Molecular Mechanisms of Changes in Homeostasis of the Dermal Extracellular Matrix: Both Involutional and Mediated by Ultraviolet Radiation
Abstract
1. Aging of Human Skin
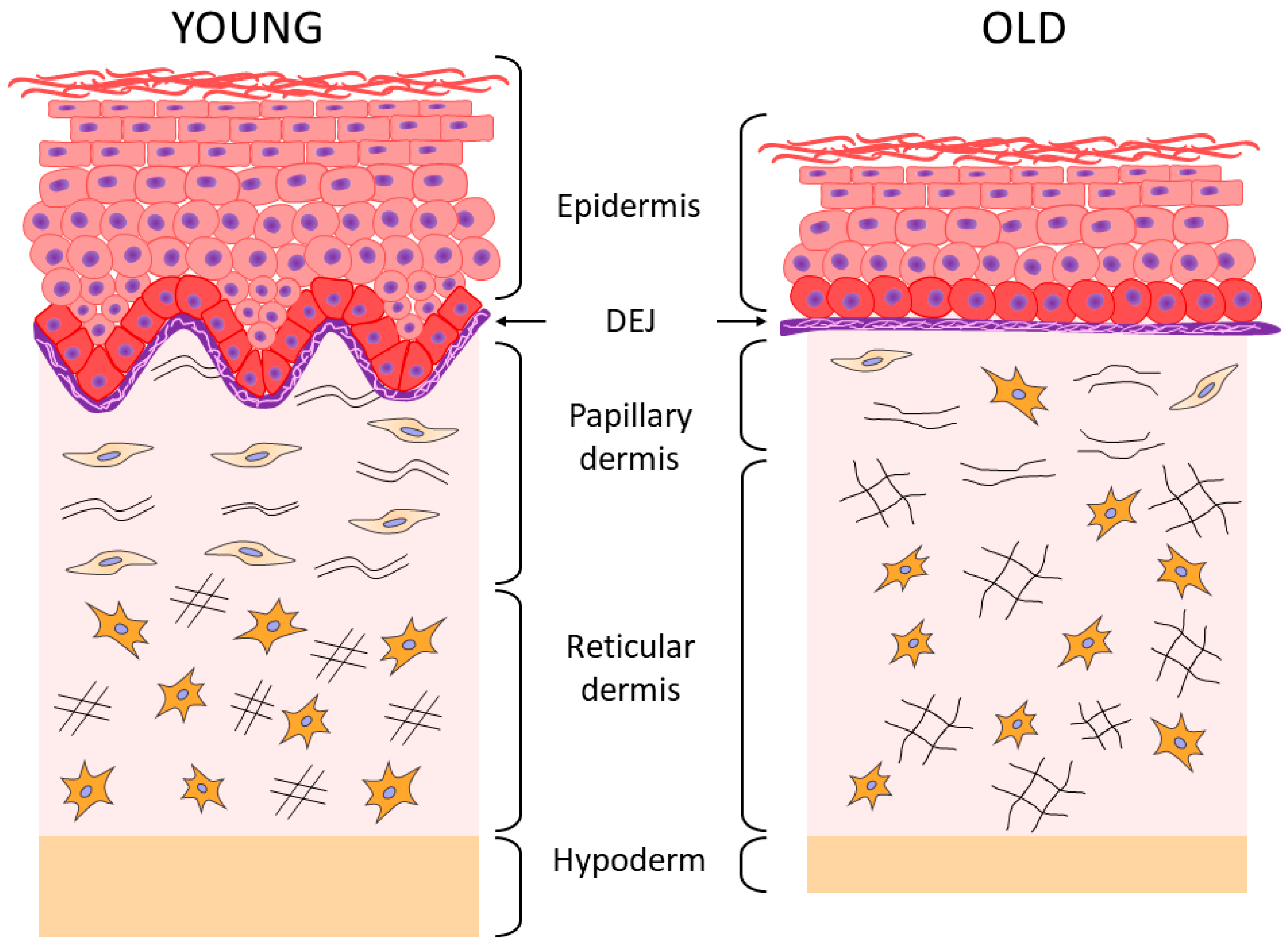
2. Aging of the Epidermis and the Dermo-Epidermal Junction
3. Aging of the Dermis
4. Aging of the Hypodermis
5. Causes of Destruction of the Extracellular Matrix in the Skin
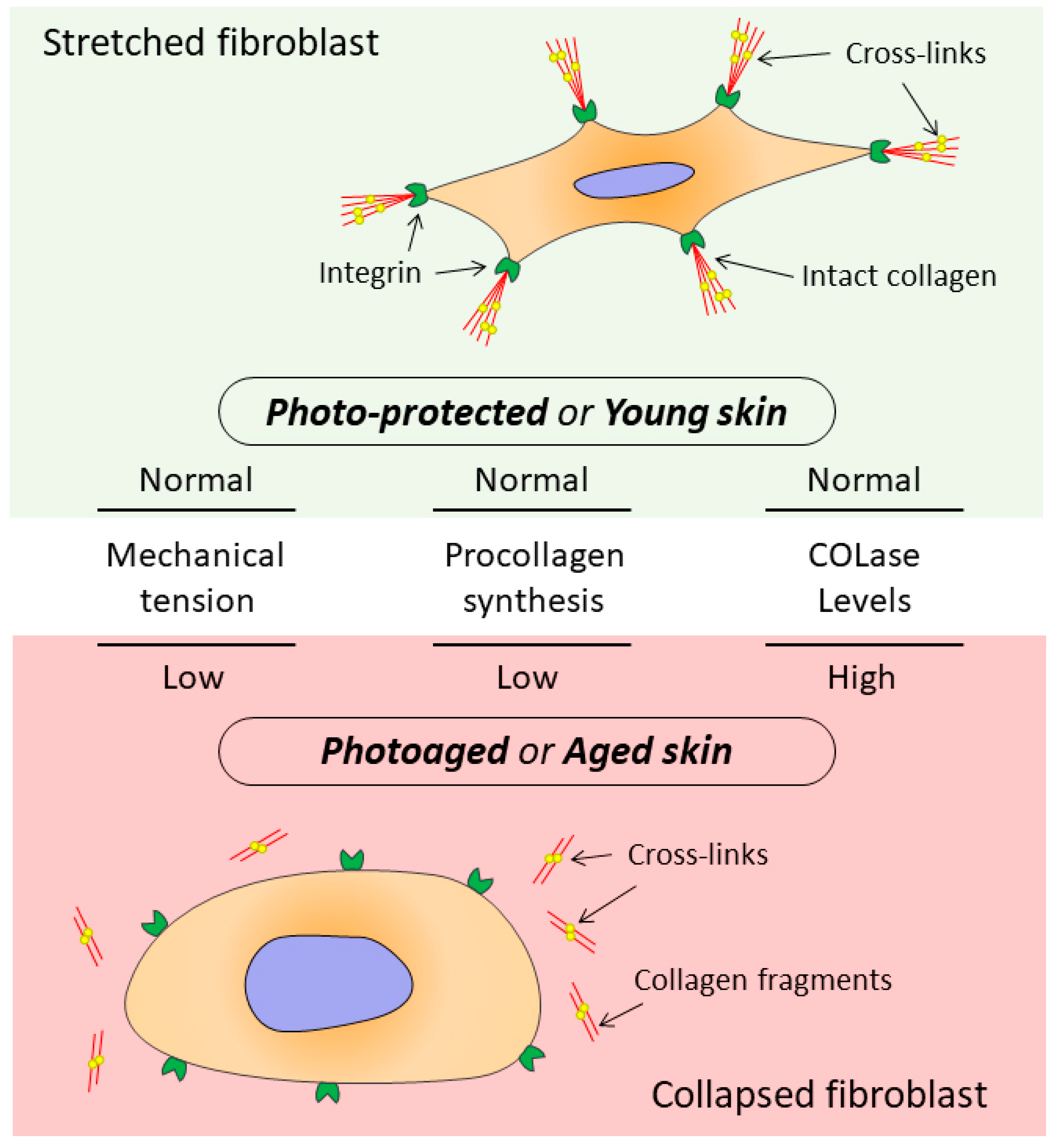
6. The Relationship between the State of Collagen Matrix and Functioning of Dermal Fibroblasts
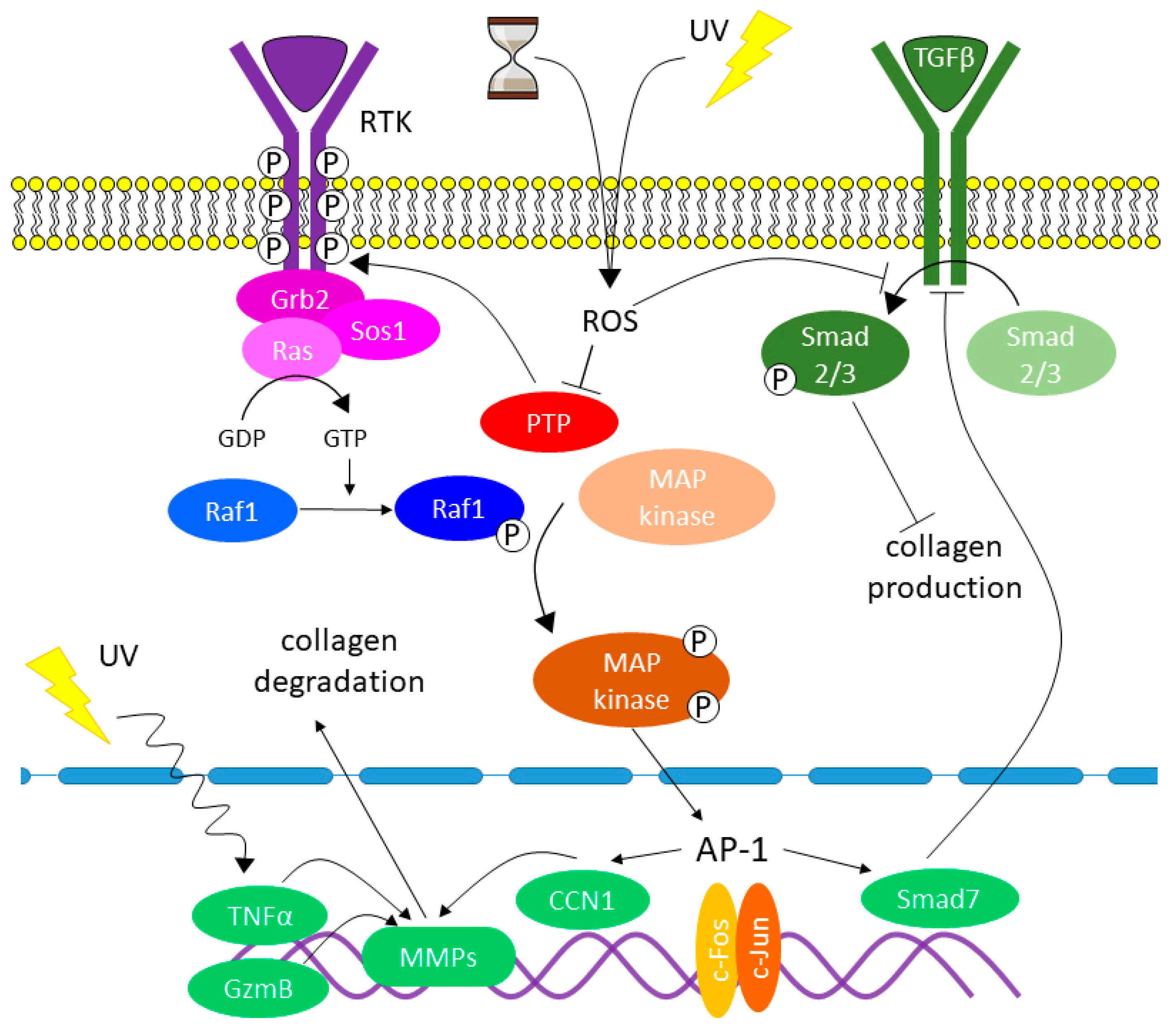
7. The Role of Oxidative Stress and Other Factors in Changing Collagen Homeostasis
- Increase in the stiffness of the dermal ECM;
- Damage of collagen in terms of its biochemical properties that are essential for the interactions between the ECM and DFs;
8. Morphological Changes in Dermal Fibroblasts Observed in the Aging Process of Dermal Collagen Matrix
9. Conclusions
Funding
Conflicts of Interest
Abbreviations
References
- Capri, M.; Salvioli, S. The genetics of human longevity. Ann. N. Y. Acad. Sci. 2006, 1067, 252–263. [Google Scholar] [CrossRef] [PubMed]
- Rittie, L.; Fisher, G.J. Natural and Sun-Induced Aging of Human Skin. Cold Spring Harb. Perspect. Med. 2015, 5, a015370. [Google Scholar] [CrossRef]
- Wang, A.S.; Dreesen, O. Biomarkers of Cellular Senescence and Skin Aging. Front. Genet. 2018, 9, 247. [Google Scholar] [CrossRef] [PubMed]
- Langton, A.K.; Graham, H.K. Ageing significantly impacts the biomechanical function and structural composition of skin. Exp. Dermatol. 2019, 28, 981–984. [Google Scholar] [CrossRef] [PubMed]
- Pedić, L.; Pondeljak, N. Recent information on photoaging mechanisms and the preventive role of topical sunscreen products. Acta Derm. APA 2020, 29, 201–207. [Google Scholar] [CrossRef]
- Zhang, W.; Qu, J. The ageing epigenome and its rejuvenation. Nat. Rev. Mol. Cell Biol. 2020, 21, 137–150. [Google Scholar] [CrossRef]
- Tewari, A.; Grage, M.M.L. UVA1 is skin deep: Molecular and clinical implications. Photochem. Photobiol. Sci. 2013, 12, 95–103. [Google Scholar] [CrossRef]
- Yaar, M.; Eller, M.S. Fifty years of skin aging. J. Investig. Dermatol. Symp. Proc. 2002, 7, 51–58. [Google Scholar] [CrossRef]
- Zhong, J.; Hua, N. A novel promising therapy for skin aging: Dermal multipotent stem cells against photoaged skin by activation of TGF-b/Smad and p38 MAPK signaling pathway. Med. Hypotheses 2011, 76, 343–346. [Google Scholar] [CrossRef]
- Naylor, E.; Watson, R. Molecular aspects of skin ageing. Maturitas 2011, 69, 249–256. [Google Scholar] [CrossRef]
- Fisher, G.; Kang, S. Mechanism of photoaging and chronological skin aging. Arch. Dermatol. 2002, 138, 1462–1467. [Google Scholar] [CrossRef] [PubMed]
- Miyamura, Y.; Coelho, S. Regulation of human skin pigmentation and responses to ultraviolet radiation. Pigment. Cell Res. 2007, 20, 2–13. [Google Scholar] [CrossRef]
- Fisher, G.; Varani, J. Looking older: Fibroblast Collapse and Therapeutic Implications. Arch. Dermatol. 2008, 144, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Varani, J.; Schuger, L. Reduced fibroblast interaction with intact collagen as a mechanism for depressed collagen synthesis in photodamaged skin. J. Investig. Dermatol. 2004, 122, 1471–1479. [Google Scholar] [CrossRef] [PubMed]
