Evidence for Dual Activation of IK(M) and IK(Ca) Caused by QO-58 (5-(2,6-Dichloro-5-fluoropyridin-3-yl)-3-phenyl-2-(trifluoromethyl)-1H-pyrazolol[1,5-a]pyrimidin-7-one)
Abstract
:1. Introduction
2. Results
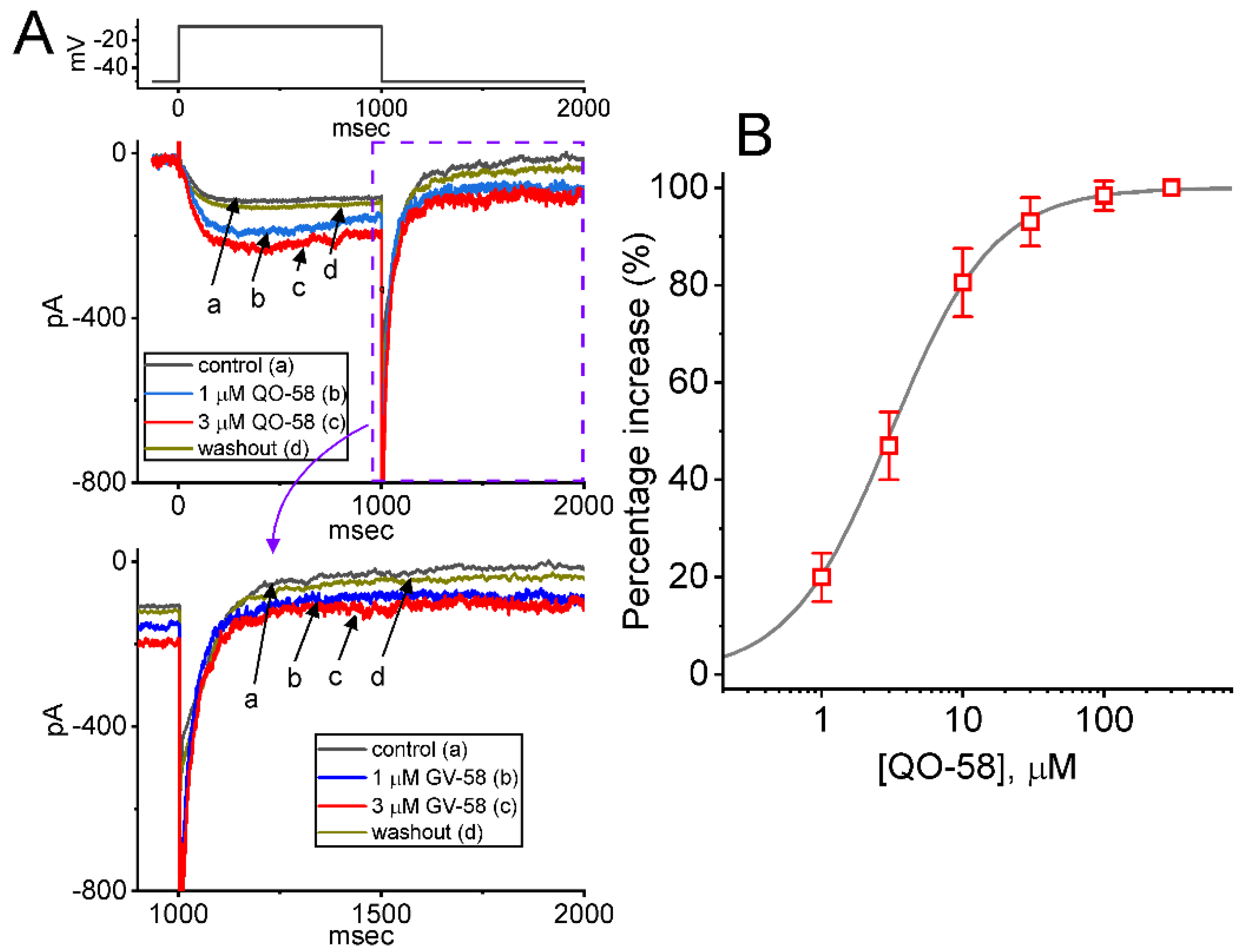
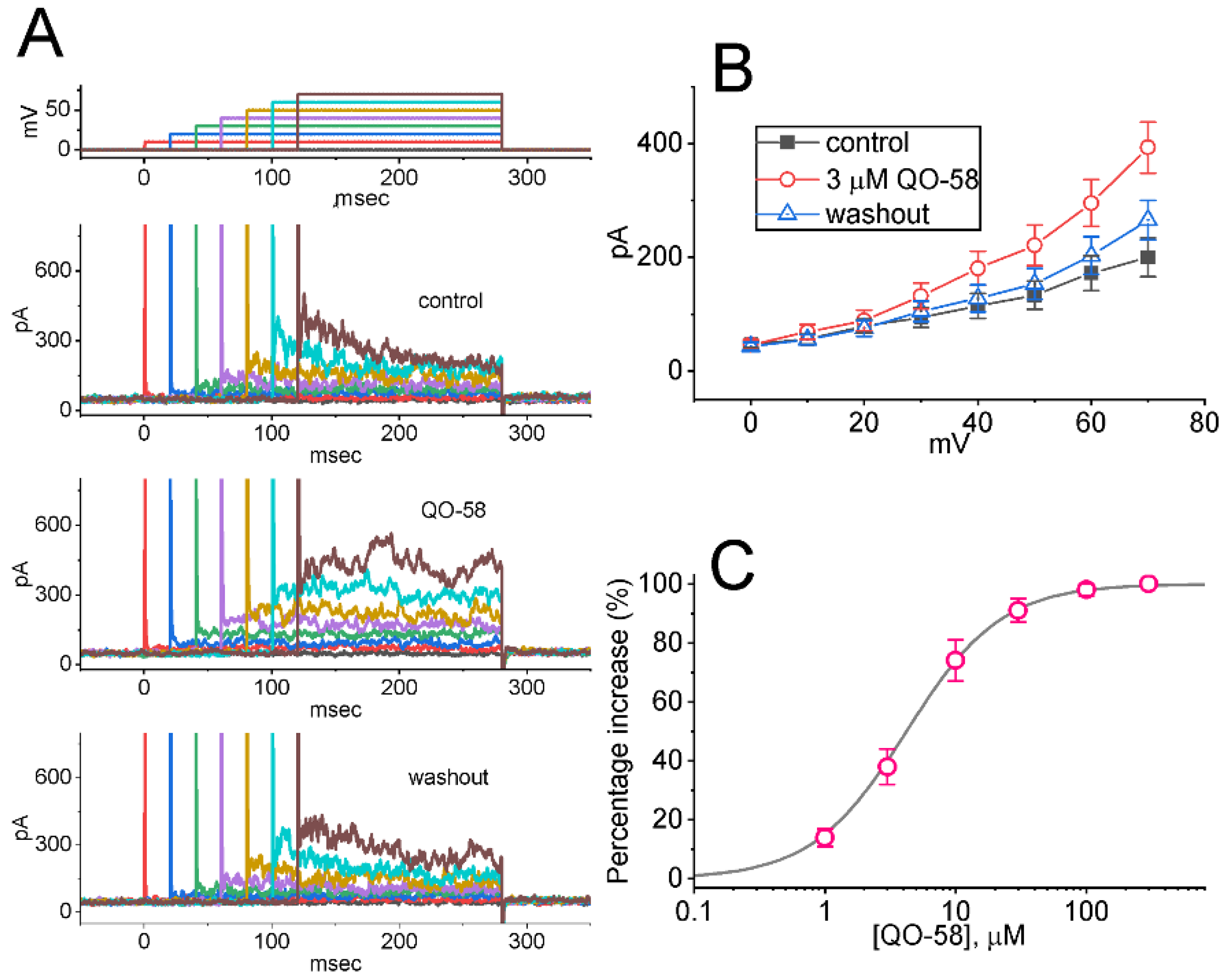
2.1. Stimulatory Effect of QO-58 on M-Type K+ Current (IK(M)) Recorded from Pituitary GH3 Cells
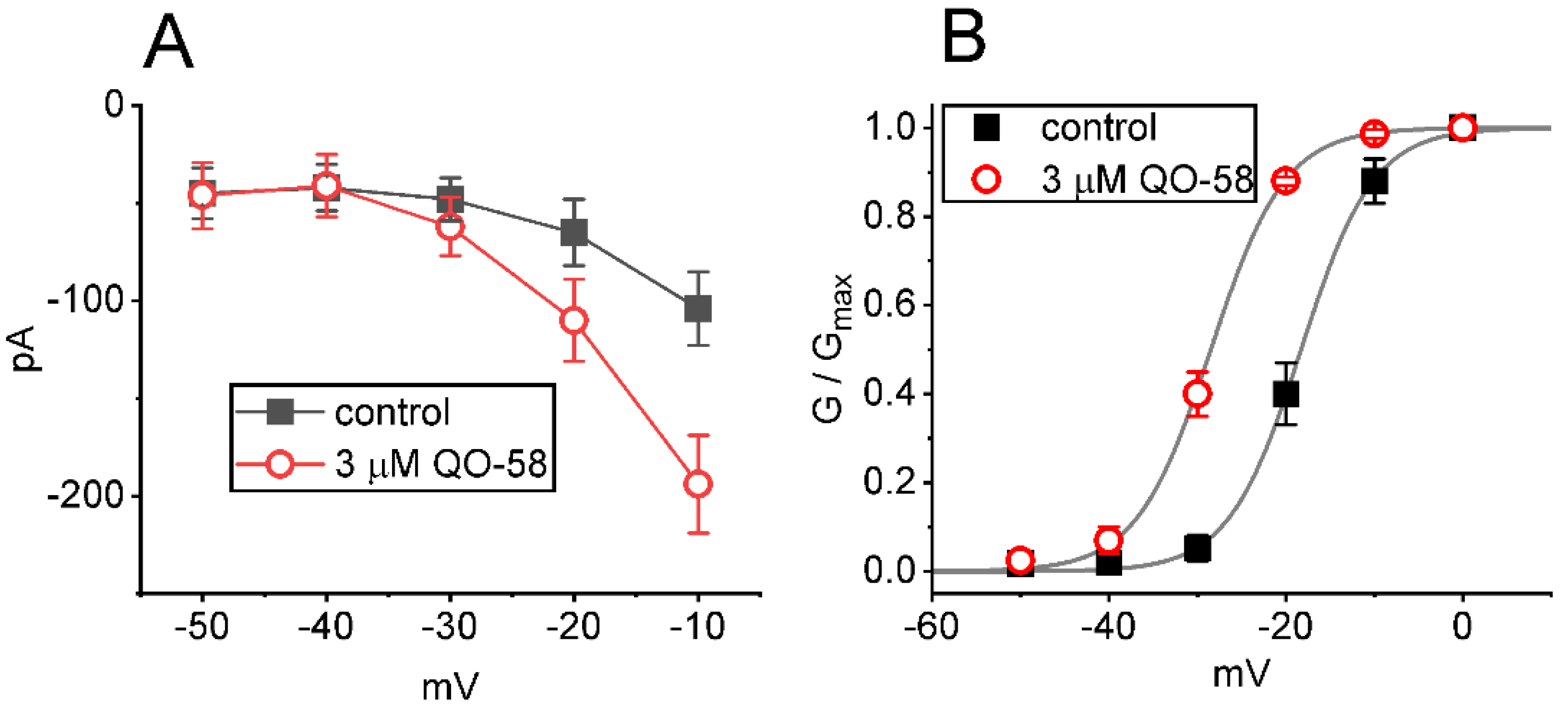
2.2. Effect of QO-58 on Average Current Versus Voltage (I-V) Relationship and Steady-State Activation Curve of IK(M)
2.3. Effect of QO-58 on IK(M) Triggered by Triangular Ramp Pulse (Vramp) with Varying Durations
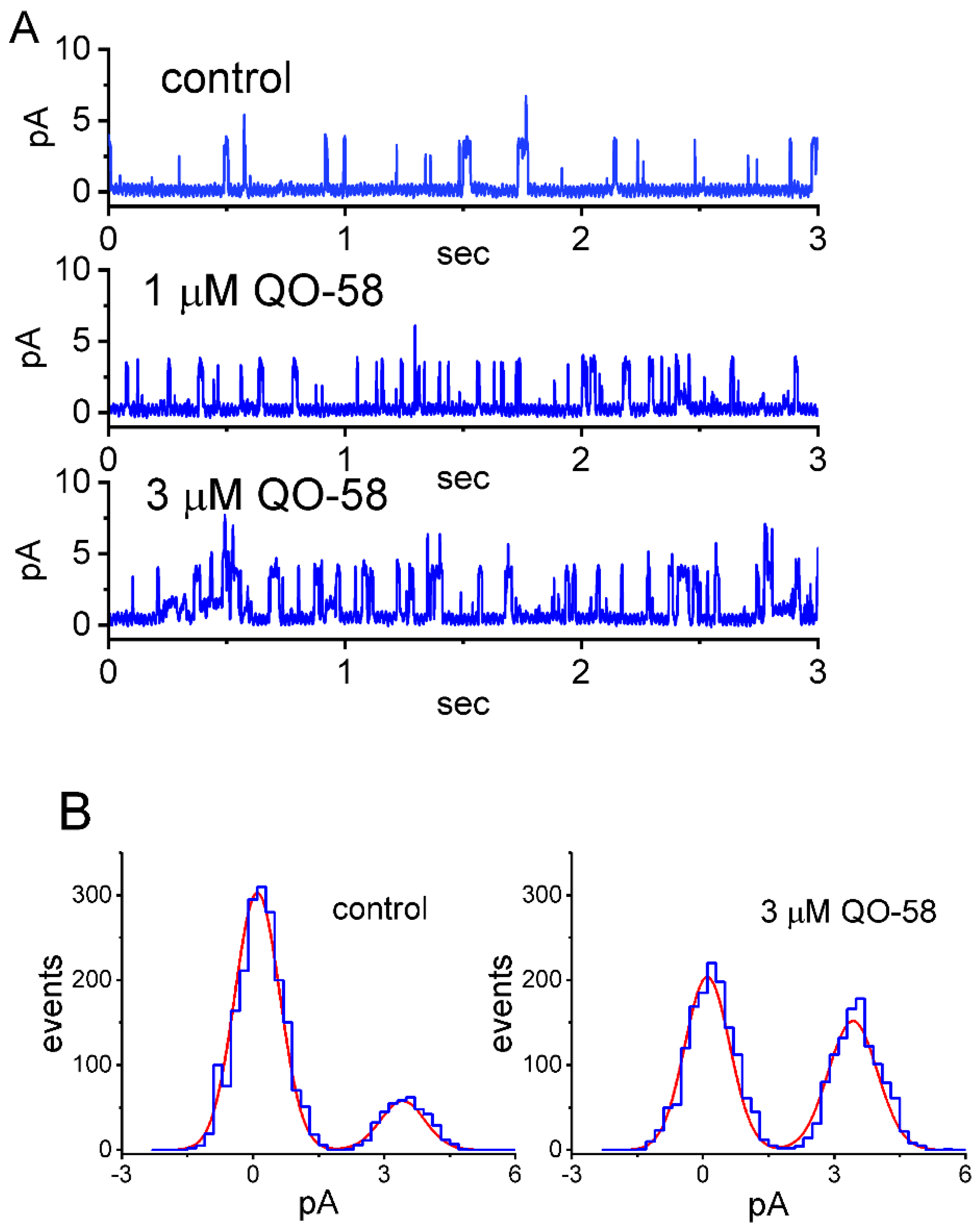
2.4. Effect of QO-58 on M-Type K+ Channel (KM) Channels Measured from GH3 Cells
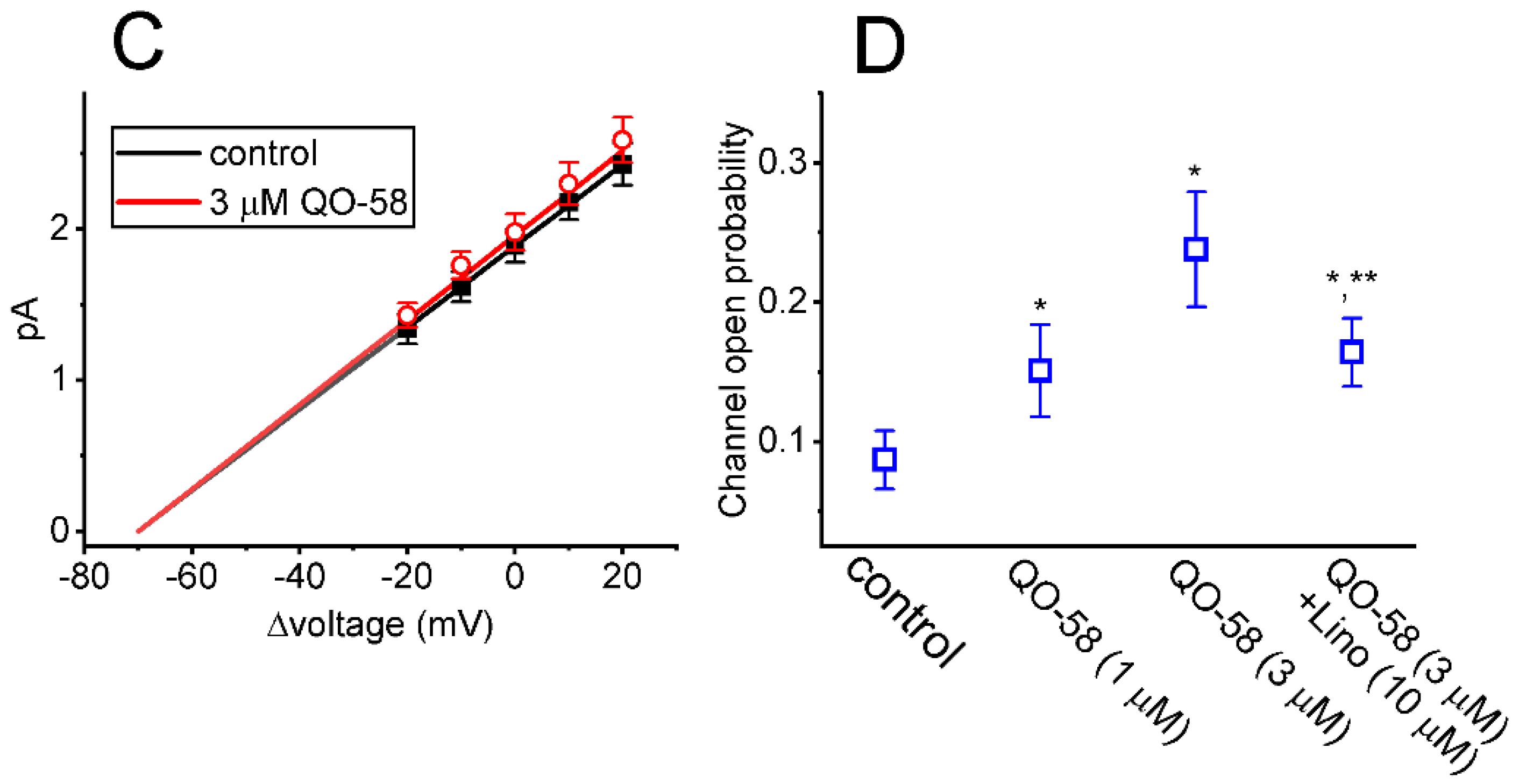
2.5. Effect of QO-58 on the Single-Channel Conductance and Activation Curve of KM Channels
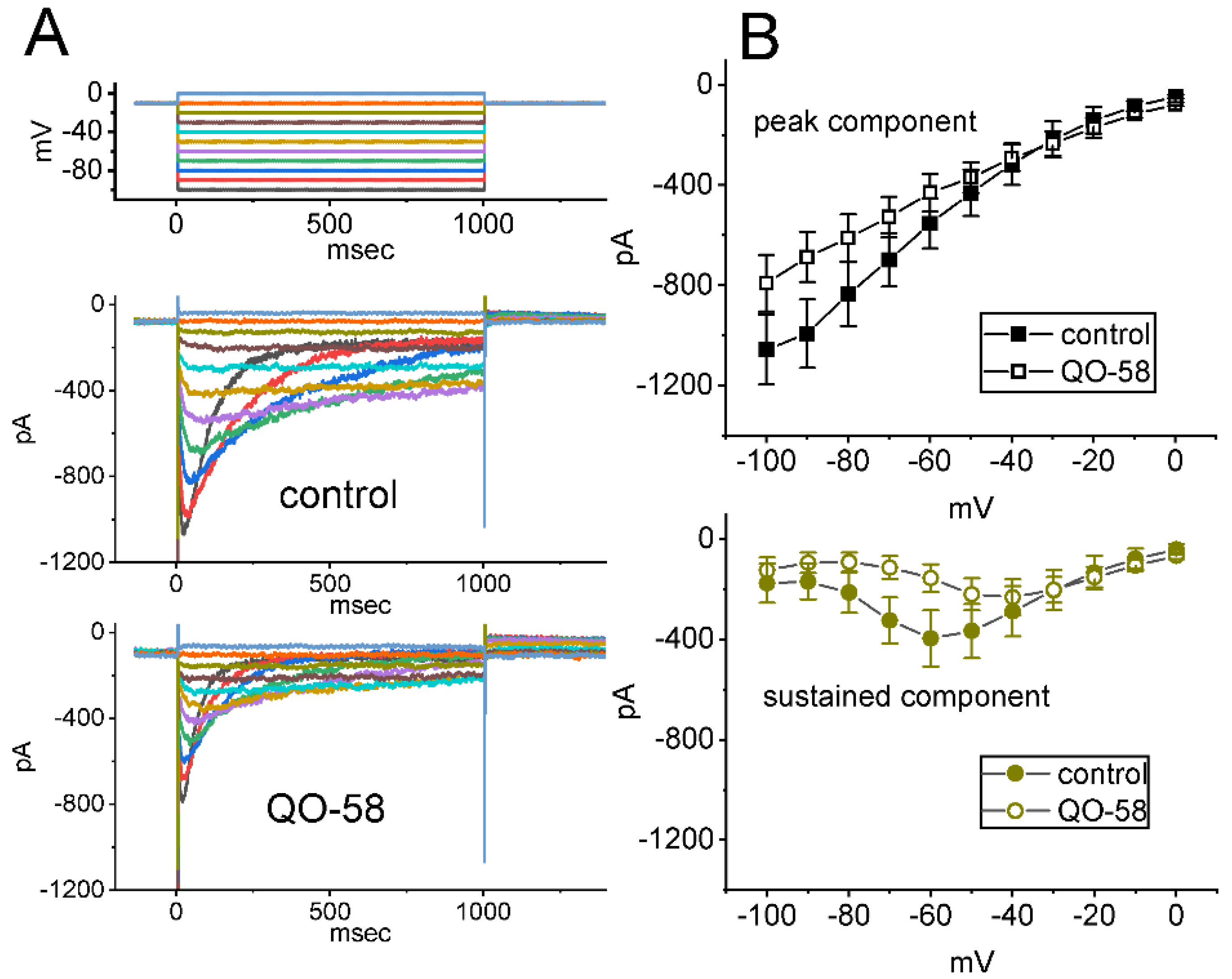
2.6. QO-58-Mediated Stimulation of Ca2+-Activated K+ Currents (IK(Ca)) by the Presence of QO-58
2.7. Stimulatory Effect of QO-58 on the Activity of Large-Conductance Ca2+-Activated K+ (BKCa) Channels Identified in GH3 Cells
2.8. Minor Inhibitory Effect of QO-58 on Erg-Mediated K+ Current (IK(erg)) Seen in GH3 Cells
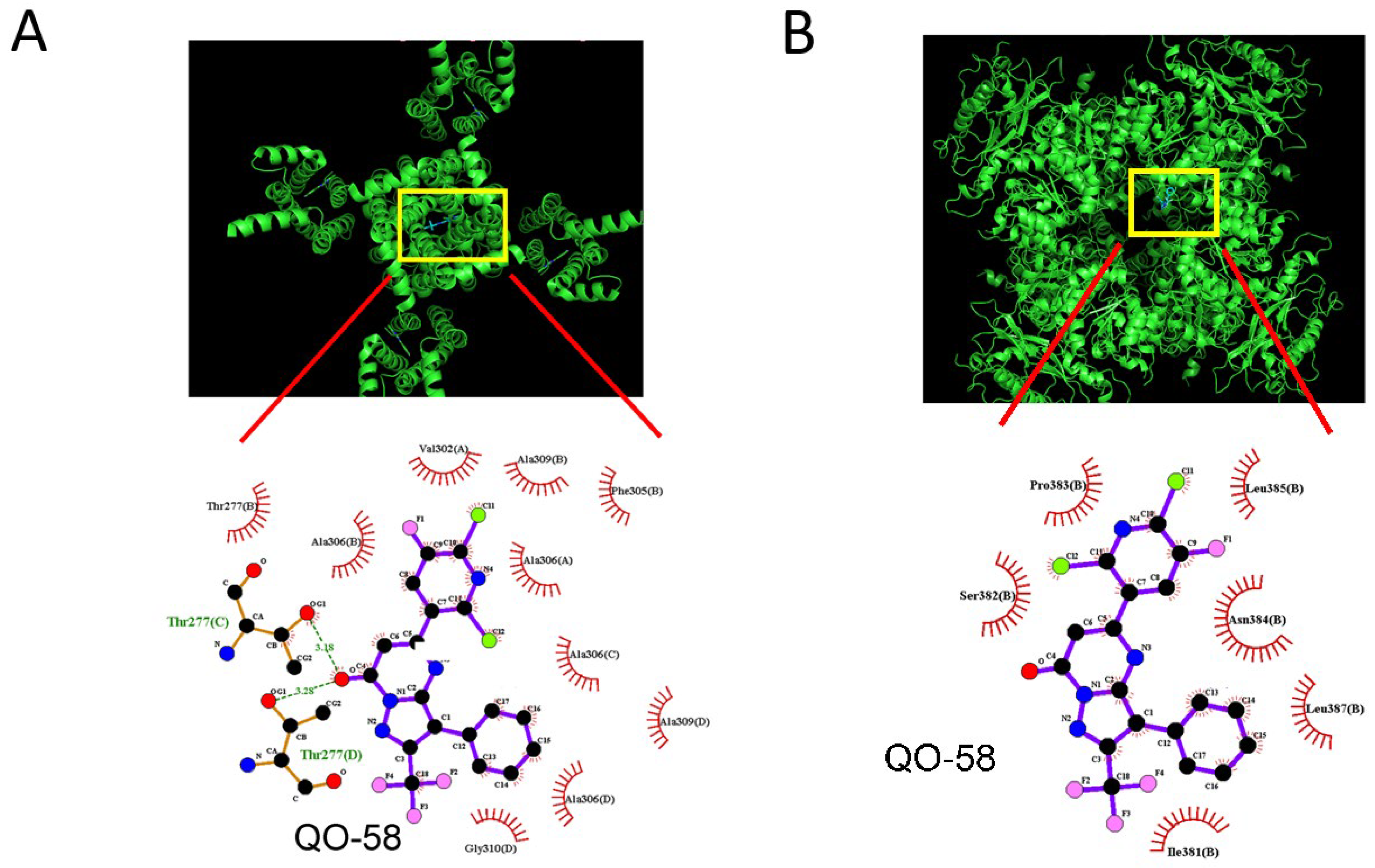
2.9. Docking Results on Interaction between KCa1.1 Channel and QO-58 or between KCNQ2 and QO-58
3. Discussion
4. Materials and Methods
4.1. Chemicals and Solution in This Work
4.2. Cell Preparations
4.3. Electrophysiological Measurements
4.4. Data Recordings and Analyses
4.5. Curve-Fitting Approximations and Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BKCa channel | large-conductance Ca2+-activated K+ channel |
| EC50 | concentration required for 50% stimulation |
| I-V | current versus voltage |
| IK(Ca) | Ca2+-activated K+ current |
| IK(erg) | erg-mediated K+ current |
| IK(M) | M-type K+ current |
| KM channel | M-type K+ channel |
| QO-58 | 5-(2,6-dichloro-5-fluoropyridin-3-yl)-3-phenyl-2-(trifluoromethyl)-1H-pyrazolol[1,5-a]pyrimidin-7-one/5-(2,6-dichloro-5-fluoro-3-pyridinyl)-3-phenyl-2-(trifluoromethyl)-pyrazolo[1,5-a]pyrimidin-7(4H)-one) |
| SEM | standard error of the mean |
| TRH | thyrotropin releasing hormone |
| TTX | tetrodotoxin |
| τact | activation time constant |
