Crosstalk between Schizophrenia and Metabolic Syndrome: The Role of Oxytocinergic Dysfunction
Abstract
1. Schizophrenia: A Complex Disorder
2. Metabolic Syndrome: Definition, Prevalence, and Pathophysiology
3. Schizophrenia and Metabolic Syndrome
3.1. Vulnerability to Metabolic Syndrome in Persons with Schizophrenia
3.2. Neurotransmitters and Hormones of Metabolic Syndrome in Persons with Schizophrenia Treated with Antipsychotics
3.3. Neurotransmitters and Hormones of Metabolic Syndrome in Antipsychotic-Naïve Persons with First-Episode Psychosis
3.4. Co-Shared Genetics Pathway in Schizophrenia and Metabolic Syndrome
4. Oxytocin
4.1. Love Hormone: Oxytocin
4.2. The Implication of Oxytocin in Schizophrenia
4.3. Metabolic Effects of Oxytocin
5. Oxytocin Dysfunction as a Common Mechanism Underlying Schizophrenia and Metabolic Syndrome
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jauhar, S.; Johnstone, M.; McKenna, P.J. Schizophrenia. Lancet 2022, 399, 473–486. [Google Scholar] [CrossRef]
- McGrath, J.; Saha, S.; Chant, D.; Welham, J. Schizophrenia: A Concise Overview of Incidence, Prevalence, and Mortality. Epidemiol. Rev. 2008, 30, 67–76. [Google Scholar] [CrossRef] [PubMed]
- van Os, J.; Kapur, S. Schizophrenia. Lancet 2009, 374, 635–645. [Google Scholar] [CrossRef]
- Laursen, T.M.; Nordentoft, M.; Mortensen, P.B. Excess early mortality in schizophrenia. Annu. Rev. Clin. Psychol. 2014, 10, 425–448. [Google Scholar] [CrossRef] [PubMed]
- McCutcheon, R.A.; Reis Marques, T.; Howes, O.D. Schizophrenia-An Overview. JAMA Psychiatry 2020, 77, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Samochowiec, J.; Misiak, B. Gut microbiota and microbiome in schizophrenia. Curr. Opin. Psychiatry 2021, 34, 503–507. [Google Scholar] [CrossRef]
- Stahl, S.M. Beyond the dopamine hypothesis of schizophrenia to three neural networks of psychosis: Dopamine, serotonin, and glutamate. CNS Spectr. 2018, 23, 187–191. [Google Scholar] [CrossRef]
- Goh, K.K.; Chen, C.H.; Lane, H.Y. Oxytocin in Schizophrenia: Pathophysiology and Implications for Future Treatment. Int. J. Mol. Sci. 2021, 22, 2146. [Google Scholar] [CrossRef]
- Yang, A.C.; Tsai, S.J. New Targets for Schizophrenia Treatment beyond the Dopamine Hypothesis. Int. J. Mol. Sci. 2017, 18, 1689. [Google Scholar] [CrossRef]
- Oakley, P.; Kisely, S.; Baxter, A.; Harris, M.; Desoe, J.; Dziouba, A.; Siskind, D. Increased mortality among people with schizophrenia and other non-affective psychotic disorders in the community: A systematic review and meta-analysis. J. Psychiatr. Res. 2018, 102, 245–253. [Google Scholar] [CrossRef]
- Hjorthøj, C.; Stürup, A.E.; McGrath, J.J.; Nordentoft, M. Years of potential life lost and life expectancy in schizophrenia: A systematic review and meta-analysis. Lancet Psychiatry 2017, 4, 295–301. [Google Scholar] [CrossRef]
- Haro, J.M.; Altamura, C.; Corral, R.; Elkis, H.; Evans, J.; Krebs, M.O.; Zink, M.; Malla, A.; Méndez, J.I.; Bernasconi, C.; et al. Understanding the course of persistent symptoms in schizophrenia: Longitudinal findings from the pattern study. Psychiatry Res. 2018, 267, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Laursen, T.M.; Plana-Ripoll, O.; Andersen, P.K.; McGrath, J.J.; Toender, A.; Nordentoft, M.; Canudas-Romo, V.; Erlangsen, A. Cause-specific life years lost among persons diagnosed with schizophrenia: Is it getting better or worse? Schizophr. Res. 2019, 206, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Tanskanen, A.; Tiihonen, J.; Taipale, H. Mortality in schizophrenia: 30-year nationwide follow-up study. Acta Psychiatr. Scand. 2018, 138, 492–499. [Google Scholar] [CrossRef]
- Stroup, T.S.; Gray, N. Management of common adverse effects of antipsychotic medications. World Psychiatry 2018, 17, 341–356. [Google Scholar] [CrossRef]
- Correll, C.U.; Detraux, J.; De Lepeleire, J.; De Hert, M. Effects of antipsychotics, antidepressants and mood stabilizers on risk for physical diseases in people with schizophrenia, depression and bipolar disorder. World Psychiatry 2015, 14, 119–136. [Google Scholar] [CrossRef]
- Mazereel, V.; Detraux, J.; Vancampfort, D.; van Winkel, R.; De Hert, M. Impact of Psychotropic Medication Effects on Obesity and the Metabolic Syndrome in People With Serious Mental Illness. Front. Endocrinol. 2020, 11, 573479. [Google Scholar] [CrossRef]
- Taipale, H.; Tanskanen, A.; Mehtälä, J.; Vattulainen, P.; Correll, C.U.; Tiihonen, J. 20-year follow-up study of physical morbidity and mortality in relationship to antipsychotic treatment in a nationwide cohort of 62,250 patients with schizophrenia (FIN20). World Psychiatry 2020, 19, 61–68. [Google Scholar] [CrossRef]
- Perry, B.I.; McIntosh, G.; Weich, S.; Singh, S.; Rees, K. The association between first-episode psychosis and abnormal glycaemic control: Systematic review and meta-analysis. Lancet Psychiatry 2016, 3, 1049–1058. [Google Scholar] [CrossRef]
- Pillinger, T.; Beck, K.; Gobjila, C.; Donocik, J.G.; Jauhar, S.; Howes, O.D. Impaired Glucose Homeostasis in First-Episode Schizophrenia: A Systematic Review and Meta-analysis. JAMA Psychiatry 2017, 74, 261–269. [Google Scholar] [CrossRef]
- Firth, J.; Siddiqi, N.; Koyanagi, A.; Siskind, D.; Rosenbaum, S.; Galletly, C.; Allan, S.; Caneo, C.; Carney, R.; Carvalho, A.F.; et al. The Lancet Psychiatry Commission: A blueprint for protecting physical health in people with mental illness. Lancet Psychiatry 2019, 6, 675–712. [Google Scholar] [CrossRef]
- Chan, J.K.N.; Wong, C.S.M.; Or, P.C.F.; Chen, E.Y.H.; Chang, W.C. Risk of mortality and complications in patients with schizophrenia and diabetes mellitus: Population-based cohort study. Br. J. Psychiatry 2021, 219, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.H.; Daumit, G.L.; Dua, T.; Aquila, R.; Charlson, F.; Cuijpers, P.; Druss, B.; Dudek, K.; Freeman, M.; Fujii, C.; et al. Excess mortality in persons with severe mental disorders: A multilevel intervention framework and priorities for clinical practice, policy and research agendas. World Psychiatry 2017, 16, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Sánchez, M.; Escurriola, M.F.; Sanmartín, M.I.F.; Solntseva, I.; Baquero, D.B.; Arno, A.G. Cardiovascular disease and mortality in people with schizophrenia or antipsychotic treatment: A cohort study in primary care. Psychiatry Res. 2021, 306, 114233. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.; Zimmet, P.Z. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet. Med. 1998, 15, 539–553. [Google Scholar] [CrossRef]
- Balkau, B.; Charles, M.A. Comment on the provisional report from the WHO consultation. European Group for the Study of Insulin Resistance (EGIR). Diabet. Med. 1999, 16, 442–443. [Google Scholar] [CrossRef]
- Expert Panel on Detection, Evaluation; Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001, 285, 2486–2497. [Google Scholar] [CrossRef]
- Einhorn, D.; Reaven, G.M.; Cobin, R.H.; Ford, E.; Ganda, O.P.; Handelsman, Y.; Hellman, R.; Jellinger, P.S.; Kendall, D.; Krauss, R.M.; et al. American College of Endocrinology position statement on the insulin resistance syndrome. Endocr. Pract. 2003, 9, 237–252. [Google Scholar] [CrossRef]
- Alberti, K.G.; Zimmet, P.; Shaw, J. The metabolic syndrome—A new worldwide definition. Lancet 2005, 366, 1059–1062. [Google Scholar] [CrossRef]
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.; Loria, C.M.; Smith, S.C., Jr. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef]
- Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z. The metabolic syndrome. Lancet 2005, 365, 1415–1428. [Google Scholar] [CrossRef]
- Mitchell, A.J.; Vancampfort, D.; Sweers, K.; van Winkel, R.; Yu, W.; De Hert, M. Prevalence of metabolic syndrome and metabolic abnormalities in schizophrenia and related disorders—A systematic review and meta-analysis. Schizophr. Bull. 2013, 39, 306–318. [Google Scholar] [CrossRef] [PubMed]
- Goossens, G.H. The Metabolic Phenotype in Obesity: Fat Mass, Body Fat Distribution, and Adipose Tissue Function. Obes. Facts 2017, 10, 207–215. [Google Scholar] [CrossRef]
- Moore, J.X.; Chaudhary, N.; Akinyemiju, T. Metabolic Syndrome Prevalence by Race/Ethnicity and Sex in the United States, National Health and Nutrition Examination Survey, 1988–2012. Prev. Chronic Dis. 2017, 14, E24. [Google Scholar] [CrossRef] [PubMed]
- Hirode, G.; Wong, R.J. Trends in the Prevalence of Metabolic Syndrome in the United States, 2011–2016. JAMA 2020, 323, 2526–2528. [Google Scholar] [CrossRef] [PubMed]
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens Rep. 2018, 20, 12. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Park, S.Y.; Choi, C.S. Insulin Resistance: From Mechanisms to Therapeutic Strategies. Diabetes Metab. J. 2022, 46, 15–37. [Google Scholar] [CrossRef]
- Fahed, G.; Aoun, L.; Bou Zerdan, M.; Allam, S.; Bou Zerdan, M.; Bouferraa, Y.; Assi, H.I. Metabolic Syndrome: Updates on Pathophysiology and Management in 2021. Int. J. Mol. Sci. 2022, 23, 786. [Google Scholar] [CrossRef]
- Unamuno, X.; Gómez-Ambrosi, J.; Rodríguez, A.; Becerril, S.; Frühbeck, G.; Catalán, V. Adipokine dysregulation and adipose tissue inflammation in human obesity. Eur. J. Clin. Investig. 2018, 48, e12997. [Google Scholar] [CrossRef]
- de Oliveira Dos Santos, A.R.; de Oliveira Zanuso, B.; Miola, V.F.B.; Barbalho, S.M.; Santos Bueno, P.C.; Flato, U.A.P.; Detregiachi, C.R.P.; Buchaim, D.V.; Buchaim, R.L.; Tofano, R.J.; et al. Adipokines, Myokines, and Hepatokines: Crosstalk and Metabolic Repercussions. Int. J. Mol. Sci. 2021, 22, 2639. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Li, X.; Adams, H.; Kubena, K.; Guo, S. Etiology of Metabolic Syndrome and Dietary Intervention. Int. J. Mol. Sci. 2018, 20, 128. [Google Scholar] [CrossRef] [PubMed]
- Kerem, L.; Lawson, E.A. The Effects of Oxytocin on Appetite Regulation, Food Intake and Metabolism in Humans. Int. J. Mol. Sci. 2021, 22, 7737. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Lind, L.; Sundström, J.; Ärnlöv, J.; Risérus, U.; Lampa, E. A longitudinal study over 40 years to study the metabolic syndrome as a risk factor for cardiovascular diseases. Sci. Rep. 2021, 11, 2978. [Google Scholar] [CrossRef]
- Sundström, J.; Risérus, U.; Byberg, L.; Zethelius, B.; Lithell, H.; Lind, L. Clinical value of the metabolic syndrome for long term prediction of total and cardiovascular mortality: Prospective, population based cohort study. BMJ 2006, 332, 878–882. [Google Scholar] [CrossRef]
- Olfson, M.; Gerhard, T.; Huang, C.; Crystal, S.; Stroup, T.S. Premature Mortality Among Adults with Schizophrenia in the United States. JAMA Psychiatry 2015, 72, 1172–1181. [Google Scholar] [CrossRef]
- Vancampfort, D.; Wampers, M.; Mitchell, A.J.; Correll, C.U.; De Herdt, A.; Probst, M.; De Hert, M. A meta-analysis of cardio-metabolic abnormalities in drug naïve, first-episode and multi-episode patients with schizophrenia versus general population controls. World Psychiatry 2013, 12, 240–250. [Google Scholar] [CrossRef] [PubMed]