- Proshkina, E.N.; Solovev, I.A. Key Molecular Mechanisms of Aging, Biomarkers, and Potential Interventions. Mol. Biol. 2020, 54, 883–921. [Google Scholar] [CrossRef]
- Freemont, A.; Hoyland, J. Morphology, mechanisms and pathology of musculoskeletal ageing. J. Pathol. 2007, 211, 252–259. [Google Scholar] [CrossRef]
- Farage, M.; Miller, K. Characteristics of the aging skin. Adv. Wound Care 2013, 2, 5–10. [Google Scholar] [CrossRef]
- Mizukoshi, K.; Yonekura, K. Changes in dermal papilla structures due to aging in the facial cheek region. Ski. Res. Technol. 2015, 21, 224–231. [Google Scholar] [CrossRef]
- Lynch, M.D.; Watt, F.M. Fibroblast heterogeneity: Implications for human disease. J. Clin. Investig. 2018, 128, 26–35. [Google Scholar] [CrossRef]
- Langton, A.K.; Halai, P. The impact of intrinsic ageing on the protein composition of the dermal-epidermal junction. Mech. Ageing Dev. 2016, 156, 14–16. [Google Scholar] [CrossRef]
- Mine, S.; Fortunel, N. Aging Alters Functionally Human Dermal Papillary Fibroblasts but Not Reticular Fibroblasts: A New View of Skin Morphogenesis and Aging. PLoS ONE 2008, 3, e4066. [Google Scholar] [CrossRef] [PubMed]
- Gritsenko, D.A.; Orlova, O.A. Transcription factor p53 and skin aging. Adv. Gerontol. 2017, 30, 10–16. (In Russian) [Google Scholar] [CrossRef] [PubMed]
- Alonso, L.; Fuchs, E. Stem cells of the skin epithelium. Proc. Natl. Acad. Sci. USA 2003, 100, 11830–11835. [Google Scholar] [CrossRef] [PubMed]
- Dreesen, M.A.O.; Chojnowsk, A. Lamin B1 fluctuations have differential effects on cellular proliferation and senescence. J. Cell Biol. 2013, 200, 605–617. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef]
- Katoh, N.; Tennstedt, D. Gerontodermatology: The fragility of the epidermis in older adults. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 1–20. [Google Scholar] [CrossRef]
- Blume-Peytavi, U.; Kottner, J. Age-associated skin conditions and diseases: Current perspectives and future options. Gerontologist 2016, 56, 230–242. [Google Scholar] [CrossRef]
- Barrandon, Y.; Green, H. Three clonal types of keratinocyte with different capacities for multiplication. Proc. Natl. Acad. Sci. USA 1987, 84, 2302–2306. [Google Scholar] [CrossRef]
- Liu, N.; Matsumura, H. Stem cell competition orchestrates skin homeostasis and ageing. Nature 2019, 568, 344–350. [Google Scholar] [CrossRef]
- Watanabe, M.; Natsuga, K. Type XVII collagen coordinates proliferation in the interfollicular epidermis. Elife 2017, 6, e26635. [Google Scholar] [CrossRef]
- Farage, M.A.; Miller, K.W. Structural characteristics of the aging skin: A review. Cutan. Ocul. Toxicol. 2007, 26, 343–357. [Google Scholar] [CrossRef] [PubMed]
- Hendi, A. Melanocytes in nonlesional sun-exposed skin: A multicenter comparative study. J. Am. Acad. Dermatol. 2011, 65, 1186–1193. [Google Scholar] [CrossRef] [PubMed]
- Chang, A. Geriatric Dermatology Review: Major Changes in Skin Function in Older Patients and Their Contribution to Common Clinical Challenges. J. Am. Med. Dir. Assoc. 2013, 14, 724–730. [Google Scholar] [CrossRef] [PubMed]
