functional Adiponectin Receptor 1 Are Expressed by Airway Epithelial Cells in Chronic Obstructive Pulmonary Disease. J. Immunol. 2008, 182, 684–691. [Google Scholar] [CrossRef] [PubMed]
- Jasinski-Bergner, S.; Büttner, M.; Quandt, D.; Seliger, B.; Kielstein, H. Adiponectin and Its Receptors Are Differentially Expressed in Human Tissues and Cell Lines of Distinct Origin. Obes. Facts 2017, 10, 569–583. [Google Scholar] [CrossRef] [PubMed]
- Achari, A.E.; Jain, S.K. Adiponectin, a Therapeutic Target for Obesity, Diabetes, and Endothelial Dysfunction. Int. J. Mol. Sci. 2017, 18, 1321. [Google Scholar] [CrossRef]
- Fu, Y.; Luo, N.; Klein, R.L.; Garvey, W.T. Adiponectin promotes adipocyte differentiation, insulin sensitivity, and lipid accumulation. J. Lipid Res. 2005, 46, 1369–1379. [Google Scholar] [CrossRef]
- Frühbeck, G.; Catalán, V.; Rodríguez, A.; Ramírez, B.; Becerril, S.; Salvador, J.; Colina, I.; Gómez-Ambrosi, J. Adiponectin-leptin Ratio is a Functional Biomarker of Adipose Tissue Inflammation. Nutrients 2019, 11, 454. [Google Scholar] [CrossRef]
- Frühbeck, G.; Catalán, V.; Rodríguez, A.; Ramírez, B.; Becerril, S.; Salvador, J.; Portincasa, P.; Colina, I.; Gómez-Ambrosi, J. Involvement of the leptin-adiponectin axis in inflammation and oxidative stress in the metabolic syndrome. Sci. Rep. 2017, 7, 6619. [Google Scholar] [CrossRef]
- Favre, G.; Legueult, K.; Pradier, C.; Raffaelli, C.; Ichai, C.; Iannelli, A.; Redheuil, A.; Lucidarme, O.; Esnault, V. Visceral fat is associated to the severity of COVID-19. Metabolism 2020, 115, 154440. [Google Scholar] [CrossRef]
- Colleluori, G.; Graciotti, L.; Pesaresi, M.; Di Vincenzo, A.; Perugini, J.; Di Mercurio, E.; Caucci, S.; Bagnarelli, P.; Zingaretti, C.M.; Nisoli, E.; et al. Visceral fat inflammation and fat embolism are associated with lung’s lipidic hyaline membranes in subjects with COVID-19. Int. J. Obes. 2022, 46, 1009–1017. [Google Scholar] [CrossRef]
- Di Filippo, L.; De Lorenzo, R.; Sciorati, C.; Capobianco, A.; Lorè, N.I.; Giustina, A.; Manfredi, A.A.; Rovere-Querini, P.; Conte, C. Adiponectin to leptin ratio reflects inflammatory burden and survival in COVID-19. Diabetes Metab. 2021, 47, 101268. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.E.; Omar, M.M.; Hibah, N.A.A.; Issa, H.A. Leptin hormone in obese and non-obese stable and exacerbated cases of chronic obstructive pulmonary disease. Egypt. J. Chest Dis. Tuberc. 2015, 64, 557–565. [Google Scholar] [CrossRef]
- Oraby, S.S.; Ahmed, E.S.; Farag, T.S.; Zayed, A.E.; Ali, N.K. Adiponectin as inflammatory biomarker of chronic obstructive pulmonary disease. Egypt. J. Chest Dis. Tuberc. 2014, 63, 583–587. [Google Scholar] [CrossRef]
- Wang, L. The Potential Mechanism of Th17/Treg Imbalance in the Microenvironments of Chronic Inflammation and Allergic Asthma. Eur. Respir. J. 2012, 40, P1950. [Google Scholar]
- Ramos-Ramírez, P.; Malmhäll, C.; Tliba, O.; Rådinger, M.; Bossios, A. Adiponectin/AdipoR1 Axis Promotes IL-10 Release by Human Regulatory T Cells. Front. Immunol. 2021, 12, 1796. [Google Scholar] [CrossRef]
- Bruzzaniti, S.; Bocchino, M.; Santopaolo, M.; Calì, G.; Stanziola, A.A.; D’Amato, M.; Esposito, A.; Barra, E.; Garziano, F.; Micillo, T.; et al. An immunometabolic pathomechanism for chronic obstructive pulmonary disease. Proc. Natl. Acad. Sci. USA 2019, 116, 15625–15634. [Google Scholar] [CrossRef]
- Zheng, H.; Zhang, X.; Castillo, E.F.; Luo, Y.; Liu, M.; Yang, X.O. Leptin Enhances TH2 and ILC2 Responses in Allergic Airway Disease. J. Biol. Chem. 2016, 291, 22043–22052. [Google Scholar] [CrossRef]