| Vhys | voltage-dependent hysteresis |
| Vramp | ramp voltage |
References
- Qi, J.; Zhang, F.; Mi, Y.; Fu, Y.; Xu, W.; Zhang, D.; Wu, Y.; Du, X.; Jia, Q.; Wang, K.; et al. Design, synthesis and biological activity of pyrazolo[1,5-a]pyrimidin-7(4H)-ones as novel Kv7/KCNQ potassium channel activators. Eur. J. Med. Chem. 2011, 46, 934–943. [Google Scholar] [CrossRef]
- Zhang, F.; Mi, Y.; Qi, J.L.; Li, J.W.; Si, M.; Guan, B.C.; Du, X.N.; An, H.L.; Zhang, H.L. Modulation of K(v)7 potassium channels by a novel opener pyrazolo[1,5-a]pyrimidin-7(4H)-one compound QO-58. Br. J. Pharmacol. 2013, 168, 1030–1042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Jia, Q.; Qi, J.; Zhang, H.; Wu, Y.; Shi, X. Exploring in vivo metabolism and excretion of QO-58L using ultra-high-performance liquid chromatography coupled with tandem mass spectrometry. Eur. J. Pharm. Sci. 2018, 117, 379–391. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, Y.; Zhang, D.; Fan, X.; Shao, D.; Li, H. Suppression of KCNQ/M Potassium Channel in Dorsal Root Ganglia Neurons Contributes to the Development of Osteoarthritic Pain. Pharmacology 2019, 103, 257–262. [Google Scholar] [CrossRef]
- Teng, B.-C.; Song, Y.; Zhang, F.; Ma, T.-Y.; Qi, J.-L.; Zhang, H.-L.; Li, G.; Wang, K. Activation of neuronal Kv7/KCNQ/M-channels by the opener QO58-lysine and its anti-nociceptive effects on inflammatory pain in rodents. Acta Pharmacol. Sin. 2016, 37, 1054–1062. [Google Scholar] [CrossRef] [Green Version]
- Du, M.Z.; Duan, R.; Shao, D.C.; Zhang, X.Y.; Zhang, F.; Li, H. Antinociceptive efficacy of QO-58 in the monosodium lodoacetate rat model for osteoarthritis pain. Zhonghua Yi Xue Za Zhi 2017, 97, 1333–1336. [Google Scholar]
- Liu, C.-F.; Qi, J.; Zhang, H.-L.; Jia, Q.-Z. Pharmacokinetic study of QO-58: A new potassium channel opener. Chin. Pharmacol. Bull. 2014, 30, 574–577. [Google Scholar]
- Sheng, Z.-F.; Zhang, H.; Zheng, P.; Chen, S.; Gu, Z.; Zhou, J.-J.; Phaup, J.G.; Chang, H.-M.; Yeh, E.T.H.; Pan, H.-L.; et al. Impaired Kv7 channel activity in the central amygdala contributes to elevated sympathetic outflow in hypertension. Cardiovasc. Res. 2022, 118, 585–596. [Google Scholar] [CrossRef]
