Abstract
Epinephelus coioides is a fish species with high economic value due to its delicious meat, high protein content, and rich fatty acid nutrition. It has become a high-economic fish in southern parts of China and some other Southeast Asian countries. In this study, the myostatin nucleic acid vaccine was constructed and used to immunize E. coioides. The results from body length and weight measurements indicated the myostatin nucleic acid vaccine promoted E. coioides growth performance by increasing muscle fiber size. The results from RT-qPCR analysis showed that myostatin nucleic acid vaccine upregulated the expression of myod, myog and p21 mRNA, downregulated the expression of smad3 and mrf4 mRNA. This preliminary study is the first report that explored the role of myostatin in E. coioides and showed positive effects of autologous nucleic acid vaccine on the muscle growth of E. coioides. Further experiments with increased numbers of animals and different doses are needed for its application to E. coiodes aquaculture production.
1. Introduction
Myostatin, also known as growth differentiation factor-8 (GDF-8), belongs to the transforming growth factor β superfamily. The human myostatin gene encodes 376 amino acids, including 3 exons and 2 introns. It is expressed in large amounts in skeletal muscle and its expression is also detected in other tissues and organs [1,2,3,4,5,6]. In addition, myostatin is present in mammals such as mice, rats, cattle, pigs, and goats [7,8,9,10,11]. The myostatin gene has been cloned and sequenced in a variety of fish including Danio rerio [12,13,14,15,16]. Past research indicates that myostatin is highly conserved among species, and its gene structure remains highly consistent across mammals [7]. Sequence alignment data show that the myostatin gene is conserved in the evolutionary processes. Myostatin in Morone saxatilis, Oreochromis mossambicus, Sciaeops ocellatus, mammals, and birds have the same amino acid ratio in the overall sequence from 60% to 65%; while the ratio in fish is the same. The ratios of amino acids are higher, and the similarities between striped seabass and tilapia are 93.4% and 96.8%, respectively. The conservation of this sequence implies that the fish myostatin gene may have a certain degree of similarity with mammalian species in function [17,18]. However, unlike mammals which only have one myostatin gene, bony fishes experience one more genome-wide duplication. Genome doubling has made some fishes have at least two myostatin genes [19]. In D. rerio, myostatin-1 and myostatin-2 exist in a variety of tissues, including skeletal and heart muscles, brain, liver, intestines, and ovary. The level of myostatin-1 mRNA rises steadily throughout the development of the body, while the level of myostatin-2 mRNA reaches its peak in the early stage of body development. The expression of myostatin-1 is higher than myostatin-2 in most tissues [20].
Myostatin negatively regulates muscle growth to a large degree [7]. “Double muscle phenotype,” caused by mutations of the myostatin gene, has been found in cattle, dogs, sheep, rabbits, and other mammals [8,21,22,23,24,25,26]. In D. rerio, knockout of the myostatin gene increased weight by 1.4 times in the experimental group after 4 months [27]. Researchers have a keen interest in regulating the muscle growth of related animals through the suppression of myostatin activity. Treatment with antibodies against myostatin resulted in increased skeletal muscle mass and grip strength in adult mice [28]. Skeletal muscle mass and endurance of mice can be enhanced through inoculation with a myostatin DNA vaccine (nucleic acid vaccine) [29]. Nucleic acid vaccines directly recombine the exogenous or autologous gene encoding an antigen protein with plasmid by DNA recombination technology, and then directly introduce the recombinant DNA into animal cells. This synthesizes the antigen protein through the transcription system of host cells to induce the host to produce an immune response to the antigen protein, to achieve the purpose of preventing and treating diseases [30]. Because fish autologous myostatin is weakly immunogenic, the antigen needs to be fused with the immune enhancement vector gene [31]. High immunogenicity fusion protein can be expressed by fusing genes such as follicular inhibin and somatostatin into immune enhancement vector genes such as HBsAg [32,33]. Therefore, based on our previous studies and experiences, we constructed a highly immunogenic myostatin autologous nucleic acid with HBsAg foreign gene. It is expected that this construct can stimulate the fish immune system to produce the corresponding antibody, which will inhibit the function of myostatin mature peptide, so as to promote muscle development and growth.
To understand the effects of the vaccine on muscle growth, we selectively studied myogenic regulatory proteins. Myogenic regulatory factors (mrfs) are a member of basic helix-loop-helix (bHLH) family of transcription factors [34], including myogenic factor 5 (myf5), myogenic differentiation (myod), myogenin (myog), and myogenic regulatory factor 4 (mrf4). These transcription factors control the myogenic commitment and differentiation of skeletal muscle cells during embryonic development and postpartum myogenesis. They act on multiple points in the muscle lineage by regulating proliferation, the irreversible cell cycle arrest of precursor cells, sarcomeres, and activation of muscle-specific genes to promote differentiation and sarcomere assembly, and jointly establishes skeletal muscle phenotype [35,36].
This study aims to develop a biological agent for making the myostatin nucleic acid vaccine to promote muscle growth and development, which will certainly decrease the cost of feeding, and improve the fillet meat yield. It can be developed as an alternative to transgenic animals. The effects of myostatin depression by its nucleic acid vaccine were studied on muscle growth of E. coioides and myofibers and related myogenic regulator factors. This is the first application of fish nucleic acid vaccine research to economically valuable aquatic species in the field.
