Regulation and Release of Vasoactive Endoglin by Brain Endothelium in Response to Hypoxia/Reoxygenation in Stroke
Abstract
1. Introduction
2. Results
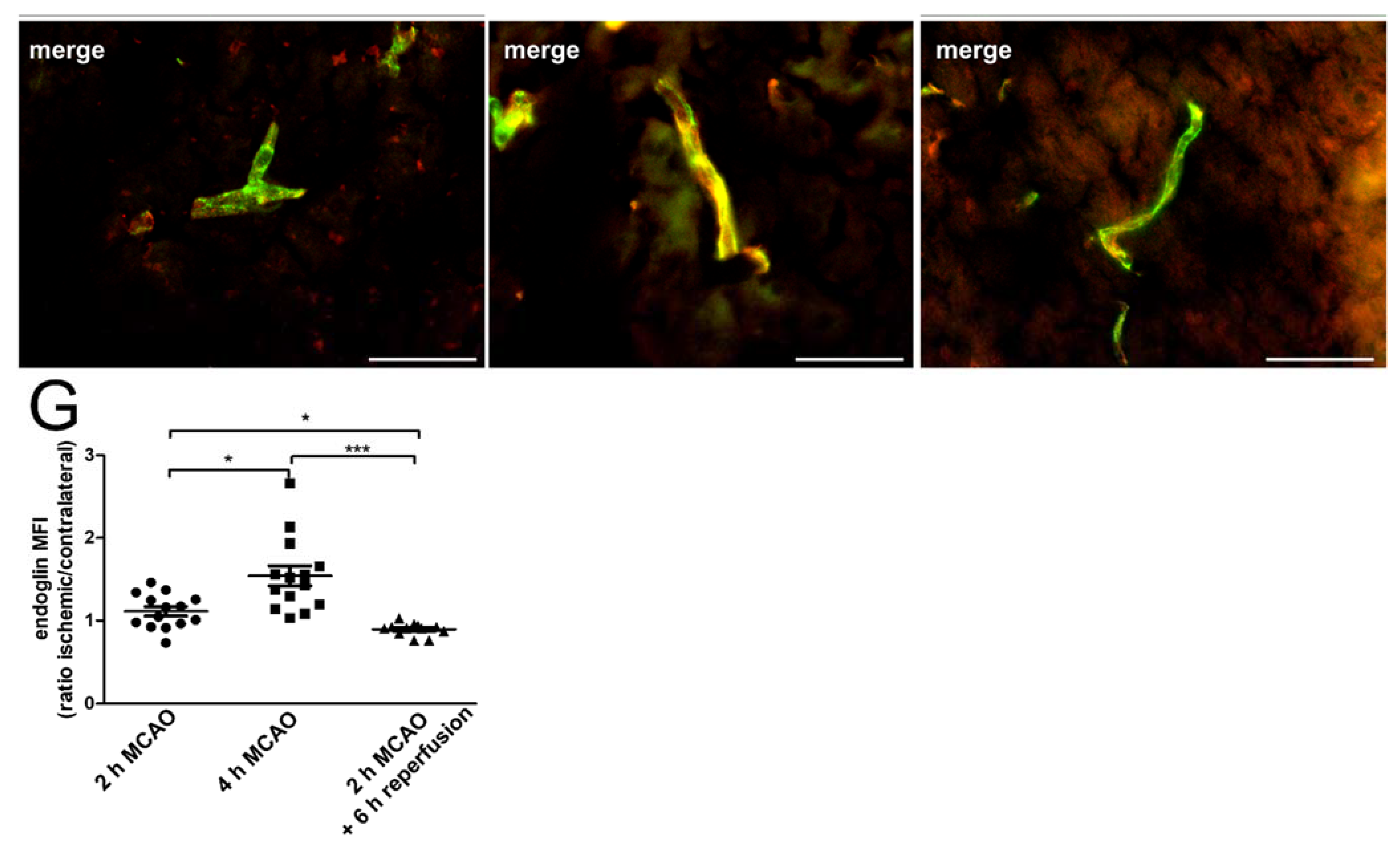
2.1. Hypoxia Induces Endoglin Expression in Brain Endothelium
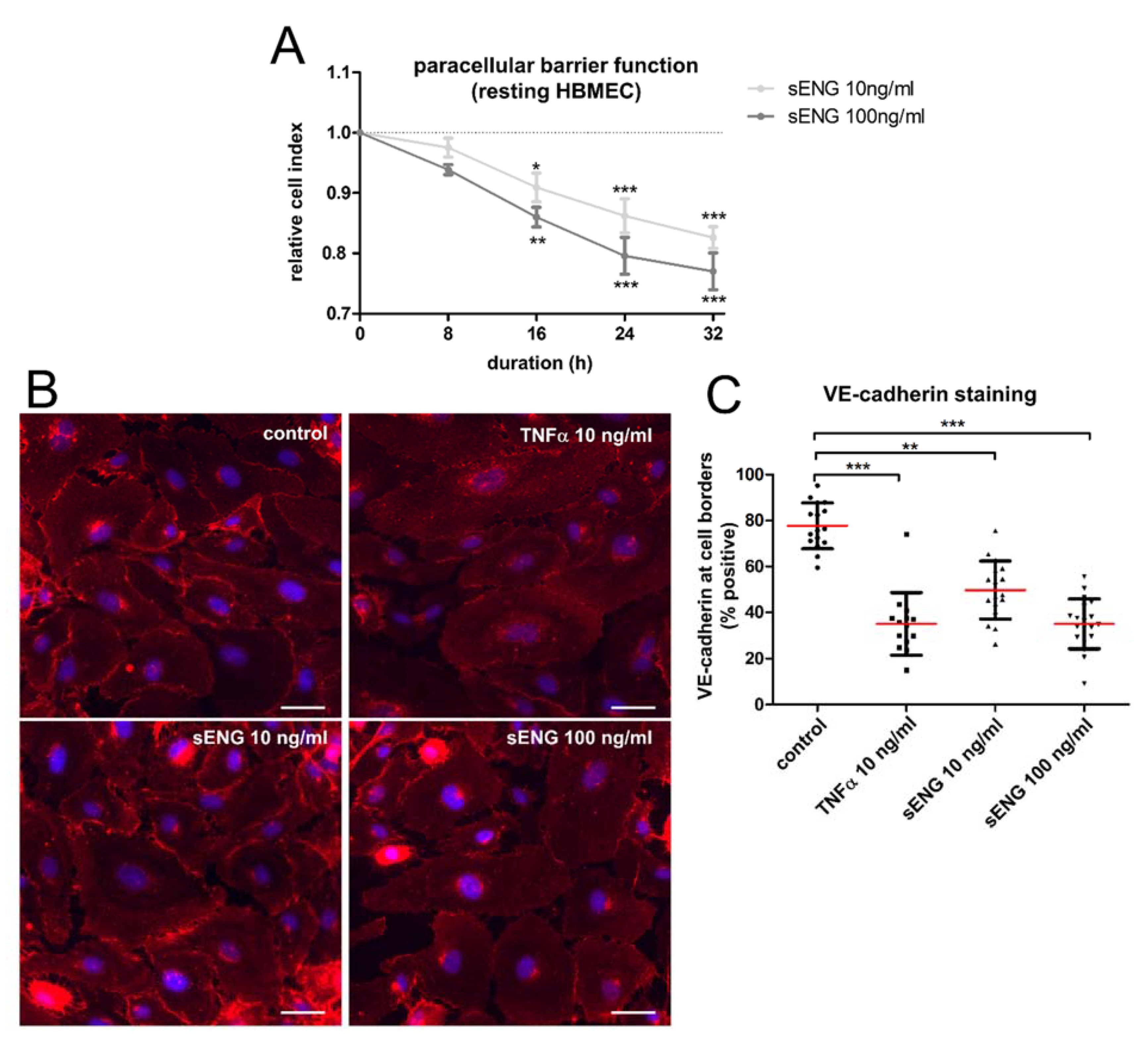
2.2. Brain Endothelium Sheds Soluble ENG in Response to Reoxygenation
2.3. Soluble ENG Induces an Inflammatory Phenotype in Brain Endothelial Cells
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Western Blotting
4.3. Quantitative Real-Time PCR
4.4. xCELLigence Assay
4.5. Immunocytochemistry
4.6. Animals
4.7. Ischemia Model
4.8. Immunohistochemistry
4.9. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Mueller-Kronast, N.H.; Zaidat, O.O.; Froehler, M.T.; Jahan, R.; Aziz-Sultan, M.A.; Klucznik, R.P.; Saver, J.L.; Hellinger, F.R., Jr.; Yavagal, D.R.; Yao, T.L.; et al. Systematic Evaluation of Patients Treated With Neurothrombectomy Devices for Acute Ischemic Stroke: Primary Results of the STRATIS Registry. Stroke 2017, 48, 2760–2768. [Google Scholar] [CrossRef] [PubMed]
- Knowland, D.; Arac, A.; Sekiguchi, K.J.; Hsu, M.; Lutz, S.E.; Perrino, J.; Steinberg, G.K.; Barres, B.A.; Nimmerjahn, A.; Agalliu, D. Stepwise recruitment of transcellular and paracellular pathways underlies blood-brain barrier breakdown in stroke. Neuron 2014, 82, 603–617. [Google Scholar] [CrossRef] [PubMed]
- Klohs, J.; Steinbrink, J.; Bourayou, R.; Mueller, S.; Cordell, R.; Licha, K.; Schirner, M.; Dirnagl, U.; Lindauer, U.; Wunder, A. Near-infrared fluorescence imaging with fluorescently labeled albumin: A novel method for non-invasive optical imaging of blood-brain barrier impairment after focal cerebral ischemia in mice. J. Neurosci. Methods 2009, 180, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Jickling, G.C.; Liu, D.; Stamova, B.; Ander, B.P.; Zhan, X.; Lu, A.; Sharp, F.R. Hemorrhagic transformation after ischemic stroke in animals and humans. J. Cereb. Blood Flow Metab. 2014, 34, 185–199. [Google Scholar] [CrossRef]