- De Hert, M.; Schreurs, V.; Vancampfort, D.; Van Winkel, R. Metabolic syndrome in people with schizophrenia: A review. World Psychiatry 2009, 8, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.J.; Jang, M.H. Risk Factors of Metabolic Syndrome in Community-Dwelling People with Schizophrenia. Int. J. Environ. Res. Public Health 2020, 17, 6700. [Google Scholar] [CrossRef]
- Sicras-Mainar, A.; Maurino, J.; Ruiz-Beato, E.; Navarro-Artieda, R. Prevalence of metabolic syndrome according to the presence of negative symptoms in patients with schizophrenia. Neuropsychiatr. Dis. Treat. 2015, 11, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Simonelli-Muñoz, A.J.; Fortea, M.I.; Salorio, P.; Gallego-Gomez, J.I.; Sánchez-Bautista, S.; Balanza, S. Dietary habits of patients with schizophrenia: A self-reported questionnaire survey. Int. J. Ment. Health Nurs. 2012, 21, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Bresee, L.C.; Majumdar, S.R.; Patten, S.B.; Johnson, J.A. Utilization of general and specialized cardiac care by people with schizophrenia. Psychiatr. Serv. 2012, 63, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Ward, H.B.; Beermann, A.; Nawaz, U.; Halko, M.A.; Janes, A.C.; Moran, L.V.; Brady, R.O., Jr. Evidence for Schizophrenia-Specific Pathophysiology of Nicotine Dependence. Front. Psychiatry 2022, 13, 804055. [Google Scholar] [CrossRef] [PubMed]
- Rummel-Kluge, C.; Komossa, K.; Schwarz, S.; Hunger, H.; Schmid, F.; Lobos, C.A.; Kissling, W.; Davis, J.M.; Leucht, S. Head-to-head comparisons of metabolic side effects of second generation antipsychotics in the treatment of schizophrenia: A systematic review and meta-analysis. Schizophr. Res. 2010, 123, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.H.; Lee, N.Y.; Kim, S.H.; Chung, I.W.; Youn, T.; Kang, U.G.; Ahn, Y.M.; You, H.Y.; Kim, Y.S. Long-Term Evolution of Metabolic Status in Patients with Schizophrenia Stably Maintained on Second-Generation Antipsychotics. Psychiatry Investig. 2018, 15, 628–637. [Google Scholar] [CrossRef]
- Huhn, M.; Nikolakopoulou, A.; Schneider-Thoma, J.; Krause, M.; Samara, M.; Peter, N.; Arndt, T.; Bäckers, L.; Rothe, P.; Cipriani, A.; et al. Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: A systematic review and network meta-analysis. Lancet 2019, 394, 939–951. [Google Scholar] [CrossRef]
- Pillinger, T.; McCutcheon, R.A.; Vano, L.; Mizuno, Y.; Arumuham, A.; Hindley, G.; Beck, K.; Natesan, S.; Efthimiou, O.; Cipriani, A.; et al. Comparative effects of 18 antipsychotics on metabolic function in patients with schizophrenia, predictors of metabolic dysregulation, and association with psychopathology: A systematic review and network meta-analysis. Lancet Psychiatry 2020, 7, 64–77. [Google Scholar] [CrossRef]
- Bak, M.; Fransen, A.; Janssen, J.; van Os, J.; Drukker, M. Almost All Antipsychotics Result in Weight Gain: A Meta-Analysis. PLoS ONE 2014, 9, e94112. [Google Scholar] [CrossRef] [PubMed]
- Vandenberghe, F.; Gholam-Rezaee, M.; Saigí-Morgui, N.; Delacrétaz, A.; Choong, E.; Solida-Tozzi, A.; Kolly, S.; Thonney, J.; Gallo, S.F.; Hedjal, A.; et al. Importance of early weight changes to predict long-term weight gain during psychotropic drug treatment. J. Clin. Psychiatry 2015, 76, e1417–e1423. [Google Scholar] [CrossRef] [PubMed]
- Dayabandara, M.; Hanwella, R.; Ratnatunga, S.; Seneviratne, S.; Suraweera, C.; de Silva, V.A. Antipsychotic-associated weight gain: Management strategies and impact on treatment adherence. Neuropsychiatr. Dis. Treat. 2017, 13, 2231–2241. [Google Scholar] [CrossRef] [PubMed]
- Khandker, R.; Chekani, F.; Limone, B.; Thiel, E. Cardiometabolic outcomes among schizophrenia patients using antipsychotics: The impact of high weight gain risk vs low weight gain risk treatment. BMC Psychiatry 2022, 22, 133. [Google Scholar] [CrossRef] [PubMed]
- Grajales, D.; Ferreira, V.; Valverde, Á.M. Second-Generation Antipsychotics and Dysregulation of Glucose Metabolism: Beyond Weight Gain. Cells 2019, 8, 1336. [Google Scholar] [CrossRef]
- Parks, K.A.; Parks, C.G.; Yost, J.P.; Bennett, J.I.; Onwuameze, O.E. Acute Blood Pressure Changes Associated with Antipsychotic Administration to Psychiatric Inpatients. Prim. Care Companion CNS Disord. 2018, 20, 26587. [Google Scholar] [CrossRef] [PubMed]
- Ballon, J.S.; Pajvani, U.; Freyberg, Z.; Leibel, R.L.; Lieberman, J.A. Molecular pathophysiology of metabolic effects of antipsychotic medications. Trends Endocrinol. Metab. 2014, 25, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.; Rostrup, E.; Wulff, S.; Glenthøj, B.; Ebdrup, B.H. Striatal Reward Activity and Antipsychotic-Associated Weight Change in Patients with Schizophrenia Undergoing Initial Treatment. JAMA Psychiatry 2016, 73, 121–128. [Google Scholar] [CrossRef]
- Carli, M.; Kolachalam, S.; Longoni, B.; Pintaudi, A.; Baldini, M.; Aringhieri, S.; Fasciani, I.; Annibale, P.; Maggio, R.; Scarselli, M. Atypical Antipsychotics and Metabolic Syndrome: From Molecular Mechanisms to Clinical Differences. Pharmaceuticals 2021, 14, 238. [Google Scholar] [CrossRef] [PubMed]
- Siafis, S.; Tzachanis, D.; Samara, M.; Papazisis, G. Antipsychotic Drugs: From Receptor-binding Profiles to Metabolic Side Effects. Curr. Neuropharmacol. 2018, 16, 1210–1223. [Google Scholar] [CrossRef] [PubMed]
- Manu, P.; Dima, L.; Shulman, M.; Vancampfort, D.; De Hert, M.; Correll, C.U. Weight gain and obesity in schizophrenia: Epidemiology, pathobiology, and management. Acta Psychiatr. Scand. 2015, 132, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.C.; Goh, K.K.; Lu, M.L. Metabolic disturbances associated with antipsychotic drug treatment in patients with schizophrenia: State-of-the-art and future perspectives. World J. Psychiatry 2021, 11, 696–710. [Google Scholar] [CrossRef] [PubMed]
- Borovcanin, M.M.; Vesic, K.; Jovanovic, M.; Mijailovic, N.R. Galectin-3 possible involvement in antipsychotic-induced metabolic changes of schizophrenia: A minireview. World J. Diabetes 2021, 12, 1731–1739. [Google Scholar] [CrossRef] [PubMed]
- Lis, M.; Stańczykiewicz, B.; Liśkiewicz, P.; Misiak, B. Impaired hormonal regulation of appetite in schizophrenia: A narrative review dissecting intrinsic mechanisms and the effects of antipsychotics. Psychoneuroendocrinology 2020, 119, 104744. [Google Scholar] [CrossRef] [PubMed]
- Popovic, V.; Doknic, M.; Maric, N.; Pekic, S.; Damjanovic, A.; Miljic, D.; Popovic, S.; Miljic, N.; Djurovic, M.; Jasovic-Gasic, M.; et al. Changes in neuroendocrine and metabolic hormones induced by atypical antipsychotics in normal-weight patients with schizophrenia. Neuroendocrinology 2007, 85, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Venkatasubramanian, G.; Chittiprol, S.; Neelakantachar, N.; Shetty, T.; Gangadhar, B.N. Effect of antipsychotic treatment on Insulin-like Growth Factor-1 and cortisol in schizophrenia: A longitudinal study. Schizophr. Res. 2010, 119, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.C.; Zhang, Z.Y.; Mulvey, T.; Petersen, N.; Lach, S.; Xiu, P.; Phillips, A.; Han, W.; Wang, M.W.; Shepherd, P.R. Clozapine directly increases insulin and glucagon secretion from islets: Implications for impairment of glucose tolerance. Schizophr. Res. 2014, 157, 128–133. [Google Scholar] [CrossRef]
- Ebdrup, B.H.; Knop, F.K.; Madsen, A.; Mortensen, H.B.; Søgaard, B.; Holst, J.J.; Szecsi, P.B.; Lublin, H. Glucometabolic hormones and cardiovascular risk markers in antipsychotic-treated patients. J. Clin. Psychiatry 2014, 75, e899–e905. [Google Scholar] [CrossRef] [PubMed]
- Klemettilä, J.P.; Solismaa, A.; Seppälä, N.; Hämäläinen, M.; Moilanen, E.; Leinonen, E.; Kampman, O. Glucagon-like peptide-1 serum levels are associated with weight gain in patients treated with clozapine. Psychiatry Res. 2021, 306, 114227. [Google Scholar] [CrossRef] [PubMed]
- Basoglu, C.; Oner, O.; Ates, A.M.; Algul, A.; Semiz, U.B.; Ebrinc, S.; Cetin, M.; Ozcan, O.; Ipcioglu, O.M. Association between symptom improvement and change of body mass index, lipid profile, and leptin, ghrelin, and cholecystokinin levels during 6-week olanzapine treatment in patients with first-episode psychosis. J. Clin. Psychopharmacol. 2010, 30, 636–638. [Google Scholar] [CrossRef] [PubMed]
- van der Zwaal, E.M.; Merkestein, M.; Lam, Y.K.; Brans, M.A.; Luijendijk, M.C.; Bok, L.I.; Verheij, E.R.; La Fleur, S.E.; Adan, R.A. The acute effects of olanzapine on ghrelin secretion, CCK sensitivity, meal size, locomotor activity and body temperature. Int. J. Obes. 2012, 36, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, A.K.; Rodgers, J.B.; Sicard, M.; Zai, C.C.; Likhodi, O.; Freeman, N.; Meltzer, H.Y.; Lieberman, J.A.; Kennedy, J.L.; Müller, D.J. Association study of polymorphisms in cholecystokinin gene and its receptors with antipsychotic induced weight gain in schizophrenia patients. Prog. Neuropsychopharmacol. Biol. Psychiatry 2010, 34, 1484–1490. [Google Scholar] [CrossRef]
- Bartoli, F.; Lax, A.; Crocamo, C.; Clerici, M.; Carrà, G. Plasma adiponectin levels in schizophrenia and role of second-generation antipsychotics: A meta-analysis. Psychoneuroendocrinology 2015, 56, 179–189. [Google Scholar] [CrossRef]
- Tay, Y.H.; Lee, J. The relationship between serum adiponectin levels, cardiometabolic indices and metabolic syndrome in schizophrenia. Asian J. Psychiatr. 2019, 43, 1–6. [Google Scholar] [CrossRef]
- Sapra, M.; Lawson, D.; Iranmanesh, A.; Varma, A. Adiposity-independent hypoadiponectinemia as a potential marker of insulin resistance and inflammation in schizophrenia patients treated with second generation antipsychotics. Schizophr. Res. 2016, 174, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Kelesidis, T.; Kelesidis, I.; Chou, S.; Mantzoros, C.S. Narrative review: The role of leptin in human physiology: Emerging clinical applications. Ann. Intern. Med. 2010, 152, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Petrikis, P.; Karampas, A.; Leondaritis, G.; Markozannes, G.; Archimandriti, D.T.; Spyrou, P.; Georgiou, G.; Skapinakis, P.; Voulgari, P.V. Adiponectin, leptin and resistin levels in first-episode, drug-naïve patients with psychosis before and after short-term antipsychotic treatment. J. Psychosom. Res. 2022, 157, 110789. [Google Scholar] [CrossRef]
- Stubbs, B.; Wang, A.K.; Vancampfort, D.; Miller, B.J. Are leptin levels increased among people with schizophrenia versus controls? A systematic review and comparative meta-analysis. Psychoneuroendocrinology 2016, 63, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Endomba, F.T.; Tankeu, A.T.; Nkeck, J.R.; Tochie, J.N. Leptin and psychiatric illnesses: Does leptin play a role in antipsychotic-induced weight gain? Lipids Health Dis. 2020, 19, 22. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, X.; Yao, X.; Yang, Y.; Ning, X.; Zhao, T.; Xia, L.; Zhang, Y.; Zhang, K.; Liu, H. Do Leptin Play a Role in Metabolism-Related Psychopathological Symptoms? Front. Psychiatry 2021, 12, 710498. [Google Scholar] [CrossRef]
- Obradovic, M.; Sudar-Milovanovic, E.; Soskic, S.; Essack, M.; Arya, S.; Stewart, A.J.; Gojobori, T.; Isenovic, E.R. Leptin and Obesity: Role and Clinical Implication. Front. Endocrinol. 2021, 12, 585887. [Google Scholar] [CrossRef]