- Pinkerton, J.; Kim, R.; Brown, A.; Rae, B.; Mayall, J.; Ali, K.; Starkey, M.; Robertson, A.; Wood, L.; Cooper, M.; et al. IL-5/IL-13 Drive NLRP3 Inflammasome-Mediated, Steroid-Resistant AHR in a Model of Obesity-Associated Asthma. Eur. Respir. J. 2019, 54, PA3345. [Google Scholar] [CrossRef]
- Wani, K.; Alharthi, H.; Alghamdi, A.; Sabico, S.; Al-Daghri, N.M. Role of NLRP3 Inflammasome Activation in Obesity-Mediated Metabolic Disorders. Int. J. Environ. Res. Public Health 2021, 18, 511. [Google Scholar] [CrossRef]
- López-Reyes, A.; Martinez-Armenta, C.; Espinosa-Velázquez, R.; Vázquez-Cárdenas, P.; Cruz-Ramos, M.; Palacios-Gonzalez, B.; Gomez-Quiroz, L.E.; Martínez-Nava, G.A. NLRP3 Inflammasome: The Stormy Link Between Obesity and COVID-19. Front. Immunol. 2020, 11, 570251. [Google Scholar] [CrossRef]
- Kim, H.Y.; Lee, H.J.; Chang, Y.-J.; Pichavant, M.; Shore, S.A.; Fitzgerald, K.A.; Iwakura, Y.; Israel, E.; Bolger, K.; Faul, J.; et al. Interleukin-17–producing innate lymphoid cells and the NLRP3 inflammasome facilitate obesity-associated airway hyperreactivity. Nat. Med. 2014, 20, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.; Gris, D.; Lei, Y.; Jha, S.; Zhang, L.; Huang, M.T.-H.; Brickey, W.J.; Ting, J.P.-Y. Fatty acid–induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat. Immunol. 2011, 12, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Camell, C.; Goldberg, E.; Dixit, V.D. Regulation of Nlrp3 inflammasome by dietary metabolites. In Seminars in Immunology; Academic Press: Cambridge, MA, USA, 2015; pp. 334–342. [Google Scholar]
- Park, S.-H.; Ham, S.; Lee, A.; Möller, A.; Kim, T.S. NLRP3 negatively regulates Treg differentiation through Kpna2-mediated nuclear translocation. J. Biol. Chem. 2019, 294, 17951–17961. [Google Scholar] [CrossRef] [PubMed]
- Pan, P.; Shen, M.; Yu, Z.; Ge, W.; Chen, K.; Tian, M.; Xiao, F.; Wang, Z.; Wang, J.; Jia, Y.; et al. SARS-CoV-2 N protein promotes NLRP3 inflammasome activation to induce hyperinflammation. Nat. Commun. 2021, 12, 4664. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lv, C.; Wang, S.; Ying, H.; Weng, Y.; Yu, W. NLRP3 Inflammasome Involves in the Acute Exacerbation of Patients with Chronic Obstructive Pulmonary Disease. Inflammation 2018, 41, 1321–1333. [Google Scholar] [CrossRef]
- Simpson, J.L.; Phipps, S.; Baines, K.; Oreo, K.M.; Gunawardhana, L.; Gibson, P. Elevated expression of the NLRP3 inflammasome in neutrophilic asthma. Eur. Respir. J. 2013, 43, 1067–1076. [Google Scholar] [CrossRef]
- Vandanmagsar, B.; Youm, Y.-H.; Ravussin, A.; Galgani, J.E.; Stadler, K.; Mynatt, R.L.; Ravussin, E.; Stephens, J.M.; Dixit, V.D. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat. Med. 2011, 17, 179–188. [Google Scholar] [CrossRef]
- Shah, D.; Torres, C.; Bhandari, V. Adiponectin deficiency induces mitochondrial dysfunction and promotes endothelial activation and pulmonary vascular injury. FASEB J. 2019, 33, 13617–13631. [Google Scholar] [CrossRef]
- Chong, L.; Li, H.; Zhu, L.; Yu, G. Regulatory effect of mitoQ on the mtROS-NLRP3 inflammasome pathway in leptin-pretreated BEAS-2 cells. Exp. Ther. Med. 2021, 21, 466. [Google Scholar] [CrossRef]
- Chaudhuri, R.; Thompson, M.A.; Pabelick, C.; Agrawal, A.; Prakash, Y. Obesity, mitochondrial dysfunction, and obstructive lung disease. In Mechanisms and Manifestations of Obesity in Lung Disease; Academic Press: Cambridge, MA, USA, 2018; pp. 143–167. [Google Scholar] [CrossRef]