- Zouboulis, C.; Ganceviciene, R. Aesthetic aspects of skin aging, prevention, and local treatment. Clin. Dermatol. 2019, 37, 365–372. [Google Scholar] [CrossRef]
- Luebberding, S.; Krueger, N. Age-related changes in skin barrier function—Quantitative evaluation of 150 female subjects. Int. J. Cosmet. Sci. 2013, 35, 183–190. [Google Scholar] [CrossRef]
- Meyer, L.J.; Stern, R. Age-dependent changes of hyaluronan in human skin. J. Investig. Dermatol. 1994, 102, 385–389. [Google Scholar] [CrossRef]
- Oh, J.H.; Kim, Y.K. Intrinsic aging and photoagingdependent level changes of glycosaminoglycans and their correlation with water content in human skin. J. Dermatol. Sci. 2011, 62, 192–201. [Google Scholar] [CrossRef]
- Lee, D.H.; Oh, J.-H. Glycosaminoglycan and proteoglycan in skin aging. J. Dermatol. Sci. 2016, 83, 174–181. [Google Scholar] [CrossRef]
- Makrantonaki, E.; Zouboulis, C.C. Characteristics and pathomechanisms of endogenously aged skin. Dermatology 2007, 214, 352–360. [Google Scholar] [CrossRef]
- Vazquez, F.; Palacios, S. Changes of the basement membrane and type IV collagen in human skin during aging. Maturitas 1996, 25, 209–215. [Google Scholar] [CrossRef]
- Lavker, R.M.; Zheng, P.S. Morphology of aged skin. Clin. Geriatr. Med. 1989, 5, 53–67. [Google Scholar] [CrossRef]
- Výbohová, D.; Mellová, Y. Qualitative changes of the capillary bed in aging human skin. Histol. Histopathol. 2012, 27, 961–967. [Google Scholar] [CrossRef] [PubMed]
- El-Domyati, M.; Attia, S. Intrinsic aging vs. photoaging: A comparative histopathological, immunohistochemical, and ultrastructural study of skin. Exp. Dermatol. 2002, 11, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.-W.; Kwon, S.-H. Molecular Mechanisms of Dermal Aging and Antiaging Approaches. Int. J. Mol. Sci. 2019, 20, 2126. [Google Scholar] [CrossRef] [PubMed]
- Marcos-Garces, V.; Aguilar, P.M. Age-related dermal collagen changes during development, maturation and ageing—A morphometric and comparative study. J. Anat. 2014, 225, 98–108. [Google Scholar] [CrossRef]
- Haydont, V.; Bernard, B. Age-related evolutions of the dermis: Clinical signs, fibroblast and extracellular matrix dynamics. Mech. Ageing Dev. 2019, 177, 150–156. [Google Scholar] [CrossRef]
- Baumann, L. Skin ageing and its treatment. J. Pathol. 2007, 211, 241–251. [Google Scholar] [CrossRef]
- Oh, J.H.; Kim, Y.K. Changes in glycosaminoglycans and related proteoglycans in intrinsically aged human skin in vivo. Exp. Dermatol. 2011, 5, 454–456. [Google Scholar] [CrossRef]
- Ahmed, T.; Nash, A. Combining nano-physical and computational investigations to understand the nature of aging in dermal collagen. Int. J. Nanomed. 2017, 12, 3303–3314. [Google Scholar] [CrossRef]
- Cole, M.A.; Quan, T. Extracellular matrix regulation of fibroblast function: Redefining our pe rspective on skin aging. J. Cell Commun. Signal. 2018, 12, 35–43. [Google Scholar] [CrossRef]
- Rittié, L.; Fisher, G.J. UV-light-induced signal cascades and skin aging. Ageing Res. Rev. 2002, 1, 705–720. [Google Scholar] [CrossRef]