- Rossi, E.; Pericacho, M.; Bachelot-Loza, C.; Pidard, D.; Gaussem, P.; Poirault-Chassac, S.; Blanco, F.J.; Langa, C.; Gonzalez-Manchon, C.; Novoa, J.M.L.; et al. Human endoglin as a potential new partner involved in platelet-endothelium interactions. Cell. Mol. Life Sci. 2018, 75, 1269–1284. [Google Scholar] [CrossRef]
- Matsubara, S.; Bourdeau, A.; terBrugge, K.G.; Wallace, C.; Letarte, M. Analysis of endoglin expression in normal brain tissue and in cerebral arteriovenous malformations. Stroke 2000, 31, 2653–2660. [Google Scholar] [CrossRef]
- Li, C.; Issa, R.; Kumar, P.; Hampson, I.N.; Lopez-Novoa, J.M.; Bernabeu, C.; Kumar, S. CD105 prevents apoptosis in hypoxic endothelial cells. J. Cell Sci. 2003, 116, 2677–2685. [Google Scholar] [CrossRef]
- Tian, F.; Zhou, A.X.; Smits, A.M.; Larsson, E.; Goumans, M.J.; Heldin, C.H.; Boren, J.; Akyurek, L.M. Endothelial cells are activated during hypoxia via endoglin/ALK-1/SMAD1/5 signaling in vivo and in vitro. Biochem. Biophys. Res. Commun. 2010, 392, 283–288. [Google Scholar] [CrossRef]
- Zhu, W.; Ma, L.; Zhang, R.; Su, H. The roles of endoglin gene in cerebrovascular diseases. Neuroimmunol. Neuroinflamm. 2017, 4, 199–210. [Google Scholar] [CrossRef]
- McAllister, K.A.; Grogg, K.M.; Johnson, D.W.; Gallione, C.J.; Baldwin, M.A.; Jackson, C.E.; Helmbold, E.A.; Markel, D.S.; McKinnon, W.C.; Murrell, J.; et al. Endoglin, a TGF-beta binding protein of endothelial cells, is the gene for hereditary haemorrhagic telangiectasia type 1. Nat. Genet. 1994, 8, 345–351. [Google Scholar] [CrossRef]
- Torsney, E.; Charlton, R.; Parums, D.; Collis, M.; Arthur, H.M. Inducible expression of human endoglin during inflammation and wound healing in vivo. Inflamm. Res. 2002, 51, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Lastres, P.; Martin-Perez, J.; Langa, C.; Bernabeu, C. Phosphorylation of the human-transforming-growth-factor-beta-binding protein endoglin. Biochem. J. 1994, 301 Pt 3, 765–768. [Google Scholar] [CrossRef] [PubMed]