2. Results
2.1. Construction of Nucleic Acid Vaccine
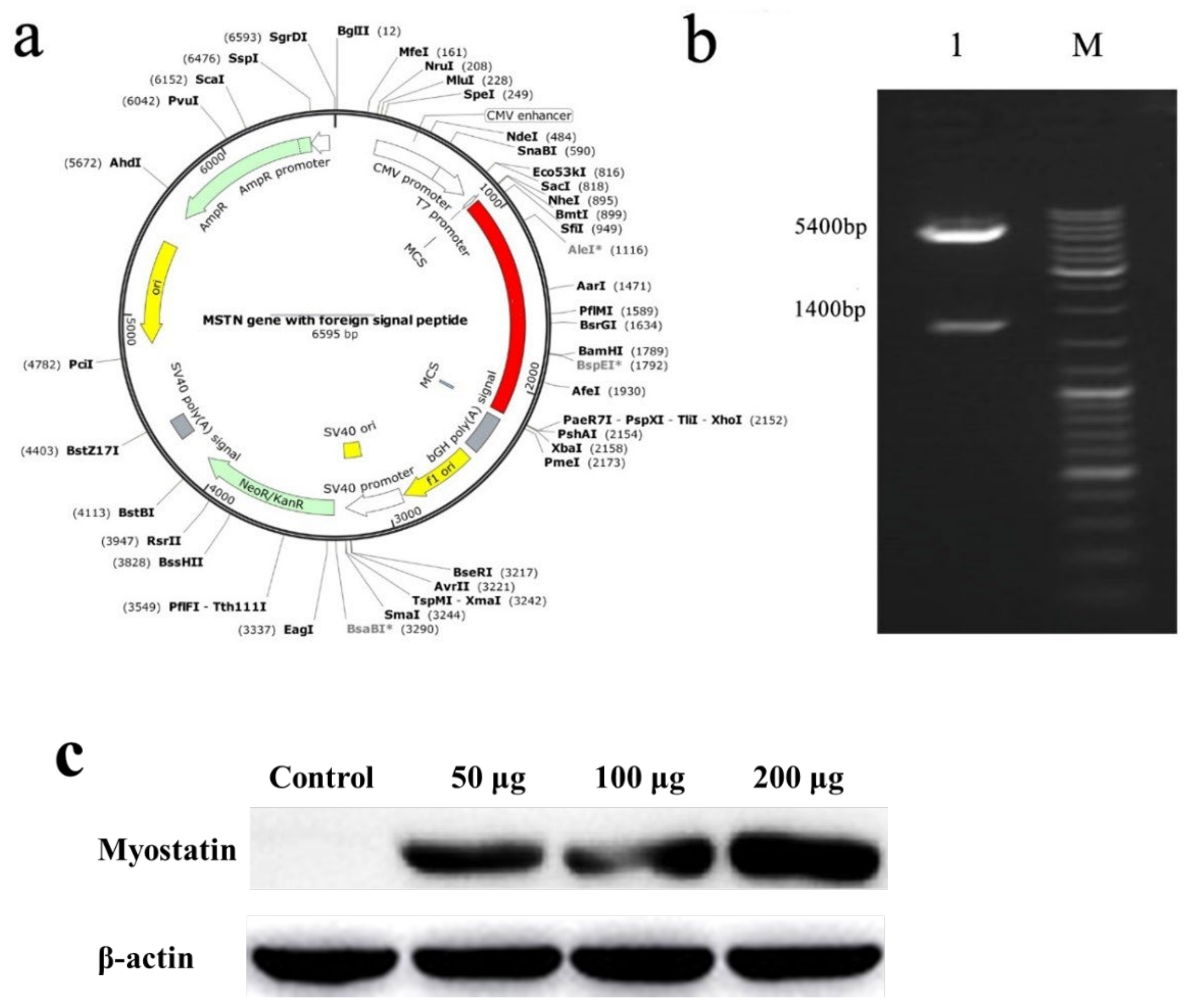
The obtained exogenous gene (indicated by the red in Figure 1) was cloned into pcDNA3.1+ to obtain the recombinant plasmid, which was the nucleic acid vaccine (Figure 1a), and Nhe I and Xho I were used for double enzyme digestion identification. The final expectation was to obtain bands of 1400 and 5400 bp, which indicated that the plasmid was the target plasmid (Figure 1b). To confirm the expression of recombinant plasmid in muscle in vaccinated E. coioides, we performed western blot with anti-HBSAg antibody. As shown in Figure 1c. The result confirmed the expression of recombinant plasmid in muscle of vaccinated E. coioides. Furthermore, the HBSAg expressing in muscle increased along with the dose of recombinant plasmid injected, and the highest expression level of HBSAg was seen in the 200 μg vaccine group.
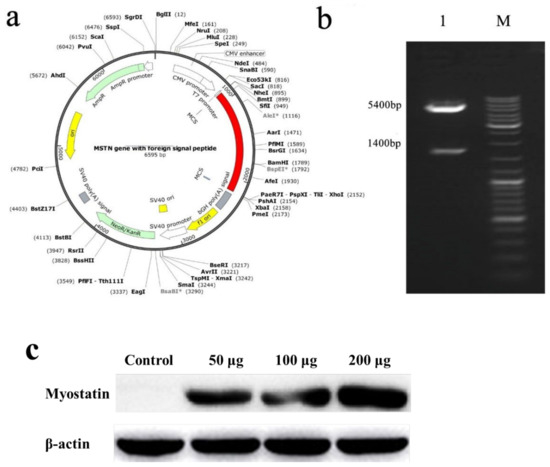
Figure 1.
(a) Schematic diagram of nucleic acid vaccine. (b) Double enzyme digestion electropherogram. 1: Plasmid digestion band, 1400 and 5400 bp; M: DNA marker. (c) Western blotting analysis of HBSAg protein in muscle of vaccinated E. coioides.
2.2. Effects of Myostatin Autologous Nucleic Acid Vaccine on Body Weight and Length
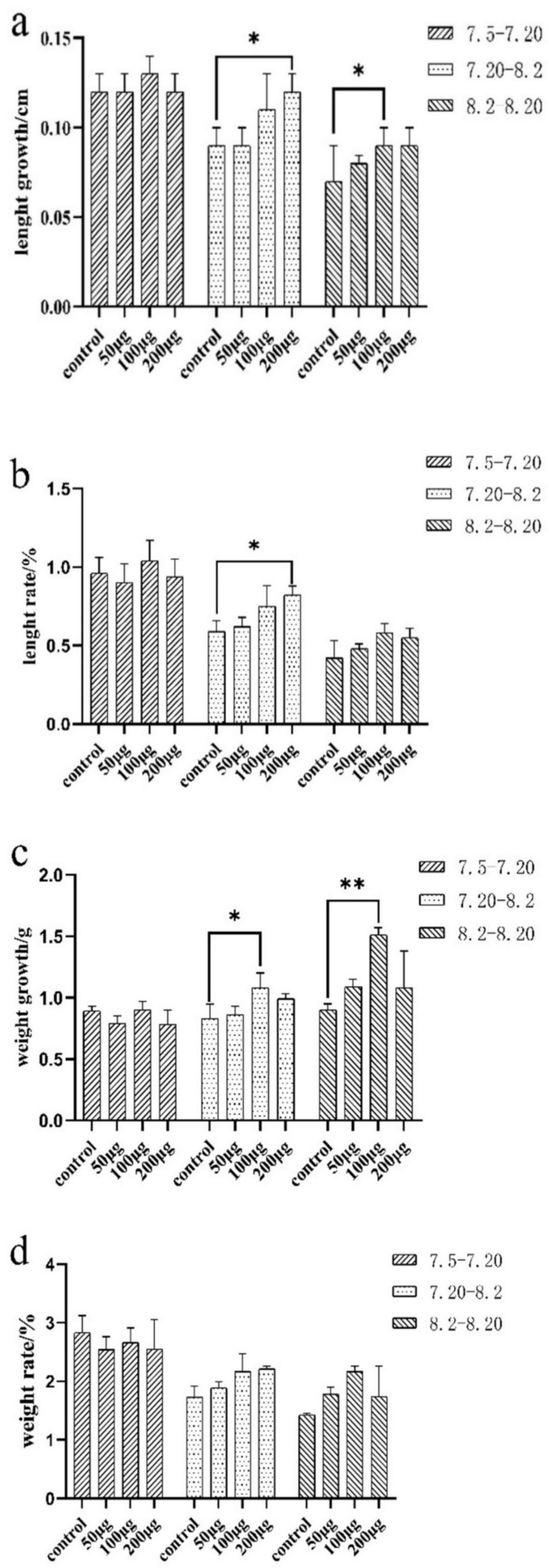
The body weight and length of E. coioides at each immunization and final sampling were recorded and analyzed. The results showed that body weight and length of the three experimental groups of E. coioides did not change significantly from the first immunization to the second immunization (7.5–7.20) when compared with the control group. From the second to the third immunization (7.20–8.2) and the third immunization to the sampling period (8.2–8.20), the growth and rate of the body length and weight of the three experimental groups of E. coioides showed an upward trend with the increase in the injection dose in comparison with the control group. After statistical analysis, from the second injection to the third injection, the length growth and weight rate of the experimental group and the control group with a dose of 200 μg were significantly different. There was a significant difference in the weight growth between the experimental and control groups with an injection dose of 100 μg. From the third injection to the sampling period, the length and weight growth of the experimental group and the control group with an injection dose of 100 μg were significantly different (Figure 2).