- Chang, W.T.; Wu, S.N. Effective Activation of BK(Ca) Channels by QO-40 (5-(Chloromethyl)-3-(Naphthalen-1-yl)-2-(Trifluoromethyl)Pyrazolo [1,5-a]pyrimidin-7(4H)-one), Known to Be an Opener of KCNQ2/Q3 Channels. Pharmaceuticals 2021, 14, 388. [Google Scholar] [CrossRef]
- Wang, H.-S.; Pan, Z.; Shi, W.; Brown, B.S.; Wymore, R.S.; Cohen, I.S.; Dixon, J.E.; McKinnon, D. KCNQ2 and KCNQ3 potassium channel subunits: Molecular correlates of the M-channel. Science 1998, 282, 1890–1893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gribkoff, V.K. The therapeutic potential of neuronal KCNQ channel modulators. Expert Opin. Ther. Targets 2003, 7, 737–748. [Google Scholar] [CrossRef]
- Wulfsen, I.; Hauber, H.P.; Schiemann, D.; Bauer, C.K.; Schwarz, J.R. Expression of mRNA for voltage-dependent and inward-rectifying K channels in GH3/B6 cells and rat pituitary. J. Neuroendocrinol. 2000, 12, 263–272. [Google Scholar] [CrossRef]
- Yue, C.; Yaari, Y. KCNQ/M channels control spike afterdepolarization and burst generation in hippocampal neurons. J. Neurosci. 2004, 24, 4614–4624. [Google Scholar] [CrossRef] [Green Version]
- Hernández, C.C.; Zaika, O.; Tolstykh, G.P.; Shapiro, M.S. Regulation of neural KCNQ channels: Signalling pathways, structural motifs and functional implications. J. Physiol. 2008, 586, 1811–1821. [Google Scholar] [CrossRef]
- Brown, D.A.; Passmore, G.M. Neural KCNQ (Kv7) channels. Br. J. Pharmacol. 2009, 156, 1185–1195. [Google Scholar] [CrossRef]
- Greene, D.L.; Hoshi, N. Modulation of Kv7 channels and excitability in the brain. Cell Mol. Life Sci. 2017, 74, 495–508. [Google Scholar] [CrossRef] [Green Version]
- Hu, B.; Cilz, N.I.; Lei, S. Somatostatin depresses the excitability of subicular bursting cells: Roles of inward rectifier K+ channels, KCNQ channels and Epac. Hippocampus 2017, 27, 971–984. [Google Scholar] [CrossRef]
- Li, T.; Wu, K.; Yue, Z.; Wang, Y.; Zhang, F.; Shen, H. Structural Basis for the Modulation of Human KCNQ4 by Small-Molecule Drugs. Mol. Cell 2021, 81, 25–37.e4. [Google Scholar] [CrossRef]
- Ramakrishna, Y.; Manca, M.; Glowatzki, E.; Sadeghi, S.G. Cholinergic Modulation of Membrane Properties of Calyx Terminals in the Vestibular Periphery. Neuroscience 2021, 452, 98–110. [Google Scholar] [CrossRef]
- Revill, A.L.; Katzell, A.; Del Negro, C.A.; Milsom, W.K.; Funk, G.D. KCNQ Current Contributes to Inspiratory Burst Termination in the Pre-Bötzinger Complex of Neonatal Rats in vitro. Front. Physiol. 2021, 12, 626470. [Google Scholar] [CrossRef]