- Müller, T.D.; Nogueiras, R.; Andermann, M.L.; Andrews, Z.B.; Anker, S.D.; Argente, J.; Batterham, R.L.; Benoit, S.C.; Bowers, C.Y.; Broglio, F.; et al. Ghrelin. Mol. Metab. 2015, 4, 437–460. [Google Scholar] [CrossRef]
- Chao, A.M.; Jastreboff, A.M.; White, M.A.; Grilo, C.M.; Sinha, R. Stress, cortisol, and other appetite-related hormones: Prospective prediction of 6-month changes in food cravings and weight. Obesity 2017, 25, 713–720. [Google Scholar] [CrossRef]
- Klok, M.D.; Jakobsdottir, S.; Drent, M.L. The role of leptin and ghrelin in the regulation of food intake and body weight in humans: A review. Obes. Rev. 2007, 8, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Otto, B.; Cuntz, U.; Fruehauf, E.; Wawarta, R.; Folwaczny, C.; Riepl, R.L.; Heiman, M.L.; Lehnert, P.; Fichter, M.; Tschöp, M. Weight gain decreases elevated plasma ghrelin concentrations of patients with anorexia nervosa. Eur. J. Endocrinol. 2001, 145, 669–673. [Google Scholar] [CrossRef] [PubMed]
- Goetz, R.L.; Miller, B.J. Meta-analysis of ghrelin alterations in schizophrenia: Effects of olanzapine. Schizophr. Res. 2019, 206, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; He, M.; Deng, C.; Wang, H.; Lian, J.; Huang, X.F. Hypothalamic ghrelin signalling mediates olanzapine-induced hyperphagia and weight gain in female rats. Int. J. Neuropsychopharmacol. 2014, 17, 807–818. [Google Scholar] [CrossRef]
- Zhang, Q.; Deng, C.; Huang, X.F. The role of ghrelin signalling in second-generation antipsychotic-induced weight gain. Psychoneuroendocrinology 2013, 38, 2423–2438. [Google Scholar] [CrossRef]
- Wu, T.H.; Chiu, C.C.; Goh, K.K.; Chen, P.Y.; Huang, M.C.; Chen, C.H.; Lu, M.L. Relationship between metabolic syndrome and acylated/desacylated ghrelin ratio in patients with schizophrenia under olanzapine medication. J. Psychopharmacol. 2020, 34, 86–92. [Google Scholar] [CrossRef]
- Lu, J.; Huang, M.L.; Li, J.H.; Jin, K.Y.; Li, H.M.; Mou, T.T.; Fronczek, R.; Duan, J.F.; Xu, W.J.; Swaab, D.; et al. Changes of Hypocretin (Orexin) System in Schizophrenia: From Plasma to Brain. Schizophr. Bull. 2021, 47, 1310–1319. [Google Scholar] [CrossRef]
- Chen, P.Y.; Chen, C.H.; Chang, C.K.; Kao, C.F.; Lu, M.L.; Lin, S.K.; Huang, M.C.; Hwang, L.L.; Mondelli, V. Orexin-A Levels in Relation to the Risk of Metabolic Syndrome in Patients with Schizophrenia Taking Antipsychotics. Int. J. Neuropsychopharmacol. 2019, 22, 28–36. [Google Scholar] [CrossRef]
- Basoglu, C.; Oner, O.; Gunes, C.; Semiz, U.B.; Ates, A.M.; Algul, A.; Ebrinc, S.; Cetin, M.; Ozcan, O.; Ipcioglu, O. Plasma orexin A, ghrelin, cholecystokinin, visfatin, leptin and agouti-related protein levels during 6-week olanzapine treatment in first-episode male patients with psychosis. Int. Clin. Psychopharmacol. 2010, 25, 165–171. [Google Scholar] [CrossRef]
- Chen, P.Y.; Chang, C.K.; Chen, C.H.; Fang, S.C.; Mondelli, V.; Chiu, C.C.; Lu, M.L.; Hwang, L.L.; Huang, M.C. Orexin-a elevation in antipsychotic-treated compared to drug-free patients with schizophrenia: A medication effect independent of metabolic syndrome. J. Formos. Med. Assoc. 2022. [Google Scholar] [CrossRef]
- Schwetz, V.; Librizzi, R.; Trummer, C.; Theiler, G.; Stiegler, C.; Pieber, T.R.; Obermayer-Pietsch, B.; Pilz, S. Treatment of hyperprolactinaemia reduces total cholesterol and LDL in patients with prolactinomas. Metab. Brain Dis. 2017, 32, 155–161. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gragnoli, C.; Reeves, G.M.; Reazer, J.; Postolache, T.T. Dopamine-prolactin pathway potentially contributes to the schizophrenia and type 2 diabetes comorbidity. Transl. Psychiatry 2016, 6, e785. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhang, C.; Siafis, S.; Zhuo, K.; Zhu, D.; Wu, H.; Liu, D.; Jiang, K.; Wang, J.; Leucht, S.; et al. Prolactin levels influenced by antipsychotic drugs in schizophrenia: A systematic review and network meta-analysis. Schizophr. Res. 2021, 237, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Oberweis, B.; Gragnoli, C. Potential role of prolactin in antipsychotic-mediated association of schizophrenia and type 2 diabetes. J. Cell. Physiol. 2012, 227, 3001–3006. [Google Scholar] [CrossRef]
- Dasgupta, A.; Singh, O.P.; Rout, J.K.; Saha, T.; Mandal, S. Insulin resistance and metabolic profile in antipsychotic naïve schizophrenia patients. Prog. Neuropsychopharmacol. Biol. Psychiatry 2010, 34, 1202–1207. [Google Scholar] [CrossRef]
- Quintana, D.S.; Dieset, I.; Elvsåshagen, T.; Westlye, L.T.; Andreassen, O.A. Oxytocin system dysfunction as a common mechanism underlying metabolic syndrome and psychiatric symptoms in schizophrenia and bipolar disorders. Front. Neuroendocrinol. 2017, 45, 1–10. [Google Scholar] [CrossRef]
- Lang, X.; Liu, Q.; Fang, H.; Zhou, Y.; Forster, M.T.; Li, Z.; Zhang, X. The prevalence and clinical correlates of metabolic syndrome and cardiometabolic alterations in 430 drug-naive patients in their first episode of schizophrenia. Psychopharmacology 2021, 238, 3643–3652. [Google Scholar] [CrossRef]
- Garrido-Torres, N.; Rocha-Gonzalez, I.; Alameda, L.; Rodriguez-Gangoso, A.; Vilches, A.; Canal-Rivero, M.; Crespo-Facorro, B.; Ruiz-Veguilla, M. Metabolic syndrome in antipsychotic-naïve patients with first-episode psychosis: A systematic review and meta-analysis. Psychol. Med. 2021, 51, 2307–2320. [Google Scholar] [CrossRef]
- Gardner-Sood, P.; Lally, J.; Smith, S.; Atakan, Z.; Ismail, K.; Greenwood, K.E.; Keen, A.; O’Brien, C.; Onagbesan, O.; Fung, C.; et al. Cardiovascular risk factors and metabolic syndrome in people with established psychotic illnesses: Baseline data from the IMPaCT randomized controlled trial. Psychol. Med. 2015, 45, 2619–2629. [Google Scholar] [CrossRef]
- Smith, J.; Griffiths, L.A.; Band, M.; Horne, D. Cardiometabolic Risk in First Episode Psychosis Patients. Front. Endocrinol. 2020, 11, 564240. [Google Scholar] [CrossRef]
- Kucukgoncu, S.; Kosir, U.; Zhou, E.; Sullivan, E.; Srihari, V.H.; Tek, C. Glucose metabolism dysregulation at the onset of mental illness is not limited to first episode psychosis: A systematic review and meta-analysis. Early Interv. Psychiatry 2019, 13, 1021–1031. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; He, H.; Bai, X.; Jiang, L.; Chen, W.; Zeng, X.; Li, Y.; Teixeira, A.L.; Dai, J. Unveiling the Metabolic Profile of First-Episode Drug-Naïve Schizophrenia Patients: Baseline Characteristics of a Longitudinal Study among Han Chinese. Front. Psychiatry 2021, 12, 702720. [Google Scholar] [CrossRef] [PubMed]
- Tomasik, J.; Lago, S.G.; Vázquez-Bourgon, J.; Papiol, S.; Suárez-Pinilla, P.; Crespo-Facorro, B.; Bahn, S. Association of Insulin Resistance with Schizophrenia Polygenic Risk Score and Response to Antipsychotic Treatment. JAMA Psychiatry 2019, 76, 864–867. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, M.; Du, X.; Liao, W.; Chen, D.; Fan, F.; Xiu, M.; Jia, Q.; Ning, Y.; Huang, X.; et al. Glucose disturbances, cognitive deficits and white matter abnormalities in first-episode drug-naive schizophrenia. Mol. Psychiatry 2020, 25, 3220–3230. [Google Scholar] [CrossRef] [PubMed]
- Correll, C.U.; Robinson, D.G.; Schooler, N.R.; Brunette, M.F.; Mueser, K.T.; Rosenheck, R.A.; Marcy, P.; Addington, J.; Estroff, S.E.; Robinson, J.; et al. Cardiometabolic risk in patients with first-episode schizophrenia spectrum disorders: Baseline results from the RAISE-ETP study. JAMA Psychiatry 2014, 71, 1350–1363. [Google Scholar] [CrossRef] [PubMed]
- Misiak, B.; Bartoli, F.; Stramecki, F.; Samochowiec, J.; Lis, M.; Kasznia, J.; Jarosz, K.; Stańczykiewicz, B. Appetite regulating hormones in first-episode psychosis: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2019, 102, 362–370. [Google Scholar] [CrossRef]
- Chouinard, V.A.; Henderson, D.C.; Dalla Man, C.; Valeri, L.; Gray, B.E.; Ryan, K.P.; Cypess, A.M.; Cobelli, C.; Cohen, B.M.; Öngür, D. Impaired insulin signaling in unaffected siblings and patients with first-episode psychosis. Mol. Psychiatry 2019, 24, 1513–1522. [Google Scholar] [CrossRef]
- Misiak, B.; Wiśniewski, M.; Lis, M.; Samochowiec, J.; Stańczykiewicz, B. Glucose homeostasis in unaffected first-degree relatives of schizophrenia patients: A systematic review and meta-analysis. Schizophr. Res. 2020, 223, 2–8. [Google Scholar] [CrossRef]
- Erion, K.A.; Corkey, B.E. Hyperinsulinemia: A Cause of Obesity? Curr. Obes. Rep. 2017, 6, 178–186. [Google Scholar] [CrossRef]
- Chen, D.C.; Du, X.D.; Yin, G.Z.; Yang, K.B.; Nie, Y.; Wang, N.; Li, Y.L.; Xiu, M.H.; He, S.C.; Yang, F.D.; et al. Impaired glucose tolerance in first-episode drug-naïve patients with schizophrenia: Relationships with clinical phenotypes and cognitive deficits. Psychol. Med. 2016, 46, 3219–3230. [Google Scholar] [CrossRef]
- Soontornniyomkij, V.; Lee, E.E.; Jin, H.; Martin, A.S.; Daly, R.E.; Liu, J.; Tu, X.M.; Eyler, L.T.; Jeste, D.V. Clinical Correlates of Insulin Resistance in Chronic Schizophrenia: Relationship to Negative Symptoms. Front. Psychiatry 2019, 10, 251. [Google Scholar] [CrossRef]
- Harris, L.W.; Guest, P.C.; Wayland, M.T.; Umrania, Y.; Krishnamurthy, D.; Rahmoune, H.; Bahn, S. Schizophrenia: Metabolic aspects of aetiology, diagnosis and future treatment strategies. Psychoneuroendocrinology 2013, 38, 752–766. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.M.; Caravaggio, F.; Costa-Dookhan, K.A.; Castellani, L.; Kowalchuk, C.; Asgariroozbehani, R.; Graff-Guerrero, A.; Hahn, M. Brain insulin action in schizophrenia: Something borrowed and something new. Neuropharmacology 2020, 163, 107633. [Google Scholar] [CrossRef] [PubMed]
- Misiak, B.; Pruessner, M.; Samochowiec, J.; Wiśniewski, M.; Reginia, A.; Stańczykiewicz, B. A meta-analysis of blood and salivary cortisol levels in first-episode psychosis and high-risk individuals. Front. Neuroendocrinol. 2021, 62, 100930. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.C.; Sharifi, N.; Condren, R.; Thakore, J.H. Evidence of basal pituitary-adrenal overactivity in first episode, drug naïve patients with schizophrenia. Psychoneuroendocrinology 2004, 29, 1065–1070. [Google Scholar] [CrossRef] [PubMed]
- Spelman, L.M.; Walsh, P.I.; Sharifi, N.; Collins, P.; Thakore, J.H. Impaired glucose tolerance in first-episode drug-naïve patients with schizophrenia. Diabet. Med. 2007, 24, 481–485. [Google Scholar] [CrossRef]
- Han, Y.; Ji, H.; Liu, L.; Zhu, Y.; Jiang, X. The Relationship of Functional Status of Cortisol, Testosterone, and Parameters of Metabolic Syndrome in Male Schizophrenics. Biomed. Res. Int. 2020, 2020, 9124520. [Google Scholar] [CrossRef]
- Balõtšev, R.; Haring, L.; Koido, K.; Leping, V.; Kriisa, K.; Zilmer, M.; Vasar, V.; Piir, A.; Lang, A.; Vasar, E. Antipsychotic treatment is associated with inflammatory and metabolic biomarkers alterations among first-episode psychosis patients: A 7-month follow-up study. Early Interv. Psychiatry 2019, 13, 101–109. [Google Scholar] [CrossRef]
- Lis, M.; Stańczykiewicz, B.; Pawlik-Sobecka, L.; Samochowiec, A.; Reginia, A.; Misiak, B. Assessment of Appetite-Regulating Hormones Provides Further Evidence of Altered Adipoinsular Axis in Early Psychosis. Front. Psychiatry 2020, 11, 480. [Google Scholar] [CrossRef]