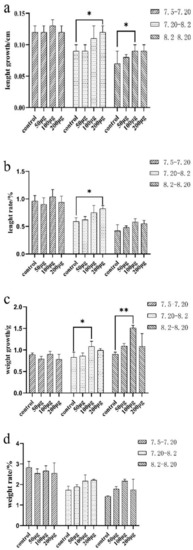
Figure 2.
Growth and rate of length and weight of E. coioides. (a) Growth of the length of E. coioides; (b) rate of the length of E. coioides; (c) growth of the weight of E. coioides; (d) rate of the weight of E. coioides. Bar: mean ± SD. * p < 0.05 and ** p < 0.01 unpaired t test, n = 3.
2.3. Effects of Myostatin Autologous Nucleic Acid Vaccine on the Muscle Tissue
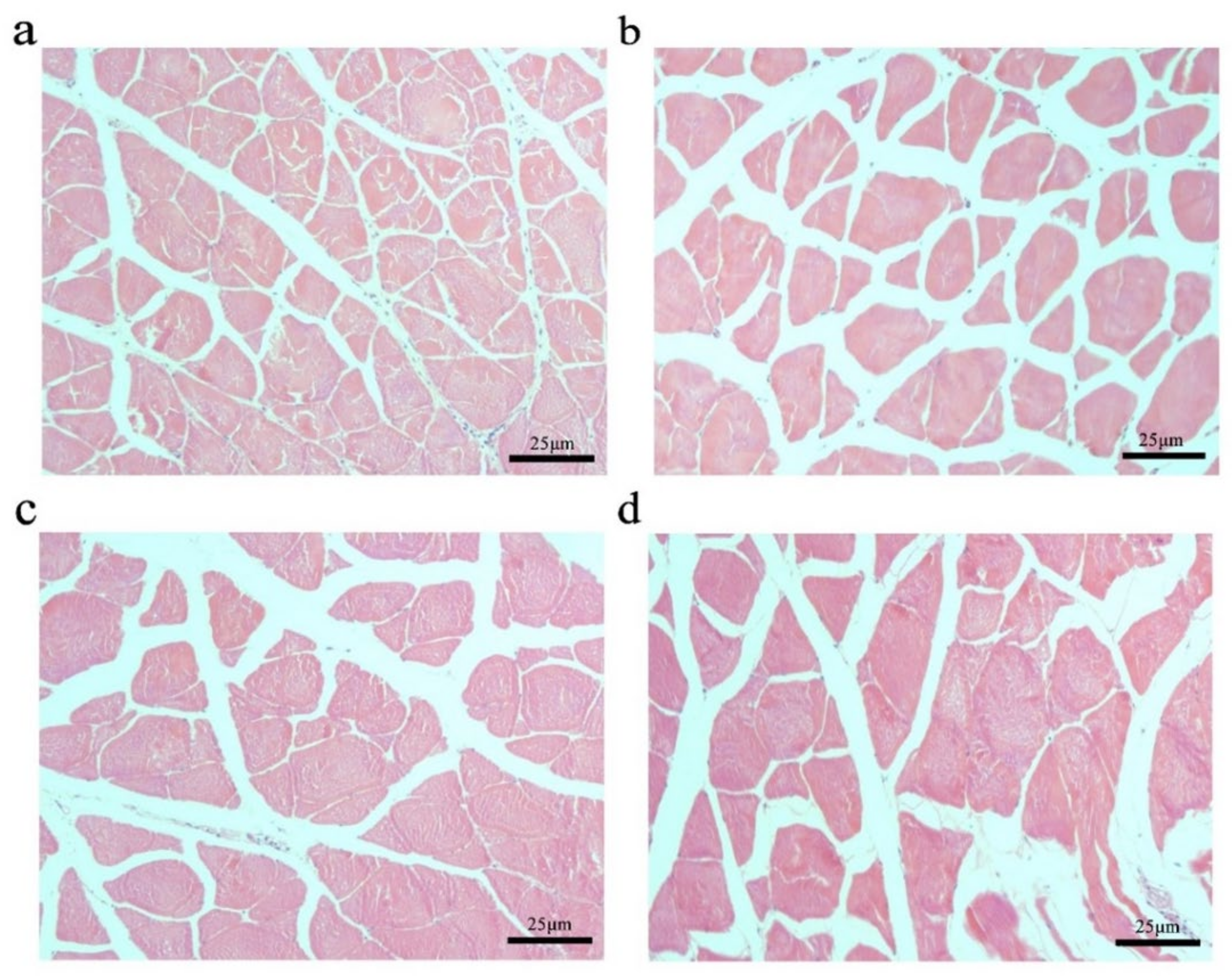
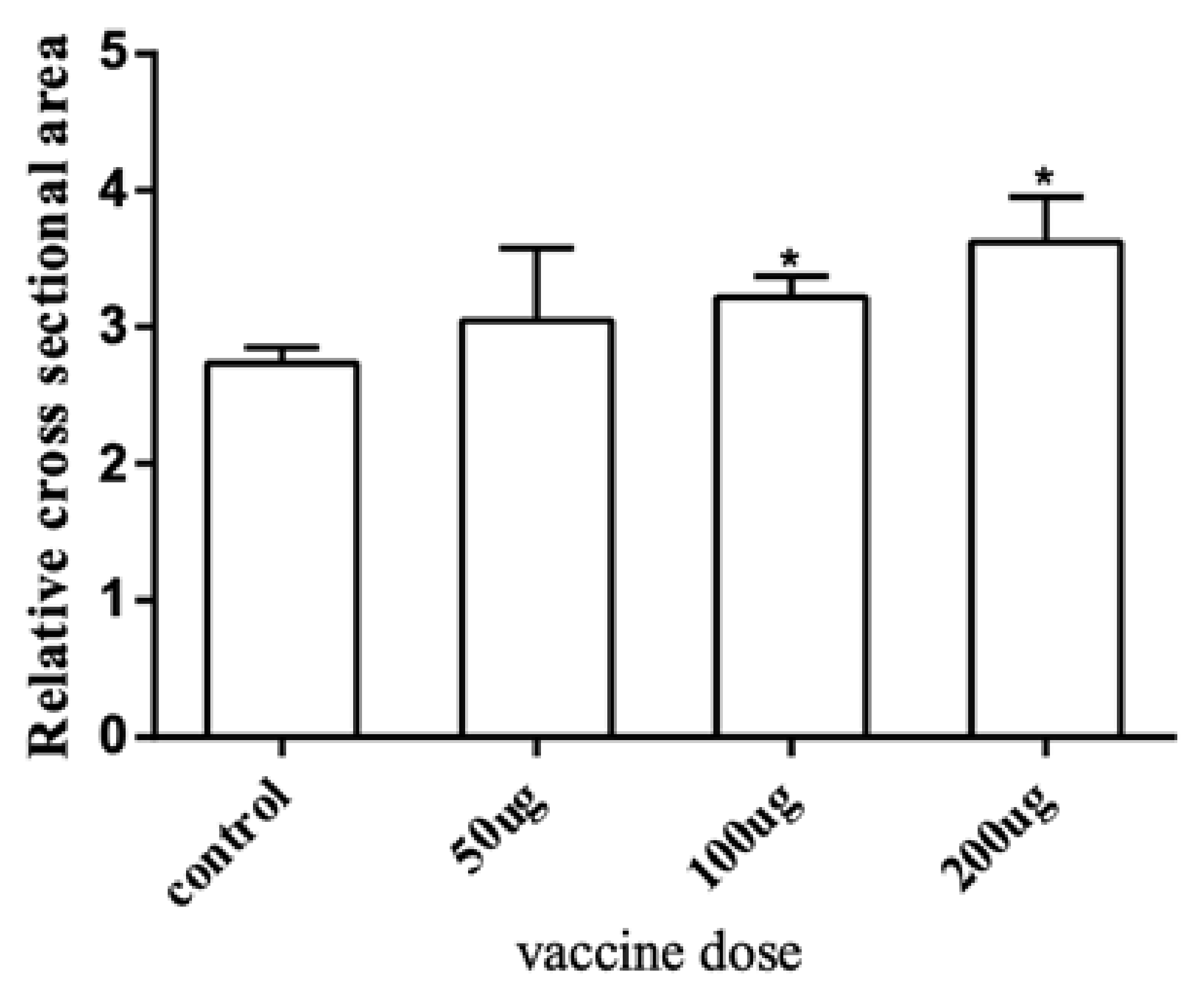
To further explore the effect of the myostatin autologous nucleic acid vaccine on the muscle tissue of E. coioides, the muscle fibers of E. coioides were made into paraffin sections, stained with H&E, and photographed under a microscope (Figure 3). Image processing was performed by Adobe Photoshop CS6 software, and average relative cross-sectional area of muscle fibers in the control group and the three experimental groups was calculated. It was found that the average relative cross-sectional area of muscle fiber in the experimental group was larger than that of the control group. With the increase in the immunization dose of the experimental group, the relative cross-sectional area of the average muscle fibers increased. Statistical analysis found that compared with the control group, the experimental group with an injection dose of 100 and 200 μg had significantly larger muscle fibers (Figure 4).
