Near-Infrared Fluorescence Imaging of Renal Cell Carcinoma with ASP5354 in a Mouse Model for Intraoperative Guidance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Preparation of Orthotopic Renal Cell Carcinoma Mouse Model
2.3. Materials
2.4. Instruments
2.5. ASP5354 NIRF Imaging
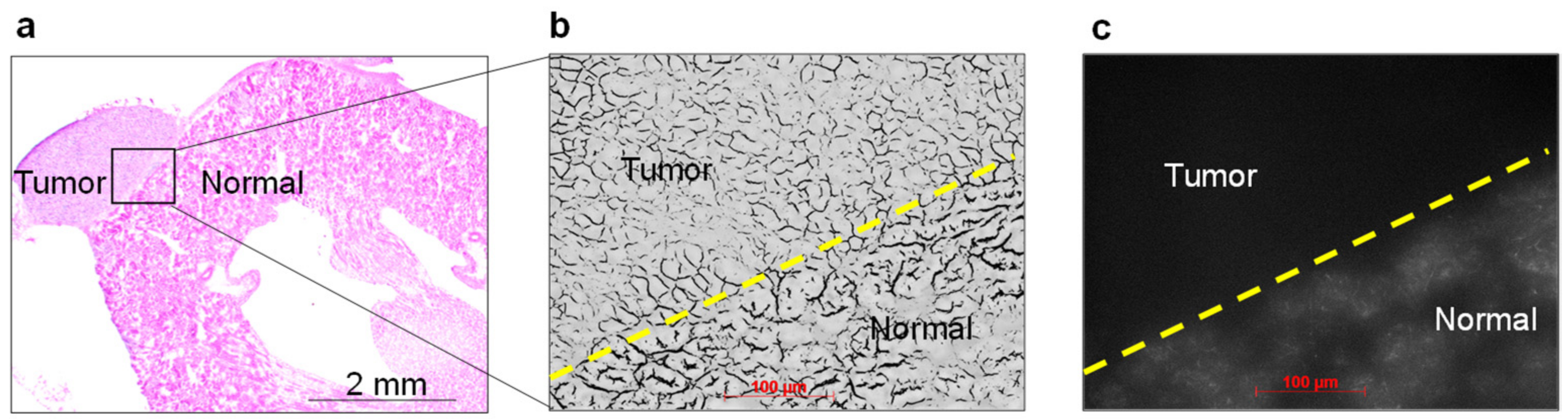
2.6. Histopathological Analysis and NIRF Imaging of Cancerous Kidney Sections
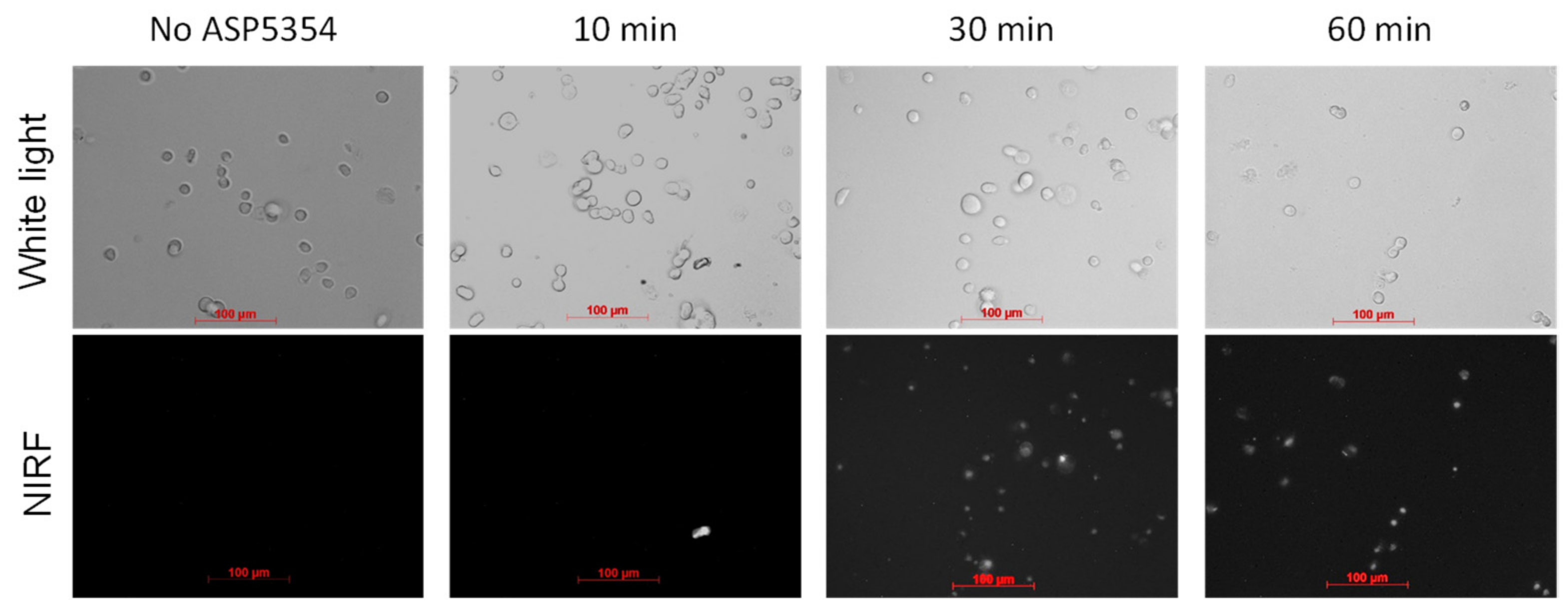
2.7. NIRF Imaging of Cellular Uptake of ASP5354
2.8. ICG NIRF Imaging
2.9. Statistical Analysis