- Wua, Y.J.; Dworetzky, S.I. Recent developments on KCNQ potassium channel openers. Curr. Med. Chem. 2005, 12, 453–460. [Google Scholar] [CrossRef]
- Munro, G.; Dalby-Brown, W. Kv7 (KCNQ) channel modulators and neuropathic pain. J. Med. Chem. 2007, 50, 2576–2582. [Google Scholar] [CrossRef]
- Sun, J.; Kapur, J. M-type potassium channels modulate Schaffer collateral-CA1 glutamatergic synaptic transmission. J. Physiol. 2012, 590, 3953–3964. [Google Scholar] [CrossRef] [Green Version]
- Allen, N.M.; Mannion, M.; Conroy, J.; Lynch, S.A.; Shahwan, A.; Lynch, B.; King, M.D. The variable phenotypes of KCNQ-related epilepsy. Epilepsia 2014, 55, e99–e105. [Google Scholar] [CrossRef]
- Wang, J.-J.; Li, Y. KCNQ potassium channels in sensory system and neural circuits. Acta Pharmacol. Sin. 2015, 37, 25–33. [Google Scholar] [CrossRef] [Green Version]
- Barrese, V.; Stott, J.B.; Greenwood, I.A. KCNQ-Encoded Potassium Channels as Therapeutic Targets. Annu. Rev. Pharmacol. Toxicol. 2018, 58, 625–648. [Google Scholar] [CrossRef]
- Vanhoof-Villalba, S.L.; Gautier, N.M.; Mishra, V.; Glasscock, E. Pharmacogenetics of KCNQ channel activation in 2 potassium channelopathy mouse models of epilepsy. Epilepsia 2017, 59, 358–368. [Google Scholar] [CrossRef] [Green Version]
- Lezmy, J.; Gelman, H.; Katsenelson, M.; Styr, B.; Tikochinsky, E.; Lipinsky, M.; Peretz, A.; Slutsky, I.; Attali, B. M-Current Inhibition in Hippocampal Excitatory Neurons Triggers Intrinsic and Synaptic Homeostatic Responses at Different Temporal Scales. J. Neurosci. 2020, 40, 3694–3706. [Google Scholar] [CrossRef]
- Cho, H.Y.; Chuang, T.H.; Wu, S.N. The Effectiveness in Activating M-Type K+ Current Produced by Solifenacin ([(3R)-1-azabicyclo[2.2.2]octan-3-yl] (1S)-1-phenyl-3,4-dihydro-1H-isoquinoline-2-carboxylate): Independent of Its Antimuscarinic Action. Int. J. Mol. Sci. 2021, 22, 12399. [Google Scholar] [CrossRef]
- Jones, F.; Gamper, N.; Gao, H. Kv7 Channels and Excitability Disorders. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2021; pp. 185–230. [Google Scholar]