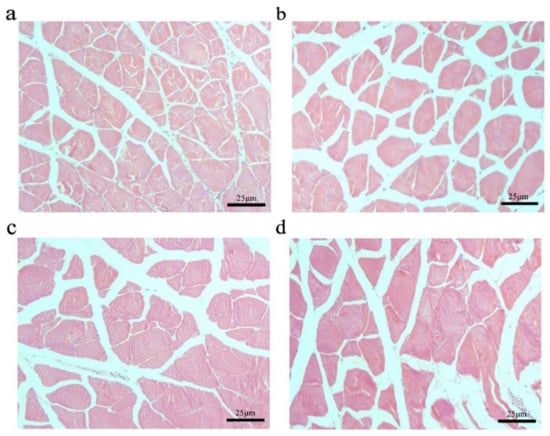
Figure 3.
Changes in muscle fibers of E. coioides after immunization. (a) HE staining of paraffin sections of muscle fibers in the control group; (b) HE staining of paraffin sections of muscle fibers in the immunized 50 μg vaccine group; (c) HE staining of paraffin sections of muscle fibers in the immunized 100 μg vaccine group; (d) HE staining of paraffin sections of muscle fibers in the immunized 200 μg vaccine group.
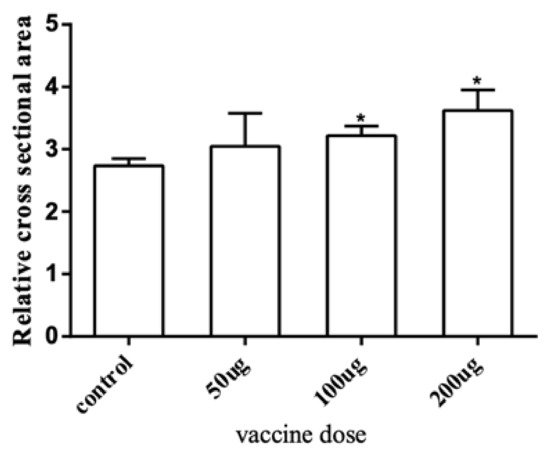
Figure 4.
The average relative area of a single muscle fiber. The cross-section range of muscle fiber cells was accurately selected and the relative cross-sectional area of a single muscle fiber was calculated through Adobe Photoshop CS6 software. Bar: mean ± SE. * p < 0.05 unpaired t test, n = 4.
2.4. Effects of Myostatin Autologous Nucleic Acid Vaccine on the Downstream and Related Genes of the Myostatin Signaling Pathway
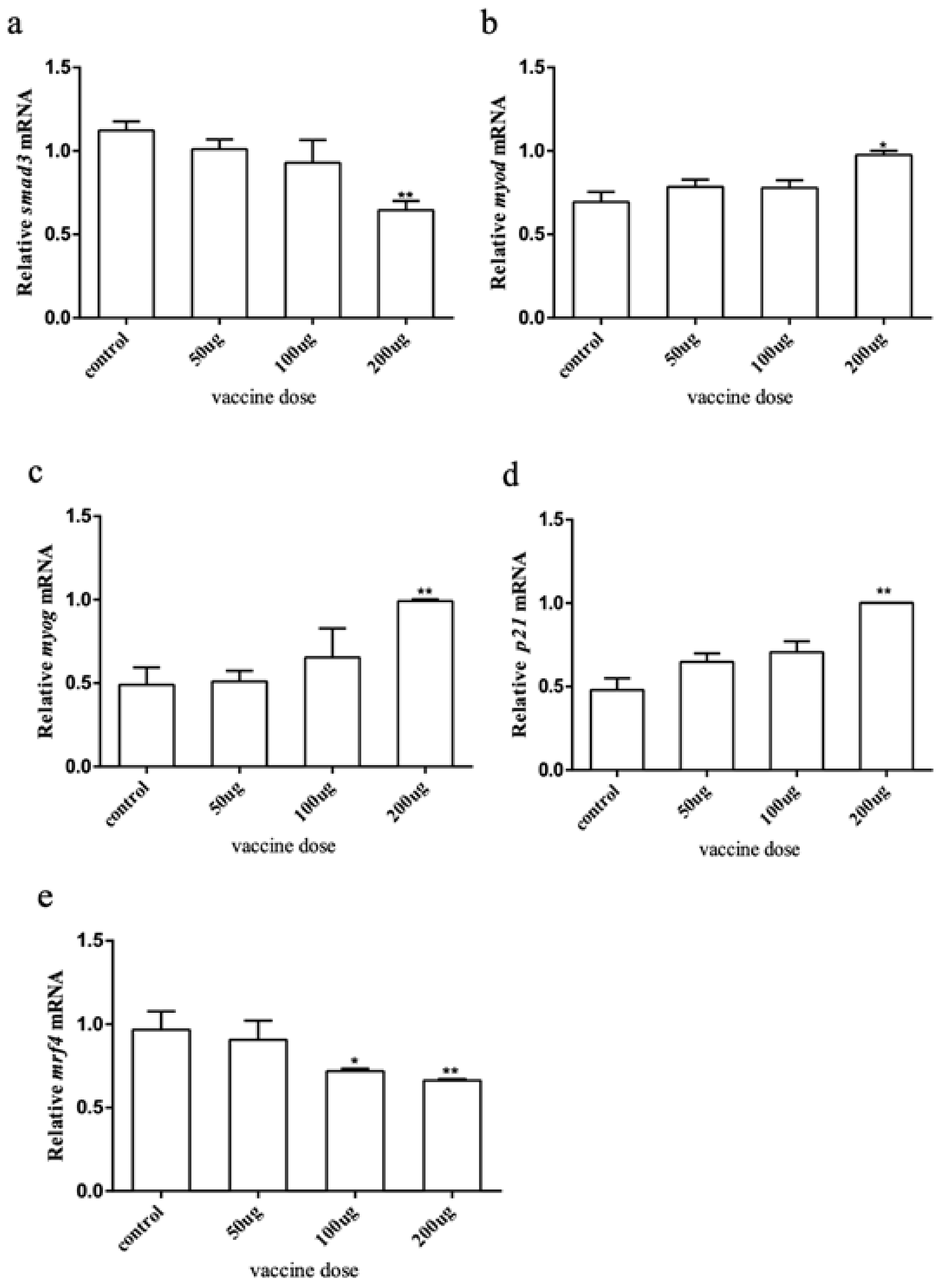
To explore how the myostatin autologous nucleic acid vaccine works on the muscles of E. coioides, muscle samples were taken from the experimental and control fish, and the total RNA was extracted and reverse transcribed into cDNA. RT-qPCR was used to detect the downstream genes of the myostatin signaling pathway and myogenic regulatory genes smad3, myod, myog, p21, and mrf4. The results showed that after myostatin autologous nucleic acid vaccine immunization, the mRNA expression of smad3 and mrf4 in the three doses of the experimental group showed a downward trend (Figure 5). After statistical analysis, there was no significant difference in smad3 when the dose was 50 and 100 μg, and there was a significant difference when the dose was 200 μg. There was no significant difference in mrf4 when the dose was 50 μg, and there was a significant difference when the dose was 100 and 200 μg. The mRNA expression levels of myod, myog, and p21 all showed an upward trend, but there were significant differences between treatment and control groups when the dose was 200 μg.