3. Results
3.1. In Vivo NIRF Imaging of ASP5354 Clearance
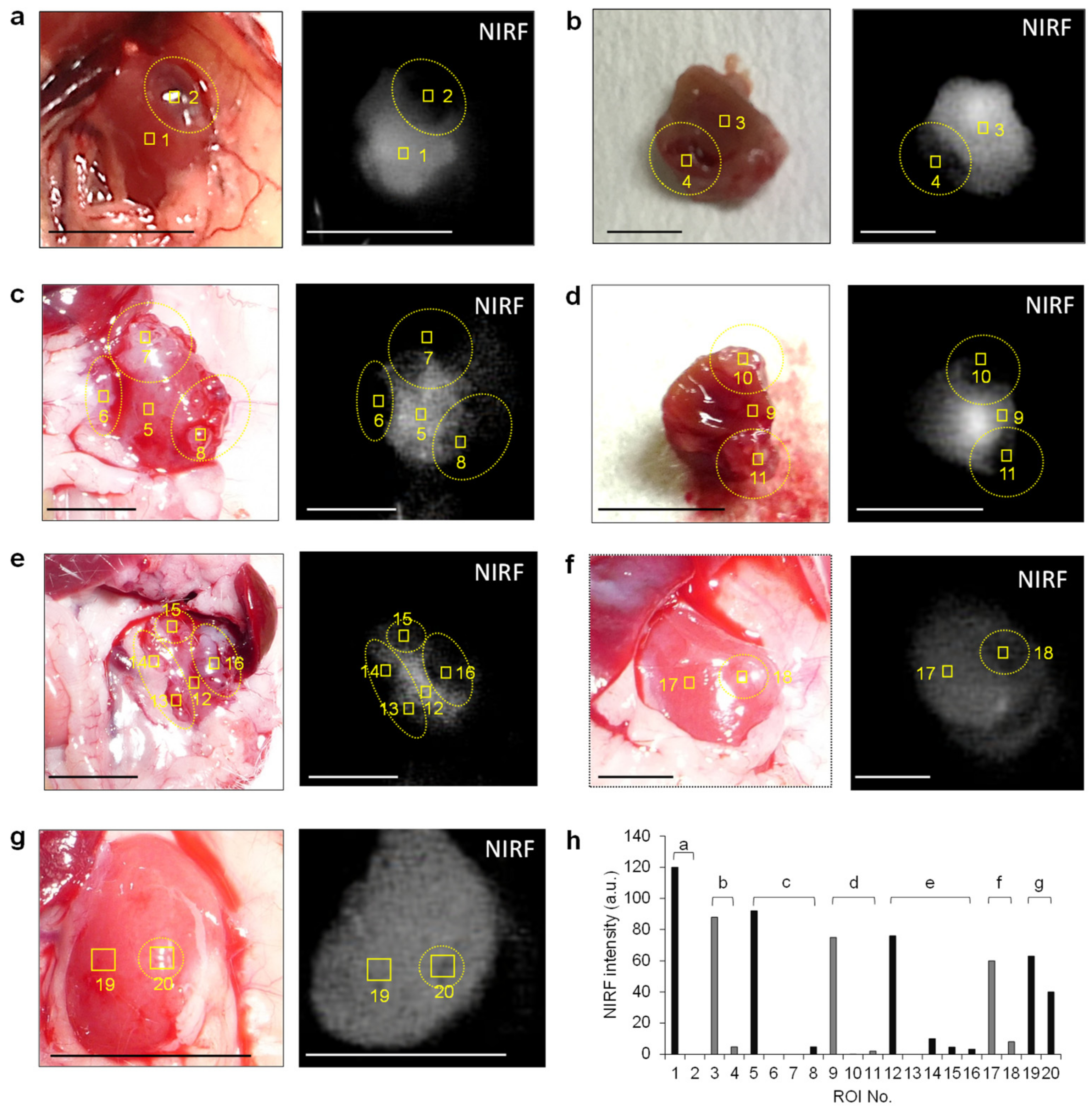
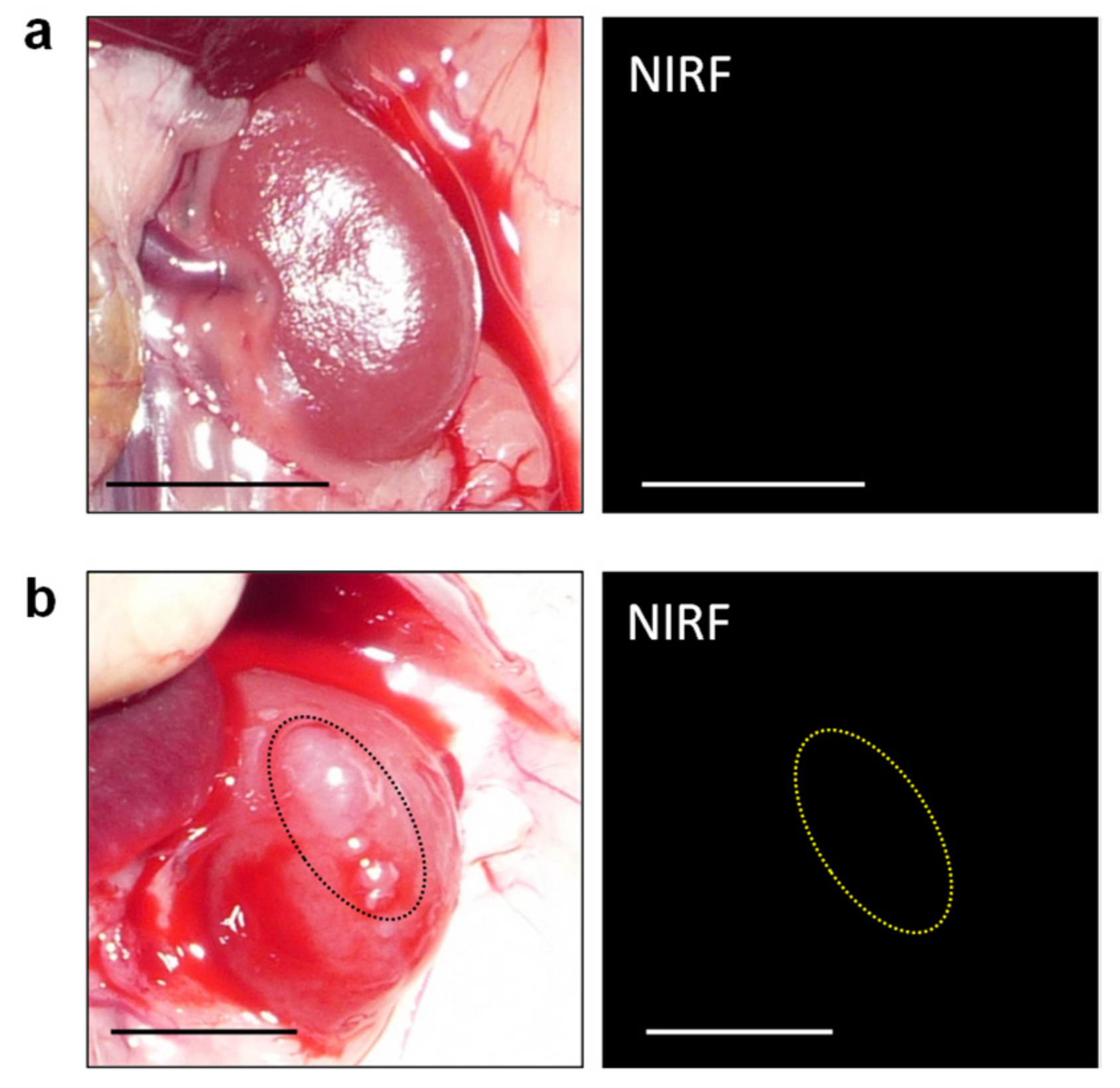
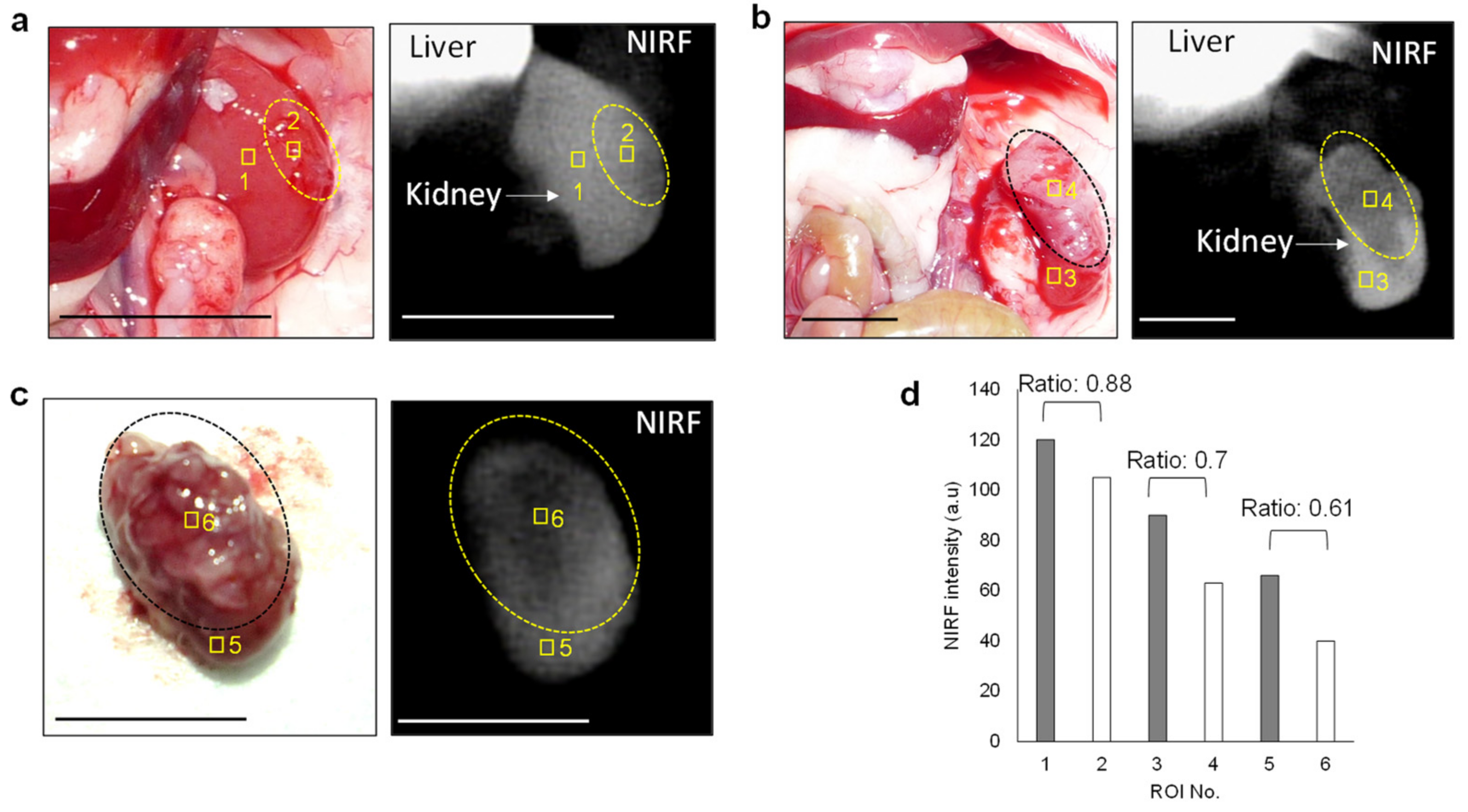
3.2. In Vivo and Ex Vivo NIRF Imaging of Renal Cell Carcinoma with ASP5354
3.3. NIRF Imaging of Cellar Uptake of ASP5354
3.4. In Vivo NIRF Imaging of Renal Cell Carcinoma with ICG
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- GLOBOCAN. 2020. Available online: https://www.uicc.org/news/globocan-2020-new-global-cancer-data (accessed on 8 June 2022).