- Lo, Y.-C.; Lin, C.-L.; Fang, W.-Y.; Lőrinczi, B.; Szatmári, I.; Chang, W.-H.; Fülöp, F.; Wu, S.-N. Effective Activation by Kynurenic Acid and Its Aminoalkylated Derivatives on M-Type K+ Current. Int. J. Mol. Sci. 2021, 22, 1300. [Google Scholar] [CrossRef]
- Costi, S.; Han, M.-H.; Murrough, J.W. The Potential of KCNQ Potassium Channel Openers as Novel Antidepressants. CNS Drugs 2022, 36, 207–216. [Google Scholar] [CrossRef]
- Flunker, L.; Nutter, T.; Bowers, C.; Cooper, B. Development of KVO treatment strategies for chronic pain in a rat model of Gulf War Illness. Toxicol. Appl. Pharmacol. 2021, 434, 115821. [Google Scholar] [CrossRef]
- Li, S.-B.; Damonte, V.M.; Chen, C.; Wang, G.X.; Kebschull, J.M.; Yamaguchi, H.; Bian, W.-J.; Purmann, C.; Pattni, R.; Urban, A.E.; et al. Hyperexcitable arousal circuits drive sleep instability during aging. Science 2022, 375, eabh3021. [Google Scholar] [CrossRef]
- Osuma, A.T.; Xu, X.; Wang, Z.; Van Camp, J.A.; Freiberg, G.M. Design and evaluation of pyrazolopyrimidines as KCNQ channel modulators. Bioorganic Med. Chem. Lett. 2019, 29, 126603. [Google Scholar] [CrossRef]
- Redford, K.E.; Abbott, G.W. KCNQ Potassium Channels as Targets of Botanical Folk Medicines. Annu. Rev. Pharmacol. Toxicol. 2022, 62, 447–464. [Google Scholar] [CrossRef]
- Selyanko, A.A.; Hadley, J.K.; Wood, I.C.; Abogadie, F.C.; Delmas, P.; Buckley, N.J.; London, B.; Brown, D.A. Two Types of K+ Channel Subunit, Erg1 and KCNQ2/3, Contribute to the M-Like Current in a Mammalian Neuronal Cell. J. Neurosci. 1999, 19, 7742–7756. [Google Scholar] [CrossRef] [Green Version]
- Hsiao, H.T.; Liu, Y.C.; Liu, P.Y.; Wu, S.N. Concerted suppression of I(h) and activation of I(K(M)) by ivabradine, an HCN-channel inhibitor, in pituitary cells and hippocampal neurons. Brain Res. Bull. 2019, 149, 11–20. [Google Scholar] [CrossRef]
- So, E.C.; Liu, P.Y.; Wu, S.N. Effectiveness in the inhibition of dapagliflozin and canagliflozin on M-type K+ current and α-methylglucoside-induced current in pituitary tumor (GH3) and pheochromocytoma PC12 cells. Eur. J. Pharmacol. 2020, 879, 173141. [Google Scholar] [CrossRef]
- Kis, A.; Krick, S.; Baumlin, N.; Salathe, M. Airway Hydration, Apical K+ Secretion, and the Large-Conductance, Ca2+-activated and Voltage-dependent Potassium (BK) Channel. Ann. Am. Thorac. Soc. 2016, 13, S163–S168. [Google Scholar]