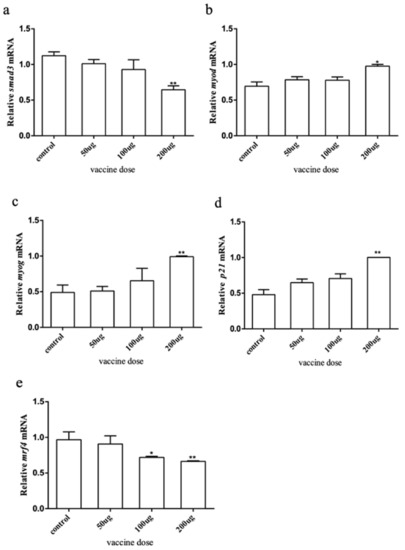
Figure 5.
The expression of myostatin signaling pathway downstream and related genes by immune myostatin autologous nucleic acid vaccine. (a) Expression level of smad3; (b) expression level of myod; (c) expression level of myog; (d) expression level of p21; (e) expression level of mrf4. Bar: mean ± SE. * p < 0.05 and ** p < 0.01 unpaired t test, n = 4.
3. Discussion
In this study, we explored the nucleic acid vaccine approach to depress the biological functions of myostatin in E. coioides. E. coioides was used as the experimental animal, and the immune experiment was carried out. Since there is currently no product on the market that can detect whether myostatin antibodies are produced in the body of E. coioides, the immune effects are analyzed in animal growth performances, myofiber and gene expression levels, and the antibodies were not analyzed. The growth rate of length and weight in one of the experimental groups had an upward trend compared with the control group. After statistical analysis, there were significant differences. After the immunization experiment, the experimental group and the control group were sampled, and muscle sections were made. Microscopic observation revealed that the average relative cross-sectional area of a single muscle fiber in the experimental group was larger than that of the control group, and there were significant differences. In previous studies, it had been demonstrated in a variety of animals that inhibiting the expression of myostatin could lead to an increase in the number of muscle fibers or hypertrophy and a significant increase in body weight [7,8,27,37,38]. Additionally, a study using zebrafish as a model organism for myostatin knockout showed that after interference with myostatin, the weight and body length growth rate of the experimental group showed an upward trend and the phenomenon of thick muscle fibers appeared. This is the same as most other research results. Therefore, it could be determined that the myostatin autologous nucleic acid vaccine promotes muscle growth of E. coioides. However, in-depth analysis of the growth data of the body length and weight of this research object revealed that there were only some significant differences. There were several speculations about this: first, referring to the period during the first injection to the second injection in this experiment, the growth rate of body weight and length of the three experimental groups did not change significantly compared with the control group. With the extension of time, after repeated immunizations during the subsequent farming period, the rate began to show an upward trend. Therefore, in future studies, we will consider extending the breeding time and increasing the immunization times to better promote muscle growth of E. coioides. Secondly, most of the past studies focused on mammals, and the types and numbers of fish were relatively few. However, this study is the first to use the E. coioides as the research object and it was speculated that the difference in the species causes the function of myostatin to be not completely the same. Studies have shown that in some animals myostatin was specifically expressed only in muscle tissue [7], while weak expression of myostatin was detected in some tissues such as the heart, spleen, kidney, and breast, the expression of direct homolog of myostatin was detected in almost all tissues of fish [39,40,41]. The different expression patterns of myostatin in fish and mammals means that its function may also be different. Fish myostatin-related articles also point out that there was no clear correlation between fish muscle growth rate and the expected myostatin expression level, which makes us doubt its role in the regulation of fish muscle growth. Fish myostatin could be used as a general inhibitor of cell proliferation and cell growth to control tissue quality, but it could not be used as a strong muscle regulator [17].
The muscle samples were taken from all groups, and the RNA was extracted and reverse transcribed into cDNA to detect the expression of the myostatin signaling pathway downstream and related genes. As mentioned above, myostatin was mediated by Smads [42,43,44,45,46], and then regulated the expression of mrfs. The expression of Smad3 decreased, the expression of myog and myod were upregulated, and the expression of mrf4 decreased after immunization with myostatin autologous nucleic acid. It was speculated that myostatin autologous nucleic acid vaccine can produce corresponding protein antibody in E. coioides, neutralizing endogenous protein and reducing the expression of Smad3 in the pathway; thus, upregulating the downstream myod and myog, which was consistent with the experimental results of sheep and mice immunized with myostatin autologous nucleic acid vaccine [47]. In this study, the body length/body weight growth speed, length/weight growth rate and cross-sectional area of muscle fiber increased after immunization with myostatin nucleic acid vaccine, which indicated that myostatin nucleic acid vaccine played a certain role in the growth of E. coioides in this study. On the other hand, the expression of p21 in this experiment showed an upward trend, while that of mrf4 showed a downward trend, which was different from the results of other studies that inhibited the function of myostatin. According to the existing literature, we speculated that there were several possibilities. First, in this study, pcDNA 3.1+ vector was used to construct myostatin nucleic acid vaccine and to inject it into E. coioides, while previous studies mostly used siRNA or lentiviral vector to treat cells. Secondly, the primary muscle cells of E. coioides were used in this study, but previous studies were mostly found in goats, mice, pigs, and other species. Finally, it may be that differentiation of experimental cells was triggered in the late growth stage, leading to the upregulation of p21 expression. When the myoblasts gradually fused to form myotubes, and the myotubes gradually differentiated into myofibers, mrf4 expression tended to be stable and gradually decreased. The higher the maturity and integrality of muscle fibers, the lower the expression level of mrf4 [47,48]. In another study we are preparing to publish, we found that Myostatin recombinant protein regulates cell differentiation by regulating the expression of smad3, mrf4, myod, and myog; at the same time, Myostatin recombinant protein regulates the expression of p21 to inhibit cell proliferation. The biological function of Myostatin at the level of muscle cells was verified.