- Bocchio-Chiavetto, L.; Zanardini, R.; Tosato, S.; Ventriglia, M.; Ferrari, C.; Bonetto, C.; Lasalvia, A.; Giubilini, F.; Fioritti, A.; Pileggi, F.; et al. Immune and metabolic alterations in first episode psychosis (FEP) patients. Brain Behav. Immun. 2018, 70, 315–324. [Google Scholar] [CrossRef]
- Graham, K.A.; Cho, H.; Brownley, K.A.; Harp, J.B. Early treatment-related changes in diabetes and cardiovascular disease risk markers in first episode psychosis subjects. Schizophr. Res. 2008, 101, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Martorell, L.; Muntané, G.; Porta-López, S.; Moreno, I.; Ortega, L.; Montalvo, I.; Sanchez-Gistau, V.; Monseny, R.; Labad, J.; Vilella, E. Increased levels of serum leptin in the early stages of psychosis. J. Psychiatr. Res. 2019, 111, 24–29. [Google Scholar] [CrossRef]
- Hommel, J.D.; Trinko, R.; Sears, R.M.; Georgescu, D.; Liu, Z.W.; Gao, X.B.; Thurmon, J.J.; Marinelli, M.; DiLeone, R.J. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron 2006, 51, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, D.; Del Olmo, N.; Ruiz-Gayo, M. Desensitization of leptin receptors is coincident with the upregulation of dopamine-related genes in the prefrontal cortex of adolescent mice. Neuroreport 2016, 27, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.Q.; Li, S.X.; Chen, F.B.; Zhang, Y.; Li, P.; Jin, M.; Sun, Y.; Wang, F.; Mi, W.F.; Shi, L.; et al. Diurnal neurobiological alterations after exposure to clozapine in first-episode schizophrenia patients. Psychoneuroendocrinology 2016, 64, 108–116. [Google Scholar] [CrossRef]
- Garcia-Rizo, C.; Fernandez-Egea, E.; Oliveira, C.; Justicia, A.; Parellada, E.; Bernardo, M.; Kirkpatrick, B. Prolactin concentrations in newly diagnosed, antipsychotic-naïve patients with nonaffective psychosis. Schizophr. Res. 2012, 134, 16–19. [Google Scholar] [CrossRef]
- Tosato, S.; Bonetto, C.; Tomassi, S.; Zanardini, R.; Faravelli, C.; Bruschi, C.; D’Agostino, A.; Minelli, A.; Scocco, P.; Lasalvia, A.; et al. Childhood trauma and glucose metabolism in patients with first-episode psychosis. Psychoneuroendocrinology 2020, 113, 104536. [Google Scholar] [CrossRef]
- Song, X.; Fan, X.; Song, X.; Zhang, J.; Zhang, W.; Li, X.; Gao, J.; Harrington, A.; Ziedonis, D.; Lv, L. Elevated levels of adiponectin and other cytokines in drug naïve, first episode schizophrenia patients with normal weight. Schizophr. Res. 2013, 150, 269–273. [Google Scholar] [CrossRef]
- Fernandez-Egea, E.; Bernardo, M.; Donner, T.; Conget, I.; Parellada, E.; Justicia, A.; Esmatjes, E.; Garcia-Rizo, C.; Kirkpatrick, B. Metabolic profile of antipsychotic-naive individuals with non-affective psychosis. Br. J. Psychiatry 2009, 194, 434–438. [Google Scholar] [CrossRef]
- van Nimwegen, L.J.; Storosum, J.G.; Blumer, R.M.; Allick, G.; Venema, H.W.; de Haan, L.; Becker, H.; van Amelsvoort, T.; Ackermans, M.T.; Fliers, E.; et al. Hepatic insulin resistance in antipsychotic naive schizophrenic patients: Stable isotope studies of glucose metabolism. J. Clin. Endocrinol. Metab. 2008, 93, 572–577. [Google Scholar] [CrossRef]
- Cohn, T.A.; Remington, G.; Zipursky, R.B.; Azad, A.; Connolly, P.; Wolever, T.M. Insulin resistance and adiponectin levels in drug-free patients with schizophrenia: A preliminary report. Can. J. Psychiatry 2006, 51, 382–386. [Google Scholar] [CrossRef]
- Riecher-Rössler, A.; Rybakowski, J.K.; Pflueger, M.O.; Beyrau, R.; Kahn, R.S.; Malik, P.; Fleischhacker, W.W. Hyperprolactinemia in antipsychotic-naive patients with first-episode psychosis. Psychol. Med. 2013, 43, 2571–2582. [Google Scholar] [CrossRef] [PubMed]
- Petrikis, P.; Tigas, S.; Tzallas, A.T.; Archimandriti, D.T.; Skapinakis, P.; Mavreas, V. Prolactin levels in drug-naïve patients with schizophrenia and other psychotic disorders. Int. J. Psychiatry Clin. Pract. 2016, 20, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Del Cacho, N.; Butjosa, A.; Vila-Badia, R.; Cuadras, D.; Kaplan, M.; Rubio-Abadal, E.; Pardo, M.; Muñoz-Samons, D.; Cuevas-Esteban, J.; Saenz-Navarrete, G.; et al. Prolactin levels in drug-naïve first episode nonaffective psychosis patients compared with healthy controls. Sex differences. Psychiatry Res. 2019, 276, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Albayrak, Y.; Beyazyüz, M.; Beyazyüz, E.; Kuloğlu, M. Increased serum prolactin levels in drug-naive first-episode male patients with schizophrenia. Nord. J. Psychiatry 2014, 68, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Petruzzelli, M.G.; Margari, M.; Peschechera, A.; de Giambattista, C.; De Giacomo, A.; Matera, E.; Margari, F. Hyperprolactinemia and insulin resistance in drug naive patients with early onset first episode psychosis. BMC Psychiatry 2018, 18, 246. [Google Scholar] [CrossRef] [PubMed]
- Smeland, O.B.; Frei, O.; Dale, A.M.; Andreassen, O.A. The polygenic architecture of schizophrenia—Rethinking pathogenesis and nosology. Nat. Rev. Neurol. 2020, 16, 366–379. [Google Scholar] [CrossRef] [PubMed]
- Postolache, T.T.; Del Bosque-Plata, L.; Jabbour, S.; Vergare, M.; Wu, R.; Gragnoli, C. Co-shared genetics and possible risk gene pathway partially explain the comorbidity of schizophrenia, major depressive disorder, type 2 diabetes, and metabolic syndrome. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2019, 180, 186–203. [Google Scholar] [CrossRef] [PubMed]
- Andreassen, O.A.; Djurovic, S.; Thompson, W.K.; Schork, A.J.; Kendler, K.S.; O’Donovan, M.C.; Rujescu, D.; Werge, T.; van de Bunt, M.; Morris, A.P.; et al. Improved detection of common variants associated with schizophrenia by leveraging pleiotropy with cardiovascular-disease risk factors. Am. J. Hum. Genet. 2013, 92, 197–209. [Google Scholar] [CrossRef]
- Lane, J.M.; Liang, J.; Vlasac, I.; Anderson, S.G.; Bechtold, D.A.; Bowden, J.; Emsley, R.; Gill, S.; Little, M.A.; Luik, A.I.; et al. Genome-wide association analyses of sleep disturbance traits identify new loci and highlight shared genetics with neuropsychiatric and metabolic traits. Nat. Genet. 2017, 49, 274–281. [Google Scholar] [CrossRef]
- Cao, H.; Chen, J.; Meyer-Lindenberg, A.; Schwarz, E. A polygenic score for schizophrenia predicts glycemic control. Transl. Psychiatry 2017, 7, 1295. [Google Scholar] [CrossRef] [PubMed]
- Hackinger, S.; Prins, B.; Mamakou, V.; Zengini, E.; Marouli, E.; Brčić, L.; Serafetinidis, I.; Lamnissou, K.; Kontaxakis, V.; Dedoussis, G.; et al. Evidence for genetic contribution to the increased risk of type 2 diabetes in schizophrenia. Transl. Psychiatry 2018, 8, 252. [Google Scholar] [CrossRef] [PubMed]
- Nagalski, A.; Kozinski, K.; Wisniewska, M.B. Metabolic pathways in the periphery and brain: Contribution to mental disorders? Int. J. Biochem. Cell Biol. 2016, 80, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.I.; Shuldiner, A.R. Rethinking the genetic basis for comorbidity of schizophrenia and type 2 diabetes. Schizophr. Res. 2010, 123, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Bellivier, F. Schizophrenia, antipsychotics and diabetes: Genetic aspects. Eur. Psychiatry 2005, 20 (Suppl. 4), S335–S339. [Google Scholar] [CrossRef]
- Mizuki, Y.; Sakamoto, S.; Okahisa, Y.; Yada, Y.; Hashimoto, N.; Takaki, M.; Yamada, N. Mechanisms Underlying the Comorbidity of Schizophrenia and Type 2 Diabetes Mellitus. Int. J. Neuropsychopharmacol. 2021, 24, 367–382. [Google Scholar] [CrossRef] [PubMed]
- Malan-Müller, S.; Kilian, S.; van den Heuvel, L.L.; Bardien, S.; Asmal, L.; Warnich, L.; Emsley, R.A.; Hemmings, S.M.; Seedat, S. A systematic review of genetic variants associated with metabolic syndrome in patients with schizophrenia. Schizophr. Res. 2016, 170, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.Y.; Chen, P.; Zhu, H.W.; Yin, X.L.; Ye, G.; Chi, Y.Y.; Kang, Z.P.; Sun, H.Y.; Hou, W.L.; Guan, L.Y.; et al. The type 2 diabetes mellitus susceptibility gene CDKAL1 polymorphism is associated with depressive symptom in first-episode drug-naive schizophrenic patients. Hum. Psychopharmacol. 2021, 36, e2790. [Google Scholar] [CrossRef] [PubMed]
- Fattakhov, N.; Smirnova, L.; Atochin, D.; Parshukova, D.; Skuratovskaia, D.; Painter, Q.; Zatolokin, P.; Semke, A.; Litvinova, L.; Ivanova, S. Haplotype analysis of endothelial nitric oxide synthase (NOS3) genetic variants and metabolic syndrome in healthy subjects and schizophrenia patients. Int. J. Obes. 2018, 42, 2036–2046. [Google Scholar] [CrossRef]
- Perry, B.I.; Bowker, N.; Burgess, S.; Wareham, N.J.; Upthegrove, R.; Jones, P.B.; Langenberg, C.; Khandaker, G.M. Evidence for Shared Genetic Aetiology between Schizophrenia, Cardiometabolic, and Inflammation-Related Traits: Genetic Correlation and Colocalization Analyses. Schizophr. Bull. Open 2022, 3, sgac001. [Google Scholar] [CrossRef]
- Lawford, B.R.; Barnes, M.; Morris, C.P.; Noble, E.P.; Nyst, P.; Heslop, K.; Young, R.M.; Voisey, J.; Connor, J.P. Dopamine 2 Receptor Genes Are Associated with Raised Blood Glucose in Schizophrenia. Can. J. Psychiatry 2016, 61, 291–297. [Google Scholar] [CrossRef]
- Hansen, T.; Ingason, A.; Djurovic, S.; Melle, I.; Fenger, M.; Gustafsson, O.; Jakobsen, K.D.; Rasmussen, H.B.; Tosato, S.; Rietschel, M.; et al. At-risk variant in TCF7L2 for type II diabetes increases risk of schizophrenia. Biol. Psychiatry 2011, 70, 59–63. [Google Scholar] [CrossRef]
- Jurczyk, A.; Nowosielska, A.; Przewozniak, N.; Aryee, K.E.; DiIorio, P.; Blodgett, D.; Yang, C.; Campbell-Thompson, M.; Atkinson, M.; Shultz, L.; et al. Beyond the brain: Disrupted in schizophrenia 1 regulates pancreatic β-cell function via glycogen synthase kinase-3β. FASEB J. 2016, 30, 983–993. [Google Scholar] [CrossRef]
- Dahoun, T.; Trossbach, S.V.; Brandon, N.J.; Korth, C.; Howes, O.D. The impact of Disrupted-in-Schizophrenia 1 (DISC1) on the dopaminergic system: A systematic review. Transl. Psychiatry 2017, 7, e1015. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Liao, X.; Zhong, Y.; Wu, Y.; Lai, X.; Jiao, H.; Yan, M.; Zhang, Y.; Ma, C.; Wang, S. The Candidate Schizophrenia Risk Gene Tmem108 Regulates Glucose Metabolism Homeostasis. Front. Endocrinol. 2021, 12, 770145. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Sun, Y.; Zhang, X.; Li, S.; Hu, D.; Xiao, L.; Chen, Y.; He, L.; Wang, D.W. Integrated Analysis of Summary Statistics to Identify Pleiotropic Genes and Pathways for the Comorbidity of Schizophrenia and Cardiometabolic Disease. Front. Psychiatry 2020, 11, 256. [Google Scholar] [CrossRef] [PubMed]
- Nadalin, S.; Buretić-Tomljanović, A. An association between the BanI polymorphism of the PLA2G4A gene for calcium-dependent phospholipase A2 and plasma glucose levels among females with schizophrenia. Prostaglandins Leukot. Essent. Fat. Acids 2018, 135, 39–41. [Google Scholar] [CrossRef] [PubMed]