- Barkai, O.; Goldstein, R.H.; Caspi, Y.; Katz, B.; Lev, S.; Binshtok, A.M. The Role of Kv7/M Potassium Channels in Controlling Ectopic Firing in Nociceptors. Front. Mol. Neurosci. 2017, 10, 181. [Google Scholar] [CrossRef] [Green Version]
- Latorre, R.; Castillo, K.; Carrasquel-Ursulaez, W.; Sepulveda, R.V.; Gonzalez-Nilo, F.; Gonzalez, C.; Alvarez, O. Molecular Determinants of BK Channel Functional Diversity and Functioning. Physiol. Rev. 2017, 97, 39–87. [Google Scholar] [CrossRef] [PubMed]
- Al-Karagholi, M.A.; Gram, C.; Nielsen, C.A.W.; Ashina, M. Targeting BK(Ca) Channels in Migraine: Rationale and Perspectives. CNS Drugs 2020, 34, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.L.; Gao, Z.H.; Li, S.W.; Wu, S.N. High Efficacy by GAL-021: A Known Intravenous Peripheral Chemoreceptor Modulator that Suppresses BK(Ca)-Channel Activity and Inhibits I(K(M)) or I(h). Biomolecules 2020, 10, 188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, J. BK Channel Gating Mechanisms: Progresses Toward a Better Understanding of Variants Linked Neurological Diseases. Front. Physiol. 2021, 12, 762175. [Google Scholar] [CrossRef]
- Niday, Z.; Bean, B.P. BK Channel Regulation of Afterpotentials and Burst Firing in Cerebellar Purkinje Neurons. J. Neurosci. 2021, 41, 2854–2869. [Google Scholar] [CrossRef] [PubMed]
- Schrøder, R.L.; Strøbaek, D.; Olesen, S.P.; Christophersen, P. Voltage-independent KCNQ4 currents induced by (+/−)BMS-204352. Pflug. Arch. 2003, 446, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.T.; Tseng, Y.T.; Lo, Y.C.; Wu, S.N. Ability of naringenin, a bioflavonoid, to activate M-type potassium current in motor neuron-like cells and to increase BKCa-channel activity in HEK293T cells transfected with α-hSlo subunit. BMC Neurosci. 2014, 15, 135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sankaranarayanan, S.; Simasko, S. Characterization of an M-like current modulated by thyrotropin-releasing hormone in normal rat lactotrophs. J. Neurosci. 1996, 16, 1668–1678. [Google Scholar] [CrossRef]
- Schwarz, J.R.; Bauer, C.K. Functions of erg K+ channels in excitable cells. J. Cell Mol. Med. 2004, 8, 22–30. [Google Scholar] [CrossRef]
- Vandenberg, J.I.; Perry, M.D.; Perrin, M.J.; Mann, S.A.; Ke, Y.; Hill, A.P. hERG K+ channels: Structure, function, and clinical significance. Physiol. Rev. 2012, 92, 1393–1478. [Google Scholar] [CrossRef] [Green Version]