- Jang, H.A.; Kim, J.W.; Byun, S.S.; Hong, S.H.; Kim, Y.J.; Park, Y.H.; Yang, K.S.; Cho, S.; Cheon, J.; Kang, S.H. Oncologic and Functional Outcomes after Partial Nephrectomy Versus Radical Nephrectomy in T1b Renal Cell Carcinoma: A Multicenter, Matched Case-Control Study in Korean Patients. Cancer Res. Treat. 2016, 48, 612–620. [Google Scholar] [CrossRef] [Green Version]
- Thompson, R.H.; Lane, B.R.; Lohse, C.M.; Leibovich, B.C.; Fergany, A.; Frank, I.; Gill, I.S.; Blute, M.L.; Campbell, S.C. Renal Function After Partial Nephrectomy: Effect of Warm Ischemia Relative to Quantity and Quality of Preserved Kidney. Urology 2012, 79, 356–360. [Google Scholar] [CrossRef]
- Kaczmarek, B.F.; Sukumar, S.; Petros, F.; Trinh, Q.-D.; Mander, N.; Chen, R.; Menon, M.; Rogers, C.G. Robotic ultrasound probe for tumor identification in robotic partial nephrectomy: Initial series and outcomes. Int. J. Urol. 2013, 20, 172–176. [Google Scholar] [CrossRef]
- Venigalla, S.; Wu, G.; Miyamoto, H. The Impact of Frozen Section Analysis During Partial Nephrectomy on Surgical Margin Status and Tumor Recurrence: A Clinicopathologic Study of 433 Cases. Clin. Genitourin. Cancer 2013, 11, 527–536. [Google Scholar] [CrossRef]
- Tobis, S.; Knopf, J.K.; Silvers, C.; Messing, E.; Yao, J.; Rashid, H.; Wu, G.; Golijanin, D. Robot-Assisted and Laparoscopic Partial Nephrectomy with Near Infrared Fluorescence Imaging. J. Endourol. 2012, 26, 797–802. [Google Scholar] [CrossRef]
- Slooter, M.D.; Janssen, A.; Bemelman, W.A.; Tanis, P.J.; Hompes, R. Currently available and experimental dyes for intraoper-ative near-infrared fluorescence imaging of the ureters: A systematic review. Tech. Coloproctol. 2019, 23, 305–313. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Pu, K. Near-infrared fluorescent molecular probes for imaging and diagnosis of nephro-urological diseases. Chem. Sci. 2021, 12, 3379–3392. [Google Scholar] [CrossRef]
- Weissleder, R. A clearer vision for in vivo imaging. Nat. Biotechnol. 2001, 19, 316–317. [Google Scholar] [CrossRef]
- Reinhart, M.B.; Huntington, C.R.; Blair, L.J.; Heniford, B.T.; Augenstein, V.A. Indocyanine green: Historical context, current applications, and future considerations. Surg. Innov. 2016, 23, 166–175. [Google Scholar] [CrossRef]
- Gathje, J.; Steuer, R.R.; Nicholes, K.R. Stability studies on indocyanine green dye. J. Appl. Physiol. 1970, 29, 181–185. [Google Scholar] [CrossRef]
- Ishizawa, T.; Fukushima, N.; Shibahara, J.; Masuda, K.; Tamura, S.; Aoki, T.; Hasegawa, K.; Beck, Y.; Fukayama, M.; Kokudo, N. Real-time identification of liver cancers by using indocyanine green fluorescent imaging. Cancer 2009, 115, 2491–2504. [Google Scholar] [CrossRef]
- Gotoh, K.; Yamada, T.; Ishikawa, O.; Takahashi, H.; Eguchi, H.; Yano, M.; Ohigashi, H.; Tomita, Y.; Miyamoto, Y.; Imaoka, S. A novel image-guided surgery of hepatocellular carcinoma by indocyanine green fluorescence imaging navigation. J. Surg. Oncol. 2009, 100, 75–79. [Google Scholar] [CrossRef]
- Tobis, S.; Knopf, J.K.; Silvers, C.R.; Marshall, J.; Cardin, A.; Wood, R.W.; Reeder, J.; Erturk, E.; Madeb, R.; Yao, J.; et al. Near Infrared Fluorescence Imaging After Intravenous Indocyanine Green: Initial Clinical Experience with Open Partial Nephrectomy for Renal Cortical Tumors. Urology 2012, 79, 958–964. [Google Scholar] [CrossRef]