- Sasayama, D.; Hori, H.; Teraishi, T.; Hattori, K.; Ota, M.; Tatsumi, M.; Higuchi, T.; Amano, N.; Kunugi, H. Possible impact of ADRB3 Trp64Arg polymorphism on BMI in patients with schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 2012, 38, 341–344. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zhang, J.; Jin, C.; Mi, W.; Wang, F.; Ma, W.; Ma, C.; Yang, Y.; Li, W.; Zhang, H.; et al. Association study of NRXN3 polymorphisms with schizophrenia and risperid.done-induced bodyweight gain in Chinese Han population. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 43, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Kuo, P.H.; Kao, C.F.; Chen, P.Y.; Chen, C.H.; Tsai, Y.S.; Lu, M.L.; Huang, M.C. Polymorphisms of INSIG2, MC4R, and LEP are associated with obesity- and metabolic-related traits in schizophrenic patients. J. Clin. Psychopharmacol. 2011, 31, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Ozsoy, F.; Karakus, N.B.; Yigit, S.; Kulu, M. Effect of AUTS2 gene rs6943555 variant in male patients with schizophrenia in a Turkish population. Gene 2020, 756, 144913. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; You, Y.; Fan, Y.; Shen, C.; Xue, Y. Association between ApoA1 Gene Polymorphisms and Antipsychotic Drug-Induced Dyslipidemia in Schizophrenia. Neuropsychiatr. Dis. Treat. 2021, 17, 1289–1297. [Google Scholar] [CrossRef] [PubMed]
- Itokawa, M.; Arai, M.; Kato, S.; Ogata, Y.; Furukawa, A.; Haga, S.; Ujike, H.; Sora, I.; Ikeda, K.; Yoshikawa, T. Association between a novel polymorphism in the promoter region of the neuropeptide Y gene and schizophrenia in humans. Neurosci. Lett. 2003, 347, 202–204. [Google Scholar] [CrossRef]
- Rybakowski, J.K.; Dmitrzak-Weglarz, M.; Kapelski, P.; Hauser, J. Functional-1149 g/t polymorphism of the prolactin gene in schizophrenia. Neuropsychobiology 2012, 65, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.R.; Islam, T.; Nicoletti, F.; Petralia, M.C.; Ciurleo, R.; Fisicaro, F.; Pennisi, M.; Bramanti, A.; Demirtas, T.Y.; Gov, E.; et al. Identification of Common Pathogenetic Processes between Schizophrenia and Diabetes Mellitus by Systems Biology Analysis. Genes 2021, 12, 237. [Google Scholar] [CrossRef]
- García-Gutiérrez, M.S.; Navarrete, F.; Sala, F.; Gasparyan, A.; Austrich-Olivares, A.; Manzanares, J. Biomarkers in Psychiatry: Concept, Definition, Types and Relevance to the Clinical Reality. Front. Psychiatry 2020, 11, 432. [Google Scholar] [CrossRef]
- Gimpl, G.; Fahrenholz, F. The oxytocin receptor system: Structure, function, and regulation. Physiol. Rev. 2001, 81, 629–683. [Google Scholar] [CrossRef]
- Iovino, M.; Messana, T.; Tortora, A.; Giusti, C.; Lisco, G.; Giagulli, V.A.; Guastamacchia, E.; De Pergola, G.; Triggiani, V. Oxytocin Signaling Pathway: From Cell Biology to Clinical Implications. Endocr. Metab. Immune Disord. Drug Targets 2021, 21, 91–110. [Google Scholar] [CrossRef]
- Rosenfeld, A.J.; Lieberman, J.A.; Jarskog, L.F. Oxytocin, dopamine, and the amygdala: A neurofunctional model of social cognitive deficits in schizophrenia. Schizophr. Bull. 2011, 37, 1077–1087. [Google Scholar] [CrossRef]
- Lefevre, A.; Mottolese, R.; Dirheimer, M.; Mottolese, C.; Duhamel, J.R.; Sirigu, A. A comparison of methods to measure central and peripheral oxytocin concentrations in human and non-human primates. Sci. Rep. 2017, 7, 17222. [Google Scholar] [CrossRef]
- Rault, J.L. Effects of positive and negative human contacts and intranasal oxytocin on cerebrospinal fluid oxytocin. Psychoneuroendocrinology 2016, 69, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Liang, M.; Munesue, S.; Deguchi, K.; Harashima, A.; Furuhara, K.; Yuhi, T.; Zhong, J.; Akther, S.; Goto, H.; et al. Vascular RAGE transports oxytocin into the brain to elicit its maternal bonding behaviour in mice. Commun. Biol. 2019, 2, 76. [Google Scholar] [CrossRef] [PubMed]
- McCormack, S.E.; Blevins, J.E.; Lawson, E.A. Metabolic Effects of Oxytocin. Endocr. Rev. 2020, 41, 121–145. [Google Scholar] [CrossRef] [PubMed]
- Kagerbauer, S.M.; Debus, J.M.; Martin, J.; Gempt, J.; Jungwirth, B.; Hapfelmeier, A.; Podtschaske, A.H. Absence of a diurnal rhythm of oxytocin and arginine-vasopressin in human cerebrospinal fluid, blood and saliva. Neuropeptides 2019, 78, 101977. [Google Scholar] [CrossRef]
- Lawson, E.A.; Ackerman, K.E.; Estella, N.M.; Guereca, G.; Pierce, L.; Sluss, P.M.; Bouxsein, M.L.; Klibanski, A.; Misra, M. Nocturnal oxytocin secretion is lower in amenorrheic athletes than nonathletes and associated with bone microarchitecture and finite element analysis parameters. Eur. J. Endocrinol. 2013, 168, 457–464. [Google Scholar] [CrossRef]
- Gajdosechova, L.; Krskova, K.; Segarra, A.B.; Spolcova, A.; Suski, M.; Olszanecki, R.; Zorad, S. Hypooxytocinaemia in obese Zucker rats relates to oxytocin degradation in liver and adipose tissue. J. Endocrinol. 2014, 220, 333–343. [Google Scholar] [CrossRef]
- Cid-Jofré, V.; Moreno, M.; Reyes-Parada, M.; Renard, G.M. Role of Oxytocin and Vasopressin in Neuropsychiatric Disorders: Therapeutic Potential of Agonists and Antagonists. Int. J. Mol. Sci. 2021, 22, 12077. [Google Scholar] [CrossRef]
- Ferreira, A.C.; Osório, F.L. Peripheral oxytocin concentrations in psychiatric disorders—A systematic review and methanalysis: Further evidence. Prog. Neuropsychopharmacol. Biol. Psychiatry 2022, 117, 110561. [Google Scholar] [CrossRef]
- Peled-Avron, L.; Abu-Akel, A.; Shamay-Tsoory, S. Exogenous effects of oxytocin in five psychiatric disorders: A systematic review, meta-analyses and a personalized approach through the lens of the social salience hypothesis. Neurosci. Biobehav. Rev. 2020, 114, 70–95. [Google Scholar] [CrossRef]
- Abramova, O.; Zorkina, Y.; Ushakova, V.; Zubkov, E.; Morozova, A.; Chekhonin, V. The role of oxytocin and vasopressin dysfunction in cognitive impairment and mental disorders. Neuropeptides 2020, 83, 102079. [Google Scholar] [CrossRef]
- Bakos, J.; Srancikova, A.; Havranek, T.; Bacova, Z. Molecular Mechanisms of Oxytocin Signaling at the Synaptic Connection. Neural Plast. 2018, 2018, 4864107. [Google Scholar] [CrossRef] [PubMed]
- Brambilla, M.; Manenti, R.; de Girolamo, G.; Adenzato, M.; Bocchio-Chiavetto, L.; Cotelli, M. Effects of Intranasal Oxytocin on Long-Term Memory in Healthy Humans: A Systematic Review. Drug Dev. Res. 2016, 77, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Becker, B.; Yao, S.; Ma, X.; Kou, J.; Kendrick, K.M. Oxytocin Enhancement of the Placebo Effect May Be a Novel Therapy for Working Memory Impairments. Psychother. Psychosom. 2019, 88, 125–126. [Google Scholar] [CrossRef]
- Ditzen, B.; Schaer, M.; Gabriel, B.; Bodenmann, G.; Ehlert, U.; Heinrichs, M. Intranasal oxytocin increases positive communication and reduces cortisol levels during couple conflict. Biol. Psychiatry 2009, 65, 728–731. [Google Scholar] [CrossRef] [PubMed]
- Aulinas, A.; Pulumo, R.L.; Asanza, E.; Mancuso, C.J.; Slattery, M.; Tolley, C.; Plessow, F.; Thomas, J.J.; Eddy, K.T.; Miller, K.K.; et al. Endogenous Oxytocin Levels in Relation to Food Intake, Menstrual Phase, and Age in Females. J. Clin. Endocrinol. Metab. 2019, 104, 1348–1356. [Google Scholar] [CrossRef]
- Stefanska, A.; Bergmann, K.; Sypniewska, G. Chapter One—Metabolic Syndrome and Menopause: Pathophysiology, Clinical and Diagnostic Significance. In Advances in Clinical Chemistry; Makowski, G.S., Ed.; Elsevier: Amsterdam, The Netherlands, 2015; Volume 72, pp. 1–75. [Google Scholar]
- González-Rodríguez, A.; Seeman, M.V. The association between hormones and antipsychotic use: A focus on postpartum and menopausal women. Ther. Adv. Psychopharmacol. 2019, 9, 2045125319859973. [Google Scholar] [CrossRef]
- Acevedo-Rodriguez, A.; Mani, S.K.; Handa, R.J. Oxytocin and Estrogen Receptor β in the Brain: An Overview. Front. Endocrinol. 2015, 6, 160. [Google Scholar] [CrossRef]
- Adamowicz, K.; Kucharska-Mazur, J. Dietary Behaviors and Metabolic Syndrome in Schizophrenia Patients. J. Clin. Med. 2020, 9, 537. [Google Scholar] [CrossRef]
- Huffmeijer, R.; van Ijzendoorn, M.H.; Bakermans-Kranenburg, M.J. Ageing and oxytocin: A call for extending human oxytocin research to ageing populations—A mini-review. Gerontology 2013, 59, 32–39. [Google Scholar] [CrossRef]
- Roux, C.H.; Pisani, D.F.; Gillet, P.; Fontas, E.; Yahia, H.B.; Djedaini, M.; Ambrosetti, D.; Michiels, J.F.; Panaia-Ferrari, P.; Breuil, V.; et al. Oxytocin Controls Chondrogenesis and Correlates with Osteoarthritis. Int. J. Mol. Sci. 2020, 21, 3966. [Google Scholar] [CrossRef]
- Donadon, M.F.; Martin-Santos, R.; Osório, F.L. The Associations Between Oxytocin and Trauma in Humans: A Systematic Review. Front. Pharmacol. 2018, 9, 154. [Google Scholar] [CrossRef] [PubMed]
- Barker, V.; Walker, R.M.; Evans, K.L.; Lawrie, S.M. Methylation of glucocorticoid receptor (NR3C1), BDNF and oxytocin receptor genes in association with childhood maltreatment in schizophrenia and schizoaffective disorder. Schizophr. Res. 2020, 216, 529–531. [Google Scholar] [CrossRef] [PubMed]
- Kennett, J.E.; McKee, D.T. Oxytocin: An emerging regulator of prolactin secretion in the female rat. J. Neuroendocrinol. 2012, 24, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.M.; Hsu, T.M.; Suarez, A.N.; Subramanian, K.S.; Fatemi, R.A.; Cortella, A.M.; Noble, E.E.; Roitman, M.F.; Kanoski, S.E. Central oxytocin signaling inhibits food reward-motivated behaviors and VTA dopamine responses to food-predictive cues in male rats. Horm. Behav. 2020, 126, 104855. [Google Scholar] [CrossRef]
- Hung, L.W.; Neuner, S.; Polepalli, J.S.; Beier, K.T.; Wright, M.; Walsh, J.J.; Lewis, E.M.; Luo, L.; Deisseroth, K.; Dölen, G.; et al. Gating of social reward by oxytocin in the ventral tegmental area. Science 2017, 357, 1406–1411. [Google Scholar] [CrossRef]
- Zhang, B.; Nakata, M.; Nakae, J.; Ogawa, W.; Yada, T. Central insulin action induces activation of paraventricular oxytocin neurons to release oxytocin into circulation. Sci. Rep. 2018, 8, 10415. [Google Scholar] [CrossRef]
- Hurlemann, R.; Grinevich, V. Behavioral Pharmacology of Neuropeptides: Oxytocin; Springer International Publishing: New York City, NY, USA, 2018. [Google Scholar]
- Binay, Ç.; Paketçi, C.; Güzel, S.; Samancı, N. Serum Irisin and Oxytocin Levels as Predictors of Metabolic Parameters in Obese Children. J. Clin. Res. Pediatr. Endocrinol. 2017, 9, 124–131. [Google Scholar] [CrossRef]
- Akalu, Y.; Molla, M.D.; Dessie, G.; Ayelign, B. Physiological Effect of Ghrelin on Body Systems. Int. J. Endocrinol. 2020, 2020, 1385138. [Google Scholar] [CrossRef]
- Rich, M.E.; Caldwell, H.K. A Role for Oxytocin in the Etiology and Treatment of Schizophrenia. Front. Endocrinol. 2015, 6, 90. [Google Scholar] [CrossRef]
- Kiss, A.; Osacka, J. The effect of amisulpride, olanzapine, quetiapine, and aripiprazole single administration on c-Fos expression in vasopressinergic and oxytocinergic neurons of the rat hypothalamic paraventricular nucleus. Neuropeptides 2021, 87, 102148. [Google Scholar] [CrossRef]