- Varani, J.; Spearman, D. Inhibition of type I procollagen synthesis by damaged collagen in photoaged skin and by collagenase-degraded collagen in vitro. Am. J. Pathol. 2001, 158, 931–942. [Google Scholar] [CrossRef]
- Varani, J.; Dame, M.K. Decreased collagen production in chronologically aged skin: Roles of age-dependent alteration in fibroblast function and defective mechanical stimulation. Am. J. Pathol. 2006, 168, 1861–1868. [Google Scholar] [CrossRef]
- Varani, J.; Warner, R.L. Vitamin A antagonizes decreased cell growth and elevated collagen-degrading matrix metalloproteinases and stimulates collagen accumulation in naturally aged human skin. J. Investig. Dermatol. 2000, 114, 480–486. [Google Scholar] [CrossRef]
- Carrino, D.; Sorrell, J. Age-reiated changes in the proteogiycans of human skin. Arch. Biochem. Biophys. 2002, 373, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Shuster, S.; Black, M.M. The influence of age and sex on skin thickness, skin collagen and density. Br. J. Dermatol. 1975, 93, 639–643. [Google Scholar] [CrossRef]
- Baroni, E.; Biondo-Simões, M. Influence of aging on the quality of the skin of white women: The role of collagen. Acta. Cir. Bras. 2012, 27, 736–740. [Google Scholar] [CrossRef][Green Version]
- Lovel, C.R.; Smolenski, K.A. Type I and III collagen content and fibre distribution in normal human skin during ageing. Br. J. Dermatol. 1987, 117, 419–428. [Google Scholar] [CrossRef]
- Gunin, A.G.; Petrov, V.V. Age-related changes in angiogenesis in human dermis. Exp. Gerontol. 2014, 55, 143–151. [Google Scholar] [CrossRef]
- Helmbold, P.; Lautenschläger, C. Detection of a physiological juvenile phase and the central role of pericytes in human dermal microvascular aging. J. Investig. Dermatol. 2006, 126, 1419–1421. [Google Scholar] [CrossRef]
- Zorina, A.; Zorin, V. Age-Related Changes in the Fibroblastic Differon of the Dermis: Role in Skin Aging. Int. J. Mol. Sci. 2022, 23, 6135. [Google Scholar] [CrossRef] [PubMed]
- Picard, F.; Gehin, M. SRC-1 and TIF-2 control energy balance between white and brown adipose tissues. Cell 2002, 111, 931–941. [Google Scholar] [CrossRef]
- Karagiannides, I.; Tchkonia, T. Altered expression of C/EBP family members results in decreased adipogenesis with aging. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001, 280, 1772–1780. [Google Scholar] [CrossRef] [PubMed]
- Cartwright, V.; Tchkonia, T. Aging in adipocytes: Potential impact of inherent, depot-specific mechanisms. Exp. Gerontol. 2007, 42, 463–471. [Google Scholar] [CrossRef]
- Frasca, D.; Blomberg, B.B. Adipose tissue, immune aging, and cellular senescence. Semin. Immunopathol. 2020, 42, 573–587. [Google Scholar] [CrossRef] [PubMed]
- Cawthorn, W.P.; Heyd, F. Tumour necrosis factor-alpha inhibits adipogenesis via a beta-catenin/TCF4(TCF7L2)-dependent pathway. Cell Death Differ. 2007, 14, 1361–1373. [Google Scholar] [CrossRef]
- Xu, M.; Palmer, A.K. Targeting senescent cells enhances adipogenesis and metabolic function in old age. Elife 2015, 4, 12997. [Google Scholar] [CrossRef]
- Tchkonia, T.; Morbeck, D. Fat tissue, aging, and cellular senescence. Aging Cell 2010, 9, 667–684. [Google Scholar] [CrossRef]