- Zhou, R.; Yazdi, A.S.; Menu, P.; Tschopp, J. A role for mitochondria in NLRP3 inflammasome activation. Nature 2011, 469, 221–225. [Google Scholar] [CrossRef]
- Shimada, K.; Crother, T.R.; Karlin, J.; Dagvadorj, J.; Chiba, N.; Chen, S.; Ramanujan, V.K.; Wolf, A.J.; Vergnes, L.; Ojcius, D.M.; et al. Oxidized Mitochondrial DNA Activates the NLRP3 Inflammasome during Apoptosis. Immunity 2012, 36, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Han, M.W.; Kim, S.H.; Oh, I.; Kim, Y.H.; Lee, J. Obesity Can Contribute to Severe Persistent Allergic Rhinitis in Children through Leptin and Interleukin-1β. Int. Arch. Allergy Immunol. 2021, 182, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Thomas, R.; Kochumon, S.; Sindhu, S. Increased adipose tissue expression of IL-18R and its ligand IL-18 associates with inflammation and insulin resistance in obesity. Immun. Inflamm. Dis. 2017, 5, 318–335. [Google Scholar] [CrossRef] [PubMed]
- Rovina, N.; Dima, E.; Gerassimou, C.; Kollintza, A.; Gratziou, C.; Roussos, C. Interleukin-18 in induced sputum: Association with lung function in chronic obstructive pulmonary disease. Respir. Med. 2009, 103, 1056–1062. [Google Scholar] [CrossRef] [PubMed]
- Todoric, K.; Zhou, H.; Zhang, H.; Mills, K.; Peden, D.B.; Hernandez, M.L. Body mass index correlates with pollutant-induced interleukin-1β in sputum and blood. Ann. Allergy Asthma Immunol. 2014, 114, 251–253. [Google Scholar] [CrossRef]
- Carreras, A.; Zhang, S.X.L.; Almendros, I.; Wang, Y.; Peris, E.; Qiao, Z.; Gozal, D. Resveratrol Attenuates Intermittent Hypoxia-Induced Macrophage Migration to Visceral White Adipose Tissue and Insulin Resistance in Male Mice. Endocrinology 2014, 156, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Becari, C.; Somers, K.R.; Polonis, K.; Pfeifer, M.A.; Prachi, S. Acute Effects of Intermittent Hypoxia on Leptin-Mediated Increases in Adiponectin Expression. FASEB J. 2016, 30, 759.1. [Google Scholar] [CrossRef]
- Fitzpatrick, S.F.; King, A.D.; O’Donnell, C.; Roche, H.M.; Ryan, S. Mechanisms of intermittent hypoxia-mediated macrophage activation—Potential therapeutic targets for obstructive sleep apnoea. J. Sleep Res. 2020, 30, e13202. [Google Scholar] [CrossRef]
- Li, X.; He, J. The Association Between Serum/Plasma Leptin Levels and Obstructive Sleep Apnea Syndrome: A Meta-Analysis and Meta-Regression. Front. Endocrinol. 2021, 12, 1209. [Google Scholar] [CrossRef]
- Díaz-García, E.; García-Tovar, S.; Alfaro, E.; Jaureguizar, A.; Casitas, R.; Sánchez-Sánchez, B.; Zamarrón, E.; Fernández-Lahera, J.; López-Collazo, E.; Cubillos-Zapata, C.; et al. Inflammasome Activation: A Keystone of Proinflammatory Response in Obstructive Sleep Apnea. Am. J. Respir. Crit. Care Med. 2022, 205, 1337–1348. [Google Scholar] [CrossRef]
- Wu, X.; Gong, L.; Xie, L.; Gu, W.; Wang, X.; Liu, Z.; Li, S. NLRP3 Deficiency Protects Against Intermittent Hypoxia-Induced Neuroinflammation and Mitochondrial ROS by Promoting the PINK1-Parkin Pathway of Mitophagy in a Murine Model of Sleep Apnea. Front. Immunol. 2021, 12, 628168. [Google Scholar] [CrossRef] [PubMed]
- Dheda, K.; Chang, J.-S.; Lala, S.; Huggett, J.; Zumla, A.; Rook, G. Gene expression of IL17 and IL23 in the lungs of patients with active tuberculosis. Thorax 2008, 63, 566–568. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Perret, J.; McDonald, C.; Apostolopoulos, V. Elevated serum interleukin-5 levels in severe chronic obstructive pulmonary disease. Acta Biochim. Biophys. Sin. 2017, 49, 560–563. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.K.; Ho, C.Y.; Ko, F.W.S.; Chan, C.H.S.; Ho, A.S.S.; Hui, D.S.C.; Lam, C.W.K. Proinflammatory cytokines (IL-17, IL-6, IL-18 and IL-12) and Th cytokines (IFN-γ, IL-4, IL-10 and IL-13) in patients with allergic asthma. Clin. Exp. Immunol. 2001, 125, 177–183. [Google Scholar] [CrossRef]