- Gallardo-Vara, E.; Blanco, F.J.; Roque, M.; Friedman, S.L.; Suzuki, T.; Botella, L.M.; Bernabeu, C. Transcription factor KLF6 upregulates expression of metalloprotease MMP14 and subsequent release of soluble endoglin during vascular injury. Angiogenesis 2016, 19, 155–171. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Sun, Y.; Xie, L.; Jin, K.; Sheibani, N.; Greenberg, D.A. Hypoxic induction of endoglin via mitogen-activated protein kinases in mouse brain microvascular endothelial cells. Stroke 2003, 34, 2483–2488. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.H.; Zhang, H.Y.; Lu, S.; Jiang, L.L.; Wu, J.; Yang, Y.L.; Zhang, S.A. MMP-14 aggravates onset of severe preeclampsia by mediating soluble endoglin release. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 1209–1215. [Google Scholar] [CrossRef] [PubMed]
- Hawinkels, L.J.; Kuiper, P.; Wiercinska, E.; Verspaget, H.W.; Liu, Z.; Pardali, E.; Sier, C.F.; ten Dijke, P. Matrix metalloproteinase-14 (MT1-MMP)-mediated endoglin shedding inhibits tumor angiogenesis. Cancer Res. 2010, 70, 4141–4150. [Google Scholar] [CrossRef] [PubMed]
- Aristorena, M.; Gallardo-Vara, E.; Vicen, M.; de Las Casas-Engel, M.; Ojeda-Fernandez, L.; Nieto, C.; Blanco, F.J.; Valbuena-Diez, A.C.; Botella, L.M.; Nachtigal, P.; et al. MMP-12, Secreted by Pro-Inflammatory Macrophages, Targets Endoglin in Human Macrophages and Endothelial Cells. Int. J. Mol. Sci. 2019, 20, 3107. [Google Scholar] [CrossRef]
- Sanchez-Elsner, T.; Botella, L.M.; Velasco, B.; Langa, C.; Bernabeu, C. Endoglin expression is regulated by transcriptional cooperation between the hypoxia and transforming growth factor-beta pathways. J. Biol. Chem. 2002, 277, 43799–43808. [Google Scholar] [CrossRef]
- Krupinski, J.; Kaluza, J.; Kumar, P.; Kumar, S.; Wang, J.M. Role of angiogenesis in patients with cerebral ischemic stroke. Stroke 1994, 25, 1794–1798. [Google Scholar] [CrossRef]
- Lambertsen, K.L.; Biber, K.; Finsen, B. Inflammatory cytokines in experimental and human stroke. J. Cereb. Blood Flow Metab. 2012, 32, 1677–1698. [Google Scholar] [CrossRef]
- Singh, R.J.; Mason, J.C.; Lidington, E.A.; Edwards, D.R.; Nuttall, R.K.; Khokha, R.; Knauper, V.; Murphy, G.; Gavrilovic, J. Cytokine stimulated vascular cell adhesion molecule-1 (VCAM-1) ectodomain release is regulated by TIMP-3. Cardiovasc. Res. 2005, 67, 39–49. [Google Scholar] [CrossRef]