- Wu, L.; Meng, J.; Shen, Q.; Zhang, Y.; Pan, S.; Chen, Z.; Zhu, L.Q.; Lu, Y.; Huang, Y.; Zhang, G. Caffeine inhibits hypothalamic A(1)R to excite oxytocin neuron and ameliorate dietary obesity in mice. Nat. Commun. 2017, 8, 15904. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.M.; Zhao, G.Y.; Zhang, B.B.; Xu, Q.; Chu, C.P.; Jin, H.; Qiu, D.L. Nicotine enhances GABAergic inhibition of oxytocin mRNA-expressing neuron in the hypothalamic paraventricular nucleus in vitro in rats. Neurosci. Lett. 2017, 638, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Carter, C.S.; Kenkel, W.M.; MacLean, E.L.; Wilson, S.R.; Perkeybile, A.M.; Yee, J.R.; Ferris, C.F.; Nazarloo, H.P.; Porges, S.W.; Davis, J.M.; et al. Is Oxytocin “Nature’s Medicine”? Pharmacol. Rev. 2020, 72, 829. [Google Scholar] [CrossRef] [PubMed]
- Cochran, D.M.; Fallon, D.; Hill, M.; Frazier, J.A. The role of oxytocin in psychiatric disorders: A review of biological and therapeutic research findings. Harv. Rev. Psychiatry 2013, 21, 219–247. [Google Scholar] [CrossRef]
- Nakata, Y.; Kanahara, N.; Kimura, A.; Niitsu, T.; Komatsu, H.; Oda, Y.; Nakamura, M.; Ishikawa, M.; Hasegawa, T.; Kamata, Y.; et al. Oxytocin system dysfunction in patients with treatment-resistant schizophrenia: Alterations of blood oxytocin levels and effect of a genetic variant of OXTR. J. Psychiatr. Res. 2021, 138, 219–227. [Google Scholar] [CrossRef]
- Hernández-Díaz, Y.; González-Castro, T.B.; Tovilla-Zárate, C.A.; López-Narváez, M.L.; Genis-Mendoza, A.D.; Castillo-Avila, R.G.; Ramos-Méndez, M.Á.; Juárez-Rojop, I.E. Oxytocin levels in individuals with schizophrenia are high in cerebrospinal fluid but low in serum: A systematic review and meta-analysis. Metab. Brain Dis. 2021, 36, 2415–2424. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Ma, X.; Geng, Y.; Zhao, W.; Zhou, F.; Wang, J.; Markett, S.; Biswal, B.B.; Ma, Y.; Kendrick, K.M.; et al. Oxytocin differentially modulates specific dorsal and ventral striatal functional connections with frontal and cerebellar regions. Neuroimage 2019, 184, 781–789. [Google Scholar] [CrossRef]
- Tully, J.; Gabay, A.S.; Brown, D.; Murphy, D.G.M.; Blackwood, N. The effect of intranasal oxytocin on neural response to facial emotions in healthy adults as measured by functional MRI: A systematic review. Psychiatry Res. Neuroimaging 2018, 272, 17–29. [Google Scholar] [CrossRef]
- Rubin, L.H.; Li, S.; Yao, L.; Keedy, S.K.; Reilly, J.L.; Hill, S.K.; Bishop, J.R.; Sue Carter, C.; Pournajafi-Nazarloo, H.; Drogos, L.L.; et al. Peripheral oxytocin and vasopressin modulates regional brain activity differently in men and women with schizophrenia. Schizophr. Res. 2018, 202, 173–179. [Google Scholar] [CrossRef]
- Abram, S.V.; De Coster, L.; Roach, B.J.; Mueller, B.A.; van Erp, T.G.M.; Calhoun, V.D.; Preda, A.; Lim, K.O.; Turner, J.A.; Ford, J.M.; et al. Oxytocin Enhances an Amygdala Circuit Associated With Negative Symptoms in Schizophrenia: A Single-Dose, Placebo-Controlled, Crossover, Randomized Control Trial. Schizophr. Bull. 2020, 46, 661–669. [Google Scholar] [CrossRef]
- Wigton, R.; Tracy, D.K.; Verneuil, T.M.; Johns, M.; White, T.; Michalopoulou, P.G.; Averbeck, B.; Shergill, S. The importance of pro-social processing, and ameliorating dysfunction in schizophrenia. An FMRI study of oxytocin. Schizophr. Res. Cogn. 2022, 27, 100221. [Google Scholar] [CrossRef]
- Montag, C.; Brockmann, E.-M.; Bayerl, M.; Rujescu, D.; Müller, D.J.; Gallinat, J. Oxytocin and oxytocin receptor gene polymorphisms and risk for schizophrenia: A case–control study. World J. Biol. Psychiatry 2013, 14, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Kaneko, N.; Nunokawa, A.; Shibuya, M.; Egawa, J.; Someya, T. Oxytocin receptor (OXTR) gene and risk of schizophrenia: Case-control and family-based analyses and meta-analysis in a Japanese population. Psychiatry Clin. Neurosci. 2012, 66, 622. [Google Scholar] [CrossRef] [PubMed]
- Souza, R.P.; de Luca, V.; Meltzer, H.Y.; Lieberman, J.A.; Kennedy, J.L. Schizophrenia severity and clozapine treatment outcome association with oxytocinergic genes. Int. J. Neuropsychopharmacol. 2010, 13, 793–798. [Google Scholar] [CrossRef]
- Giralt-López, M.; Miret, S.; Soler, J.; Campanera, S.; Parellada, M.; Fañanás, L.; Fatjó-Vilas, M. The role of schizotypal traits and the OXTR gene in theory of mind in schizophrenia: A family-based study. Eur. Psychiatry 2020, 63, e15. [Google Scholar] [CrossRef] [PubMed]
- Haram, M.; Tesli, M.; Bettella, F.; Djurovic, S.; Andreassen, O.A.; Melle, I. Association between Genetic Variation in the Oxytocin Receptor Gene and Emotional Withdrawal, but not between Oxytocin Pathway Genes and Diagnosis in Psychotic Disorders. Front. Hum. Neurosci. 2015, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo-Figueroa, M.; Salazar, A.; Romero-López-Alberca, C.; MacDowell, K.S.; García-Bueno, B.; Bioque, M.; Bernardo, M.; Parellada, M.; González-Pinto, A.; García Portilla, M.P.; et al. The influence of oxytocin and prolactin during a first-episode of psychosis: The implication of sex differences, clinical features and cognitive performance. Int. J. Neuropsychopharmacol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tao, H.; Yang, X.; Huang, K.; Zhang, X.; Li, C. Decreased Serum Oxytocin and Increased Homocysteine in First-Episode Schizophrenia Patients. Front. Psychiatry 2019, 10, 217. [Google Scholar] [CrossRef]
- Strauss, G.P.; Chapman, H.C.; Keller, W.R.; Koenig, J.I.; Gold, J.M.; Carpenter, W.T.; Buchanan, R.W. Endogenous oxytocin levels are associated with impaired social cognition and neurocognition in schizophrenia. J. Psychiatr. Res. 2019, 112, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Aydın, O.; Lysaker, P.H.; Balıkçı, K.; Ünal-Aydın, P.; Esen-Danacı, A. Associations of oxytocin and vasopressin plasma levels with neurocognitive, social cognitive and meta cognitive function in schizophrenia. Psychiatry Res. 2018, 270, 1010–1016. [Google Scholar] [CrossRef] [PubMed]
- Rubin, L.H.; Wehring, H.J.; Demyanovich, H.; Sue Carter, C.; Pournajafi-Nazarloo, H.; Feldman, S.M.; Earl, A.K.; August, S.; Gold, J.M.; Kelly, D.L. Peripheral oxytocin and vasopressin are associated with clinical symptom severity and cognitive functioning in midlife women with chronic schizophrenia. Schizophr. Res. 2018, 195, 409–411. [Google Scholar] [CrossRef] [PubMed]
- Kéri, S.; Kiss, I.; Kelemen, O. Sharing secrets: Oxytocin and trust in schizophrenia. Soc. Neurosci. 2009, 4, 287–293. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, E.; Dadds, M.R.; Brennan, J.L.; Williams, K.; Levy, F.; Cauchi, A.J. A review of safety, side-effects and subjective reactions to intranasal oxytocin in human research. Psychoneuroendocrinology 2011, 36, 1114–1126. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Zhu, X.M.; Zhang, Q.E.; Yang, X.H.; Cai, D.B.; Li, L.; Li, X.B.; Ng, C.H.; Ungvari, G.S.; Ning, Y.P.; et al. Adjunctive intranasal oxytocin for schizophrenia: A meta-analysis of randomized, double-blind, placebo-controlled trials. Schizophr. Res. 2019, 206, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Andari, E.; Massa, N.M.; Fargotstein, M.D.; Taylor, N.B.; Halverson, D.M.; Owens, A.V.; Currin, D.L.; Bhattacharya, A.; Gitman, D.; Cuthbert, B.C.; et al. Effects of Oxytocin on Emotion Recognition in Schizophrenia: A Randomized Double-Blind Pilot Study. J. Clin. Psychopharmacol. 2021, 41, 103–113. [Google Scholar] [CrossRef] [PubMed]
- De Coster, L.; Lin, L.; Mathalon, D.H.; Woolley, J.D. Neural and behavioral effects of oxytocin administration during theory of mind in schizophrenia and controls: A randomized control trial. Neuropsychopharmacology 2019, 44, 1925–1931. [Google Scholar] [CrossRef] [PubMed]
- Abu-Akel, A.; Fischer-Shofty, M.; Levkovitz, Y.; Decety, J.; Shamay-Tsoory, S. The role of oxytocin in empathy to the pain of conflictual out-group members among patients with schizophrenia. Psychol. Med. 2014, 44, 3523–3532. [Google Scholar] [CrossRef] [PubMed]
- Balikci, K.; Aydin, O.; Tas, C.; Esen Danaci, A. Oxytocin and social cognition in patients with schizophrenia: Comparison with healthy siblings and healthy controls. Psychiatry Clin. Psychopharmacol. 2018, 28, 123–130. [Google Scholar] [CrossRef]
- Rubin, L.H.; Carter, C.S.; Bishop, J.R.; Pournajafi-Nazarloo, H.; Harris, M.S.; Hill, S.K.; Reilly, J.L.; Sweeney, J.A. Peripheral vasopressin but not oxytocin relates to severity of acute psychosis in women with acutely-ill untreated first-episode psychosis. Schizophr. Res. 2013, 146, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Bradley, E.R.; Tai, M.; Hankin, M.; Woolley, J.D. Preliminary evidence that oxytocin does not improve mentalizing in women with schizophrenia. Horm. Behav. 2021, 128, 104915. [Google Scholar] [CrossRef] [PubMed]
- Strauss, G.P.; Granholm, E.; Holden, J.L.; Ruiz, I.; Gold, J.M.; Kelly, D.L.; Buchanan, R.W. The effects of combined oxytocin and cognitive behavioral social skills training on social cognition in schizophrenia. Psychol. Med. 2019, 49, 1731–1739. [Google Scholar] [CrossRef] [PubMed]
- Sabe, M.; Zhao, N.; Crippa, A.; Strauss, G.P.; Kaiser, S. Intranasal Oxytocin for Negative Symptoms of Schizophrenia: Systematic Review, Meta-Analysis, and Dose-Response Meta-Analysis of Randomized Controlled Trials. Int. J. Neuropsychopharmacol. 2021, 24, 601–614. [Google Scholar] [CrossRef] [PubMed]
- Horta, M.; Kaylor, K.; Feifel, D.; Ebner, N.C. Chronic oxytocin administration as a tool for investigation and treatment: A cross-disciplinary systematic review. Neurosci. Biobehav. Rev. 2020, 108, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Martins, D.; Paduraru, M.; Paloyelis, Y. Heterogeneity in response to repeated intranasal oxytocin in schizophrenia and autism spectrum disorders: A meta-analysis of variance. Br. J. Pharmacol. 2022, 179, 1525–1543. [Google Scholar] [CrossRef]
- Kou, J.; Zhang, Y.; Zhou, F.; Sindermann, C.; Montag, C.; Becker, B.; Kendrick, K.M. A randomized trial shows dose-frequency and genotype may determine the therapeutic efficacy of intranasal oxytocin. Psychol. Med. 2020, 1–10. [Google Scholar] [CrossRef]
- Macdonald, K.; Feifel, D. Oxytocin in schizophrenia: A review of evidence for its therapeutic effects. Acta Neuropsychiatr. 2012, 24, 130–146. [Google Scholar] [CrossRef] [PubMed]
- Perello, M.; Raingo, J. Leptin activates oxytocin neurons of the hypothalamic paraventricular nucleus in both control and diet-induced obese rodents. PLoS ONE 2013, 8, e59625. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Qiu, L.; Xiao, W.; Ni, H.; Chen, L.; Wang, F.; Mai, W.; Wu, J.; Bao, A.; Hu, H.; et al. Reconstruction of the Hypothalamo-Neurohypophysial System and Functional Dissection of Magnocellular Oxytocin Neurons in the Brain. Neuron 2021, 109, 331–346.e337. [Google Scholar] [CrossRef]
- Roberts, Z.S.; Wolden-Hanson, T.; Matsen, M.E.; Ryu, V.; Vaughan, C.H.; Graham, J.L.; Havel, P.J.; Chukri, D.W.; Schwartz, M.W.; Morton, G.J.; et al. Chronic hindbrain administration of oxytocin is sufficient to elicit weight loss in diet-induced obese rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017, 313, R357–R371. [Google Scholar] [CrossRef] [PubMed]
- Leng, G.; Sabatier, N. Oxytocin—The Sweet Hormone? Trends Endocrinol. Metab. 2017, 28, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Lawson, E.A.; Olszewski, P.K.; Weller, A.; Blevins, J.E. The role of oxytocin in regulation of appetitive behaviour, body weight and glucose homeostasis. J. Neuroendocrinol. 2020, 32, e12805. [Google Scholar] [CrossRef] [PubMed]