- Al Heialy, S.; Gaudet, M.; Ramakrishnan, R.K.; Mogas, A.; Salameh, L.; Mahboub, B.; Hamid, Q. Contribution of IL-17 in Steroid Hyporesponsiveness in Obese Asthmatics Through Dysregulation of Glucocorticoid Receptors α and β. Front. Immunol. 2020, 11, 1724. [Google Scholar] [CrossRef]
- Desai, D.; Newby, C.; Symon, F.A.; Haldar, P.; Shah, S.; Gupta, S.; Bafadhel, M.; Singapuri, A.; Siddiqui, S.; Woods, J.; et al. Elevated Sputum Interleukin-5 and Submucosal Eosinophilia in Obese Individuals with Severe Asthma. Am. J. Respir. Crit. Care Med. 2013, 188, 657–663. [Google Scholar] [CrossRef]
- Marijsse, G.S.; Seys, S.F.; Schelpe, A.S.; Dilissen, E.; Goeminne, P.; Dupont, L.J.; Ceuppens, J.L.; Bullens, D.M.A. Obese Individualswith Asthma Preferentially Have a High IL-5/IL-17A/IL-25 Sputum Inflammatory Pattern. Am. J. Respir. Crit. Care Med. 2014, 189, 1284–1285. [Google Scholar] [CrossRef]
- Bhatt, S.P.; Guleria, R.; Kabra, S.K. Metabolic alterations and systemic inflammation in overweight/obese children with obstructive sleep apnea. PLoS ONE 2021, 16, e0252353. [Google Scholar] [CrossRef]
- Savchenko, L.; Mykytiuk, M.; Cinato, M.; Tronchere, H.; Kunduzova, O.; Kaidashev, I. IL-26 in the induced sputum is associated with the level of systemic inflammation, lung functions and body weight in COPD patients. Int. J. Chronic Obstr. Pulm. Dis. 2018, 13, 2569–2575. [Google Scholar] [CrossRef]
- Peres, A.; Dorneles, G.P.; Dias, A.S.; Vianna, P.; Chies, J.A.B.; Monteiro, M.B. T-cell profile and systemic cytokine levels in overweight-obese patients with moderate to very-severe COPD. Respir. Physiol. Neurobiol. 2018, 247, 74–79. [Google Scholar] [CrossRef]
- Leon-Cabrera, S.; Arana-Lechuga, Y.; Esqueda-León, E.; Terán-Pérez, G.; Gonzalez-Chavez, A.; Escobedo, G.; Moctezuma, J.V. Reduced Systemic Levels of IL-10 Are Associated with the Severity of Obstructive Sleep Apnea and Insulin Resistance in Morbidly Obese Humans. Mediat. Inflamm. 2015, 2015, 493409. [Google Scholar] [CrossRef] [PubMed]
- Besnard, A.-G.; Guillou, N.; Tschopp, J.; Erard, F.; Couillin, I.; Iwakura, Y.; Quesniaux, V.; Ryffel, B.; Togbe, D. NLRP3 inflammasome is required in murine asthma in the absence of aluminum adjuvant. Allergy 2011, 66, 1047–1057. [Google Scholar] [CrossRef] [PubMed]
- Gasse, P.; Riteau, N.; Vacher, R.; Michel, M.-L.; Fautrel, A.; Di Padova, F.; Fick, L.; Charron, S.; Lagente, V.; Eberl, G.; et al. IL-1 and IL-23 Mediate Early IL-17A Production in Pulmonary Inflammation Leading to Late Fibrosis. PLoS ONE 2011, 6, e23185. [Google Scholar] [CrossRef] [PubMed]
- Weiss, D.I.; Ma, F.; Merleev, A.A.; Maverakis, E.; Gilliet, M.; Balin, S.J.; Bryson, B.D.; Ochoa, M.T.; Pellegrini, M.; Bloom, B.R.; et al. IL-1β Induces the Rapid Secretion of the Antimicrobial Protein IL-26 from Th17 Cells. J. Immunol. 2019, 203, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Wen, Z.; Shi, X.; Li, Y.; Fan, L.; Xiang, M.; Li, A.; Scott, M.J.; Xiao, G.; Li, S.; et al. Hemorrhagic Shock Augments Nlrp3 Inflammasome Activation in the Lung through Impaired Pyrin Induction. J. Immunol. 2013, 190, 5247–5255. [Google Scholar] [CrossRef]
- Poli, C.; Augusto, J.F.; Dauvé, J.; Adam, C.; Preisser, L.; Larochette, V.; Pignon, P.; Savina, A.; Blanchard, S.; Subra, J.F.; et al. IL-26 Confers Proinflammatory Properties to Extracellular DNA. J. Immunol. 2017, 198, 3650–3661. [Google Scholar] [CrossRef]