- Leeuwenberg, J.F.; Smeets, E.F.; Neefjes, J.J.; Shaffer, M.A.; Cinek, T.; Jeunhomme, T.M.; Ahern, T.J.; Buurman, W.A. E-selectin and intercellular adhesion molecule-1 are released by activated human endothelial cells in vitro. Immunology 1992, 77, 543–549. [Google Scholar]
- Kumar, S.; Pan, C.C.; Bloodworth, J.C.; Nixon, A.B.; Theuer, C.; Hoyt, D.G.; Lee, N.Y. Antibody-directed coupling of endoglin and MMP-14 is a key mechanism for endoglin shedding and deregulation of TGF-beta signaling. Oncogene 2014, 33, 3970–3979. [Google Scholar] [CrossRef] [PubMed]
- Ota, T.; Fujii, M.; Sugizaki, T.; Ishii, M.; Miyazawa, K.; Aburatani, H.; Miyazono, K. Targets of transcriptional regulation by two distinct type I receptors for transforming growth factor-beta in human umbilical vein endothelial cells. J. Cell Physiol. 2002, 193, 299–318. [Google Scholar] [CrossRef] [PubMed]
- Shen, F.; Degos, V.; Chu, P.L.; Han, Z.; Westbroek, E.M.; Choi, E.J.; Marchuk, D.; Kim, H.; Lawton, M.T.; Maze, M.; et al. Endoglin deficiency impairs stroke recovery. Stroke 2014, 45, 2101–2106. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Slevin, M.; Kumar, P.; Gaffney, J.; Kumar, S.; Krupinski, J. Can angiogenesis be exploited to improve stroke outcome? Mechanisms and therapeutic potential. Clin. Sci. 2006, 111, 171–183. [Google Scholar] [CrossRef]
- Venkatesha, S.; Toporsian, M.; Lam, C.; Hanai, J.; Mammoto, T.; Kim, Y.M.; Bdolah, Y.; Lim, K.H.; Yuan, H.T.; Libermann, T.A.; et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat. Med. 2006, 12, 642–649. [Google Scholar] [CrossRef]
- Varejckova, M.; Gallardo-Vara, E.; Vicen, M.; Vitverova, B.; Fikrova, P.; Dolezelova, E.; Rathouska, J.; Prasnicka, A.; Blazickova, K.; Micuda, S.; et al. Soluble endoglin modulates the pro-inflammatory mediators NF-kappaB and IL-6 in cultured human endothelial cells. Life Sci. 2017, 175, 52–60. [Google Scholar] [CrossRef]