- Mitsui, Y.; Shiina, H.; Arichi, N.; Hiraoka, T.; Inoue, S.; Sumura, M.; Honda, S.; Yasumoto, H.; Igawa, M. Indocyanine green (ICG)-based fluorescence navigation system for discrimination of kidney cancer from normal parenchyma: Application during partial nephrectomy. Int. Urol. Nephrol. 2012, 44, 753–759. [Google Scholar] [CrossRef]
- Angell, J.E.; Khemees, T.A.; Abaza, R. Optimization of Near Infrared Fluorescence Tumor Localization during Robotic Partial Nephrectomy. J. Urol. 2013, 190, 1668–1673. [Google Scholar] [CrossRef]
- Manny, T.B.; Krane, L.S.; Hemal, A.K. Indocyanine Green Cannot Predict Malignancy in Partial Nephrectomy: Histopathologic Correlation with Fluorescence Pattern in 100 Patients. J. Endourol. 2013, 27, 918–921. [Google Scholar] [CrossRef] [Green Version]
- Harke, N.; Schoen, G.; Schiefelbein, F.; Heinrich, E. Selective clamping under the usage of near-infrared fluorescence imaging with indocyanine green in robot-assisted partial nephrectomy: A single-surgeon matched-pair study. World J. Urol. 2014, 32, 1259–1265. [Google Scholar] [CrossRef]
- Simone, G.; Tuderti, G.; Anceschi, U.; Ferriero, M.; Costantini, M.; Minisola, F.; Vallati, G.; Pizzi, G.; Guaglianone, S.; Misuraca, L.; et al. “Ride the Green Light”: Indocyanine green-marked off-clamp robotic partial nephrectomy for totally endo-phytic renal masses. Eur. Urol. 2019, 75, 1008–1014. [Google Scholar] [CrossRef]
- Hope-Ross, M.; Yannuzzi, L.A.; Gragoudas, E.S.; Guyer, D.R.; Slakter, J.S.; Sorenson, J.A.; Krupsky, S.; Orlock, D.A.; Puliafito, C.A. Adverse Reactions due to Indocyanine Green. Ophthalmology 1994, 101, 529–533. [Google Scholar] [CrossRef]
- Chu, W.; Chennamsetty, A.; Toroussian, R.; Lau, C. Anaphylactic Shock After Intravenous Administration of Indocyanine Green During Robotic Partial Nephrectomy. Urol. Case Rep. 2017, 12, 37–38. [Google Scholar] [CrossRef]
- Bjurlin, M.A.; McClintock, T.R.; Stifelman, M.D. Near-Infrared Fluorescence Imaging with Intraoperative Administration of Indocyanine Green for Robotic Partial Nephrectomy. Curr. Urol. Rep. 2015, 16, 20. [Google Scholar] [CrossRef]
- Soga, N.; Inoko, A.; Furusawa, J.; Ogura, Y. Evaluation to Differentiate between Tumor Lesions and the Parenchyma in Partial Nephrectomies for Renal Tumors Based on Quantitative Fluorescence Imaging Using Indocyanine Green Dye. Curr. Urol. 2019, 13, 74–81. [Google Scholar] [CrossRef]
- Guzzo, T.J.; Jiang, J.; Keating, J.; DeJesus, E.; Judy, R.; Nie, S.; Low, P.; Lal, P.; Singhal, S. Intraoperative Molecular Diagnostic Imaging Can Identify Renal Cell Carcinoma. J. Urol. 2016, 195, 748–755. [Google Scholar] [CrossRef]
- Shum, C.F.; Bahler, C.D.; Low, P.S.; Ratliff, T.L.; Kheyfets, S.V.; Natarajan, J.P.; Sandusky, G.E.; Sundaram, C.P. Novel Use of Folate-Targeted Intraoperative Fluorescence, OTL38, in Robot-Assisted Laparoscopic Partial Nephrectomy: Report of the First Three Cases. J. Endourol. Case Rep. 2016, 2, 189–197. [Google Scholar] [CrossRef] [Green Version]