- Youssef, D.; Elbehidy, R.M.; Shokry, D.M.; Elbehidy, E.M. The influence of leptin on Th1/Th2 balance in obese children with asthma. J. Bras. De Pneumol. 2013, 39, 562–568. [Google Scholar] [CrossRef]
- Kasahara, D.I.; Kim, H.Y.; Williams, A.S.; Verbout, N.; Tran, J.; Si, H.; Wurmbrand, A.P.; Jastrab, J.; Hug, C.; Umetsu, D.T.; et al. Pulmonary Inflammation Induced by Subacute Ozone Is Augmented in Adiponectin-Deficient Mice: Role of IL-17A. J. Immunol. 2012, 188, 4558–4567. [Google Scholar] [CrossRef]
- Ardila-Gatas, J.; Sharma, G.; Hanipah, Z.N.; Tu, C.; Brethauer, S.A.; Aminian, A.; Tolle, L.; Schauer, P.R. Bariatric surgery in patients with interstitial lung disease. Surg. Endosc. 2018, 33, 1952–1958. [Google Scholar] [CrossRef]
- Sekine, A.; Wasamoto, S.; Hagiwara, E.; Yamakawa, H.; Ikeda, S.; Okabayashi, H.; Oda, T.; Okuda, R.; Kitamura, H.; Baba, T.; et al. Beneficial impact of weight loss on respiratory function in interstitial lung disease patients with obesity. Respir. Investig. 2020, 59, 247–251. [Google Scholar] [CrossRef]
- Dias-Júnior, S.A.; Reis, M.; de Carvalho-Pinto, R.M.; Stelmach, R.; Halpern, A.; Cukier, A. Effects of weight loss on asthma control in obese patients with severe asthma. Eur. Respir. J. 2013, 43, 1368–1377. [Google Scholar] [CrossRef] [PubMed]
- Van Brakel, L.; Mensink, R.P.; Wesseling, G.; Plat, J. Nutritional Interventions to Improve Asthma-Related Outcomes through Immunomodulation: A Systematic Review. Nutrients 2020, 12, 3839. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.E.; Gibson, P.; Collins, C.E.; Hilton, J.M.; Wood, L. Diet-induced weight loss in obese children with asthma: A randomized controlled trial. Clin. Exp. Allergy 2013, 43, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, S.; Rochet, N.; Gautier, N.; Chabry, J.; Pisani, D.F. The adiponectin receptor agonist AdipoRon normalizes glucose metabolism and prevents obesity but not growth retardation induced by glucocorticoids in young mice. Metabolism 2019, 103, 154027. [Google Scholar] [CrossRef]
- Okada-Iwabu, M.; Yamauchi, T.; Iwabu, M.; Honma, T.; Hamagami, K.-I.; Matsuda, K.; Yamaguchi, M.; Tanabe, H.; Kimura-Someya, T.; Shirouzu, M.; et al. A small-molecule AdipoR agonist for type 2 diabetes and short life in obesity. Nature 2013, 503, 493–499. [Google Scholar] [CrossRef]
- Onodera, T.; Zadeh, E.G.; Xu, P.; Gordillo, R.; Guo, Z.; Joffin, N.; Yu, B.; Scherer, P.E.; Li, W.-H. PEGylated AdipoRon derivatives improve glucose and lipid metabolism under insulinopenic and high-fat diet conditions. J. Lipid Res. 2021, 62, 100095. [Google Scholar] [CrossRef]
- Chen, S.; Wang, Y.; Zhang, H.; Chen, R.; Lv, F.; Li, Z.; Jiang, T.; Lin, D.; Zhang, H.; Yang, L.; et al. The Antioxidant MitoQ Protects Against CSE-Induced Endothelial Barrier Injury and Inflammation by Inhibiting ROS and Autophagy in Human Umbilical Vein Endothelial Cells. Int. J. Biol. Sci. 2019, 15, 1440–1451. [Google Scholar] [CrossRef]
- Wiegman, C.H.; Michaeloudes, C.; Haji, G.; Narang, P.; Clarke, C.J.; Russell, K.E.; Bao, W.; Pavlidis, S.; Barnes, P.J.; Kanerva, J.; et al. Oxidative stress–induced mitochondrial dysfunction drives inflammation and airway smooth muscle remodeling in patients with chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2015, 136, 769–780. [Google Scholar] [CrossRef]
- MitoQ for the Treatment of Metabolic Dysfunction in Asthma—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04026711?term=mitoq&draw=2&rank=4 (accessed on 25 February 2022).