- Conley, B.A.; Smith, J.D.; Guerrero-Esteo, M.; Bernabeu, C.; Vary, C.P. Endoglin, a TGF-beta receptor-associated protein, is expressed by smooth muscle cells in human atherosclerotic plaques. Atherosclerosis 2000, 153, 323–335. [Google Scholar] [CrossRef]
- Kim, S.J.; Moon, G.J.; Cho, Y.H.; Kang, H.Y.; Hyung, N.K.; Kim, D.; Lee, J.H.; Nam, J.Y.; Bang, O.Y. Circulating mesenchymal stem cells microparticles in patients with cerebrovascular disease. PLoS ONE 2012, 7, e37036. [Google Scholar] [CrossRef]
- Simak, J.; Gelderman, M.P.; Yu, H.; Wright, V.; Baird, A.E. Circulating endothelial microparticles in acute ischemic stroke: A link to severity, lesion volume and outcome. J. Thromb. Haemost. 2006, 4, 1296–1302. [Google Scholar] [CrossRef] [PubMed]
- Rossi, E.; Sanz-Rodriguez, F.; Eleno, N.; Duwell, A.; Blanco, F.J.; Langa, C.; Botella, L.M.; Cabanas, C.; Lopez-Novoa, J.M.; Bernabeu, C. Endothelial endoglin is involved in inflammation: Role in leukocyte adhesion and transmigration. Blood 2013, 121, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Stoll, G.; Nieswandt, B. Thrombo-inflammation in acute ischaemic stroke—Implications for treatment. Nat. Rev. Neurol. 2019, 15, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Schuhmann, M.K.; Stoll, G.; Bieber, M.; Vogtle, T.; Hofmann, S.; Klaus, V.; Kraft, P.; Seyhan, M.; Kollikowski, A.M.; Papp, L.; et al. CD84 Links T Cell and Platelet Activity in Cerebral Thrombo-Inflammation in Acute Stroke. Circ. Res. 2020, 127, 1023–1035. [Google Scholar] [CrossRef]
- Essig, F.; Kollikowski, A.M.; Mullges, W.; Stoll, G.; Haeusler, K.G.; Schuhmann, M.K.; Pham, M. Local Cerebral Recombinant Tissue Plasminogen Activator Concentrations During Acute Stroke. JAMA Neurol. 2021, 78, 615–617. [Google Scholar] [CrossRef]
- Silwedel, C.; Speer, C.P.; Haarmann, A.; Fehrholz, M.; Claus, H.; Buttmann, M.; Glaser, K. Novel insights into neuroinflammation: Bacterial lipopolysaccharide, tumor necrosis factor alpha, and Ureaplasma species differentially modulate atypical chemokine receptor 3 responses in human brain microvascular endothelial cells. J. Neuroinflamm. 2018, 15, 156. [Google Scholar] [CrossRef]