In this study, the body weight and length of E. coioides increased to a certain extent after immunization with nucleic acid vaccine, and the cross-sectional area of muscle fiber also increased significantly. At the same time, the expression of related genes downstream of the myostatin signaling pathway changed in previous studies. This shows that the myostatin nucleic acid vaccine could promote the muscle growth of E. coioides. However, considering that the increase in body weight and body length was not as significant as that of mammals, and the sampling location of muscle slices is the immune site, it was impossible to determine whether the increase in cross-sectional area of muscle fibers has an overall effect. Additionally, the change in body weight and body length might be caused by the change in immune site. Therefore, it was not confirmed that this promotion effect was very strong at present, which needs further exploration.
4. Materials and Methods
4.1. Construction of Nucleic Acid Vaccine
The DNA construct (Figure 1a) consists of KOZAK sequences, signal peptide of Japanese encephalitis virus, Surface core antigen of hepatitis B virus, Th epitope, somatostatin, linker, and myostatin mature peptide, which was designed according to the vector specification and existing research results. KOZAK sequence increases the expression of foreign protein; signal peptide of Japanese encephalitis virus correctly guides foreign proteins and improves protein biological efficiency; Th epitope improves protein immunogenicity; Surface core antigen of hepatitis B virus makes the myostatin mature peptide exogenous; Somatostatin and myostatin are genes that inhibit muscle growth; linker sequence helps hepatitis B surface antigen protein and myostatin mature peptide protein appropriately linked together. After being synthesized by Sangon Biotech, it was cloned into pcDNA3.1+ vector, and finally, the recombinant plasmid and positive bacteria were obtained. The recombinant plasmids were identified by double digestion with Nhe I and Xho I. See Table 1 for gene sequence.

Table 1.
Gene sequence used to construct nucleic acid vaccine.
4.2. Vaccine Production
The positive bacteria obtained above were inoculated into a large amount of LB medium for expanded culture and plasmid was extracted by alkali lysis. After obtaining a large number of crude extract particles, the plasmid was purified by tangential flow of hollow fiber filtration system Akta flux, CFP-4-E-2U hollow fiber through the filter column, tangential flow of hollow fiber filtration system Akta flux, and UFP-300-C-2U hollow fiber through the filter column. The specific steps are described above [49].
4.3. Animals
E. coioides were collected from the Guangdong Marine Fisheries Test Center (22°705093′ N, 114°541433′ E) China, on 4–6 March 2020. Weight 31.6 ± 6.7 g, length 12.8 ± 1.0 cm. Before the experiment, the fish were cultured in a mariculture system at 25 °C for two weeks. After the culture, the obtained samples were placed in the centrifuge tubes with Sample Protector for RNA/DNA (TAKARA, Kyoto, Japan), and then stored at −80 °C. The other part of the sample was placed in Bouin’s Fixative Solution (PHYGENE, Fuzhou, China). Voucher specimens were deposited at the Department of Aquatic Animal Medicine, College of Marine Sciences, South China Agricultural University, Guangzhou, China (Accession number 20200824). All animal experiments were conducted by the guidelines and approval of the Animal Research and Ethics Committees of South China Agricultural University (#2019-0136).
4.4. Immunization Protocol and Sample Collection
A total of 180 E. coioides about 13 cm in length were randomly divided into four groups, with three parallel groups in each group. One group was injected with 100 μL of saline as the control group, and the other three groups were injected with 100 μL of myostatin autologous nucleic acid vaccine at concentrations of 500, 1000, and 2000 ng/μL as the experimental group. All E. coioides were fed under the same conditions and ate freely. The first immunization was completed three times, and each group was immunized three times with an interval of 2 weeks. After the third immunization, the animals were cultured for 2–4 weeks, fasting for one day before sampling. When sampled, the weight and length of all subjects were recorded. Then, the muscle tissue was taken and one part stored in Bouin’s Fixative Solution (PHYGENE, Fuzhou, China) and the other part in Sample Protector for RNA/DNA (TAKARA, Kyoto, Japan).
4.5. Muscle Histology
The muscles were fixed overnight in Bouin’s Fixative Solution (PHYGENE, Fuzhou, China) at room temperature, and then serially sectioned after dehydration in an ethanol gradient, transparent in xylene, and embedded in paraffin. The slice thickness was 5 μm. The chips were then dipped in two cylinders of xylene for 2 min, 10 times each time. After de-waxing, gradient concentration ethanol rehydration was carried out, and H&E staining was carried out after rehydration. The slides were dehydrated and dried, and finally sealed with neutral gum. Slides were observed and photographed under a Motic optical microscope.
4.6. RNA Extraction, cDNA Synthesis and qRT-PCR
Total RNA from tissue was extracted by using Trizol reagent (Invitrogen, CA, California, USA). Reverse transcription reactions were performed using a ReverTra Ace® qPCR RT Kit (TOYOBO, Osaka, Japan) using the protocol provided by the manufacturer. RT-qPCR was performed using a SYBR® Green Realtime PCR Master Mix (TOYOBO, Osaka, Japan) using the protocol provided by the manufacturer. The reference genes β-actin were used, and qRT-PCR gene targets and primers are given in Table 2.

Table 2.
Primers used in this study.
4.7. Western Blot Analysis
To demonstrate that the recombinant plasmid was really expressed in muscle cells of injected fish, we perform a western blot with anti-HBSAg antibody (10-1323, Fitzgerald Industries International, North Acton, MA, USA).The mixed muscle tissues from three fish of each group were used for Western blot analysis. Protein was extracted from muscle tissue and the protein concentration was evaluated using a BCA protein assay. Equal amounts of protein were subjected to SDS-PAGE and transferred onto polyvinylidene difluoride (PVDF) membranes (Millipore, Burlington, MA, USA). Immunoblotting was carried out using a 1:1000 dilution of antibodies anti-HBSAg (Abcam, Cambridge, UK) and anti-β-actin (Abcam) according to the manufacturer’s instructions. Primary antibodies were visualized using the enhanced chemiluminescent development reagent (Amersham Pharmacia Biotech Ltd., Little Chalfont, UK) following the peroxidase-conjugated secondary antibody (Santa Cruz Biotechnology, Inc., Dallas, TX, USA).
4.8. Statistical Analysis
The experimental data was statistically analyzed using Graphpad Prism v8.0.2.263 (GraphPad Software, San Diego, CA, USA), with β-actin as an internal parameter and analyzed by 2−ΔΔCt method. Data are expressed as mean ± standard error (Mean ± SE) or mean ± standard deviation (Mean ± SD). Statistical significance was defined as p < 0.05. The cross-sectional image of muscle fiber cells was used to accurately select the cross-section range of muscle fiber cells and calculate the relative cross-sectional area of a single muscle fiber through Adobe Photoshop CS6 software. The calculation formula is as follows. Then, statistical analysis was performed by Graphpad Prism 8.0 software.