- Takayanagi, Y.; Kasahara, Y.; Onaka, T.; Takahashi, N.; Kawada, T.; Nishimori, K. Oxytocin receptor-deficient mice developed late-onset obesity. Neuroreport 2008, 19, 951–955. [Google Scholar] [CrossRef]
- Camerino, C. Low sympathetic tone and obese phenotype in oxytocin-deficient mice. Obesity 2009, 17, 980–984. [Google Scholar] [CrossRef] [PubMed]
- Grinevich, V.; Knobloch-Bollmann, H.S.; Eliava, M.; Busnelli, M.; Chini, B. Assembling the Puzzle: Pathways of Oxytocin Signaling in the Brain. Biol. Psychiatry 2016, 79, 155–164. [Google Scholar] [CrossRef]
- Douglass, A.M.; Kucukdereli, H.; Ponserre, M.; Markovic, M.; Gründemann, J.; Strobel, C.; Alcala Morales, P.L.; Conzelmann, K.-K.; Lüthi, A.; Klein, R. Central amygdala circuits modulate food consumption through a positive-valence mechanism. Nat. Neurosci. 2017, 20, 1384–1394. [Google Scholar] [CrossRef] [PubMed]
- Blevins, J.E.; Graham, J.L.; Morton, G.J.; Bales, K.L.; Schwartz, M.W.; Baskin, D.G.; Havel, P.J. Chronic oxytocin administration inhibits food intake, increases energy expenditure, and produces weight loss in fructose-fed obese rhesus monkeys. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 308, R431–R438. [Google Scholar] [CrossRef] [PubMed]
- Blevins, J.E.; Baskin, D.G. Translational and therapeutic potential of oxytocin as an anti-obesity strategy: Insights from rodents, nonhuman primates and humans. Physiol. Behav. 2015, 152, 438–449. [Google Scholar] [CrossRef] [PubMed]
- Blevins, J.E.; Thompson, B.W.; Anekonda, V.T.; Ho, J.M.; Graham, J.L.; Roberts, Z.S.; Hwang, B.H.; Ogimoto, K.; Wolden-Hanson, T.; Nelson, J.; et al. Chronic CNS oxytocin signaling preferentially induces fat loss in high-fat diet-fed rats by enhancing satiety responses and increasing lipid utilization. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 310, R640–R658. [Google Scholar] [CrossRef]
- Maejima, Y.; Aoyama, M.; Sakamoto, K.; Jojima, T.; Aso, Y.; Takasu, K.; Takenosihita, S.; Shimomura, K. Impact of sex, fat distribution and initial body weight on oxytocin’s body weight regulation. Sci. Rep. 2017, 7, 8599. [Google Scholar] [CrossRef] [PubMed]
- Yi, K.J.; So, K.H.; Hata, Y.; Suzuki, Y.; Kato, D.; Watanabe, K.; Aso, H.; Kasahara, Y.; Nishimori, K.; Chen, C.; et al. The regulation of oxytocin receptor gene expression during adipogenesis. J. Neuroendocrinol. 2015, 27, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Oldfield, B.J.; Giles, M.E.; Watson, A.; Anderson, C.; Colvill, L.M.; McKinley, M.J. The neurochemical characterisation of hypothalamic pathways projecting polysynaptically to brown adipose tissue in the rat. Neuroscience 2002, 110, 515–526. [Google Scholar] [CrossRef]
- Noble, E.E.; Billington, C.J.; Kotz, C.M.; Wang, C. Oxytocin in the ventromedial hypothalamic nucleus reduces feeding and acutely increases energy expenditure. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 307, R737–R745. [Google Scholar] [CrossRef]
- Welch, M.G.; Margolis, K.G.; Li, Z.; Gershon, M.D. Oxytocin regulates gastrointestinal motility, inflammation, macromolecular permeability, and mucosal maintenance in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 307, G848–G862. [Google Scholar] [CrossRef]
- Wu, C.L.; Doong, M.L.; Wang, P.S. Involvement of cholecystokinin receptor in the inhibition of gastrointestinal motility by oxytocin in ovariectomized rats. Eur. J. Pharmacol. 2008, 580, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wang, S.C.; Yang, H.; Lv, C.; Jia, S.; Liu, X.; Wang, X.; Meng, D.; Qin, D.; Zhu, H.; et al. Therapeutic Potential of Oxytocin in Atherosclerotic Cardiovascular Disease: Mechanisms and Signaling Pathways. Front. Neurosci. 2019, 13, 454. [Google Scholar] [CrossRef] [PubMed]
- Jameson, H.; Bateman, R.; Byrne, P.; Dyavanapalli, J.; Wang, X.; Jain, V.; Mendelowitz, D. Oxytocin neuron activation prevents hypertension that occurs with chronic intermittent hypoxia/hypercapnia in rats. Am. J. Physiol. Heart Circ. Physiol. 2016, 310, H1549–H1557. [Google Scholar] [CrossRef] [PubMed]
- Iovino, M.; Messana, T.; De Pergola, G.; Iovino, E.; Guastamacchia, E.; Licchelli, B.; Vanacore, A.; Giagulli, V.A.; Triggiani, V. Brain Angiotensinergic Regulation of the Immune System: Implications for Cardiovascular and Neuroendocrine Responses. Endocr. Metab. Immune Disord. Drug Targets 2020, 20, 15–24. [Google Scholar] [CrossRef]
- Davis, C.; Patte, K.; Zai, C.; Kennedy, J.L. Polymorphisms of the oxytocin receptor gene and overeating: The intermediary role of endophenotypic risk factors. Nutr. Diabetes 2017, 7, e279. [Google Scholar] [CrossRef]
- Damen, L.; Grootjen, L.N.; Juriaans, A.F.; Donze, S.H.; Huisman, T.M.; Visser, J.A.; Delhanty, P.J.D.; Hokken-Koelega, A.C.S. Oxytocin in young children with Prader-Willi syndrome: Results of a randomized, double-blind, placebo-controlled, crossover trial investigating 3 months of oxytocin. Clin. Endocrinol. 2021, 94, 774–785. [Google Scholar] [CrossRef]
- Kabasakalian, A.; Ferretti, C.J.; Hollander, E. Oxytocin and Prader-Willi Syndrome. Curr. Top. Behav. Neurosci. 2018, 35, 529–557. [Google Scholar] [CrossRef]
- Swaab, D.F.; Purba, J.S.; Hofman, M.A. Alterations in the hypothalamic paraventricular nucleus and its oxytocin neurons (putative satiety cells) in Prader-Willi syndrome: A study of five cases. J. Clin. Endocrinol. Metab. 1995, 80, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Du, F.-M.; Kuang, H.-Y.; Duan, B.-H.; Liu, D.-N.; Yu, X.-Y. Associations of oxytocin with metabolic parameters in obese women of childbearing age. Endokrynol. Pol. 2019, 70, 417–422. [Google Scholar] [CrossRef]
- Maestrini, S.; Mele, C.; Mai, S.; Vietti, R.; Di Blasio, A.; Castello, L.; Surico, D.; Aimaretti, G.; Scacchi, M.; Marzullo, P. Plasma Oxytocin Concentration in Pre- and Postmenopausal Women: Its Relationship with Obesity, Body Composition and Metabolic Variables. Obes. Facts 2018, 11, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, S.A.P.; Amiri, P.; Saidpour, A.; Hosseinzadeh, N.; Abolhasani, M.; Ghorbani, A. The prevalence of food addiction and its associations with plasma oxytocin level and anthropometric and dietary measurements in Iranian women with obesity. Peptides 2019, 122, 170151. [Google Scholar] [CrossRef]
- Qian, W.; Zhu, T.; Tang, B.; Yu, S.; Hu, H.; Sun, W.; Pan, R.; Wang, J.; Wang, D.; Yang, L.; et al. Decreased circulating levels of oxytocin in obesity and newly diagnosed type 2 diabetic patients. J. Clin. Endocrinol. Metab. 2014, 99, 4683–4689. [Google Scholar] [CrossRef]
- Weingarten, M.F.J.; Scholz, M.; Wohland, T.; Horn, K.; Stumvoll, M.; Kovacs, P.; Tönjes, A. Circulating Oxytocin Is Genetically Determined and Associated with Obesity and Impaired Glucose Tolerance. J. Clin. Endocrinol. Metab. 2019, 104, 5621–5632. [Google Scholar] [CrossRef]
- Szulc, P.; Amri, E.Z.; Varennes, A.; Panaia-Ferrari, P.; Fontas, E.; Goudable, J.; Chapurlat, R.; Breuil, V. High serum oxytocin is associated with metabolic syndrome in older men—The MINOS study. Diabetes Res. Clin. Pract. 2016, 122, 17–27. [Google Scholar] [CrossRef]
- Skinner, J.A.; Garg, M.L.; Dayas, C.V.; Burrows, T.L. Is weight status associated with peripheral levels of oxytocin? A pilot study in healthy women. Physiol. Behav. 2019, 212, 112684. [Google Scholar] [CrossRef]
- Brede, S.; Fehr, S.; Dalla-Man, C.; Cobelli, C.; Lehnert, H.; Hallschmid, M.; Klement, J. Intranasal oxytocin fails to acutely improve glucose metabolism in obese men. Diabetes Obes. Metab. 2019, 21, 424–428. [Google Scholar] [CrossRef]
- Xiao, C.; Dash, S.; Stahel, P.; Lewis, G.F. Effects of intranasal insulin on endogenous glucose production in insulin-resistant men. Diabetes Obes. Metab. 2018, 20, 1751–1754. [Google Scholar] [CrossRef]
- Burmester, V.; Higgs, S.; Terry, P. Rapid-onset anorectic effects of intranasal oxytocin in young men. Appetite 2018, 130, 104–109. [Google Scholar] [CrossRef]
- Burmester, V.; Gibson, E.L.; Butler, G.; Bailey, A.; Terry, P. Oxytocin reduces post-stress sweet snack intake in women without attenuating salivary cortisol. Physiol. Behav. 2019, 212, 112704. [Google Scholar] [CrossRef] [PubMed]
- Lawson, E.A.; Marengi, D.A.; DeSanti, R.L.; Holmes, T.M.; Schoenfeld, D.A.; Tolley, C.J. Oxytocin reduces caloric intake in men. Obesity 2015, 23, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Ott, V.; Finlayson, G.; Lehnert, H.; Heitmann, B.; Heinrichs, M.; Born, J.; Hallschmid, M. Oxytocin reduces reward-driven food intake in humans. Diabetes 2013, 62, 3418–3425. [Google Scholar] [CrossRef] [PubMed]
- Striepens, N.; Schröter, F.; Stoffel-Wagner, B.; Maier, W.; Hurlemann, R.; Scheele, D. Oxytocin enhances cognitive control of food craving in women. Hum. Brain Mapp. 2016, 37, 4276–4285. [Google Scholar] [CrossRef] [PubMed]
- Thienel, M.; Fritsche, A.; Heinrichs, M.; Peter, A.; Ewers, M.; Lehnert, H.; Born, J.; Hallschmid, M. Oxytocin’s inhibitory effect on food intake is stronger in obese than normal-weight men. Int. J. Obes. 2016, 40, 1707–1714. [Google Scholar] [CrossRef]
- Ding, C.; Leow, M.K.; Magkos, F. Oxytocin in metabolic homeostasis: Implications for obesity and diabetes management. Obes. Rev. 2019, 20, 22–40. [Google Scholar] [CrossRef]
- Klement, J.; Ott, V.; Rapp, K.; Brede, S.; Piccinini, F.; Cobelli, C.; Lehnert, H.; Hallschmid, M. Oxytocin Improves β-Cell Responsivity and Glucose Tolerance in Healthy Men. Diabetes 2017, 66, 264–271. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, C.; Chen, Q.; Chen, X.; Xu, Z.; Wu, J.; Cai, D. Treatment of obesity and diabetes using oxytocin or analogs in patients and mouse models. PLoS ONE 2013, 8, e61477. [Google Scholar] [CrossRef]
- Altirriba, J.; Poher, A.L.; Rohner-Jeanrenaud, F. Chronic Oxytocin Administration as a Treatment Against Impaired Leptin Signaling or Leptin Resistance in Obesity. Front. Endocrinol. 2015, 6, 119. [Google Scholar] [CrossRef]
- Chen, C.Y.; Chiang, Y.C.; Kuo, T.C.; Tam, K.W.; Loh, E.W. Effects of intranasal oxytocin in food intake and craving: A meta-analysis of clinical trials. Clin. Nutr. 2021, 40, 5407–5416. [Google Scholar] [CrossRef]
- Plessow, F.; Marengi, D.A.; Perry, S.K.; Felicione, J.M.; Franklin, R.; Holmes, T.M.; Holsen, L.M.; Makris, N.; Deckersbach, T.; Lawson, E.A. Effects of Intranasal Oxytocin on the Blood Oxygenation Level-Dependent Signal in Food Motivation and Cognitive Control Pathways in Overweight and Obese Men. Neuropsychopharmacology 2018, 43, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Kerem, L.; Hadjikhani, N.; Holsen, L.; Lawson, E.A.; Plessow, F. Oxytocin reduces the functional connectivity between brain regions involved in eating behavior in men with overweight and obesity. Int. J. Obes. 2020, 44, 980–989. [Google Scholar] [CrossRef] [PubMed]
- van der Klaauw, A.A.; Ziauddeen, H.; Keogh, J.M.; Henning, E.; Dachi, S.; Fletcher, P.C.; Farooqi, I.S. Oxytocin administration suppresses hypothalamic activation in response to visual food cues. Sci. Rep. 2017, 7, 4266. [Google Scholar] [CrossRef] [PubMed]