- So, E.C.; Foo, N.P.; Ko, S.Y.; Wu, S.N. Bisoprolol, Known to Be a Selective β1-Receptor Antagonist, Differentially but Directly Suppresses IK(M) and IK(erg) in Pituitary Cells and Hippocampal Neurons. Int. J. Mol. Sci. 2019, 20, 657. [Google Scholar] [CrossRef] [Green Version]
- Männikkö, R.; Pandey, S.; Larsson, H.P.; Elinder, F. Hysteresis in the voltage dependence of HCN channels: Conversion between two modes affects pacemaker properties. J. Gen. Physiol. 2005, 125, 305–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fürst, O.; D’Avanzo, N. Isoform dependent regulation of human HCN channels by cholesterol. Sci. Rep. 2015, 5, 14270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selyanko, A.; Brown, D. Regulation of M-type Potassium Channels in Mammalian Sympathetic Neurons: Action of Intracellular Calcium on Single Channel Currents. Neuropharmacology 1996, 35, 933–947. [Google Scholar] [CrossRef]
- Wu, S.N.; Lo, Y.K.; Li, H.F.; Shen, A.Y. Functional coupling of voltage-dependent L-type Ca2+ current to Ca2+-activated K+ current in pituitary GH3 cells. Chin. J. Physiol. 2001, 44, 161–167. [Google Scholar] [PubMed]
- Plante, A.E.; Whitt, J.P.; Meredith, A.L. BK channel activation by L-type Ca2+ channels CaV1.2 and CaV1.3 during the subthreshold phase of an action potential. J. Neurophysiol. 2021, 126, 427–439. [Google Scholar] [CrossRef]
- Knaus, H.G.; McManus, O.B.; Lee, S.H.; Schmalhofer, W.A.; Garcia-Calvo, M.; Helms, L.M.; Sanchez, M.; Giangiacomo, K.; Reuben, J.P.; Smith, A.B., 3rd; et al. Tremorgenic Indole Alkaloids Potently Inhibit Smooth Muscle High-Conductance Calcium-Activated Potassium Channels. Biochemistry 1994, 33, 5819–5828. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-N.; Chern, J.-H.; Shen, S.; Chen, H.-H.; Hsu, Y.-T.; Lee, C.-C.; Chan, M.-H.; Lai, M.-C.; Shie, F.-S. Stimulatory actions of a novel thiourea derivative on large-conductance, calcium-activated potassium channels. J. Cell. Physiol. 2017, 232, 3409–3421. [Google Scholar] [CrossRef]
- Zhou, J.-J.; Gao, Y.; Kosten, T.A.; Zhao, Z.; Li, D.-P. Acute stress diminishes M-current contributing to elevated activity of hypothalamic-pituitary-adrenal axis. Neuropharmacology 2016, 114, 67–76. [Google Scholar]