- Mitterberger, M.C.; Lechner, S. Adipogenic differentiation is impaired in replicative senescent human subcutaneous adipose-derived stromal/progenitor cells. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 13–24. [Google Scholar] [CrossRef]
- Shapiro, S.D.; Endicott, S.K. Marked longevity of human lung parenchymal elastic fibers deduced from prevalence of D-aspartate and nuclear weapons-related radiocarbon. J. Clin. Investig. 1991, 87, 1828–1834. [Google Scholar] [CrossRef]
- Rosenbloom, J.; Abrams, W.R. Extracellular matrix 4: The elastic fiber. FASEB J. 1993, 7, 1208–1218. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.P.; Hansch, C. Matrix metalloproteinases (mmps): Chemical-biological functions and (q) sars. Bioorg. Med. Chem. 2007, 15, 2223–2268. [Google Scholar] [CrossRef] [PubMed]
- Pittayapruek, P.; Meephansan, J. Role of matrix metalloproteinases in photoaging and photocarcinogenesis. Int. J. Mol. Sci. 2016, 17, 868. [Google Scholar] [CrossRef] [PubMed]
- Sternlicht, M.D.; Werb, Z. How matrix metalloproteinases regulate cell behavior. Annu. Rev. Cell. Dev. Biol. 2001, 17, 463–516. [Google Scholar] [CrossRef]
- Fisher, G.J.; Datta, S.C. Molecular basis of sun-induced premature skin ageing and retinoid antagonism. Nature 1996, 379, 335–339. [Google Scholar] [CrossRef]
- Brennan, M.; Bhatti, H. Matrix metalloproteinase-1 is the major collagenolytic enzyme responsible for collagen damage in uv-irradiated human skin. Photochem. Photobiol. 2003, 78, 43–48. [Google Scholar] [CrossRef]
- Newby, A.C. Dual role of matrix metalloproteinases (matrixins) in intimal thickening and atherosclerotic plaque rupture. Physiol. Rev. 2005, 85, 1–31. [Google Scholar] [CrossRef]
- Nagase, H.; Visse, R. Structure and function of matrix metalloproteinases and timps. Cardiovasc. Res. 2006, 69, 562–573. [Google Scholar] [CrossRef]
- Quan, T.; Little, E. Elevated matrix metalloproteinases and collagen fragmentation in photodamaged human skin: Impact of altered extracellular matrix microenvironment on dermal fibroblast function. J. Investig. Dermatol. 2013, 133, 1362–1366. [Google Scholar] [CrossRef]
- Qin, Z.; Balimunkwe, R.M. Age-related reduction of dermal fibroblast size upregulates multiple matrix metalloproteinases as observed in aged human skin in vivo. Br. J. Dermatol. 2017, 177, 1337–1348. [Google Scholar] [CrossRef]
- Burridge, K.; Chrzanowska-Wodnicka, M. Focal adhesions, contractility, and signaling. Annu. Rev. Cell Dev. Biol. 1996, 12, 463–518. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Turnbull, J. Extracellular matrix and cell signalling: The dynamic cooperation of integrin, proteoglycan and growth factor receptor. J. Endocrinol. 2011, 209, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Silver, F.; DeVore, D. Role of mechanophysiology in aging of ECM: Effects in mechanochemical transduction. J. Appl. Physiol. 2003, 95, 2134–2141. [Google Scholar] [CrossRef] [PubMed]
- Grinnell, F. Fibroblast biology in three-dimensional collagen matrices. Trends Cell Biol. 2003, 13, 264–269. [Google Scholar] [CrossRef]