- Spetter, M.S.; Feld, G.B.; Thienel, M.; Preissl, H.; Hege, M.A.; Hallschmid, M. Oxytocin curbs calorie intake via food-specific increases in the activity of brain areas that process reward and establish cognitive control. Sci. Rep. 2018, 8, 2736. [Google Scholar] [CrossRef]
- Warren, K.R.; Wehring, H.J.; Liu, F.; McMahon, R.P.; Chen, S.; Chester, C.; Kelly, D.L. Effects of intranasal oxytocin on satiety signaling in people with schizophrenia. Physiol. Behav. 2018, 189, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Haase Alasantro, L.; Hicks, T.H.; Green-Krogmann, E.; Murphy, C. Metabolic syndrome and cognitive performance across the adult lifespan. PLoS ONE 2021, 16, e0249348. [Google Scholar] [CrossRef] [PubMed]
- Koch, E.; Rosenthal, B.; Lundquist, A.; Chen, C.H.; Kauppi, K. Interactome overlap between schizophrenia and cognition. Schizophr. Res. 2020, 222, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Bora, E.; Akdede, B.B.; Alptekin, K. The relationship between cognitive impairment in schizophrenia and metabolic syndrome: A systematic review and meta-analysis. Psychol. Med. 2017, 47, 1030–1040. [Google Scholar] [CrossRef]
- Bahchevanov, K.M.; Dzhambov, A.M.; Chompalov, K.A.; Massaldjieva, R.I.; Atanassova, P.A.; Mitkov, M.D. Contribution of Components of Metabolic Syndrome to Cognitive Performance in Middle-Aged Adults. Arch. Clin. Neuropsychol. 2021, 36, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhan, G.; Rao, S.; Zhang, H. Metabolic syndrome and its factors affect cognitive function in chronic schizophrenia complicated by metabolic syndrome. J. Nerv. Ment. Dis. 2014, 202, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Tahmi, M.; Palta, P.; Luchsinger, J.A. Metabolic Syndrome and Cognitive Function. Curr. Cardiol. Rep. 2021, 23, 180. [Google Scholar] [CrossRef] [PubMed]
- Michalopoulou, P.G.; Averbeck, B.B.; Kalpakidou, A.K.; Evans, S.; Bobin, T.; Kapur, S.; Shergill, S.S. The effects of a single dose of oxytocin on working memory in schizophrenia. Schizophr. Res. 2015, 162, 62–63. [Google Scholar] [CrossRef] [PubMed]
- Thorp, A.A.; Schlaich, M.P. Relevance of Sympathetic Nervous System Activation in Obesity and Metabolic Syndrome. J. Diabetes Res. 2015, 2015, 341583. [Google Scholar] [CrossRef] [PubMed]
- Stogios, N.; Gdanski, A.; Gerretsen, P.; Chintoh, A.F.; Graff-Guerrero, A.; Rajji, T.K.; Remington, G.; Hahn, M.K.; Agarwal, S.M. Autonomic nervous system dysfunction in schizophrenia: Impact on cognitive and metabolic health. NPJ Schizophr. 2021, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Carnethon, M.R.; Prineas, R.J.; Temprosa, M.; Zhang, Z.M.; Uwaifo, G.; Molitch, M.E. The association among autonomic nervous system function, incident diabetes, and intervention arm in the Diabetes Prevention Program. Diabetes Care 2006, 29, 914–919. [Google Scholar] [CrossRef]
- Lundqvist, M.H.; Almby, K.; Wiklund, U.; Abrahamsson, N.; Kamble, P.G.; Pereira, M.J.; Eriksson, J.W. Altered hormonal and autonomic nerve responses to hypo- and hyperglycaemia are found in overweight and insulin-resistant individuals and may contribute to the development of type 2 diabetes. Diabetologia 2021, 64, 641–655. [Google Scholar] [CrossRef]
- Daniels, N.; Prinsen, J.; Soriano, J.R.; Alaerts, K. Oxytocin enhances the recovery of eye-contact induced autonomic arousal: A treatment mechanism study with placebo-controlled design. Eur. Neuropsychopharmacol. 2020, 39, 87–98. [Google Scholar] [CrossRef]
- Norman, G.J.; Cacioppo, J.T.; Morris, J.S.; Malarkey, W.B.; Berntson, G.G.; Devries, A.C. Oxytocin increases autonomic cardiac control: Moderation by loneliness. Biol. Psychol. 2011, 86, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Quintana, D.S.; Kemp, A.H.; Alvares, G.A.; Guastella, A.J. A role for autonomic cardiac control in the effects of oxytocin on social behavior and psychiatric illness. Front. Neurosci. 2013, 7, 48. [Google Scholar] [CrossRef] [PubMed]
- Morgan, V.A.; Waterreus, A.; Carr, V.; Castle, D.; Cohen, M.; Harvey, C.; Galletly, C.; Mackinnon, A.; McGorry, P.; McGrath, J.J.; et al. Responding to challenges for people with psychotic illness: Updated evidence from the Survey of High Impact Psychosis. Aust. N. Z. J. Psychiatry 2017, 51, 124–140. [Google Scholar] [CrossRef]
- Liao, H.-Y.; Lee, Y.; Hsu, S.-T.; Yen, C.-F. Loneliness in patients with schizophrenia. Taiwan J. Psychiatry 2021, 35, 59–63. [Google Scholar] [CrossRef]
- Badcock, J.C.; Mackinnon, A.; Waterreus, A.; Watts, G.F.; Castle, D.; McGrath, J.J.; Morgan, V.A. Loneliness in psychotic illness and its association with cardiometabolic disorders. Schizophr. Res. 2019, 204, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Foret, J.T.; Dekhtyar, M.; Birdsill, A.C.; Tanaka, H.; Haley, A.P. Metabolic syndrome components moderate the association between executive function and functional connectivity in the default mode network. Brain Imaging Behav. 2021, 15, 2139–2148. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.L.; Zong, X.F.; Mann, J.J.; Zheng, J.J.; Liao, Y.H.; Li, Z.C.; He, Y.; Chen, X.G.; Tang, J.S. A Review of the Functional and Anatomical Default Mode Network in Schizophrenia. Neurosci. Bull. 2017, 33, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Zeev-Wolf, M.; Levy, J.; Ebstein, R.P.; Feldman, R. Cumulative Risk on Oxytocin-Pathway Genes Impairs Default Mode Network Connectivity in Trauma-Exposed Youth. Front. Endocrinol. 2020, 11, 335. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Braskie, M.N.; Hafzalla, G.W.; Faskowitz, J.; McMahon, K.L.; de Zubicaray, G.I.; Wright, M.J.; Yu, C.; Thompson, P.M. Relationship of a common OXTR gene variant to brain structure and default mode network function in healthy humans. Neuroimage 2017, 147, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Godoy, L.C.; Frankfurter, C.; Cooper, M.; Lay, C.; Maunder, R.; Farkouh, M.E. Association of Adverse Childhood Experiences with Cardiovascular Disease Later in Life: A Review. JAMA Cardiol. 2021, 6, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Stanton, K.J.; Denietolis, B.; Goodwin, B.J.; Dvir, Y. Childhood Trauma and Psychosis: An Updated Review. Child. Adolesc. Psychiatr. Clin. N. Am. 2020, 29, 115–129. [Google Scholar] [CrossRef] [PubMed]
- Womersley, J.S.; Nothling, J.; Toikumo, S.; Malan-Müller, S.; van den Heuvel, L.L.; McGregor, N.W.; Seedat, S.; Hemmings, S.M.J. Childhood trauma, the stress response and metabolic syndrome: A focus on DNA methylation. Eur. J. Neurosci. 2022, 55, 2253–2296. [Google Scholar] [CrossRef] [PubMed]
- Lunding, S.H.; Simonsen, C.; Aas, M.; Rødevand, L.; Werner, M.C.F.; Laskemoen, J.F.; Hjell, G.; Ringen, P.A.; Lagerberg, T.V.; Melle, I.; et al. Childhood trauma and cardiometabolic risk in severe mental disorders: The mediating role of cognitive control. Eur. Psychiatry 2021, 64, e24. [Google Scholar] [CrossRef]
- Richetto, J.; Meyer, U. Epigenetic Modifications in Schizophrenia and Related Disorders: Molecular Scars of Environmental Exposures and Source of Phenotypic Variability. Biol. Psychiatry 2021, 89, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Quintana, D.S.; Guastella, A.J. An Allostatic Theory of Oxytocin. Trends Cogn. Sci. 2020, 24, 515–528. [Google Scholar] [CrossRef] [PubMed]
- Winterton, A.; Bettella, F.; de Lange, A.G.; Haram, M.; Steen, N.E.; Westlye, L.T.; Andreassen, O.A.; Quintana, D.S. Oxytocin-pathway polygenic scores for severe mental disorders and metabolic phenotypes in the UK Biobank. Transl. Psychiatry 2021, 11, 599. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.H.; Chang, W.H.; Chi, M.H.; Peng, Y.C.; Huang, C.C.; Yang, Y.K.; Chen, P.S. The OXTR Polymorphism Stratified the Correlation of Oxytocin and Glucose Homeostasis in Non-Diabetic Subjects. Diabetes Metab. Syndr. Obes. 2019, 12, 2707–2713. [Google Scholar] [CrossRef]
- Jacondino, C.B.; Borges, C.A.; Rosemberg, L.S.; da Silva, I.G.; da Luz Correa, B.; Valle Gottlieb, M.G. Association of oxytocin levels and oxytocin receptor gene polymorphism (rs2254298) with cardiovascular risk factors in Brazilian elderly from Primary Health Care. Arch. Gerontol. Geriatr. 2019, 84, 103903. [Google Scholar] [CrossRef]
- Pillinger, T.; D’Ambrosio, E.; McCutcheon, R.; Howes, O.D. Is psychosis a multisystem disorder? A meta-review of central nervous system, immune, cardiometabolic, and endocrine alterations in first-episode psychosis and perspective on potential models. Mol. Psychiatry 2019, 24, 776–794. [Google Scholar] [CrossRef]
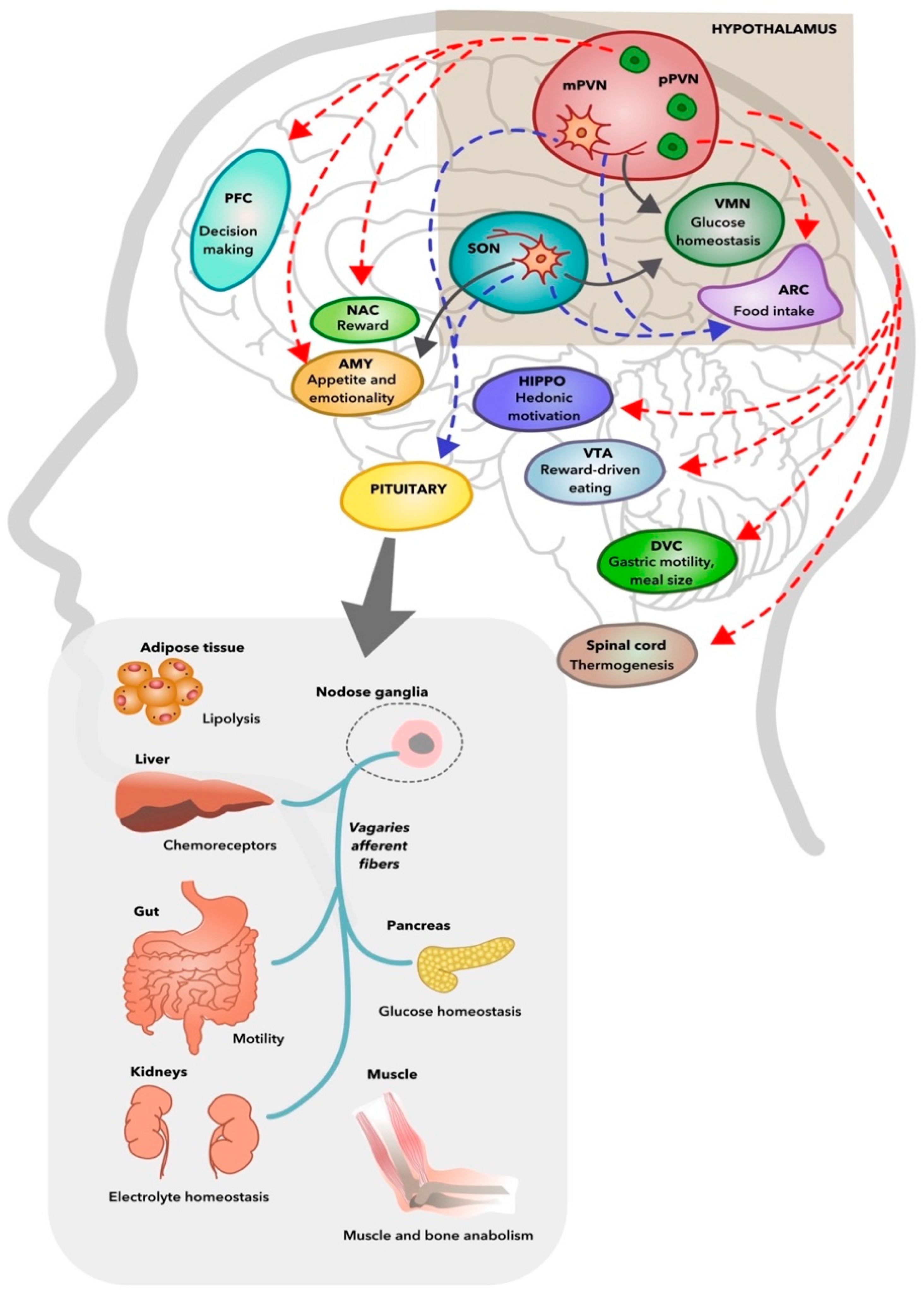
| Criteria | WHO (1998) | EGIR (1999) | NCEP: ATP III (2001) | AACE (2003) | IDF (2005) | AHA/NHLBI (2009) | |
|---|---|---|---|---|---|---|---|
| Central obesity | WC | - | ≥94 cm (M) ≥80 cm (W) | >102 cm (M) >88 cm (W) | - | ≥94 cm (M) ≥80 cm (W) * | ≥94 cm (M) ≥80 cm (W) * |
| BMI | >30 kg/m2 | - | - | - | >30 kg/m2 | - | |
| WTH | >0.90 (M) >0.85 (W) | - | - | - | - | - | |
| Increased TG | TG | ≥150 mg/dL # | >177 mg/dL or under Tx # | ≥150 mg/dL | ≥150 mg/dL | ≥150 mg/dL or under Tx | ≥150 mg/dL or under Tx |
| Reduced HDL | HDL | <35 mg/dL (M) <39 mg/dL (W) # | <39 mg/dL or under Tx # | <40 mg/dL (M) <50 mg/dL (W) | <40 mg/dL (M) <50 mg/dL (W) | <40 mg/dL (M), <50 mg/dL (W), or under Tx | <40 mg/dL (M), <50 mg/dL (W), or under Tx |
| Increased BP | BP | ≥160/90 mmHg | ≥140/90 mmHg or under Tx | ≥130/85 mmHg | >130/85 mmHg | ≥130/85 mmHg or under Tx | ≥130/85 mmHg or under Tx |
| Increased glucose concentration | IFG | ≥110 mg/dL | ≥110 mg/dL (2 measures) | ≥110 mg/dL | 110–125 mg/dL | ≥100 mg/dL or under Tx | ≥100 mg/dL or under Tx |
| IGT ¶ | 140–200 mg/dL | - | - | 140–200 mg/dL | - | - | |
| IR | Yes † | Yes ‡ | - | - | - | - | |
| DM | ≥126 mg/dL | - | - | - | Yes | Yes | |
| Other | MA | UAE ≥ 20 ug/min UACR ≥ 20 mg/g | - | - | - | - | - |
| Diagnosis | IGR §, DM, or IR + ≥2 other criteria | IGR § or IR + ≥2 other criteria | ≥3 criteria | ≥2 criteria | Central obesity + ≥2 other criteria | ≥3 criteria | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goh, K.K.; Chen, C.Y.-A.; Wu, T.-H.; Chen, C.-H.; Lu, M.-L. Crosstalk between Schizophrenia and Metabolic Syndrome: The Role of Oxytocinergic Dysfunction. Int. J. Mol. Sci. 2022, 23, 7092. https://doi.org/10.3390/ijms23137092
Goh KK, Chen CY-A, Wu T-H, Chen C-H, Lu M-L. Crosstalk between Schizophrenia and Metabolic Syndrome: The Role of Oxytocinergic Dysfunction. International Journal of Molecular Sciences. 2022; 23(13):7092. https://doi.org/10.3390/ijms23137092
Chicago/Turabian StyleGoh, Kah Kheng, Cynthia Yi-An Chen, Tzu-Hua Wu, Chun-Hsin Chen, and Mong-Liang Lu. 2022. "Crosstalk between Schizophrenia and Metabolic Syndrome: The Role of Oxytocinergic Dysfunction" International Journal of Molecular Sciences 23, no. 13: 7092. https://doi.org/10.3390/ijms23137092
APA StyleGoh, K. K., Chen, C. Y.-A., Wu, T.-H., Chen, C.-H., & Lu, M.-L. (2022). Crosstalk between Schizophrenia and Metabolic Syndrome: The Role of Oxytocinergic Dysfunction. International Journal of Molecular Sciences, 23(13), 7092. https://doi.org/10.3390/ijms23137092