- Bauer, C.K.; Schwarz, J.R. Ether-à-go-go K+ channels: Effective modulators of neuronal excitability. J. Physiol. 2018, 596, 769–783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bauer, C.K.; Schwarz, J.R. Evidence for the Effectiveness of Remdesivir (GS-5734), a Nucleoside-Analog Antiviral Drug in the Inhibition of IK(M) or IK(DR) and in the Stimulation of I (MEP). Front. Pharmacol. 2020, 11, 1091. [Google Scholar]
- Chen, L.; Cho, H.-Y.; Chuang, T.-H.; Ke, T.-L.; Wu, S.-N. The Effectiveness of Isoplumbagin and Plumbagin in Regulating Amplitude, Gating Kinetics, and Voltage-Dependent Hysteresis of erg-mediated K+ Currents. Biomedicines 2022, 10, 780. [Google Scholar] [CrossRef] [PubMed]
- Hung, T.-Y.; Wu, S.-N.; Huang, C.-W. The Integrated Effects of Brivaracetam, a Selective Analog of Levetiracetam, on Ionic Currents and Neuronal Excitability. Biomedicines 2021, 9, 369. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, C.-L.; Fu, P.; Cho, H.-Y.; Chuang, T.-H.; Wu, S.-N. Evidence for Dual Activation of IK(M) and IK(Ca) Caused by QO-58 (5-(2,6-Dichloro-5-fluoropyridin-3-yl)-3-phenyl-2-(trifluoromethyl)-1H-pyrazolol[1,5-a]pyrimidin-7-one). Int. J. Mol. Sci. 2022, 23, 7042. https://doi.org/10.3390/ijms23137042
Wu C-L, Fu P, Cho H-Y, Chuang T-H, Wu S-N. Evidence for Dual Activation of IK(M) and IK(Ca) Caused by QO-58 (5-(2,6-Dichloro-5-fluoropyridin-3-yl)-3-phenyl-2-(trifluoromethyl)-1H-pyrazolol[1,5-a]pyrimidin-7-one). International Journal of Molecular Sciences. 2022; 23(13):7042. https://doi.org/10.3390/ijms23137042
Chicago/Turabian StyleWu, Chao-Liang, Poyuan Fu, Hsin-Yen Cho, Tzu-Hsien Chuang, and Sheng-Nan Wu. 2022. "Evidence for Dual Activation of IK(M) and IK(Ca) Caused by QO-58 (5-(2,6-Dichloro-5-fluoropyridin-3-yl)-3-phenyl-2-(trifluoromethyl)-1H-pyrazolol[1,5-a]pyrimidin-7-one)" International Journal of Molecular Sciences 23, no. 13: 7042. https://doi.org/10.3390/ijms23137042
APA StyleWu, C.-L., Fu, P., Cho, H.-Y., Chuang, T.-H., & Wu, S.-N. (2022). Evidence for Dual Activation of IK(M) and IK(Ca) Caused by QO-58 (5-(2,6-Dichloro-5-fluoropyridin-3-yl)-3-phenyl-2-(trifluoromethyl)-1H-pyrazolol[1,5-a]pyrimidin-7-one). International Journal of Molecular Sciences, 23(13), 7042. https://doi.org/10.3390/ijms23137042