- Federal Register. Volume 81, Issue 242; 2016. Available online: https://www.govinfo.gov/content/pkg/FR-2016-12-16/html/2016-30109.htm (accessed on 19 February 2022).
- Huang, Y.; Jiang, H.; Chen, Y.; Wang, X.; Yang, Y.; Tao, J.; Deng, X.; Liang, G.; Zhang, H.; Jiang, W.; et al. Tranilast directly targets NLRP 3 to treat inflammasome-driven diseases. EMBO Mol. Med. 2018, 10, e8689. [Google Scholar] [CrossRef]
- Uno, M.; Kurita, S.; Misu, H.; Ando, H.; Ota, T.; Matsuzawa-Nagata, N.; Kita, Y.; Nabemoto, S.; Akahori, H.; Zen, Y.; et al. Tranilast, an antifibrogenic agent, ameliorates a dietary rat model of nonalcoholic steatohepatitis. Hepatology 2008, 48, 109–118. [Google Scholar] [CrossRef]
- Cao, J.; Peng, Q. NLRP3 Inhibitor Tranilast Attenuates Gestational Diabetes Mellitus in a Genetic Mouse Model. Drugs R&D 2022, 22, 105–112. [Google Scholar] [CrossRef]
- IRCT Assessment of the Effect of Tranilast (Novel NLRP3 Inflammasome Inhibitor) on the Efficacy of Antiviral Drug Regimens in the Treatment of Patients with Severe COVID19. Available online: https://en.irct.ir/trial/47265 (accessed on 19 February 2022).
- Saeedi-Boroujeni, A.; Mahmoudian-Sani, M.-R.; Nashibi, R.; Houshmandfar, S.; Gandomkari, S.T.; Khodadadi, A. Tranilast: A potential anti-Inflammatory and NLRP3 inflammasome inhibitor drug for COVID-19. Immunopharmacol. Immunotoxicol. 2021, 43, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Coll, R.C.; Hill, J.R.; Day, C.J.; Zamoshnikova, A.; Boucher, D.; Massey, N.L.; Chitty, J.; Fraser, J.A.; Jennings, M.P.; Robertson, A.A.B.; et al. MCC950 directly targets the NLRP3 ATP-hydrolysis motif for inflammasome inhibition. Nat. Chem. Biol. 2019, 15, 556–559. [Google Scholar] [CrossRef] [PubMed]
- Pinkerton, J.W.; Kim, R.Y.; Brown, A.C.; Rae, B.E.; Donovan, C.; Mayall, J.R.; Carroll, O.R.; Ali, K.; Scott, H.A.; Berthon, B.S.; et al. Relationship between type 2 cytokine and inflammasome responses in obesity-associated asthma. J. Allergy Clin. Immunol. 2021, 149, 1270–1280. [Google Scholar] [CrossRef] [PubMed]
- Wada, N.; Yamada, H.; Motoyama, S.; Saburi, M.; Sugimoto, T.; Kubota, H.; Miyawaki, D.; Wakana, N.; Kami, D.; Ogata, T.; et al. Maternal high-fat diet exaggerates diet-induced insulin resistance in adult offspring by enhancing inflammasome activation through noncanonical pathway of caspase-11. Mol. Metab. 2020, 37, 100988. [Google Scholar] [CrossRef]
- Mullard, A. NLRP3 inhibitors stoke anti-inflammatory ambitions. Nat. Rev. Drug Discov. 2019, 18, 405–407. [Google Scholar] [CrossRef]
- Marchetti, C.; Swartzwelter, B.; Gamboni, F.; Neff, C.P.; Richter, K.; Azam, T.; Carta, S.; Tengesdal, I.; Nemkov, T.; D’Alessandro, A.; et al. OLT1177, a β-sulfonyl nitrile compound, safe in humans, inhibits the NLRP3 inflammasome and reverses the metabolic cost of inflammation. Proc. Natl. Acad. Sci. USA 2018, 115, E1530–E1539. [Google Scholar] [CrossRef]
- Lunding, L.P.; Skouras, D.B.; Vock, C.; Dinarello, C.A.; Wegmann, M. The NLRP3 inflammasome inhibitor, OLT1177®, ameliorates experimental allergic asthma in mice. Allergy 2021, 77, 1035–1038. [Google Scholar] [CrossRef]
- Safety and Efficacy of Dapansutrile for Treatment of Moderate COVID-19 Symptoms and Evidence of Early Cytokine Release Syndrome—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04540120 (accessed on 22 February 2022).