- Teranishi, K. A Near-infrared fluorescent probe coated with β-cyclodextrin molecules for real-time imaging-guided intraoperative ureteral identification and diagnosis. Mol. Pharm. 2020, 17, 2672–2681. [Google Scholar] [CrossRef]
- Fushiki, H.; Yoshikawa, T.; Matsuda, T.; Sato, T.; Suwa, A. Preclinical Development and Validation of ASP5354: A Near-Infrared Fluorescent Agent for Intraoperative Ureter Visualization. Mol. Imaging Biol. 2021. [Google Scholar] [CrossRef]
- Murase, T.; Takizawa, M.; Galitz, L.; Flach, S.; Murray, V.; Gufford, B.; Suwa, A. Randomized, Double-Blind, Controlled Study to Evaluate Safety and Pharmacokinetics of Single Ascending Doses of ASP5354, an Investigational Imaging Product, in Healthy Adult Volunteers. Clin. Pharmacol. Drug Dev. 2021, 10, 1460–1468. [Google Scholar] [CrossRef]
- Kurahashi, T.; Iwatsuki, K.; Onishi, T.; Arai, T.; Teranishi, K.; Hirata, H. Near-infrared indocyanine dye permits real-time characterization of both venous and lymphatic circulation. J. Biomed. Opt. 2016, 21, 86009. [Google Scholar] [CrossRef]
- Yang, X.; Shao, C.; Wang, R.; Chu, C.-Y.; Hu, P.; Master, V.; Osunkoya, A.O.; Kim, H.L.; Zhau, H.E.; Chung, L.W.K. Optical Imaging of Kidney Cancer with Novel Near Infrared Heptamethine Carbocyanine Fluorescent Dyes. J. Urol. 2013, 189, 702–710. [Google Scholar] [CrossRef] [Green Version]
- Muselaers, C.H.; Stillebroer, A.B.; Rijpkema, M.; Franssen, G.M.; Oosterwijk, E.; Mulders, P.F.; Oyen, W.J.; Boerman, O.C. Optical Imaging of Renal Cell Carcinoma with Anti–Carbonic Anhydrase IX Monoclonal Antibody Girentuximab. J. Nucl. Med. 2014, 55, 1035–1040. [Google Scholar] [CrossRef] [Green Version]
- An, H.-W.; Hou, D.; Zheng, R.; Wang, M.-D.; Zeng, X.-Z.; Xiao, W.-Y.; Yan, T.-D.; Wang, J.-Q.; Zhao, C.-H.; Cheng, L.-M.; et al. A Near-Infrared Peptide Probe with Tumor-Specific Excretion-Retarded Effect for Image-Guided Surgery of Renal Cell Carcinoma. ACS Nano 2020, 14, 927–936. [Google Scholar] [CrossRef]
- Du, B.; Chong, Y.; Jiang, X.; Yu, M.; Lo, U.; Dang, A.; Chen, Y.; Li, S.; Hernandez, E.; Lin, J.C.; et al. Hyperfluorescence Imaging of Kidney Cancer Enabled by Renal Secretion Pathway Dependent Efflux Transport. Angew. Chem. Int. Ed. 2021, 60, 351–359. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teranishi, K. Near-Infrared Fluorescence Imaging of Renal Cell Carcinoma with ASP5354 in a Mouse Model for Intraoperative Guidance. Int. J. Mol. Sci. 2022, 23, 7228. https://doi.org/10.3390/ijms23137228
Teranishi K. Near-Infrared Fluorescence Imaging of Renal Cell Carcinoma with ASP5354 in a Mouse Model for Intraoperative Guidance. International Journal of Molecular Sciences. 2022; 23(13):7228. https://doi.org/10.3390/ijms23137228
Chicago/Turabian StyleTeranishi, Katsunori. 2022. "Near-Infrared Fluorescence Imaging of Renal Cell Carcinoma with ASP5354 in a Mouse Model for Intraoperative Guidance" International Journal of Molecular Sciences 23, no. 13: 7228. https://doi.org/10.3390/ijms23137228
APA StyleTeranishi, K. (2022). Near-Infrared Fluorescence Imaging of Renal Cell Carcinoma with ASP5354 in a Mouse Model for Intraoperative Guidance. International Journal of Molecular Sciences, 23(13), 7228. https://doi.org/10.3390/ijms23137228