Author Contributions
Conceptualization, H.Y. and J.Y.; methodology, J.X.; software, B.F.; validation, J.Y.; formal analysis, B.F.; investigation, Q.W.; resources, H.Z.; data curation, Q.W.; writing—original draft preparation, B.F., Y.Y. and Y.H.; writing—review and editing, H.Y. and J.Y.; visualization, H.Y.; supervision, H.Y.; project administration, H.Y.; funding acquisition, H.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by Provincial Marine six Industries Special project for Promoting High-quality Economic Development (Marine economic development) funded by Department of Natural Resources of Guangdong Province (GDNRC[2022]50); Guangdong Forestry Science and Technology Innovation Project (2021KJCX012, 2022KJCX019); Provincial Science and Technology Special Fund Project for Zhongshan City (major special project + Task list management mode) (2021sdr003); Rural Science and Technology Commissioner Service Team project of New Rural Development Institute, South China Agricultural University (2021XNYNYKJHZGJ022); Provincial Projects with Special Funds for Promoting Economic Development of Marine and Fisheries Department of Guangdong (SDYY-2018-05).
Institutional Review Board Statement
All animal experiments were conducted by the guidelines and approval of the Animal Research and Ethics Committees of South China Agricultural University (#2019-0136).
Informed Consent Statement
Not applicable.
Data Availability Statement
All data discussed are contained in the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Saunders, M.A.; Good, J.M.; Lawrence, E.C.; Ferrell, R.E.; Li, W.H.; Nachman, M.W. Human adaptive evolution at Myostatin (GDF-8), a regulator of muscle growth. Am. J. Hum. Genet. 2006, 79, 1089–1097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manickam, R.; Pena, R.N.; Whitelaw, C.B. Mammary gland differentiation inversely correlates with GDF-8 expression. Mol. Reprod. Dev. 2008, 75, 1783–1788. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.L.; Huang, Y.Y.; Ho, W.K.; Poon, H.K.; Cheung, P.L.; Wai, S.O.; Chow, P.H. Growth-differentiation factor-8 (GDF-8) in the uterus: Its identification and functional significance in the golden hamster. Reprod. Biol. Endocrinol. RBE 2009, 7, 134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peiris, H.N.; Mitchell, M.D. The expression and potential functions of placental myostatin. Placenta 2012, 33, 902–907. [Google Scholar] [CrossRef]
- Buehring, B.; Binkley, N. Myostatin—The holy grail for muscle, bone, and fat? Curr. Osteoporos. Rep. 2013, 11, 407–414. [Google Scholar] [CrossRef]
- Yasaka, N.; Suzuki, K.; Kishioka, Y.; Wakamatsu, J.; Nishimura, T. Laminin binds to myostatin and attenuates its signaling. Anim. Sci. J. Nihon Chikusan Gakkaiho 2013, 84, 663–668. [Google Scholar] [CrossRef]
- McPherron, A.C.; Lawler, A.M.; Lee, S.J. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature 1997, 387, 83–90. [Google Scholar] [CrossRef]
- McPherron, A.C.; Lee, S.J. Double muscling in cattle due to mutations in the myostatin gene. Proc. Natl. Acad. Sci. USA 1997, 23, 12457–12461. [Google Scholar] [CrossRef] [Green Version]
- Hua, L.S.; Zhu, X.Y.; Rong, Z.; Yun, L.A.; Yan, D.C.; Wen, J.S.; Gang, L.M.; Qin, W.Y.; Chun, C.G. Polymorphism of Porcine Myostatin Gene. Acta Genet. Sin. 2002, 29, 326–331. [Google Scholar]
- Tay, G.K.; Iaschi, S.P.A.; Bellinge, R.H.S.; Chong, F.N.; Hui, J. The development of sequence-based-typing of myostatin (GDF-8) to identify the double muscling phenotype in the goat. Small Rumin. Res. 2004, 52, 1–12. [Google Scholar] [CrossRef]
- Gu, H.; Cao, Y.; Qiu, B.; Zhou, Z.; Deng, R.; Chen, Z.; Li, R.; Li, X.; Wei, Q.; Xia, X.; et al. Establishment and phenotypic analysis of an Mstn knockout rat. Biochem. Biophys. Res. Commun. 2016, 477, 115–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ostbye, T.K.; Galloway, T.F.; Nielsen, C.; Gabestad, I.; Bardal, T.; Andersen, O. The two myostatin genes of Atlantic salmon (Salmo salar) are expressed in a variety of tissues. Eur. J. Biochem. 2001, 268, 5249–5257. [Google Scholar] [CrossRef] [PubMed]
- Rescan, P.Y.; Jutel, I.; Ralliere, C. Two myostatin genes are differentially expressed in myotomal muscles of the trout (Oncorhynchus mykiss). J. Exp. Biol. 2001, 204 Pt 20, 3523–3529. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.Q.; Chen, S.L.; Sha, Z.X.; Liu, Y. Molecular cloning and expression analysis of the myostatin gene in sea perch (Lateolabrax japonicus). Mar. Biotechnol. 2007, 9, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.; Sun, Y.; Zhang, X.; Wan, H.; Yao, C.; Liang, K.; Li, L.; Liu, B.; Zhong, J.; Zhang, Z.; et al. Characterization of two myostatin genes in pufferfish Takifugu bimaculatus: Sequence, genomic structure, and expression. PeerJ 2020, 8, e9655. [Google Scholar] [CrossRef]
- Wu, L.; Li, Y.; Xu, Y.; Wang, L.; Ma, X.; Dong, C.; Zhao, X.; Tian, X.; Li, X.; Kong, X. The roles of two myostatins and immune effects after inhibition in Qi river crucian carp (Carassius auratus). Fish Shellfish Immunol. 2020, 98, 710–719. [Google Scholar] [CrossRef]
- Gabillard, J.C.; Biga, P.R.; Rescan, P.Y.; Seiliez, I. Revisiting the paradigm of myostatin in vertebrates: Insights from fishes. Gen. Comp. Endocrinol. 2013, 194, 45–54. [Google Scholar] [CrossRef]
- Gao, Y.; Dai, Z.; Shi, C.; Zhai, G.; Jin, X.; He, J.; Lou, Q.; Yin, Z. Depletion of Myostatin b Promotes Somatic Growth and Lipid Metabolism in Zebrafish. Front. Endocrinol. 2016, 7, 88. [Google Scholar] [CrossRef] [Green Version]
- Stinckens, A.; Georges, M.; Buys, N. Mutations in the myostatin gene leading to hypermuscularity in mammals: Indications for a similar mechanism in fish? Anim. Genet. 2011, 42, 229–234. [Google Scholar] [CrossRef]
- Helterline, D.L.; Garikipati, D.; Stenkamp, D.L.; Rodgers, B.D. Embryonic and tissue-specific regulation of myostatin-1 and -2 gene expression in zebrafish. Gen. Comp. Endocrinol. 2007, 151, 90–97. [Google Scholar] [CrossRef] [Green Version]