- Xu, Y.; Gurusrddappa, S. Multiple binding sites in collagen type I for the integrins a1b1 and a2b1. J. Biol. Chem. 2000, 275, 38981–38989. [Google Scholar] [CrossRef]
- Stephens, P.; Genever, P. Non-epithelial oral mucosal progenitor cell populations. Oral Dis. 2007, 13, 1–10. [Google Scholar] [CrossRef]
- Orringer, J.S.; Hammerberg, C. Molecular Effects of Photodynamic Therapy for Photoaging. Arch. Dermatol. 2008, 144, 1296–1302. [Google Scholar] [CrossRef]
- Rinnerthaler, M.; Bischof, J. Oxidative stress in aging human skin. Biomolecules 2015, 5, 545–589. [Google Scholar] [CrossRef]
- Poljsak, B.; Milisav, I. The neglected significance of “antioxidative stress”. Oxid. Med. Cell. Longev. 2012, 2012, 480895. [Google Scholar] [CrossRef]
- Schneider, L.A.; Raizner, K. Uva-1 exposure in vivo leads to an il-6 surge within the skin. Exp. Dermatol. 2017, 26, 830–832. [Google Scholar] [CrossRef]
- Wlaschek, M.; Maity, P. Connective tissue and fibroblast senescence in skin aging. J. Investig. Dermatol. 2021, 141, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Kohl, E.; Steinbauer, J. Skin ageing. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 873–884. [Google Scholar] [CrossRef] [PubMed]
- Varga, J.; Rosenbloom, J. Transforming growth factor beta (TGF-β) causes a persistent increase in steady-state amounts of type i and type iii collagen and fibronectin mrnas in normal human dermal fibroblasts. Biochem. J. 1987, 247, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Ke, Y.; Wang, X.J. Tgf-beta signaling in photoaging and uv-induced skin cancer. J. Investig. Dermatol. 2020, 141, 1104–1110. [Google Scholar] [CrossRef]
- Quan, T.; Shao, Y. Reduced expression of connective tissue growth factor (ctgf/ccn2) mediates collagen loss in chronologically aged human skin. J. Investig. Dermatol. 2010, 130, 415–424. [Google Scholar] [CrossRef]
- Chen, C.C.; Lau, L.F. Functions and mechanisms of action of CCN matricellular proteins. Int. J. Biochem. Cell Biol. 2009, 41, 771–783. [Google Scholar] [CrossRef]
- Leask, A.; Abraham, D.J. All in the CCN family: Essential matricellular signaling modulators emerge from the bunker. J. Cell Sci. 2006, 119, 4803–4810. [Google Scholar] [CrossRef]
- Quan, T.; He, T. Connective tissue growth factor: Expression in human skin in vivo and inhibition by ultraviolet irradiation. J. Investig. Dermatol. 2002, 118, 402–408. [Google Scholar] [CrossRef]
- Quan, T.; He, T. Solar ultraviolet irradiation reduces collagen in photoaged human skin by blocking transforming growth factor-b type II receptor/Smad signaling. Am. J. Pathol. 2004, 165, 741–751. [Google Scholar] [CrossRef]
- Quan, T.H.; He, T.Y. Ultraviolet irradiation induces Smad7 via induction of transcription factor AP-1 in human skin fibroblasts. J. Biol. Chem. 2005, 280, 8079–8085. [Google Scholar] [CrossRef]
- Qin, Z.; Robichaud, P. Oxidant exposure induces cysteine-rich protein 61 (CCN1) via cjun/ap-1 to reduce collagen expression in human dermal fibroblasts. PLoS ONE 2014, 9, e115402. [Google Scholar] [CrossRef]