- Moon, J.-S.; Nakahira, K.; Chung, K.-P.; DeNicola, G.; Koo, M.J.; Pabón, M.A.; Rooney, K.T.; Yoon, J.-H.; Ryter, S.W.; Stout-Delgado, H.; et al. NOX4-dependent fatty acid oxidation promotes NLRP3 inflammasome activation in macrophages. Nat. Med. 2016, 22, 1002–1012. [Google Scholar] [CrossRef]
- Green, D.E.; Murphy, T.C.; Kang, B.-Y.; Kleinhenz, J.M.; Szyndralewiez, C.; Page, P.; Sutliff, R.L.; Hart, C.M. The Nox4 Inhibitor GKT137831 Attenuates Hypoxia-Induced Pulmonary Vascular Cell Proliferation. Am. J. Respir. Cell Mol. Biol. 2012, 47, 718–726. [Google Scholar] [CrossRef]
- Safety and Efficacy of Oral GKT137831 in Patient with Type 2 Diabetes and Albuminuria—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT02010242 (accessed on 22 February 2022).
- GKT137831 in IPF Patients with Idiopathic Pulmonary Fibrosis—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03865927 (accessed on 22 February 2022).
- Camargo, L.D.N.; Righetti, R.F.; Aristóteles, L.R.D.C.R.B.; Dos Santos, T.M.; de Souza, F.C.R.; Fukuzaki, S.; Cruz, M.M.; Alonso-Vale, M.I.C.; Saraiva-Romanholo, B.M.; Prado, C.M.; et al. Effects of Anti-IL-17 on Inflammation, Remodeling, and Oxidative Stress in an Experimental Model of Asthma Exacerbated by LPS. Front. Immunol. 2018, 8, 1835. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.Y.; Pinkerton, J.W.; Essilfie, A.T.; Robertson, A.A.B.; Baines, K.J.; Brown, A.C.; Mayall, J.R.; Ali, M.K.; Starkey, M.R.; Hansbro, N.G.; et al. Role for NLRP3 Inflammasome–mediated, IL-1β–Dependent Responses in Severe, Steroid-Resistant Asthma. Am. J. Respir. Crit. Care Med. 2017, 196, 283–297. [Google Scholar] [CrossRef] [PubMed]
- Pascoe, S.; Kanniess, F.; Bonner, J.; Lloyd, P.; Lowe, P.; Beier, J.; Woessner, R. A Monoclonal Antibody to IL-1B Attenuates the Late Asthmatic Response to Antigen Challenge in Patients with Mild Asthma. Annual Congress 2006—Novel Treatments for Asthma 2006. Available online: https://www.ers-education.org/lr/show-details/?idP=5730 (accessed on 24 February 2022).
- Safety and Efficacy of Multiple Doses of Canakinumab (ACZ885) in Chronic Obstructive Pulmonary Disease (COPD) Patients—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT00581945 (accessed on 24 February 2022).