- Grobet, L.; Martin, L.J.; Poncelet, D.; Pirottin, D.; Brouwers, B.; Riquet, J.; Schoeberlein, A.; Dunner, S.; Menissier, F.; Massabanda, J.; et al. A deletion in the bovine myostatin gene causes the double-muscled phenotype in cattle. Nat. Genet. 1997, 17, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Kambadur, R.; Sharma, M.; Smith, T.P.; Bass, J.J. Mutations in myostatin (GDF8) in double-muscled Belgian Blue and Piedmontese cattle. Genome Res. 1997, 7, 910–916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mosher, D.S.; Quignon, P.; Bustamante, C.D.; Sutter, N.B.; Mellersh, C.S.; Parker, H.G.; Ostrander, E.A. A mutation in the myostatin gene increases muscle mass and enhances racing performance in heterozygote dogs. PLoS Genet. 2007, 3, e79. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Ouyang, H.; Xie, Z.; Yao, C.; Guo, N.; Li, M.; Jiao, H.; Pang, D. Efficient Generation of Myostatin Mutations in Pigs Using the CRISPR/Cas9 System. Sci. Rep. 2015, 5, 16623. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Fujimura, T.; Matsunari, H.; Sakuma, T.; Nakano, K.; Watanabe, M.; Asano, Y.; Kitagawa, E.; Yamamoto, T.; Nagashima, H. Efficient modification of the myostatin gene in porcine somatic cells and generation of knockout piglets. Mol. Reprod. Dev. 2016, 83, 61–70. [Google Scholar] [CrossRef]
- He, Z.; Zhang, T.; Jiang, L.; Zhou, M.; Wu, D.; Mei, J.; Cheng, Y. Use of CRISPR/Cas9 technology efficiently targetted goat myostatin through zygotes microinjection resulting in double-muscled phenotype in goats. Biosci. Rep. 2018, 38, BSR20180742. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.Y.; Hu, S.Y.; Gong, H.Y.; Chen, M.H.; Lu, J.K.; Wu, J.L. Suppression of myostatin with vector-based RNA interference causes a double-muscle effect in transgenic zebrafish. Biochem. Biophys. Res. Commun. 2009, 387, 766–771. [Google Scholar] [CrossRef]
- Whittemore, L.-A.; Song, K.; Li, X.; Aghajanian, J.; Davies, M.; Girgenrath, S.; Hill, J.J.; Jalenak, M.; Kelley, P.; Knight, A.; et al. Inhibition of myostatin in adult mice increases skeletal muscle mass and strength. Biochem. Biophys. Res. Commun. 2003, 300, 965–971. [Google Scholar] [CrossRef]
- Tang, L.; Yan, Z.; Wan, Y.; Han, W.; Zhang, Y. Myostatin DNA vaccine increases skeletal muscle mass and endurance in mice. Muscle Nerve 2007, 36, 342–348. [Google Scholar] [CrossRef]
- Nelson, C.E.; Gersbach, C.A. Engineering Delivery Vehicles for Genome Editing. Annu. Rev. Chem. Biomol. Eng. 2016, 7, 637–662. [Google Scholar] [CrossRef] [Green Version]
- Chiang, Y.A.; Kinoshita, M.; Maekawa, S.; Kulkarni, A.; Lo, C.F.; Yoshiura, Y.; Wang, H.C.; Aoki, T. TALENs-mediated gene disruption of myostatin produces a larger phenotype of medaka with an apparently compromised immune system. Fish Shellfish Immunol. 2016, 48, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Mao, D.G.; Yang, L.G.; Cao, S.X.; Zhang, Z.L.; He, X.H. Construction of expression vector of follicular inhibin and HBsAg fusion gene and expression in Hela cell. Chin. J. Immunol. 2003, 19, 775–778. [Google Scholar]
- Cao, S.X.; Yang, L.G.; Zhang, W.W.; Mao, D.G.; He, X.H. Construction and Expression of Somatostatin DNA Vaccine pEGS/2SS in Hela. Chin. J. Vet. Sci. 2005, 025, 499–502. [Google Scholar]
- Comai, G.; Tajbakhsh, S. Molecular and cellular regulation of skeletal myogenesis. Curr. Top. Dev. Biol. 2014, 110, 1–73. [Google Scholar] [PubMed]
- Buckingham, M.; Rigby, P.W. Gene regulatory networks and transcriptional mechanisms that control myogenesis. Dev. Cell 2014, 28, 225–238. [Google Scholar] [CrossRef] [Green Version]
- Hernandez-Hernandez, J.M.; Garcia-Gonzalez, E.G.; Brun, C.E.; Rudnicki, M.A. The myogenic regulatory factors, determinants of muscle development, cell identity and regeneration. Semin. Cell Dev. Biol. 2017, 72, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Acosta, J.; Carpio, Y.; Borroto, I.; González, O.; Estrada, M.P. Myostatin gene silenced by RNAi show a zebrafish giant phenotype. J. Biotechnol. 2005, 119, 324–331. [Google Scholar] [CrossRef]
- Wang, X.; Niu, Y.; Zhou, J.; Zhu, H.; Ma, B.; Yu, H.; Yan, H.; Hua, J.; Huang, X.; Qu, L.; et al. CRISPR/Cas9-mediated MSTN disruption and heritable mutagenesis in goats causes increased body mass. Anim. Genet. 2018, 49, 43–51. [Google Scholar] [CrossRef]
- Ji, S.; Losinski, R.L.; Cornelius, S.G.; Frank, G.R.; Willis, G.M.; Gerrard, D.E.; Depreux, F.F.; Spurlock, M.E. Myostatin expression in porcine tissues: Tissue specificity and developmental and postnatal regulation. Am. J. Physiol. 1998, 275 Pt 2, R1265–R1273. [Google Scholar] [CrossRef]
- Lyons, J.A.; Haring, J.S.; Biga, P.R. Myostatin expression, lymphocyte population, and potential cytokine production correlate with predisposition to high-fat diet induced obesity in mice. PLoS ONE 2010, 5, e12928. [Google Scholar] [CrossRef]
- Liu, H.; Mao, H.; Dong, X.; Cao, H.; Liu, K.; Yin, Z. Expression of MSTN gene and its correlation with pectoralis muscle fiber traits in the domestic pigeons (Columba livia). Poult. Sci. 2019, 98, 5265–5271. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; McPherron, A.C. Regulation of myostatin activity and muscle growth. Proc. Natl. Acad. Sci. USA 2001, 98, 9306–9311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, D.; Black, B.L.; Derynck, R. TGF-beta inhibits muscle differentiation through functional repression of myogenic transcription factors by Smad3. Genes Dev. 2001, 15, 2950–2966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rebbapragada, A.; Benchabane, H.; Wrana, J.L.; Celeste, A.J.; Attisano, L. Myostatin signals through a transforming growth factor beta-like signaling pathway to block adipogenesis. Mol. Cell. Biol. 2003, 23, 7230–7242. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Topouzis, S.; Liang, L.F.; Stotish, R.L. Myostatin signaling through Smad2, Smad3 and Smad4 is regulated by the inhibitory Smad7 by a negative feedback mechanism. Cytokine 2004, 26, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; McFarlane, C.; Vajjala, A.; Lokireddy, S.; Ng, Z.H.; Tan, C.K.; Tan, N.S.; Wahli, W.; Sharma, M.; Kambadur, R. Smad3 signaling is required for satellite cell function and myogenic differentiation of myoblasts. Cell Res. 2011, 21, 1591–1604. [Google Scholar] [CrossRef] [Green Version]
- Du, W. Effect of Immunization with Myostatin DNA Vaccine and Propeptide on Animal Muscle Growth. Ph.D. Thesis, Shihezi University, Shihezi, China, 2018. [Google Scholar]
- Wang, H.N.; Sun, H.X.; Zhang, Y.J.; Liu, Y.Q.; Gu, Z.H.; Shi, X.F. Effects of Interfering MSTN on Proliferation and Differentiation of Sheep Myoblasts and Expression of Related Genes. Chin. J. Anim. Vet. Sci. 2018, 49, 46–54. [Google Scholar]
- Xia, J. Study on Tissues Distribution and Preparation Technology of Avian Influenza DNA Vaccine. Master’s Thesis, Xinjiang Agricultural University, Urumqi, China, 2007. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).