- Quan, T.H.; He, T.Y. Elevated cysteine-rich 61 mediates aberrant collagen homeostasis in chronologically aged and photoaged human skin. Am. J. Pathol. 2006, 169, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Quan, T.H.; Qin, Z.P. Cysteine-rich protein 61 (CCN1) mediates replicative senescence-associated aberrant collagen homeostasis in human skin fibroblasts. J. Cell Biochem. 2012, 113, 3011–3018. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.I.; Kiyono, K. Regulation of autophagy by transforming growth factor-beta (Tgf-beta) signaling. Autophagy 2010, 6, 645–647. [Google Scholar] [CrossRef]
- Jeong, D.; Qomaladewi, N.P. The role of autophagy in skin fibroblasts, keratinocytes, melanocytes, and epidermal stem cells. J. Investig. Dermatol. 2020, 140, 1691–1697. [Google Scholar] [CrossRef]
- Kalfalah, F.; Janke, L. Crosstalk of clock gene expression and autophagy in aging. Aging 2016, 8, 1876–1895. [Google Scholar] [CrossRef]
- Parkinson, L.G.; Toro, A. Granzyme B mediates both direct and indirect cleavage of extracellular matrix in skin after chronic low-dose ultraviolet light irradiation. Aging Cell 2015, 14, 67–77. [Google Scholar] [CrossRef]
- Dimri, G.P.; Lee, X. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. USA 1995, 92, 9363–9367. [Google Scholar] [CrossRef]
- Singh, K.; Maity, P. Superoxide anion radicals induce igf-1 resistance through concomitant activation of ptp1b and pten. EMBO Mol. Med. 2015, 7, 59–77. [Google Scholar] [CrossRef]
- Chen, J.-H.; Lin, X. Role of advanced glycation end products in mobility and considerations in possible dietary and nutritional intervention strategies. Nutr. Metab. 2018, 15, 72. [Google Scholar] [CrossRef]
- Fournet, M.; Bonte, F. Glycation damage: A possible hub for major pathophysiological disorders and aging. Aging Dis. 2018, 9, 880–900. [Google Scholar] [CrossRef] [PubMed]
- Ott, C.; Jacobs, K. Role of advanced glycation end products in cellular signaling. Redox. Biol. 2014, 2, 411–429. [Google Scholar] [CrossRef] [PubMed]
- Reigle, K.; Di Lullo, G. Non-enzymatic glycation of type I collagen diminishes collagen-proteoglycan binding and weakens cell adhesion. J. Cell Biochem. 2008, 104, 1684–1698. [Google Scholar] [CrossRef] [PubMed]
- Segel, M.; Neumann, B. Niche stiffness underlies the ageing of central nervous system progenitor cells. Nature 2019, 573, 130e4. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lollis, E.M. Matrix stiffness regulates vascular integrity through focal adhesion kinase activity. FASEB J. 2019, 33, 1199e208. [Google Scholar] [CrossRef] [PubMed]
- Fisher, G.J.; Quan, T. Collagen fragmentation promotes oxidative stress and elevates matrix metalloproteinase-1 in fibroblasts in aged human skin. Am. J. Pathol. 2009, 174, 101–114. [Google Scholar] [CrossRef]
- Sole’-Boldo, L.; Raddatz, G. Single-cell transcriptomes of the human skin reveal age-related loss of fibroblast priming. Commun. Biol. 2020, 3, 188. [Google Scholar] [CrossRef]
- Driskell, R.R.; Watt, F.M. Understanding fibroblast heterogeneity in the skin. Trend Cell Biol. 2015, 25, 92–99. [Google Scholar] [CrossRef]
- Zouboulis, C.; Adjaye, J. Human skin stem cells and the ageing process. Exp. Gerontol. 2008, 43, 986–997. [Google Scholar] [CrossRef]