- Calverley, P.M.A.; Sethi, S.; Dawson, M.; Ward, C.K.; Finch, D.K.; Penney, M.; Newbold, P.; Van Der Merwe, R. A randomised, placebo-controlled trial of anti–interleukin-1 receptor 1 monoclonal antibody MEDI8968 in chronic obstructive pulmonary disease. Respir. Res. 2017, 18, 153. [Google Scholar] [CrossRef] [PubMed]
- Balkhair, A.; Al-Zakwani, I.; Al Busaidi, M.; Al-Khirbash, A.; Al Mubaihsi, S.; BaTaher, H.; Al Aghbari, J.; Al Kindi, M.; Baawain, S.; Al Alawi, A.; et al. Anakinra in hospitalized patients with severe COVID-19 pneumonia requiring oxygen therapy: Results of a prospective, open-label, interventional study. Int. J. Infect. Dis. 2020, 103, 288–296. [Google Scholar] [CrossRef]
- FitzGerald, J.M.; Bleecker, E.R.; Nair, P.; Korn, S.; Ohta, K.; Lommatzsch, M.; Ferguson, G.T.; Busse, W.W.; Barker, P.; Sproule, S.; et al. Benralizumab, an anti-interleukin-5 receptor α monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2016, 388, 2128–2141. [Google Scholar] [CrossRef]
- Criner, G.J.; Celli, B.R.; Brightling, C.E.; Agusti, A.; Papi, A.; Singh, D.; Sin, D.D.; Vogelmeier, C.F.; Sciurba, F.C.; Bafadhel, M.; et al. Benralizumab for the Prevention of COPD Exacerbations. N. Engl. J. Med. 2019, 381, 1023–1034. [Google Scholar] [CrossRef]
- Staton, T.L.; Peng, K.; Owen, R.; Choy, D.F.; Cabanski, C.R.; Fong, A.; Brunstein, F.; Alatsis, K.R.; Chen, H. A phase I, randomized, observer-blinded, single and multiple ascending-dose study to investigate the safety, pharmacokinetics, and immunogenicity of BITS7201A, a bispecific antibody targeting IL-13 and IL-17, in healthy volunteers. BMC Pulm. Med. 2019, 19, 5. [Google Scholar] [CrossRef]
- Chakraborty, A.; Tannenbaum, S.; Rordorf, C.; Lowe, P.; Floch, D.; Gram, H.; Roy, S. Pharmacokinetic and Pharmacodynamic Properties of Canakinumab, a Human Anti-Interleukin-1β Monoclonal Antibody. Clin. Pharmacokinet. 2012, 51, e1–e18. [Google Scholar] [CrossRef]
- Ridker, P.M.; MacFadyen, J.G.; Thuren, T.; Libby, P. Residual inflammatory risk associated with interleukin-18 and interleukin-6 after successful interleukin-1β inhibition with canakinumab: Further rationale for the development of targeted anti-cytokine therapies for the treatment of atherothrombosis. Eur. Hear. J. 2019, 41, 2153–2163. [Google Scholar] [CrossRef]
- Kubysheva, N.; Boldina, M.; Eliseeva, T.; Soodaeva, S.; Klimanov, I.; Khaletskaya, A.; Bayrasheva, V.; Solovyev, V.; Villa-Vargas, L.A.; Ramírez-Salinas, M.A.; et al. Relationship of Serum Levels of IL-17, IL-18, TNF-α, and Lung Function Parameters in Patients with COPD, Asthma-COPD Overlap, and Bronchial Asthma. Mediat. Inflamm. 2020, 2020, 4652898. [Google Scholar] [CrossRef]
- Imaoka, H.; Hoshino, T.; Takei, S.; Kinoshita, T.; Okamoto, M.; Kawayama, T.; Kato, S.; Iwasaki, H.; Watanabe, K.; Aizawa, H. Interleukin-18 production and pulmonary function in COPD. Eur. Respir. J. 2008, 31, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.-T.; Yang, C.-M. Inflammatory Signalings Involved in Airway and Pulmonary Diseases. Mediat. Inflamm. 2013, 2013, 791231. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palma, G.; Sorice, G.P.; Genchi, V.A.; Giordano, F.; Caccioppoli, C.; D’Oria, R.; Marrano, N.; Biondi, G.; Giorgino, F.; Perrini, S. Adipose Tissue Inflammation and Pulmonary Dysfunction in Obesity. Int. J. Mol. Sci. 2022, 23, 7349. https://doi.org/10.3390/ijms23137349
Palma G, Sorice GP, Genchi VA, Giordano F, Caccioppoli C, D’Oria R, Marrano N, Biondi G, Giorgino F, Perrini S. Adipose Tissue Inflammation and Pulmonary Dysfunction in Obesity. International Journal of Molecular Sciences. 2022; 23(13):7349. https://doi.org/10.3390/ijms23137349
Chicago/Turabian StylePalma, Giuseppe, Gian Pio Sorice, Valentina Annamaria Genchi, Fiorella Giordano, Cristina Caccioppoli, Rossella D’Oria, Nicola Marrano, Giuseppina Biondi, Francesco Giorgino, and Sebastio Perrini. 2022. "Adipose Tissue Inflammation and Pulmonary Dysfunction in Obesity" International Journal of Molecular Sciences 23, no. 13: 7349. https://doi.org/10.3390/ijms23137349
APA StylePalma, G., Sorice, G. P., Genchi, V. A., Giordano, F., Caccioppoli, C., D’Oria, R., Marrano, N., Biondi, G., Giorgino, F., & Perrini, S. (2022). Adipose Tissue Inflammation and Pulmonary Dysfunction in Obesity. International Journal of Molecular Sciences, 23(13), 7349. https://doi.org/10.3390/ijms23137349