- Haarmann, A.; Nowak, E.; Deiss, A.; van der Pol, S.; Monoranu, C.M.; Kooij, G.; Muller, N.; van der Valk, P.; Stoll, G.; de Vries, H.E.; et al. Soluble VCAM-1 impairs human brain endothelial barrier integrity via integrin alpha-4-transduced outside-in signalling. Acta Neuropathol. 2015, 129, 639–652. [Google Scholar] [CrossRef]
- Bischoff, I.; Hornburger, M.C.; Mayer, B.A.; Beyerle, A.; Wegener, J.; Furst, R. Pitfalls in assessing microvascular endothelial barrier function: Impedance-based devices versus the classic macromolecular tracer assay. Sci. Rep. 2016, 6, 23671. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef]
- Schuhmann, M.K.; Bieber, M.; Franke, M.; Kollikowski, A.M.; Stegner, D.; Heinze, K.G.; Nieswandt, B.; Pham, M.; Stoll, G. Platelets and lymphocytes drive progressive penumbral tissue loss during middle cerebral artery occlusion in mice. J. Neuroinflamm. 2021, 18, 46. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haarmann, A.; Zimmermann, L.; Bieber, M.; Silwedel, C.; Stoll, G.; Schuhmann, M.K. Regulation and Release of Vasoactive Endoglin by Brain Endothelium in Response to Hypoxia/Reoxygenation in Stroke. Int. J. Mol. Sci. 2022, 23, 7085. https://doi.org/10.3390/ijms23137085
Haarmann A, Zimmermann L, Bieber M, Silwedel C, Stoll G, Schuhmann MK. Regulation and Release of Vasoactive Endoglin by Brain Endothelium in Response to Hypoxia/Reoxygenation in Stroke. International Journal of Molecular Sciences. 2022; 23(13):7085. https://doi.org/10.3390/ijms23137085
Chicago/Turabian StyleHaarmann, Axel, Lena Zimmermann, Michael Bieber, Christine Silwedel, Guido Stoll, and Michael K. Schuhmann. 2022. "Regulation and Release of Vasoactive Endoglin by Brain Endothelium in Response to Hypoxia/Reoxygenation in Stroke" International Journal of Molecular Sciences 23, no. 13: 7085. https://doi.org/10.3390/ijms23137085
APA StyleHaarmann, A., Zimmermann, L., Bieber, M., Silwedel, C., Stoll, G., & Schuhmann, M. K. (2022). Regulation and Release of Vasoactive Endoglin by Brain Endothelium in Response to Hypoxia/Reoxygenation in Stroke. International Journal of Molecular Sciences, 23(13), 7085. https://doi.org/10.3390/ijms23137085