- Wong, T.Y.; Solis, M.A. Molecular mechanism of extrinsic factors affecting antiaging of stem cells. World J. Stem. Cells 2015, 7, 512–520. [Google Scholar] [CrossRef]
- Tümpel, S.; Rudolph, K.L. Quiescence: Good and Bad of Stem Cell Aging. Trends Cell Biol. 2019, 29, 672–685. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, G. Molecular mechanisms of skin ageing. Mechanisms of Ageing and Development. Mech. Ageing Dev. 2002, 123, 801–810. [Google Scholar] [CrossRef]
- Gasek, N.S.; Kuchel, G.A. Strategies for targeting senescent cells in human disease. Nat. Aging 2021, 1, 870–879. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Tchkonia, T. “The Achilles’ heel of senescent cells: From transcriptome to senolytic drugs”. Aging Cell 2015, 14, 644–658. [Google Scholar] [CrossRef]
- Childs, B.G.; Gluscevic, M. Senescent cells: An emerging target for diseases of ageing. Nat. Rev. Drug Discov. 2017, 16, 718–735. [Google Scholar] [CrossRef]
- Fafián-Labora, J.A.; O’Loghlen, A. NF-κB/IKK activation by small extracellular vesicles within the SASP. Aging Cell 2021, 20, e13426. [Google Scholar] [CrossRef]
- Di Micco, R.; Krizhanovsky, V. Cellular senescence in ageing: From mechanisms to therapeutic opportunities. Nat. Rev. Mol. Cell Biol. 2021, 22, 75–95. [Google Scholar] [CrossRef]
- Fedintsev, A.; Moskalev, A. Stochastic non-enzymatic modification of long-lived macromolecules—A missing hallmark of aging. Age Res. Rev. 2020, 62, 101097. [Google Scholar] [CrossRef]

| Chronological Aging | Photoaging | |
|---|---|---|
| HA in theepidermis | ↓ amount | ↓ amount |
| HA in the derma | amount not changed ↓ bioavailability | ↑ amount ↓ length |
| Total content of sulphated GAGs | ↓ amount | ↑ amount |
| Versican | ↓ mRNA expression ↑ amount in males | ↓ mRNA expression ↑ amount in the solar elastosis area |
| Decorin | ↓ mRNA expression ↓ amount ↓ size | ↓ amount in the solar elastosis area |
| Biglycan | ↓ mRNA expression ↓ amount | ↓ amount |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zorina, A.; Zorin, V.; Kudlay, D.; Kopnin, P. Molecular Mechanisms of Changes in Homeostasis of the Dermal Extracellular Matrix: Both Involutional and Mediated by Ultraviolet Radiation. Int. J. Mol. Sci. 2022, 23, 6655. https://doi.org/10.3390/ijms23126655
Zorina A, Zorin V, Kudlay D, Kopnin P. Molecular Mechanisms of Changes in Homeostasis of the Dermal Extracellular Matrix: Both Involutional and Mediated by Ultraviolet Radiation. International Journal of Molecular Sciences. 2022; 23(12):6655. https://doi.org/10.3390/ijms23126655
Chicago/Turabian StyleZorina, Alla, Vadim Zorin, Dmitry Kudlay, and Pavel Kopnin. 2022. "Molecular Mechanisms of Changes in Homeostasis of the Dermal Extracellular Matrix: Both Involutional and Mediated by Ultraviolet Radiation" International Journal of Molecular Sciences 23, no. 12: 6655. https://doi.org/10.3390/ijms23126655
APA StyleZorina, A., Zorin, V., Kudlay, D., & Kopnin, P. (2022). Molecular Mechanisms of Changes in Homeostasis of the Dermal Extracellular Matrix: Both Involutional and Mediated by Ultraviolet Radiation. International Journal of Molecular Sciences, 23(12), 6655. https://doi.org/10.3390/ijms23126655







